Abstract
Steroids are an important biomolecule class for analysis due to their promise as biomarkers for various diseases and their abuse as performance enhancers in sports. Current analytical methods, including chromatography and nuclear magnetic resonance spectroscopy, fall short of being able to confidently analyze steroids, partly due to the large number of steroid isomers. Ion mobility spectrometry (IMS), a gas-phase ion separator, has shown potential for steroid analysis both in conjunction with liquid chromatography (LC) and as a stand-alone technique. This review will examine the current literature on IMS analysis of steroids. Analysis by LC-IMS will include examination of steroids and steroid glucuronides in human urine and serum samples for enhanced signal-to-noise ratios and higher confidence of identification. The stand-alone IMS analysis will examine the use of derivatization of steroids and formation of multimers to enhance resolution for steroid isomers analysis, where both methods have shown to greatly increase the separation of steroid isomer species. However, these methods have not been applied to biological mixtures to assess their applicability to medical and forensic applications, which should be a future direction of this field.
Keywords: Steroid Analysis, Ion Mobility Spectrometry, Liquid Chromatography
Graphical Abstract
INTRODUCTION
Types of Steroids and Their Functions.
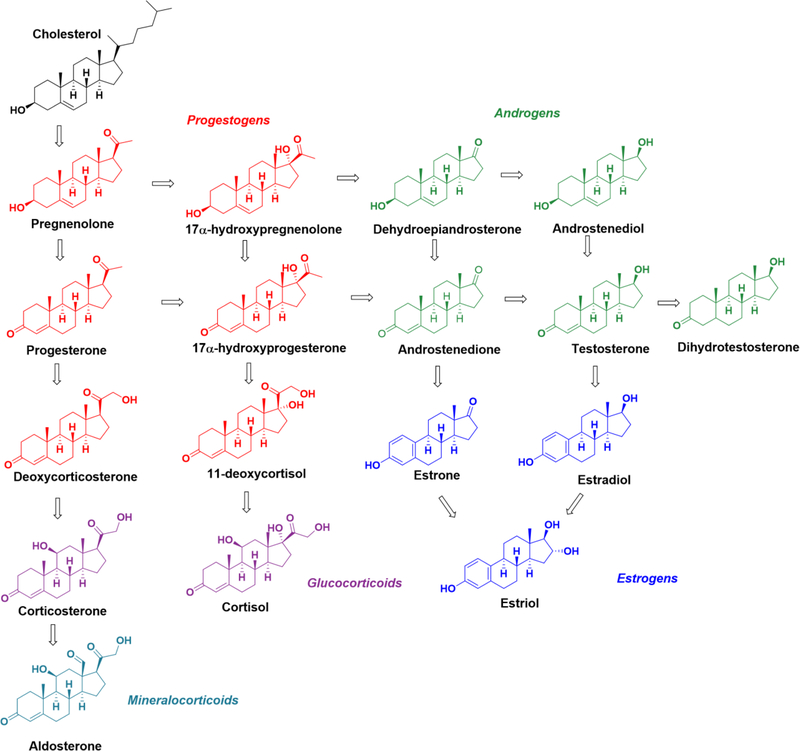
Steroids are an important class of biomolecules, closely related to lipids. All steroids are derivatives of cholesterol, where steroidogenesis, the biosynthesis of steroids, begins when the cholesterol side chain cleavage enzyme converts cholesterol to pregnenolone. From this initial conversion, pregnenolone can be converted into progestogens, mineralocorticoids, glucocorticoids, androgens, and estrogens, as shown in Figure 1. Steroids are significantly utilized in signaling through a variety of steroid receptors [1].
Figure 1.
Major steps in human steroidogenesis.
Three major groups of steroids are classified based on their functions: corticosteroids, sex steroids, and neurosteroids. Corticosteroids are produced in the adrenal cortex and serve signaling roles in a variety of biological processes including stress response, glucose metabolism, and maintenance of electrolyte levels. The corticosteroids can be separated into two major classes: glucocorticoids and mineralocorticoids. Glucocorticoids, including cortisol and cortisone, bind to the glucocorticoid receptor and are primarily involved in the regulation of glucose metabolism and the immune response. On the other hand mineralocorticoids, primarily aldosterone, maintain salt and water homeostasis in the body [2].
Sex steroid hormones are the class of steroids that are responsible for secondary sex characteristics and reproductive function. This group of steroids includes progestogens, androgens, and estrogens. Progestogens are involved in maintaining pregnancy and in the regulation of the menstrual cycle. Estrogens and androgens are responsible for female and male secondary sex characteristics, respectively. Additionally, estrogens and androgens are heavily involved in various human health concerns such as cardiovascular disease, diabetes, and infertility [2–7].
Lastly, neurosteroids, or neuroactive steroids, are an additional class of steroid hormones that are primarily active in the brain. Inhibitory and excitatory neurosteroids act as positive and negative allosteric modulators of the GABAA receptor, respectively. Furthermore, pheromones are a class of neurosteroids that function to elicit various types of behaviors or physiological responses in other members of the same species. Ultimately, the variety of steroids and their biological function results in their usefulness for medical and forensic applications, but also present an analytical challenge [2].
Analytical Challenges.
The analytical determination of steroids analysis poses a number of potential hurdles due to the high degree of structural similarity between steroids with different biological functions. For example, aldosterone and cortisone are constitutional isomers of one other; however, they have markedly different biological roles, as aldosterone is a mineralocorticoid and cortisone is a glucocorticoid. Such structural complexities generally arise from the many interconnected steroid biosynthesis pathways [1, 8–11]. This can be illustrated by examining the case of dehydroepiandrosterone (DHEA) and testosterone, two constitutionally isomeric steroids. DHEA is produced from 17α-hydroxypregnenolone by 17,20 lyase; however, DHEA is also a substrate for 3β-hydroxysteroid dehydrogenase (3β-HSD) which produces androstenedione and 17β-hydroxysteroid dehydrogenase (17β-HSD) to produce androstenediol. Subsequently, androstenedione or androstenediol can be converted to testosterone through 17β-HSD or 3β-HSD, respectively. The structural diversity of steroids can be largely attributed to other similarly coupled steroidogenic pathways, which are also able to yield subtly different structures with dramatically divergent biochemical functions [1].
Aside from constitutional isomers among steroids, there are multiple steroid stereoisomers, such as 17α-estradiol and 17β-estradiol. These two structures result from the activities of two different enzymes for the addition or hydration reaction to form an alcohol group. Specifically, 17α-estradiol and 17β-estradiol are produced by 17α-HSD and 17β-HSD, respectively. Notably, 17α-estradiol has a 58% or 11% relative binding affinity to the estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ), respectively, as compared to 17β-estradiol. This illustrates how the presence of distinct yet difficult to separate stereoisomers have different biological activities, increasing the challenge of steroid analysis [1].
