Abstract
The past three years have been exciting for both collagen biologists and human geneticists studying the disease known as osteogenesis imperfecta (OI or brittle bone disease). Functional studies on Cartilage-associated Protein (Crtap) have identified it as an essential component of a heterotrimeric, endoplasmic reticulum resident complex responsible for both collagen prolyl 3-hydroxylation and chaperone function. Importantly, human mutations in the CRTAP gene have been associated with recessive forms of OI. Although the function and in vivo biological significance of the 3-hydroxyproline modification are still poorly understood, studies on Crtap have led to the identification of additional genes in which mutations also cause recessive forms of OI. These discoveries have now focused the interest of geneticists on the endoplasmic reticulum that will require the help of biochemists to unravel the molecular dynamics and complexities of collagen folding.
Introduction
Type I collagen is the most abundant protein in the human body and a fundamental component of bone matrix [1]. It is a heterotrimeric molecule consisting of two α1(I) chains and one α2(I) chain that are synthesized in the rough endoplasmic reticulum (rER) as preprocollagen chains and undergo several post-translation modifications, including signal peptide cleavage, disulfide linkage, prolyl- and lysyl-hydroxylation and glycosylation, before folding into a triple helical structure [2]. After transport through the Golgi apparatus and secretion to the extracellular matrix, additional processing takes place, which includes N- and C-propeptide cleavage, hydroxylysine oxidation and final assembly and cross-linking of fibrils [2]. Collagen type I is the prototypical example of a protein that is post-translationally modified and destined to the secretory pathway. An increasing number of rER enzymes and protein complexes act as ‘chaperones’, such as protein disulfide isomerase, peptidyl prolyl cis-trans isomerases, Hsp47, and the recently identified prolyl 3-hydroxylation complex. By interacting with the procollagen chains, these rER components facilitate the process of chain recognition and assembly, folding of the triple helix and also inhibition of the inappropriate intracellular polymerization of collagen monomers into fibrils. Some of these molecules, like P3h1 (Leprecan) and perhaps Crtap, may accompany the collagen outside the cell and play a different function in the extracellular matrix.
Osteogenesis imperfecta (OI) is associated with mutations in the COL1A1 and COL1A2 genes but also in an increasing number of genes encoding rER-resident, collagen interacting proteins. Here we will focus our attention on Crtap, a member of the Leprecan family of genes and a key component of the collagen prolyl 3-hydroxylation complex and review its role in bone formation and human disease.
Crtap expression pattern and role during skeletal formation
Crtap was originally identified as a novel cDNA differentially expressed by chicken chondrocytes in vitro [3]. Initial evidence in the chicken confirmed by subsequent studies in the mouse identified expression of the Crtap transcript in several tissues and organs throughout development, although strongest protein expression was detected in the developing skeleton [3, 4]. The recent development of a newer Crtap polyclonal antibody allowed a better description of its distribution pattern: Crtap is expressed at all stages of mouse embryonic cartilage differentiation (especially in proliferating chondrocytes) and by osteoblastic and osteoclastic cells [5]. Furthermore its expression is detected in lung, kidney, testis, skin [6] and likely will be detected in all tissues expressing type I collagen.
To characterize its role during vertebrate development, a Crtap-null mouse was generated using a classic gene targeting inactivation strategy [5]. Crtap−/− mice from heterozygous crossings are born at the expected Mendelian ratio although they suffer some perinatal lethality; this becomes very frequent when the mutation is backcrossed in a pure C57BL/6J genetic background (Roy Morello, unpublished observations). Homozygous mutant pups are slightly smaller compared to their wild-type littermates, and both sexes remain significantly smaller in adulthood, weighing on average about 20–25% less than wild-type controls [5]. Their most striking phenotype is in the skeleton, with a profound generalized osteopenia, rhizomelia, mild cartilage dysplasia and progressive kyphosis of the spine. Craniofacial malformation and skin laxity are also present. Long-bone as well as vertebral histomorphometry showed about 50% reduction in trabecular bone volume and significant reduction in trabecular number and thickness with consequent increased trabecular separation. While both osteoblast and osteoclast numbers and surfaces were not different from wild-type controls, the bone formation rate was significantly decreased due to a reduced mineral apposition rate [5]. Moreover, all osteoid parameters including thickness, surface and volume, were reduced. Interestingly, the mineralization lag time, i.e. the time required for newly deposited matrix to mineralize, was also decreased, suggesting both a quantitative and qualitative defect of the osteoblast secreted matrix. Irrespective of the profound osteopenia, fractures were never observed in the Crtap−/− mice (Roy Morello, unpublished observations).
