Abstract
The cerebellum works as a network hub for optimizing eye movements through its mutual connections with the brainstem and beyond.
Here, we review three key areas in the cerebellum that are related to control of eye movements: (1) the flocculus/paraflocculus (tonsil) complex, primarily for high-frequency, transient vestibular responses, and also for smooth pursuit maintenance and steady gaze holding, (2) the nodulus/ventral uvula, primarily for low-frequency, sustained vestibular responses, and (3) the dorsal vermis/posterior fastigial nucleus primarily for accuracy of saccades.
Although there is no absolute compartmentalization of function within the three major ocular motor areas in the cerebellum, the structural-functional approach provides a framework for assessing ocular-motor performance in patients with disease involving the cerebellum or brainstem.
INTRODUCTION
The question, “What is a cerebellar eye sign?” has always been complicated by the unique features of the connectivity and function of the cerebellum. First, precise localization to the cerebellum can be confounded by the intimate afferent and efferent connections of the cerebellum with the brainstem, the thalamus and beyond, to the cerebral hemispheres (Figure 1). Even some of the deep “cerebellar” nuclei are displaced into the vestibular complex within the brainstem and receive direct projections from some of the cerebellar Purkinje cells that are related to vestibuloocular and vestibulospinal function. Secondly the cerebellum has a fundamental role in maintaining accurate, precisely calibrated motor performance, showing a robust adaptive capability that promptly responds to the changes required in the face of normal development and aging as well as disease and trauma. Unless you see a patient within seconds of the onset of a neurological insult, any abnormalities will reflect not only the immediate damage but also the attempt of the cerebellum to “repair” the problem. One consequence of this capability is that the effects of a new lesion in the cerebellum can reflect what the cerebellum has had to repair in the past. In other words, a previously repaired imperfection may be revealed when there is new damage to the cerebellum. The vagaries of disease and trauma, as well as the patterns of natural development and aging are idiosyncratic from patient to patient and challenged the cerebellum to have made different types of adjustments in the past. Furthermore, more than one area in the cerebellum may participate in the same function, though perhaps not to the same degree. Thus, one part of the cerebellum can attempt to substitute (and hide a defect) for another (Figures 2–5). Finally, the three major cerebellar arteries – anterior inferior, posterior inferior, and superior – each perfuse parts of both the cerebellum and the brainstem, and in the case of the anterior inferior cerebellar artery, the labyrinth, too. Thus, analyzing deficits in patients with strokes – a traditional mainstay of functional anatomical localization – is complicated by uncertainty about whether the lesion is confined to the cerebellum or also involves the brainstem or labyrinth.
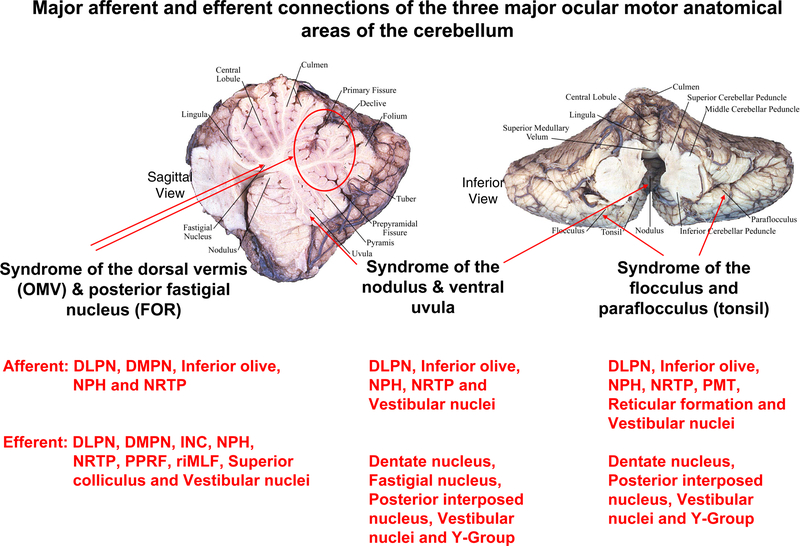
Figure 1.
Key cerebellar structures involved in the control of eye movements, sagittal view (left) and inferior view (right), and their major afferent and efferent connections. This is modified from Leigh and Zee, The Neurology of Eye Movements (Leigh and Zee, 2015)). Abbreviations: DLPN Dorsolateral Pontine Nuclei; DMPN Dorsomedial Pontine Nuclei; NPH Nucleus Prepositus Hypoglossi; NRTP Nucelus Reticularis Tegmenti Pontis; INC Interstitial Nucleus of Cajal; PPRF Paramedian Pontine Reticular Formation; riMLF Rostral Interstitial Nucleus of MLF; PMT Nuclei of the Paramedian Tract.
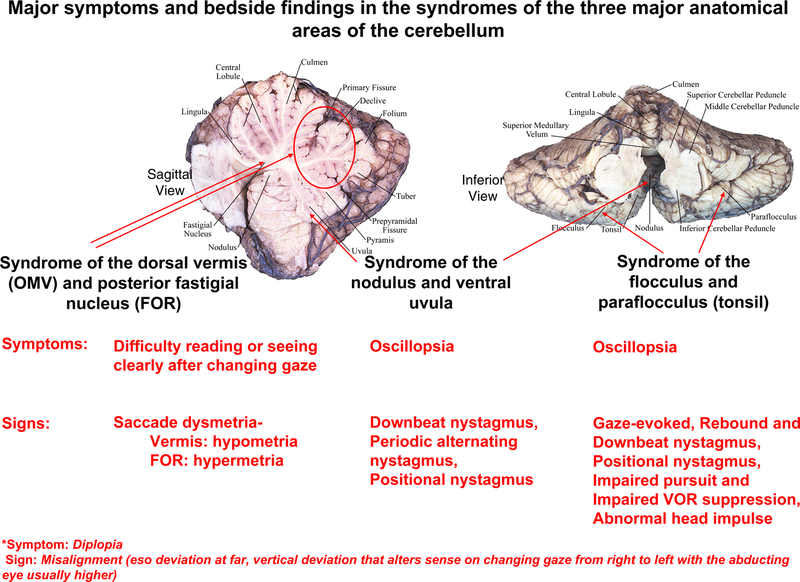
Figure 2.
Key cerebellar structures involved in the control of eye movements, sagittal view (left) and inferior view (right), and the major symptoms and signs of lesions in each. *Note: diplopia and ocular misalignment are not yet well localized and may arise from multiple areas in the cerebellum. Figure is modeled after Leigh and Zee, The Neurology of Eye Movements (Leigh and Zee, 2015).