Ion Mobility Spectrometry.
Many analytical techniques have been applied to the study of steroids, including nuclear magnetic resonance (NMR) spectroscopy, gas chromatography (GC), and liquid chromatography (LC). These approaches all tend to have acquisition times on the order of tens of minutes to hours, and in some cases are still unable to resolve and distinguish multiple steroid species in mixtures. Ion mobility spectrometry (IMS) coupled to mass spectrometry (MS) has shown promise in separating small molecules, and on the millisecond timescale [9, 12–15]. Generally speaking, IMS encompasses a collection of instrument hardware configurations and operating principles that all function as gas phase ion separators that employ an electric field and a drift gas to sort ions based on their gas-phase sizes, shapes, and charge states [16].
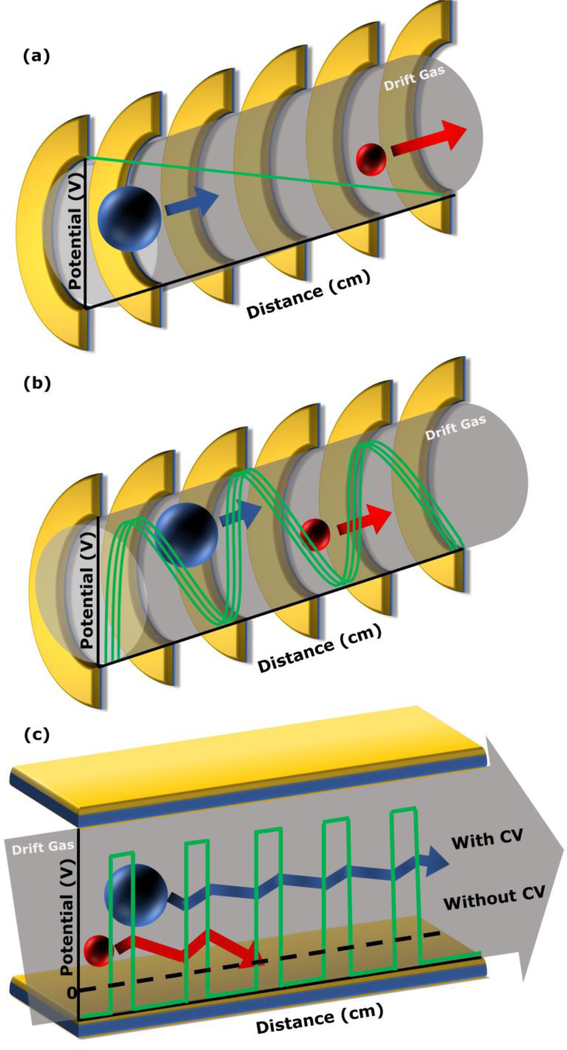
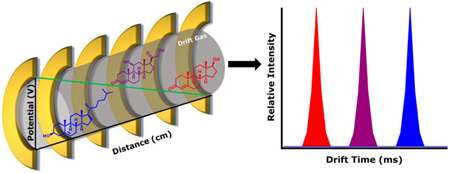
While there are many types of IMS, the scope of this chapter will focus on the three most relevant to steroid analysis: two temporal separators, drift tube ion mobility spectrometry (DTIMS) and traveling wave ion mobility spectrometry (TWIMS); and a spatial separator, differential ion mobility spectrometry (DMS), which is also commonly referred to as high-field asymmetric ion mobility spectrometry (FAIMS). A schematic for each type of IMS is presented in Figure 2. DTIMS separates ions through a static electric field propelling an ion through the drift gas. The resulting ion-neutral collisions impede the transit of ions through the drift gas, resulting in an ion drift time that is inversely proportional to the orientationally-averaged ion-neutral collision cross section (CCS). The larger the collision cross section, the lower the mobility, which translates to more ion-neural collisions impeding the movement of the ion and requiring more time for the ion to transverse the drift tube [16–18]. Using classical static-field DTIMS measurements, CCS values can be calculated directly from drift time measurements. The adoption of DTIMS has increased with the commercialization of this technique in the commercially available Agilent 6560 instrument.
Figure 2.
Schematic of DTIMS (a), TWIMS (b), and DMS (c), where two ions of high and low mobilities are represented by red and blue spheres, respectively; the electric fields are indicated with green lines; and the drift gas is represented by gray fills.
Alternatively, TWIMS separates ions by using a dynamic electric field in the form of a radially confining RF field superimposed on an axially propagating DC traveling wave. In this case, the traveling wave moves in the direction that tends to sweep ions through the drift gas; however, lower mobility ions are subject to a greater number of collisions and can eventually overcome the potential barrier of the traveling DC and roll over the wave. The more rollover events the ion encounters, the longer the ion will take to traverse the mobility tube. While the time spent in the device is correlated the collision cross section, due to the dynamic nature of the traveling wave, the TWIMS drift times must generally be calibrated to CCSs using a series of compounds with previously reported CCS values measured by DTIMS [16, 17, 19–31]. Commercial instruments incorporating TWIMS include the Waters Synapt series of instruments and the Waters Vion.
In contrast to the temporal separations carried out by DTIMS and TWIMS, DMS is a spatial separator where the drift gas propels ions forward in the presence of an asymmetric field. The electric field between the DMS electrodes oscillates such that it is twice as intense in one direction for half the time as the other direction. Because ions have field-dependent mobilities in each direction, the differential field disperses ions through the device based on the difference between the high-field and low-field mobilities. A compensation voltage (CV) can be applied to one of the electrodes to focus the ions with a specific differential mobility through the device. This compensation voltage can remain constant to select ions of only a specific differential mobility, or it can be scanned to determine the differential mobilities of different types of ions [16, 32, 33]. All three types of ion mobility spectrometry have found application to steroid analysis. DMS has been commercialized into a variety of different platforms and devices are sold to add onto a wide variety of other commercial instruments.
LIQUID CHROMATOGRAPHY COUPLED TO ION MOBILITY SPECTROMETRY
Steroids in Serum.
Previous methods to analyze steroids in biological samples include LC followed by tandem mass spectrometry (MS/MS). Ray et al. combined LC-MS/MS with DMS to increase performance in the measurement of endogenous steroids [11]. They analyzed serum samples by adding deuterated internal standards to 250 μL of serum and then extracted with 1.5 mL of methyl t-butyl ether (MTBE), mixed, and centrifuged. The organic layer was then evaporated and reconstituted with 100 μL of 50% water/methanol and analyzed by LC-MS/MS.
