Abstract
Fluorescence detection is extensively used in high throughput screening. In HTS there is a continuous migration toward higher density plates and smaller sample volumes. In the present report we describe the advantages of two-photon or multiphoton excitation for HTS. Multiphoton excitation (MPE) is the simultaneous absorption of two long-wavelength photons to excite the lowest singlet state of the fluorophore. MPE is typically accomplished with short but high-intensity laser pulses, which allows simultaneous absorption of two or more photons. The intensity of the multiphoton-induced fluorescence is proportional to the square, cube, or higher power of the instantneous photon flux. Consequently, two-photon or multiphoton excitation only occurs at the focal point of the incident beam. This property of two-photon excitation allows the excited volume to be very small and to be localized in the center of each well in the HTS plate. We show that two-photon-induced fluorescence of fluorescein can be reliably measured in microwell plates. We also show the use of 6-carboxy fluorescein as a pH probe with two-photon excitation, and measure 4′–6-diamidino-2-phenylindole (DAPI) binding and two-photon-induced fluorescence. In further studies we measure the time-dependent intensity decays of DAPI bound to DNA and of calcium-dependent fluorophores. Finally, we demonstrate the possibility of three-photon excitation of several fluorophores, including indole, in the HTS plate. These results suggest that MPE can be used in high-density multiwell plates.
INTRODUCTION
At present, there is rapid development of technology for high throughput screening and drug discovery.1–4 This development is motivated by the large number of potentially bioactive compounds that can be isolated from natural sources or that are the result of combinatorial synthesis.5,6 The large number of compounds to test and the large number of individual tests has resulted in a testing bottleneck in drug discovery. Additionally, potential drugs or bioactive compounds are typically available only in small amounts, requiring the smallest possible assay volumes. These bottlenecks are being overcome by the development of instrumentation for HTS. These instruments provide highly automated systems for manipulation of the samples, loading small volumes into high-density plates, and for optical readout of the results. The optical readout is typically done by fluorescence intensity. There is also movement toward the use of wavelength ratios, polarization, and lifetimes as the spectral observables.
During the past decade there has also been rapid growth in the field of multiphoton excitation (MPE).7 Multiphoton excitation refers to the simultaneous absorption of two or more long-wavelength photons to bring the fluorophore to the first excited singlet state. Historically, MPE has been used by chemical physicists to study the symmetry of electronic states. More recently, it has been used in biophysical chemistry8–12 and even cellular imaging.13–17 Wavelengths for MPE are typically near 800 nm, which is near the minimum absorption of water. While the instantaneous intensities are high, MPE is usually accomplished with picosecond or femtosecond pulses and little heat is delivered to the sample.18 Perhaps more importantly, MPE provides localized excitation at the focal point, providing the equivalent of confocal fluorescence detection without a confocal optical system. The long wavelengths used for MPE typically do not excite autofluorescence in the samples, and the scattered light is easily removed from optical filtering. MPE can be used for cellular imaging because there is less damage to the sensitive biological components because of the highly localized excitation.
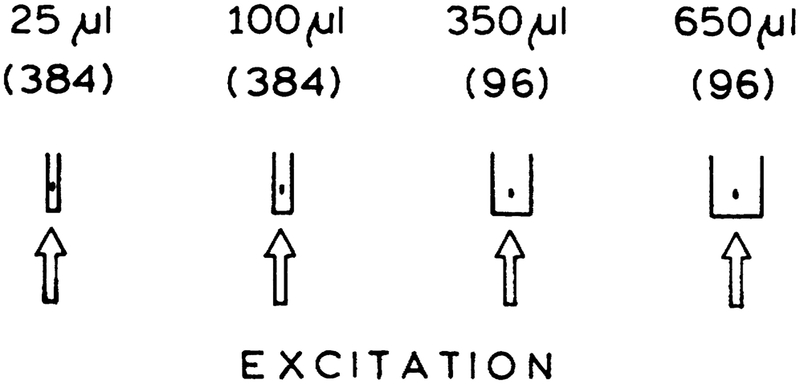
The advantages of MPE do not appear to have been considered in high throughput screening. One advantage is excitation of a small volume. This volume can be localized in the center of a well in a high-density plate (Fig. 1). Excitation will only occur in the center of the well, and the light reaching the top, bottom, or sides of the well will be too weak to excite multiphoton emission. There is less likelihood of interference from fluorescence from the plastics with long-wavelength excitation, and the longer wavelengths are easily removed with optical filters.
