Abstract
Importance:
While it has long been known that polycystic ovarian syndrome (PCOS) is associated with cardiometabolic risk factors, there is emerging evidence that other benign gynecologic conditions, such as uterine leiomyomas, endometriosis, and even hysterectomy without oophorectomy, can be associated with cardiometabolic risk factors. Understanding the evidence and mechanisms of these associations can lead to novel preventive and therapeutic interventions.
Objective:
This article discusses the evidence and the potential mechanisms mediating the association between cardiometabolic risk factors and benign gynecologic disorders.
Evidence acquisition:
We reviewed PubMed, Embase, Scopus, and Google Scholar databases to obtain plausible clinical and biological evidence, including hormonal, immunologic, inflammatory, growth factor-related, genetic, epigenetic, atherogenic, vitamin D-related, and dietary factors.
Results:
Cardiometabolic risk factors appear to contribute to uterine leiomyoma pathogenesis. For example, obesity can modulate leiomyomatous cellular proliferation and extracellular matrix deposition through hyperestrogenic states, chronic inflammation, insulin resistance, and adipokines. On the other hand, endometriosis has been shown to induce systemic inflammation, thereby increasing cardiometabolic risks, for example, through inducing atherosclerotic changes.
Conclusion and relevance:
Clinical implications of these associations are twofold. First, screening and early modification of cardiometabolic risk factors can be part of a preventive strategy for uterine leiomyomas and hysterectomy. Second, patients diagnosed with uterine leiomyomas or endometriosis can be screened and closely followed for cardiometabolic risk factors and cardiovascular disease.
Keywords: Uterine leiomyomas, fibroids, endometriosis, benign gynecologic disorders, cardiometabolic risk factors, hysterectomy, gynecology
1. Background:
Cardiometabolic risk factors (CMRFs), including obesity, hypertension, diabetes mellitus, and hyperlipidemia, stand as major contributors to atherosclerosis, ischemic heart disease, strokes, and certain cancers. Their high prevalence renders them a tremendous cause of morbidity and mortality among people of different races and backgrounds. The metabolic syndrome (MetS) is a constellation of cardiometabolic risk factors and signifies individuals at a high risk of developing cardiovascular events.
While it has long been known that polycystic ovarian syndrome (PCOS) is associated with CMRFs, there is emerging evidence that other benign gynecologic disorders (BGDs), such as uterine leiomyomas, endometriosis, and hysterectomy without oophorectomy, may be associated with cardiometabolic risk. While it is thought that CMRFs may contribute to leiomyoma pathogenesis, there is evidence that endometriosis may lead to increased cardiovascular risk (figure 1). However, it remains unclear if these associations are causal. Therefore, further experimental research is necessary to delineating the nature of this relationship. This article will discuss the evidence of association between cardiometabolic risk and benign gynecologic disorders, the underlying mechanisms, and the implications on healthcare. An overview of the biological mechanisms that underlie the association between CMRFs and BGDs is presented in figure 2.
Figure 1.
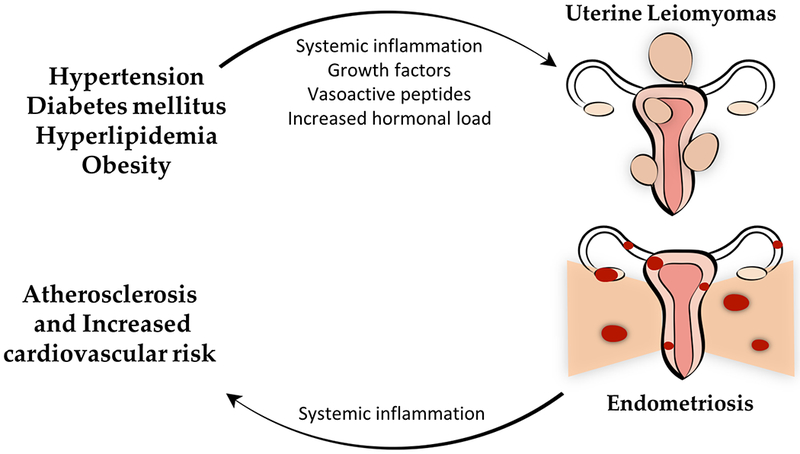
Schematic presentation of the hypothesized reciprocal relationship between cardiometabolic risk factors (CMRFs) and benign gynecologic disorders (BGDs): uterine leiomyomas and endometriosis.
Figure 2.

Overview of biological mechanisms underlying the association between CMRFs and BGDs.
2. Pathophysiologic Considerations:
2.1. Overview of Cardiometabolic risk factors:
Obesity is one of the well-recognized risk factors for cardiovascular disease. Recent evidence proposes that it is not merely the presence of excessive fatty tissue per se, but also the type of fat that determines such risk.(1) Obesity is categorized into metabolically healthy obesity (MHO) and metabolically unhealthy obesity (MUO).(1) This classification arises from the difference of type and distribution of body fat. For example, visceral adipose tissue (VAT) and intramuscular fat are the major contributors to obesity-induced systemic inflammation, insulin resistance, and increased cardiovascular risk.(1) From another perspective, fat can be classified as white adipose tissue (WAT) and brown adipose tissue (BAT).(2) While WAT increases cardiovascular and metabolic complications of obesity, BAT, on the other hand, specializes in thermogenesis, and in fact, is associated with improved cardiovascular health.(3)
2.2. Hormonal/endocrine:
Female steroid hormones have been classically implicated in the pathogenesis of multiple gynecologic disorders and were introduced as one mechanism that connects CMRFs and BGDs.(1) On one hand, obesity contributes to this association by increasing the hormonal burden in the obese female.(4) Peripheral adipose tissue can convert androgenic substrates to estrogens, a process mediated by the aromatase enzyme and hence, termed aromatization(1) (Figure 3). This conversion rate is higher in the female adipose tissue, where fibroblasts play a vital role in promoting aromatase expression.(4) Interestingly, obesity-induced inflammation, which will be separately discussed in this review, enhances the expression of aromatase by tumor necrosis factor alpha (TNF-α).(5) It is worth noting, however, that aromatase is expressed at higher levels in the adipose tissue of postmenopausal compared to premenopausal women, who in contrast, rely mostly on their ovarian follicles for aromatization and estrogen production.(4) Therefore, the obesity-mediated role in increasing the bodily hormonal load might be more pronounced in the postmenopausal female.(4)
Figure 3.
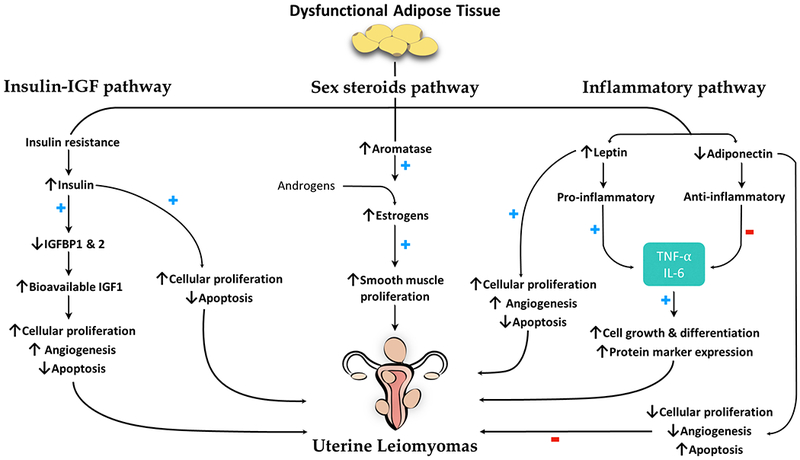
Schematic presentation of the proposed mechanistic pathways mediating the effects of obesity on development of uterine leiomyomas. ↑ and ↓ denote increased or decreased level or function, respectively. + and − denote enhancing or suppressing effect, respectively. IGF1, insulin-like growth factor I; IGFBP, IGF-binding protein; IL, interleukin; TNF, tumor necrosis factor.