In this study, there were five specific endogenous steroids of interest: corticosterone, 11-deoxycortisol, 11-deoxycorticosterone, 17-hydroxy progesterone, progesterone [11]. In these five steroids, there are two sets of isomers: corticosterone and 11-deoxycortisol and 11-deoxycortisone and 17-hydroxyprogesterone. Corticosterone and 11-deoxycortisol also coelute under the C18 reversed phase chromatography conditions used, which means that the current LC-MS method would not be able to separate these two pairs of isomers. However, with combined use of LC-DMS allowed more optimal separation between these two isomers than either technique alone.
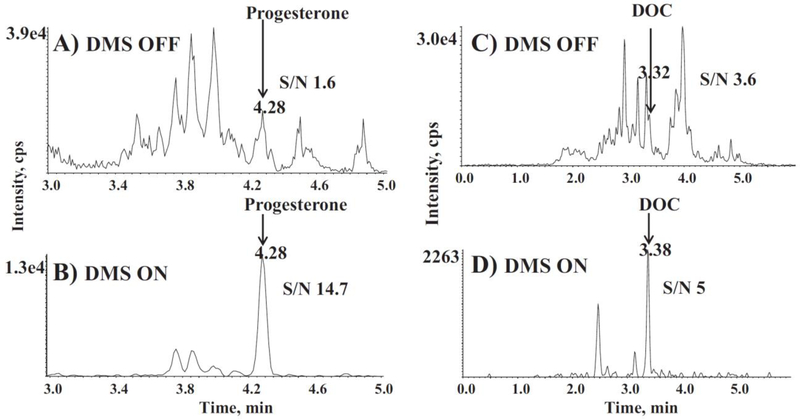
Furthermore, Ray et al. investigated how to use DMS to remove signal of coeluting peaks and chemical noise [11]. Since DMS can select mobilities of the ions that pass on to MS detection, the CV can be set to only allow ions through that match the differential mobility for the analyte of interest. Therefore, DMS can be used to remove noise, which can be viewed in Figure 3.
Figure 3.
Chromatograms of a serum sample with 2.2 nM of progesterone (a,b) and 0.3 nM of 11-deoxycorticosterone (c,d) with DMS off in the top panels (a,c) and DMS on in the bottom panels (b,d). DMS demonstrated an increase in the signal-to-noise ratio by reducing the noise through mobility selection. Reproduced with permission from Ray et al. [11], copyright 2014 Elsevier B. V.
Steroid Glucuronides in Urine.
For sports drug testing, it is typical to test the urine of athletes for steroid glucuronides. A steroid glucuronide has a glucuronide attached to the alcohol of the steroid. However, the level of steroids in urine are very low resulting in the need for cutting edge analysis techniques to identify doping in sports. Therefore, Guddat et al. studied steroids in urine by hydrolyzing the glucuronides by β-glucuronidase isolated from E. coli and extracted through MTBE [34]. The method in this case was the incorporation of DMS into a liquid chromatography combined with tandem quadrupole detection set to multiple reaction monitoring mode.
Here, Guddat et al. showed that with the addition of DMS the noise was reduced, allowing an increase in the signal-to-noise ratio for analytes and a decrease in the signal of peaks that could be detected as false positives [34]. In general, the incorporation of DMS improved the signal-to-noise ratios 5× as compared to without DMS. As a result, the incorporation of DMS allowed for a lower level of detection, higher identification confidence, and less extensive sample preparation.
Kaur-Atwal et al. furthered the use of IMS on the separation of steroid glucuronides by directly analyzing steroid glucuronides in urine by ultra-high performance liquid chromatography (UPLC)-TWIMS-MS analysis [4]. Testosterone glucuronide (TG) and epitestosterone glucuronide (ETG), carbon 17 epimers, were analyzed in standard mixtures where the retention times were 5.16 min and 8.41 min for testosterone and epitestosterone glucuronide, respectively. Additionally, both glucuronides were partially separated by TWIMS with a scan number (a quantity related to drift time in ion mobility) of 68 and 82 for epitestosterone and testosterone glucuronide, respectively. With the calibration of homo-poly-amino acids, the helium collision cross sections were measured for epitestosterone glucuronide as 137 ± 3 Å2 and for testosterone glucuronide as 152 ± 3 Å2. Due to this dual separation, the combined UPLC-TWIMS-MS analysis allowed for increased separation, identification, and sensitivity.
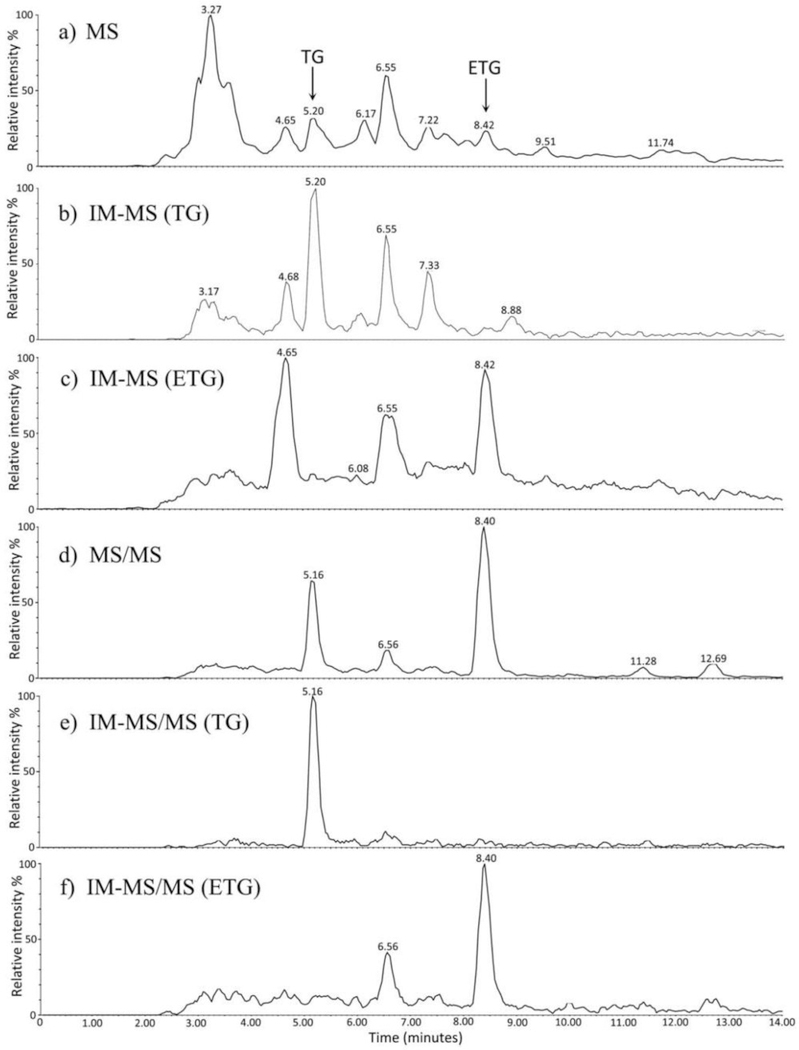
The urine samples were extracted using ethyl acetate and analyzed through UPLC-TWIMS-MS/MS analysis by Kaur-Atwal et al. [4]. The results of this analysis are shown in Figure 4, where with UPLC-MS alone the ETG and TG have low signal-to-noise ratios. However, the additional selection in the ion mobility dimension for TG and ETG increased the signal-to-noise ratio of the major peaks. Using MS/MS, these can be selected further to those producing the product ions of m/z 109.1 and 97.1, which were respectively characteristic of TG and ETG. With MS/MS alone, the signal-to-noise ratios of all peaks were increased, but these were even further increased with higher selectivity when examining the MS/MS and TWIMS results together for TG and ETG. Through this analysis, the identification confidence and signal-to-noise ratios were increased by TWIMS selection without the loss of information from ions with different mobilities.
