Abstract
In this short paper we report the interactions of silver island films with chemiluminescing species. Our findings show that silver island films can increase the detectability of chemiluminescent reactions/species, with an approximately 5-fold increase in signal intensity. This finding not only suggests the use of silver nanostructures to amplify chemiluminscent signatures in assay platforms, and therefore increase the detectability of analytes or biospecies, but more importantly, suggests that surface plasmons can be directly excited by chemically induced electronically excited molecules. This finding is of significance towards our understanding of fluorophore–metal interactions, a relatively new near-field fluorescence concept, recently named metal-enhanced fluorescence and also radiative decay engineering.
Keywords: Metal-enhanced fluorescence, Radiative decay engineering, Silver nanostructures, Metal-enhanced chemiluminescence, Radiating plasmons, Plasmon controlled fluorescence
Introduction
The use of light-producing chemical reactions for quantitative detection in biotechnology is rapidly on the increase [1–7], especially with regard to chemiluminescence based ligand-binding assays [1–7]. The attractiveness of chemiluminescence as an analytical tool lies primarily in the simplicity of detection [8]; the fact that most samples have no unwanted background luminescence, as is typically observed in fluorescence-based assays [9]; and the fact that no optical filters are required to separate the excitation wavelengths and scatter [8], as is also required for fluorescence-based detection [9]. Chemiluminescent-based detection is however currently limited by the availability of chemiluminescent probes, which is not a factor governing fluorescence based detection [9]. Both fluorescence and chemiluminescence based technologies do however suffer from an inherent need for increased sensitivity/detection limits [8,9]. For fluorescence, this is governed by the quantum yield of the tagging fluorophore, the level of unwanted background fluorescence and the photostability of the fluorophore [9], whereas, for chemiluminescence, detection is limited by the quantum efficiency of the chemiluminescence reaction or probe, and the time before depletion of the reactants [8]. For both detection systems, an increased luminescence yield would clearly benefit the overall detectability and therefore, for bioassays, the sensitivity towards a particular analyte.
In this regard our laboratories have recently developed a new technology which we have shown can increase the system quantum yield [10–13], the photostability of the fluorophore [10–13] and by using spatially localized excitation, can readily remove unwanted background fluorescence [14]. To date, we have published numerous papers on metal-enhanced fluorescence (MEF) [10–20] also called radiative decay engineering [21] and surface enhanced fluorescence (SEF) [22], by us. In all of these studies, we have mostly used nanosecond decay time fluorophores in close proximity to a variety of different sized [15] and shaped [16,17] noble metal nanostructures. Until now, we have not considered the effects of silver on chemilumescence-based reactions.
Hence, in this paper, we have used silver island films to enhance the luminescence signatures of standard “glow light stick” solutions, which are readily commercially available. Most profoundly, the lack of an external excitation source to generate metal surface plasmons, suggests that this is the first observation of surface plasmons generated from chemically induced electronically excited states.
Materials and methods
Materials
Silver nitrate (99.9%), sodium hydroxide (99.996%), ammonium hydroxide (30%), trisodium citrate, d-glucose, and premium quality APS-coated glass slides (75 mm × 25 mm) were obtained from Sigma-Aldrich (St. Loius, MO). The blue-glow chemiluminescence sticks used were the “Color Bright” light sticks, obtained from Omniglow (West Springfield, MA).
Chemiluminescence
The chemiluminescent materials used in this study were obtained from commercial light glow sticks. These glow sticks contain the necessary reacting chemicals encapsulated within a plastic tube. The plastic tube contains a phenyl oxalate ester and a fluorescent probe, where the choice of dye simply determines the color of the luminescence [9]. For our work here, this choice was arbitrary as long as the luminophore emited in the visible spectral region, consistent with previous reports [10–13]. A glass capsule containing the activating agent (hydrogen peroxide) is placed inside the plastic tube. Activation of the chemicals is accomplished with a bend, snap, and a vigorous shake of the plastic tube which breaks the glass capsule containing the peroxide and mixes the chemicals to begin the chemiluminescence reaction. The hydrogen peroxide oxidizes the phenyl oxalate ester to a peroxyacid ester and phenol. The unstable peroxyacid ester decomposes to a peroxy compound and phenol, the process chemically inducing an electronic excited state. Commercially available chemiluminescence materials were purchased and used to demonstrate the utility of the metal-enhanced chemiluminescence (MEC) approach for the general optical amplification of chemiluminescence signatures.
