Abstract
Sleep is a phenomenon in animal behavior as enigmatic as it is ubiquitous, and one deeply tied to endocrine function. Though there are still many unanswered questions about the neurochemical basis of sleep and its functions, extensive interactions have been identified between sleep and the endocrine system, in both endocrine system’s effect on sleep and sleep’s effect on the endocrine system. Unfortunately, until recent years, much research on sleep behavior largely disregarded its connections with the endocrine system. Use of both clinical studies and rodent models to investigate interactions between neuroendocrine function, including biological sex, and sleep therefore presents a promising area of further exploration. Further investigation of the neurobiological and neuroendocrine basis of sleep could have wide impact on a number of clinical and basic science fields. In this review, we summarize the state of basic sleep biology and its connections to the field of neuroendocrine biology, as well as suggest key future directions for the neuroendocrine regulation of sleep that may significantly impact new therapies for sleep disorders in women and men.
Keywords: Endocrine, Sex differences, Sleep
Despite being among life’s most common behavioral states, sleep remains a phenomenon that resists easy explanation. Sleep is generally defined as being characterized by an increased threshold for response to sensory input, a decrease in motor function, and a lack of consciousness. Though humans spend on average a third of their lifespan in the sleep state (Aminoff et al. 2011), there are still many unanswered questions about the neurochemical basis of sleep and its functions. Extensive interactions have been identified between sleep and the endocrine system, in both endocrine system’s effect on sleep and sleep’s effect on the endocrine system. Numerous endocrine factors can affect sleep quantity and quality, while studies have shown a profound effect of sleep behavior on overall endocrine function and stability (Morgan and Tsai 2015; Spiegel et al. 1999). Further investigation of the neurobiological and neuroendocrine basis of sleep could have wide impact on a number of clinical and basic science fields, from treatment of insomnia to exploration of sex-distinct sleep differences to investigation of the pathogenesis of neuro-degenerative diseases. In this review, we summarize the state of basic sleep biology and its connections to the field of neuroendocrine biology, as well as suggest key future directions for the neuroendocrine regulation of sleep that may significantly impact new therapies for sleep disorders in women and men.
1. Sleep Behavior Consists of Multiple Distinguishable States
Sleep consists of several distinct states, which can be distinguished by their patterns of brain activity (Saper et al. 2010). The most important distinction between sleep states is between Rapid Eye Movement (REM) and non-Rapid Eye Movement (non-REM) sleep. Non-REM sleep predominates at the outset of a particular sleep bout, and is distinguished by an ordering and synchronizing of brain activity (Mong and Cusmano 2016). This synchronization leads to a decrease in the frequency and increase in the amplitude of brain waves, causing waves in the delta (0–4 Hz) range to predominate; those waves are considered synonymous with slow wave activity (SWA) (Lanquart et al. 2018). During non-REM sleep, muscle activity is decreased relative to the wake state, but paralysis of skeletal muscles is not present (Mong and Cusmano 2016). In humans, further refinement of non-REM sleep can be achieved by separating it into distinct stages, numbered 1–3 in order of increasing depth of sleep. Stage 3 (redefined in 2007 from the prior stages 3 and 4) (Moser et al. 2009) is referred to as slow-wave sleep and represents the deepest sleep states.
In contrast, REM sleep, also known as paradoxical sleep, consists of highly disordered brain activity that somewhat mimics brain activity in the wake state. In this state, waves in the theta (4–8 Hz) range dominate (Hutchison and Rathore 2015), and skeletal muscles are paralyzed. REM sleep does not occur at the onset of a sleep bout in healthy animals, instead appearing later in the sleep bout after a period of slow-wave sleep has been completed (Saper et al. 2010). The differing functions of REM and non-REM sleep are poorly understood, and to date many sleep studies have focused on the aggregate time spent in sleep versus wake as their main metric. However, methods do exist for isolating REM or non-REM sleep. For example, the flowerpot method, in which an experimental animal is allowed to sleep on a small shelf, such as an upside down flowerpot, above a pool of water, selectively deprives the experimental subject of REM sleep only by prohibiting sleep during periods of muscle paralysis (Aalto and Kiianmaa 1984). These methods may become more prominent as further differences between the two states are elucidated.
2. The Circadian and Homeostatic Systems Drive Sleep Pressure
The biological circuitry of sleep is an area of intense inquiry, with many questions remaining on both the neuroanatomy and neurochemistry of the relevant pathways. This question is complicated by the existence of two distinct systems governing aspects of sleep regulation. These systems’ net output is generally described as sleep pressure. Sleep pressure has been defined as the intrinsic need for sleep of a given animal at a given time (Eban-Rothschild et al. 2017). Beyond the familiar intrinsic feeling of sleepiness as a manifestation of sleep pressure, quantitative markers derived from EEG outputs exist that can approximate sleep pressure in a reproducible fashion (Mong and Cusmano 2016). The two sleep-pressure systems, known as the circadian wake system and the homeostatic sleep-pressure system, operate in parallel and in concert to generate an overall sleep pressure that is responsive to both the animal’s intrinsic homeostatic needs as well as external factors such as the light–dark cycle.
The better-understood of the two systems which combine to govern sleep pressure is the circadian wake system. The circadian system orients sleep to the light and dark cycle, as well as consolidates sleep and wake into larger blocks. Circadian timing has two key properties. First, it has an endogenous rhythm with a period of approximately (though not exactly) 24 h (Abbott et al. 2015). Second, that rhythm can be shifted in response to external cues (Abbott et al. 2015). Light–dark cycles are both the most prevalent and potent of these cues, but other factors such as exercise, feeding, temperature, and certain pharmacological agents have been shown to entrain the system as well, in some cases maladaptively (Abbott et al. 2015). The key neurobiological regulator of the circadian sleep system is the suprachiasmatic nucleus (SCN) of the hypothalamus. Animals with lesions of the SCN have been shown to have as much total sleep time as controls, but sleep in unconsolidated random bouts unrelated to the light–dark cycle (Mouret et al. 1978). Transplantation of a donor SCN has been shown to rescue a normal phenotype in that regard (Sawaki et al. 1984). Additionally, studies of humans isolated from the natural light–dark cycle and left to sleep ad libitum show that those humans settle into a diurnal sleep pattern that approximates, but does not exactly mimic, the 24-h day, showing the intrinsic rhythmicity of the SCN. The SCN receives its principal entraining inputs from environmental light cues through specialized photosensitive ganglion cells in the retina. Importantly, the photoreceptors and cortical areas responsible for conscious vision are not involved, meaning that the circadian rhythm is reasonably well entrained in most blind animals (Squarcini et al. 2013). Importantly for the endocrine system, the circadian system serves to create “biological day” and “biological night”. Hormones and other biological properties have been shown to fluctuate on a 24-h cycle according to stereotypical patterns; lesions of the circadian system have been shown to disrupt the daily fluctuation of hormone levels such as growth hormone (Steyn and Ngo 2017), cortisol (Challet 2015), and leptin (Challet 2015), among others (see Sect. 6).
The second sleep system, quite distinct from the circadian system, is the homeostatic pressure system. As the name suggests, the homeostatic sleep system governs the amount of sleep needed after a given period of wake to maintain homeostasis (Allada et al. 2017). The total amount of sleep needed for an animal in a given period of time tends to be quite consistent, and independent of both the circadian system and the light–dark cycle (Donlea 2017). This phenomenon is further exemplified by the need for recovery sleep, which is nearly always necessary after periods of sleep deprivation (Donlea 2017). Similarly, to other homeostatic systems such as temperature, extreme loss of homeostasis (such as through prolonged sleep deprivation) is fatal (Greene and Siegel 2004). Homeostatic sleep pressure increases roughly linearly with increasing wake time, reaching a maximum at the onset of the sleep state, and then decreases roughly linearly with time spent asleep (Donlea 2017). There is debate over whether homeostatic sleep pressure has a measurable biological correlate, though studies have shown correlations with both molecular markers such as adenosine (Reichert et al. 2016; Zeitzer et al. 2006) and behavioral markers such as delta power during recovery non-REM sleep (Alam et al. 2014).
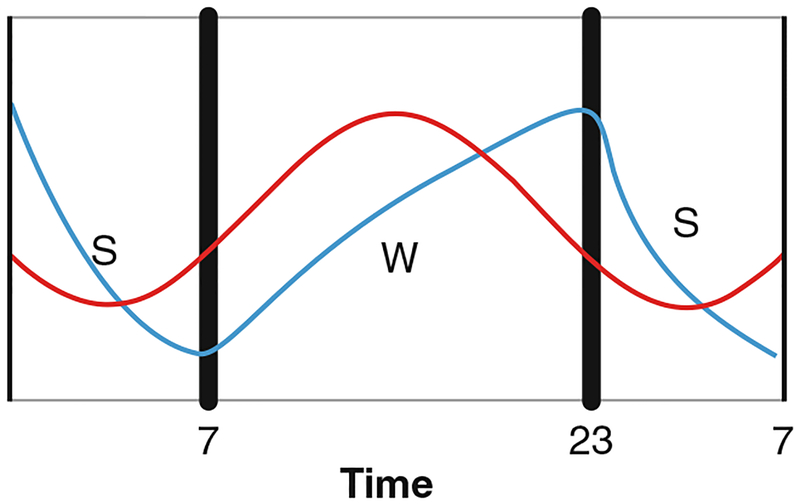
The two pathways, circadian and homeostatic, work in concert to generate an overall sleep pressure (Fig. 1). Homeostatic sleep pressure increases monotonically throughout the day; however, during the daylight hours in humans, circadian wake drive is also high and increasing. By the onset of the dark phase of the cycle, both homeostatic sleep drive and circadian wake drive are high, canceling the effect of either. However, after the onset of the dark phase, circadian wake drive begins to decrease, while homeostatic sleep drive remains high, stimulating the onset of the sleep state. By the end of the sleep state in the early morning hours, circadian wake drive is still low, but homeostatic sleep drive is low enough to compensate, causing the switch to flip again and the onset of the wake state to commence. This dual cycle has important impacts on situations where sleep occurs outside of the normal rhythm; for example, in shift workers, falling asleep in the early morning is not a problem as circadian wake drive is low and sleep pressure is high (Wagner 1999). However, remaining asleep through the day can be difficult as circadian wake drive increases while sleep pressure falls.