Figure 4.
Extracted ion chromatograms of urine extract with mass selection at m/z 465.3 (a), mass selection and mobility selection between TG (b), mass selection and mobility selection for ETG (c), tandem mass selection for the product ions m/z 109.1 and 97.1 (d), tandem mass selection and mobility selection for TG (e), and tandem mass selected and mobility selected for ETG (f). The mobility selection was found to decrease the presence of noise and spurious signals. Reproduced with permission from Kaur-Atwal et al. [4], copyright 2011 Royal Society of Chemistry.
ION MOBILITY AS A STAND-ALONE SEPARATION PLATFORM
While IMS provides a benefit when coupled to chromatography, if IMS coupled to mass spectrometry could fully address the analysis of steroids without the need for LC, the acquisition time and sample preparation would greatly decrease. Therefore, more recently, the field has moved to research examining the potential to use IMS as a stand-alone separation technique to analyze steroids. The two methods being employed to stand-alone IMS analysis are derivatization of steroids and analysis of multimer formation, such as dimer and trimers, for enhanced separation.
Derivatization.
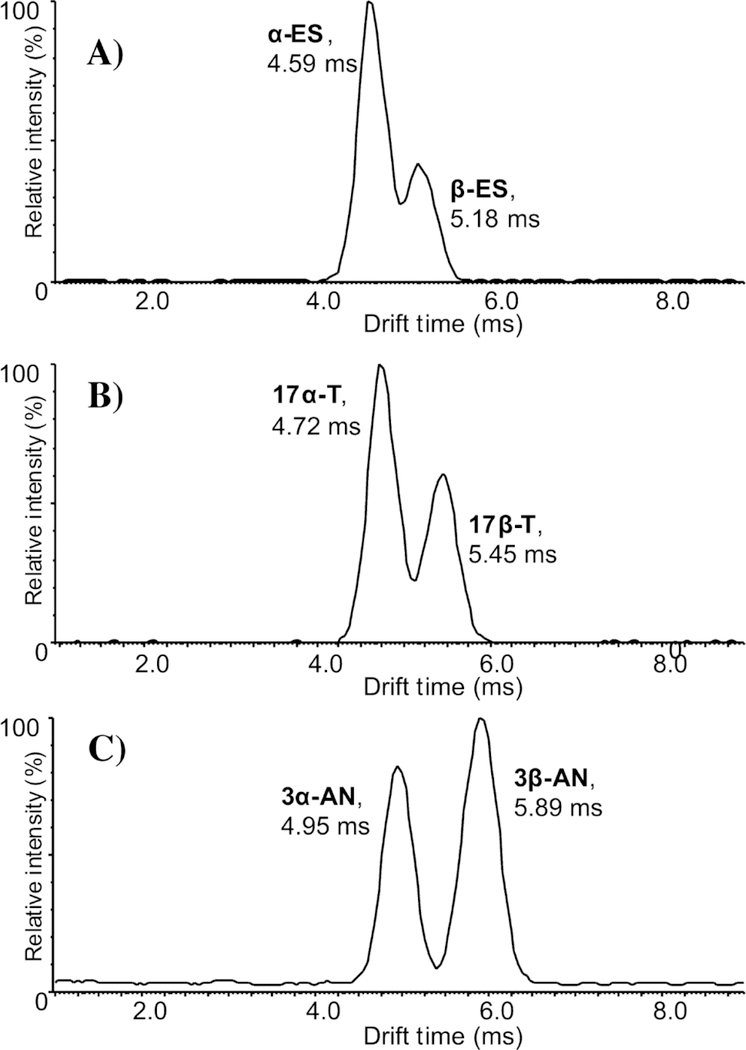
Ahonen et al. utilized TWIMS separation on the three pairs of steroid stereoisomers: α-Estradiol and β-Estradiol, testosterone and epitestosterone, and androsterone and epiandrosterone [35]. During analysis of native steroids, the arrival time distributions (ATD), traces analogous to chromatograms in LC, of all stereoisomers remained overlapped with no valuable separation. To improve the separation between the isomers, the steroids were derivatized with p-toluenesulfonyl isocyanate (PTSI), which attaches to the oxygen in the alcohol that changes stereoposition in each isomer pair. The derivatization brought about a significant change in the gas-phase structures of the stereoisomers, which altered their apparent mobilities in the TWIMS separation.
With the derivatization in Ahonen et al., the separation showed marked improvement in TWIMS analysis [35]. The estradiol stereoisomers showed partial separation at a resolution value of 0.77 at 2.5 mbar in N2 drift gas. Testosterone, which is a larger steroid, had more enhanced separation at 0.93 resolution value at the same conditions. Ultimately, Androsterone stereoisomers had the highest resolution at these conditions at 1.08 illustrated in Figure 5. These authors also examined differences at different pressures and with CO2 vs. N2 drift gas. The overall trends showed the N2 drift gas gave higher resolution, and higher pressures improved the separation of isomers. However, the limit of detection for these species is at or above the concentrations of steroids in human serum when analyzed as the derivatized species. Therefore, the ability to analyze native steroids by IMS-MS would be essential for the analysis of more complex mixtures or biological mixtures.
