Introduction and Background
Non-muscle invasive bladder cancer (NMIBC) is a challenging disease to treat. These patients are typically treated with transurethral resection (TUR) followed in most cases by intravesical therapy, to reduce the risk of disease recurrence and progression while maintaining a functional bladder.1 Unfortunately, the majority of patients remain at risk for tumor recurrence and nearly a third progress to muscle-invasive disease.2 Additional intravesical options are urgently needed to mitigate the risk of progression to secondary muscle invasive bladder cancer and early cystectomy.
Managing NMIBC with BCG
BCG was initially developed as a vaccine for tuberculosis, and it is a live attenuated drug derived from Mycobacterium bovis. The use of BCG for the treatment of cancer can be traced back to the observations of Pearl in 1929, when patients with a diagnosis of tuberculosis were noted to have lower rates of cancer at the time of their autopsies.3 In 1976, Morales and colleagues published the landmark study describing the first use of BCG to treat bladder cancer intravesically.4 The intravesical introduction of BCG causes an infection of urothelial and tumor cells through a fibronectin-mediated process.5 This, in turn, promotes a local immune response facilitated by granulocytes, macrophages, and T-helper cells. Anti-tumor responses result partly from the antigen-processing functions of these phagocytic cells. Several cytokines, including tumor necrosis factor-α (TNF), interferon (IFN), and interleukin (IL)-1, IL-2, IL-6, IL-8, IL-10, IL-12, and IL-17 participate in the immune stimulation process as demonstrated by post-BCG urine analysis.6,7 More recently, the role of autophagy in generating an epigenetic reprogramming of monocytes (“trained immunity”) has also been implicated in the mechanism of generating an intravesical BCG-stimulated host response.8
The anti-tumor activity of BCG is therefore derived from both the adaptive and innate immune systems of the patient. Intravesical administration stimulates an immune response via cytokine release, including IL-2, TNF, IFN-γ, and IL-12 among others. CD4+ T cells and CD8+ cytotoxic T-cells produce much of the adaptive anti-tumor response, while the innate immune system is driven by natural killer cells, neutrophils, dendritic cells and macrophages.
In clinical trials, intravesical BCG has demonstrated initial complete response rates that vary between 55-65% for papillary tumors and 70-75% for CIS.9,10 Despite the benefits demonstrated in both decreased progression and recurrence rates, over one-third of patients “fail” BCG therapy and an additional 40% of initial responders are found to have relapsed within 5 years of treatment.11
Understanding the genomics of non-muscle invasive bladder cancer
Advances in whole genome sequencing technology have vastly improved our insights into the complexity and heterogeneity of bladder cancer. In particular, parallel efforts to comprehensively characterize the molecular composition of muscle-invasive disease have revealed that MIBC can be broadly grouped into basal and luminal subtypes that are similar to those found in breast cancer, with distinct clinical features.12–14 Efforts to similarly characterize NMIBC have also been undertaken, with mutations identified in the TERT promoter,15 FGFR3,16–18 PIK3CA,19 and STAG220. Hurst and colleagues described two genomic subtypes for stage Ta tumors; one group (named Genomic Subtype 1) was characterized by no detectable copy-number alterations, while the second group (Genomic Subtype 2) was defined by a loss of chromosome 9q.21
Gene therapy definitions
Gene therapy refers to the delivery of nucleic acid into a host’s cell in order to produce a therapeutic effect.22 The FDA definition of gene therapy notes that they include products that “mediate their effects by transcription and/or translation of transferred genetic material and/or by integrating into the host genome…administered as nucleic acids, viruses, or genetically engineered microorganisms.”23 Gene therapy can thus either target tumor cells directly to repair mutated tumor suppressor functions, or it can modulate the host’s immune response to generate an anti-tumor response (so called “immunogene therapy”).24 Early gene therapy studies attempted to replace a defective gene with a normal copy of the affected gene and focused on treatment of genetically inherited diseases such as cystic fibrosis or muscular dystrophy.25
Most research efforts today focus on using gene therapy to treat human malignancies. The most common gene transfer vectors used in clinical trials include adenoviral, retroviral, and naked plasmid vectors, and there are currently over 1,800 approved gene therapy clinical trials worldwide.23
Early gene therapy efforts in bladder cancer
Initial gene therapy efforts focused on ways to restore the normal function of tumor suppressor genes in urothelial cell carcinoma. Specifically, p53 and retinoblastoma (RB), two tumor suppressor genes that are known to be mutated in 40-60% of bladder cancers and which confer a worse prognosis, were targeted using replication-deficient adenoviral vectors by our group at the MD Anderson Cancer Center.26–28 Both p53 and RB are involved in the regulation of normal cell cycle functions as well as programmed cell death. Human epithelial cells, including urothelial cell carcinoma cells, are uniquely susceptible to an adenoviral infection due to the presence of the coxsackie/adenovirus receptor (CAR).29 In pre-clinical studies, in vivo transfer of wild-type human p53 into urothelial cancer cells was shown to inhibit the growth of these cells, putatively through the completion of a previously defective apoptotic pathway.28 Replication-deficient adenoviral vectors work by infecting cells during the cell cycle but cannot replicate in vivo29 Transferred genes can thus persist within the infected cell for about two weeks.30