Uterine leiomyomas have been well-known to be hormone-responsive tumors, where estrogen and progesterone receptors exist in abundance. This is evidenced by their growth during reproductive years and pregnancy and tendency to regress after menopause.(6) Estrogen binds to its receptors, both nuclear and membrane-bound, and initiates a series of cellular events that culminate in enhanced smooth muscle proliferation in the leiomyoma.(7) In the most part, nuclear estrogen receptors (ERs), both ERα and ERβ, mediate transcriptional activity, while membrane-bound receptors participate in rapid signaling.(8) As opposed to normal myometrium, leiomyomas overexpress ERα and ERβ mRNA,(9) and ERα can be epigenetically modified in these tumors,(10) suggesting an enhanced but aberrant effect of estrogen on leiomyomas that may be further augmented in the obese female.
Similarly, endometriosis is another estrogen-dependent gynecologic disorder, i.e., estrogen facilitates the maintenance and progression of the ectopic endometrial tissue.(11) Intriguingly, estrogen and progesterone can additionally recruit stem cells that are derived from the bone marrow to the ectopic endometrium enhancing vasculogenesis, and eventually, thriving of the endometrial implants.(12) Theoretically, obesity should amplify the role of estrogen in endometriosis; however, studies detected an inverse relationship between BMI and stage of endometriosis, possibly suggesting the presence of other pathogenic mechanisms that could counteract the role of ovarian steroids.(13) Indeed, Goetz et al showed that endometriosis causes metabolic changes and weight loss in an animal model.(14)
Besides the effect of obesity on sex hormones, adiposity is also associated with insulin resistance. In fact, BMI and serum insulin levels share a positive correlation, consequently promoting a hyperinsulinemic state in the obese population.(1) Not only does insulin resistance predispose to diabetes mellitus, but the ensuing hyperinsulinemia additionally exerts growth-promoting properties(15) both directly and indirectly.(16) The indirect effects of insulin on cellular growth are termed the insulin-IGF hypothesis, which states that chronically elevated insulin levels can downregulate the production of insulin-like growth factor binding proteins 1 and 2 (IGFBP-1 and IGFBP-2).(1) Normally, IGFBP-1 and IGFBP-2 bind to insulin-like growth factor 1 (IGF-1) and decrease its bioavailability.(1) In the absence of this action, bioactive IGF-1 levels rise with a resultant increase in its effects on cellular mitogenesis(1) (Figure 3). Despite having no clear evidence of an association between uterine fibroids and insulin levels, and some studies, in fact, reporting an inverse association between the two,(17) IGF-1 remains as an important contributor to the pathobiology of uterine fibroids(18), which may propose an indirect role of insulin in these tumors mediated by IGF-1. Nevertheless, more experimental research needs to validate this postulated association and its biologic aspects.
2.3. Inflammation:
CMRFs promote an inflammatory milieu both locally and systemically(19), which heightens an individual’s risk of developing disorders, including cancers.(1) Inflammatory states were also demonstrated to have a role in the development of some BGDs such as uterine leiomyomas.(20) Obesity is one famous example of an inflammation-inducing CMRF(1) (Figure 3). In overweight and obese individuals, adipose tissue exerts paracrine and endocrine actions by secreting a variety of signaling molecules, including pro-inflammatory cytokines and adipokines,(4) with different mediators having different effects on promoting and suppressing inflammation.(1) On the long term, obesity is a major contributor to chronic systemic inflammation, supported by elevated levels of pro-inflammatory cytokines, TNF-α and interleukin 6 (IL-6) in particular, detected in the sera of obese patients.(21)
In vitro studies have shown that TNF-α has a role in regulating leiomyoma cell differentiation. Nair et al (20) showed increased proliferation of human leiomyoma cells cultured in adipocyte-conditioned media or co-cultured with human adipocytes. They also found increased expression of the pro-proliferative protein marker PCNA, anti-apoptosis protein BCL-2, and cell cycle division protein cyclin D1. Additionally, they demonstrated increased proliferation of human leiomyoma cells exposed to higher concentrations of TNF-α and reversal of these effects on adding anti-TNF-α-neutralizing antibodies.(20)
Similarly, endometriosis is associated with systemic inflammation. Iwabe et al have shown that women with endometriosis had a significantly higher concentration of TNF-α and IL-6 in their peritoneal fluid compared to women without endometriosis.(22) Moreover, they concluded in another experiment that TNF-α stimulated the proliferation of stromal endometriotic cells by inducing IL-8 expression, an effect that was reversed by adding anti-TNF-α and anti-IL-8 antibodies.(23) In line with these findings, Pizzo et al detected significantly higher levels of TNF-α in the sera of women with endometriosis, particularly in the initial stages, supporting the role of inflammatory mediators early on in the disease process.(24) Endometriosis is thought to create a state of systemic inflammation that can promote the development of atherosclerotic lesions (Figure 1). Therefore, endometriosis can be considered a cardiovascular risk factor.(25) Systemic inflammation may contribute to atherosclerosis directly by injuring the endothelium and indirectly by inducing insulin resistance and lipid derangements(26) (Figure 4). However, whether the association between endometriosis and cardiovascular risk is causal needs to be further investigated.
Figure 4.
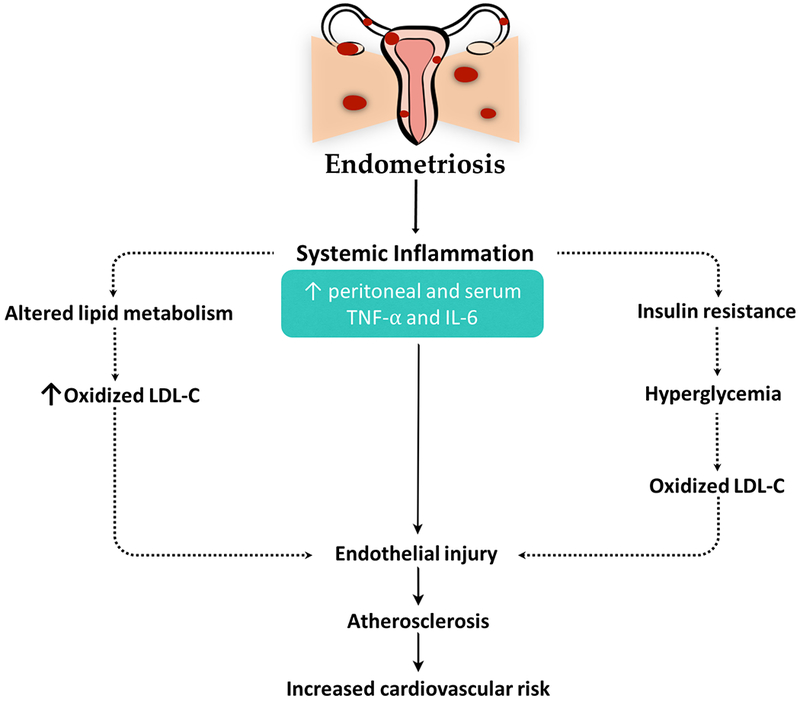
Schematic presentation of the proposed mechanistic pathways mediating the effects of endometriosis on cardiovascular risk. Dotted lines denote indirect actions. IL, interleukin; LDL-C, low density lipoprotein cholesterol; TNF, tumor necrosis factor.
Adipocytes specialize in secreting many polypeptide hormones termed adipokines, of which leptin and adiponectin are of importance when addressing obesity-related inflammation.(1) Leptin, in addition to being involved in appetite suppression, has marked pro-inflammatory properties and is found at higher serum concentrations in obese people.(1) Interestingly, leptin promotes cellular proliferation and angiogenesis and suppresses apoptosis and immune function, features that are collectively found in neoplastic processes.(1) On the contrary, obesity is accompanied by lower serum adiponectin with levels negatively correlating with BMI.(27) As opposed to leptin, adiponectin manifests anti-inflammatory properties and sensitizes cells to insulin, actions that can indirectly suppress neoplastic phenomena.(1) Besides, adiponectin can directly mediate this suppression by sequestering growth factors or binding to adiponectin receptors 1 and 2.(1) This culminates in decreased fatty acid synthesis, cellular proliferation, and DNA mutagenesis as well as increased apoptosis.(1)
Markowska et al (28) have shown that leptin genes and leptin proteins were expressed in uterine leiomyomas and surrounding myometrium but were absent in the myometrium of women without leiomyomas. Chen et al (29) found that while serum adiponectin levels inversely correlated with BMI in women with and without leiomyomas, serum adiponectin was significantly lower among women with leiomyomas.