Fig. 1.
Schematic of the circadian and homeostatic systems. The homeostatic sleep process (blue) creates sleep pressure roughly linearly in response to time awake, and decreases with time asleep. The circadian wake process (red) is most active during the daylight hours and provides a method of maintaining wake. After sunset, melatonin stimulates a breakdown in the circadian process, which allows the pro-sleep homeostatic process to become dominant, stimulating the sleep state. After a period of sleep, homeostatic pressure has lowered enough that even a low circadian wake drive becomes dominant, stimulating the wake state
3. Diverse Chemical Mechanisms and Circuitry Govern Sleep-Pressure Systems
The circadian system is highly centralized, with the SCN being the key center of its action. The SCN exerts circadian control in myriad ways, both neurologically and hormonally. Neurons in the SCN are home to a set of clock transcription factors that stimulate their own repressors (Aryal et al. 2017), forming a daily oscillating cycle of gene expression. By a mechanism not fully understood, this mechanism governs the differing release of neurotransmitters depending on the time of the cycle (Golombek and Rosenstein 2010). Thus, the SCN releases different waves of neurotransmitters, such as glutamate, GABA, and vasopressin (Shinohara et al. 1998). The main neurological projections of the SCN radiate to the medial hypothalamus, but for hormonal control, the epithalamus is a key site of SCN effects. The pineal gland of the epithalamus releases melatonin, perhaps the best-known hormonal circadian modulator. Release of melatonin is dependent on environmental inputs, most notably the light cycle; melatonin increases several hours after the onset of the dark phase of the light cycle, and remains high until the restoration of the light phase the next day (Bedrosian et al. 2013). While melatonin is not required for the initiation or maintenance of circadian rhythms overall (Gandhi et al. 2015), it serves as a key link to entrain biological circadian processes to light cues (Sack et al. 2000), and may stimulate sleep in humans and some animal species, possibly through adenosine signaling (Gandhi et al. 2015; Zhdanova et al. 2001). In humans, melatonin’s effects are the principal mediator for normal sleep onset several hours into the dark phase of the diurnal cycle. Melatonin receptors have been shown to exist in myriad tissues (Morgan et al. 1994), potentially providing a system-wide mechanism for circadian synchronicity that drives an acute need for sleep at a stereotypical time each day.
Unlike the circadian system, which is largely dependent on environmental inputs via the specialized retinal ganglion cells, the homeostatic system appears to have multiple inputs. An entire class of molecules known as somnogens have been identified that appear to increase homeostatic sleep pressure. Among the most important of these molecules is the nucleoside adenosine (Lazarus et al. 2017; Huang et al. 2014), produced in the brain both purposefully as a neurotransmitter and as a waste product of ATP metabolism. Indeed, many laypersons are familiar with the hypnogenic effects of adenosine through the widespread use of the non-specific adenosine receptor antagonist caffeine (Yanik et al. 1987). Adenosine has been shown to accumulate in the brain, particularly in the basal forebrain (Blanco-Centurion et al. 2006), with increasing wake time and decrease in the sleep state (Blanco-Centurion et al. 2006). Additionally, low (nanomolar) concentrations of adenosine have been shown to enhance wake neurotransmission due to activation of the inhibitory A1 receptor in the preoptic hypothalamus, while high (micromolar) adenosine concentrations have been shown to inhibit wake neurotransmission through activation of stimulatory A2A receptors in the same nuclei (Methippara et al. 2005; Kumar et al. 2013). Several other molecules, such as prostaglandin D2 (Zhang et al. 2017), IL-1 (Obal et al. 1990), and TNF-alpha (Kapás et al. 1992), have been hypothesized to act in similar fashions to stimulate the sleep-pressure homeostat. Additionally, an emerging area of research in mechanisms of the homeostatic sleep-pressure system may be through the process of protein phosphorylation. A family of proteins, sleep need index phosphorylation proteins (SNIPPs), have been found to become steadily phosphorylated during wake time and dephosphorylated during sleep (Wang et al. 2018). The kinase Sik3 has been shown to aid in this phosphorylation; a constitutively active mutant of this kinase has been shown to induce sleep pressure artificially, resulting in mice with higher sleep times and delta power during their non-REM sleep (Honda et al. 2018).
Downstream of these somnogen initiators, the homeostatic sleep-pressure system contains multiple neurotransmitters, including orexin, acetylcholine, monoamines, and glutamate (Stenberg 2007). The sleep circuitry is a complex and multi-faceted system from a neuroanatomical perspective, with separate wake-promoting and sleep-promoting networks. Studies of wake-promoting systems historically focused on monoaminergic and cholinergic neurons of the upper brainstem, including noradrenaline from the locus coeruleus, 5HT from the raphe, and acetyl choline from the tegmentum, among others (Saper and Fuller 2017). These systems project broadly to the cortex by way of the thalamus, ventral portions of hypothalamus, and basal forebrain. This system is also augmented by peptidergic orexin inputs joining at the hypothalamus. Paradoxically though, lesions of these pathways had little effect on total sleep and wake time (Fuller et al. 2011). Thus, in recent years, the importance of glutamatergic and GABAergic networks on the wake-promoting circuitry has grown. GABAergic inputs from the basal forebrain, lateral hypothalamus, and supramammillary nucleus have been shown to promote wake, as have glutamatergic inputs from the supramammillary, parabrachial, and pedunculopontine nuclei (Saper and Fuller 2017).
Several nuclei are thought to stimulate sleep in the homeostatic sleep-pressure system, with two nuclei of the preoptic hypothalamus of key importance. The ventral lateral preoptic nucleus (VLPO) (Wagner 1999) and median preoptic nucleus (MnPN) (Mong and Cusmano 2016) are thought to be key originators of this pathway. These nuclei send GABAergic projections to key mediators of the wake state, particularly nuclei in the lateral hypothalamus governing the orexinergic wake system (Mong and Cusmano 2016). A feed-forward loop has been identified in which the MnPN both inhibits the orexinergic wake system and stimulates the VLPO, which itself serves as an inhibitor of the orexinergic wake nuclei in the lateral hypothalamus (Szymusiak and McGinty 2008). Orexinergic wake nuclei have widespread projections to the cortex and brainstem and are primarily active during the wake phase (Fulcher et al. 2014); lesions of these nuclei have been shown to induce a narcoleptic-like phenotype (Ocampo-Garcés et al. 2011). The orexin system is important from an endocrine perspective in its dual importance to both sleep–wake circuitry and feeding behavior. Orexin projections from the lateral hypothalamus project broadly across the brain to centers important for feeding, such as the paraventricular nucleus, and centers important for maintenance of wake, such as the locus coeruleus (Grafe and Bhatnagar 2018). However, the exact structure and function relationship of these pathways, and what neuronal pathways may exist connecting between feeding and sleep behavior, is incompletely known. Beyond the orexinergic system, the MnPN and VLPO send broad GABAergic inhibitory projections to many of the same nuclei involved in the wake system, including the supramammillary, tuberomammillary, and parabrachial nuclei, as well as monoaminergic nuclei such as the raphe and locus coeruleus. Additionally, projections from the MnPN and VLPO stimulate sleep by way of other brainstem nuclei such as the ventral periaqueductal gray (Saper and Fuller 2017). The MnPN and VLPO have been shown to receive circadian inputs from the SCN by indirect projections via the dorsal medial hypothalamus and/or supraventricular zone, suggesting a potential pathway for the integration of the circadian and homeostatic systems (Deurveilher and Semba 2003; Sun et al. 2001).
4. Several Hypotheses Exist as to the Functions of Sleep
Despite its ubiquitous nature, very little is known about why sleep is necessary; from an evolutionary standpoint, the necessity for an animal to spend such a large portion of its lifespan in a position both vulnerable and seemingly of little use to the animal would seem a poor adaptation. The question of sleep’s function in animal physiology is a hotly debated one, with several working hypotheses. In particular, three well-developed hypotheses have formed of key sleep functions: as a method of brain microenvironmental homeostasis, a mechanism for memory consolidation and cognition, and as a regulator of metabolism and energy balance.
The evidence for sleep as a homeostatic process is well-established. Like other homeostatic systems, sleep pressure responds in an analog fashion to the relative distance from its homeostatic mean; in essence, sleep pressure directly increases with wake time. The recently identified glymphatic system may provide a mechanism for control of brain microenvironmental homeostasis that is sleep-dependent. The glymphatic system is a fluid-dynamic model of cerebrospinal (CSF) and interstitial (ISF) fluid flow around the brain and through the brain parenchyma. This flow has been shown to be important for clearance of metabolites and other waste products, including amyloid beta (Xie et al. 2013) from the brain. Additionally, glymphatic flow has been shown to be upregulated by as much as a factor of ten in the sleep state (Xie et al. 2013). Thus, a model has emerged in which waste products of metabolism and brain activity build up in the wake state due to inadequate glymphatic flow, but are cleared from the parenchyma in the sleep state when flow is increased (Plog and Nedergaard 2018). These findings could suggest that brain clearance is a key function of the sleep state and a key purpose of the homeostatic sleep function.
