Abstract
Animal models of stroke contribute to the development of better stroke prevention and treatment through studies investigating the pathophysiology of different stroke sub-types and by testing promising treatments before trials in humans. There are two broad types of animal models: those in which stroke is induced through artificial means, modeling the consequences of a vascular insult but not the vascular pathology itself; and those in which strokes occur spontaneously. Most animal models of stroke are in rodents due to cost, ethical considerations, availability of standardized neurobehavioral assessments, and ease of physiological monitoring. While there are similarities in cerebrovascular anatomy and pathophysiology between rodents and humans, there are also important differences, including brain size, length and structure of perforating arteries, and gray to white matter ratio, which is substantially lower in humans. The wide range of rodent models of stroke includes models of global and focal ischemia, and of intracerebral and sub-arachnoid hemorrhage. The most widely studied model of spontaneous stroke is the spontaneously hypertensive stroke-prone rat, in which the predominant lesions are small subcortical infarcts resulting from a vascular pathology similar to human cerebral small vessel disease. Important limitations of animal models of stroke - they generally model only certain aspects of the disease and do not reflect the heterogeneity in severity, pathology and comorbidities of human stroke - and key methodological issues (especially the need for adequate sample size, randomization, and blinding in treatment trials) must be carefully considered for the successful translation of pathophysiological concepts and therapeutics from bench to bedside.
Keywords: animal models, intracerebral hemorrhage, ischemic stroke, rodent, stroke subtypes, sub-arachnoid hemorrhage
Background
Pathological types and subtypes of stroke
Stroke is the second most common cause of death worldwide (after ischemic heart disease) and is a leading global cause of disability (1). Stroke is a clinical syndrome, with three main pathological types - ischemic (80% in populations of European origin, somewhat less in Chinese and other Asian populations), intracerebral hemorrhage (ICH, 15% in European populations, 30% or more in Chinese and other Asian populations) and sub-arachnoid hemorrhage (SAH, 5% in European populations) (2). There are several subtypes within each of these. Approximately 50% of ischemic strokes are attributed to large-artery atherothrombotic disease, 25% to disease of the small intracranial arteries (resulting in lacunar strokes), 20% to cardiac emboli, and 5% to various rare causes (e.g. extracranial artery dissection) (2). ICH maybe subclassified according to its location (lobar, deep, intraventricular, or combinations of these) or its cause if known (e.g. hypertension, amyloid angiopathy, arteriovenous malformation) (3). About 75% of spontaneous SAH cases are due to a ruptured aneurysm, 20% have no identifiable cause (of which at least half are due to idiopathic or non-aneurysmal perimesencephalic SAH), and the remainder are caused by a variety of rare disorders such as arteriovenous malformations of the brain or spine (4).
Current strategies for treatment and prevention
Of the millions of people worldwide each year who suffer a stroke (5), up to 50% will have died or be dependent on others six-months later (2). The prognosis is worse for hemorrhagic stroke, with a one-month case fatality of around 50% (3,6). Only a few interventions have been shown by large randomized controlled trials to improve outcome after acute stroke: stroke unit care, including early and longer term rehabilitation, is proven to save lives and reduce dependency following ischemic or hemorrhagic stroke; for ischemic stroke, early aspirin is of proven benefit and can be given to most patients, intravenous thrombolysis improves outcome for a small proportion presenting very early after symptom onset, and early hemicraniectomy benefits those very few younger patients who have malignant middle cerebral artery (MCA) territory infarction (7); randomized trials do not yet support routine use of specific interventions for ICH, but surgical evacuation is indicated for a small proportion with infratentorial hemorrhage and declining conscious level (3); and oral nimodipine improves outcome after aneurysmal SAH (4). Even if used appropriately and widely for all stroke patients in the population, these interventions collectively can only reduce the proportion of people dying or becoming disabled as a result of their stroke by <10% (7). Better and more widely applicable interventions for acute stroke are therefore needed if we are to reduce further the burden of poststroke death and disability.
After a stroke or transient ischemic attack (TIA), there is an increased risk of stroke and other serious vascular events, particularly in the early days and weeks. Secondary preventive strategies are composed of lifestyle changes such as the following: smoking cessation, healthy diet, weight loss, and exercise; blood pressure reduction; cholesterol reduction with a statin and antiplatelet treatment for those at risk of recurrent ischemic events; and carotid endarterectomy for a few patients with recently symptomatic carotid stenosis (8). These are effective when used optimally for individuals, but since only recurrent strokes, or strokes preceded by a TIA, are amenable to this type of prevention, the impact in reducing the number of strokes occurring in the population is limited. In addition, our current understanding of how the effects of these interventions differ between different drugs (e.g. different antihypertensive agents or different cholesterol lowering drugs) and between different stroke subtypes (e.g. the effects of blood pressure reduction following large-artery atherothrombotic or small vessel disease lacunar ischemic stroke) is incomplete. An improved understanding of these will lead to better and more targeted secondary prevention.
Primary prevention, i.e. preventing strokes from occurring in the first place, through targeting individuals at high risk or through mass population approaches could be highly effective. Indeed, modeling exercises suggest that around 70% of strokes could be prevented through optimization at population level of blood pressure, cholesterol levels, alcohol intake, cigarette smoking, diet, overweight, and exercise (9). Such exercises are, however, dependent on several assumptions, and in particular tend not to distinguish between the various different pathological types and subtypes of stroke. To predict the likely effects on stroke of existing primary preventive strategies, and to allow the development of better strategies for the future, a better understanding of the underlying causes of the different subtypes is needed.