FIG. 1.
Two-photon excitation in microwells with different sizes. The numbers in parentheses are the number of wells per plate. The two-photon-induced signal is not expected to depend on the size of the sample because only a central spot with a 20–30-μ, diameter is excited. MPE is compatible with the smallest HTS sample sizes used at this time.
Because of these potential advantages, we examined the possibility of MPE in microwell plates. We found that fluorophore concentrations can be reliably measured with multiphoton two-or three-photon excitation. We also accomplished pH sensing using 6-carboxy fluorescein (6-CF) with the two-photon excitation. DNA concentrations were measured by enhanced emission from probes that bind to DNA. Fluorescence intensity and anisotropy decays were measured for 4’−6-diamidino-2-phenylindole (DAPI) bound to DNA and for calcium probes. Three-photon excitation was demonstrated for several fluorophores, including indole, suggesting the possibility of measuring intrinsic protein fluorescence. The results demonstrate that intensities, anisotropies, and time-resolved measurements can all be accomplished in a HTS format with MPE.
MATERIALS AND METHODS
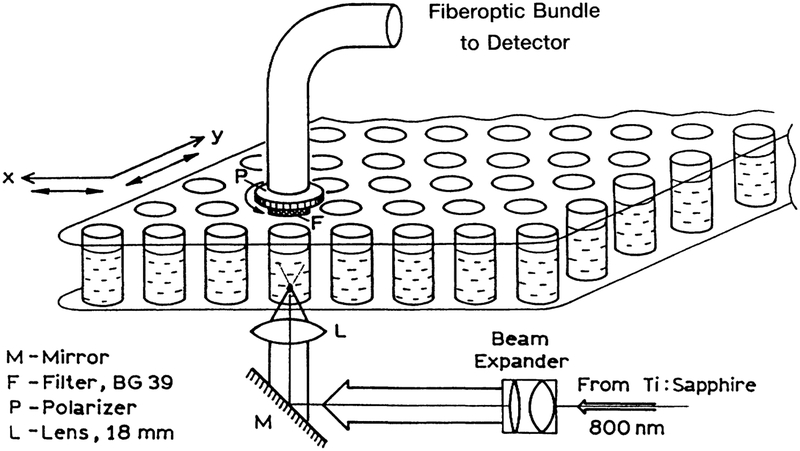
Transparent 96-well plates were obtained from Polyfiltronics, Inc. (Rockland, MA). DAPI and Ca-Green were obtained from Molecular Probes, Inc. (Eugene, OR); 6-CF was obtained from Eastman Kodak (Rochester, NY); and DPH, PPO, and indole were obtained from Aldrich (Milwaukee, WI). Fluo-3 was a gift from Dr. J. Kao (UMBI). Excitation was accomplished using the mode-locked output with a Ti:sapphire laser at 800 or 850 nm. The repetition rate was near 80 MHz and the pulse width was near 100 fs. The excitation was brought through the bottom of the plastic plate, as shown in Figure 2. The emission was brought to a detector using a fiberoptic bundle, and was isolated using the filters listed in the figure legends.
FIG. 2.
Optical arrangement for measuring two-photon-induced fluorescence in a microwell plate.
For polarization measurements, the plastic bottom was replaced with a glass microscope slide because the imperfections and/or strains in the plastic bottom depolarized the incident light. The emission was observed through a polarizer placed on the input face of the fiberoptic bundle (Fig. 2).