Formation of silver island films (SiFs) on APS-coated glass substrates
The silver island films were made according to previously published procedures employing the chemical reduction of silver nitrate on glass microscope slides using sodium hydroxide, ammonium hydroxide and glucose [10–13].
Chemiluminescence from SiFs and glass
The chemiluminescence experiments were performed using a blue emission glow stick. After chemiluminescence initiation, approximately 70 µl of the glow stick fluid was placed between two APS-coated microscope glass slides, clamped together. The glass slides contained silver island films on one end and were bare glass on the other end. The bare end of the glass served as the control sample by which to compare the benefits of using the metal-enhanced chemiluminescence phenomenon. Subsequently, the enhancement ratio, the intensity from silver/intensity from glass, could be determined.
Chemiluminescence measurements
Chemiluminescence spectra were collected using an Ocean Optics spectrometer, model SD 2000 (Dunedin, FL), connected to an Ocean Optics 1000 µm diameter fiber with a numerical aperture of 0.22 (Dunedin, FL). The fiber was positioned vertically on top of the slides containing the luminescing material. Spectra were collected with an integration time ranging between 4 and 10 s. The integration time was kept constant between the control and silver island film sample measurements.
Results
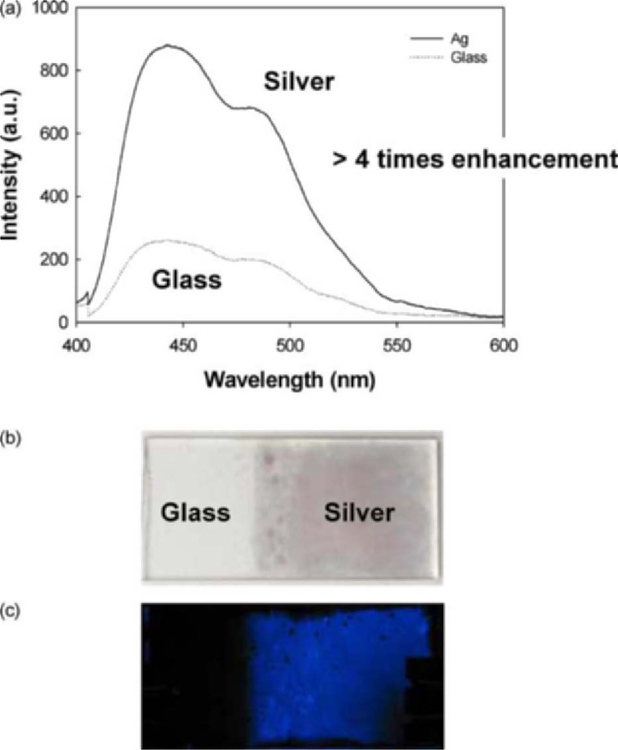
Figure 1(a) shows the luminescence emission spectra from between the silvered glass and glass plates. The emission from the silvered portion of the slide was spatially averaged to be about 4–5 times greater than the glass control side of the sample. In addition, the volume between both the sandwiched glass and silver slides was identical. Figure 1(b, c) shows the photographs of the slides, both before and after the addition of the chemiluminescent material. Approximately 70 µL of the fluid was enough to form a thin coating across both portions of the slide, held by capillary action as the slides were sandwiched. The enhanced chemiluminescence is clearly visible on the silvered portion. Interestingly, the digital camera was not able to capture the blue emission from the thin fluid layer of the glass region of the slide, since the intensity was quite weak as shown in Fig. 1(a).
Fig. 1.