2.4. Genetics:
Recent studies in certain racial groups identified single nucleotide polymorphisms (SNPs) across variable chromosomal regions that are associated with uterine leiomyomas. Of importance, some of these SNPs take place in genes that are also associated with cardiovascular disease states.(30) For example, the gene oligonucleotide/oligosaccharide-binding fold containing 1 (OBCFC1) is implicated in cardiovascular disease, while the gene sirtuin 3 (SIRT3) is involved in MetS, obesity, and exercise response.(30) Additionally, the gene blocked early in transport 1 homolog (BET1L) plays a role in glucose regulation and type 2 diabetes mellitus.(30)
Surgically-induced endometriosis in female mice was shown to dysregulate six hepatic genes involved in metabolism.(14) This dysregulation of gene expression could possibly explain the association between endometriosis and low BMI of affected females.(14) Four anorexigenic genes, Cyp2r1, Favp4, Mrc1, and Rock2, were upregulated in mice with endometriosis, while two obesogenic genes, Igfbp1 and Mmd2, were downregulated.(14) The mechanism by which endometriosis mediates its aberrant effect on hepatic genes remains unclear. However, it has been postulated that endometrial implants could possibly migrate to the liver by means of lymphatic, vascular, or transcoelomic peritoneal spread(31), directly affecting hepatic gene expression.(14) In contrast, endometriosis induces a state of peritoneal inflammation that can alter the regulation of hepatic genes (14), possibly by means of differentially-expressed circulating microRNAs found in women with endometriosis.(32)
2.5. Epigenetics:
Research has shown that there may be epigenetic associations between CMRFs and BGDs. An example that was extensively discussed is the Sp/Krüppel-like factor (KLF) family of transcription factors, which is involved in regulating genes of CYP metabolic enzymes.(33) These enzymes function to metabolize estrogen and progesterone in the endometrium, producing biologically active metabolites that play important roles in endometrial physiology.(34) Once targeted by KLF transcription factors, CYP enzymes genes can be silenced, an action that is mediated by deacetylating promoter histones, rendering transcription nonpermissible.(33)
Interestingly, Yin and colleagues(35) have found that knockdown of KLF11 was associated with increased proliferation of human leiomyoma smooth muscles, suggesting a possible protective role of this transcription factor against neoplastic growths of the myometrium. Another study done by Daftary et al (36) has concluded that KLF11 expression was drastically decreased in endometrial implants of mice with surgically-induced endometriosis, with KLF11 knockout animal models manifesting larger and densely-adhesive lesions resembling advanced human endometriosis.
Of great importance to this review, mutations in KLF11 were additionally involved in the development of human diabetes by dysregulation of insulin and glucose transport.(37) KLF11 is normally expressed in human pancreatic islets and beta cells, and in response to high serum glucose, KLF mRNA expression increases in pancreatic beta cells.(38) Functioning as a transcription factor, glucose-inducible KLF11 binds to the insulin promoter, up-regulating its levels and maintaining glucose homeostasis. In addition, a frequent polymorphic Q62R variant was identified in the KLF11 gene and was significantly associated with late-onset type 2 diabetes mellitus in people of northern-European ancestry.(38)
2.6. Diet:
Some studies have suggested an association of diet with certain BGDs. Some dietary components, namely fatty acids, can potentiate the levels of inflammatory mediators,(39) which happen to be elevated in patients with endometriosis(40) as this review previously addressed. Concomitantly, these dietary factors are found in close relation to certain CMRFs, including hyperlipidemia.(41) Prazzini et al (42) have inferred that endometriosis is positively associated with red meat consumption but negatively so with green vegetable and fruit consumption. In another study, women classified in the fifth quintile of animal fat intake demonstrated a 20% greater risk of having endometriosis when compared to those in the first quintile.(43) The same study additionally provided evidence of increased risk of endometriosis with trans-unsaturated fats and palmitic acid, a type of saturated fat found in animal products, but not with saturated and monosaturated fat, the major components of animal fat. In contrast, other dietary constituents were of possible protective effects against endometriosis. Women consuming each additional 1% of energy from long-chain omega-3 fatty acids rather than from trans fats demonstrated a 50% lower risk of endometriosis.(43)
At a cellular level, trans fatty acids can mediate downregulation of peroxisome-proliferator activated receptor-γ (PPAR-γ) expression.(44) Lebovic et al (44) concluded that this inhibitory action can promote regression of surgically-induced endometriosis in female rats. Furthermore, trans fatty acids elevate the serum levels of inflammatory mediators, such as IL-6 and markers of TNF- α activation, which are considered possible contributors to the pathogenesis of endometriosis.(39)
Relating to cardiovascular risk, a meta-analysis that included six observational studies concluded that adding 50 g serving/day of processed red meats correlated with a 42% higher risk (RR 1.42) of cardiovascular events.(45) Moreover, Skeaff and Miller(46) showed in their meta-analysis that trans fatty acid intake significantly correlated with a higher risk of cardiovascular morbidity and mortality.
2.7. Vitamin D:
Besides the numerous physiological functions of vitamin D, experimental studies demonstrated anti-tumor properties of vitamin D and its metabolites.(47) In the light of this review, vitamin D deficiency correlates with a higher risk of uterine leiomyomas.(48) This association is reinforced by the observation that uterine leiomyomas are more prevalent among African American females, who concomitantly, are known to have lower serum levels of vitamin D.(49) Furthermore, a study by Halder and colleagues (50) showed that vitamin D receptors were less expressed in 60% of fibroids compared to the normal myometrium, implying an increased risk of uterine leiomyomas with decreased vitamin D-mediated cellular signaling. Similarly, women with endometriosis were found to have lower serum vitamin D levels compared to healthy women, which can be possibly explained by the loss of the anti-proliferative actions of vitamin D, promoting the progression of endometriosis.(51)
Vitamin D is additionally implicated in cardiovascular disease.(52) Kar and Datta have demonstrated that patients with systolic-diastolic hypertension have lower serum levels of vitamin D compared to non-hypertensive individuals.(53) In line with this observation, higher serum vitamin D levels correlated with lower plasma renin activity, indicating a role for vitamin D in regulating the renin-angiotensin-aldosterone system.(54) In addition, among type 2 diabetics, a positive correlation was detected between vitamin D deficiency and Framingham score, which is used to assess cardiovascular risk, suggesting a protective role for vitamin D against cardiovascular disease.(55)
From a physiological perspective, vitamin D suppresses inflammatory responses and downregulates the production of pro-inflammatory cytokines, including TNF- α and IL-6, which are known to participate greatly in developing atherosclerosis and increasing cardiovascular risk on one hand,(56) and promoting cellular growth and differentiation on another.(4)
2.8. Growth factors:
Several growth factors have been implicated in the pathobiology of BGDs, including uterine leiomyomas. A well-recognized example is insulin-like growth factors (IGFs), which have mitogenic properties that contribute significantly to myomatous proliferation(57) (Figure 3). Both IGF-1 and IGF-2 share this association with uterine fibroids, with the former having more prominent effects as evidenced by studies.(58) In a study done by Burroughs et al,(59) IGF-1 was shown to be 7.5 times more expressed in uterine leiomyomas compared to normal tissues of Eker rats. In addition, Peng and colleagues(58) concluded a correlation between overexpression of IGF-1 and size of the leiomyoma, indicating dysregulated signaling of IGF-1 in these tumors.
