Synopsis
Hybrid closed-loop (artificial pancreas) systems have recently been introduced into clinical practice for adults with type 1 diabetes (T1D). This reflects successful translation from research studies in highly supervised settings to evaluation of the technology in free-living home settings.
We review the different closed-loop approaches and the key clinical evidence supporting adoption of hybrid closed-loop systems for adults with T1D. We also discuss the growing evidence for automated insulin delivery in pregnant women and in hospitalised patients with hyperglycaemia. We consider the psychosocial impact of closed-loop systems, and the challenges and potential future advancements for automated insulin delivery.
Keywords: Hybrid closed-loop, Artificial pancreas, Type 1 diabetes, Inpatient diabetes, Psychosocial impact
Introduction
Achieving the recommended glycaemic targets for people with type 1 diabetes (T1D) is challenging without experiencing problematic hypoglycaemia and a high burden of diabetes self-care. The benefits of intensive insulin therapy to reduce the risk of long-term complications were shown in the Diabetes Control and Complications Trial, and led to increased uptake of insulin pump therapy (continuous subcutaneous insulin infusion, CSII) to achieve improved glycaemic outcomes, reduce the risk of hypoglycaemia and improve quality of life for people with T1D.1,2
Continuous glucose monitoring (CGM) devices, measuring real-time interstitial glucose concentration have steadily improved in terms of accuracy and reliability; use of CGM is associated with improvements in glucose control and reduced hypoglycaemia in adults with T1D.3 Despite evidence of clinical benefit of these diabetes technologies, and widespread uptake (the T1D Exchange clinic registry data reports pump use in 63% of participants and CGM use in 30%), the American Diabetes Association HbA1c target of ≤7% (53mmol/mol) is achieved by less than a quarter of adults with T1D.4
The closed-loop approach
Insulin pumps can be used in conjunction with real-time CGM, allowing users to manually modify the insulin infusion rate according to CGM values (sensor augmented pump therapy, SAP). The simplest form of automated insulin delivery is the low glucose suspend (LGS) feature which automatically suspends insulin infusion when the sensor glucose reaches a pre-specified CGM threshold value or when sensor glucose is predicted to cross the pre-specified CGM threshold value (predictive low glucose management; PLGM) within a particular time frame. LGS and PLGM have been shown to be effective in reducing the frequency and duration of hypoglycaemia without any significant impact on HbA1c.5,6 The pathway of the key milestones in the development of fully automated multi-hormone artificial pancreas systems is illustrated in Figure 1.
Figure 1.
JDRF Pathway to the Artificial Pancreas. The six developmental stages of artificial pancreas systems
From JDRF. Artificial Pancreas Project. Avaliable at: https://www.jdrf.ca/our-research/treat/artificial-pancreas-project/; with permission.
Closed-loop insulin delivery is more sophisticated compared to suspend approaches. A control algorithm is either incorporated into the insulin pump or hosted on a separate device, such as a smartphone and the components of the closed-loop system communicate wirelessly (Figure 2). Real-time glucose data provided by a CGM device is received by the control algorithm, which then instructs insulin delivery via the insulin pump by automatically modifying the insulin infusion rate every 5–10 minutes based on the sensor glucose levels (single-hormone closed-loop systems). Glucagon, or other hormones, can also be delivered in a similar glucose-responsive manner within dual-hormone closed-loop systems.
Figure 2.
Automated insulin delivery (artificial pancreas). A subcutaneous continuous glucose monitor (rectangle on abdomen) transmits the interstitial glucose levels to a controller (hand held device), which hosts a control algorithm and the user interface. An insulin pump (in pocket) delivers subcutaneous rapid-acting insulin analogue. Insulin delivery is adjusted in real-time by the control algorithm. Communication between system components is wireless
From Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nature Reviews Endocrinology. 2011;7(7):385–395; with permission.
One of the key benefits of closed-loop insulin delivery over sensor augmented pump therapy is the continuous and automatic modulation of insulin delivery rates to adapt to the within day and between day variability of insulin requirements. A retrospective analysis of insulin requirements during a 12 week hybrid closed-loop study involving 32 adults with T1D under free-living conditions showed high variability of overnight insulin requirements (coefficient of variation 31%), which was higher than the variability of daytime insulin requirements (coefficient of variation 22%).7
Control algorithms
The control algorithm is the principal component of a closed-loop system, directing insulin delivery in response to sensor glucose levels and other key inputs such as meal intake while also accommodating variability of insulin requirements between and within individual users, and accounting for glucose sensor and insulin delivery limitations. Several different control algorithms have been developed and utilised in closed-loop systems.