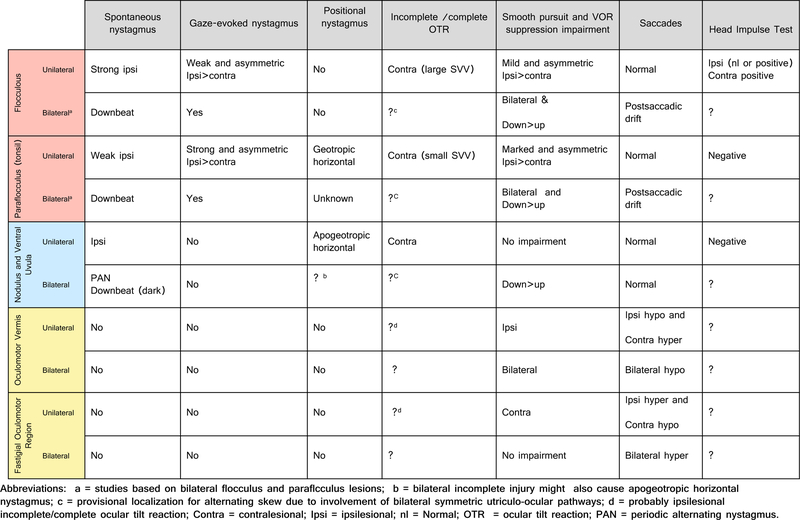
Figure 5.
Detailed ocular motor findings in key cerebellar structures involved in the control of eye movements in the cerebellum. Note the color coding corresponds to that in figure 4.
Despite these drawbacks, modern techniques of experimental and computational neuroscience including quantitative analysis of eye movements, and functional and anatomical imaging have allowed for plausible hypotheses about normal cerebellar functions, and in turn, clinically-useful guidelines about which ocular motor abnormalities reliably point to cerebellar dysfunction. Here we will review some of the better substantiated hypotheses about cerebellar eye signs, as well as touch on some of the latest updates to our knowledge of the cerebellar ocular motor syndromes. For this we will focus on abnormalities with lesions in three key areas in the cerebellum that are related to control of eye movements: the flocculus/paraflocculus (tonsil) complex, the inferior cerebellar vermis including the nodulus and ventral uvula, and the dorsal vermis and underling the fastigial nuclei. We will emphasize abnormalities that can usually be seen at the bedside on simple visual inspection.
By way of a simplified preview (Figure 2), and as an outline for the detailed presentation of localization in the cerebellum of ocular motor abnormalites, the major eye findings (and visual symptoms) in patients with cerebellar disease are seen with lesions of the vestibulocerebellum (flocculus/paraflocculus (tonsil), nodulus, ventral uvula) and with lesions of the dorsal vermis and underlying posterior portion of the fastigial nuclei (called the FOR, fastigial oculomotor region)1.
Lesions of the flocculus/paraflocculus are associated with 1) defects in relatively high-frequency, vestibular responses (such as the head-impulse test) 2) Impaired pursuit tracking and impaired suppression of the vestibulo-ocular reflex (VOR), and 3) impaired fixation including spontaneous (usually downbeat) nystagmus and gaze-evoked nystagmus, particularly in the horizontal plane. Such patients commonly complain of oscillopsia, the illusory movement of the environment.
Lesions of the nodulus/ventral uvula are associated with defects in low-frequency, sustained vestibular responses (mediated by a velocity-storage mechanism) and include spontaneous nystagmus in the vertical plane and periodic alternating nystagmus in the horizontal plane. Such patients also commonly complain of oscillopsia. Positional nystagmus has been reported with lesions in both areas of the vestibulocerebellum.
Lesions of the dorsal vermis/posterior fastigial nucleus are associated with defects in the accuracy of saccades: hypometria (undershooting) with bilateral vermal lesions and hypermetria (overshooting) with bilateral fastigial nucleus lesions. When the defect in saccades is severe such patients may complain of difficulty reading and inability to see clearly after changing gaze.
THE FLOCCULUS/PARAFLOCCULUS (TONSIL) COMPLEX
The major defects with lesions of the flocculus/paraflocculus (tonsil) complex are impaired tracking of objects moving in the environment, either with the head still or moving (VOR suppression), and an inability to hold gaze steady, both straight ahead and in eccentric positions in the orbit.
Pursuit
Experimental bilateral ablations of the flocculus and paraflocculus in monkeys provided the first firm clues to topical localization of pursuit in the cerebellum. Thus, impaired smooth pursuit, and incomplete suppression of an induced but unwanted vestibular nystagmus is a cardinal feature of the experimental floccular/parafloccular syndrome2,3,4.
“Up-down” asymmetry of vertical, gaze-velocity Purkinje cells of the flocculus that display a predilection for downward pursuit is the proposed mechanism for the asymmetrical pattern of vertical smooth pursuit impairment (downward pursuit more involved than upward pursuit) seen in monkeys with bilateral lesions of the flocculus and paraflocculus5.
Other cerebellar regions, however, are also involved in the control of smooth pursuit including the dorsal vermis, with its lateral extension in the cerebellar hemisphere around lobule VII, and the nodulus and uvula of the caudal vermis6,7,8,9. These regions may make partially, functionally-independent contributions to smooth tracking, i.e., the flocculus/paraflocculus to steady-state, sustained pursuit and the dorsal vermis to the initiation acceleration phase of pursuit.
Recent studies of patients with unilateral stroke in the flocculus or paraflocculus (tonsil) have suggested more specificity of ocular motor and vestibular functions in these regions, though, as with all patient case reports, and especially those of patients with cerebellar lesions, some variability is to be expected, even when the lesions seemingly involve the same areas. A further confound are the differences in the nomenclature and in the interpretation of functional versus anatomical homologies between the human and monkey cerebellum10. Thus, the ventral paraflocculus in monkeys corresponds to the accessory paraflocculus in humans, the dorsal paraflocculus in monkeys corresponds to the tonsil in humans and the lobulus petrosus of monkeys does not have a clear anatomical homology in humans though functionally it may be related to the tonsil. With these caveats it has been suggested that lesions in the tonsil may have greater effects on pursuit and horizontal gaze-holding than lesions in the flocculus. Vertical nystagmus is not a feature of unilateral floccular/parafloccular lesions11,12. Suppression of spontaneous nystagmus with fixation, while classically pointing to a peripheral origin for the nystagmus, may sometimes be spared in central, especially unilateral lesions13.
Gaze-holding
Lesions of the floccular/parafloccular complex impair gaze holding in eccentric positions, so that the eyes drift centripetally, generating a gaze evoked nystagmus2 (video at https://collections.lib.utah.edu/ark:/87278/s6089dz6)14. This finding implies the floccular/parafloccular complex helps to regulate the brainstem networks that mathematically transform velocity to position commands (position is an integral of velocity over time). This gaze-holding mechanism – the ocular motor integrator – holds the eye still after each conjugate eye movement. If integration is imperfect, the output of the integrator slowly dissipates (the rate of decay is reflected in the time constant of decay of the imperfect integrator) leading to centripetal drift. This requires resetting eccentric saccades to restore fixation of the desired target, producing a gaze-evoked nystagmus. The components of the integrator circuit in the brainstem include the medial vestibular nucleus and the nucleus prepositus hypoglossi (MVH-NPH) for horizontal eye position and the superior vestibular nucleus (SVN) and interstitial nucleus of Cajal (INC) for eye vertical position. Experimental work suggests that the brainstem component of the ocular motor neural integrator is inherently “leaky”, i.e., imperfect, and the floccular/parafloccular complex increases the fidelity (as reflected in the time constant) of this network by feedback of eye movement signals to the brainstem.