Intravesical instillation of adenoviral vectors was initially carried out using a reporter gene to verify successful viral-mediated transfection within animal models. Morris and colleagues intravesically delivered an adenoviral vector containing β-galactosidase into the bladders of rats, subsequently assessing the efficiency of gene transfer and the impact of this “gene therapy” on the histology of the affected cells.31 They found that not only was the adenovirus-mediated gene transfer effective, bladder architecture was histologically indistinguishable from normal controls without any evidence of cystitis or systemic spread of the infection (as determined by PCR-based assessments for the gene product within the kidneys, heart, and lung extracts from the intravesically treated rats).31
Kuball et al conducted an open-label, single-center phase I study evaluating both intratumoral injection as well as intravesical instillation of recombinant-adenovirus/p53 (SCH 58500), which encoded the wild-type p53 human cDNA.32 This was conducted in patients with invasive bladder cancer who were scheduled for radical cystectomy, so as to allow for the evaluation of post-cystectomy tissue sampling to understand treatment response. The agent was delivered intravesically through a catheter in two sequential administrations, with a dwell time of 60 minutes for each instillation. Prior to instillation the bladders were primed with a transduction-enhancing agent called Big CHAP.33 Eight patients ultimately received intravesical instillation and underwent cystectomy with tissue available for analysis. Key findings included grade 2 and 3 urethral and vesical burning reported by patients during instillation and dwell-time within the bladder, but no systemic toxicities were observed. Detectable p53 transgene expression was identified in seven of eight bladder tumors that had received an intravesical instillation. Although this study was not designed to establish clinical efficacy, it represented a novel proof-of-concept for the safety and biologic feasibility of intravesical gene therapy in humans with bladder cancer.32
Our group at MD Anderson also conducted a phase I, dose-escalation study using Ad5CMV-p53, administered intravesically to cystectomy-ineligible patients with measurable, locally advanced urothelial cell carcinoma of the bladder.34 In this study, wild-type human p53 cDNA was combined with a cytomegalovirus (CMV) promoter and a replication-defective adenoviral vector. Instillations were performed daily through a Foley catheter at different doses and patients were monitored for adverse events for at least 12 months. Thirteen patients were treated (10 with prior muscle-invasive bladder cancer and three with extensive recurrent superficial disease after BCG).34 None of the patients in this trial experienced dose-limiting toxicity, and the most common complaint was bladder spasms, observed most often in the higher dose levels. Two out of seven patients with assessable tissue samples showed vector-specific p53 expression after treatment, and both had received the higher dosing schedule.34 Overall, the low efficiency of gene transfer was in part attributed to the protective glycosoaminoglycan (GAG) layer of the bladder mucosa, as well potentially lower levels of CAR expression within the patients treated. In addition, detecting p53 staining was made more challenging by the fact that most patients in the study had abnormally high p53 staining at baseline, likely due to the advanced disease state of the included study population. Nevertheless, the study provided confidence as to the general safety of intravesical gene therapy and highlighted some important limitations that future trials would need to consider.
Priming the bladder for intravesical gene therapy
The urothelium of the bladder is a complex, multilayer surface that is tasked with providing a barrier from pathogens and urinary waste products.35 The superficial part of surface is also known as the GAG layer and is comprised of a hydrophilic, polyanionic barrier of glycoproteins and proteoglycans. The main components of the GAG layer include chondroitin sulfate and hyaluronic acid, and these in part work to provide protection against the unwanted internalization of ions, solutes, water, and urinary bacteria that may be present in excreted urine.35 Dysfunction of the GAG layer has been implicated in interstitial cystitis and the development of chronic pelvic pain syndrome.35
Successful intravesical gene therapy requires a means to permeate the GAG layer such that the underlying urothelium can be accessed for efficient viral transduction. As noted earlier, the non-ionic detergent Big CHAP was an early transduction-promoting agent used in initial intravesical adenoviral vector clinical studies, described first by Connor and colleagues.33 Further investigation of this agent led to the discovery of “Syn3,” a polyamide compound demonstrated to improve intravesical gene delivery.36 This compound would prove to be essential in overcoming the intravesical gene transduction limitations encountered in previous clinical trials.