Epidermal growth factor (EGF), another presumed contributor to the pathobiology of uterine fibroids, was shown to upregulate protein synthesis in both myomatous and normal myometrial cells.(60) In their study, Ren and colleagues(61) documented an increased EGF-mediated stimulation of DNA synthesis in leiomyomas compared to normal myometrial cells. Binding of EGF to its receptor (EGFR) results in distinctive signaling cascades between leiomyomas and the normal myometrium despite equal expression of EGFR.(61) This differential signal transmission might point to aberrancy of EGF signaling in leiomyomatous growths.(61) In an experimental study by Park et al (62), hyperglycemic milieus were shown to create epigenetic alterations in some oncogenic pathways, including the EGFR pathway, thereby promoting neoplastic activity. However, this finding has been documented in mice with breast cancer, and more research is warranted to further investigate the effect of diabetes-associated hyperglycemia in benign tumors such as uterine leiomyomas.
IGF-1 was shown to share a significant positive association with severity of coronary artery disease, as assessed by Gensini score, which is determined by the degree of coronary luminal narrowing and location.(63) These observations could be explained by the actions of IGF-1 on the vascular smooth muscles, which as a result, show enhanced proliferation and migration into the intima, predisposing to higher risk of atherosclerotic vessel disease.(64) Association of EGF with cardiovascular disease was similarly addressed in the literature.(65) EGFR and its ligands, which are found in vascular smooth muscles and endothelial cells, modulate several functions that can predispose to atherosclerosis, including cellular proliferation, differentiation, and inflammation.(65) In fact, they are highly expressed in vascular smooth muscles of intimal atherosclerotic lesions(66) and are implicated in vascular dysfunction associated with diabetes mellitus.(67)
2.9. Atherogenic hypothesis:
A body of evidence suggests that atherosclerosis and uterine leiomyomas may possibly share common pathogenic features along their development.(68) Atherosclerotic plaques are primarily composed of smooth muscles that have proliferated and migrated from the vascular media following intimal injury.(68) When compared to atherosclerotic plaques, uterine leiomyomas similarly represent a proliferating population of smooth muscles that originally resides in the uterine myometrium, creating a plausible analogy between the two.(68) Furthermore, experiments that analyzed the components of coronary plaques concluded a high possibility of their monoclonality, an inherent feature of benign smooth muscle tumors such as uterine leiomyomas.(69) Intriguingly, cells from both atheromatous plaques and uterine leiomyomas showed identical behavior when cultured in vitro.(69) Additionally, both atheromatous plaques and uterine leiomyomas can undergo fibrosis and calcification on the long run.(68) Aksoy and colleagues(70) have, in fact, concluded that carotid intima-media thickness, a reliable indicator for atherosclerosis, significantly differed among patients with and without uterine leiomyomas.
2.10. Immune mechanisms:
The immune system is thought to contribute, in part, to the pathogenesis of some BGDs. Initial lesions of endometriosis are associated with activation of the innate immune system following retrograde menstruation.(71) This is supported by documenting an increased number of innate immune cells, including macrophages and natural killer cells, in the peritoneal fluid of patients with endometriosis as well as in the lesions themselves.(72) These immune cells are rather dysfunctional and in fact, considered contributors to the progression of endometriosis by secreting inflammatory cytokines and angiogenic factors.(73) This initial aberrant response is believed to determine which females are at higher risk of developing endometriosis when retrograde menstruation takes place.(74) Interestingly, gut microbiota was demonstrated to be a key factor in initiating such inflammatory responses. Studies depicted its role in priming neutrophils that mount immune reactions in the peritoneal cavities of female mice with endometriosis.(75)
Gut microbiota seems to undergo structural and functional changes in patients with obesity.(76) This may modulate, in part, obesity-induced inflammation, which, in turn, predisposes to atherosclerotic cardiovascular disease.(77) Whether this modulatory effect of gut microbiota on systemic inflammation is contributory to the pathobiology of uterine leiomyomas needs to be further evaluated. In a study done by Le Chatelier et al,(78) individuals with low gene counts, which indicate decreased richness of the gut microbiome, were at higher risk of having abnormal lipid profiles, insulin insensitivity, and pro-inflammatory markers compared to those with higher gene counts. This points to a role for the changes in gut microbiota in promoting metabolic and inflammatory derangements.(77)
3. Evidence of associations of CMRFs and BGDs:
3.1. Uterine leiomyomas:
Studies have documented associations of some components of the MetS with uterine leiomyomas (Figure 1). For instance, hypertensive females were at higher risk of being diagnosed with uterine leiomyomas compared to their non-hypertensive counterparts.(79–81) Faerstein and colleagues(79) concluded that this risk is higher among females with a first diagnosis of hypertension before 35 years of age and among hypertensives requiring medications. Additionally, they found that myomatous females had a higher mean duration of hypertension compared to controls, suggesting an increased risk with a longer duration of hypertension.(79) Reverse-causality was the popular explanation for this association, where myomatous growths result in urinary tract obstruction.(79) Nevertheless, hypertension may be involved in inducing smooth muscle injury and inflammatory milieus that promote myomatous proliferation, possibly by actions of transforming growth factor-β (TGF- β),(82) in a mechanism similar to that of atheromatous plaque formation.(80)
Uterine leiomyomas were shown to share a positive association with diabetes mellitus by some studies. Tak et al (81) showed that females with uterine leiomyomas were more likely to be diabetic compared to non-myomatous females, and that women with three or more myomas had a significantly higher fasting plasma glucose than those with one myoma. In contrast, Velez Edwards et al (83) detected a protective role for type 2 diabetes mellitus against uterine leiomyomas, more pronounced among European Americans and those treated with insulin. In line with this finding, a study by Baird et al (17) showed an inverse relationship between IGF-1 and insulin, which are elevated in diabetes mellitus, and uterine fibroids despite their hypothesis predicting a positive correlation. This possibly suggests that vascular pathologies contributed to by diabetes mellitus might hinder the development of myomatous growths. (17)
A linkage between uterine leiomyomas and obesity has been reported by some studies. Takeda et al (80) have shown that overweight is significantly associated with uterine leiomyomas among Japanese women, a finding that was reiterated by Tak et al, (81) who found that myomatous South Korean women had significantly higher waist circumferences and body fat levels than their controls, and that the number and size of leiomyomas correlated positively with BMI. Nevertheless, Sato et al(84) inferred that women with occult obesity, i.e., body fat over 30% and BMI under 24, were at highest risk for developing uterine leiomyomas. This implies that BMI might not be an inclusive indicator of obesity and associated risks, and that parameters of central adiposity such as waist-to-hip ratio correlate more strongly with visceral fat implicated in systemic inflammation and cardiometabolic complications.(1)
The relationship between uterine fibroids and hyperlipidemia was documented in the literature. Tak et al (81) found higher low-density lipoprotein cholesterol (LDL-C) and lower high-density lipoprotein cholesterol (HDL-C) levels among women with uterine leiomyomas. In addition, they concluded that the number of myomas correlated positively with triglyceride levels but negatively with HDL-C levels. A study done by Uimari et al (85) demonstrated that the risk of uterine leiomyomas increases for each 1 mmol/L increase in LDL-C, triglycerides, and total cholesterol (TC) levels. Estrogen is a known modulator of lipid metabolism, and aberrant estrogen signaling is hypothesized to contribute to derangement of lipids and development of uterine fibroids.(85) However, Takeda et al failed to document a significant association between hypertriglyceridemia and uterine leiomyomas.(80)
The risk of uterine leiomyomas correlated with the MetS. Takeda et al (80) found that myomatous risk rose significantly with the number of MetS risk factors, while Tak et al (81) also demonstrated that MetS was more prevalent among women with multiple leiomyomas. Despite the available evidence of the relationship between uterine fibroids and CMRFs, more inclusive studies and mechanistic research is essential to decide on whether this association is causal or rather observational.