Spontaneous nystagmus
Downbeat nystagmus is characteristic of monkeys with bilateral lesions in the flocculus/paraflocculus2 and a frequent feature of many cerebellar disorders in human beings (video at https://collections.lib.utah.edu/ark:/87278/s6dj8q9h)15. Various hypotheses on its mechanism have been proposed including 1) an imbalance in central vestibular tone caused by disinhibition of the VOR pathways mediated by the anterior semicircular canals (note the flocculus inhibits anterior but not posterior semicircular pathways in the brainstem)16, 2) “up-down” asymmetry of vertical, gaze-velocity Purkinje cells of the flocculus that display a predilection for downward pursuit17,18 and 3) disinhibition of inherent gravitational pathways modulated by the otolith signals, which cause the eyes to drift up19,20.
For horizontal eccentric gaze in monkeys, lesions of the flocculus/paraflocculus always induce a gaze-evoked nystagmus with a velocity-decreasing waveform, implying that the brainstem component of the ocular motor integrator is “leaky” or imperfect. For vertical gaze, lesions of the flocculus/paraflocculus always induce downbeat nystagmus, but, from animal to animal the waveform of the slow phase is variable. In some animals the waveform of the slow phase is velocity-decreasing, implying a “leaky” vertical neural integrator in which case output of the integrator decreases over time; in other animals the waveform of the slow phase is velocity-increasing, implying an “unstable” vertical neural integrator in which case the output of the integrator increases with time. Hence, lesions of the floccular/parafloccular complex unveil the inherent imperfections of the brainstem components of the ocular motor neural integrators. The exact pattern of abnormality that emerges will depend on the ocular motor history of the animal (e.g., injury or disease) and inborn propensities2.
In patients who have downbeat nystagmus with velocity-increasing waveforms, the intensity of the nystagmus increases when the position of the eye is moved in the direction of the slow phase, the opposite of Alexander’s law for a vestibular nystagmus that states the intensity of the nystagmus increases when the position of the eye is moved in the direction of the quick phase.
The potassium channel blockers, 4-Aminopyidine (4-AP) and 3,4-Diaminopyridine (3,4-DAP) can reduce downbeat nystagmus in many patients with cerebellar disease21,22. The probable mechanism is prolongation of action potentials in Purkinje cells which restores their precision of pacemaking23,24.
When the eyes return to the straight-ahead position after sustained horizontal eccentric gaze, a ‘rebound nystagmus’ commonly occurs with slow phases directed toward the previously held eccentric eye position (video at https://collections.lib.utah.edu/ark:/87278/s6vx45m7)25. On sustained eccentric gaze, the amplitude of the gaze-evoked nystagmus may reverse direction, such that the eyes drift centrifugally (away from the straight-ahead position), leading to a centripetal nystagmus, beating toward the straight-ahead position26,27. Both rebound and centripetal nystagmus reflect how neuronal circuits within the brainstem or in undamaged parts of the cerebellum try to counteract the centripetal drift precipitated by gaze-evoked nystagmus.
Postsaccadic drift
Postsaccadic drift, a brief movement lasting a few hundred milliseconds following each saccade, is also characteristic of the floccular/parafloccular syndrome in monkeys. It results from a mismatch in the relative amplitudes of the saccade pulse (phasic innervation, generated by brainstem “burst” neurons to rapidly move the eye to a new position) and the step (tonic innervation, the output of the neural integrator, to hold the eye steady in its new position). The direction of postsaccadic drift, backwards or onwards, is idiosyncratic from animal to animal, and depends on the strength (less or more, respectively) of the step output of the ocular motor integrator relative to the size of the pulse2.
Vestibulo-ocular reflexes (VOR)
Ablation of the flocculus/paraflocculus in monkeys variably affects the gain (amplitude of response (eye motion) relative to the amplitude of the stimulus (head motion)) of the VOR. It may increase, decrease or stay the same. What is consistent among all lesioned animals is impaired adaptation; a loss of the ability to optimize VOR performance by changing the gain, direction, or phase of the compensatory response as needed2,3,28,29. We note that in monkeys with floccular/parafloccular lesions only the rotational VOR was measured. We have no data, in monkeys or in human patients on how lesions of the floccular/parafloccular complex affect the translational VOR (side-to side, up and down or fore and aft). Lesions of the flocculus in patients, however, can produce abnormalities in the otolith-ocular response to the change in linear acceleration from the pull of gravity when the head is tilted laterally. For example, some features of the ocular tilt reaction (OTR) emerge with unilateral lesions in the floccular/parafloccular complex. The OTR is the pathological response to a faulty central estimate of the lateral (toward the shoulder) position of the head with respect to gravity. The findings include skew deviation, ocular counterroll, abnormal head tilt and altered perception of upright. The consistent feature is a contraversive tilt of the subjective visual vertical30,31. One can consider that the OTR reflects an imbalance in static otolith-ocular reflex tone analogous to the spontaneous nystagmus that reflects an imbalance in semicircular canal-ocular reflex tone. As we will show, components of the OTR as well as spontaneous nystagmus also occur with lesions in the nodulus.
Changes in vestibular responses measured with head impulses and with caloric stimulation have been reported in several patients with isolated infarctions of the flocculus though again with some variability30,31. The consistent vestibular finding, however, was an impaired quantitative response to high-velocity impulses with the head rotating toward the intact side. At 24 weeks follow up of a single patient, the head impulse response almost completely recovered, with the slow-phase gain of the VOR returning to the normal range. There were, however, residual corrective saccades following head impulses directed away from the side of the floccular lesion, revealing persistent mild hypofunction31. Cross-coupled responses, e.g., an unwanted vertical component of the response, and an increased gain of the slow phase with backup corrective saccades are also signs on horizontal head-impulse testing in patients with diffuse cerebellar dysfunction32. Experimental lesions suggest a localization to the flocculus/paraflocculus but data for human patients is not conclusive33. Cross-coupling in some cerebellar patients also occurs during low-frequency, vestibular stimulation (caloric irrigations or constant-velocity head rotations) but it likely is related to lesions in the cerebellar nodulus/ventral uvula.
Positional nystagmus
More recently a geotropic, direction-changing, horizontal positional nystagmus (beating toward the ground in both the right ear and left ear down positions), which can mimic the benign form of peripheral paroxysmal positional vertigo from the lateral semicircular canal, has been associated with lesions in the cerebellar tonsil34. Note that these patients also had markedly impaired horizontal pursuit and some had positional downbeat nystagmus; findings that are not seen in benign positional vertigo from the lateral semicircular canal. This will be discussed further below.
NODULUS/VENTRAL UVULA
The nodulus/ventral uvula and immediate paravermal areas have important connections to brainstem areas that are concerned with low-frequency vestibular responses, especially the so-called “velocity-storage mechanism” within the brainstem. During low-frequency horizontal (yaw-axis) rotation, e.g., a sustained constant-velocity rotation, the time taken for the response of the vestibular nerve (which reflects the position of the cupula) to decline to 37% of its initial time (defined as the time constant) is about 5 seconds. The central velocity-storage mechanism, however, extends the duration of the nystagmus response threefold (time constant becomes about 15 seconds), thus improving the performance of the VOR at low-frequency rotations35.