Metal-Enhanced Chemiluminescence spectra (MEC) on a silvered surface (a) and photographs showing the enhanced luminescence (b) and (c)
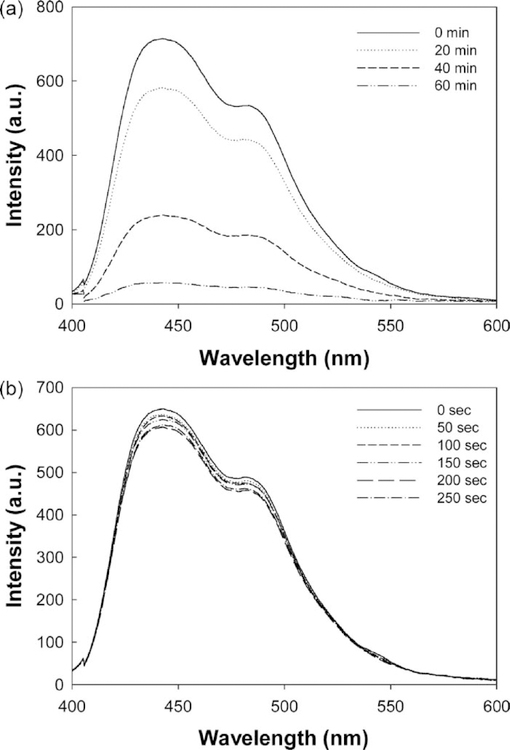
We also undertook several control experiments to determine the loss of chemiluminescent intensity due to the depletion of the reactants, (Fig. 2). After a period of 60 min, most of the emission from the silvered plates had disappeared. Fig. 2(a). Interestingly, the luminescence emission intensity changed very little in several tens of seconds, Fig. 2(b), which was the time needed to measure the intensity on both silver and glass shown in Fig. 1, making the comparison between silver and glass a valid one.
Fig. 2.
Metal-enhanced chemiluminescence on a silvered surface as a function of time (a) and the intensity of luminescence in terms of seconds (b)
Finally, while not shown here, we also measured the rate of loss of luminescence from both the silvered and glass portions of the slide. For both, the rate of chemiluminescence was almost identical, suggesting that no chemical interaction between the chemiluminescent reagents and silver occurred, the enhanced luminescence signals observed being due to interactions with surface plasmons as discussed below [23]. Further details in this regard will be published in a full paper elsewhere.
Discussion
Until recently [23], the emission of fluorophores and luminescent species in close proximity to metallic nanostructures was thought to originate solely from the fluorophore, the excited plasmons interacting with fluorophores and changing their free-space spectral characteristics [10–22]. However, only recently our interpretation of metal-enhanced fluorescence has changed to one whereby excited fluorophores can nonradiatively transfer energy and couple to surface plasmons, which in turn, affect the fluorophores photophysical characteristics, in essence, the fluorophore–metal system radiates energy [23]. With the chemiluminescence species shown here, it is thought that a similar effect occurs, where excited species couple to surface plasmons, which in turn affect the photophysical properties of the chemically excited state (Fig. 3). While we present no direct evidence for chemiluminescence induced plasmon emission, the characteristics of the luminophore near the metallic particles observed here are very similar to those observed with nanosecond decay time fluorophores, strongly suggesting the same mechanism for enhanced emission [23].
Fig. 3.
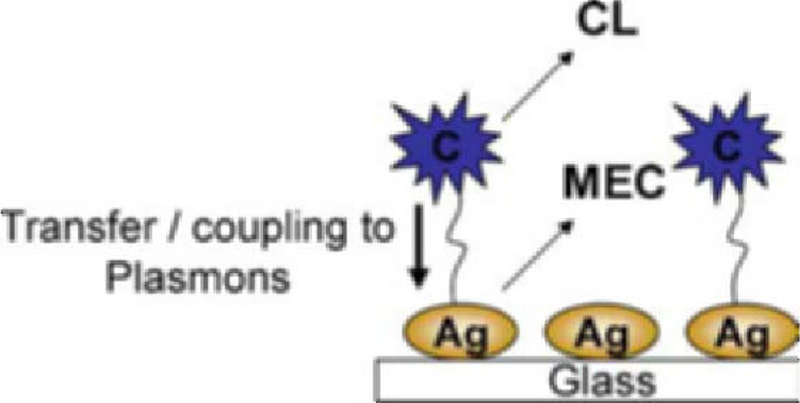
Proposed model for Metal-Enhanced Chemiluminescence (MEC). The chemically induced electronically excited luminophore (C) transfers energy to silver plasmons (a resonance coupling interaction), which themselves radiate the photophysical properties of the excited species. CL—Chemiluminescence, MEC—Metal-Enhanced Chemiluminescence, Ag—Silver
Interestingly, it is known that surface plasmons can be created by illumination of thin continuous metal films under very unique optical conditions, such as through a prism, or a medium of high dielectric constant and with plane-polarized light [24–25]. However, surface plasmons can also be created by direct illumination of metallic solution based colloids or nanostructures, or even by nanostructures bound to surfaces [23]. With fluorophores near metallic surfaces, surface plasmons can therefore be generated by both a close-proximity excited fluorophore and by direct illumination. With our chemiluminescent system here, there is no external excitation source for direct illumination, and therefore no possible direct mode of excitation of the surface plasmons. This suggests that the surface plasmons are indeed excited from a chemically induced electronically excited state of a luminophore. To the best of our knowledge this is the first observation of a chemically induced electronic excitation of surface plasmons.