3.2. Endometriosis:
CMRFs have not only been implicated in the pathogenesis of endometriosis, but, in fact, endometriosis was shown to exert systemic effects and create dysfunctional inflammatory milieus that could significantly contribute to cardiovascular risk(25) (Figures 1 and 4). In a cross-sectional study conducted by Melo et al,(86) women with endometriosis had higher levels of TC, LDL-C, triglycerides, and HDL-C and a lower HDL-C:TC ratio. The documentation of an atherogenic lipid profile in endometriosis patients can raise multiple hypotheses. On one hand, endometriosis induces a state of systemic inflammation, which, in turn, can alter lipid metabolism leading to deranged lipid parameters.(26) In the presence of other CMRFs, oxidized lipids, namely LDL-C, create endothelial injuries and perpetuate a cascade of events that results in atherosclerotic plaque formation(86) (Figure 4). From another perspective, oxidization of exudated lipids in the peritoneal cavity can enhance the growth of endometrial implants and promote the development of advanced adhesive disease, suggesting a mutual etiopathogenic mechanism for both endometriosis and atherosclerosis mediated by abnormal lipids and their injurious properties.(87)
In a prospective cohort study done by Mu et al,(87) women with laparoscopically-confirmed endometriosis demonstrated a higher risk of hypercholesterolemia and hypertension compared to women without endometriosis. In addition, they concluded that women with either hypercholesterolemia or hypertension had a significantly higher risk of laparoscopically-confirmed endometriosis than women with neither CMRF.
3.3. Hysterectomy:
Many studies have discussed the association of hysterectomy and cardiovascular risk. The Women’s Health Initiative (WHI) Observational Study(88) demonstrated that women with history of hysterectomy were more likely to be obese, diabetic, hypertensive, and hypercholesterolemic at baseline compared to women with no such history. In addition, they were less likely to engage in physical activity and had higher intake of saturated fat. Hysterectomized women in the study were more likely to report prior cardiovascular events, including myocardial infarctions, congestive heart failure, and coronary interventions, compared to their non-hysterectomized counterparts. Upon following them up, women with history of hysterectomy with or without oophorectomy suffered more cardiovascular events, both fatal and non-fatal, compared to those with intact uteri. The increased prevalence of self-reported cardiovascular events at baseline and after follow-up among hysterectomized females can be explained by the more prevalent CMRFs these women had, which can possibly be attributed to their lower socioeconomic status and poorer access to health care.(88) CMRFS may additionally contribute to BGDs, such as uterine leiomyomas and endometriosis, as this article previously discussed, and hence, to a higher chance of undergoing a hysterectomy(88) (figures 5). On the other hand, hysterectomy is known to compromise the blood supply to the preserved ovaries through anastomotic vessels, and in turn, predisposes to an earlier onset of menopause. Surgical menopause may possibly thereby deprive a previously premenopausal female of the protective effects of estrogen against cardiovascular disease.(89)
Figure 5.
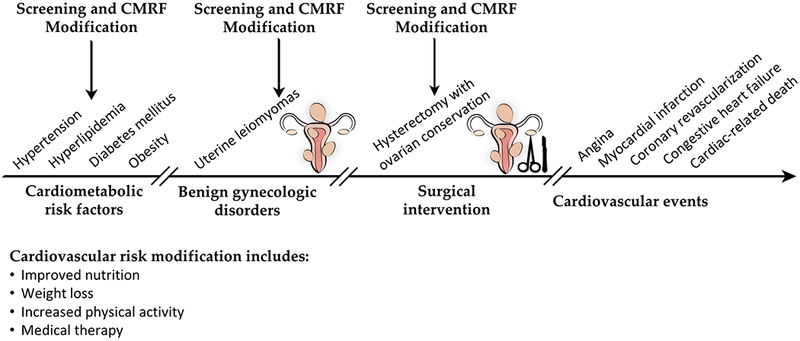
Schematic presentation of a proposed temporal relationship between cardiometabolic risk factors (CMRFs), benign gynecologic disorders (BGDs), surgical intervention, and cardiovascular events. Screening and preventive opportunities are also demonstrated.
Ingelsson et al (89) have documented an increased cardiovascular risk upon following up hysterectomized females younger than 50 years of age at the time of operation, but a lower risk among women older than 50 years of age when compared to their controls. This differential risk between the two age groups might be due to selection bias, where women undergoing hysterectomy for benign indications at an older age are of a better baseline health than those who do not. On the other hand, the hormonal effects of hysterectomy might be more prominent in premenopausal females compared to women who have already gone through menopause as well as dependent on whether a concomitant bilateral oophorectomy was performed.(89)
On the contrary, the Study of Women’s Health across the Nation (SWAN)(90) inferred that hysterectomy with ovarian conservation is not a predictor of subsequent increased cardiovascular risk. In addition, the Coronary Artery Risk Development in Young Adults (CARDIA) Study(91) concluded that postmenopausal levels of CMRFs were not influenced by hysterectomy status and rather reflect the Cardiometabolic profile prior to surgery. Nevertheless, the presence of multiple hypotheses underlying the relationship between CMRFs, hysterectomy, and cardiovascular events necessitates more comprehensive studies to further explore the exact nature of this relationship and its temporal component.
Laughlin-Tommaso et al (92) have found that obesity shares a significant association with hysterectomy performed for benign indications with ovarian conservation. However, this finding may, in part, be attributed to selecting comorbid patients for surgical treatment to avoid the adverse effects of oral contraceptives on cardiovascular risk. In a study done by Ding et al,(93) hysterectomized females were at higher risk of receiving a diagnosis of hypertension during the follow-up period. The exact mechanism that connects hypertension and hysterectomy remains largely undetermined. It is hypothesized that the development of hypertension is associated with the hormonal changes following hysterectomy.(94) This can be supported by the observation that postmenopausal women have higher blood pressure than their premenopausal counterparts, suggesting a possible modulatory role for ovarian hormones on blood pressure control.(95)
4. Clinical Implications:
The body of evidence this review presents of the association between CMRFs and BGDs creates novel preventive and therapeutic approaches that gynecologists and other healthcare providers can put into practice. These include screening measures, cardiovascular risk modification, and medical therapy (figure 5).
4.1. Screening:
Screening of patients with CMRFs for BGDs and vice versa would have a remarkable role in the prevention of either disease state. Not only does early detection of CMRFs halt the occurrence of cardiovascular events, but early modification of these factors can, perhaps, prevent the development of uterine leiomyomas and hysterectomy. On the other hand, screening of myomatous and hysterectomized women as well as females with endometriosis for CMRFs and associated complications such as ischemic heart disease can substantially aid in primary and secondary prevention of Cardiometabolic phenomena. For instance, carotid intima-media thickness can possibly be more liberally used as a screening measure for subclinical atherosclerosis among women with uterine leiomyomas knowing it significantly differs among myomatous and non-myomatous females.(70) Office screening for cardiovascular disease using classic CMRF assessment can be more liberally applied as well. The research evidence this review provides should stimulate gynecologists and other healthcare providers to engage in preventive strategies against BGDs and cardiovascular disease.