Concomitant to the phenotype delineation of the Crtap-null animals, Vranka et al. published the identification and initial characterization of the collagen binding protein prolyl 3-hydroxylase 1 (P3h1) [7]. P3h1 was obtained to near purity from a chicken rER extract, but it always co-eluted with two ‘contaminant’ proteins, namely Crtap and Cyclophilin B (CypB). These were shown not to be required for the enzymatic activity of P3h1 in vitro [7]. Whether Crtap was necessary or not for collagen prolyl 3-hydroxylation in vivo was unclear and the Crtap−/− mice represented a useful model to evaluate this. Cyanogen bromide peptides derived from α1(I) and α1(II) chains extracted from bone and cartilage of Crtap−/− and wild type mice, respectively, were analyzed by tandem mass spectrometry for 3-hydroxyproline (3Hyp) content. A difference in peptide mass between Crtap−/− and wild type mice was revealed by mass spectrometry for the peptide sequence known to contain the primary proline (residue 986 of the triple helix) that becomes 3-hydroxylated in both α1(I) and α1(II) chains. These data showed a complete lack of the 3-hydroxyl group at Pro986 in α1(I) chains from bone or skin collagen and in α1(II) chains from cartilage of Crtap−/− mice and demonstrated that Crtap is required in vivo for fibrillar collagen proyl 3-hydroxylation [5].
In vitro collagen synthetic studies in Crtap−/− mouse osteoblasts revealed over-modification of procollagen chains, suggesting a slowing of triple-helix winding and an increased hydroxylation of proline and lysine residues in the rER. Ultrastructural studies showed significantly increased collagen fibril diameter in Crtap−/− skin and cartilage compared to wild type values. Consistent with a potential interaction with P3h1 which had been previously shown to be a rER resident protein [7, 8], the majority of Crtap was also localized by immunofluorescence within the rER in multiple cell lines, with only minor staining in the extracellular matrix shown in mouse tissue sections. Finally, several lines of experimental evidence, including co-sedimentation on a sucrose gradient, laser light scattering and Western analysis on affinity chromatography eluates, demonstrated that in the rER Crtap can complex with P3h1 and Cyclophilin B (CypB) in a 1:1:1 ratio [5].
At the amino acid level, Crtap and P3h1 are about 30% identical, even though Crtap completely lacks the half, C-terminal portion of P3h1 which contains the enzymatic domain. Together with SC65 (coding for synaptonemal complex protein 65), Leprel1 (coding for prolyl 3-hydroxylase 2) and Leprel2 (coding for prolyl 3-hydroxylase 3), Crtap (or Leprel3) and Lepre1 constitute the Leprecan family of genes.
CRTAP mutations cause recessive OI
OI is a hereditary disease characterized by bone fragility and short stature [9]. The clinical spectrum ranges from perinatal lethality to nearly asymptomatic individuals with occasional fractures and normal stature. The majority of individuals with a clinical diagnosis of OI have an identifiable mutation in COL1A1 or COL1A2, the genes that encode the two type I collagen alpha chains [9]. OI patients with collagen type I mutations can be classified into four clinically defined types with varying severity, named OI types I to IV.