Conclusions
In this paper we have shown the favorable interactions of silvered surfaces with chemiluminescent materials. The presence of the silver nanostructures increases the spatially averaged luminescent intensity approximately 5-fold, with no accelerated depletion of reactants observed, as compared to a sample employing no silver. While we have used commercially available glow sticks as a source of chemiluminescence material and to subsequently project our radiating plasmon hypothesis, silvered surfaces could also readily be used with the many other chemiluminescent reactions and systems employed in biosciences today, to similarly amplify luminescent signal intensities. In this regard, work is underway by our laboratories and will be reported in due course.
Acknowledgements
This work was supported by the NIH GM070929 and the National Center for Research Resources, RR008119. Partial salary support to CDG and JRL from UMBI is also acknowledged.
Abbreviations
- APS
3-(Aminopropyl)triethoxysilane
- MEC
Metal-enhanced chemiluminescence
- MEF
Metal-enhanced fluorescence
- RDE
Radiative decay engineering
- SEF
Surface-enhanced fluorescence
- SiFs
Silver island films
Contributor Information
Mustafa H. Chowdhury, Center for Fluorescence Spectroscopy, Medical Biotechnology Center, University of Maryland School of Medicine, 725 West Lombard St, Baltimore, MD, 21201 USA
Kadir Aslan, Institute of Fluorescence, Laboratory for Advanced Medical Plasmonics, Medical Biotechnology Center, University of Maryland Biotechnology Institute, 725 West Lombard St., Baltimore, MD, 21201 USA.
Stuart N. Malyn, Institute of Fluorescence, Laboratory for Advanced Medical Plasmonics, Medical Biotechnology Center, University of Maryland Biotechnology Institute, 725 West Lombard St., Baltimore, MD, 21201 USA
Joseph R. Lakowicz, Center for Fluorescence Spectroscopy, Medical Biotechnology Center, University of Maryland School of Medicine, 725 West Lombard St, Baltimore, MD, 21201 USA
Chris D. Geddes, Center for Fluorescence Spectroscopy, Medical Biotechnology Center, University of Maryland School of Medicine, 725 West Lombard St, Baltimore, MD, 21201 USA Institute of Fluorescence, Laboratory for Advanced Medical Plasmonics, Medical Biotechnology Center, University of Maryland Biotechnology Institute, 725 West Lombard St., Baltimore, MD, 21201 USA.
References
- 1.Hofmann O, Miller P, Sullivan P, Jones TS, deMello JC, Bradley DDC, deMello AJ (2005) Thin-film organic photodiodes as integrated detectors for microscale chemiluminescence assays. Sens Actuators B-Chem. 106(2):878–884 [Google Scholar]
- 2.Myhre O, Andersen JM, Aarnes H, Fonnum F (2003) Evaluation of the probes 2’,7’-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol 65(10):1575–1582 [DOI] [PubMed] [Google Scholar]
- 3.Bronstein I, Martin CS, Fortin JJ, Olesen CEM, Voyta JC (1996) Chemiluminescence: Sensitive detection technology for reporter gene assays. Clin Chem 42(9):1542–1546 [PubMed] [Google Scholar]
- 4.Moris P, Alexandre I, Roger M, Remacle J (1995) Chemiluminescence Assays of Organophosphorus and Carbamate Pesticides. Analytica Chimica Acta 302(1):53–59 [Google Scholar]
- 5.Garcia-Campana AM, Baeyens Willy R (2001) Chemiluminescence in analytical chemistry, Marcel Dekker, New York [Google Scholar]
- 6.Wampler JE (1985) Instrumentation: Seeing the light and measuring it In Burr JG (ed.) Chemi- and Bioluminescence, Marcel Dekker, New York, pp 1–44 [Google Scholar]
- 7.Berthold F (1990) Instrumentation for chemilunescence immunoassays In Van Dyke K, Van Dyke R. (eds) Luminescence immunoassays and molecular applications, CRC Press, Boca Raton, pp 11–25 [Google Scholar]
- 8.Nieman T (1995) Chemiluminescence: Theory and Instrumentation, Overview In Encyclopedia of analytical science, Academic Press, Orlando, pp 608–613 [Google Scholar]