The velocity-storage mechanism is also instrumental in solving the tilt-translation ambiguity of otolith signals: Am I tilted or am I translating? Each requires a different response, ocular counterroll (torsion) and a sustained horizontal nystagmus, respectively, to the same change in linear acceleration (gravity or translation) that is signaled by the otolith organs. The velocity-storage mechanism is used to calculate an internal estimate of tilt of the head with respect to gravity by integrating signals encoding rotational velocity of the head from the vertical semicircular canals during lateral head tilt. This internal estimate of head tilt is then subtracted from the gravito-inertial (linear acceleration) signal that is transmitted directly from the otoliths to calculate an internal estimate of the translation of the head. This signal, in turn, drives the horizontal translational vestibulo-ocular reflex36.
Vestibulo-ocular reflexes (VOR)
Lesions of the nodulus/uvula remove the inhibition normally derived from the projections from its Purkinje cells to the velocity-storage mechanism in the vestibular nuclei, thus unmasking a potential for instability and oscillations such as periodic alternating nystagmus (see below). For the horizontal VOR, lesions in the nodulus prolong the VOR response to a constant-velocity rotation (time constant is increased)37.
Patients also lose the ability to “dump” (rapidly discharge, also called tilt-suppression) the velocity-storage mechanism when pitching the head forward immediately following the end of a constant-velocity rotation of the head or following a sustained period of head-shaking in patients with unilateral vestibular imbalance37,38,39. There is also a loss of the normal decrease in the VOR time constant after repetitive stimulation in the dark (habituation)40. Cross-coupling of responses from horizontal to vertical during sustained horizontal rotations of the head and a downbeat nystagmus following sustained horizontal head shaking reflect interference with the normal functions of the nodulus to assure that eye responses are appropriately aligned with the plane of rotation of the body41,42,43.
As indicated above the nodulus/uvula also process signals arising from the utricle and saccule to drive the translational VOR (t-VOR). Ablation of the nodulus/uvula in monkeys causes a deficit in the integration of linear head acceleration to sustain eye velocity during constant-velocity head translation44. For horizontal translation the reduction in t-VOR occurs only in the dark, whereas for vertical translation the reduction in t-VOR occurs both in the dark and light45 implying a more specific role for the nodulus/uvula in vertical translation. No data is available from human patients on the effects of focal lesions in the flocculus/paraflocculus or nodulus/uvula regions on the translational VOR.
As with the flocculus/paraflocculus complex, some patients with lesions in the nodulus/uvula have abnormalities of some features of the OTR, again a contraversive tilt of the subjective sense of upright accompanied by ocular torsion46. Lesions in other parts of the cerebellum but sparing the nodulus/uvula/dentate nucleus may lead to an ipsiversive OTR47, 48.
With degenerative lesions, patients often show a skew deviation that alternates sense on far left and far right gaze such that the abducting eye is usually higher. Evolutionary hypotheses predict these abnormal patterns of eye alignment reflect the emergence of static otolith-ocular reflexes in frontal-eyed animals that would be “appropriate” for lateral-eyed animals with their heads pitched backwards or forwards while their eyes were directed to the right or left49. In the case of human patients, a faulty sense of the direction of the pull of gravity, backwards or forwards, could lead to the peculiar disorder of eye alignment with the abducting or adducting eye being higher, respectively. As described below, a faulty sense of the direction of the pull of gravity (pitched forward or backward) also contributes to the patterns of central positional nystagmus that mimic a lateral canal benign positional vertigo.
Pursuit
Experimental lesions of the nodulus/uvula also reproduce the “up-down” asymmetry pattern of vertical smooth pursuit impairment seen in floccular/paraflocculus injury (downward pursuit is impaired with little effect on upward pursuit)9. There is no tight relationship, however, between the deficit in vertical pursuit and any spontaneous vertical nystagmus.
Spontaneous nystagmus
Periodic alternating nystagmus (PAN) – a horizontal crescendo-decrescendo jerk nystagmus that reverses direction every 90 seconds –may emerge in the dark following ablation of the nodulus/uvula37 (video at https://collections.lib.utah.edu/ark:/87278/s62k013r)50. Underlying the pathogenesis of PAN in humans in whom the nystagmus is also seen in the light are (1) a disinhibited unstable velocity-storage mechanism in the brainstem, (2) an intact adaptive network – probably located within the brainstem – that nulls sustained, inappropriate nystagmus, and (3) an inability to use motion of images on the retina as error signals to suppress the nystagmus51. This last finding implies that for PAN to be seen in the light, other regions of the cerebellum, such as the flocculus/paraflocculus, involving mechanisms that suppress unwanted nystagmus during fixation, must also be affected.
Inhibition by Purkinje cells from the nodulus/uvula on the velocity-storage mechanism is mediated through GABA-receptors, metabotropic G-protein channels that cause slow postsynaptic inhibition by closing Ca + and opening of K + channels (slow IPSP). Disengagement of the disinhibited velocity-storage mechanism using Baclofen, a surrogate GABA-B agonist, abolishes PAN52.
Animals with experimental lesions of the nodulus/uvula also have downbeat nystagmus. Contrary to the downbeat nystagmus seen with flocculus/paraflocculus lesions, the nystagmus can be suppressed with visual fixation and is seen only in darkness, and slow-phase velocity is not increased in lateral gaze or decreased in up gaze. These findings imply the downbeat nystagmus with nodulus/uvula lesions is unlikely related to an abnormal vertical ocular motor integrator but rather due to a central vestibular imbalance, possibly in saccular otolith-ocular pathways that mediate the vertical translational VOR53. In contrast, the downbeat nystagmus associated with floccular/nodular lesions is probably related to effects upon anterior semicircular canal pathways that mediate the vertical rotational VOR.
Positional nystagmus
Positional nystagmus also occurs with lesions in the nodulus and can mimic benign positional vertigo from the lateral canal. But, in contrast to the geotropic horizontal beating nystagmus with lesions in the tonsil, in the case of the nodulus the mimic is of an apogeotropic (beats toward the sky) horizontal nystagmus. A recent model for central positional nystagmus predicted that the nystagmus with lesions in the nodulus/uvula arises from a mismatch between gravito-inertial acceleration (GIA) signals provided by the otolith and a central faulty estimate of the direction of gravity derived from the velocity-storage mechanism. Depending upon whether the faulty estimate of gravity is pitched backward or forward one can get a geotropic or apogeotropic direction-changing, horizontal positional nystagmus54.
DORSAL CEREBELLAR VERMIS AND POSTERIOR FASTIGIAL NUCLEUS
Saccades
Experimental bilateral lesions in the dorsal cerebellar vermis (OMV, ocular motor vermis) lead to acute abnormalities of saccades including the initiation time (latency), accuracy (trajectory, amplitude and direction), velocity and acceleration55,56,57. The main enduring change, however, is inaccuracy (hypometria) of saccades. Purkinje cells in the OMV are active before saccades, and electrical stimulation of this region evokes saccades58,59. Observations in monkeys were confirmed in human studies with stimulation using transcranial magnetic stimulation (TMS) over the posterior cerebellum and with functional magnetic resonance imaging (fMRI)60,61.
Lesions of the OMV also impair saccade adaptation, an error-detector mechanism that normally adjusts premotor commands in the long-term to maintain or restore the accuracy of saccades. This defect may be related to impaired learning per se, or when error signals are inconsistent because of variability of the amplitude of saccades55,56,62,63. The error signals that trigger cerebellar learning probably arrive on climbing fiber projections to the vermis from the inferior olive64,65,66.