Apart from these ‘classical’ types of OI, three conditions, called OI types V, VI and VII, have been identified over the past decade [9]. OI types V to VII largely resemble OI types I, III or IV on clinical grounds, but also have some distinguishing features, and are not caused by COL1A1 or COL1A2 mutations. OI type VII was initially identified as an autosomal recessive disorder in a Native (‘First Nations’) Canadian community in which eight members were affected with a moderately severe form of OI [10]. The disease locus was mapped to the short arm of chromosome 3, which contains the CRTAP gene [11]. When it became apparent that the phenotype of the Crtap−/− mouse model shared some characteristics with OI type VII, CRTAP emerged as an obvious candidate gene for OI type VII. Indeed, sequence analysis of the CRTAP gene in patients with OI type VII revealed a mutation in intron 1 that led to the introduction of a cryptic splice site and caused a marked reduction in the expression of CRTAP protein (see ‘hypomorphic mutation’ section below) [5]. Subsequently, CRTAP loss of function mutations were discovered in newborns and fetuses with lethal forms of OI [12–15].
As of now, 25 individuals with CRTAP mutations have been described in the literature [5, 10, 12–15]. The database of CRTAP mutations currently contains a total of 17 different mutations (http://www.le.ac.uk/ge/collagen/). These mutations affect either exon/intron 1 or exon/intron 4 of the CRTAP gene. All but the initially discovered ‘hypomorphic mutation’ in intron 1 are thought to lead to a complete inactivation of CRTAP [12, 13, 15]. More than half of the mutations are nonsense or frameshift mutations that introduce a premature termination codon. Three missense mutations have been described but only one reported patient is homozygous for a missense mutation (Table 1). Barnes et al. estimated that CRTAP mutations are responsible for 2–3% of cases of lethal OI [12]. This estimate is roughly in line with the observations by Bodian et al., who found CRTAP mutations in 1 out of 62 cases with lethal OI [14].
Table 1.
Characteristics of reported live-born individuals with CRTAP mutations
| Source | Mutations | Sex | Gestational age | Birth Weight (g) | Status |
|---|---|---|---|---|---|
| Van Dijk, 2009 | nonsense/missense | M | term | Died at 5 days | |
| Van Dijk, 2009 | nonsense/nonsense | F | term | 2725 | Died at 24 days |
| Van Dijk, 2009 | nonsense/nonsense | M | term | 2570 | Died at 1 month |
| Van Dijk, 2009 | nonsense/splice site | F | term | 2710 | Died at 2 months |
| Barnes, 2006 | nonsense/nonsense | F | 36 weeks | 2435 | Died at 3 months |
| Barnes, 2006 | start codon/frameshift | F | term | 2600 | Died at 10 months |
| Barnes, 2006 | splice site/splice site | M | 35 weeks | 2370 | Died at 10 months |
| Baldridge, 2008 | Frameshift/frameshift | F | NA | NA | Alive at 12 months |
| Van Dijk, 2009 | splice site/splice site | F | term | 3245 | Alive at 2 years |
| Van Dijk, 2009 | splice site/splice site | M | term | 3110 | Alive at 4 years |
| Baldridge, 2008 | missense/missense | F | NA | NA | Alive at 9 years |
| Ward, 2002 / unpublished | Cryptic splice site/cryptic splice site | F | term | 4005 | Alive at 16 years |
| Ward, 2002 / unpublished | Cryptic splice site/cryptic splice site | F | term | 2900 | Alive at 18 years |
| Ward, 2002 / unpublished | Cryptic splice site/cryptic splice site | F | term | 3830 | Alive at 19 years |
| Ward, 2002 / unpublished | Cryptic splice site/cryptic splice site | F | term | 3770 | Alive at 20 years |
| Ward, 2002 / unpublished | Cryptic splice site/cryptic splice site | 2F, 2M | NA | NA | Alive at 28–33 years |
Clinical features of recessive OI due to CRTAP mutations
Hypomorphic Mutation.
Patients with hypomorphic mutations in CRTAP present the least severe phenotype associated with CRTAP mutations until now [10]. Patients were born with multiple fractures but had a normal birth weight (Table 1). During early childhood, all patients had recurrent fractures which led to bowing of femora and tibiae and necessitated intramedullary rodding surgery. In the four adult patients, fractures were rare after puberty, but by then they required wheelchairs for mobility. The four younger patients who received intravenous pamidronate treatment during childhood and adolescence were ambulatory without assistance at the end of puberty [16]. None of the patients with the hypomorphic mutation developed respiratory problems in the postnatal period and none was diagnosed with cardiac or other anomalies. Also, these patients did not have dentinogenesis imperfecta, ligamentous laxity, or facial dysmorphism. Skin and hearing were normal, and the sclerae were white.