Immunogene therapy using interferon
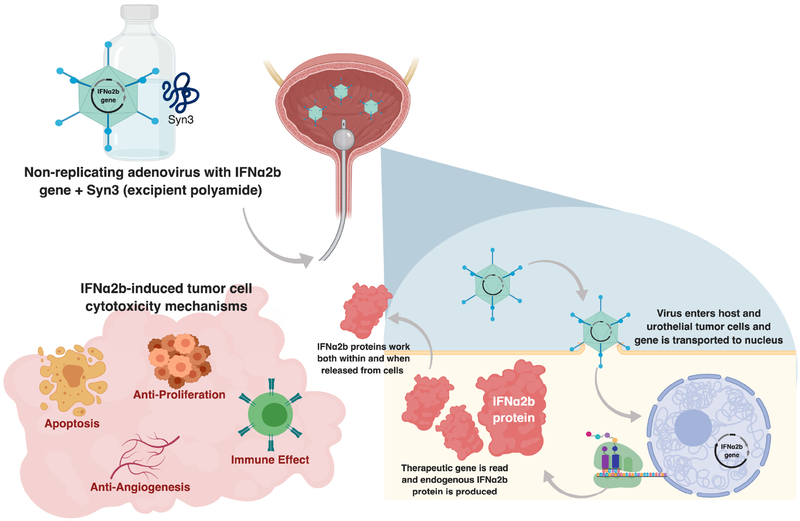
Immunogene therapy refers to the delivery of genetic material for the purpose of modulating a host immune response. In immunogene therapy, tumor-induced immunosuppression can be altered, and antigen-specific antitumor responses can be stimulated. Therapies such as high-dose IL-2 have been used effectively in treating renal cell carcinoma, albeit with significant systemic adverse events. Interferon-α (IFNα) belongs to the family of cytokine proteins and works to pleiotropically impede tumor cell growth. IFNα has been demonstrated to augment the response of T helper type 1 immune responses when combined with BCG, and as such its use as combination therapy with BCG has been explored in several trials. In a national multi-institutional phase II trial evaluate the combination of IFNα and BCG in patients with non-muscle invasive disease, 59% of BCG-naïve and 45% of prior BCG unresponsive patients remained disease free at 24-months.37 A hypothetical way to potentiate the immunogene effect of IFNα may be to deliver it within a gene therapy/adenoviral construct, ideally increasing transfection rate of IFNα into urothelial cells and stimulating an immune response by virtue of the adenovirus vehicle itself (Figure 1). Unlike the delivery of systemic cytokines such as IL-2 therapy, intravesically delivered IFNα has been established as well tolerated by patients and is likely well tolerated even through gene therapy.
Figure 1.
Mechanisms of action of intravesical rAdIFNα2b/Syn3 gene therapy.
Pre-clinical studies conducted by Iqbal and colleagues in 2001 found that recombinant adenovirus expressing human IFNα2b could generate biologically active interferon expression in both rat models as well as in vitro.38 Recombinant AdIFNα2b was also able to generate an anti-tumor response in xenografts. Benedict and colleagues subsequently demonstrated that intravesical instillation of adenovirus-encoding IFNα along with Syn3 into athymic mouse models growing KY7/GFP human bladder tumors led to marked tumor regression.39 High levels of IFN protein were identified in both urine and bladder tissue following intravesical exposure. Cytotoxicity to bladder cancer cell lines were demonstrated following Ad-IFN instillation, and IFN levels were noted for at least 7 days following treatment. This data provided some of the initial steps justifying further evaluation of utilizing intravesical Ad-IFNα/Syn3 to treat non-muscle invasive bladder cancer (Figure 1).39
Several questions remained. First, how did IFN-based gene therapy generate tumor cell cytotoxicity? Izawa and colleagues answered this question in part with data that demonstrated the fact that IFN triggered an indirect antiangiogenic effect, caused in part by downregulating the expression of the angiogenesis factors bFGF and MMP-9 within tumor cells.40 Neovascularization of tumor cells was therefore restricted, and secondarily led to tumor cell apoptosis. A second component of IFN-stimulated cytotoxicity is driven by TRAIL-mediated apoptosis, which Papageorgiou and colleagues reported occurred through an IRF-1 dependent mechanism.41 Indeed, elevated TRAIL levels have also been noted in patients with detectable urinary IFNα levels following treatment.42 A second question related to selection of the appropriate dosing strategy when using Ad-IFNα intravesically. Tao and colleagues showed that single instillations were just as effective as sequential instillations and identified a dosing floor using an orthotopic bladder cancer model. A dose of 1 x 1011 particles/mL along with Syn3 was found to be effective in reducing the size of tumors.43,44
Human trials using Adenovirus-mediated interferon-based gene therapy
The results of the first phase I clinical trial evaluating the safety and toxicity of intravesical rAd- IFNα/Syn3 was published in 2013.45 In the study, patients with non-muscle invasive (Ta, Tis, T1) disease who had recurred following at least 2 cycles of BCG were enrolled to receive one of five dosing cohorts, using a standard dose-escalation design. Patients were monitored daily for adverse events up to 5 days after treatment, and after 12 weeks of therapy, underwent cystoscopy with bladder biopsy and urine cytology. A total of 17 patients were enrolled in the study. No dose limiting toxicities were identified, nor were there any significant treatment-related adverse events. The most common complaints following therapy included urinary urgency, headache, fatigue, and nausea. Lower urinary tract symptoms, which were encountered by 88% of patients, were well managed with anticholinergic therapy. Efficiency of gene therapy was assessed by detecting levels of IFNα protein in the urine. All but the lowest dose were able to generate detectable IFNα protein levels. Out of the 14 patients treated with effective dosing and who had confirmed gene transfer (as evidenced by detectable urinary IFNα), six (43%) had a complete response (CR) at 3 months with 2 remaining disease free at 29 and 39 months.