Potential uses of animal models of stroke
Improved understanding of etiology
Animal models of stroke can contribute to an improved understanding of the underlying etiology of different subtypes of stroke and so to the development of better prevention and treatment strategies. In considering how animal models can contribute to our understanding of stroke, it is important to appreciate that most animal models of disease, including stroke, do not attempt to model the whole disease process but aim to enable detailed study of specific aspects of this in carefully controlled circumstances. For stroke, different animal models have been developed that model aspects of the underlying vascular pathology, the parenchymal pathology that results from a vascular insult, or sometimes both.
Testing new interventions
Animal models are also useful in testing promising therapeutic interventions, both pharmacological and nonpharmacological (e.g. hypothermia or rehabilitation strategies) before trials in humans are carried out. These are usually experiments testing the effects of interventions in induced stroke models, in particular models of ischemic stroke resulting from large-artery occlusion. Such experiments have considerable potential to inform the design of clinical trials in human stroke, but there is a substantial gap between apparent demonstrations of efficacy of treatments for acute stroke in animal models and evidence for efficacy in human stroke (10). While part of this failure of translation from the laboratory to the clinic may be explained by shortcomings in the human clinical trials, other explanations include methodological flaws in the design and conduct of animal studies (e.g. lack of proper allocation concealment, randomization, blinding), lack of generalizability of some animal models of stroke (i.e. they do not sufficiently reflect the disease in humans, e.g. due to use of young animals with no comorbidities, and/or use of unrealistic time-to-treatment windows), and publication bias, whereby neutral or negative animal studies are more likely to remain unpublished than neutral clinical trials, leading to the (false) impression that the first are more often positive than the second (11).
Overview of specific animal models
Most animal models of stroke use rodents, but rabbits, pigs, dogs, and primates have also been used (12). The animal models currently used can be usefully divided into two broad types: models in which stroke is induced in animals through artificial means and animals in which strokes occur spontaneously. An essential feature of the former is that they model the consequences of various types of vascular insult through acute vessel injury but do not model the vascular pathology itself. Our focus in this overview is on models in which the animals develop stroke lesions and/or neurological deficits. The table gives a brief summary of induced and spontaneous rodent models of adult stroke together with some important advantages and disadvantages of each (Table 1). Further details of these and of the few nonrodent models are provided later.
Table 1.
Rodent models of adult stroke
| Etiology | Model | Advantages | Disadvantages |
|---|---|---|---|
| Induced stroke models | |||
| Global ischemia - acute | Four-vessel occlusion (13–15) | Reversible forebrain ischemia may be induced in awake animals. | Two-stage operative procedure. Occurrence of seizures. Variable outcomes in different rat strains. |
| Two-vessel occlusion (16,17) | One-stage operative procedure. Reversible ischemic forebrain injury. | Necessity of systemic hypotension (exsanguination). | |
| Asphyxial cardiac arrest (18,19) | Whole brain ischemia followed by related systemic changes such as hypoxemia, acidosis, systemic inflammation, hypercortisolemia, hyperglycemia. | Intensive postsurgery care (assisted ventilation, supplemental fluids). Body temperature during first days of recovery strongly influences outcome. | |
| Global ischemia - chronic | Bilateral common carotid artery ligation (20) | Produces white matter changes similar to leukoa raiosis seen on CT and MR brain scans in humans. | Approximately 20% case fatality within one-week of procedure. Damage to visual pathway may compromise neurobehavioral assessment. |
| Bilateral common carotid artery stenosis (21) | Produces milder reduction in cerebral blood flow than ligation model earlier, with white matter lesions but sparing of visual pathways and gray matter | Lesions take two-weeks to develop | |
| Focal ischemia | Endovascular MCAO (22–24) | Most frequently used method in rodents. Easy to perform permanent or transient ischemia. | Risk of vessel rupture (SAH). Postsurgical hyperthermia. |
| Surgical MCAO (25–27) | Better control of occlusion site and therefore less variability. | Necessity of craniotomy. | |
| Thromboembolic MCAO (28) | Mimics most common cause of ischemic stroke in humans. Suitable to investigate thrombolytic therapies. | Higher variability in lesion size, location, and occur rence of spontaneous reperfusion. | |
| Photothrombosis (29–31) | Ability to induce infarct in variable cortical location. Less invasive procedure. | Less relevance to human condition. Occurrence of spontaneous reperfusion. | |
| Intracarotid injection of SDS detergent (32) | Selective perforating artery occlusion caused by in situ small vessel injury including endothelial damage and fibrin thrombus. | Unpredictable distribution of infarcts. | |
| Cortical pial artery occlusion (12) | Arterial occlusion with forceps or photochemical irradiation produces small cortical infarcts. | ||
| Subcortical injection of endothelin-1 (12) | Production of subcortical ischemic lesions in dose-dependent manner. | Several microvessels affected simultaneously - cannot accurately target single perforating vessel. | |
| Selective intraluminal thread occlusion of anterior choroidal/hypothalamic artery (12) | Production of precise area of subcortical infarction in defined arterial territory | Difficult to place thread accurately - MCAO occurs inadvertently in significant proportion of animals. | |
| SAH | Endovascular perforation (33,34) | Rupture of intracranial vessels reflects the clinical condition of aneurysmal SAH in humans. | Mortality reaches 37–5-50% within 24 h after SAH induction. Variable severities. |
| Intracisternal blood injection (35,36) | SAH severity may be regulated by blood quantity and number of injections. | Nonphysiological blood distribution. | |
| ICH | Intracerebral injection of bacterial collagenase (37) | Rupture of intracerebral vessels mimics ICH in humans. Allows investigations of delayed hematoma expansion and hemostasis. | Bacterial collagenase may induce inflammatory responses. Variability in hematoma size. |
| Intracerebral blood injection (38,39) | Injection of variable blood compositions. No confounding inflammation. | Matched to hematoma size, neurofunctional deficits resolve more rapidly compared with the collagenase injection model. | |
| Spontaneous stroke models | |||
| Focal ischemia | SHRSP (40) | Similar vascular and parenchymal pathological processes to human small vessel disease and lacunar stroke. | Spontaneous strokes do not occur until 20 weeks although can be hastened by high salt diet. |
| IHR (41) | Animals given saline rapidly develop scattered microscopic areas of cortical infarction. | Vascular pathology not extensively studied. | |
| ICH | R+/A+ mice (42) | Animals given high-salt diet and L-NAME develop spontaneous intracerebral hemorrhage | Strokes take several weeks to develop. Vascular pathology not much studied. |
CT, computed topography; ICH, intracerebral hemorrhage; IHR, inducible hypertensive rat; MCAO, middle cerebral artery occlusion; R+/A+ mice, transgenic mice expressing human rennin and human angiotensinogen; SAH, sub-arachnoid hemorrhage; SHRSP, spontaneously hypertensive stroke prone rat; L-NAME, N(omega)-nitro-L-arginine methyl ester.