Radiographically, the most striking findings were the presence of rhizomelia (selective shortening of proximal limb segments) and coxa vara. The presence of scoliosis and vertebral compression was variable. Skull radiographs revealed Wormian bones (accessory skull bones completely surrounded by a suture line) in half of the patients. Lumbar spine bone mineral density was within the normal range in the first year of life, but was below the normal reference range after the age of four years in all patients. There were no abnormalities in biochemical markers of bone and mineral metabolism, and bone histomorphometric results in OI type VII were similar to those of OI type I, with a decreased amount of both cancellous and cortical bone and elevated bone turnover.
Loss-of-Function Mutations.
Apart from the individuals described in the preceding section, all other subjects with CRTAP mutations had presumed loss-of-function mutations and had a severe or lethal form of OI. Of the 17 cases with inactivating CRTAP mutations reported so far, five were aborted fetuses and eleven were born alive [12, 15, 17]. In one case, no information was provided on pregnancy and birth, but a diagnosis of lethal OI was reported [14]. Seven of the live-born children died of respiratory failure in the first year of life (Table 1). At the time of reporting, the oldest of the four surviving patients was nine years of age.
In most of the reported cases, birth weight was in the lower part of the reference range (Table 1). All children were born with multiple fractures, mostly of long bones and ribs. In a few cases, rhizomelia (short humeri and femora) was reported, but whether this was caused by a primary growth delay in the proximal limb segments or secondary to fractures is not clear. Apart from fractures and bowing, radiographic features included thin ribs and Wormian bones (Figure 1). Sclerae were white.
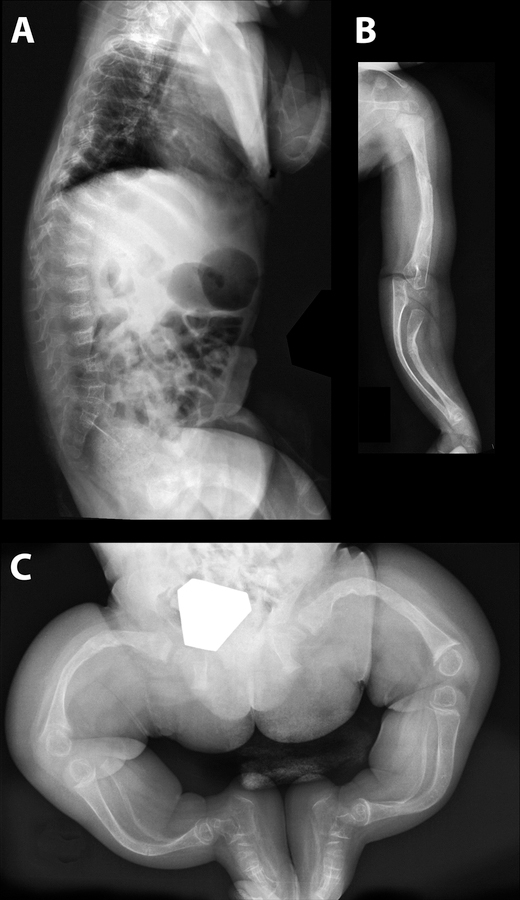
Figure 1.
Radiographs of a 15-months old girl with a homozygous p.Glu269_Val270del mutation in CRTAP. A. The lateral view of the spine shows multiple vertebral compression fractures. B. The long bones of the right forearm are deformed. C. There are severe deformities of the long bones of the lower extremities and cortices are very thin.