Model predictive control (MPC) algorithms employ a personalised model of glucose regulation to predict glycaemic excursions based on inputs including subcutaneous insulin. The insulin infusion rate is determined by minimizing the difference between model-predicted glucose-concentrations and the target glucose levels over a specified prediction time perspective.
Proportional-integral-derivative (PID) controllers continuously modify insulin infusion rates by evaluating glucose excursions from three perspectives, deviation from target glucose (proportional component), area-under-the-curve between measured and target glucose level (integral component), and rate of change of measured glucose levels (derivative component).
The fuzzy logic control approach modulates insulin delivery on the basis of rules which reflect the experiential knowledge of diabetes practitioners.
Direct comparison of the performance of PID and MPC algorithms in a crossover study observed additional benefits of MPC over PID controllers.8 Furthermore, meta-analysis data suggests that MPC and fuzzy logic control algorithms may be associated with increased time spent with glucose levels in target range (70–180 mg/dL; 3.9–10mmol/L) compared with PID algorithms, although analysis of subgroup differences was not significant.9
Most control algorithms incorporate safety modules to restrict insulin delivery, limiting the amount of insulin on board or the maximum rate of insulin delivery, and suspending insulin delivery when sensor glucose levels are low or decreasing rapidly.
User specific parameters such as body weight and insulin requirements are usually required for initialisation of closed-loop systems. However, adaptation of the algorithm to changes in physiological parameters with real-time adjustment of closed-loop control parameters is essential for optimal performance.
Hybrid and fully closed-loop systems
Hybrid closed-loop systems require the user to manually initiate mealtime boluses while fully closed-loop systems automatically dose insulin without information about meals with the advantage of reduced user intervention. Most closed-loop systems adopt a hybrid approach as glucose control is compromised with fully closed-loop systems due to delayed absorption of subcutaneous insulin. Fully closed-loop approaches are associated with significant postprandial hyperglycaemic excursions and late post-prandial hypoglycaemia. Ultra-rapid insulin analogues or adjuncts are needed to improve the performance of fully closed-loop systems in managing post-prandial hyperglycaemia.
Dual hormone closed-loop systems
Both single- and dual-hormone approaches (delivering insulin and glucagon or another hormone) are being pursued for clinical use. Addition of glucagon is an attractive option to more closely mimic pancreatic islet physiology, but also increases complexity and cost of the system. The potential role of glucagon is to further reduce the risk of hypoglycemia, or to buffer more aggressive insulin delivery with higher glucagon doses. A lack of room-temperature stable glucagon formulations is limiting progress at present, as available formulations require reconstitution prior to use and exchange of the glucagon reservoir every 24 hours. Novel glucagon analogues are being developed but the full pharmacokinetic and safety profile is yet to be established. A more detailed discussion regarding the role of glucagon in automated insulin delivery can be found in this issue.
Clinical evidence of automated insulin delivery in adults
Clinical trials evaluating hybrid closed-loop systems have progressed from short duration studies undertaken in highly supervised research facility settings to studies lasting several months in unsupervised, free-living conditions. Hybrid closed-loop insulin delivery has demonstrated efficacy and safety in the outpatient setting in children, adolescents, and adults with T1D.9,10
Meta-analysis data
Two recent meta-analyses have evaluated the safety and efficacy of outpatient hybrid closed-loop therapy in non-pregnant individuals with TID.9,10 The larger of these included 40 studies (1,042 participants); 31 used a single-hormone approach and nine evaluated a dual-hormone system. The data favour hybrid closed-loop systems, compared with sensor augmented pump therapy or standard pump therapy, across a number of glycaemic metrics including the proportion of time spent with sensor glucose in target range and time spent above and below target glucose range. However, most of the randomised controlled trials included in these meta-analyses had a relatively small sample size, including around 20–30 participants and short intervention period often less than one week. Only two studies were of long enough duration to report on HbA1c outcomes. In addition, outcome reporting across studies was not consistent. The JDRF Artificial Pancreas Project Consortium advocate the use of standardised CGM metrics in addition to HbA1c, safety and technical metrics as outcome measures in artificial pancreas clinical trials to align outcome reporting.11
Time in target glucose range
Meta-analysis data have shown that outpatient use of single hormone hybrid closed-loop systems increases the time spent in target glucose range (70–180 mg/dL; 3.9–10.0 mmol/L) over 24h by approximately 10% compared with control therapy. This equates to over two additional hours each day in normoglycaemia.10 The benefit of automated insulin delivery is most pronounced overnight, when time in target glucose range is around 15 percentage points greater than with control therapy. Increased time in target glucose range associated with closed-loop systems is attributable to reduced time spent in hyperglycaemia.