Induced stroke models
Various induced models of stroke have been developed in the past decade, mainly in rodents (43–47). These include models of global and focal ischemia, and of both intracerebral and SAH.
Induced cerebral ischemia
Models of global ischemia
These models do not mimic the focal condition of ischemic stroke as defined by the World Health Organization. However, acute global ischemia models may mimic the cerebral damage that occurs after cardiac arrest, while chronic cerebral hypoperfusion models may produce subcortical white matter damage similar to white matter hyperintensities seen on magnetic resonance (MR) brain scans in humans that are thought to be due to underlying cerebral small vessel disease. These models have the potential to be clinically informative since around 50% of all patients that survive sudden cardiac arrest have persistent severe motor and cognitive deficits (48), while white matter hyperintensities in humans are associated with progressive cognitive decline, gait difficulties, and increased risk of stroke and myocardial infarction (49).
Acute global ischemic damage in rodents is commonly induced by permanent occlusion of both vertebral arteries and temporary ligation of the two common carotid arteries (four-vessel occlusion model) (13–15) or by temporary occlusion of the common carotid arteries combined with induced systemic hypotension (two-vessel occlusion model) (16,17,50). Both methods cause extensive bilateral forebrain injury (44). A model of asphyxial cardiac arrest in rats has also been established (18,19). Compared with four- or two-vessel occlusion models, asphyxial cardiac arrest causes temporary complete ischemia of the whole brain, requiring resuscitation thereafter.
Chronic global hypoperfusion models in rodents include ligation of both common carotid arteries (20) and bilateral common carotid artery stenosis using external microcoils (21). Both models have been shown to produce mainly white matter lesions.
Models of MCA occlusion (MCA)
The majority of ischemic stroke in humans occurs in the vascular territory of the MCA (51), and models of MCAO were developed to mimic the consequences of this clinical situation. MCAO in rodents has been reported to induce long-term sensorimotor and cognitive deficits as well as impairments of postural and sensory reflexes (52–55). This has made MCAO models suitable for assessing the effects of physical interventions to promote recovery (56,57).
Endovascular or surgical approaches are commonly used to produce vessel occlusions (22–26) and may be performed as either permanent or transient vessel blockade. Transient MCAO allows the investigation of ischemia related brain injury as well as cerebral consequences of reperfusion. The late onset and inconsistency of spontaneous reperfusion in stroke patients (27) have led to suggestions that prospective acute ischemic stroke therapies should be tested in transient as well as in permanent MCAO models before entering clinical trials in humans (58).
Thromboembolic occlusion of the MCA or its distal branches is commonly induced via injection of autologous blood clots into the internal carotid artery (ICA) (28,59–62). Although producing a higher variability in lesion size and location than the temporary and permanent vessel occlusion models described earlier, this model allows the investigation of thrombolytic agents or combinations of thrombolytic and neuroprotective drugs (63,64). Other models of induced thromboembolic stroke include delivery of photoactivated thrombogenic agents, which deliver a shower of thromboemboli to a relatively restricted arterial territory producing small foci of cortical ischemia (29–31), and intracarotid injection of sodium dodecyl sulfate (SDS) detergent, resulting in endothelial damage with subsequent thromboemboli, and producing multiple small infarcts in hippocampus, cortex, and thalamus (32).
Models of perforating artery territory ischemic stroke
Several methods of inducing ischemic stroke lesions in the territory of perforating arteries have also been described (12). Occlusion of a small cortical pial artery on the surface of the rat brain with either forceps or photochemical irradiation produces small cortical infarcts. Occlusion by crushing or photocoagulation of thalamoperforators or lenticulostriate arteries in primates produces infarcts resembling striatocapsular ischemic lesions (12). Injection of the powerful vasoconstrictor endothelin-1 into the subcortical tissues of the rat brain, probably affecting several microvessels, has also been used to induce subcortical ischemic lesions (12). Finally, selective occlusions of the anterior choroidal or hypothalamic artery using a precisely placed intraluminal thread in rats or craniotomy and electrocoagulation in the miniature pig have been reported to produce stroke-like neurological signs and subcortical striatocapsular or lacunar-type infarcts (12,65).
Induced SAH
Models of SAH produce intracranial bleeds in the subarachnoid space between the arachnoid membrane and piamater, most frequently via endovascular perforation of the ICA with a monofilament suture (33,34), or via injection of blood or blood components into the cisterna magna (35,36). The intracisternal injection model allows close control of subarachnoid blood volumes; the perforation model, however, better imitates the actual clinical condition of SAH in humans. Preclinical rodent studies of induced SAH have demonstrated a close correlation between the volume of sub-arachnoid blood, the extent of subsequent ischemic brain injury, and functional impairments (66). Sensorimotor and cognitive impairments have been shown to develop following both blood injection and perforation models of SAH, but only cognitive deficits persisted over a time span of five weeks post-surgery (66–68). This is probably due to the diffuse cortical and subcortical brain injury with lack of direct injury to the descending motor tracts. Analogously, lasting cognitive and psychological deficits are the most important problem for patients surviving SAH (69–72).