Another hypothesis for the function of sleep involves the process of memory consolidation (Cellini 2017). Multiple studies have shown that memories are enhanced during sleep; in particular, declarative memories have been shown to be enhanced after non-REM sleep (Krause et al. 2017; Ackermann and Rasch 2014), while non-declarative or emotional memories have shown enhancement after REM sleep (Sun et al. 2001). Sleep has also been shown to be important for synaptic downscaling, in which synapses are uniformly lessened in strength during sleep (Raven et al. 2018). This uniform downscaling prevents or relieves the saturation of synapse receptor patches. Relief of saturation allows for further long-term potentiation and depression at the same synapses, in order for more differentiation of synaptic strength (and thus memory formation) to proceed.
Perhaps most importantly from an endocrine perspective, sleep has been hypothesized as an important mechanism for the regulation of metabolism and systemic energy balance. Overall metabolism declines only modestly in sleep (Fraser and Nordin 1955), suggesting that energy conservation per se is not a key function of the sleep state. One working hypothesis for the purpose of slower metabolism is that it may allow free radical scavengers more freedom to reduce reactive molecules that can damage the brain (Villafuerte et al. 2015). More broadly though, sleep has been shown to induce the fluctuation of hormones important for the regulation of normal metabolism. Sleep disruption has been shown to enhance ghrelin (Copinschi et al. 2014) and decrease leptin (Allada et al. 2017) levels, stimulating appetite; it is also associated with a state similar to insulin resistance, possibly due to dysregulation of growth hormone (GH) levels (Rasmussen et al. 2008). As a result, disruptions in sleep have been shown to be a risk factor and exacerbating factor for metabolic syndrome and related disorders (Rasmussen et al. 2008).
5. Sleep Disorders Have a Broad Clinical Impact
Sleep disorders are both quite prevalent and generally thought to be underdiagnosed. While we will not attempt to give an exhaustive overview of all sleep disorders here, this section will profile some common conditions to illustrate the myriad interactions with the endocrine system. Sleep disorders can take two forms: disorders of sleep quantity and quality per se, and related comorbidities which can be introduced or exacerbated by sleep disruption.
Insomnia is the most common sleep disorder; it is defined as the persistent inability to sleep despite the opportunity to do so. It has been estimated that insomnia is clinically present in 6% of the population, and as many as a third of individuals may show some symptoms (Ohayon 2002). Insomnia may be a primary condition, or secondary to a multitude of other neurological, psychiatric, and physical disorders. A multitude of pharmaceutical and non-pharmaceutical interventions exists to combat insomnia, of varying effectiveness. Importantly for the endocrine field, insomnia shows a sex difference, as it is significantly more common in women than men (Mong and Cusmano 2016). Additionally, insomnia pharmacology is an arena where sex differences have become a prominent issue. One of the most common anti-insomnia medications, the benzodiazepine-like drug zolpidem (popularly known by the brand name Ambien), was also the first major drug to show a strong interaction with the patient’s biological sex, as in 2013 the FDA reduced the recommended dose for women to half that of men. Though the case of zolpidem sexual interaction was due to differing rates of liver metabolism (Greenblatt et al. 2014), the brain has shown an interaction between pharmacology and biological sex in the context of insomnia as well. A study administering olanzapine (a second-generation anti-psychotic) showed sex differences in its effect on sleep, as slow-wave sleep increased in women and decreased in men (Giménez et al. 2011).
Another prominent sleep disorder is restless legs syndrome (RLS). RLS is the uncontrollable urge to move one’s legs when at rest, which leads to an inhibition of deep sleep states (Gamaldo and Earley 2006). RLS is quite common, with estimates of between 2 and 15% of the population displaying symptoms. The causes are unclear, but several underlying diseases and medication side-effects, most notably iron deficiency (Trotti and Becker 2019), have been speculated as potential causes. Similar to insomnia, RLS is far more prevalent in women than men, for reasons that remain unclear (Berger et al. 2004).
A prominent sleep disorder about which more is known surrounding the etiology is obstructive sleep apnea (OSA). OSA is marked by closure of the airway during the night, leading to periods of cessation or attenuation of breathing and hypoxia (White 2017). OSA is often underdiagnosed, as it presents with very non-specific symptoms, such as daytime sleepiness, fatigue, and impaired cognition, to the sufferer. As such, it is often only noticed by bed partners due to nighttime snoring (Punjabi 2008). OSA is often a comorbidity of obesity, due to the presence of additional fatty tissue in structures surrounding the airway (Schwab et al. 2015). Unlike many sleep disorders, OSA is more commonly diagnosed in males (Punjabi 2008), though there is speculation that it may simply be underdiagnosed in women. OSA is usually managed mechanically through the use of continuous positive airway pressure (CPAP) machines or mandibular splint devices, which both physically open the airway (Bratton et al. 2015).
Disorders of circadian regulation are also widespread. Delayed sleep phase disorder (DSPD) is a chronic circadian dysregulation that pushes the onset of sleep and the onset of wake much later relative to societal norms (Pavlova 2017), often due to genetics (Matheson and Hainer 2017). It is a form of “social jet lag”, a broader term that also encompasses a delayed circadian phase due to behavioral and environmental factors (Kayaba et al. 2018). DSPD and social jet lag are relatively rare in adults, with a prevalence of under 2 in 1000, but are common in adolescents, with studies showing a prevalence of 5% (Danielsson et al. 2016) and some estimates being even higher. Treatments of DSPD with melatonin (Auld et al. 2017) and analogues have shown some success in combating the symptoms, though relapse can be a concern (Micic et al. 2016). The converse of this condition, advanced sleep phase disorder, is significantly rarer, though more common in the elderly. Both DSPD and ASPD have shown a strong genetic component in familial studies (Matheson and Hainer 2017).
Mood disruptions have shown a sleep and circadian component. Disruption of the circadian rhythms of melatonin has been linked to seasonal affective disorder (SAD), a common form of depression. SAD is prevalent in the winter months, when daylight cycles are shorter and cause disruption of melatonin secretion (Wirz-Justice 2018); some SAD patients experience melatonin secretion well into the morning hours, when levels should be low. Morning bright-light therapy to resynchronize melatonin levels has been shown to mitigate some effects of SAD (Wirz-Justice 2018). Major depression has also been linked to melatonin release, and some melatonin receptor agonists have been approved to treat depression (Hickie and Rogers 2011).
Sleep disruption has been shown to be tied to many serious pathologies, both cognitive and physical. Sleep changes have been shown to be correlated with Alzheimer’s pathology as well as a potential leading sign of the disease (Peter-Derex et al. 2015). Alzheimer’s patients have shown decreased sleep at night and increased sleep during the day, as well as an overall loss of REM sleep (Pase et al. 2017). Additionally, self-reported sleep problems, most notably sleep fragmentation, have been associated with a higher risk of Alzheimer’s years later (Macedo et al. 2017). The loss of sleep-dependent microenvironmental homeostasis may be a contributing factor to the accumulation of brain metabolites in such dementia. Amyloid Beta, the key protein which aggregates in Alzheimer’s, has been shown to display a diurnal rhythm that increases during wake time and decreases during sleep (Lucey and Bateman 2014). The impact of sleep on memory formation and consolidation may also explain portions of this connection.
The strong endocrine connections between sleep and metabolism also present a possible explanation for population-level correlations between sleep disruption and the key public health issue of metabolic syndrome. Obesity is a key public health concern, and sleep has been shown by multiple studies to both impact and have an impact on metabolic and feeding behaviors. Most notably, obesity is a major risk factor for OSA (Schwab et al. 2015) as described above. Conversely, sleep loss has been shown to have a stimulating effect on appetite (Schmid et al. 2015) and has been correlated with increased obesity. However, the molecular mechanisms of these interactions, particularly the connections between clinical phenotypes and neuroendocrine mechanisms, are still ill defined and multi-faceted. Sleep disruption has been shown to increase oxidative stress (Villafuerte et al. 2015), enhance pro-inflammatory mediators such as IL-1 and TNF-alpha (Obal et al. 1990; Kapás et al. 1992), activate the sympathetic nervous system (Schlaich et al. 2015), and stimulate cortisol secretion (Wright et al. 2015). Activation of these pathways has been shown to be risk factors for obesity, metabolic syndrome, and sequelae such as type 2 diabetes.
6. Sleep Behavior and Circadian Timing Impact Multiple Hormonal Functions
Multiple different hormones have been shown to be impacted by the sleep–wake cycle, though it remains something of an open question what roles the intrinsic circadian timekeeper and sleep behavioral cycle play in governing the differential release. Indeed, there is evidence to suggest that different hormones may display different mechanisms of diurnal entrainment vis-à-vis homeostatic sleep pressure, circadian timing, and sleep behavior (Pietrowsky et al. 1994).