While dual-hormone systems have demonstrated greater improvements in time in target glucose range compared with single-hormone systems, almost all studies of dual-hormone systems have been of a short duration and compared to standard pump therapy. This is in contrast to studies of single-hormone systems where the comparator is usually sensor-augmented pump therapy. Direct comparison of single- and dual-hormone closed-loop systems in adults observed no difference in the time spent in target glucose range over a 24h period under supervised conditions.12
Hypoglycaemia
Hybrid closed-loop insulin delivery is associated with a reduction in the proportion of time spent in hypoglycaemia (<70 mg/dL; 3.9 mmol/L) by 1.5 percentage points (approximately 20 minutes/24h period) compared to control therapy.10 Night time spent in hypoglycaemia is also reduced with hybrid closed-loop by 2.2 percentage points compared to standard therapy. As the incidence of severe hypoglycaemia in clinical studies is very low, there is insufficient evidence to determine benefit of hybrid closed-loop insulin delivery on reducing the risk of severe hypoglycaemia. However, hybrid closed-loop use was associated with a decrease in low blood glucose index (a measure of the risk of severe hypoglycaemia) overnight compared to control therapy.
Mean glucose and HbA1c
Compared to standard or sensor augmented pump therapy, hybrid closed-loop systems have a favourable effect on mean sensor glucose concentration, with a reduction of 9 mg/dL (0.5 mmol/L).10 This is consistent with a 0.3% to 0.4% reduction in HbA1c observed with closed-loop systems compared with control therapy in studies with a duration per intervention of more than eight weeks.10,13 While the effect of hybrid closed-loop on lowering of HbA1c is modest, this is despite the reduction in hypoglycemia observed in these studies.
Insulin requirements
There are conflicting results between individual studies regarding the effect of hybrid closed-loop systems on total daily insulin dose. Meta-analysis data suggests there is no difference between closed-loop and control interventions in the total daily insulin requirement.9,10
Key outpatient hybrid closed-loop clinical studies in adults
Assessments of efficacy from larger clinical trials under free-living conditions in representative populations are critical to support reimbursement and wider adoption of hybrid closed-loop systems.
The first study exploring the feasibility of prolonged home use of a single-hormone hybrid closed-loop system compared overnight closed-loop glucose control with sensor-augmented pump therapy in 24 subjects over 6 weeks with remote monitoring.14 Overnight closed-loop insulin delivery increased the time spent with sensor glucose in the target range (4.4 v 3.1 hours/night) and reduced hypoglycemia compared with control therapy (median 3.8 v 48.7 minutes/night).
Clinical evidence to support 24h (day and night) hybrid closed-loop insulin-delivery at home under free living conditions without remote monitoring came from a randomized, controlled crossover study comparing 12 weeks of hybrid closed-loop insulin delivery using the Cambridge algorithm, with sensor-augmented pump therapy in 33 adults with T1D.15 Time spent with sensor glucose in the target range was 11 percentage points greater with the hybrid closed-loop system than with the control intervention (67.7% v 56.8%) and hybrid closed-loop also reduced hypoglycaemia. Mean HbA1c after 12 weeks was 0.3% lower with hybrid closed-loop than with sensor-augmented pump therapy. This study realised the potential for hybrid closed-loop systems in real-world settings.