Figure 5.
Arrival time distributions of PTSI derivatized steroid mixtures illustrating partial to near baseline resolution of steroid stereoisomers. Reproduced with permission from Ahonen et al. [35], copyright 2013 Elsevier B. V.
Formation of Multimers.
For native steroid analysis, Chouinard et al. identified the formation of dimer and trimer species that could alter their isomeric separation in IMS [12]. The carbon 3 epimers androsterone and epiandrosterone were analyzed via DTIMS analysis, where extracted ATDs at the monomer mass show two peaks for each isomer. The first peak for each isomer were overlapped, but separation was observed for the remaining, non-overlapped features. The second peak in the ATD mirrored the ATD extracted at the dimer mass. These results were attributed to the unstable nature of the dimers whereby they fragment into monomers after DTIMS separation, but before MS analysis.
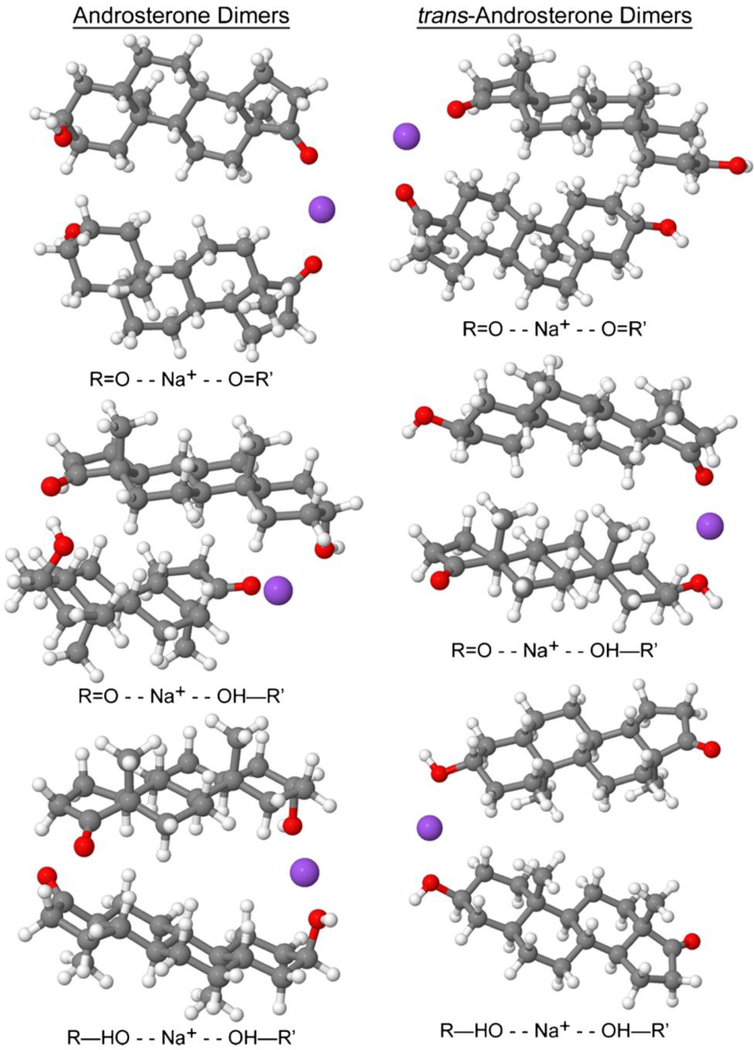
These authors further examined both the experimental and theoretical aspects of dimer and trimer formation [12]. Experimentally, the CCS for androsterone and epiandrosterone as sodiated monomers and dimers were calculated. These experimental CCSs were then compared to the theoretical CCSs. Optimized geometries were calculated for each theoretical CCS, in this case by placing the sodium ion either at the ketone or at the alcohol. The energies for these optimized geometries were then compared to find the most likely placement of the sodium adduct. Each geometry had CCS calculated using the trajectory method. These CCS were then compared to the experimental CCS to determine the most likely geometries these molecules form as monomers and dimers. The energy differences were calculated to determine the most likely adduction pathway of the monomers and dimers [12]. For the monomers, both androsterone and epiandrosterone had the lowest energy structures when the sodium adducts at the ketone, which would result in very little difference in the gas phase shape. However, for the dimers, the lowest energy structures for epiandrosterone involves the sodium adduct between the ketone of each molecule. For androsterone, the lowest energy structure results from the adduction of the sodium between a ketone of one androsterone and the alcohol of the other. The modeled structures, shown in Figure 6, illustrate that the different placements of the sodium in the dimer results in very different geometries, where androsterone is clearly more compact than epiandrosterone or trans-androsterone. When the CCSs for these geometries are compared, the magnitudes of the CCS have discrepancies from the experimental CCS, but the relative differences between the CCS are very similar. For androsterone, the optimized geometry’s CCS value for the monomer and dimer are 186.9 Å2 and 234.7 Å2, respectively, where the experimental values are 197.1 Å2 and 242.6 Å2, respectively. For trans-androsterone, the theoretical values for the monomer and dimer were 186.2 Å2 and 245.7 Å2, respectively, where the experimental values are 196.8 Å2 and 256.3 Å2, respectively. From these results, the theoretical CCS continuously underestimate the experimental values.
Figure 6.
Optimized gas-phase geometries for androsterone and trans-androsterone as their sodiated dimers where the sodium is adducted between two ketones, one ketone and one alcohol, and two alcohols. The lowest energy structures are for between two ketones for trans-androsterone and between one ketone and one alcohol for androsterone. Reproduced with permission from Chouinard et al. [12], copyright 2016 American Society for Mass Spectrometry.
The use of multimers to further steroid analysis through IMS was continued by Chouinard et al., when they expanded their initial study to eighteen different steroids [9]. Through DTIMS analysis, most protonated species of the steroid isomers were overlapped, except in the case of pregnenolone and 5α-dihydroprogesterone. Additionally, most sodiated monomer steroid isomers were overlapped, apart from aldosterone and cortisone which had a resolution value of 1.95. However, the sodiated dimers of different steroid isomers showed promise in the separation of steroid isomers, where five steroid isomer pairs had a resolution value above 1.5 as their sodiated dimer. The sodiated dimer was the optimum species to separate androsterone and epiandrosterone, as previously seen, but also showed separation of etiocholanolone and epietiocholanolone, its carbon 5 epimer. However, in this case, these four isomers were separated by the position of the hydroxyl group at carbon 3, where the position of the proton at carbon 5 does not alter the ATDs substantially. For example, androsterone and etiocholanolone, which are carbon 5 epimers, had essentially overlapped ATDs.