Induced ICH
Enzymatically induced vessel rupture as well as intraparenchymal injection of blood or blood products are widespread methods used in mice and rats to mimic the consequences of human spontaneous ICH (37–39). To mimic the effects of hemorrhage in a particular targeted brain area (e.g. the basal ganglia), a small craniotomy (burr hole) is needed, followed by a stereotactically guided injection of bacterial collagenase or blood. Blood injection models are often used to study the pathological mechanisms of hemorrhagic brain damage and edema formation. Hemostatic aspects are, however, better investigated with the collagenase injection model since collagenase disrupts the basal lamina of vessels, causing spontaneous bleeding into the surrounding brain tissue (73,74). Patients with supratentorial ICH involving basal ganglia and thalamus are often left with sensorimotor deficits of varying severities, which can be imitated in rodent models of experimental ICH (75,76). The collagenase injection model, in particular, generates long-term neurofunctional deficits and so has been used more extensively than the blood injection model for studying rehabilitation approaches such as forced running and skilled reaching training after ICH (77–79).
Spontaneous stroke models
Spontaneously hypertensive stroke-prone rat (SHRSP)
Probably the most widely studied spontaneous animal model of stroke is the SHRSP. This rat was selectively bred from a substrain of the spontaneously hypertensive rat (SHR), which was itself developed by selective cross breeding of outbred Wistar Kyoto Rats. While the SHR has elevated blood pressure but rarely shows signs of stroke, the SHRSP develops increasing levels of blood pressure from six-weeks of age, malignant hypertension by 12 weeks, and stroke-like symptoms, including circling, drooling, and limb weakness, by 20 weeks. The SHRSP has mainly been used in MCAO models to produce large MCA territory infarcts. The spontaneous strokes suffered by the SHRSP have been less extensively studied. They include cortical infarcts and cerebral hemorrhages, but the predominant lesions are small subcortical infarcts, which result from pathology affecting the small blood vessels that appear very similar to the human small vessel disease underlying lacunar ischemic strokes (12,40,80).
The subcortical infarcts suffered by the SHRSP are generally assumed to be a direct consequence of elevated blood pressure. However, a careful systematic review of the relevant literature and a recent immunohistochemical study suggest a primary causative role for endothelial dysfunction, blood-brain barrier (BBB) leakage and inflammation, with evidence of increased BBB permeability in the SHRSP preceding the development of hypertension and well before the appearance of any stroke lesions (40,81), similar to the endothelial dysfunction and BBB leak demonstrated in human small vessel disease (82,83).
Thus, the SHRSP is not only an animal in which strokes maybe induced, but also has spontaneous strokes, which seem set to be highly informative for understanding the underlying pathological processes of human stroke. This applies particularly to lacunar ischemic stroke, for which the SHRSP is by far the most relevant animal model (12,40,80), and which has low case fatality, making the small vessel pathology underlying this subtype of stroke very difficult to study in humans. Furthermore, important mechanistic insights may also be obtained from detailed study of the spontaneous cortical infarcts and cerebral hemorrhages suffered by this animal.
Other spontaneous stroke animal models
Male inducible hypertensive rats carry the mouse renin gene on the Y chromosome under the control of an inducible promoter. Induction of the transgene in adult life produces overactivation of the renin-angiotensin system and hypertension. When these induced animals were given 0–9% saline in addition to drinking water, they developed stroke-like signs, with unsteadiness of gait by seven-days. Neuropathological examination of animals sacrificed at 14 days revealed areas of microscopic cerebral infarction scattered throughout the cortex, hemorrhages in association with some areas of tissue necrosis, and hemorrhages on the brain surface in some animals (41). Transgenic mice expressing human renin and human angiotensinogen (R+/A+) treated with high-salt diet and N(omega)-nitro-L-arginine methyl ester have been reported to develop progressively increasing blood pressure, neurological signs, death within 10 weeks, and evidence of ICH in the brainstem, cerebellum and basal ganglia (42). Elderly dogs also sustain spontaneous strokes with neurological deficits (hemiparesis, facial hypalgesia, and hemianopia) but have been far less extensively studied (84,85).
Transgenic models
A number of rare forms of stroke occur in humans as a result of single gene disorders (86). These include several conditions that cause specific vasculopathies of the intracranial small arteries and can give rise to lacunar ischemic strokes and leukoaraiosis on brain imaging. The most common of these is cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL), resulting from mutations in the Notch3 gene. Several Notch3 mutant mouse models have been developed, which reproduce the features of human CADASIL vascular pathology accompanied by progressive white matter damage (but no infarcts) in one of the most recently developed models (87,88). These and other transgenic models of various other monogenic forms of stroke not only have the potential to provide insights into the disease mechanisms of these rare forms of stroke, but may also prove useful for testing new treatments for the rare monogenic and - perhaps - for some more common subtypes of stroke. A full description and discussion of transgenic mouse models of stroke is beyond the scope of this paper (and so they are not individually listed in the table) (Table 1), yet this is a rapidly developing and exciting area (86).
Choosing the right model
Clearly, the choice of model must depend on the research question being addressed. Acute stroke presents a highly variable clinical condition. Variation in the affected brain area, the severity and preexisting comorbidities contribute to a wide variation in outcomes in patients (89). Animal models, by contrast, tend to eliminate confounding variables in order to prevent misleading interpretations. Additionally, no animal model entirely imitates the complexity of ischemic or hemorrhagic stroke. These limitations must be considered carefully when translating from model to patient.