It is important for successful implementation to understand the target populations where hybrid closed-loop therapy may be most beneficial, and to ensure its efficacy and safety benefits are generalisable. Many early studies demonstrated efficacy of hybrid closed-loop in individuals with good glycaemic control at recruitment, who may not be representative of the wider population with T1D. A recent multicentre study compared hybrid closed-loop with sensor-augmented pump therapy in a more diverse population of 86 children, adolescents and adults with sub-optimal glycaemic control despite pump therapy (baseline HbA1c 7.5%−10.0% at recruitment) over a period of 12 weeks of free-living.13 The time with glucose in target range was 10.8 percentage points higher with closed-loop compared with control therapy (65% v 54%). Time spent in hypoglycaemia (<70mg/dL; 3.9 mmol/L) was also significantly reduced with closed-loop (2.6% v 3.9%; P=0.0130). In the closed-loop group, HbA1c was reduced from a screening value of 8.3% (67 mmol/mol) to 8.0% (64 mmol/mol) after the 4-week run-in, and to 7.4% (57 mmol/mol) following the 12-week intervention period. In the control group, the HbA1c values were 8.2% (66 mmol/mol) at screening, 7.8% (62 mmol/mol) after run-in, and 7.7% (61 mmol/mol) after the intervention period; adjusting for baseline HbA1c, the reduction in HbA1c was significantly greater by 0.36% (4.0mmol/mol) in the closed-loop group compared with the control group (P<0.0001).
The performance of a hybrid closed-loop system incorporating a patch pump (Omnipod) has been evaluated in two small supervised studies in adults with T1D with challenges including overestimated and missed meal boluses and moderate intensity exercise.16,17 After the overestimated bolus (130%), 4 hour postprandial percentage time <70 mg/dL (3.9mmol/L) was 0%. After the missed bolus, postprandial percentage time ≥250 mg/dL (14mmol/L) was 10.3% compared with 0.2% with the delivered bolus. In the 12-h period after 30 minutes of moderate exercise, percentage time <70 mg/dL (3.9mmol/L) was low using either a raised glucose set point at 150mg/dL rather than 130mg/dL (1.4%), or using a reduced temporary basal rate of 50% (1.6%) started 90 min pre-exercise.
The largest hybrid closed-loop crossover study to date compared the Diabeloop DBLG1 hybrid closed-loop system with sensor-augmented pump therapy for 12 weeks in 68 adults with T1D (baseline HbA1c 7.6%, 59.4mmol/mol) in the home setting with remote monitoring.18 The proportion of time with sensor glucose in the target range was 9.2 percentage points greater with closed-loop than with control therapy (68.5% v 59.4%). Time spent in hypoglycaemia (<70mg/dL; 3.9 mmol/L) was significantly lower with closed-loop than during the control period. Mean HbA1c was reduced by 0.29% with closed-loop compared with 0.14% in the control group without reaching significance for between group comparison. During the study, five severe hypoglycaemic episodes occurred in the closed-loop group and three in the sensor-augmented pump therapy group; these were attributed to pump hardware malfunctions or human error. The DBLG1 hybrid closed-loop system has received CE mark for use in adults with T1D.
Commercially available hybrid closed-loop systems
The first commercially available hybrid closed-loop system, the 670G pump (Medtronic, Northridge, CA, USA), approved by the US Food and Drug Administration in September 2016, and with CE mark since June 2018, has been shown to be safe in people with T1D over 7 years of age. The 670G is a single-hormone hybrid closed-loop system with the control algorithm embedded in the insulin pump. The pump basal rate is automated based on a PID algorithm with insulin on board feedback. This system has a fixed target sensor glucose concentration of 6.7 (120 mg/dL) or 8.3 mmol/l (150 mg/dL) with the higher target designed for exercise. Clinical evaluation to assess safety was non-randomised and lacked a control arm introducing selection bias therefore evidence regarding its efficacy is uncertain.19 Ninety four adults and 30 adolescents used the closed-loop system day-and-night for 12 weeks. No episodes of severe hypoglycaemia or ketoacidosis were observed. From baseline run-in to the end of the 3 month study phase, adult HbA1c levels decreased from 7.3% (56mmo/mol) to 6.8% (51 mmol/mol) (P < 0.001), respectively. The time in target increased from 68.8% at baseline to 73.8% (P < 0.001) in adults.
Safety and efficacy has since been demonstrated in the real world setting in an observational study of over 3,000 patients who completed 3 months using the 670G system in Auto Mode during the commercial launch.20 Individuals used Auto Mode 80.8% of the time. The time spent in target glucose range (70–180 mg/dL; 3.9–10 mmol/L) was 66.0% during a baseline period using Manual Mode compared with 73.3% during Auto Mode. Time spent in hypoglycaemia (<70mg/dL; 3.9mmol/L) reduced from 2.7% to 2.1% with Auto Mode. A small increase in total insulin delivered of 2.1 units/day was noted with Auto Mode, attributable to increased bolus delivery.