In the phase II setting, conducted between 2012 and 2015 by the Society of Urologic Oncology Clinical Trials Consortium (SUO-CTC), 40 patients from 13 centers with a diagnosis of high-grade BCG-refractory or relapsed non-muscle invasive bladder cancer (Ta, Tis, T1) were randomized to receive either a low-dose (1 x 1011 viral particles/mL) or high-dose (3 x 1011 viral particles/ml) of rAdIFNα/Syn3 intravescially.46 The study was multi-institutional, and patients were treated every 3 months for 1 year, at which point they received a study-mandated biopsy from the location of the index tumor and at least 5 random bladder biopsies from protocol-specified locations. The primary endpoint of the phase II trial was 25% freedom from high-grade recurrence at 12 months, and evidence of effective gene transfer was found in all trial patients as determined by measurable levels of IFNα-2b in the urine of patients. There appeared to be no significant difference between the two dosing arms, although the median time to recurrence slightly favored the high-dose group (6.5 mos vs 3.5 mos). Importantly, rAdIFNα/Syn3 appeared to act on both papillary and CIS BCG-unresponsive lesions; a subset analysis demonstrated a 50% high-grade recurrence free survival (HG RFS) rate for papillary tumors at 12 months, and a 30% HG RFS rate for CIS lesions. The latter is particularly important given the paucity of treatment options available for patients with CIS; the only approved agent aside from BCG remains valrubicin, which has only a 10% 12-month RFS rate.47 Long-term follow-up data from the phase II trial remains pending.
In late 2016, a multi-institutional phase III trial for rAdIFNα/Syn3 (Adstiladrin®, previously Instiladrin®) was initiated to evaluate the efficacy of the therapy in treating patients with high-grade BCG unresponsive non-muscle invasive disease, 100 of whom must have CIS. In addition to determining the CR rate as well as CR-durability among patients with CIS, the trial seeks to understand the percent of high-grade recurrence free survival at 3-month intervals up to 1 year in patients with Ta/T1 lesions without CIS. This trial completed accrual in the second quarter of 2018 and data is maturing.
Ongoing efforts to optimize interferon-based gene therapy
The role of combination therapy with gene therapy constructs is currently unknown and an area of ongoing area interest to our group. Plote et al evaluated the immunologic mechanisms that mediated interferon-stimulated immune responses using poly:IC, a toll-like receptor 3 agonist that acts as a synthetic analogue of interferon.48 Among the key findings included the fact that interferon drives an inflammatory response driven by both innate and adaptive immune cells, including increased CD8 T cells, NK cells, and CD11b+Ly6G+ cells.48 In addition, mice containing MB49 urothelial tumors that were treated with a combination of poly:IC and anti-PD-1 monoclonal antibody showed prolonged survival, decreased angiogenesis, and enhanced MAPK/AKT signaling.48 Thus efforts to combine immune checkpoint inhibitors along with intravesical viral-mediated interferon may further potentiate an anti-tumor response and warrant additional study.
Our lab is also investigating the role of urine-derived biomarkers following treatment with intravesical gene therapy to better predict which patients may most benefit from intravesical gene therapy. Analysis of the immune cells that are shed within the urine of patients with NMIBC may provide an opportunity to identify patients who would most benefit from a cytokine-based intravesical treatment versus an alternative form of therapy. Correlative data from the Phase I and II rAdIFNα/Syn3 studies are being used to explore the role of urinary biomarkers further.