At birth, the clinical picture of patients with inactivating CRTAP mutations thus seems to be quite similar to that of patients with OI type II or III caused by structural collagen type I mutations. Possibly, the two disorders differ somewhat in the shape of the face, which is reported to be round in patients with CRTAP mutations and triangular in patients with collagen type I mutations. However, according to our experience, patients with severe OI caused by collagen type I mutations mostly develop a triangular face only after infancy. Scleral hue seems to be of some use in the differential diagnosis, as neonates with severe OI due to collagen type I mutations usually have grey sclerae whereas those with CRTAP mutations have white sclerae.
Regarding postnatal development, most patients with inactivating CRTAP mutations suffered from respiratory problems during infancy that eventually resulted in death in the majority of cases. These respiratory difficulties may be the consequence of thoracic cage skeletal deformities, but as Crtap is expressed in lung tissue it is also conceivable that there is a more direct effect of CRTAP mutations on lung function [5, 6].
Even though patients with CRTAP mutations reportedly have a normal skull shape at birth, it appears from the published cases that skull deformities can develop during infancy. As in other forms of severe OI, this is probably an indication of decreased mechanical resistance of the skull bone.
Reported children with CRTAP mutations have had severe growth failure. In addition, ‘popcorn epiphyses’, thought to contain material from disintegrated growth plates, were observed in an 8-year old girl with a homozygous missense mutation in CRTAP [13]. Possibly, growth plates are more severely affected in patients with CRTAP mutations than in patients with collagen type I mutations, because CRTAP is expressed in growth plates, whereas collagen type I is not. Nevertheless, growth failure and popcorn epiphyses are also commonly observed in children with severe OI caused by collagen type I mutations and therefore are by no means specific signs of CRTAP mutations [18, 19].
Treatment
No treatment studies have been published in patients with inactivating CRTAP mutations, but it is clear that the lives of such patients are threatened by respiratory failure. They therefore require supportive respiratory therapy and aggressive treatment of pulmonary infections. Physiotherapy and, for children surviving infancy, orthopedic surgery, may also be required.
The manifestations of OI are mostly secondary to the extreme bone weakness. Bisphosphonate treatment is widely used to strengthen the bones of growing children with moderate to severe forms of OI [9]. The most frequently used approach is intravenous pamidronate, which has been reported to lead to increased lumbar spine bone mineral density and cortical thickness, improvements in vertebral shape, stronger muscles, and better mobility function. A recent study suggests that the effects of intravenous pamidronate treatment are similar in patients with hypomorphic CRTAP mutations as in patients who have OI of similar severity caused by collagen type I mutations [16]. Treatment with intravenous bisphosphonates is clearly beneficial in infants with OI caused by collagen type I mutations, and therefore might reasonably be used in infants with inactivating CRTAP mutations as well. It should be noted, though, that respiratory distress has occurred in infants receiving the first intravenous cycle of pamidronate [20], which may be a cause of concern in children with CRTAP mutations who already have respiratory problems.
Pathogenesis of recessive OI and collagen prolyl 3-hydroxylation
The identification of CRTAP and then LEPRE1 mutations in probands affected with recessive OI has catalyzed a great interest in the function of collagen prolyl 3-hydroxylation. Until recently, lack of such modification at Pro986 of α1(I) in Crtap−/− mice as well as patients with CRTAP or LEPRE1 mutations was held responsible for the dramatic osteopenia and bone defects. However, new evidence has demonstrated that the Crtap/P3h1/CypB prolyl 3-hydroxylation complex also exerts peptidyl-prolyl cis-trans isomerase (PPIase) activity, which is the ability to catalyze the bond preceding a proline residue between its cis and trans form, as well as collagen chaperone function in the rER [24]. Thus, a functional dysregulation of the complex not only affects collagen prolyl 3-hydroxylation but also collagen folding kinetics and/or ability which results in over-modification of additional lysine and proline residues. The importance of the latter versus the former function has now been demonstrated by the identification of mutations in PPIB (encoding Cyclophilin B) in severe recessive OI cases negative for mutations in COL1A1, COL1A2, CRTAP or LEPRE1 [25]. Unlike CRTAP or LEPRE1 mutations which cause a >90% reduction of prolyl 3-hydroxylation at Pro986 in α1(I), lack of PPIB causes a less severe reduction in 3Hyp at the same location but similar over-modification of type I procollagen chains made by cultured skin fibroblasts [25]. This suggests that the collagen chaperone function of the prolyl 3-hydroxylation complex may be more relevant as the cause of human disease than prolyl 3-hydroxylation itself. Moreover, homozygous PPIB mutations do not affect the stability of CRTAP or P3H1 proteins while null mutations in CRTAP cause instability and lack of P3H1 protein and vice versa, but without affecting CypB [6, 25]. Hence CypB appears to enjoy a higher degree of ‘independence’ from the trimeric complex but the requirement for its prolyl isomerase activity in normal collagen folding is still unclear. Whether the stable CRTAP/P3H1 dimer associates with CypB and brings it to its substrate, the nascent triple-helix, at a position later marked by the 3-Hyp, is a possibility and will be the subject of further studies.