A recent real-world observational study including 93 children and young adults using the 670G Auto Mode for a mean of 8 months follow-up demonstrated high discontinuation rates. Over one third (38%) of individuals with previous experience in pump therapy and CGM discontinued Auto Mode because of technical difficulties including frequent alarms, excessive calibration requirements, premature sensor failure and the device exiting Auto Mode. The remaining users’ time in Auto Mode varied from 10% to 90%. Auto Mode utilisation correlated with HbA1c; a mean decrease in HbA1c of 0.27% after approximately 3 months using Auto Mode (P=0.025) was observed in a subset of 58 users who continued to use Auto Mode.21
Subsequent iterations of the 670G system are being developed with broader glucose and insulin delivery parameters to reduce the alarm burden and the number of Auto Mode exits to improve usability of the system.
Open APS, Loop and AndroidAPS closed-loop systems
The OpenAPS (Open Artificial Pancreas System), Loop, and AndroidAPS communities have developed alternative non-commercial closed-loop systems. Individuals within this movement have built their own hybrid closed-loop systems from commercially available insulin pumps (although sometimes out of warranty), CGM devices, and open source algorithms. The lack of regulatory approvals for these systems allows for a rapid innovation cycle and more options for customisation. These systems appeal to increasing numbers of people with T1D, with reportedly several thousand users to date worldwide. The responsibility of healthcare professionals in supporting users of these non-regulatory approved systems remains controversial, and the FDA have recently released a safety communication warning against using these DIY systems. A comprehensive review of this approach can be found in this issue.
Automated insulin delivery during pregnancy
The benefits of maintaining near-normoglycaemia during pregnancy are clear, with increased rates of stillbirth, neonatal death, preterm delivery, and macrosomia associated with maternal hyperglycemia during pregnancy in women with T1D. Maintaining glucose within the recommended tight target range in pregnancy is particularly challenging for women with T1D: insulin requirements typically increase 2–3 fold throughout pregnancy, with high day-to-day variability of insulin needs. Despite intensive insulin therapy with frequent glucose monitoring and HbA1c levels <7% (53 mmol/mol), pregnant women with T1D spend approximately 50% of the time with glucose levels above the target range, and experience high rates of hypoglycaemia (<70mg/dL; 3.9mmol/l), with glucose levels below target range for approximately 15% of the day (3.5 hours per day).22
Automated insulin delivery using the Cambridge hybrid closed-loop system has been shown to be safe and effective in pregnant women with T1D.23 In a randomized, crossover study, overnight hybrid closed-loop therapy at home for 4 weeks led to increased time spent in the tighter target glucose range for pregnancy (63–140 mg/dL; 3.5–7.8 mmol/l) by 15 percentage points (74.7% vs. 59.5%; P=0.002) compared with sensor augmented pump therapy, without increasing hypoglycaemia. During a continuation phase applying hybrid closed-loop therapy day-and-night until delivery (up to 14.6 additional weeks which included antenatal hospitalisations, labour, and delivery), sensor glucose levels were within the target range for pregnancy (63–140 mg/dL; 3.5–7.8 mmol/l) 68.7% of the time. The impact of hybrid closed-loop glucose control on perinatal outcomes remains to be determined but larger outcome studies are planned.