Neurons in the fastigial oculomotor region (FOR) generate presaccadic bursts for contralateral saccades (facilitate contraversive saccades) and a late saccadic burst for ipsiversive saccades (terminate ipsiversive saccades)67. Projections from the FOR to the brainstem are through the contralateral hooked bundle of Russell (uncinate fasciculus of the cerebellum) that runs aside the superior cerebellar peduncle. They impinge on inhibitory burst neurons that normally act to stop saccades directed contralaterally (ipsilateral to the FOR). Note the output pathway from the FOR crosses immediately to the other side, traversing the contralateral FOR before exiting along the contralateral superior cerebellar peduncle. This anatomical arrangement means that a unilateral structural lesion of the FOR will always be, in effect, a bilateral lesion. A unilateral functional lesion of the FOR, however, is possible if the overlying cerebellar vermis is affected on one side. Furthermore, experimental lesions of just one FOR (created by injections of neurotoxins that affect cell bodies only) lead to ipsilesional hypermetric and contralesional hypometric saccades. Bilateral FOR lesions produce hypermetria for saccades in all directions68.
The OMV monitors the premotor commands that generate saccades as they unfold, and via its inhibitory projections can adjust FOR activity to assure the saccade stops on target69,70. Similarly, but conversely to the FOR, each side of the OMV facilitates ipsiversive saccades and helps terminate contraversive saccades. Consequently, lesions of the OMV cause ipsilesional hypometric and contralesional hypermetric saccades. Bilateral OMV lesions cause hypometric saccades in both horizontal directions55.
One important clinical example of a unilateral, “functional” lesion of the FOR is seen in Wallenberg’s syndrome with infarction of the dorsal lateral medulla. In this case inputs from climbing fibers, which run in the inferior cerebellar peduncle and impinge upon the Purkinje cells of the dorsal vermis, are interrupted, causing increased simple-spike activity of Purkinje cells, which in turn inhibits the underlying FOR on that side. This leads to hypermetria of saccades, so-called ipsipulsion, to the side of the lesion (see video at https://collections.lib.utah.edu/ark:/87278/s65176w6)71.
Another area within the cerebellum related to the generation of saccades is the caudal dentate nucleus. In particular the generation of anti-saccades, i.e., saccades directed willfully to the opposite, mirror location of a suddenly-appearing target in one hemifield, are influenced by activity in this area of the cerebellum72.
Pursuit
In human studies, stimulation of the OMV by focal TMS over the posterior cerebellum modulates smooth pursuit acceleration and maintenance73. Studies in monkeys revealed that Purkinje cells in the OMV and FOR have directional selectivity that is related to smooth pursuit acceleration (the initial 100 ms of tracking, prior to the onset of corrective feedback), to maintenance (steady-state tracking) and to termination of pursuit. Neurons in the FOR generate an early burst for contralateral pursuit (facilitate contraversive pursuit) and a late burst for ipsilateral pursuit (terminate ipsiversive pursuit), equivalent to the pattern of control over saccades by the FOR. Similarly, but conversely to the FOR (accounted for by the inhibitory nature of Purkinje cells), each side of the OMV acts to facilitate ipsiversive pursuit and mediates the termination of contraversive pursuit74,75.
Purkinje cells in the flocculus/paraflocculus may be engaged more with smooth pursuit maintenance, whereas Purkinje cell in the vermis more with smooth pursuit initiation and termination. In addition, the Purkinje cells in the vermis modulates their discharge in response to visual stimuli (retinal slip velocity) and oculomotor stimuli (predictive feedback mechanisms), whereas Purkinje cells in the flocculus/paraflocculus modulate their activity only related to eye movements75,76. More recently, it was shown that the Purkinje cells in the flocculus/paraflocculus play a major role in directional learning, while the Purkinje cells in the vermis have a larger impact in speed learning77. These new physiological findings await correlates in human patients with various cerebellar lesions.
With a lesion in the FOR, contralateral acceleration for pursuit is decreased and ipsilateral acceleration is increased. The contralateral pursuit gain in the maintenance phase is also impaired.
Bilateral lesions in the FOR, however, restore the balance between the two FOR and horizontal pursuit is preserved, indicating pursuit can be generated outside the FOR.
Apropos the inhibitory nature of the OMV on FOR, ipsilesional acceleration is decreased and bilateral lesions impair horizontal pursuit acceleration in both directions6,78. Thus far, however, no simple scheme for smooth pursuit has been developed to incorporate different roles of different parts of the cerebellum in pursuit tracking. There is even evidence that the cerebellar hemispheres play a role in pursuit79.
OCULAR MISALIGNMENT
Patients with cerebellar lesions may also have an esodeviation (eyes turn inward) at distance viewing that simulates a divergence palsy (Figure 2, bottom). This pattern of strabismus has been created experimentally in monkeys with dorsal vermal lesions but may also arise from involvement of other areas of the cerebellum80. As noted above an alternating hyperdeviation, usually with the abducting eye higher, on changing horizontal gaze from right to left, is also a feature of cerebellar disease. Its localization is uncertain.
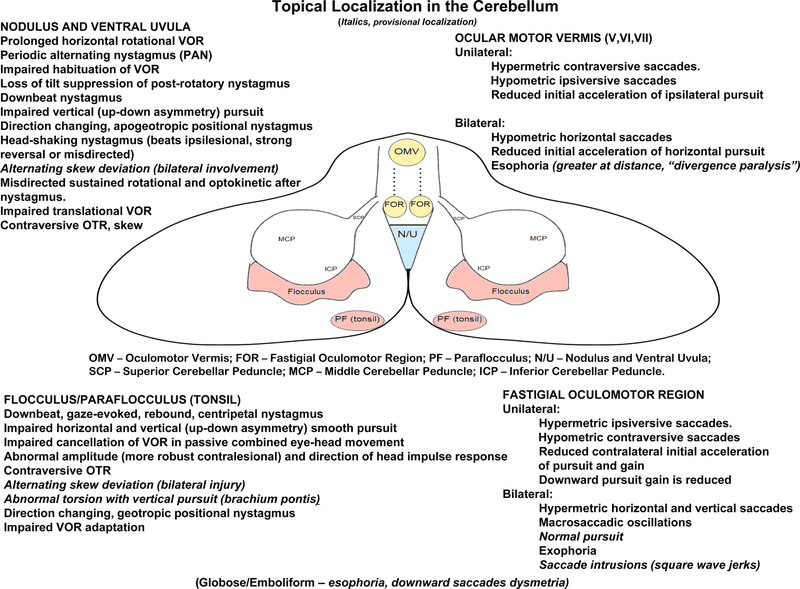
Figure 3.