4.2. Cardiovascular risk modification:
Given the multiple mechanisms by which obesity may mediate its role in the pathogenesis of many BGDs, weight loss stands as a significant measure to prevent cardiovascular and gynecologic diseases. Systemic inflammation can, at least in part, be improved with weight loss. Reduction of IL-6 and C-reactive protein (CRP) in the sera of subjects who lost weight(4) through caloric restriction and exercise had been documented. Estrogen levels were similarly demonstrated to decrease with intentional weight loss.(96)
Barry et al found that high-intensity interval training and moderate-intensity continuous training can have immunomodulatory actions on IL-6 and IL-10, which suggests a potential role for exercise in obesity-induced systemic inflammation.(97) In fact, athletic females were shown to have a lower lifetime risk of benign tumors of the reproductive tract, including those of the uterus, compared to their non-athletic counterparts.(98)
Dietary modifications are also introduced to counteract CMRFs.(99) As previously mentioned in this review, decreased richness of gut microbiota was shown to influence the development of CMRFs, such as obesity, hyperlipidemia, insulin resistance, and systemic inflammation.(78) These changes in the gut microbiota were shown to be greatly modulated by dietary factors, particularly fat intake;(99) for example, high-fat diets can alter the composition of gut microbiota by reducing the quantity of beneficial species. Consequently, this increases the serum levels of lipopolysaccharides, promoting a systemic inflammatory milieu.(100) As opposed to the unfavorable effects of saturated fatty acids on gut microbiota, ingestion of oleic acid and omega-3 polyunsaturated fatty acid can, in fact, have remarkably beneficial modulatory effects on gut microbiota, thereby decreasing the risk of obesity and its complications.(99)
4.3. Medical therapy:
4.3.1. Statins:
In a large database analysis, statin use was found to be associated with a lower risk of having uterine leiomyomas and leiomyoma-associated symptoms.(101) This can possibly be mediated by the anti-mitogenic and pro-apoptotic actions of statins on leiomyoma cells and tumor growth inhibition in leiomyoma animal model.(102,103) In addition, simvastatin was demonstrated to inhibit production of extracellular matrix at clinically relevant levels.(104) These results support the potential therapeutic use of statins for uterine leiomyomas and related symptoms.(105,106) Similarly, statins have shown promise in the treatment of endometriosis in animal models.(107,108)
4.3.2. Probiotics/synbiotics:
To counteract the adverse outcomes associated with high-fat intake on gut microbiota, probiotics or synbiotics can presumably be administered.(99) Depending on the type, probiotic use has been associated with prevention of weight gain, regulation of glucose metabolism, and suppression of inflammatory states associated with obesity.(99)
5. Future Directions:
Future research is needed to further investigate whether the association between CMRFs and BGDs is more than observational. More mechanistic research will enhance our understanding of the underlying cellular and molecular mechanisms involved.
Target audience:
Obstetricians and gynecologists, family physicians.
Learning objectives:
After participating in this activity, the learner should be better able to:
Identify the association between cardiometabolic risk factors and benign gynecologic disorders;
Explain the potential underlying mechanisms of such association; and
Discuss their clinical implications on health and health care
CME Test Questions.
-
Which of the following statements accurately describes obesity-associated inflammation?
It can be mediated by adiponectin secreted from adipose tissue
It is accompanied by lower levels of leptin compared to non-obese individuals
It can promote mitogenic phenomena in obese individuals
It is associated with low serum levels of TNF- α and IL-6
-
What supports the pathologic resemblance between atherosclerosis and leiomyomas?
Both lesions develop following intimal injury
They contain similar soft tissue components
Both lesions are considered significant precursors for malignancy
Composite cells of both lesions have a polyclonal origin
-
By which of the following postulated mechanisms can endometriosis directly mediate increased cardiovascular risk?
Subclinical inflammation
Lipid derangements
Direct invasion
Hyperestrogenism
-
What is the most likely relation between cardiometabolic risk factors (CMRFs) and uterine leiomyomas and endometriosis?
CMRFs likely contribute to pathogenesis of uterine leiomyoma
CMFRs likely contribute to pathogenesis of endometriosis
Endometriosis likely contributes to cardiometabolic risks.
A and C
-
What are the potential clinical applications of the relation of CMRFs and Benign Gynecologic Disorders (BGDs) including uterine leiomyomas, endometriosis?
May screen patients with BGDs for CMRFs
May screen patients with CMRFs for BGDs
Early cardiovascular risk modification for patients with BGDs
All of the above
Acknowledgments
This work was supported, in part, by NIH grant 1R01HD094380-01 to Mostafa A. Borahay.
Footnotes
The authors have no conflict of interest to declare.
References
- 1.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15(8):484–498. [DOI] [PubMed] [Google Scholar]
- 2.van Dam AD, Boon MR, Berbee JFP, et al. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur J Pharmacol. 2017;816:82–92. [DOI] [PubMed] [Google Scholar]
- 3.Aldiss P, Davies G, Woods R, et al. ‘Browning’ the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. Int J Cardiol. 2017;228:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park J, Morley TS, Kim M, et al. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10(8):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulun SE, Chen D, Moy I, et al. Aromatase, breast cancer and obesity: a complex interaction. Trends in endocrinology and metabolism: TEM. 2012;23(2):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruo T, Ohara N, Wang J, et al. Sex steroidal regulation of uterine leiomyoma growth and apoptosis. Hum Reprod Update. 2004;10(3):207–220. [DOI] [PubMed] [Google Scholar]
- 7.Borahay MA, Asoglu MR, Mas A, et al. Estrogen Receptors and Signaling in Fibroids: Role in Pathobiology and Therapeutic Implications. Reproductive sciences. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borahay MA, Al-Hendy A, Kilic GS, et al. Signaling Pathways in Leiomyoma: Understanding Pathobiology and Implications for Therapy. Molecular medicine. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maekawa R, Sato S, Yamagata Y, et al. Genome-wide DNA methylation analysis reveals a potential mechanism for the pathogenesis and development of uterine leiomyomas. PLoS One. 2013;8(6):e66632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovacs KA, Oszter A, Gocze PM, et al. Comparative analysis of cyclin D1 and oestrogen receptor (alpha and beta) levels in human leiomyoma and adjacent myometrium. Mol Hum Reprod. 2001;7(11):1085–1091. [DOI] [PubMed] [Google Scholar]
- 11.Susheelamma CJ, Pillai SM, Asha Nair S. Oestrogen, progesterone and stem cells: the discordant trio in endometriosis? Expert Rev Mol Med. 2018;20:e2. [DOI] [PubMed] [Google Scholar]
- 12.Laschke MW, Giebels C, Nickels RM, et al. Endothelial progenitor cells contribute to the vascularization of endometriotic lesions. Am J Pathol. 2011;178(1):442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi KW, Shin JH, Park MS, et al. Association of body mass index with severity of endometriosis in Korean women. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2009;105(1):39–42. [DOI] [PubMed] [Google Scholar]
- 14.Goetz LG, Mamillapalli R, Taylor HS. Low Body Mass Index in Endometriosis Is Promoted by Hepatic Metabolic Gene Dysregulation in Mice. Biology of reproduction. 2016;95(6):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKeown-Eyssen G Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev. 1994;3(8):687–695. [PubMed] [Google Scholar]
- 16.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6(2):164–179. [DOI] [PubMed] [Google Scholar]
- 17.Baird DD, Travlos G, Wilson R, et al. Uterine leiomyomata in relation to insulin-like growth factor-I, insulin, and diabetes. Epidemiology. 2009;20(4):604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gkioka E, Msaouel P, Philippou A, et al. Review: The Role of Insulin-like Growth Factor-1 Signaling Pathways in Uterine Leiomyoma. In Vivo. 2015;29(6):637–649. [PubMed] [Google Scholar]