Automated insulin delivery in the inpatient setting
Achieving recommended glucose levels during a hospital admission is challenging. The effect of the current illness, medication changes and alterations to meal timings and intake in hospital all contribute to sub-optimal glucose control. Attempts to attain target glucose levels with current insulin therapy (multiple daily insulin injections) can increase the risk of hypoglycaemia and increase workload for healthcare professionals. Hyper- and hypoglycaemia in hospital are associated with increased risk of infection, length of stay, admission to the intensive care unit and mortality.24
The feasibility of using fully automated closed-loop insulin delivery, i.e. without meal announcement, in the inpatient setting has been shown to be safe and effective in achieving near-normal glucose control.25 A randomised controlled trial involving 136 adults with hyperglycaemia on the general wards compared fully closed-loop insulin delivery with standard insulin therapy for up to 15 days.26 Time spent with sensor glucose in the target range (100–180 mg/dL; 5.6–10mmol/l) was 65.8% with closed-loop compared with 41.5% with standard insulin therapy, a difference of 24 percentage points which equates to almost six additional hours each day with glucose levels in target range (P<0.001). There was no difference in the duration of hypoglycaemia (<54 mg/dL; 3.0mmol/L) or the amount of insulin delivered. Closed-loop insulin delivery was associated with significantly better glycaemic control without a higher risk of hypoglycaemia. A post-hoc analysis compared fully closed-loop insulin delivery with standard insulin therapy in patients undergoing hemodialysis while in hospital. Diabetes management in people receiving hemodialysis can be very challenging. Patients using closed-loop therapy spent more time with glucose levels in target range than the control group (69.0% v 31.5% respectively; P<0.001), without increasing the risk of hypoglycemia. Closed-loop insulin delivery offers a novel approach to manage glucose in this vulnerable patient population and further outpatient studies are warranted.27
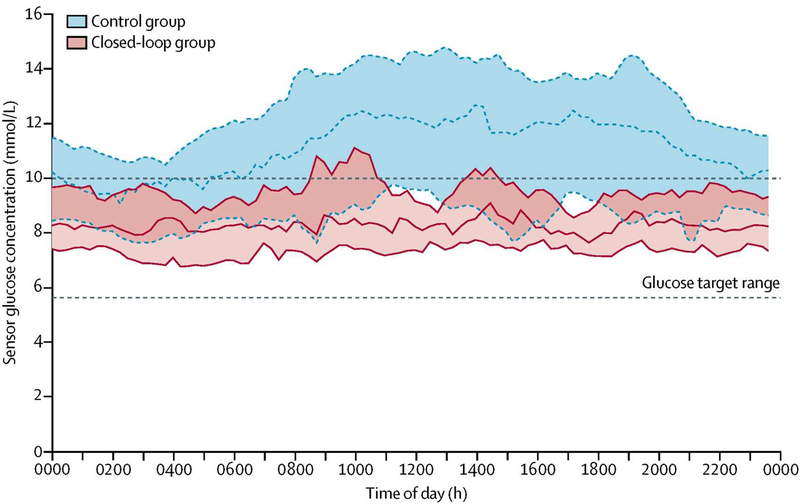
Fully automated closed-loop insulin delivery using faster-acting insulin aspart in patients requiring nutritional support (enteral/parenteral nutrition), was associated with superior glucose control compared to standard insulin therapy in a study of 43 inpatients in the general wards with hyperglycaemia.28 The closed-loop group spent approximately eight additional hours each day with glucose levels in target range (100–180 mg/dL; 5.6–10mmol/l) compared with those receiving standard insulin therapy (68·4% v 36·4%; P<0.001) without an increase in hypoglycaemia (Figure 3). Automated insulin delivery is a safe and effective tool to improve glycaemic control in hospitalised patients receiving nutritional support, where glucose management can be particularly challenging.
Figure 3.
Fully closed-loop insulin delivery in inpatients receiving enteral and/or parenteral nutrition. Sensor glucose concentration during closed-loop (red) and control (blue) interventions from midnight to midnight (lines indicate median, shaded areas indicate IQRs). The glucose target range is 5.6–10.0 mmol/l (100–180mg/dL)
From Boughton CK, Bally L, Martignoni F, et al. Fully closed-loop insulin delivery in inpatients receiving nutritional support: a two-centre, open-label, randomised controlled trial. The Lancet Diabetes & Endocrinology. 2019;7(5):368–377; with permission.
The closed-loop approach is an attractive option to change the management of inpatient diabetes; larger studies are required to determine if the observed improved glucose control with closed-loop can lead to improved clinical outcomes for patients, reduce staff work burden and healthcare costs.
The psychosocial impact of automated insulin delivery
The glycaemic benefits of hybrid closed-loop systems have been clearly demonstrated but depend on intensive use of the technology. Uptake and long-term usage of closed-loop systems will be heavily influenced by the user experience. Hybrid closed-loop systems still require user interaction for delivery of mealtime boluses, in addition to the standard insulin pump and CGM related tasks. Therefore managing user expectations at the outset is important to promote effective long-term usage and realise the clinical benefits.
Qualitative evaluations of the impact of closed-loop technology on human factors and quality of life measures have been explored in several studies.29 User reported benefits of automated insulin delivery, aside from improved glycaemic outcomes include reduced fear of hypoglycaemia particularly overnight, increased reassurance and reduced anxiety, improved sleep and confidence, more time off from the demands of T1D, empowerment, and freedom to participate in exercise and unplanned activity. Remote monitoring systems which allow closed-loop data sharing with selected individuals will likely improve user satisfaction, particularly amongst parents of young children with T1D.