Illustration of cerebellar structures involved in control of eye movements (inferior view) and topical oculomotor localization with cerebellar lesions. Figure is modeled after Leigh and Zee, The Neurology of Eye Movements (Leigh and Zee, 2015)
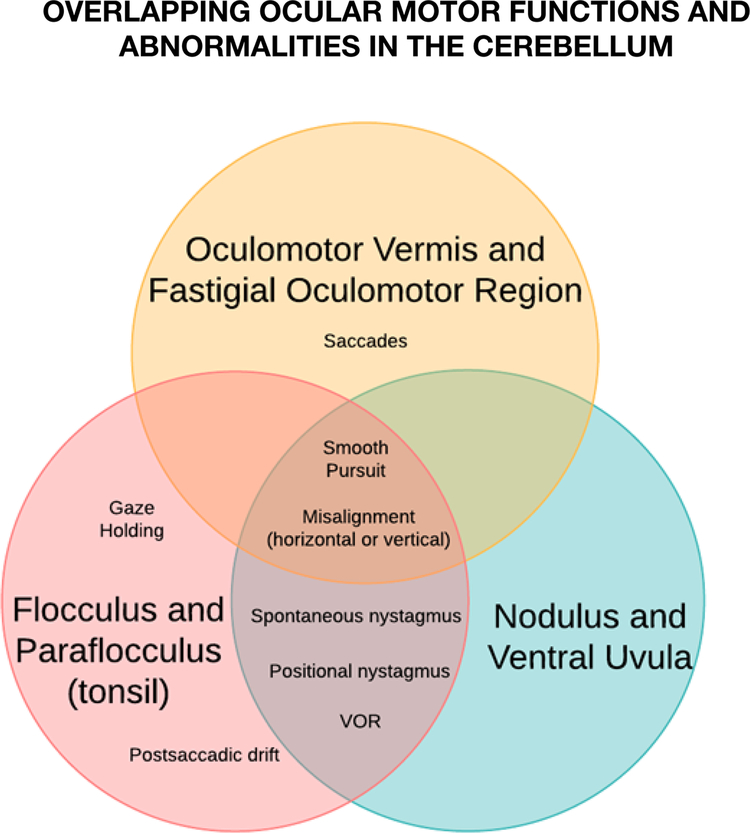
Figure 4.
Illustration of overlapping ocular motor functions and abnormalities in the cerebellum (demonstrated in three-set Venn diagram). VOR, vestibulo-ocular reflex. *Abnormalities of ocular alignment are not yet well localized
TAKE HOME MESSAGES.
Eye movements abnormalities are easy to observe clinically and to measure and quantify, making them excellent markers for assessing diseases that involve the cerebellum81.
Lesions in the flocculus and paraflocculus (tonsil) lead to spontaneous downbeat nystagmus, defects in eccentric gaze holding, impaired smooth pursuit, and abnormalities of high-frequency, high-velocity brief head rotations (head impulses) (figures 2–3).
Lesions in the nodulus and ventral uvula lead to spontaneous downbeat nystagmus, periodic alternating nystagmus, and changes in the response to low-frequency, sustained head rotations (Figures 2–3).
Lesions in the dorsal vermis and underlying fastigial nuclei lead to inaccurate saccades: hypermetria with bilateral fastigial nucleus lesions and hypometria with bilateral dorsal vermis lesions (Figures 2–3).
There is no absolute compartmentalization of function within the three major ocular motor areas in the cerebellum; however, this redundancy is beneficial as part of the essential role that the cerebellum plays in maintaining movements accurate in the face of disease, trauma, natural development and aging (Figure 4).
New technology – for example, quantitative bedside video-oculography, high-resolution structural and functional imaging, and transcranial direct current stimulation82 – enables better localization and characterization of cerebellar deficits. This information will assist in developing better diagnostic algorithms, and novel treatments, including medications and rehabilitation programs that can take advantage of the central role of the cerebellum in monitoring and adjusting movements to keep them accurate.
Footnotes
Disclosure: No conflicts of interest
References
- 1.Daniel R Gold DO Techniques for the clinical ocular motor examination can be found on The Dan Gold Neuro-Ophthalmology Collection. [Neuro-Ophthalmology Virtual Education Library: NOVEL Web Site]. 2016. Available at: https://novel.utah.edu/Gold/collection.php. Accessed January 18, 2019. [Google Scholar]
- 2.Zee DS, Yamazaki A, Butler PH, Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981;46(4):878–99. [DOI] [PubMed] [Google Scholar]
- 3.Rambold H, Churchland A, Selig Y, Jasmin L, Lisberger SG. Partial ablations of the flocculus and ventral paraflocculus in monkeys cause linked deficits in smooth pursuit eye movements and adaptive modification of the VOR. J Neurophysiol. 2002;87(2):912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takemori S, Cohen B. Loss of visual suppression of vestibular nystagmus after flocculus lesions. Brain Res. 1974;72(2):213–24. [DOI] [PubMed] [Google Scholar]
- 5.Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol. 1990;63(5):1241–61. [DOI] [PubMed] [Google Scholar]
- 6.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol. 2000;83(4):2047–62. [DOI] [PubMed] [Google Scholar]
- 7.Ohki M, Kitazawa H, Hiramatsu T, Kaga K, Kitamura T, Yamada J, et al. Role of primate cerebellar hemisphere in voluntary eye movement control revealed by lesion effects. J Neurophysiol. 2009;101(2):934–47. [DOI] [PubMed] [Google Scholar]
- 8.Heinen SJ, Keller EL. The function of the cerebellar uvula in monkey during optokinetic and pursuit eye movements: single-unit responses and lesion effects. Exp Brain Res. 1996;110(1):1–14. [DOI] [PubMed] [Google Scholar]
- 9.Walker MF, Tian J, Shan X, Tamargo RJ, Ying H, Zee DS. Lesions of the cerebellar nodulus and uvula impair downward pursuit. J Neurophysiol. 2008;100(4):1813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voogd J, Marani E. Essentials of Cerebellum and Cerebellar Disorders: Springer; 2016. 656 p. [Google Scholar]
- 11.Lee SH, Park SH, Kim JS, Kim HJ, Yunusov F, Zee DS. Isolated unilateral infarction of the cerebellar tonsil: ocular motor findings. Ann Neurol. 2014;75(3):429–34. [DOI] [PubMed] [Google Scholar]
- 12.Choi JY, Kim JS. Nystagmus and central vestibular disorders. Curr Opin Neurol. 2017;30(1):98–106. [DOI] [PubMed] [Google Scholar]
- 13.Kim HA, Yi HA, Lee H. Failure of fixation suppression of spontaneous nystagmus in cerebellar infarction: frequency, pattern, and a possible structure. Cerebellum. 2016;15(2):182–9. [DOI] [PubMed] [Google Scholar]
- 14.Daniel R Gold DO Gaze-evoked and rebound nystagmus in a cerebellar syndrome. Video. [Neuro-Ophthalmology Virtual Education Library: NOVEL Web Site]. 2016. Available at: https://collections.lib.utah.edu/ark:/87278/s6089dz6. Accessed January 18, 2019.