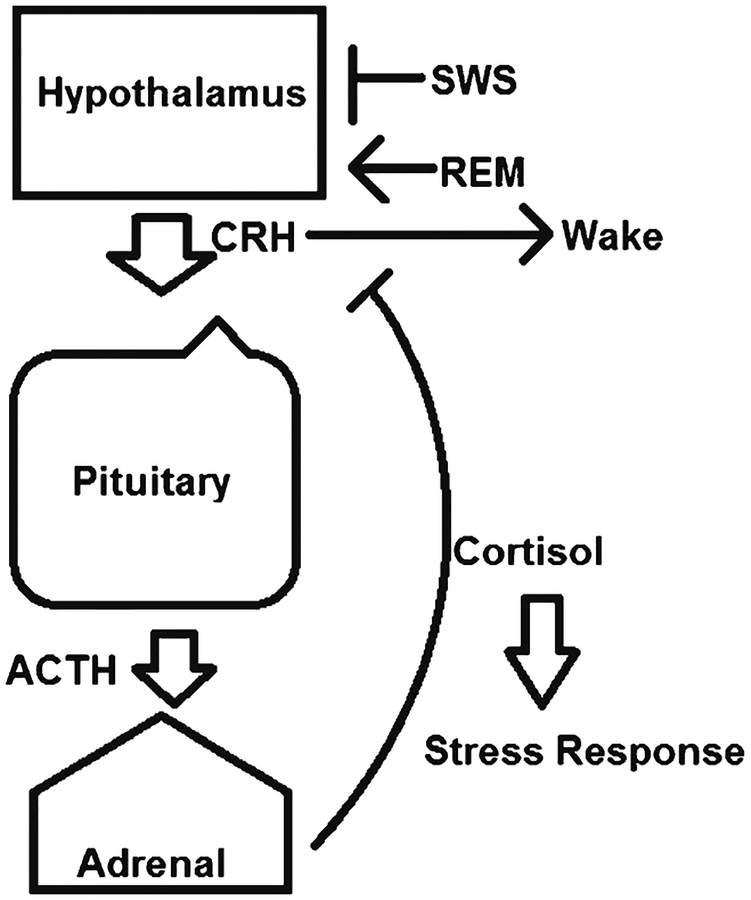
Among the most important hormonal pathways regulated by sleep is the hypothalamic–pituitary–adrenal (HPA) axis (Fig. 2). The SCN sends projections to the pituitary, which result in an oscillating hormone secretion rhythm in line with the diurnal cycle. The SCN also sends direct neuronal projections to the pituitary, further entraining the release of glucocorticoids in a stereotypical diurnal rhythm. In humans, cortisol levels peak in the early morning just after the onset of the wake phase (Allada et al. 2017), a phenomenon thought adapted to prepare the body for wake-time activity. This cortisol rhythm is light-entrained, meaning that significant disruptions have been shown in shift workers and others with circadian rhythm disruption (Allada et al. 2017; James et al. 2004).
Fig. 2.
Schematic of sleep impacts on the HPA axis. Slow-wave sleep is an inhibitor of the HPA axis stress pathway, while REM sleep stimulates cortisol production. Corticotropin releasing hormone (CRH) has been shown to stimulate wake, while exogenous glucocorticoids have been shown to counteract this effect and stimulate sleep by feedback inhibition
The HPA axis and cortisol play a major role in regulation of the stress response, and sleep has long been shown to have a potent inhibitory influence on this pathway. Sympathetic nervous activity and its downstream effects, including cardiovascular function, display a dependence on sleep state (Miki and Yoshimoto 2013). Slow-wave sleep in particular is an inhibitor of HPA axis activity; consequently, cortisol is elevated in the later portions of a sleep bout and during REM sleep (Born and Wagner 2004). Furthermore, sleep displays a modulation of the adrenal response of cortisol production to the action of adrenocorticotropic hormone; adrenal ACTH sensitivity has also been shown to vary with the diurnal cycle (Späth-Schwalbe et al. 1991). Further downstream, cortisol rhythms have been shown to affect the immune system; as cortisol is a potent immune and inflammatory suppressor, circadian disruption has been shown to increase inflammatory cytokines and inflammatory pathologies, including cancers (Schlaich et al. 2015; Born and Wagner 2004).
Insulin is another hormone entrained rhythmically. Insulin, a pancreatic hormone, has a principal function of promoting the absorption of glucose from the blood. However, in experiments of clamping glucose concentration, insulin still rises in the late phase of the sleep cycle (Copinschi et al. 2014), most likely to restrain hepatic glucose production in order to prevent a glycemic peak. This rhythm appears to be SCN-based by studies of dysregulated feeding in SCN-lesioned rats (Marchant and Mistlberger 1997); however, there is also evidence that the pancreatic beta cells responsible for insulin secretion have their own set of clock transcription factors that may operate independently (Perelis et al. 2016). Insulin response is also circadian-modulated, with insulin sensitivity in adipose tissue being significantly higher in daytime hours (Carrasco-Benso et al. 2016). Sleep deprivation has been shown to be sufficient to induce insulin resistance by multiple pathways (Donga et al. 2010).
Similarly, another key hormone for metabolism, growth hormone (GH), which promotes lipolysis and muscle growth, displays cycles entrained to sleep activity (Steyn and Ngo 2017). GH is elevated in the earlier portions of a sleep bout, particularly in slow-wave sleep, and decreases in later sleep phases (van Cauter and Plat 1996). These hormonal changes, as well as similar sleep-dependent fluctuation in the appetite stimulating and repressing hormones ghrelin (Rasmussen et al. 2008) and leptin (Challet 2015), may in part explain the correlation between sleep disruption and obesity and metabolic syndrome.
Circadian factors have also been shown to have an effect on reproductive hormones. However, studies of the circadian cycle and reproductive-related hormones have been complicated by the fact that the connection appears much stronger in rodents than in humans (Mong and Cusmano 2016). For example, in rodents, the surge of luteinizing hormone occurs just before the onset of the dark phase (and thus mating activity), a surge that is mediated by the SCN (Ramírez et al. 2017). Thus, circadian rhythms are very stereotypical in rodent mating behaviors; however, humans do not display any equivalent circadian rhythm in mating activity. Despite that phenomenon, there is clinical evidence to suggest that sleep–wake and circadian disruption may play a role in certain human reproductive disorders. For example, shiftwork in pregnant women may be associated with a greater risk of preterm birth (Nurminen 1998), though other analyses dispute this conclusion (van Melick et al. 2014).
Sleep has also been shown to have an effect on sex hormones, particularly testosterone. Testosterone secretion is linked to sleep cycles, with peak levels occurring in the middle of the sleep cycle, often near the time of REM sleep onset (Wittert 2014). Insufficient or fragmented sleep, which reduces the amount of REM, blocks the nocturnal increase in testosterone. Therefore, sleep disruption could be a risk factor for low testosterone levels (Wittert 2014).
7. Non-gonadal Endocrine Factors Impact Sleep
A multitude of different hormones have been shown to impact the sleep state, as well as the quantity and quality of sleep achieved. The key circadian mediator melatonin is one of the best-known and most directly sleep-impacting hormones; it is classically low during the daytime and increases after the onset of darkness, stimulating sleep in humans (Saper and Fuller 2017). Melatonin administration, which is available as an over-the-counter pharmaceutical, has been shown to increase total sleep time and sleep maintenance (White 2017). Though melatonin may be sedative in some species such as zebrafish (Zhdanova et al. 2001), it appears to not have direct sedative effects in humans (Azeez et al. 2018). Instead, melatonin in humans appears to be a link between environmental cues, most notably light cues, and the circadian synchronization of biological processes (Gandhi et al. 2015). This effect can be recapitulated pharmacologically, as exogenous melatonin has also been shown to replicate EEG changes from the circadian pacemaker (Dijk and Cajochen 1997).
Beyond melatonin, the HPA axis has been shown to have an impact on sleep. Administration of corticotropin releasing hormone (CRH), the first hormone in the HPA axis, increases wake time (Held et al. 2005), and conversely, administration of a specific CRH receptor blockade decreases wake time (Chang and Opp 1999). Similarly, insomnia has been associated with an all-day increase in HPA axis activity and cortisol secretion (D’Aurea et al. 2015). However, paradoxically, exogenous glucocorticoids have been shown to be stimulators of slow-wave sleep and inhibitors of REM sleep, particularly in the context of the very high cortisol levels of late pregnancy (Santiago et al. 2001). It has been hypothesized that this effect is due to feedback inhibition by cortisol of CRH release (Steiger 2002).
8. Biological Sex Differences and Ovarian Steroids Impact Sleep Behavior
As sleep is highly evolutionarily conserved, the suggestion of biological sex differences may be counterintuitive, but reproductive and sex hormones have also been shown to interact with the sleep–wake system. Women and men have long been clinically shown to have differing sleep patterns (Mong and Cusmano 2016). In particular, women paradoxically sleep longer than men, but generally self-report a lower sleep quality (Mong and Cusmano 2016). Objective data suggest that women should have higher sleep quality than men; women have longer total sleep time and less total wake time, a shorter latency to sleep onset, and higher sleep efficiency than men (Bixler et al. 2009; Goel et al. 2005). EEG studies have also shown a higher proportion of deep slow-wave sleep (stage 3) and less light sleep (stage 1 and 2) in women than men (Redline et al. 2004). However, clinical evidence is not in agreement with those findings, showing that women have been consistently diagnosed with insomnia and other sleep disorders, including RLS, at a markedly higher rate than men (Mong and Cusmano 2016; Berger et al. 2004). It is unclear if male sex steroids, mainly testosterone, affect sleep in men, as paradoxically both low testosterone levels and testosterone replacement have been shown to be risk factors for sleep deprivation (Wittert 2014). In animal studies, castration does not significantly change sleep time in male rodents, suggesting little impact of testosterone levels on male sleep (Cusmano et al. 2014). However, there is a larger complement of scientific literature suggesting an impact of female hormones on sleep behavior.
Overall sex differences in sleep patterns may be due to sleep-independent factors, including psychosocial ones, which may be tied to a higher presence of anxiety in females (Mong and Cusmano 2016); inversely, sleep loss may also be more potently anxiogenic in females as well (Goldstein-Piekarski et al. 2018). However, there is strong evidence that hormonal complement plays a role; most strikingly, the sex difference in sleep quality emerges in females with puberty (Johnson et al. 2006) and disappears at menopause. There are specific distinctions in the circadian timing of sleep with biological sex and female hormonal state as well. The endogenous circadian rhythm may have an interaction with biological sex, though the mechanism has not been fully explored (Eastman et al. 2017). However, there is clinical evidence for a sex difference in circadian timing. Premenopausal women go to bed earlier than men and have much earlier melatonin peaks (Cain et al. 2010) until menopause, when the sex difference in sleep onset disappears but melatonin peaks become even earlier (Mong and Cusmano 2016). The mechanisms of these circadian differences are incompletely understood.