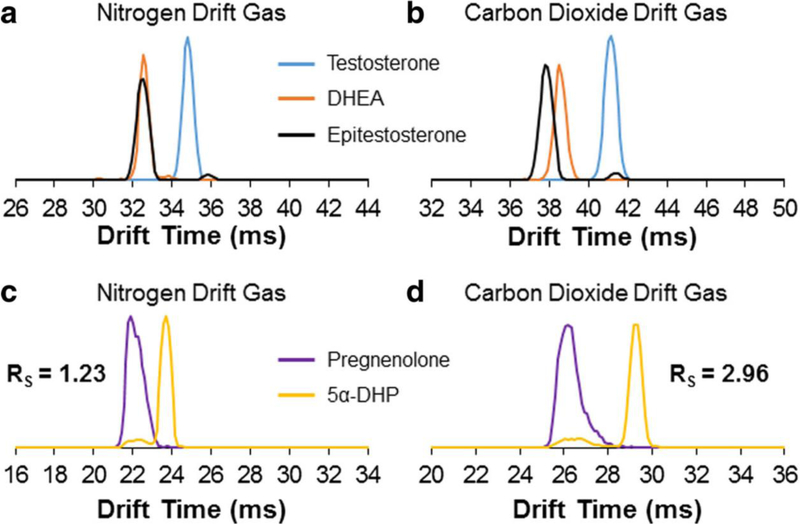
To further the separation of dimers, Chouinard et al. examined the differences that alternate drift gases and different metal adducts on the separation of these steroid isomers [9]. For the different drift gases, the resolution generally increased as more polarizable drift gases were used, where helium was the least polarizable drift gas and carbon dioxide as the most polarizable drift gas, as shown in Figure 7. Additionally, for only androsterone and epiandrosterone, the separation was examined with different metal adducts. The results for different metal adducts showed that alkali metals and alkaline earth metals have similar separation to sodium. However, manganese, cobalt, copper, and zinc did show some enhancement of the separation as the [2M+XII+Acetate]+ adduct, where X represents the metal. Although, these transition metal adducts all had lower signal than the alkali and alkaline earth metals adducts. Therefore, this study demonstrated the ability to analyze steroid isomer standards using DTIMS by examining their multimer adducts.
Figure 7.
Overlaid ATDs of testosterone, epitestosterone, and DHEA in nitrogen drift gas (a) and carbon dioxide drift gas (b), and overlaid ATDs of pregnenolone and 5α-dihydroprogesterone in nitrogen drift gas (c) and carbon dioxide drift gas (d). Reproduced with permission from Chouinard et al. [9], copyright 2016 Springer-Verlag.
Metal adducted multimer research was furthered by Rister et al., which examined five sets of steroid isomers with group I metal adducts as monomers and multimers through TWIMS-MS analysis [36]. Through this technique, each set of isomers achieved a resolution above 1 in the optimum adduct, which was not consistently a sodiated dimer. Androsterone and epidandrosterone had the highest resolution as a lithiated dimer. Furthermore, corticosterone and 11-deoxycortisol maintained a resolution above 1 for the lithiated, sodiated, and potassiated dimeric species. Aldosterone formed multiple different conformers as a monomer and dimer; therefore, aldosterone and cortisone were best resolved as potassiated trimers. Similarly, ɑ-estradiol and β-estradiol were separated best as the potassiated dimer. Finally, both testosterone/epitestosterone and testosterone/DHEA pairs was shown to have resolution above 1.5 as lithiated and sodiated dimer adducts, whereas epitestosterone and DHEA have low resolution despite multimer or metal adduct. Overall, these results show that altering the metal adduct could increase the TWIMS resolution of steroid isomer multimers.
The first work on the employment of metal adducted multimers for the separation of steroid isomers in a mixture was reported in Rister et al. [37]. This work showed that similar separation and arrival time distributions existed between the standard individual solution and multi-component standard solutions. However, the combining of multiple components allowed for the formation of heterodimers for certain species. These heterodimers decrease the resolution between isomers and complicate the ability to analyze and quantify steroids by this method. However, there still exists beneficial separation between isomers through this method alone. The value of this rapid separation technique is shown in Figure 8, where a mixture of all the steroids studied were combined and extracted arrival time distributions are shown for a variety of steroids and adducts that show valuable separation. Overall, this study highlights the limitations that are experienced by employing this method in a mixture and the potential value of this method despite the challenges.
Figure 8.
Mass spectrum of a mixture of steroids (a) and extracted ATDs for the testosterone isobars as lithiated monomers (b), aldosterone and cortisone as potassiated monomers (c), potassiated estradiol isomer dimers and lithiated testosterone isomer dimers (d), lithiated dimers of androsterone and epiandrosterone (e), and corticosterone and 11-deoxycortisol as lithiated dimers. Reproduced with permission from Rister et al. [37], copyright 2019 John Wiley & Sons, Ltd.
Finally, the use of group I metal adduction in ion mobility separation was applied to estradiol glucuronide isomers by Rister and Dodds [36]. Estradiol can undergo glucuronidation at either carbon 3, near the aromatic ring, or carbon 17, near the five-membered ring. After analysis of group I metals, the results showed that a dimeric species adducted to sodium provided a resolution above 1.00 in both one-component and two-component solutions. Furthermore, this work was followed using energy resolved collision-induced dissociation (CID) to discriminate the isomers by the different percentage of loss of the glucuronic acid. Therefore, the separation of steroid conjugates using IMS and metal adduction demonstrated here shows the potential applicability of these techniques in stand-alone analysis of steroids and steroid conjugates.
While the use of multimer formation has been shown to increase the separation of steroid isomers, there are practical concerns when incorporating multimer analysis in biological samples. Two major limitations were presented by Rister et al. and are shown in Figure 8 [37]. First, when certain isomers are presented in a mixture, a heterodimer of the combined isomers can form, which decreases the separation of isomers. Additionally, as shown in Figure 8, dimers have lower signal-to-noise ratios than monomer species. Given the low biological concentration of steroids, the dimer species would decrease the signal-to-noise ratios even further than studying the monomer species. However, formations of multimers could allow for more rapid and specific analyses when IMS is combined with prior chromatographic separation, such as LC. In this case, LC could separate species that form heterodimers and concentrates analytes prior to ionization.