For preclinical studies of potential acute stroke treatments, several important issues need to be considered. First, it is important to consider for which types or subtypes of human stroke the treatment under investigation may be relevant and ensure that the appropriate model or range of models is used. Second, since the majority of stroke patients are advanced in age and suffer from a range of comorbidities, such as hypertension and diabetes, it may be appropriate to incorporate such conditions into animal models. Third, experimental lesions must be reproducible and operative procedures should be minimally invasive and of short duration. Fourth, it should be possible to monitor physiological parameters and, where appropriate, maintain them within normal range (90). Fifth, a model that allows assessment of a relevant, functional, neurobehavioral outcome is preferable to the sole measurement of a surrogate such as infarct size. Finally, it is important to use a model for which sufficient numbers of animals for adequate statistical power can be assembled at reasonable cost (11).
For better understanding of pathophysiology, the choice of model will depend on its representativeness of the pathology concerned. Spontaneous stroke models will sometimes be most appropriate for such purposes, in particular because they allow the study of the vascular pathology causing stroke lesions as well as the lesions themselves.
Rodent stroke models are frequently the choice both for preclinical studies and pathophysiological investigations. As described earlier, a wide range of rodent stroke models exists that mimic several different pathological stroke subtypes, and rodents with comorbidities are available as well as young, healthy animals. Rats have substantial similarities to humans in terms of cerebrovascular anatomy and physiology (90), and in addition, their reasonably small size allows continuous monitoring of physiological parameters (91). A number of standardized neurobehavioral assessments have been established for rodents, allowing the integration of relevant, functional outcomes, which greatly improves the potential for translation of preclinical stroke studies (75,92–95). Use of rodents allows extensive and comprehensive evaluations of brain specimens at lower cost compared with larger animals. In addition, the use of rodents is generally considered more acceptable from an ethical perspective than use of larger animals.
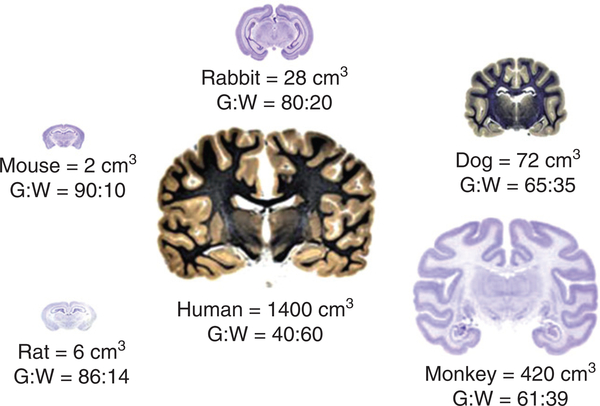
There are limitations to the use of rodent models. Notwithstanding the similarities in cerebrovascular anatomy and pathophysiology, there are also important differences, including differences in brain size, length and structure of perforating arteries, and the ratio of gray to white matter, which is substantially lower in humans than in rodents (Fig. 1) (12,80). This last difference is considerable and must be borne in mind particularly for studies of small vessel disease, which in humans causes lacunar stroke lesions predominantly in the deep white matter.
Fig. 1.
Approximate brain volume (cm3) and gray : white (G : W) matter ratio in healthy (young) animals shown relative to the human brain.
Summary
Animal - and in particular, rodent - models of induced and spontaneous strokes present a plethora of opportunities to investigate pathophysiological mechanisms and responses of stroke and its various pathological types and subtypes. Furthermore, they are used to develop and test acute therapies for ischemic and hemorrhagic brain injury. The addition of neurobehavioral assessments to morphological and mechanistic outcomes has been an important advance in preclinical stroke studies over the past decade (92). However, the limitations of these models must be carefully considered for the successful translation of pathophysiological concepts and therapeutics from bench to bedside (96,97).
Footnotes
Conflict of interest: None declared.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006; 367:1747–57. [DOI] [PubMed] [Google Scholar]
- 2.Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P. Stroke (Lancet seminar series). Lancet 2003; 362:1211–24. [DOI] [PubMed] [Google Scholar]
- 3.Al-Shahi Salman R, Labovitz DL, Stapf C. Spontaneous intracerebral haemorrhage. BMJ 2009; 339:b2586. [DOI] [PubMed] [Google Scholar]
- 4.Al-Shahi R, White PM, Davenport RJ, Lindsay KW. Subarachnoid haemorrhage. BMJ 2006; 333:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003; 2:43–53. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwkamp DJ, Setz LE, Algra A, Linn FHH, de Rooij NK, Rinkel GJE. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 2009; 8:635–42. [DOI] [PubMed] [Google Scholar]
- 7.Sudlow C, Warlow C. Getting the priorities right for stroke care. BMJ 2009; 338:b2083. [DOI] [PubMed] [Google Scholar]
- 8.Sudlow C. Preventing further vascular events after a stroke or transient ischaemic attack: an update on medical management. Pract Neurol 2008; 8:141–57. [DOI] [PubMed] [Google Scholar]
- 9.Ezzati M, Van der Hoorn S, Rodgers A, Lopez AD, Mathers CD, Murray CJL, Comparative Risk Assessment Collaborating Group. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet 2003; 362:271–80. [DOI] [PubMed] [Google Scholar]