As a further sign of the complexity and importance of proper collagen folding versus prolyl 3-hydroxylation in OI pathogenesis, mutations in another rER resident PPIase with collagen chaperone activity, named FKBP65, have been reported in additional cases of recessive OI [26]. Furthermore, mutations affecting the SERPINH1 gene, coding for HSP47, another collagen chaperone molecule, have also been associated with a recessive form of OI in dogs [27]. These data altogether suggest that any dysregulation of the highly regulated collagen folding process is a common mechanism for all recessive forms of OI and perhaps even for the classic dominant forms caused by COL1A1 and COL1A2 mutations.
Given the above evidence, the current role and significance of collagen prolyl 3-hydroxylation remain uncertain. Unlike prolyl 4-hydroxylation, which is a very common modification of proline residues in the Y position of the Gly-Xaa-Yaa triplet repeat, and confers stability to the triple helix [28], prolyl 3-hydroxylation in the X position is rarer. It is established at a single residue (Pro986 of the triple helix domain) in α1(I) and α1(II) chains but is more common in type IV collagen [5, 29]. While biophysical studies on synthetic peptides containing (Gly-3Hyp-4Hyp)(n) initially suggested that 3Hyp could confer a slight instability to the collagen triple helix [30], later studies actually showed that a 3Hyp in the Xaa position increases its stability [31]. The identification and characterization of primary (almost always fully 3-hydroxylated) and secondary (less frequently 3-hydroxylated) 3Hyp sites in various procollagen α-chains is a matter of active research. Several of these have being identified and are under further investigation (David Eyre, personal communication). From the study of the Crtap−/− mice we have now shown that a 3Hyp in α2(V) at the same P986 site as in α1(I) and α1(II) chains is >95% hydroxylated in wild type mice but unmodified in Crtap −/− bone or skin type V collagen [6]. Considering the role of type V collagen in regulating type I collagen fibril formation [32, 33] and its association with Ehlers-Danlos syndrome (EDS) when mutated [34], an abnormal α2(V) chain may explain the irregular fibrillogenesis and paucity of osteoid observed in Crtap−/− mice and also their skin laxity, a typical feature of EDS. These data also indicate that Crtap is required for the 3-hydroxylation of canonical proline sites within clade A (types I, II and V) collagen chains. Finally, given the normal 3-hydroxylation status of a previously described 3Hyp locus in α1(IV) extracted from Crtap−/− kidney [6] and the existence of three P3H genes in mice and humans, it is possible that multiple mechanisms of collagen 3-hydroxylation exist, some dependent and some independent of the Crtap function.
Conclusion
The discovery of CRTAP mutations as the cause of recessive OI was a major step forward in understanding the molecular basis of this rare disease. It led to four new genes (CRTAP, LEPRE1, PPIB and FKBP65) being linked to recessive human OI (potentially five with SERPINH1). These findings are shifting current genetic research from the matrix to the rER compartment and highlight the complex cellular task of synthesizing and folding a collagen trimeric molecule. Clearly a range of rER resident chaperone proteins are essential.