- 15.Daniel R Gold DO Cerebellar eye signs in SCA8. Video. [Neuro-Ophthalmology Virtual Education Library: NOVEL Web Site]. 2016. Available at: https://collections.lib.utah.edu/ark:/87278/s6dj8q9h. Accessed January 18, 2019. [Google Scholar]
- 16.Ito M, Nisimaru N, Yamamoto M. Specific patterns of neuronal connexions involved in the control of the rabbit’s vestibulo-ocular reflexes by the cerebellar flocculus. J Physiol. 1977;265(3):833–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marti S, Straumann D, Glasauer S. The origin of downbeat nystagmus: an asymmetry in the distribution of on-directions of vertical gaze-velocity Purkinje cells. Ann N Y Acad Sci. 2005;1039:548–53. [DOI] [PubMed] [Google Scholar]
- 18.Marti S, Straumann D, Büttner U, Glasauer S. A model-based theory on the origin of downbeat nystagmus. Exp Brain Res. 2008;188(4):613–31. [DOI] [PubMed] [Google Scholar]
- 19.Marti S, Palla A, Straumann D. Gravity dependence of ocular drift in patients with cerebellar downbeat nystagmus. Ann Neurol. 2002;52(6):712–21. [DOI] [PubMed] [Google Scholar]
- 20.Bremova T, Glasauer S, Strupp M. Downbeat nystagmus: evidence for enhancement of utriculo-ocular pathways by ocular vestibular evoked myogenic potentials? Eur Arch Otorhinolaryngol. 2015;272(11):3575–83. [DOI] [PubMed] [Google Scholar]
- 21.Strupp M, Schüler O, Krafczyk S, Jahn K, Schautzer F, Büttner U, et al. Treatment of downbeat nystagmus with 3,4-diaminopyridine: a placebo-controlled study. Neurology. 2003;61(2):165–70. [DOI] [PubMed] [Google Scholar]
- 22.Kalla R, Glasauer S, Büttner U, Brandt T, Strupp M. 4-aminopyridine restores vertical and horizontal neural integrator function in downbeat nystagmus. Brain. 2007;130(Pt 9):2441–51. [DOI] [PubMed] [Google Scholar]
- 23.Alvina K, Khodakhah K. The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J. Neurosci 2010;30:7258–7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalla R, Strupp M. Aminopyridines and acetyl-DL-leucine: new therapies in cerebellar disorders. Curr Neuropharmacol. Epub 2018. September 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel R Gold DO Ocular motor signs in SCA 6. Video. [Neuro-Ophthalmology Virtual Education Library: NOVEL Web Site]. 2017. Available at: https://collections.lib.utah.edu/ark:/87278/s6vx45m7. Accessed January 18, 2019. [Google Scholar]
- 26.Lin CY, Young YH. Clinical significance of rebound nystagmus. Laryngoscope. 1999;109(11):1803–5. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto T, Sasaki O, Yoshida K, Takei Y, Ikeda S. Periodic alternating nystagmus and rebound nystagmus in spinocerebellar ataxia type 6. Mov Disord. 2003;18(10):1201–4. [DOI] [PubMed] [Google Scholar]
- 28.Miles FA, Lisberger SG. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–99. [DOI] [PubMed] [Google Scholar]
- 29.Lisberger SG, Miles FA, Zee DS. Signals used to compute errors in monkey vestibuloocular reflex: possible role of flocculus. J Neurophysiol. 1984;52(6):1140–53. [DOI] [PubMed] [Google Scholar]
- 30.Park HK, Kim JS, Strupp M, Zee DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol. 2013;260(6):1576–82. [DOI] [PubMed] [Google Scholar]
- 31.Yacovino DA, Akly MP, Luis L, Zee DS. The floccular syndrome: dynamic changes in eye movements and vestibulo-ocular reflex in isolated infarction of the cerebellar flocculus. Cerebellum. 2018;17(2):122–31. [DOI] [PubMed] [Google Scholar]
- 32.Choi JY, Kim HJ, Kim JS. Recent advances in head impulse test findings in central vestibular disorders. Neurology. 2018;90(13):602–12. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Kim HJ, Kim JS. Perverted downward corrective saccades during horizontal head impulses in Chiari malformation. Cerebellum. Epub 2019. January 4. [DOI] [PubMed] [Google Scholar]
- 34.Choi SY, Jang JY, Oh EH, Choi JH, Park JY, Lee SH, et al. Persistent geotropic positional nystagmus in unilateral cerebellar lesions. Neurology. 2018;91(11):e1053–e7. [DOI] [PubMed] [Google Scholar]
- 35.Leigh J, Zee D. The Neurology of Eye Movements. 5th ed. New York, NY: Oxford University Press; 2015. 1136 p. [Google Scholar]
- 36.Laurens J, Angelaki DE. The functional significance of velocity storage and its dependence on gravity. Exp Brain Res. 2011;210(3–4):407–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waespe W, Cohen B, Raphan T. Dynamic modification of the vestibulo-ocular reflex by the nodulus and uvula. Science. 1985;228(4696):199–202. [DOI] [PubMed] [Google Scholar]
- 38.Lee SU, Choi JY, Kim HJ, Park JJ, Zee DS, Kim JS. Impaired tilt suppression of post-rotatory nystagmus and cross-coupled head-shaking nystagmus in cerebellar lesions: image mapping study. Cerebellum. 2017;16(1):95–102. [DOI] [PubMed] [Google Scholar]
- 39.Zuma E Maia FC, Cal R, D’Albora R, Carmona S, Schubert MC. Head-shaking tilt suppression: a clinical test to discern central from peripheral causes of vertigo. J Neurol. 2017;264(6):1264–70. [DOI] [PubMed] [Google Scholar]
- 40.Cohen H, Cohen B, Raphan T, Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res. 1992;90(3):526–38. [DOI] [PubMed] [Google Scholar]
- 41.Cohen B, John P, Yakushin SB, Buettner-Ennever J, Raphan T. The nodulus and uvula: source of cerebellar control of spatial orientation of the angular vestibulo-ocular reflex. Ann N Y Acad Sci. 2002;978:28–45. [DOI] [PubMed] [Google Scholar]
- 42.Walker MF, Zee DS. Directional abnormalities of vestibular and optokinetic responses in cerebellar disease. Ann N Y Acad Sci. 1999;871:205–20. [DOI] [PubMed] [Google Scholar]
- 43.Moon IS, Kim JS, Choi KD, Kim MJ, Oh SY, Lee H, et al. Isolated nodular infarction. Stroke. 2009;40(2):487–91. [DOI] [PubMed] [Google Scholar]
- 44.Walker MF, Tian J, Shan X, Tamargo RJ, Ying H, Zee DS. The cerebellar nodulus/uvula integrates otolith signals for the translational vestibulo-ocular reflex. PLoS One. 2010;5(11):e13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker MF, Tian J, Shan X, Tamargo RJ, Ying H, Zee DS. Lesions of the cerebellar nodulus and uvula in monkeys: effect on otolith-ocular reflexes. Prog Brain Res. 2008;171:167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarnutzer AA, Wichmann W, Straumann D, Bockisch CJ. The cerebellar nodulus: perceptual and ocular processing of graviceptive input. Ann Neurol. 2015;77(2):343–7. [DOI] [PubMed] [Google Scholar]
- 47.Wong AM, Sharpe JA. Cerebellar skew deviation and the torsional vestibuloocular reflex. Neurology. 2005;65(3):412–9. [DOI] [PubMed] [Google Scholar]
- 48.Baier B, Bense S, Dieterich M. Are signs of ocular tilt reaction in patients with cerebellar lesions mediated by the dentate nucleus? Brain. 2008;131(Pt 6):1445–54. [DOI] [PubMed] [Google Scholar]
- 49.Zee DS. Considerations on the mechanisms of alternating skew deviation in patients with cerebellar lesions. J Vestib Res. 1996;6(6):395–401. [PubMed] [Google Scholar]