- 10.O’Collins VE, Macleod MR, Donnan GA et al. 1,026 experimental treatments in acute stroke. Ann Neurol 2006; 59:467–77. [DOI] [PubMed] [Google Scholar]
- 11.van der Worp HB, Howells DW, Sena ES et al. Can animal models of disease reliably inform human studies? PLoS Med 2010; 7:e1000245. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey EL, McCulloch J, Sudlow CL, Wardlaw JM. Potential animal models of lacunar stroke. A systematic review. Stroke 2009; 40:e451–8. [DOI] [PubMed] [Google Scholar]
- 13.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 1979; 10:267–72. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Kastner R, Paschen W, Ophoff BG, Hossmann KA. A modified four-vessel occlusion model for inducing incomplete forebrain ischemia in rats. Stroke 1989; 20:938–46. [DOI] [PubMed] [Google Scholar]
- 15.Xu ZC, Pulsinelli WA. Responses of CA1 pyramidal neurons in rat hippocampus to transient forebrain ischemia: an in vivo intracellular recording study. Neurosci Lett 1994; 171:187–91. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi M, Calvert JW, Kusaka G, Zhang JH. One-stage anterior approach for four-vessel occlusion in rat. Stroke 2005; 36:2212–14. [DOI] [PubMed] [Google Scholar]
- 17.Smith ML, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjo BK. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand 1984; 69:385–401. [DOI] [PubMed] [Google Scholar]
- 18.Hendrickx HH, Rao GR, Safar P, Gisvold SE. Asphyxia, cardiac arrest and resuscitation in rats. I. Short term recovery. Resuscitation 1984; 12:97–116. [DOI] [PubMed] [Google Scholar]
- 19.Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab 1995; 15:1032–9. [DOI] [PubMed] [Google Scholar]
- 20.Wakita H, Tomimoto H, Akiguchi I, Kimura J. Dose-dependent, protective effect of FK506 against white matter changes in the rat brain after chronic cerebral ischemia. Brain Res 1998; 792:105–13. [DOI] [PubMed] [Google Scholar]
- 21.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke 2004; 35:2598–603. [DOI] [PubMed] [Google Scholar]
- 22.Kuge Y, Minematsu K, Yamaguchi T, Miyake Y. Nylon monofilament for intraluminal middle cerebral artery occlusion in rats. Stroke 1995; 26:1655–8. [DOI] [PubMed] [Google Scholar]
- 23.Hata R, Mies G, Wiessner C et al. A reproducible model of middle cerebral artery occlusion in mice: hemodynamic, biochemical, and magnetic resonance imaging. J Cereb Blood Flow Metab 1998; 18:367–75. [DOI] [PubMed] [Google Scholar]
- 24.Tureyen K, Vemuganti R, Sailor KA, Dempsey RJ. Ideal suture diameter is critical for consistent middle cerebral artery occlusion in mice. Neurosurgery 2005; 56:196–200; discussion 196–200. [DOI] [PubMed] [Google Scholar]
- 25.Robinson RG, Shoemaker WJ, Schlumpf M, Valk T, Bloom FE. Effect of experimental cerebral infarction in rat brain on catecholamines and behaviour. Nature 1975; 255:332–4. [DOI] [PubMed] [Google Scholar]
- 26.Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab 1981; 1:53–60. [DOI] [PubMed] [Google Scholar]
- 27.Ringelstein EB, Biniek R, Weiller C, Ammeling B, Nolte PN, Thron A. Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology 1992; 42:289–98. [DOI] [PubMed] [Google Scholar]
- 28.Kilic E, Hermann DM, Hossmann KA. A reproducible model of thromboembolic stroke in mice. Neuroreport 1998; 9:2967–70. [DOI] [PubMed] [Google Scholar]
- 29.Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol 1985; 17:497–504. [DOI] [PubMed] [Google Scholar]
- 30.Futrell N, Watson BD, Dietrich WD, Prado R, Millikan C, Ginsberg MD. A new model of embolic stroke produced by photochemical injury to the carotid artery in the rat. Ann Neurol 1988; 23:251–7. [DOI] [PubMed] [Google Scholar]
- 31.Nagai N, Zhao BQ, Suzuki Y, Ihara H, Urano T, Umemura K. Tissue-type plasminogen activator has paradoxical roles in focal cerebral ischemic injury by thrombotic middle cerebral artery occlusion with mild or severe photochemical damage in mice. J Cereb Blood Flow Metab 2002; 22:648–51. [DOI] [PubMed] [Google Scholar]
- 32.Toshima Y, Satoh S, Ikegaki I, Asano T. A new model of cerebral microthrombosis in rats and the neuroprotective effect of a Rhokinase inhibitor. Stroke 2000; 31:2245–50. [DOI] [PubMed] [Google Scholar]
- 33.Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke 1995; 26:1086–92. [DOI] [PubMed] [Google Scholar]
- 34.Veelken JA, Laing RJ, Jakubowski J. The Sheffield model of subarachnoid hemorrhage in rats. Stroke 1995; 26:1279–84. [DOI] [PubMed] [Google Scholar]
- 35.Solomon RA, Antunes JL, Chen RY, Bland L, Chien S. Decrease in cerebral blood flow in rats after experimental subarachnoid hemorrhage: a new animal model. Stroke 1985; 16:58–64. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H, Kanamaru K, Tsunoda H et al. Heme oxygenase-1 gene induction as an intrinsic regulation against delayed cerebral vasospasm in rats. J Clin Invest 1999; 104:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke 1990; 21:801–7. [DOI] [PubMed] [Google Scholar]
- 38.Bullock R, Mendelow AD, Teasdale GM, Graham DI. Intracranial haemorrhage induced at arterial pressure in the rat. Part 1: description of technique, ICP changes and neuropathological findings. Neurol Res 1984; 6:184–8. [DOI] [PubMed] [Google Scholar]
- 39.Belayev L, Saul I, Curbelo K et al. Experimental intracerebral hemorrhage in the mouse: histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke 2003; 34: 2221–7. [DOI] [PubMed] [Google Scholar]