There is little evidence to suggest major primary changes in sleep patterns in women between the different phases of the menstrual cycle (Baker and Lee 2018). However, there are some minor cyclic differences in sleep attributable to differing hormone levels; for example, a specific type of non-REM sleep waveform, sleep spindles, is elevated in the post-ovulatory luteal phase of the menstrual cycle (Baker and Lee 2018). High progesterone and estrogen levels also correlate with a lower amount of REM sleep (Lancel et al. 1996). Clinically, the luteal phase has also been shown to be a time of particularly pronounced sleep disruption during menopause (De Zambotti et al. 2015). Finally, another sexually differentiated hormone, prolactin, has also been shown to increase slow-wave sleep based on limited studies of patients with hyperprolactinemia (Frieboes et al. 1998). The broader significance of these changes in humans has not fully been established.
Sleep disorders have also been shown to be particularly prevalent in women at times of hormonal flux, including puberty, pregnancy, and menopause. Exogenous hormones, most notably oral contraception, have been shown to affect sleep in young women; oral contraceptive use increases REM sleep and light sleep, while reducing deep slow-wave sleep (Baker et al. 2001; Burdick et al. 2002); the mechanism for this change is unclear. Pregnancy has been shown to be a time of high levels of sleep change and disruption, though it is difficult to differentiate direct effects of hormonal change from physiological changes due to growth and development of the fetus. While there appear to be few changes in melatonin levels or circadian rhythms during pregnancy (Santiago et al. 2001), changes in the levels of estrogen, progesterone, cortisol, and oxytocin may contribute to disruption in sleep, particularly the consistent finding of lower REM sleep times in the third trimester (Santiago et al. 2001). Many women report sleep disruption in the perimenopausal period. Studies on using hormone replacement therapies to combat sleep disruption in menopause have shown improvement in self-reporting of subjective sleep quality. There is evidence that hormones play a role in consolidating sleep at night (Mong and Cusmano 2016), possibly leading to increased sleep quality with their replacement. However, more objective metrics have shown differing effects, with inconsistent findings in objective sleep quality measures with hormonal therapy (Cintron et al. 2017). These situations may be due to differing hormonal formulations between studies; alternatively, hormone therapy may exert its main impact in relieving the non-sleep symptoms of menopause, particularly vasomotor symptoms (Cintron et al. 2017), making women more comfortable and sleep easier to obtain.
Apart from the clinical finding that sex steroids may affect sleep behavior and architecture, the mechanisms underlying how sex steroids influence the sleep circuitry remain a significant gap in our knowledge. The use of animal models is critical for advancing our understanding of the potential endocrine–sleep nexus.
9. Diverse Models Exist for the Investigation of Endocrine Regulation of Sleep Behavior
As with many investigations of neurobiological behavior phenomena, animal models are a key tool in the sleep field. Rats are the most commonly used model of sleep behavior, with mice as a secondary rodent organism. Rats are widespread models for sleep studies as the circuitry and neurochemistry of sleep share similarities with humans, and pharmacologic manipulation and EEG measurement of sleep states are both feasible. Use of mice in the sleep field is generally limited to exploration of sleep in the context of specific genetic backgrounds (Mong and Cusmano 2016), which are more readily available in mice than rats. A key consideration in the use of models for sleep is the different patterns of sleep between animals. Sleep is generally entrained to the light–dark cycle through the circadian sleep system, but the specific pattern of sleep differs dramatically between species. Under normal conditions, humans are monophasic sleepers, with a single consolidated period of 7–8 h per day, concentrated in the dark portion of the light/dark cycle. Conversely, however, rodents are polyphasic sleepers, with many periods of sleep and wake throughout the day (Acerbi et al. 2008). While rodents do have some periods of consolidated wake or sleep of an hour or more, they can also experience bouts as short as a few seconds in duration (Simasko and Mukherjee 2009). Additionally, while rodents do preferentially sleep during the light period of the light–dark cycle, they exhibit significant periods of both wake and sleep throughout the entire light–dark cycle. Unlike rodents, the other major animal model system for sleep, drosophila, are largely monophasic sleepers, with a single consolidated period of sleep entrained to the dark portion of the cycle (Dubowy and Sehgal 2017). As a result, drosophila may be a more useful model of some aspects of the circadian system where light–dark dependence and sleep consolidation are key points of experimental investigation.
Historically, the majority of sleep studies have been performed in men or male animals (Mong and Cusmano 2016), a deeply unfortunate occurrence that has led the impact female animal models can have on illuminating ties between ovarian steroids and sleep. The paucity of basic studies investigating sex differences in sleep has resulted in an unclear picture on the nature of those differences. Gonadally intact female rodents generally spend less time in sleep states compared with males (Paul et al. 2006), but females, despite accumulating less total sleep, have more consolidated sleep bouts, consisting of longer bout durations with less state transitions and fewer arousals (Paul et al. 2006). Moreover, delta power, a quantitative measure of sleep intensity, is higher in females during baseline sleep as well as in recovery sleep following deprivation, a finding in agreement with human clinical data (Paul et al. 2006).
Perhaps more striking is that in the absence of circulating sex steroids, these sex differences in sleep behavior and architecture are eliminated, suggesting that sex differences in sleep are in part dependent on sex steroids. Sleep patterns in female rats are exquisitely sensitive to the natural fluctuations of ovarian steroids (Paul et al. 2006; Koehl et al. 2006). Multiple studies in rats show that during proestrus, when estrogen and progesterone are elevated, sleep time is significantly reduced compared with other phases of the estrous cycle (Schwartz and Mong 2013; Schwierin et al. 1998). Exogenous hormone replacement is observed to recapitulate this phenotype (Cusmano et al. 2014) in both rats and mice. In these studies, estrogen predominately suppresses dark phase sleep and has little or no effect in the light phase. Thus, a key paradigm for studies of hormonal modulation of sleep has been the use of hormone replacement in oophorectomized rodents (Mong and Cusmano 2016) which can provide hormonal stability that bypasses the rapid hormonal changes inherent to the 4-day menstrual cycle in rats.
10. Animal Studies Illuminate Mechanisms Connecting Female Gonadal Hormones and Sleep
In rodents, estrogens have been broadly shown to increase wake and decrease spontaneous sleep, particularly in the active phase of the light–dark cycle; exogenous replacement of estrogen in females decreases dark phase sleep by 55% (Mong and Cusmano 2016). Furthermore, estrogens have been shown to consolidate wake and fragment sleep. However, estrogen-treated rats also have more consolidated slow-wave sleep following sleep deprivation by gentle handling (Schwartz and Mong 2013), and thus estrogen may be acting to facilitate recovery from sleep deprivation. Estradiol, the most potent estrogen, may interact with the circadian system, as it is shown to have a time-of-day-dependent effect; estradiol has been shown to decrease sleep in the active phase and increase it following deprivation in the stereotypical sleep phase (Mong and Cusmano 2016; Cusmano et al. 2014). Thus, hormonal impact may be to improve circadian fealty. Supporting that contention, aromatase knockout mice, which are deficient in the formation of estrogen from testosterone, have similar duration of sleep but sleep that is more fragmented and less entrained to the stereotypical (light) phase of the light–dark cycle (Vyazovskiy et al. 2006).
The molecular mechanisms of hormone impact on sleep are poorly understood, and studies investigating where and how female steroids act on the brain are only an emerging area of investigation. Sexual differentiation of the rodent brain occurs during a brief window of early development. Exposure to sex steroids around the day of birth results in the masculinization and defeminization of the rodent brain, while absence of sex steroids leads to a feminization process (Nugent et al. 2015; Sato et al. 2004). Production of sex steroids in adults cements appropriate behaviors specific to the sex of the animals. Studies in rats have suggested that estradiol effects on sleep are established by the first phase of this process, the early programming effects of sex steroids (Cusmano et al. 2014). Female rats exposed to a masculinizing dose of testosterone during the sensitive window for brain sexual differentiation exhibit male-like responsivity to estradiol and testosterone in adulthood and exhibited male signatures in the sleep-active VLPO nucleus (Saper et al. 2010).
Steroid receptors, particularly for estrogen and progesterone, are present throughout the brain and prevalent on multiple sleep-regulating nuclei such as the hypothalamus (Rønnekleiv and Kelly 2005) and basal forebrain (Donahue et al. 2000). Previous work in rodents implicates the VLPO in particular as a key site of mediating estradiol actions over sleep. In adult oophorectomized females, estradiol decreases activation of sleep-active VLPO neurons (Hadjimarkou et al. 2008) and downregulates levels of lipocalin-type prostaglandin D synthase (L-PGDS), the enzyme responsible for the production of prostaglandin D2 that potently promotes sleep (Devidze et al. 2010; Mong et al. 2003). Estrogens also decrease expression of wake-inhibiting adenosine 2A receptors (Ribeiro et al. 2009), suggesting a potential alternate mechanism for an inhibitory impact on homeostatic sleep pressure. However, these findings are complicated by studies showing that the VLPO is not a major site of estrogen sensitivity (Bailey and Silver 2014). Instead, it is an upstream nucleus, the median preoptic (MnPN), which may be most responsible for mediating estradiol action over homeostatic sleep pressure. Blocking estradiol action directly in the median preoptic nucleus of female rats attenuates estradiol suppression of sleep (Hadjimarkou et al. 2008). Downstream, the orexinergic wake-promoting system of the lateral hypothalamus receives inputs from the MnPN and VLPO and is highly sensitive to fluctuations in endogenous and exogenous ovarian steroids (Mong and Cusmano 2016), suggesting that this section of the homeostatic sleep/wake circuitry may be a key site for estrogen action.
While estrogen receptors are present in the key circadian nucleus of the SCN (Vida et al. 2008), there is not a great deal of evidence suggesting an important function for estrogen in circadian rhythms. By contrast, androgens appear to be important to the activity of the SCN, increasing the fealty of certain behaviors to the circadian clock by mediating its response to light (Karatsoreos et al. 2011; Model et al. 2015). Finally, it is important to note that certain sex differences may be more impacted by chromosomal complement than hormonal status. Female mice had a higher level of slow-wave activity in their active phase than male mice when both were ovariectomized or gonadectomized, respectively (Ehlen et al. 2013). Additionally, anatomically female mice engineered to have an XY chromosomal compliment in the “four core genotypes” model acquire more sleep during their active phase and have higher NREM delta power than XX females, suggesting processes mediating recovery from sleep loss are partially dependent on sex chromosomes (Arnold 2004).