- 50.Daniel R Gold DO Periodic alternating nystagmus and perverted head-shaking nystagmus in cerebellar degeneration. Video. [Neuro-Ophthalmology Virtual Education Library: NOVEL Web Site]. 2017. Available at: https://collections.lib.utah.edu/ark:/87278/s62k013r. Accessed January 18, 2019. [Google Scholar]
- 51.Leigh RJ, Robinson DA, Zee DS. A hypothetical explanation for periodic alternating nystagmus: instability in the optokinetic-vestibular system. Ann N Y Acad Sci. 1981;374:619–35. [DOI] [PubMed] [Google Scholar]
- 52.Halmagyi GM, Rudge P, Gresty MA, Leigh RJ, Zee DS. Treatment of periodic alternating nystagmus. Ann Neurol. 1980;8(6):609–11. [DOI] [PubMed] [Google Scholar]
- 53.Walker MF, Tian J, Shan X, Ying H, Tamargo RJ, Zee DS. Enhancement of the bias component of downbeat nystagmus after lesions of the nodulus and uvula. Ann N Y Acad Sci. 2009;1164:482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi JY, Glasauer S, Kim JH, Zee DS, Kim JS. Characteristics and mechanism of apogeotropic central positional nystagmus. Brain. 2018. [DOI] [PubMed] [Google Scholar]
- 55.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998;80(4):1911–31. [DOI] [PubMed] [Google Scholar]
- 56.Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci. 1999;19(24):10931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kojima Y, Soetedjo R, Fuchs AF. Effects of GABA agonist and antagonist injections into the oculomotor vermis on horizontal saccades. Brain Res. 2010;1366:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujikado T, Noda H. Saccadic eye movements evoked by microstimulation of lobule VII of the cerebellar vermis of macaque monkeys. J Physiol. 1987;394:573–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noda H, Fujikado T. Involvement of Purkinje cells in evoking saccadic eye movements by microstimulation of the posterior cerebellar vermis of monkeys. J Neurophysiol. 1987;57(5):1247–61. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto M, Ohtsuka K. Transcranial magnetic stimulation over the posterior cerebellum during visually guided saccades in man. Brain. 1995;118 (Pt 5):1185–93. [DOI] [PubMed] [Google Scholar]
- 61.Hayakawa Y, Nakajima T, Takagi M, Fukuhara N, Abe H. Human cerebellar activation in relation to saccadic eye movements: a functional magnetic resonance imaging study. Ophthalmologica. 2002;216(6):399–405. [DOI] [PubMed] [Google Scholar]
- 62.Jenkinson N, Miall RC. Disruption of saccadic adaptation with repetitive transcranial magnetic stimulation of the posterior cerebellum in humans. Cerebellum. 2010;9(4):548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colnaghi S, Ramat S, D’Angelo E, Cortese A, Beltrami G, Moglia A, et al. θ-burst stimulation of the cerebellum interferes with internal representations of sensory-motor information related to eye movements in humans. Cerebellum. 2011;10(4):711–9. [DOI] [PubMed] [Google Scholar]
- 64.Shaikh AG, Wong AL, Optican LM, Zee DS. Impaired motor learning in a disorder of the inferior olive: is the cerebellum confused? Cerebellum. 2017;16(1):158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herzfeld DJ, Kojima Y, Soetedjo R, Shadmehr R. Encoding of error and learning to correct that error by the Purkinje cells of the cerebellum. Nat Neurosci. 2018;21(5):736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raymond JL, Medina JF. Computational principles of supervised learning in the cerebellum. Annu Rev Neurosci. 2018;41:233–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fuchs AF, Robinson FR, Straube A. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge pattern. J Neurophysiol. 1993;70(5):1723–40. [DOI] [PubMed] [Google Scholar]
- 68.Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993;70(5):1741–58. [DOI] [PubMed] [Google Scholar]
- 69.Goffart L, Chen LL, Sparks DL. Saccade dysmetria during functional perturbation of the caudal fastigial nucleus in the monkey. Ann N Y Acad Sci. 2003;1004:220–8. [DOI] [PubMed] [Google Scholar]
- 70.Straube A, Scheuerer W, Robinson FR, Eggert T. Temporary lesions of the caudal deep cerebellar nucleus in nonhuman primates. Gain, offset, and ocular alignment. Ann N Y Acad Sci. 2009;1164:119–26. [DOI] [PubMed] [Google Scholar]
- 71.Daniel R Gold DO Saccadic dysmetria and ocular lateropulsion in lateral medullary stroke. Video. [Neuro-Ophthalmology Virtual Education Library: NOVEL Web Site]. 2016. Available at: https://collections.lib.utah.edu/ark:/87278/s65176w6. Accessed January 18, 2019. [Google Scholar]
- 72.Kunimatsu J, Suzuki TW, Tanaka M. Implications of Lateral Cerebellum in Proactive Control of Saccades. J Neurosci. 2016;36(26):7066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohtsuka K, Enoki T. Transcranial magnetic stimulation over the posterior cerebellum during smooth pursuit eye movements in man. Brain. 1998;121 (Pt 3):429–35. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki DA, Keller EL. The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. I. Eye and head movement-related activity. J Neurophysiol. 1988;59(1):1–18. [DOI] [PubMed] [Google Scholar]
- 75.Fuchs AF, Robinson FR, Straube A. Participation of the caudal fastigial nucleus in smooth-pursuit eye movements. I. Neuronal activity. J Neurophysiol. 1994;72(6):2714–28. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki DA, Noda H, Kase M. Visual and pursuit eye movement-related activity in posterior vermis of monkey cerebellum. J Neurophysiol. 1981;46(5):1120–39. [DOI] [PubMed] [Google Scholar]
- 77.Raghavan RT, Lisberger SG. Responses of Purkinje cells in the oculomotor vermis of monkeys during smooth pursuit eye movements and saccades: comparison with floccular complex. J Neurophysiol. 2017;118(2):986–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson FR, Straube A, Fuchs AF. Participation of caudal fastigial nucleus in smooth pursuit eye movements. II. Effects of muscimol inactivation. J Neurophysiol. 1997;78(2):848–59. [DOI] [PubMed] [Google Scholar]
- 79.Lindner A, Haarmeier T, Erb M, Grodd W, Thier P. Cerebrocerebellar circuits for the perceptual cancellation of eye-movement-induced retinal image motion. J Cogn Neurosci. 2006;18(11):1899–912. [DOI] [PubMed] [Google Scholar]
- 80.Nitta T, Akao T, Kurkin S, Fukushima K. Involvement of the cerebellar dorsal vermis in vergence eye movements in monkeys. Cereb Cortex. 2008;18(5):1042–57. [DOI] [PubMed] [Google Scholar]
- 81.Huh YE, Kim JS, Kim HJ, Park SH, Jeon BS, Kim JM, et al. Vestibular Performance During High-Acceleration Stimuli Correlates with Clinical Decline in SCA6. Cerebellum. 2015;14(3):284–91. [DOI] [PubMed] [Google Scholar]
- 82.Oldrati V, Schutter DJLG. Targeting the Human Cerebellum with Transcranial Direct Current Stimulation to Modulate Behavior: a Meta-Analysis. Cerebellum. 2018;17(2):228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]