Finally, the use of vectors other than adenovirus are also under investigation. Lentivirus in particular holds promise. Lentivirus is more efficient than adenovirus at viral transfection, in part due to its ability to infect non-dividing cells. Further, lentiviral IFN constructs can integrate into the host’s genome to allow for the continual production and sustained release of IFNα. Preliminary data, recently presented at the 2019 Genitourinary Cancers symposium, showed that LV-IFNα effectively increases the expression of IFNα in vitro and in vivo along with concomitant TRAIL-mediated cytotoxicity to infected urothelial cancer cells. Efforts at further elucidating the mechanism of this agent remain ongoing.49
Other intravesical gene therapy agents under investigation
CG0070 is a replication-competent adenovirus that selectively replicates in Rb-pathway-defective bladder tumor cells, in addition to stimulating the expression of granulocyte-macrophage colony-stimulating factor (GM-CSF). Burke et al reported the results of a phase 1 study in which CG0070 was administered to 35 patients with recurrent NMIBC following at least one prior course of intravesical BCG.50 Patients received either a single dose or multi-dose intravesical course, and safety was assessed. The agent was found to have no dose-limiting toxicities with the most frequently reported adverse event being dysuria. CR was achieved in only 50% of patients with CIS (4 of 8 patients), and in 41.2% of patients with CIS +/− Ta/T1 (7 of 17 patients).50 All patients had high detectable levels of GM-CSF in the urine following treatment.50
In the phase II setting, CG0070 was administered 14 days following the most recent biopsy in patients with NMIBC (Ta, T1, CIS) who were BCG-unresponsive and had refused cystectomy.51 The agent was co-instilled with 0.1% dodecyl maltoside (DDM), a nonionic surfactant used to enhance transduction (similar to the role played by Syn3 in the rAdIFN studies). Therapy was administered intravesically every week for 6 weeks for both induction and maintenance courses. At 3 months, two patients had progressed to muscle-invasive disease, 1 patient had baseline persistence of CIS, and the other had T1 disease.51 At 6 months the CR rate was 47%, which included a 58% CR rate for pure CIS.51 12-month data has yet to be published, but interim results were reported at the 2018 American Urological Association Annual Meeting; the CR rate was noted to be 30%, with CIS-containing tumors having a 27% CR (n=45) while pure Ta/T1 tumors had a 38% CR (n=16). Ten patients underwent cystectomy, of whom 6 patients were found to have progressed to muscle-invasive disease.52 Table 1 summarizes the key clinical studies published to date investigating the use of intravesical gene therapy for bladder cancer.
Table 1.
Key clinical studies involving intravesical gene therapy
| Author | Year | Setting | Agent | Patient Population |
|---|---|---|---|---|
| Kuball et al32 | 2002 | Phase I | rAd-p53 (SCH-58500) | MIBC patients scheduled for RC |
| Pagliaro et al34 | 2003 | Phase I | rAd5-CMV-p53 | Cystectomy-ineligible locally advanced UCC |
| Burke et al50 | 2012 | Phase I | CG0070 | Recurrent NMIBC after at least 1 prior intravesical BCG dose |
| Dinney at al45 | 2013 | Phase I | rAdIFNα2b/Syn3 | BCG-unresponsive patients with NMIBC |
| Shore et al46 | 2017 | Phase II | rAdIFNα2b/Syn3 | BCG-unresponsive patients with NMIBC |
| Packiam et al51 | 2018 | Phase II | CG0070 | BCG-unresponsive patients with NMIBC who had refused cystectomy |
MIBC: muscle-invasive bladder cancer; NMIBC: non-muscle invasive bladder cancer; RC: radical cystectomy; UCC: urothelial cell carcinoma
Conclusions
BCG unresponsive non-muscle invasive bladder cancer remains a challenging disease to treat. Intravesical gene therapy represents a promising novel approach to management and will likely expand to include combination therapies. Additional insights into the mechanisms that underlie intravesical gene transduction and immune modulation, along with a more granular understanding of the molecular defects that occur in NMIBC tumor biology are also likely to provide us with additional therapeutic targets in the future.
KEY POINTS.
Non-muscle invasive bladder cancer (NMIBC) is usually treated with transurethral resection followed by intravesical bacillus Calmette-Guerin (BCG).
For patients who develop BCG-unresponsive disease but cannot tolerate surgery, there are few effective salvage intravesical options, with valrubicin only conferring a 10% complete response rate at 12 months.
Gene therapy refers to the delivery of nucleic acid into a host’s cell in order to produce a therapeutic effect, and intravesical gene therapy can modulate both the host’s immune response and generate an anti-tumor response (“immunogene therapy”).
Adenoviral vectors can be used to efficiently deliver gene therapy into both normal and tumor cells within the urinary bladder, and recombinant Ad-IFNα2b has demonstrated encouraging results in both the phase I and phase II settings for patients with BCG-unresponsive NMIBC.
Combination of other agents with intravesical gene therapy constructs and the identification of novel biomarkers to aid in treatment selection remains an area of ongoing research.
SYNOPSIS.