11. Conclusion
Sleep behavior demonstrates myriad neuroendocrine interactions and has broad implications for human health. While there is much that is unknown about the reasons for sleep, evidence exists that it is important for homeostasis of a diverse array of biological functions, including memory process, brain microenvironment homeostasis, and systemic metabolic function. Disorders of sleep regulation are extremely prevalent and are both a major cause of primary morbidity and an exacerbating factor to many health conditions. Sleep and the endocrine system exhibit a bi-directional interaction, with sleep behavior having a strong influence on endocrine factors and endocrine factors reciprocally influencing sleep behavior. In particular, biological sex and sex hormones have been shown to have a significant impact on sleep function. Unfortunately, until recent years much research on sleep behavior largely disregarded its connections with the endocrine system. Use of both clinical studies and rodent models to investigate interactions between neuroendocrine function, including biological sex, and sleep therefore presents a promising area of further exploration.
References
- Aalto J, Kiianmaa K (1984) Increased voluntary alcohol drinking concurrent with REM-sleep deprivation. Alcohol 1(1):77–79. [DOI] [PubMed] [Google Scholar]
- Abbott SM, Reid KJ, Zee PC (2015) Circadian rhythm sleep-wake disorders. Psychiatr Clin North Am 38(4):805–823. 10.1016/j.psc.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Acerbi A, McNamara P, Nunn CL (2008) To sleep or not to sleep: the ecology of sleep in artificial organisms. BMC Ecol 8:10 10.1186/1472-6785-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann S, Rasch B (2014) Differential effects of non-REM and REM sleep on memory consolidation? Curr Neurol Neurosci Rep 14(2):430 10.1007/s11910-013-0430-8. [DOI] [PubMed] [Google Scholar]
- Alam MA, Kumar S, McGinty D, Alam MN, Szymusiak R (2014) Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J Neurophysiol 111(2):287–299. 10.1152/jn.00504.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, Cirelli C, Sehgal A (2017) Molecular mechanisms of sleep homeostasis in flies and mammals. Cold Spring Harb Perspect Biol 9(8):a027730 10.1101/cshperspect.a027730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff MJ, Boller F, Swaab DF (2011) Handb Clin Neurol 98:vii 10.1016/B978-0-444-52006-7.00047-2. [DOI] [PubMed] [Google Scholar]
- Arnold AP (2004) Sex chromosomes and brain gender. Nat Rev Neurosci 5(9):701–708. 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Aryal RP, Kwak PB, Tamayo AG, Gebert M, Chiu PL, Walz T et al. (2017) Macromolecular assemblies of the mammalian circadian clock. Mol Cell 67(5):770–782.e6. 10.1016/j.molcel.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL (2017) Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev 34:10–22. 10.1016/j.smrv.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Azeez IA, Del Gallo F, Cristino L, Bentivoglio M (2018) Daily fluctuation of orexin neuron activity and wiring: the challenge of “chronoconnectivity”. Front Pharmacol 9:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M, Silver R (2014) Sex differences in circadian timing systems: implications for disease.Front Neuroendocrinol 35(1):111–139. 10.1016/j.yfrne.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Lee KA (2018) Menstrual cycle effects on sleep. Sleep Med Clin 13(3):283–294. 10.1016/j.jsmc.2018.04.002. [DOI] [PubMed] [Google Scholar]
- Baker FC, Mitchell D, Driver HS (2001) Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch 442(5):729–737. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Herring KL, Walton JC, Fonken LK, Weil ZM, Nelson RJ (2013) Evidence for feedback control of pineal melatonin secretion. Neurosci Lett 542:123–125. 10.1016/j.neulet.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Berger K, Luedemann J, Trenkwalder C, John U, Kessler C (2004) Sex and the risk of restless legs syndrome in the general population. Arch Intern Med 164(2):196–202. 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Papaliaga MN, Vgontzas AN, Lin HM, Pejovic S, Karataraki M et al. (2009) Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res 18(2):221–228. 10.1111/j.1365-2869.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ et al. (2006) Adenosine and sleep homeostasis in the basal forebrain. J Neurosci 26 (31):8092–8100. 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Wagner U (2004) Memory consolidation during sleep: role of cortisol feedback. Ann N Y Acad Sci 1032:198–201. 10.1196/annals.1314.020. [DOI] [PubMed] [Google Scholar]
- Bratton DJ, Gaisl T, Wons AM, Kohler M (2015) CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA 314(21):2280–2293. 10.1001/jama.2015.16303. [DOI] [PubMed] [Google Scholar]
- Burdick RS, Hoffmann R, Armitage R (2002) Short note: oral contraceptives and sleep in depressed and healthy women. Sleep 25(3):347–349. [PubMed] [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N et al. (2010) Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythm 25(4):288–296. 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco-Benso MP, Rivero-Gutierrez B, Lopez-Minguez J, Anzola A, Diez-Noguera A, Madrid JA et al. (2016) Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity. FASEB J 30(9):3117–3123. 10.1096/fj.201600269RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellini N (2017) Memory consolidation in sleep disorders. Sleep Med Rev 35:101–112. 10.1016/j.smrv.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Challet E(2015) Keeping circadian time with hormones. Diabetes Obes Metab 17:176–183. 10.1111/dom.12516. [DOI] [PubMed] [Google Scholar]
- Chang FC, Opp MR (1999) Pituitary CRH receptor blockade reduces waking in the rat. Physiol Behav 67(5):691–696. [DOI] [PubMed] [Google Scholar]
- Cintron D, Lipford M, Larrea-Mantilla L, Spencer-Bonilla G, Lloyd R, Gionfriddo MR et al. (2017) Efficacy of menopausal hormone therapy on sleep quality: systematic review and meta-analysis. Endocrine 55(3):702–711. 10.1007/s12020-016-1072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copinschi G, Leproult R, Spiegel K (2014) The important role of sleep in metabolism. Front Horm Res 42:59–72. 10.1159/000358858. [DOI] [PubMed] [Google Scholar]
- Cusmano DM, Hadjimarkou MM, Mong JA (2014) Gonadal steroid modulation of sleep and wakefulness in male and female rats is sexually differentiated and neonatally organized by steroid exposure. Endocrinology 155(1):204–214. 10.1210/en.2013-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aurea C, Poyares D, Piovezan RD, Passos G, Tufik S, Mello MT (2015) Objective short sleep duration is associated with the activity of the hypothalamic-pituitary-adrenal axis in insomnia. Arq Neuropsiquiatr 73(6):516–519. 10.1590/0004-282X20150053. [DOI] [PubMed] [Google Scholar]
- Danielsson K, Markström A, Broman JE, von Knorring L, Jansson-Fröjmark M (2016) Delayed sleep phase disorder in a Swedish cohort of adolescents and young adults: prevalence and associated factors. Chronobiol Int 33(10):1331–1339. 10.1080/07420528.2016.1217002. [DOI] [PubMed] [Google Scholar]
- De Zambotti M, Willoughby AR, Sassoon SA, Colrain IM, Baker FC (2015) Menstrual cycle-related variation in physiological sleep in women in the early menopausal transition. J Clin Endocrinol Metab 100(8):2918–2926. 10.1210/jc.2015-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, Semba K (2003) Indirect projections from the suprachiasmatic nucleus to the median preoptic nucleus in rat. Brain Res 987(1):100–106. [DOI] [PubMed] [Google Scholar]
- Devidze N, Fujimori K, Urade Y, Pfaff DW, Mong JA (2010) Estradiol regulation of lipocalin-type prostaglandin D synthase promoter activity: evidence for direct and indirect mechanisms. Neurosci Lett 474(1):17–21. 10.1016/j.neulet.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Cajochen C (1997) Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J Biol Rhythm 12(6):627–635. 10.1177/074873049701200618. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Stopa EG, Chorsky RL, King JC, Schipper HM, Tobet SA et al. (2000) Cells containing immunoreactive estrogen receptor-alpha in the human basal forebrain. Brain Res 856(1–2):142–151. [DOI] [PubMed] [Google Scholar]
- Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW et al. (2010) A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab 95(6):2963–2968. 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- Donlea JM (2017) Neuronal and molecular mechanisms of sleep homeostasis. Curr Opin Insect Sci 24:51–57. 10.1016/j.cois.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Dubowy C, Sehgal A (2017) Circadian rhythms and sleep in Drosophila melanogaster. Genetics 205(4):1373–1397. 10.1534/genetics.115.185157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Tomaka VA, Crowley SJ (2017) Sex and ancestry determine the free-running circadian period. J Sleep Res 26(5):547–550. 10.1111/jsr.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eban-Rothschild A, Giardino WJ, de Lecea L (2017) To sleep or not to sleep: neuronal and ecological insights. Curr Opin Neurobiol 44:132–138. 10.1016/j.conb.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlen JC, Hesse S, Pinckney L, Paul KN (2013) Sex chromosomes regulate nighttime sleep propensity during recovery from sleep loss in mice. PLoS One 8(5):e62205 10.1371/journal.pone.0062205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser R, Nordin BE (1955) The basal metabolic rate during sleep. Lancet 268(6863):532–533. [DOI] [PubMed] [Google Scholar]
- Frieboes RM, Murck H, Stalla GK, Antonijevic IA, Steiger A (1998) Enhanced slow wave sleep in patients with prolactinoma. J Clin Endocrinol Metab 83(8):2706–2710. 10.1210/jcem.83.8.5016. [DOI] [PubMed] [Google Scholar]