- 19.Sverdlov AL, Figtree GA, Horowitz JD, et al. Interplay between Oxidative Stress and Inflammation in Cardiometabolic Syndrome. Mediators Inflamm. 2016;2016:8254590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair S, Al-Hendy A. Adipocytes enhance the proliferation of human leiomyoma cells via TNF-alpha proinflammatory cytokine. Reproductive sciences. 2011;18(12):1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69(1):29–35. [DOI] [PubMed] [Google Scholar]
- 22.Iwabe T, Harada T, Terakawa N. Role of cytokines in endometriosis-associated infertility. Gynecol Obstet Invest. 2002;53 Suppl 1:19–25. [DOI] [PubMed] [Google Scholar]
- 23.Iwabe T, Harada T, Tsudo T, et al. Tumor necrosis factor-alpha promotes proliferation of endometriotic stromal cells by inducing interleukin-8 gene and protein expression. J Clin Endocrinol Metab. 2000;85(2):824–829. [DOI] [PubMed] [Google Scholar]
- 24.Pizzo A, Salmeri FM, Ardita FV, et al. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol Obstet Invest. 2002;54(2):82–87. [DOI] [PubMed] [Google Scholar]
- 25.Alderman MH 3rd, Yoder N, Taylor HS. The Systemic Effects of Endometriosis. Semin Reprod Med. 2017;35(3):263–270. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PW. Evidence of systemic inflammation and estimation of coronary artery disease risk: a population perspective. Am J Med. 2008;121(10 Suppl 1):S15–20. [DOI] [PubMed] [Google Scholar]
- 27.Poniedzialek-Czajkowska E, Mierzynski R, Slodzinska M, et al. Adipokines and C-peptide in overweight and obese pregnant women. Ginekologia polska. 2018;89(8):442–448. [DOI] [PubMed] [Google Scholar]
- 28.Markowska A, Rucinski M, Drews K, et al. Further studies on leptin and leptin receptor expression in myometrium and uterine myomas. European journal of gynaecological oncology. 2005;26(5):517–525. [PubMed] [Google Scholar]
- 29.Chen HS, Chan TF, Chung YF, et al. Aberrant serum adiponectin levels in women with uterine leiomyomas. Gynecol Obstet Invest. 2004;58(3):160–163. [DOI] [PubMed] [Google Scholar]
- 30.Edwards TL, Michels KA, Hartmann KE, et al. BET1L and TNRC6B associate with uterine fibroid risk among European Americans. Hum Genet. 2013;132(8):943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu K, Zhang W, Liu S, et al. Hepatic endometriosis: a rare case and review of the literature. Eur J Med Res. 2015;20:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho S, Mutlu L, Grechukhina O, et al. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril. 2015;103(5):1252–1260 e1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y, Tabbaa ZM, Khan Z, et al. Epigenetic regulation of uterine biology by transcription factor KLF11 via posttranslational histone deacetylation of cytochrome p450 metabolic enzymes. Endocrinology. 2014;155(11):4507–4520. [DOI] [PubMed] [Google Scholar]
- 34.Slaughter RL, Edwards DJ. Recent advances: the cytochrome P450 enzymes. Ann Pharmacother. 1995;29(6):619–624. [DOI] [PubMed] [Google Scholar]
- 35.Yin P, Lin Z, Reierstad S, et al. Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells. Cancer Res.70(4):1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daftary GS, Zheng Y, Tabbaa ZM, et al. A novel role of the Sp/KLF transcription factor KLF11 in arresting progression of endometriosis. PLoS One. 2013;8(3):e60165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutierrez-Aguilar R, Froguel P, Hamid YH, et al. Genetic analysis of Kruppel-like zinc finger 11 variants in 5864 Danish individuals: potential effect on insulin resistance and modified signal transducer and activator of transcription-3 binding by promoter variant −1659G>C. J Clin Endocrinol Metab. 2008;93(8):3128–3135. [DOI] [PubMed] [Google Scholar]
- 38.Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, et al. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A. 2005;102(13):4807–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mozaffarian D, Rimm EB, King IB, et al. trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004;80(6):1521–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril. 2001;75(1):1–10. [DOI] [PubMed] [Google Scholar]
- 41.Monguchi T, Hara T, Hasokawa M, et al. Excessive intake of trans fatty acid accelerates atherosclerosis through promoting inflammation and oxidative stress in a mouse model of hyperlipidemia. J Cardiol. 2017;70(2):121–127. [DOI] [PubMed] [Google Scholar]
- 42.Parazzini F, Chiaffarino F, Surace M, et al. Selected food intake and risk of endometriosis. Human reproduction. 2004;19(8):1755–1759. [DOI] [PubMed] [Google Scholar]
- 43.Missmer SA, Chavarro JE, Malspeis S, et al. A prospective study of dietary fat consumption and endometriosis risk. Human reproduction. 2010;25(6):1528–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-gamma induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. 2004;82 Suppl 3:1008–1013. [DOI] [PubMed] [Google Scholar]
- 45.Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes--an updated review of the evidence. Curr Atheroscler Rep. 2012;14(6):515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skeaff CM, Miller J. Dietary fat and coronary heart disease: summary of evidence from prospective cohort and randomised controlled trials. Ann Nutr Metab. 2009;55(1–3):173–201. [DOI] [PubMed] [Google Scholar]
- 47.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. [DOI] [PubMed] [Google Scholar]
- 48.Sabry M, Halder SK, Allah AS, et al. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. International journal of women’s health. 2013;5:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Catherino WH, Eltoukhi HM, Al-Hendy A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin Reprod Med. 2013;31(5):370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halder SK, Osteen KG, Al-Hendy A. 1,25-dihydroxyvitamin d3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biology of reproduction. 2013;89(6):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anastasi E, Fuggetta E, De Vito C, et al. Low levels of 25-OH vitamin D in women with endometriosis and associated pelvic pain. Clin Chem Lab Med. 2017;55(12):e282–e284. [DOI] [PubMed] [Google Scholar]
- 52.Nitsa A, Toutouza M, Machairas N, et al. Vitamin D in Cardiovascular Disease. In Vivo. 2018;32(5):977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kar A, Datta S. A study of serum Vitamin D level and its association with hypertension. J Family Med Prim Care. 2018;7(3):546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaidya A, Forman JP, Hopkins PN, et al. 25-Hydroxyvitamin D is associated with plasma renin activity and the pressor response to dietary sodium intake in Caucasians. J Renin Angiotensin Aldosterone Syst. 2011;12(3):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sivritepe R, Basat S, Ortaboz D. Association of vitamin D status and the risk of cardiovascular disease as assessed by various cardiovascular risk scoring systems in patients with type 2 diabetes mellitus. Aging Male. 2018:1–7. [DOI] [PubMed] [Google Scholar]
- 56.Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. 2014;114(2):379–393. [DOI] [PubMed] [Google Scholar]
- 57.Dixon D, He H, Haseman JK. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ Health Perspect. 2000;108 Suppl 5:795–802. [DOI] [PubMed] [Google Scholar]
- 58.Peng L, Wen Y, Han Y, et al. Expression of insulin-like growth factors (IGFs) and IGF signaling: molecular complexity in uterine leiomyomas. Fertil Steril. 2009;91(6):2664–2675. [DOI] [PubMed] [Google Scholar]
- 59.Burroughs KD, Howe SR, Okubo Y, et al. Dysregulation of IGF-I signaling in uterine leiomyoma. J Endocrinol. 2002;172(1):83–93. [DOI] [PubMed] [Google Scholar]
- 60.Fayed YM, Tsibris JC, Langenberg PW, et al. Human uterine leiomyoma cells: binding and growth responses to epidermal growth factor, platelet-derived growth factor, and insulin. Lab Invest. 1989;60(1):30–37. [PubMed] [Google Scholar]
- 61.Ren Y, Yin H, Tian R, et al. Different effects of epidermal growth factor on smooth muscle cells derived from human myometrium and from leiomyoma. Fertil Steril. 2011;96(4):1015–1020. [DOI] [PubMed] [Google Scholar]
- 62.Park J, Sarode VR, Euhus D, et al. Neuregulin 1-HER axis as a key mediator of hyperglycemic memory effects in breast cancer. Proc Natl Acad Sci U S A. 2012;109(51):21058–21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yousefzadeh G, Masoomi M, Emadzadeh A, et al. The association of insulin-like growth factor-1 with severity of coronary artery disease. J Cardiovasc Med (Hagerstown). 2013;14(6):416–420. [DOI] [PubMed] [Google Scholar]
- 64.Ruotolo G, Bavenholm P, Brismar K, et al. Serum insulin-like growth factor-I level is independently associated with coronary artery disease progression in young male survivors of myocardial infarction: beneficial effects of bezafibrate treatment. J Am Coll Cardiol. 2000;35(3):647–654. [DOI] [PubMed] [Google Scholar]