Non-muscle invasive bladder cancer (NMIBC) is a challenging disease to treat, with few effective salvage intravesical options available for patients who develop BCG-unresponsive disease. Although radical cystectomy with pelvic lymphadenectomy remains the gold standard treatment for these patients, there remains an unmet need for other options for those who are unable or unwilling to undergo surgery. To this end, intravesical gene therapy is emerging as a potential alternative with promising early data and ongoing efforts to better understand the mechanisms of action to optimize therapy.
Acknowledgments
Funding: The University of Texas MD Anderson SPORE in Genitourinary Cancer P50CA091846, NIH/NCI under P30CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Vikram M. Narayan: declares no conflicts of interest. Colin P.N. Dinney: Independent Chairman of the Adstiladrin Phase III trial for FKD Therapies Oy, and research for NCI, and the University of Eastern Finland, Faculty of Health Sciences (UEHFS). The remaining authors have nothing to disclose.
Contributor Information
Vikram M. Narayan, University of Texas MD Anderson Cancer Center, Houston, TX USA.
Colin P.N. Dinney, University of Texas MD Anderson Cancer Center, Houston, TX USA.
References
- 1.van den Bosch S, Alfred Witjes J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur Urol. 2011;60(3):493–500. doi: 10.1016/j.eururo.2011.05.045 [DOI] [PubMed] [Google Scholar]
- 2.Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119(17):3219–3227. doi: 10.1002/cncr.28147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearl R. Cancer and Tuberculosis. Am J Hyg. 1929;9:97–159. [Google Scholar]
- 4.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180–183. http://www.ncbi.nlm.nih.gov/pubmed/820877. [DOI] [PubMed] [Google Scholar]
- 5.Ratliff TL, Kavoussi LR, Catalona WJ. Role of fibronectin in intravesical BCG therapy for superficial bladder cancer. J Urol. 1988;139(2):410–414. http://www.ncbi.nlm.nih.gov/pubmed/3276931. [DOI] [PubMed] [Google Scholar]
- 6.Jackson AM, Alexandroff AB, Kelly RW, et al. Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after bacillus Calmette-Guérin (BCG) immunotherapy. Clin Exp Immunol. 1995;99(3):369–375. http://www.ncbi.nlm.nih.gov/pubmed/7882559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuge O, Vasdev N, Allchorne P, Green JS. Immunotherapy for bladder cancer. Res reports Urol. 2015;7:65–79. doi: 10.2147/RRU.S63447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buffen K, Oosting M, Quintin J, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014;10(10):e1004485. doi: 10.1371/journal.ppat.1004485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales A, Ottenhof P, Emerson L. Treatment of residual, non-infiltrating bladder cancer with bacillus Calmette-Guerin. J Urol. 1981; 125(5):649–651. http://www.ncbi.nlm.nih.gov/pubmed/7014931. [DOI] [PubMed] [Google Scholar]
- 10.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163(4):1124– 1129. http://www.ncbi.nlm.nih.gov/pubmed/10737480. [PubMed] [Google Scholar]
- 11.Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992;19(3):573–580. http://www.ncbi.nlm.nih.gov/pubmed/1636241. [PubMed] [Google Scholar]
- 12.Choi W, Porten S, Kim S, et al. Identification of Distinct Basal and Luminal Subtypes of Muscle-Invasive Bladder Cancer with Different Sensitivities to Frontline Chemotherapy. Cancer Cell. 2014;25(2):152–165. doi: 10.1016/j.ccr.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci. 2014;111(8):3110–3115. doi: 10.1073/pnas.1318376111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–322. doi: 10.1038/nature12965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allory Y, Beukers W, Sagrera A, et al. Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol. 2014;65(2):360–366. doi: 10.1016/j.eururo.2013.08.052 [DOI] [PubMed] [Google Scholar]
- 16.Cappellen D, De Oliveira C, Ricol D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999;23(1):18–20. doi: 10.1038/12615 [DOI] [PubMed] [Google Scholar]
- 17.Zieger K, Dyrskjøt L, Wiuf C, et al. Role of activating fibroblast growth factor receptor 3 mutations in the development of bladder tumors. Clin Cancer Res. 2005;11(21):7709– 7719. doi: 10.1158/1078-0432.CCR-05-1130 [DOI] [PubMed] [Google Scholar]