Acknowledgements
The authors would like to thank David R. Eyre (Seattle, WA) and Dustin Baldridge (Houston, TX) for their insightful comments. This research was supported in part by the National Institute of Health grant AR051459 (R.M.), the Osteogenesis Imperfecta Foundation and the Rolanette and Berdon Lawrence Bone Disease Program of Texas (R.M.) and by the Shriners of North America (F.R.). F.R. is a Chercheur-Boursier Clinicien of the Fonds de la Recherche en Santé du Québec.
Footnotes
Disclosure
The Authors have no conflicts of interest to disclose.
References and Recommended Reading
- 1.Gehron Robey P, and Boskey A: The composition of Bone. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Edited by Rosen CJ. American Society for Bone and Mineral Research; 2008: 32–38. [Google Scholar]
- 2.Myllyharju J and Kivirikko KI, Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet, 2004. 20(1): p. 33–43. [DOI] [PubMed] [Google Scholar]
- 3.Castagnola P, et al. , Cartilage associated protein (CASP) is a novel developmentally regulated chick embryo protein. J Cell Sci, 1997. 110 ( Pt 12): p. 1351–9. [DOI] [PubMed] [Google Scholar]
- 4.Morello R, et al. , cDNA cloning, characterization and chromosome mapping of Crtap encoding the mouse cartilage associated protein. Matrix Biol, 1999. 18(3): p. 319–24. [DOI] [PubMed] [Google Scholar]
- 5.••.Morello R, et al. , CRTAP is required for prolyl 3- hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell, 2006. 127(2): p. 291–304.This article characterizes the phenotype of Crtap−/− mice, the role of Crtap during skeletal formation, its interaction with P3h1 and CypB in the rER and the identification of the first CRTAP human mutations.
- 6.Morello R, Baldridge D, Lennington J, et al. : Comparative Phenotypic and Biochemical Analyses of Crtap−/− Mice and Patients with Recessive Osteogenesis Imperfecta. [abstract FR0141]. Presented at the ASBMR 31st Annual Meeting Denver, CO; September 11–15, 2009. [Google Scholar]
- 7.Vranka JA, Sakai LY, and Bachinger HP, Prolyl 3-hydroxylase 1: Enzyme characterization and identification of a novel family of enzymes. J Biol Chem, 2004. [DOI] [PubMed]
- 8.Jarnum S, et al. , LEPREL1, a novel ER and Golgi resident member of the Leprecan family. Biochem Biophys Res Commun, 2004. 317(2): p. 342–51. [DOI] [PubMed] [Google Scholar]
- 9.Rauch F and Glorieux FH, Osteogenesis imperfecta. Lancet, 2004. 363(9418): p. 1377–85. [DOI] [PubMed] [Google Scholar]
- 10.Ward LM, et al. , Osteogenesis imperfecta type VII: an autosomal recessive form of brittle bone disease. Bone, 2002. 31(1): p. 12–8. [DOI] [PubMed] [Google Scholar]
- 11.Labuda M, et al. , Osteogenesis imperfecta type VII maps to the short arm of chromosome 3. Bone, 2002. 31(1): p. 19–25. [DOI] [PubMed] [Google Scholar]
- 12.•.Barnes AM, et al. , Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med, 2006. 355(26): p. 2757–64.This article describes the finding of additional CRTAP mutations and confirms the role of CRTAP in recessive OI.
- 13.•.Baldridge D, et al. , CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat, 2008. 29(12): p. 1435–42This article provides clinical and molecular information of patients with recessive OI and expands the phenotype of the disorder.
- 14.Bodian DL, et al. , Mutation and polymorphism spectrum in osteogenesis imperfecta type II: implications for genotype-phenotype relationships. Hum Mol Genet, 2009. 18(3): p. 463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.•.Van Dijk FS, et al. , CRTAP mutations in lethal and severe osteogenesis imperfecta: the importance of combining biochemical and molecular genetic analysis. Eur J Hum Genet, 2009.This article describes the phenotype of recessive OI due to CRTAP mutations and discusses the diagnostic work-up in such patients.