- Fulcher BD, Phillips AJ, Postnova S, Robinson PA (2014) A physiologically based model of orexinergic stabilization of sleep and wake. PLoS One 9(3):e91982 10.1371/journal.pone.0091982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller PM, Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J (2011) Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 519(5):933–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaldo CE, Earley CJ (2006) Restless legs syndrome: a clinical update. Chest 130(5):1596–1604. 10.1378/chest.130.5.1596. [DOI] [PubMed] [Google Scholar]
- Gandhi AV, Mosser EA, Oikonomou G, Prober DA (2015) Melatonin is required for the circadian regulation of sleep. Neuron 85(6):1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez S, Romero S, Gich I, Clos S, Grasa E, Antonijoan RM et al. (2011) Sex differences in sleep after a single oral morning dose of olanzapine in healthy volunteers. Hum Psychopharmacol 26 (7):498–507. 10.1002/hup.1232. [DOI] [PubMed] [Google Scholar]
- Goel N, Kim H, Lao RP (2005) Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiol Int 22(5):905–915. 10.1080/07420520500263235. [DOI] [PubMed] [Google Scholar]
- Goldstein-Piekarski AN, Greer SM, Saletin JM, Harvey AG, Williams LM, Walker MP (2018) Sex, sleep deprivation, and the anxious brain. J Cogn Neurosci 30(4):565–578. 10.1162/jocn_a_01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek DA, Rosenstein RE (2010) Physiology of circadian entrainment. Physiol Rev 90 (3):1063–1102. 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- Grafe LA, Bhatnagar S (2018) Orexins and stress. Front Neuroendocrinol 51:132–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt DJ, Harmatz JS, Singh NN, Steinberg F, Roth T, Moline ML et al. (2014) Gender differences in pharmacokinetics and pharmacodynamics of zolpidem following sublingual administration. J Clin Pharmacol 54(3):282–290. 10.1002/jcph.220. [DOI] [PubMed] [Google Scholar]
- Greene R, Siegel J (2004) Sleep: a functional enigma. NeuroMolecular Med 5(1):59–68. 10.1385/NMM:5:1:059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA (2008) Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci 27(7):1780–1792. 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- Held K, Antonijevic I, Murck H, Künzel H, Steiger A (2005) Alpha-helical CRH exerts CRH agonistic effects on sleep-endocrine activity in humans. Neuropsychobiology 52(2):62–67. 10.1159/000086606. [DOI] [PubMed] [Google Scholar]
- Hickie IB, Rogers NL (2011) Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet 378(9791):621–631. 10.1016/S0140-6736(11)60095-0. [DOI] [PubMed] [Google Scholar]
- Honda T, Fujiyama T, Miyoshi C, Ikkyu A, Hotta-Hirashima N, Kanno S et al. (2018) A single phosphorylation site of SIK3 regulates daily sleep amounts and sleep need in mice. Proc Natl Acad Sci U S A 115(41):10458–10463. 10.1073/pnas.1810823115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZL, Zhang Z, Qu WM (2014) Roles of adenosine and its receptors in sleep-wake regulation. Int Rev Neurobiol 119:349–371. 10.1016/B978-0-12-801022-8.00014-3. [DOI] [PubMed] [Google Scholar]
- Hutchison IC, Rathore S (2015) The role of REM sleep theta activity in emotional memory. Front Psychol 6:1439 10.3389/fpsyg.2015.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James FO, Walker CD, Boivin DB (2004) Controlled exposure to light and darkness realigns the salivary cortisol rhythm in night shift workers. Chronobiol Int 21(6):961–972. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Roth T, Schultz L, Breslau N (2006) Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics 117(2): e247–e256. 10.1542/peds.2004-2629. [DOI] [PubMed] [Google Scholar]
- Kapás L, Hong L, Cady AB, Opp MR, Postlethwaite AE, Seyer JM et al. (1992) Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-alpha and TNF-alpha fragments. Am J Phys 263(3 Pt 2):R708–R715. 10.1152/ajpregu.1992.263.3.R708. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Butler MP, Lesauter J, Silver R (2011) Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology 152(5):1970–1978. 10.1210/en.2010-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaba M, Sasai-sakuma T, Inoue Y (2018) Clinical significance of social jetlag in patients with excessive daytime sleepiness. Chronobiol Int 35(12):1637–1646 [DOI] [PubMed] [Google Scholar]
- Koehl M, Battle S, Meerlo P (2006) Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep 29(9):1224–1231. [DOI] [PubMed] [Google Scholar]
- Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN et al. (2017) The sleep-deprived human brain. Nat Rev Neurosci 18(7):404–418. 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Rai S, Hsieh KC, McGinty D, Alam MN, Szymusiak R (2013) Adenosine A (2A) receptors regulate the activity of sleep regulatory GABAergic neurons in the preoptic hypothalamus. Am J Physiol Regul Integr Comp Physiol 305(1):R31–R41. 10.1152/ajpregu.00402.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Holsboer F, Rupprecht R (1996) Progesterone induces changes in sleep comparable to those of agonistic GABAA receptor modulators. Am J Phys 271(4 Pt 1):E763–E772. 10.1152/ajpendo.1996.271.4.E763. [DOI] [PubMed] [Google Scholar]
- Lanquart JP, Nardone P, Hubain P, Loas G, Linkowski P (2018) The dichotomy between low frequency and delta waves in human sleep: a reappraisal. J Neurosci Methods 293:234–246. 10.1016/j.jneumeth.2017.09.019. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Chen JF, Huang ZL, Urade Y, Fredholm BB (2017) Adenosine and sleep. Handb Exp Pharmacol 7:238–245. 10.1007/164_2017_36. [DOI] [PubMed] [Google Scholar]
- Lucey BP, Bateman RJ (2014) Amyloid-β diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging 35(Suppl 2):S29–S34. 10.1016/j.neurobiolaging.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Macedo AC, Balouch S, Tabet N (2017) Is sleep disruption a risk factor for Alzheimer’s disease? J Alzheimers Dis 58(4):993–1002. 10.3233/JAD-161287. [DOI] [PubMed] [Google Scholar]
- Marchant EG, Mistlberger RE (1997) Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain Res 765(2):273–282. [DOI] [PubMed] [Google Scholar]
- Matheson E, Hainer BL (2017) Insomnia: pharmacologic therapy. Am Fam Physician 96(1):29–35 [PubMed] [Google Scholar]
- Methippara MM, Kumar S, Alam MN, Szymusiak R, McGinty D (2005) Effects on sleep of microdialysis of adenosine A1 and A2a receptor analogs into the lateral preoptic area of rats. Am J Physiol Regul Integr Comp Physiol 289(6):R1715–R1723. 10.1152/ajpregu.00247.2005. [DOI] [PubMed] [Google Scholar]
- Micic G, Lovato N, Gradisar M, Ferguson SA, Burgess HJ, Lack LC (2016) The etiology of delayed sleep phase disorder. Sleep Med Rev 27:29–38. 10.1016/j.smrv.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Miki K, Yoshimoto M (2013) Sympathetic nerve activity during sleep, exercise, and mental stress. Auton Neurosci 174(1–2):15–20. 10.1016/j.autneu.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Model Z, Butler MP, LeSauter J, Silver R (2015) Suprachiasmatic nucleus as the site of androgen action on circadian rhythms. Horm Behav 73:1–7. 10.1016/j.yhbeh.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Cusmano DM (2016) Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond Ser B Biol Sci 371(1688):20150110 10.1098/rstb.2015.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Devidze N, Frail DE, O’Connor LT, Samuel M, Choleris E et al. (2003) Estradiol differentially regulates lipocalin-type prostaglandin D synthase transcript levels in the rodent brain: evidence from high-density oligonucleotide arrays and in situ hybridization. Proc Natl Acad Sci U S A 100(1):318–323. 10.1073/pnas.262663799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Tsai SC (2015) Sleep and the endocrine system. Crit Care Clin 31(3):403–418. 10.1016/j.ccc.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Morgan PJ, Barrett P, Howell HE, Helliwell R (1994) Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int 24(2):101–146. [DOI] [PubMed] [Google Scholar]
- Moser D, Anderer P, Gruber G, Parapatics S, Loretz E, Boeck M et al. (2009) Sleep classification according to AASM and Rechtschaffen & Kales: effects on sleep scoring parameters. Sleep 32 (2):139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouret J, Coindet J, Debilly G, Chouvet G (1978) Suprachiasmatic nuclei lesions in the rat: alterations in sleep circadian rhythms. Electroencephalogr Clin Neurophysiol 45(3):402–408. [DOI] [PubMed] [Google Scholar]
- Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A et al. (2015) Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci 18(5):690–697. 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen T (1998) Shift work and reproductive health. Scand J Work Environ Health 24(Suppl 3):28–34. [PubMed] [Google Scholar]
- Obal F, Opp M, Cady AB, Johannsen L, Postlethwaite AE, Poppleton HM et al. (1990) Interleukin 1 alpha and an interleukin 1 beta fragment are somnogenic. Am J Phys 259(3 Pt 2):R439–R446. 10.1152/ajpregu.1990.259.3.R439. [DOI] [PubMed] [Google Scholar]
- Ocampo-Garcés A, Ibáñez F, Perdomo G, Torrealba F (2011) Orexin-B-saporin lesions in the lateral hypothalamus enhance photic masking of rapid eye movement sleep in the albino rat. J Sleep Res 20(1 Pt 1):3–11. 10.1111/j.1365-2869.2010.00864.x. [DOI] [PubMed] [Google Scholar]
- Ohayon MM (2002) Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 6(2):97–111. [DOI] [PubMed] [Google Scholar]
- Pase MP, Himali JJ, Grima NA, Beiser AS, Satizabal CL, Aparicio HJ et al. (2017) Sleep architecture and the risk of incident dementia in the community. Neurology 89 (12):1244–1250. 10.1212/WNL.0000000000004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul KN, Dugovic C, Turek FW, Laposky AD (2006) Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep 29(9):1211–1223. [DOI] [PubMed] [Google Scholar]
- Pavlova M (2017) Circadian rhythm sleep-wake disorders. Continuum 23(4):1051–1063. 10.1212/CON.0000000000000499. [DOI] [PubMed] [Google Scholar]