CONCLUSIONS
The current literature on steroid analysis through ion mobility spectrometry illustrates the ability for use in conjunction with LC for enhanced signal-to-noise and confidence, but also its emergence as a stand-alone separation technique to the direct analysis of steroids. For instance, Ray et al. and Guddat et al. employed an LC-DMS-MS workflow for enhanced signal-to-noise for steroids in serum and hydrolyzed steroid glucuronides in urine, respectively [11, 34]. Furthermore, Kaur-Atwal et al. utilized TWIMS coupled to LC-MS to increase confidence and enhance signal-to-noise ratios for native testosterone and epitestosterone glucuronides directly from urine [4]. In stand-alone IMS analysis, Ahonen et al. showed the potential of TWIMS as a substantial separator on PTSI derivatized steroids, where the larger the steroid the higher the resolution [35]. Chouinard et al. illustrated the promise of native steroid analysis through the separation by sodiated dimer species [9, 12]. Furthermore, Rister et al. expanded this research to group I metal adducts, showing some increases in resolution, and highlights the potential value and limitations for mixture analysis [36, 37]. Despite the advancements made by the scientists in the field, steroids are still not able to be fully separated and very high confidence applied to the analysis.
The future direction of this field should focus on stand-alone analysis of steroid mixtures and rapid LC-IMS-MS workflows of large steroid and lipid mixtures with CCS calculations for increased identification confidence. While the formation of multimers has been shown to increase separation, the formation of these multimers in a mixture could result in the formation of heterodimers or diminished resolution. Additionally, while LC-IMS-MS has been explored, it has not been employed in a high throughput analysis approach that can characterize lipids and steroids simultaneously without stable isotope labeled internal standards for each steroid in the mixture. With research conducted on these fronts, IMS-MS could easily become a rapid, high throughput method for the analysis of steroids in medical and sports performance settings.
ACKNOWLEDGEMENTS
This work was supported in part by funding from the National Science Foundation, Division of Chemistry, through the Chemical Measurement and Imaging Program (grant number 1507989). Funding from the National Institutes of Health, National Institute of General Medical Sciences, was received through a Maximizing Investigators’ Research Award to E.D.D. (grant number R35GM128926) and a fellowship to A.L.R. from the Molecular Mechanisms of Disease Predoctoral Training Program (grant number T32GM107001). This work was carried out using core facilities supported in part by the National Institutes of Health, National Institute of General Medical Sciences through the Nebraska Center for Integrated Biomolecular Communication (grant number P20GM113126). Finally, the authors thank Ms. Jessica L. Minnick for constructive comments on a draft of the manuscript.
REFERENCES
- [1].Hu J, Zhang Z, Shen WJ, Azhar S, Cellular Cholesterol Delivery, Intracellular Processing and Utilization for Biosynthesis of Steroid Hormones, Nutr. Metab. 7 (2010) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nozaki O, Steroid Analysis for Medical Diagnosis, J. Chromat. A 935 (2001) 267–278. [DOI] [PubMed] [Google Scholar]
- [3].Haupt HA, Rovere GD, Anabolic Steroids: A Review of the Literature, Am. J. Sports Med. 12(6) (1984) 469–484. [DOI] [PubMed] [Google Scholar]
- [4].Kaur-Atwal G, Reynolds JC, Mussell C, Champarnaud E, Knapman TW, Ashcroft AE, O’Connor G, Christie SD, Creaser CS, Determination of Testosterone and Epitestosterone Glucuronides in Urine by Ultra Performance Liquid Chromatography-Ion Mobility-Mass Spectrometry, Analyst 136(19) (2011) 3911–6. [DOI] [PubMed] [Google Scholar]
- [5].Lewis J, Steroid Analysis in Saliva: An Overview, Clin. Biochem. Rev. 27 (2006) 139–146. [PMC free article] [PubMed] [Google Scholar]
- [6].Severi G, Morris HA, MacInnis RJ, English DR, Tilley W, Hopper JL, Boyle P, Giles GG, Circulating Steroid Hormones and the Risk of Prostate Cancer, Cancer Epidemiol., Biomarkers Prev. 15(1) (2006) 86–91. [DOI] [PubMed] [Google Scholar]
- [7].Van Renterghem P, Van Eenoo P, Sottas PE, Saugy M, Delbeke F, A Pilot Study on Subject-Based Comprehensive Steroid Profiling: Novel Biomarkers to Detect Testosterone Misuse in Sports, Clin. Endocrinol. 75(1) (2011) 134–40. [DOI] [PubMed] [Google Scholar]
- [8].Arlt W, Biehl M, Taylor AE, Hahner S, Libe R, Hughes BA, Schneider P, Smith DJ, Stiekema H, Krone N, Porfiri E, Opocher G, Bertherat J, Mantero F, Allolio B, Terzolo M, Nightingale P, Shackleton CH, Bertagna X, Fassnacht M, Stewart PM, Urine Steroid Metabolomics as a Biomarker Tool for Detecting Malignancy in Adrenal Tumors, J. Clin. Endocrinol. Metab. 96(12) (2011) 3775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chouinard CD, Beekman CR, Kemperman RHJ, King HM, Yost RA, Ion Mobility-Mass Spectrometry Separation of Steroid Structural Isomers and Epimers, Int. J. Ion Mobility Spectrom. 20(1–2) (2017) 31–39. [Google Scholar]
- [10].Giese RW, Measurement of Endogenous Estrogens: Analytical Challenges and Recent Advances, J. Chromatogr. A 1000(1–2) (2003) 401–412. [DOI] [PubMed] [Google Scholar]
- [11].Ray JA, Kushnir MM, Yost RA, Rockwood AL, Meikle Wayne A, Performance Enhancement in the Measurement of 5 Endogenous Steroids by LC-MS/MS Combined with Differential Ion Mobility Spectrometry, Clin. Chim. Acta 438 (2015) 330–6. [DOI] [PubMed] [Google Scholar]
- [12].Chouinard CD, Cruzeiro VW, Roitberg AE, Yost RA, Experimental and Theoretical Investigation of Sodiated Multimers of Steroid Epimers with Ion Mobility-Mass Spectrometry, J. Am. Soc. Mass Spectrom. 28(2) (2017) 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Settlage J, Oglesby T, Rajasekaran A, Williard C, Scott G, The Importance of Chromatographic Resolution when Analyzing Steroid Biomarkers, Steroids 99(Pt A) (2015) 45–8. [DOI] [PubMed] [Google Scholar]