- 40.Bailey EL, Smith C, Sudlow CLM, Wardlaw JM. Is the spontaneously hypertensive stroke prone rat a pertinent model of subcortical ischaemic stroke? A systematic review. Int J Stroke 2011; 6:434–44. [DOI] [PubMed] [Google Scholar]
- 41.Collidge TA, Lammie GA, Fleming S, Mullins JJ. The role of the renin-angiotensin system in malignant vascular injury affecting the systemic and cerebral circulations. Prog Biophys Mol Biol 2004; 84:301–19. [DOI] [PubMed] [Google Scholar]
- 42.Iida S, Baumbach GL, Lavoie JL, Faraci FM, Sigmund CD, Heistad DD. Spontaneous stroke in a genetic model of hypertension in mice. Stroke 2005; 36:1253–8. [DOI] [PubMed] [Google Scholar]
- 43.James ML, Warner DS, Laskowitz DT. Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care 2008; 9:139–52. [DOI] [PubMed] [Google Scholar]
- 44.Green AR, Cross AJ. Techniques for examining neuroprotective drugs in vivo. Int Rev Neurobiol 1997; 40:47–68. [DOI] [PubMed] [Google Scholar]
- 45.Traystman RJ. Animal models of focal and global cerebral ischemia. ILAR J 2003; 44:85–95. [DOI] [PubMed] [Google Scholar]
- 46.Titova E, Ostrowski RP, Zhang JH, Tang J. Experimental models of subarachnoid hemorrhage for studies of cerebral vasospasm. Neurol Res 2009; 31:568–81. [DOI] [PubMed] [Google Scholar]
- 47.Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke 1989; 20:1627–2. [DOI] [PubMed] [Google Scholar]
- 48.Lim C, Alexander MP, LaFleche G, Schnyer DM, Verfaellie M. The neurological and cognitive sequelae of cardiac arrest. Neurology 2004; 63:1774–8. [DOI] [PubMed] [Google Scholar]
- 49.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010; 341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wellons JC 3rd, Sheng H, Laskowitz DT, Burkhard Mackensen G, Pearlstein RD, Warner DS. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res 2000; 868:14–21. [DOI] [PubMed] [Google Scholar]
- 51.del Zoppo GJ, Poeck K, Pessin MS et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992; 32:78–86. [DOI] [PubMed] [Google Scholar]
- 52.Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol 2007; 203:555–67. [DOI] [PubMed] [Google Scholar]
- 53.Freret T, Bouet V, Leconte C et al. Behavioral deficits after distal focal cerebral ischemia in mice: usefulness of adhesive removal test. Behav Neurosci 2009; 123:224–30. [DOI] [PubMed] [Google Scholar]
- 54.Winter B, Bert B, Fink H, Dirnagl U, Endres M. Dysexecutive syndrome after mild cerebral ischemia? Mice learn normally but have deficits in strategy switching. Stroke 2004; 35:191–5. [DOI] [PubMed] [Google Scholar]
- 55.Gerlai R, Thibodeaux H, Palmer JT, van Lookeren Campagne M, Van Bruggen N. Transient focal cerebral ischemia induces sensorimotor deficits in mice. Behav Brain Res 2000; 108:63–71. [DOI] [PubMed] [Google Scholar]
- 56.Lee SU, Kim DY, Park SH, Choi DH, Park HW, Han TR. Mild to moderate early exercise promotes recovery from cerebral ischemia in rats. Can J Neurol Sci 2009; 36:443–9. [DOI] [PubMed] [Google Scholar]
- 57.Hu X, Zheng H, Yan T et al. Physical exercise induces expression of cd31 and facilitates neural function recovery in rats with focal cerebral infarction. Neurol Res 2010; 32:397–402. [DOI] [PubMed] [Google Scholar]
- 58.Stroke TherapyAcademic Industry Roundtable (STAIR). Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999; 30:2752–8. [DOI] [PubMed] [Google Scholar]
- 59.Kudo M, Aoyama A, Ichimori S, Fukunaga N. An animal model of cerebral infarction. Homologous blood clot emboli in rats. Stroke 1982; 13:505–8. [DOI] [PubMed] [Google Scholar]
- 60.Kaneko D, Nakamura N, Ogawa T. Cerebral infarction in rats using homologous blood emboli: development of a new experimental model. Stroke 1985; 16:76–84. [DOI] [PubMed] [Google Scholar]
- 61.Orset C, Macrez R, Young AR et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke 2007; 38:2771–8. [DOI] [PubMed] [Google Scholar]
- 62.Brinker G, Franke C, Hoehn M, Uhlenkuken U, Hossmann KA. Thrombolysis of cerebral clot embolism in rat: effect of treatment delay. Neuroreport 1999; 10:3269–72. [DOI] [PubMed] [Google Scholar]
- 63.Overgaard K. Thrombolytic therapy in experimental embolic stroke. Cerebrovasc Brain Metab Rev 1994; 6:257–86. [PubMed] [Google Scholar]
- 64.Zhang L, Zhang ZG, Zhang C, Zhang RL, Chopp M. Intravenous administration of a GPIIb/IIIa receptor antagonist extends the therapeutic window of intra-arterial tenecteplase-tissue plasminogen activator in a rat stroke model. Stroke 2004; 35:2890–5. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka Y, Imai H, Konno K et al. Experimental model of lacunar infarction in the gyrencephalic brain of the miniature pig: neurological assessment and histological, immunohistochemical, and physiological evaluation of dynamic corticospinal tract deformation. Stroke 2008; 39:205–12. [DOI] [PubMed] [Google Scholar]
- 66.Thal SC, Mebmer K, Schmid-Elsaesser R, Zausinger S. Neurological impairment in rats after subarachnoid hemorrhage - a comparison of functional tests. J Neurol Sci 2008; 268:150–9. [DOI] [PubMed] [Google Scholar]
- 67.Germano AF, Dixon CE, d’Avella D, Hayes RL, Tomasello F. Behavioral deficits following experimental subarachnoid hemorrhage in the rat. J Neurotrauma 1994; 11:345–53. [DOI] [PubMed] [Google Scholar]