- 16.Cheung MS, Glorieux FH, and Rauch F, Intravenous pamidronate in osteogenesis imperfecta type VII. Calcif Tissue Int, 2009. 84(3): p. 203–9. [DOI] [PubMed] [Google Scholar]
- 17.Baldridge D, et al. , CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat, 2008. 29(12): p. 1435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeitlin L, et al. , Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics, 2003. 111(5 Pt 1): p. 1030–6. [DOI] [PubMed] [Google Scholar]
- 19.Obafemi AA, et al. , Popcorn calcification in osteogenesis imperfecta: incidence, progression, and molecular correlation. Am J Med Genet A, 2008. 146A(21): p. 2725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munns CF, et al. , Respiratory distress with pamidronate treatment in infants with severe osteogenesis imperfecta. Bone, 2004. 35(1): p. 231–4. [DOI] [PubMed] [Google Scholar]
- 21.Glorieux FH, et al. , Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res, 2000. 15(9): p. 1650–8. [DOI] [PubMed] [Google Scholar]
- 22.Glorieux FH, et al. , Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res, 2002. 17(1): p. 30–8. [DOI] [PubMed] [Google Scholar]
- 23.••.Cabral WA, et al. , Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet, 2007. 39(3): p. 359–65.This is the first article describing LEPRE1 mutations in recessive OI patients.
- 24.••.Ishikawa Y, et al. , Biochemical characterization of the prolyl 3-hydroxylase 1.cartilage-associated protein.cyclophilin B complex. J Biol Chem, 2009. 284(26): p. 17641–7.This study demonstrates that the prolyl 3-hydroxylation complex, formed by Crtap/P3h1/CypB also has collagen chaperone activity.
- 25.••.van Dijk FS, et al. , PPIB mutations cause severe osteogenesis imperfecta. Am J Hum Genet, 2009. 85(4): p. 521–7.This study demonstrates that mutations in the gene encoding the third component of the prolyl 3-hydroxylation complex,Cyclophilin B, also cause recessive OI.
- 26.•.Alanay Y, Avaygan H, Camacho N, et al. : Mutations in a gene encoding a rough endoplasmic reticulum protein causes autosomal recessive progressive deforming osteogenesis imperfecta. [Abstract 212]. Presented at the ASHG 59th Annual Meeting Honolulu, HI; October 20–24, 2009 27.Identification of FKBP65 mutations, another collagen chaperone molecule, in recessive OI cases negative for mutation in COL1A1, COL1A2, CRTAP and LEPRE1 demonstrates the importance of collagen chaperone molecules in human disease.
- 27.•.Drogemuller C, et al. , A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta. PLoS Genet, 2009. 5(7): p. e1000579.This article identifies the first mutations in the gene coding HSP47, a collagen specific chaperone, in a dog strain affected with recessive OI.
- 28.Myllyharju J, Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol, 2003. 22(1): p. 15–24. [DOI] [PubMed] [Google Scholar]
- 29.Kefalides NA, Structure and biosynthesis of basement membranes. Int Rev Connect Tissue Res, 1973. 6: p. 63–104. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins CL, et al. , Effect of 3-hydroxyproline residues on collagen stability. J Am Chem Soc, 2003. 125(21): p. 6422–7. [DOI] [PubMed] [Google Scholar]
- 31.Mizuno K, et al. , Effect of the -Gly-3(S)-hydroxyprolyl-4(R)-hydroxyprolyl- tripeptide unit on the stability of collagen model peptides. Febs J, 2008. 275(23): p. 5830–40. [DOI] [PubMed] [Google Scholar]
- 32.Birk DE, et al. , Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci, 1990. 95 ( Pt 4): p. 649–57. [DOI] [PubMed] [Google Scholar]
- 33.Wenstrup RJ, et al. , Type V collagen controls the initiation of collagen fibril assembly. J Biol Chem, 2004. 279(51): p. 53331–7. [DOI] [PubMed] [Google Scholar]
- 34.Wenstrup RJ, et al. , A splice-junction mutation in the region of COL5A1 that codes for the carboxyl propeptide of pro alpha 1(V) chains results in the gravis form of the Ehlers-Danlos syndrome (type I). Hum Mol Genet, 1996. 5(11): p. 1733–6. [DOI] [PubMed] [Google Scholar]