There are also important challenges reported by users which need to be considered, including variable levels of trust in automated insulin delivery, intrusiveness of alarms and the associated sleep interruptions, technical difficulties, the size and appearance of the component devices causing limitations around exercise, and perceptions of deskilling or obsession with data.
Healthcare professional attitudes to closed-loop systems are yet to be reported.
Challenges and future directions of automated insulin delivery
Usability and wearability of current closed-loop system devices can be demanding and efforts to minimise device burden are paramount. Non-calibrating sensors with increased accuracy and longer wear time are likely to improve user acceptability. Insulin pumps are getting smaller with the user interface being transferred to smart devices (smartphones, watches).
Interoperable automated insulin delivery devices with ‘open protocol’ communications, which allow seamless secure connectivity with other devices, are underway including the development of iCGM (interoperable continuous glucose monitoring) systems and ACE (alternate controller enabled) pumps as defined by the US Food and Drug Administration. The flexibility to choose different combinations of devices and create personalised ‘closed-loop ecosystems’ will improve user choice and experience. Data management platforms will be important in making data from different closed-loop systems readily accessible to both users and healthcare professionals, and may be used to generate automated personal clinical insights to support optimal usage of this technology and potentially reduce workload of healthcare professionals.
Progress from hybrid closed-loop to fully closed-loop systems without meal announcement may be possible with newer ultra-fast insulin analogies or adjuncts (oral or co-infused) to manage post-prandial hyperglycaemia. Integration of additional inputs other than glucose, such as heart rate, to more accurately reflect rapidly changing insulin requirements during exercise, are also being investigated and may permit tighter glucose regulation without increasing hypoglycaemia.
Understanding user and healthcare professional training and support needs will be key to ensuring that the clinical benefits of closed-loop systems are realised and will be important for health economic analyses to support implementation and reimbursement.
Summary
Comprehensive clinical evidence supporting automated insulin delivery systems as a safe and efficacious approach, has led to the introduction of commercially available hybrid closed-loop systems into clinical practice for adults with T1D. Evidence for application of the closed-loop approach for pregnant women and for hospitalised patients with hyperglycaemia is growing.
There is an ongoing need to improve the performance and acceptability, and reduce device burden of automated insulin delivery systems. Adoption of the closed-loop approach as the standard of care in diabetes management requires an understanding of the training and support needs for both users and healthcare professionals to ensure successful implementation.
Key points.
Hybrid closed-loop systems have recently been introduced into clinical practice for adults with type 1 diabetes.
Studies show superior glucose control with hybrid closed-loop systems in adults with type 1 diabetes.
There is growing evidence for closed-loop systems in pregnant women with type 1 diabetes and in inpatients with hyperglycaemia.
Understanding the user experience and training requirements are key to successful implementation in order to realise the glycaemic benefits.
Acknowledgments:
Supported by the National Institute of Health Research Cambridge Biomedical Research Centre, Efficacy and Mechanism Evaluation National Institute for Health Research, The Leona M. & Harry B. Helmsley Charitable Trust, JDRF, National Institute of Diabetes and Digestive and Kidney Diseases and Diabetes UK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: RH reports having received speaker honoraria from Eli Lilly and Novo Nordisk, serving on advisory panel for Eli Lilly and Novo Nordisk, receiving license fees from B. Braun and Medtronic; having served as a consultant to B. Braun, patents and patent applications related to closed-loop, and being shareholder of CamDiab. CB declares no duality of interest associated with this manuscript.