- 65.Makki N, Thiel KW, Miller FJ Jr. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int J Mol Sci. 2013;14(10):20597–20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dreux AC, Lamb DJ, Modjtahedi H, et al. The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis. Atherosclerosis. 2006;186(1):38–53. [DOI] [PubMed] [Google Scholar]
- 67.Benter IF, Yousif MH, Griffiths SM, et al. Epidermal growth factor receptor tyrosine kinase-mediated signalling contributes to diabetes-induced vascular dysfunction in the mesenteric bed. British journal of pharmacology. 2005;145(6):829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moss NS, Benditt EP. Human atherosclerotic plaque cells and leiomyoma cells. Comparison of in vitro growth characteristics. Am J Pathol. 1975;78(2):175–190. [PMC free article] [PubMed] [Google Scholar]
- 69.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973;70(6):1753–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aksoy Y, Sivri N, Karaoz B, et al. Carotid intima-media thickness: a new marker of patients with uterine leiomyoma. European journal of obstetrics, gynecology, and reproductive biology. 2014;175:54–57. [DOI] [PubMed] [Google Scholar]
- 71.Laux-Biehlmann A, d’Hooghe T, Zollner TM. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends in pharmacological sciences. 2015;36(5):270–276. [DOI] [PubMed] [Google Scholar]
- 72.Khoufache K, Michaud N, Harir N, et al. Anomalies in the inflammatory response in endometriosis and possible consequences: a review. Minerva Endocrinol. 2012;37(1):75–92. [PubMed] [Google Scholar]
- 73.Lin YJ, Lai MD, Lei HY, et al. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006;147(3):1278–1286. [DOI] [PubMed] [Google Scholar]
- 74.Laschke MW, Menger MD. The gut microbiota: a puppet master in the pathogenesis of endometriosis? Am J Obstet Gynecol. 2016;215(1):68 e61–64. [DOI] [PubMed] [Google Scholar]
- 75.Karmarkar D, Rock KL. Microbiota signalling through MyD88 is necessary for a systemic neutrophilic inflammatory response. Immunology. 2013;140(4):483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Munckhof ICL, Kurilshikov A, Ter Horst R, et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes Rev. 2018;19(12):1719–1734. [DOI] [PubMed] [Google Scholar]
- 78.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. [DOI] [PubMed] [Google Scholar]
- 79.Faerstein E, Szklo M, Rosenshein NB. Risk factors for uterine leiomyoma: a practice-based case-control study. II. Atherogenic risk factors and potential sources of uterine irritation. Am J Epidemiol. 2001;153(1):11–19. [DOI] [PubMed] [Google Scholar]
- 80.Takeda T, Sakata M, Isobe A, et al. Relationship between metabolic syndrome and uterine leiomyomas: a case-control study. Gynecol Obstet Invest. 2008;66(1):14–17. [DOI] [PubMed] [Google Scholar]
- 81.Tak YJ, Lee SY, Park SK, et al. Association between uterine leiomyoma and metabolic syndrome in parous premenopausal women: A case-control study. Medicine (Baltimore). 2016;95(46):e5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimura C, Konishi S, Hasegawa M, et al. Development of vascular smooth muscle contractility by endothelium-derived transforming growth factor beta proteins. Pflugers Archiv : European journal of physiology. 2014;466(2):369–380. [DOI] [PubMed] [Google Scholar]
- 83.Velez Edwards DR, Hartmann KE, Wellons M, et al. Evaluating the role of race and medication in protection of uterine fibroids by type 2 diabetes exposure. BMC Womens Health. 2017;17(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sato F, Nishi M, Kudo R, et al. Body fat distribution and uterine leiomyomas. J Epidemiol. 1998;8(3):176–180. [DOI] [PubMed] [Google Scholar]
- 85.Uimari O, Auvinen J, Jokelainen J, et al. Uterine fibroids and cardiovascular risk. Human reproduction. 2016;31(12):2689–2703. [DOI] [PubMed] [Google Scholar]
- 86.Melo AS, Rosa-e-Silva JC, Rosa-e-Silva AC, et al. Unfavorable lipid profile in women with endometriosis. Fertil Steril. 2010;93(7):2433–2436. [DOI] [PubMed] [Google Scholar]
- 87.Mu F, Rich-Edwards J, Rimm EB, et al. Association Between Endometriosis and Hypercholesterolemia or Hypertension. Hypertension. 2017;70(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Howard BV, Kuller L, Langer R, et al. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women’s Health Initiative Observational Study. Circulation. 2005;111(12):1462–1470. [DOI] [PubMed] [Google Scholar]
- 89.Ingelsson E, Lundholm C, Johansson AL, et al. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750. [DOI] [PubMed] [Google Scholar]
- 90.Matthews KA, Gibson CJ, El Khoudary SR, et al. Changes in cardiovascular risk factors by hysterectomy status with and without oophorectomy: Study of Women’s Health Across the Nation. J Am Coll Cardiol. 2013;62(3):191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Appiah D, Schreiner PJ, Bower JK, et al. Is Surgical Menopause Associated With Future Levels of Cardiovascular Risk Factor Independent of Antecedent Levels? The CARDIA Study. Am J Epidemiol. 2015;182(12):991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laughlin-Tommaso SK, Khan Z, Weaver AL, et al. Cardiovascular risk factors and diseases in women undergoing hysterectomy with ovarian conservation. Menopause. 2016;23(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ding DC, Tsai IJ, Hsu CY, et al. Risk of hypertension after hysterectomy: a population-based study. BJOG : an international journal of obstetrics and gynaecology. 2018;125(13):1717–1724. [DOI] [PubMed] [Google Scholar]
- 94.Levin G, Rottenstreich A. Re: Risk of hypertension after hysterectomy: a population-based study. BJOG : an international journal of obstetrics and gynaecology. 2018;125(13):1779–1780. [DOI] [PubMed] [Google Scholar]
- 95.Dubey RK, Oparil S, Imthurn B, et al. Sex hormones and hypertension. Cardiovasc Res. 2002;53(3):688–708. [DOI] [PubMed] [Google Scholar]
- 96.Byers T, Sedjo RL. Does intentional weight loss reduce cancer risk? Diabetes Obes Metab. 2011;13(12):1063–1072. [DOI] [PubMed] [Google Scholar]
- 97.Barry JC, Simtchouk S, Durrer C, et al. Short-term exercise training reduces anti-inflammatory action of interleukin-10 in adults with obesity. Cytokine. 2018;111:460–469. [DOI] [PubMed] [Google Scholar]
- 98.Wyshak G, Frisch RE, Albright NL, et al. Lower prevalence of benign diseases of the breast and benign tumours of the reproductive system among former college athletes compared to non-athletes. British journal of cancer. 1986;54(5):841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Candido FG, Valente FX, Grzeskowiak LM, et al. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: mechanisms and clinical implications on obesity. Int J Food Sci Nutr. 2018;69(2):125–143. [DOI] [PubMed] [Google Scholar]
- 100.Cani PD, Delzenne NM. The gut microbiome as therapeutic target. Pharmacol Ther. 2011;130(2):202–212. [DOI] [PubMed] [Google Scholar]
- 101.Borahay MA, Fang X, Baillargeon JG, et al. Statin use and uterine fibroid risk in hyperlipidemia patients: a nested case-control study. Am J Obstet Gynecol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borahay MA, Kilic GS, Yallampalli C, et al. Simvastatin Potently Induces Calcium-Dependent Apoptosis of Human Leiomyoma Cells. J Biol Chem. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borahay MA, Vincent K, Motamedi M, et al. Novel Effects of Simvastatin on Uterine Fibroids: In vitro and Patient-Derived Xenograft Mouse Model Study. Am J Obstet Gynecol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malik M, Britten J, Borahay M, et al. Simvastatin, at clinically relevant concentrations, affects human uterine leiomyoma growth and extracellular matrix production. Fertil Steril. 2018;110(7):1398–1407 e1391. [DOI] [PubMed] [Google Scholar]
- 105.Zeybek B, Costantine M, Kilic GS, et al. Therapeutic Roles of Statins in Gynecology and Obstetrics: The Current Evidence. Reproductive sciences. 2018:1933719117750751. [DOI] [PubMed] [Google Scholar]
- 106.Fritton K, Borahay MA. New and Emerging Therapies for Uterine Fibroids. Semin Reprod Med. 2017;35(6):549–559. [DOI] [PubMed] [Google Scholar]
- 107.Taylor HS, Alderman Iii M, D’Hooghe TM, et al. Effect of simvastatin on baboon endometriosis. Biology of reproduction. 2017;97(1):32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taylor HS, Osteen KG, Bruner-Tran KL, et al. Novel therapies targeting endometriosis. Reproductive sciences. 2011;18(9):814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]