- 9.Lakowicz JR (1999) Principles of fluorescence spectroscopy, Kluwer, New York [Google Scholar]
- 10.Aslan K, Gryczynski I, Malicka J, Matveeva E, Lakowicz JR, Geddes CD (2005) Metal-enhanced fluorescence: An emerging tool in biotechnology. Curr Opin Biotechnol 16(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aslan K, Lakowicz JR, Geddes CD (2005) Plasmon Light Scattering in Biology and Medicine: New Sensing Approaches, Visions and Perspectives. Curr Opin Chem Biol Anal Tech. 9:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geddes CD, Aslan K, Gryczynski I, Malicka J, Lakowicz JR (2005) In Geddes CD, Lakowicz JR (eds) Radiative decay engineering, review chapter for topics in fluorescence spectroscopy, Kluwer Academic/Plenum Publishers, New York, USA, pp 405–448 [Google Scholar]
- 13.Geddes CD, Aslan K, Gryczynski I, Malicka J, Matveeva E, Lakowicz JR (2005) In Geddes CD, Lakowicz JR (eds) Topics in fluorescence spectroscopy, Kluwer Academic/Plenum Publishers, New York, USA, pp 401–448 [Google Scholar]
- 14.Lakowicz JR, Gryczynski I, Malicka J, Gryczynski Z, Geddes CD (2002) Enhanced and localized multiphoton excited fluorescence near metallic silver islands: Metallic islands can increase probe photostability. J Fluoresc 12:299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geddes CD, Cao H, Gryczynski I, Gryczynski Z, Fang J, Lakowicz JR (2003) Metal-enhanced fluorescence due to silver colloids on a planar surface: Potential applications of Indocyanine green to in vivo imaging. J Phys Chem A 107:3443–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslan K, Lakowicz JR, Geddes CD (2005) Rapid deposition of triangular silver nanoplates on planar surfaces: Application to metal-enhanced fluorescence. J Phys Chem B 109:6247–6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aslan K, Leonenko Z, Lakowicz JR, Geddes CD (2005) Fast and slow deposition of silver nanorods on planar surfaces: Application to metal-enhanced fluorescence. J Phys Chem B 109(8):3157–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geddes CD, Parfenov A, Roll D, Fang J, Lakowicz JR (2003) Electrochemical and laser deposition of silver for use in metal enhanced fluorescence. Langmuir 19:6236–6241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aslan K, Badugu R, Lakowicz JR, Geddes CD (2005) Metal-enhanced fluorescence from plastic substrates. J Fluoresc 15(2):99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aslan K, Geddes CD. Microwave-Accelerated Metal-Enhanced Fluorescence (MAMEF): A new platform technology for ultra fast and ultra bright assays. Anal Chem 77(24):8057–8067 [DOI] [PubMed] [Google Scholar]
- 21.Lakowicz JR (2001) Radiative decay engineering: Biophysical and biomedical applications. Anal Biochem 298:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakowicz JR, Geddes CD, Gryczynski I, Malicka J, Gryczynski Z, Aslan K, Lukomska J, Matveeva E, Zhang J, Badugu R, Huang J (2004) Advances in surface-enhanced fluorescence. J. Fluoresc 14(4):425–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aslan K, Leonenko Z, Lakowicz JR, Geddes CD (2005) Annealed silver-island films for applications in metal-enhanced fluorescence: Interpretation in terms of radiating plasmons. J Fluoresc 15(5):643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gryczynski I, Malicka J, Gryczynski Z, Lakowicz JR (2004) Radiative decay engineering 4. experimental studies of surface plasmon-coupled directional emission. Anal Biochem 324:170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geddes CD, Gryczynski I, Malicka J, Gryczynski Z, Lakowicz JR (2004) Directional surface plasmon coupled emission. J Fluoresc 14:119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]