- 18.Hernández S, López-Knowles E, Lloreta J, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol. 2006;24(22):3664–3671. doi: 10.1200/JCO.2005.05.1771 [DOI] [PubMed] [Google Scholar]
- 19.Platt FM, Hurst CD, Taylor CF, Gregory WM, Harnden P, Knowles MA. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res. 2009;15(19):6008–6017. doi: 10.1158/1078-0432.CCR-09-0898 [DOI] [PubMed] [Google Scholar]
- 20.Taylor CF, Platt FM, Hurst CD, Thygesen HH, Knowles MA. Frequent inactivating mutations of STAG2 in bladder cancer are associated with low tumour grade and stage and inversely related to chromosomal copy number changes. Hum Mol Genet. 2014;23(8):1964–1974. doi: 10.1093/hmg/ddt589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurst CD, Alder O, Platt FM, et al. Genomic Subtypes of Non-invasive Bladder Cancer with Distinct Metabolic Profile and Female Gender Bias in KDM6A Mutation Frequency. Cancer Cell. 2017;32(5):701–715.e7. doi: 10.1016/j.ccell.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulligan RC. The basic science of gene therapy. Science. 1993;260(5110):926–932. http://www.ncbi.nlm.nih.gov/pubmed/8493530. [DOI] [PubMed] [Google Scholar]
- 23.Wirth T, Parker N, Ylä-Herttuala S. History of gene therapy. Gene. 2013;525(2):162–169. doi: 10.1016/j.gene.2013.03.137 [DOI] [PubMed] [Google Scholar]
- 24.Duplisea JJ, Mokkapati S, Plote D, et al. The development of interferon-based gene therapy for BCG unresponsive bladder cancer: from bench to bedside. World J Urol. November 2018. doi: 10.1007/s00345-018-2553-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaji EH, Leiden JM. Gene and stem cell therapies. JAMA. 2001;285(5):545–550. http://www.ncbi.nlm.nih.gov/pubmed/11176856. [DOI] [PubMed] [Google Scholar]
- 26.Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331(19):1259–1264. doi: 10.1056/NEJM199411103311903 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi R, Hashimoto T, Xu HJ, et al. The retinoblastoma gene functions as a growth and tumor suppressor in human bladder carcinoma cells. Proc Natl Acad Sci U S A. 1991;88(12):5257–5261. http://www.ncbi.nlm.nih.gov/pubmed/2052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagliaro LC, Keyhani A, Liu B, Perrotte P, Wilson D, Dinney CP. Adenoviral p53 gene transfer in human bladder cancer cell lines: cytotoxicity and synergy with cisplatin. Urol Oncol. 21(6):456–462. http://www.ncbi.nlm.nih.gov/pubmed/14693272. [DOI] [PubMed] [Google Scholar]
- 29.Pagliaro LC. Gene therapy for bladder cancer. World J Urol. 2000;18(2):148–151. http://www.ncbi.nlm.nih.gov/pubmed/10854151. [DOI] [PubMed] [Google Scholar]
- 30.Zhang WW, Fang X, Mazur W, French BA, Georges RN, Roth JA. High-efficiency gene transfer and high-level expression of wild-type p53 in human lung cancer cells mediated by recombinant adenovirus. Cancer Gene Ther. 1994;1(1):5–13. http://www.ncbi.nlm.nih.gov/pubmed/7621238. [PubMed] [Google Scholar]
- 31.Morris BD, Drazan KE, Csete ME, et al. Adenoviral-mediated gene transfer to bladder in vivo. J Urol. 1994;152(2 Pt 1):506–509. http://www.ncbi.nlm.nih.gov/pubmed/8015103. [DOI] [PubMed] [Google Scholar]
- 32.Kuball J, Wen SF, Leissner J, et al. Successful adenovirus-mediated wild-type p53 gene transfer in patients with bladder cancer by intravesical vector instillation. J Clin Oncol. 2002;20(4):957–965. doi: 10.1200/JCO.2002.20.4.957 [DOI] [PubMed] [Google Scholar]
- 33.Connor RJ, Engler H, Machemer T, et al. Identification of polyamides that enhance adenovirus-mediated gene expression in the urothelium. Gene Ther. 2001; 8(1):41–48. doi: 10.1038/sj.gt.3301348 [DOI] [PubMed] [Google Scholar]
- 34.Pagliaro LC, Keyhani A, Williams D, et al. Repeated intravesical instillations of an adenoviral vector in patients with locally advanced bladder cancer: a phase I study of p53 gene therapy. J Clin Oncol. 2003;21(12):2247–2253. doi: 10.1200/JCO.2003.09.138 [DOI] [PubMed] [Google Scholar]
- 35.Klingler CH. Glycosaminoglycans: how much do we know about their role in the bladder? Urologia. 2016;83 Suppl 1:11–14. doi: 10.5301/uro.5000184 [DOI] [PubMed] [Google Scholar]
- 36.Yamashita M, Rosser CJ, Zhou J-H, et al. Syn3 provides high levels of intravesical adenoviral-mediated gene transfer for gene therapy of genetically altered urothelium and superficial bladder cancer. Cancer Gene Ther. 2002;9(8):687–691. doi: 10.1038/sj.cgt.7700488 [DOI] [PubMed] [Google Scholar]