- Perelis M, Ramsey KM, Marcheva B, Bass J (2016) Circadian transcription from beta cell function to diabetes pathophysiology. J Biol Rhythm 31(4):323–336. 10.1177/0748730416656949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter-Derex L, Yammine P, Bastuji H, Croisile B (2015) Sleep and Alzheimer’s disease. Sleep Med Rev 19:29–38. 10.1016/j.smrv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Pietrowsky R, Meyrer R, Kern W, Born J, Fehm HL (1994) Effects of diurnal sleep on secretion of cortisol, luteinizing hormone, and growth hormone in man. J Clin Endocrinol Metab 78 (3):683–687. 10.1210/jcem.78.3.8126142. [DOI] [PubMed] [Google Scholar]
- Plog BA, Nedergaard M (2018) The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 13:379–394. 10.1146/annurev-pathol-051217-111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punjabi NM (2008) The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 5 (2):136–143. 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez DA, Vieyra E, González AI, Morán C, Domínguez R, Morales-Ledesma L (2017) Both the suprachiasmatic nucleus and the superior ovarian nerve contribute to the processes of ovulation and steroid hormone secretion on proestrus. Reprod Sci 24(6):844–855. 10.1177/1933719116670307. [DOI] [PubMed] [Google Scholar]
- Rasmussen MH, Wildschiødtz G, Juul A, Hilsted J (2008) Polysomnographic sleep, growth hormone insulin-like growth factor-I axis, leptin, and weight loss. Obesity (Silver Spring) 16 (7):1516–1521. 10.1038/oby.2008.249. [DOI] [PubMed] [Google Scholar]
- Raven F, Van der Zee EA, Meerlo P, Havekes R (2018) The role of sleep in regulating structural plasticity and synaptic strength: implications for memory and cognitive function. Sleep Med Rev 39:3–11. 10.1016/j.smrv.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A (2004) The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med 164 (4):406–418. 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- Reichert CF, Maire M, Schmidt C, Cajochen C (2016) Sleep-wake regulation and its impact on working memory performance: the role of adenosine. Biology 5(1):E11 10.3390/biology5010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AC, Pfaff DW, Devidze N (2009) Estradiol modulates behavioral arousal and induces changes in gene expression profiles in brain regions involved in the control of vigilance. Eur J Neurosci 29(4):795–801. 10.1111/j.1460-9568.2009.06620.x. [DOI] [PubMed] [Google Scholar]
- Rønnekleiv OK, Kelly MJ (2005) Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol 26(2):65–84. 10.1016/j.yfrne.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Sack RL, Brandes RW, Kendall AR, Lewy AJ (2000) Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med 343(15):1070–1077 [DOI] [PubMed] [Google Scholar]
- Santiago JR, Nolledo MS, Kinzler W, Santiago TV (2001) Sleep and sleep disorders in pregnancy. Ann Intern Med 134(5):396–408 [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM (2017) Wake-sleep circuitry: an overview. Curr Opin Neurobiol 44:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE (2010) Sleep state switching. Neuron 68 (6):1023–1042. 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K et al. (2004) Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A 101(6):1673–1678. 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki Y, Nihonmatsu I, Kawamura H (1984) Transplantation of the neonatal suprachiasmatic nuclei into rats with complete bilateral suprachiasmatic lesions. Neurosci Res 1(1):67–72. [DOI] [PubMed] [Google Scholar]
- Schlaich M, Straznicky N, Lambert E, Lambert G (2015) Metabolic syndrome: a sympathetic disease? Lancet Diabetes Endocrinol 3(2):148–157. 10.1016/S2213-8587(14)70033-6. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Schultes B (2015) The metabolic burden of sleep loss. Lancet Diabetes Endocrinol 3(1):52–62. 10.1016/S2213-8587(14)70012-9. [DOI] [PubMed] [Google Scholar]
- Schwab RJ, Kim C, Bagchi S, Keenan BT, Comyn FL, Wang S et al. (2015) Understanding the anatomic basis for obstructive sleep apnea syndrome in adolescents. Am J Respir Crit Care Med 191(11):1295–1309. 10.1164/rccm.201501-0169OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD, Mong JA (2013) Estradiol modulates recovery of REM sleep in a time-of-day-dependent manner. Am J Physiol Regul Integr Comp Physiol 305(3):R271–R280. 10.1152/ajpregu.00474.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwierin B, Borbély AA, Tobler I (1998) Sleep homeostasis in the female rat during the estrous cycle. Brain Res 811(1–2):96–104. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma K (1998) Circadian release of amino acids in the suprachiasmatic nucleus in vitro. Neuroreport 9(1):137–140. [DOI] [PubMed] [Google Scholar]
- Simasko SM, Mukherjee S (2009) Novel analysis of sleep patterns in rats separates periods of vigilance cycling from long-duration wake events. Behav Brain Res 196(2):228–236. 10.1016/j.bbr.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Späth-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL (1991) Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry 29(6):575–584. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E (1999) Impact of sleep debt on metabolic and endocrine function. Lancet 354(9188):1435–1439. 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Squarcini CF, Pires ML, Lopes C, Benedito-Silva AA, Esteves AM, Cornelissen-Guillaume G et al. (2013) Free-running circadian rhythms of muscle strength, reaction time, and body temperature in totally blind people. Eur J Appl Physiol 113(1):157–165. 10.1007/s00421-012-2415-8. [DOI] [PubMed] [Google Scholar]
- Steiger A (2002) Sleep and the hypothalamo-pituitary-adrenocortical system. Sleep Med Rev 6 (2):125–138. [DOI] [PubMed] [Google Scholar]
- Stenberg D (2007) Neuroanatomy and neurochemistry of sleep. Cell Mol Life Sci 64 (10):1187–1204. 10.1007/s00018-007-6530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn FJ, Ngo ST (2017) Endocrine rhythms of growth hormone release: insights from animal studies. Best Pract Res Clin Endocrinol Metab 31(6):521–533. 10.1016/j.beem.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Sun X, Whitefield S, Rusak B, Semba K (2001) Electrophysiological analysis of suprachiasmatic nucleus projections to the ventrolateral preoptic area in the rat. Eur J Neurosci 14(8):1257–1274. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D (2008) Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci 1129:275–286. 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- Trotti LM, Becker LA (2019) Iron for the treatment of restless legs syndrome. Cochrane Database Syst Rev 1:CD007834 10.1002/14651858.CD007834.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Cauter E, Plat L (1996) Physiology of growth hormone secretion during sleep. J Pediatr 128 (5 Pt 2):S32–S37. [DOI] [PubMed] [Google Scholar]
- Van Melick MJ, Van Beukering MD, Mol BW, Frings-Dresen MH, Hulshof CT (2014) Shift work, long working hours and preterm birth: a systematic review and meta-analysis. Int Arch Occup Environ Health 87(8):835–849. 10.1007/s00420-014-0934-9. [DOI] [PubMed] [Google Scholar]
- Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kalló I (2008) Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol 20(11):1270–1277. 10.1111/j.1365-2826.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- Villafuerte G, Miguel-Puga A, Rodríguez EM, Machado S, Manjarrez E, Arias-Carrión O (2015) Sleep deprivation and oxidative stress in animal models: a systematic review. Oxid Med Cell Longev 2015:234952 10.1155/2015/234952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Kopp C, Wigger E, Jones ME, Simpson ER, Tobler I (2006) Sleep and rest regulation in young and old oestrogen-deficient female mice. J Neuroendocrinol 18(8):567–576. 10.1111/j.1365-2826.2006.01452.x. [DOI] [PubMed] [Google Scholar]
- Wagner DR (1999) Circadian rhythm sleep disorders. Curr Treat Options Neurol 1(4):299–308. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma J, Miyoshi C, Li Y, Sato M, Ogawa Y et al. (2018) Quantitative phosphoproteomic analysis of the molecular substrates of sleep need. Nature 558(7710):435–439. 10.1038/s41586-018-0218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DP (2017) Advanced concepts in the pathophysiology of obstructive sleep apnea. Adv Otorhinolaryngol 80:7–16. 10.1159/000470522. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A (2018) Seasonality in affective disorders. Gen Comp Endocrinol 258:244–249. 10.1016/j.ygcen.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Wittert G (2014) The relationship between sleep disorders and testosterone in men. Asian J Androl 16(2):262–265. 10.4103/1008-682X.122586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C et al. (2015) Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun 47:24–34. 10.1016/j.bbi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M et al. (2013) Sleep drives metabolite clearance from the adult brain. Science 342(6156):373–377. 10.1126/science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik G, Glaum S, Radulovacki M (1987) The dose-response effects of caffeine on sleep in rats. Brain Res 403(1):177–180. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Morales-Villagran A, Maidment NT, Behnke EJ, Ackerson LC, Lopez-Rodriguez F et al. (2006) Extracellular adenosine in the human brain during sleep and sleep deprivation: an in vivo microdialysis study. Sleep 29(4):455–461. [DOI] [PubMed] [Google Scholar]
- Zhang BJ, Huang ZL, Chen JF, Urade Y, Qu WM (2017) Adenosine A2A receptor deficiency attenuates the somnogenic effect of prostaglandin D2 in mice. Acta Pharmacol Sin 38 (4):469–476. 10.1038/aps.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanova IV, Wang SY, Leclair OU, Danilova NP (2001) Melatonin promotes sleep-like state in zebrafish. Brain Res 903(1–2):263–268 [DOI] [PubMed] [Google Scholar]