- [14].Alda MJ, Barceló D, Review of Analytical Methods for the Determination of Estrogens and Progestogens in Waste Waters, Anal. Chem. 371(4) (2001) 437–447. [DOI] [PubMed] [Google Scholar]
- [15].Gouveia MJ, Brindley PJ, Santos LL, da Costa Correia JM, Gomes P, Vale N, Mass Spectrometry Techniques in the Survey of Steroid Metabolites as Potential Disease Biomarkers: A Review, Metabolism 62(9) (2013) 1206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kanu AB, Dwivedi P, Tam M, Matz L, Hill HH Jr., Ion mobility-mass spectrometry, J. Mass Spectrom. 43(1) (2008) 1–22. [DOI] [PubMed] [Google Scholar]
- [17].Lapthorn C, Pullen F, Chowdhry BZ, Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: separating and assigning structures to ions, Mass Spectrom. Rev. 32(1) (2013) 43–71. [DOI] [PubMed] [Google Scholar]
- [18].Zheng X, Aly NA, Zhou Y, Dupuis KT, Bilbao A, Paurus Vanessa L., Orton DJ, Wilson R, Payne SH, Smith RD, Baker ES, A Structural Examination and Collision Cross Section Database for Over 500 Metabolites and Xenobiotics using Drift Tube Ion Mobility Spectrometry, Chem. Sci. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bush MF, Campuzano ID, Robinson CV, Ion mobility mass spectrometry of peptide ions: effects of drift gas and calibration strategies, Anal. Chem. 84(16) (2012) 7124–30. [DOI] [PubMed] [Google Scholar]
- [20].Forsythe JG, Petrov AS, Walker CA, Allen SJ, Pellissier JS, Bush MF, Hud NV, Fernandez FM, Collision Cross Section Calibrants for Negative Ion Mode Traveling Wave Ion Mobility-Mass Spectrometry, Analyst 140(20) (2015) 6853–61. [DOI] [PubMed] [Google Scholar]
- [21].Gelb AS, Jarratt RE, Huang Y, Dodds ED, A study of calibrant selection in measurement of carbohydrate and peptide ion-neutral collision cross sections by traveling wave ion mobility spectrometry, Anal. Chem. 86(22) (2014) 11396–402. [DOI] [PubMed] [Google Scholar]
- [22].Giles K, Williams JP, Campuzano I, Enhancements in travelling wave ion mobility resolution, Rapid Commun. Mass Spectrom. 25(11) (2011) 1559–1566. [DOI] [PubMed] [Google Scholar]
- [23].Henderson SC, Li J, Countermann AE, Clemmer DE, Intrinsic Size Parameters for Val, Ile, Leu, Gln, Thr, Phe, and Trp Residues from Ion Mobility Measurements of Polyamino Acid Ions, J. Phys. Chem. B 103 (1999) 8780–8785. [Google Scholar]
- [24].Hines KM, May JC, McLean JA, Xu L, Evaluation of Collision Cross Section Calibrants for Structural Analysis of Lipids by Traveling Wave Ion Mobility-Mass Spectrometry, Anal. Chem. 88(14) (2016) 7329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hofmann J, Struwe WB, Scarff CA, Scrivens JH, Harvey DJ, Pagel K, Estimating collision cross sections of negatively charged N-glycans using traveling wave ion mobility-mass spectrometry, Anal. Chem. 86(21) (2014) 10789–95. [DOI] [PubMed] [Google Scholar]
- [26].Shvartsburg AA, Smith RD, Fundamentals of Traveling Wave Ion Mobility Spectrometry, Anal. Chem. 80 (2008) 9689–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Campuzano I, Bush MF, Robinson CV, Beaumont C, Richardson K, Kim H, Kim HI, Structural characterization of drug-like compounds by ion mobility mass spectrometry: comparison of theoretical and experimentally derived nitrogen collision cross sections, Anal. Chem. 84(2) (2012) 1026–33. [DOI] [PubMed] [Google Scholar]
- [28].Huang Y, Dodds ED, Ion mobility studies of carbohydrates as group I adducts: isomer specific collisional cross section dependence on metal ion radius, Anal. Chem. 85(20) (2013) 9728–35. [DOI] [PubMed] [Google Scholar]
- [29].Pagel K, Harvey DJ, Ion Mobility-Mass Spectrometry of Complex Carbohydrates: Collision Cross Sections of Sodiated N-Linked Glycans, Anal. Chem. 85(10) (2013) 5138–45. [DOI] [PubMed] [Google Scholar]
- [30].Paglia G, Williams JP, Menikarachchi L, Thompson JW, Tyldesley-Worster R, Halldorsson S, Rolfsson O, Moseley A, Grant D, Langridge J, Palsson BO, Astarita G, Ion mobility derived collision cross sections to support metabolomics applications, Anal. Chem. 86(8) (2014) 3985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ruotolo BT, Benesch JL, Sandercock AM, Hyung SJ, Robinson CV, Ion Mobility-Mass Spectrometry Analysis of Large Protein Complexes, Nat. Protoc. 3(7) (2008) 1139–52. [DOI] [PubMed] [Google Scholar]
- [32].Cumeras R, Figueras E, Davis CE, Baumbach JI, Gracia I, Review on Ion Mobility Spectrometry. Part 1: Current Instrumentation, Analyst 140(5) (2015) 1376–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kolakowski BM, Mester Z, Review of Applications of High-Field Asymmetric Waveform Ion Mobility Spectrometry (FAIMS) and Differential Mobility Spectrometry (DMS), Analyst 132(9) (2007) 842–64. [DOI] [PubMed] [Google Scholar]
- [34].Guddat S, Thevis M, Kapron J, Thomas A, Schanzer W, Application of FAIMS to Anabolic Androgenic Steroids in Sport Drug Testing, Drug Test Anal. 1(11–12) (2009) 545–53. [DOI] [PubMed] [Google Scholar]
- [35].Ahonen L, Fasciotti M, Gennas GB, Kotiaho T, Daroda RJ, Eberlin M, Kostiainen R, Separation of Steroid Isomers by Ion Mobility Mass Spectrometry, J. Chromatogr. A 1310 (2013) 133–7. [DOI] [PubMed] [Google Scholar]
- [36].Rister AL, Dodds ED, Ion Mobility Spectrometry and Tandem Mass Spectrometry Analysis of Estradiol Glucuronide Isomers, Journal of the American Society for Mass Spectrometry (2019) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rister AL, Martin TL, Dodds ED, Formation of multimeric steroid metal adducts and implications for isomer mixture separation by traveling wave ion mobility spectrometry, J Mass Spectrom 54(5) (2019) 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]