- 68.Silasi G, Colbourne F. Long-term assessment of motor and cognitive behaviours in the intraluminal perforation model of subarachnoid hemorrhage in rats. Behav Brain Res 2009; 198:380–7. [DOI] [PubMed] [Google Scholar]
- 69.Bailes JE, Spetzler RF, Hadley MN, Baldwin HZ. Management morbidity and mortality of poor-grade aneurysm patients. J Neurosurg 1990; 72:559–66. [DOI] [PubMed] [Google Scholar]
- 70.Frazer D, Ahuja A, Watkins L, Cipolotti L. Coiling versus clipping for the treatment of aneurysmal subarachnoid hemorrhage: a longitudinal investigation into cognitive outcome. Neurosurgery 2007; 60:434–42. [DOI] [PubMed] [Google Scholar]
- 71.Lanzino G, Kassell NF, Germanson TP et al. Age and outcome after aneurysmal subarachnoid hemorrhage: why do older patients fare worse? J Neurosurg 1996; 85:410–8. [DOI] [PubMed] [Google Scholar]
- 72.Hackett ML, Anderson CS. Health outcomes 1 year after subarachnoid hemorrhage: an international population-based study. Neurology 2000; 55:658–62. [DOI] [PubMed] [Google Scholar]
- 73.MacLellan CL, Silasi G, Poon CC et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab 2008; 28:516–25. [DOI] [PubMed] [Google Scholar]
- 74.XiG Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg 1998; 89:991–6. [DOI] [PubMed] [Google Scholar]
- 75.Hartman R, Lekic T, Rojas H, Tang J, Zhang JH. Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res 2009; 1280:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med 2001; 344:1450–60. [DOI] [PubMed] [Google Scholar]
- 77.MacLellan CL, Silasi G, Auriat AM, Colbourne F. Rodent models of intracerebral hemorrhage. Stroke 2010; 41:S95–8. [DOI] [PubMed] [Google Scholar]
- 78.Auriat AM, Colbourne F. Delayed rehabilitation lessens brain injury and improves recovery after intracerebral hemorrhage in rats. Brain Res 2009; 1251:262–8. [DOI] [PubMed] [Google Scholar]
- 79.Auriat AM, Wowk S, Colbourne F. Rehabilitation after intracerebral hemorrhage in rats improves recovery with enhanced dendritic complexity but no effect on cell proliferation. Behav Brain Res 2010; 214:42–7. [DOI] [PubMed] [Google Scholar]
- 80.Hainsworth AH, Markus HS. Do in vivo experimental models reflect human cerebral small vessel disease? A systematic review. J Cereb Blood FlowMetab 2008; 28:1877–91. [DOI] [PubMed] [Google Scholar]
- 81.Bailey EL, Wardlaw JM, Graham D, Dominiczak AF, Sudlow CLM, Smith C. Cerebral small vessel endothelial structural changes predate hypertension in stroke prone spontaneously hypertensive rats: a blinded, controlled immunohistochemical study of 5–21 weeks old rats. Neuropathol Appl Neurobiol 2011; 37:711–26. [DOI] [PubMed] [Google Scholar]
- 82.Wardlaw J, Doubal F, Armitage P et al. Lacunar stroke is associated with diffuse blood brain barrier dysfunction. Ann Neurol 2009; 65:194–202. [DOI] [PubMed] [Google Scholar]
- 83.Hassan A, Hunt BJ, O’Sullivan M et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 2003; 126:424–32. [DOI] [PubMed] [Google Scholar]
- 84.Garosi L, McConnell JF, Platt SR et al. Clinical and topographic magnetic resonance characteristics of suspected brain infarction in 40 dogs. J Vet Intern Med 2006; 20:311–21. [DOI] [PubMed] [Google Scholar]
- 85.Rossmeisl JH Jr, Rohleder JJ, Pickett JP, Duncan R, Herring IP. Presumed and confirmed striatocapsular brain infarctions in six dogs. Vet Ophthalmol 2007; 10:23–36. [DOI] [PubMed] [Google Scholar]
- 86.Markus HS. Stroke genetics. Hum Mol Genet 2011; 20:R124–31. [DOI] [PubMed] [Google Scholar]
- 87.Ayata C. CADASIL: experimental insights from animal models. Stroke 2010; 41:S129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joutel A, Monet-Lepretre M, Gosele C et al. Cerebrovascular dysfunction and microcirculaton rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest 2010; 120:433–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wiebers DO, Adams HP Jr, Whisnant JP. Animal models of stroke: are they relevant to human disease? Stroke 1990; 21:1–3. [DOI] [PubMed] [Google Scholar]
- 90.Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 2007; 87:179–97. [DOI] [PubMed] [Google Scholar]
- 91.Takizawa S, Hogan M, Hakim AM. The effects of a competitive NMDA receptor antagonist (CGS-19755) on cerebral blood flow and pH in focal ischemia. J Cereb Blood Flow Metab 1991; 11:786–93. [DOI] [PubMed] [Google Scholar]
- 92.Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx 2006; 3:497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DeVries AC, Nelson RJ, Traystman RJ, Hurn PD. Cognitive and behavioral assessment in experimental stroke research: will it prove useful? Neurosci Biobehav Rev 2001; 25:325–42. [DOI] [PubMed] [Google Scholar]
- 94.Jeon H, Ai J, Sabri M et al. Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci 2009; 10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacLellan CL, Auriat AM, McGie SC et al. Gauging recovery after hemorrhagic stroke in rats: implications for cytoprotection studies. J Cereb Blood Flow Metab 2006; 26:1031–42. [DOI] [PubMed] [Google Scholar]
- 96.NINDS. Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke 2005; 36:e23–41. [DOI] [PubMed] [Google Scholar]
- 97.STAIR. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999; 30:2752–8. [DOI] [PubMed] [Google Scholar]