- 37.Joudi FN, Smith BJ, O’Donnell MA, National BCG-Interferon Phase 2 Investigator Group. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guérin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol. 24(4):344–348. doi: 10.1016/j.urolonc.2005.11.026 [DOI] [PubMed] [Google Scholar]
- 38.Iqbal Ahmed CM, Johnson DE, Demers GW, et al. Interferon alpha2b gene delivery using adenoviral vector causes inhibition of tumor growth in xenograft models from a variety of cancers. Cancer Gene Ther. 2001;8(10):788–795. doi: 10.1038/sj.cgt.7700364 [DOI] [PubMed] [Google Scholar]
- 39.Benedict WF, Tao Z, Kim C-S, et al. Intravesical Ad-IFNalpha causes marked regression of human bladder cancer growing orthotopically in nude mice and overcomes resistance to IFN-alpha protein. Mol Ther. 2004;10(3):525–532. doi: 10.1016/j.ymthe.2004.05.027 [DOI] [PubMed] [Google Scholar]
- 40.Izawa JI, Sweeney P, Perrotte P, et al. Inhibition of tumorigenicity and metastasis of human bladder cancer growing in athymic mice by interferon-beta gene therapy results partially from various antiangiogenic effects including endothelial cell apoptosis. Clin Cancer Res. 2002;8(4):1258–1270. http://www.ncbi.nlm.nih.gov/pubmed/11948141. [PubMed] [Google Scholar]
- 41.Papageorgiou A, Dinney CPN, McConkey DJ. Interferon-alpha induces TRAIL expression and cell death via an IRF-1-dependent mechanism in human bladder cancer cells. Cancer Biol Ther. 2007;6(6):872–879. http://www.ncbi.nlm.nih.gov/pubmed/17617740. [DOI] [PubMed] [Google Scholar]
- 42.Benedict WF, Fisher M, Zhang X-Q, Yang Z, Munsell MF, Dinney CNP. Use of monitoring levels of soluble forms of cytokeratin 18 in the urine of patients with superficial bladder cancer following intravesical Ad-IFNα/Syn3 treatment in a phase l study. Cancer Gene Ther. 2014;21(3):91–94. doi: 10.1038/cgt.2014.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tao Z, Connor RJ, Ashoori F, et al. Efficacy of a single intravesical treatment with Ad-IFN/Syn 3 is dependent on dose and urine IFN concentration obtained: implications for clinical investigation. Cancer Gene Ther. 2006;13(2):125–130. doi: 10.1038/sj.cgt.7700865 [DOI] [PubMed] [Google Scholar]
- 44.Connor RJ, Anderson JM, Machemer T, Maneval DC, Engler H. Sustained intravesical interferon protein exposure is achieved using an adenoviral-mediated gene delivery system: a study in rats evaluating dosing regimens. Urology. 2005;66(1):224–229. doi: 10.1016/j.urology.2005.02.015 [DOI] [PubMed] [Google Scholar]
- 45.Dinney CPN, Fisher MB, Navai N, et al. Phase I trial of intravesical recombinant adenovirus mediated interferon-α2b formulated in Syn3 for Bacillus Calmette-Guérin failures in nonmuscle invasive bladder cancer. J Urol. 2013;190(3):850–856. doi: 10.1016/j.juro.2013.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shore ND, Boorjian SA, Canter DJ, et al. Intravesical rAd-IFNα/Syn3 for Patients With High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J Clin Oncol. 2017;35(30):3410–3416. doi: 10.1200/JCO.2017.72.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinney CPN, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol. 2013;31(8):1635–1642. doi: 10.1016/j.urolonc.2012.04.010 [DOI] [PubMed] [Google Scholar]
- 48.Plote D, Choi W, Mokkapati S, et al. Inhibition of urothelial carcinoma through targeted type I interferon-mediated immune activation. Oncoimmunology. 2019;8(5):e1577125. doi: 10.1080/2162402X.2019.1577125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mokkapati S, Duplisea JJ, Plote D, et al. Lentiviral interferon: A novel method for gene therapy in bladder cancer. J Clin Oncol. 2019;Suppl 7S:Abstract 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burke JM, Lamm DL, Meng MV, et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol. 2012;188(6):2391–2397. doi: 10.1016/j.juro.2012.07.097 [DOI] [PubMed] [Google Scholar]
- 51.Packiam VT, Lamm DL, Barocas DA, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol. 2018;36(10):440–447. doi: 10.1016/j.urolonc.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 52.Packiam VT, Barocas DA, Chamie K, et al. LBA24 CG0070, AN ONCOLYTIC ADENOVIRUS, FOR BCG-UNRESPONSIVE NON-MUSCLE-INVASIVE BLADDER CANCER (NMIBC): 12 MONTH INTERIM RESULTS FROM A MULTICENTER PHASE II TRIAL. J Urol. 2018;199(4S). doi: 10.1016/j.juro.2018.03.096 [DOI] [PubMed] [Google Scholar]