References
- 1.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993;329(14):977–986. [DOI] [PubMed] [Google Scholar]
- 2.Pickup JC. Insulin-Pump Therapy for Type 1 Diabetes Mellitus. New England Journal of Medicine. 2012;366(17):1616–1624. [DOI] [PubMed] [Google Scholar]
- 3.Rodbard D. Continuous Glucose Monitoring: A Review of Recent Studies Demonstrating Improved Glycemic Outcomes. Diabetes Technology & Therapeutics. 2017;19(Suppl 3):S-25–S-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster NC, Beck RW, Miller KM, et al. State of Type 1 Diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. New England Journal of Medicine. 2013;369(3):224–232. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun PM, Buckingham BA, Maahs DM, et al. Efficacy of an overnight predictive low-glucose suspend system in relation to hypoglycemia risk factors in youth and adults with type 1 diabetes. Journal of Diabetes Science and Technology. 2016;10(6):1216–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan Y, Thabit H, Leelarathna L, et al. Variability of insulin requirements over 12 weeks of closed-loop insulin delivery in adults with type 1 diabetes. Diabetes Care. 2016;39(5):830–832. [DOI] [PubMed] [Google Scholar]
- 8.Pinsker JE, Lee JB, Dassau E, et al. Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care. 2016;39(7):1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. The Lancet Diabetes & Endocrinology. 2017;5(7):501–512. [DOI] [PubMed] [Google Scholar]
- 10.Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maahs DM, Buckingham BA, Castle JR, et al. Outcome measures for artificial pancreas clinical trials: A Consensus Report. Diabetes Care. 2016;39(7):1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. The Lancet Diabetes & Endocrinology. 2015;3(1):17–26. [DOI] [PubMed] [Google Scholar]
- 13.Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392(10155):1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimri R, Muller I, Atlas E, et al. Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis. Pediatric Diabetes. 2014;15(2):91–99. [DOI] [PubMed] [Google Scholar]
- 15.Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. New England Journal of Medicine. 2015;373(22):2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckingham BA, Christiansen MP, Forlenza GP, et al. Performance of the Omnipod personalized model predictive control algorithm with meal bolus challenges in adults with type 1 diabetes. Diabetes Technol Ther. 2018;20(9):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forlenza GP, Buckingham BA, Christiansen MP, et al. Performance of Omnipod personalized model predictive control algorithm with moderate intensity exercise in adults with type 1 diabetes. Diabetes Technol Ther. 2019;21(5):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benhamou PY, Franc S, Reznik Y, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. The Lancet Digital Health. 2019;1(1):E17–E25. [DOI] [PubMed] [Google Scholar]
- 19.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with Type 1 Diabetes. Diabetes Technol Ther. 2017;19(3):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone MP, Agrawal P, Chen X, et al. Retrospective analysis of 3-Month real-world glucose data after the MiniMed 670G System commercial launch. Diabetes Technol Ther. 2018;20(10):689–692. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin GWG, Lyons J, Oladunjoye A, Steil G. Challenges in implementing hybrid closed loop insulin pump therapy (Medtronic 670G) in a ‘real world’ clinical setting. Journal of the Endocrine Society. 2019;3(Supplement 1). [Google Scholar]
- 22.Murphy HR, Rayman G, Duffield K, et al. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care. 2007;30(11):2785–2791. [DOI] [PubMed] [Google Scholar]
- 23.Stewart ZA, Wilinska ME, Hartnell S, et al. Closed-Loop insulin delivery during pregnancy in women with type 1 diabetes. New England Journal of Medicine. 2016;375(7):644–654. [DOI] [PubMed] [Google Scholar]
- 24.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. The Journal of Clinical Endocrinology and Metabolism. 2002;87(3):978–982. [DOI] [PubMed] [Google Scholar]
- 25.Thabit H, Hartnell S, Allen JM, et al. Closed-loop insulin delivery in inpatients with type 2 diabetes: a randomised, parallel-group trial. The Lancet Diabetes & Endocrinology. 2017;5(2):117–124. [DOI] [PubMed] [Google Scholar]
- 26.Bally L, Thabit H, Hartnell S, et al. Closed-loop insulin delivery for glycemic control in noncritical care. New England Journal of Medicine. 2018;379(6):547–556. [DOI] [PubMed] [Google Scholar]
- 27.Bally L, Gubler P, Thabit H, et al. Fully closed-loop insulin delivery improves glucose control of inpatients with type 2 diabetes receiving hemodialysis. Kidney International. In Press. [DOI] [PubMed] [Google Scholar]
- 28.Boughton CK, Bally L, Martignoni F, et al. Fully closed-loop insulin delivery in inpatients receiving nutritional support: a two-centre, open-label, randomised controlled trial. The Lancet Diabetes & Endocrinology. 2019;7(5):368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrington C. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: a review. Diabetic Medicine. 2018;35(4):436–449. [DOI] [PubMed] [Google Scholar]
- 30.Hovorka R. Closed-loop insulin delivery: from bench to clinical practice. Nature Reviews Endocrinology. 2011;7(7):385–395. [DOI] [PubMed] [Google Scholar]