Frequency measurements were performed with electronics from ISS, Inc. (Urbana, IL). The data were analyzed using nonlinear least squares.19–21
RESULTS
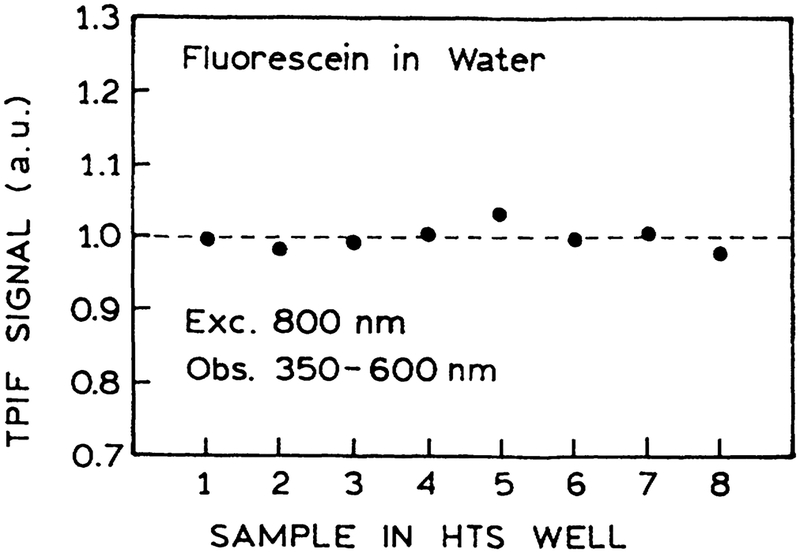
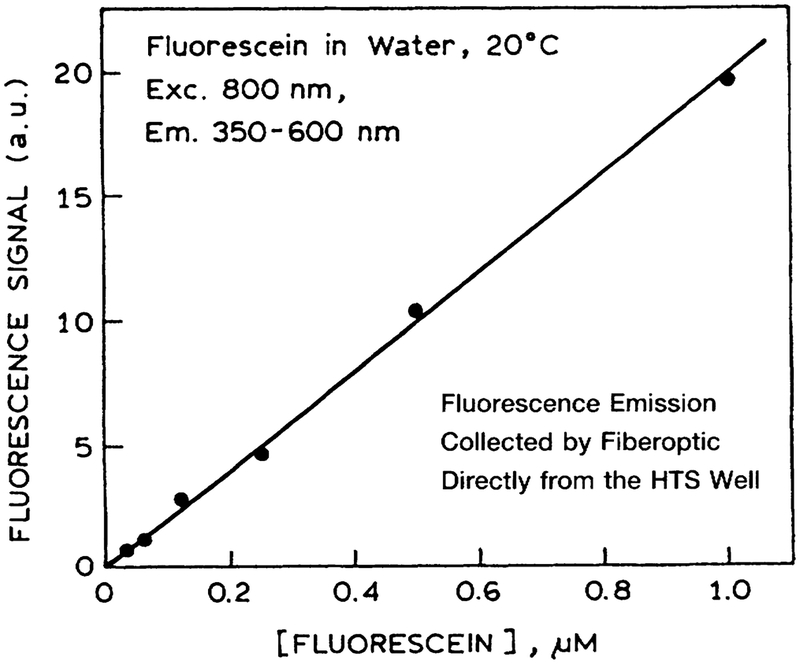
Multiphoton-induced fluorescence is highly dependent on the intensity, shape, and width of the incident pulse. Any factors that distort the pulse will result in different multiphoton-induced intensities. Hence, we examined the two-photon-induced fluorescence (TPIF) of fluorescein in water in the eight wells across the plastic 96-well plate. These intensities were equivalent to within experimental variations (Fig. 3). Hence MPE can be accomplished with the usual HTS plates, which apparently do not distort the excitation pulses enough to invalidate the intensities. We also found that the TPIF signal was linear with the fluorescein concentration (Fig. 4).
FIG. 3.
Two-photon-induced fluorescence (TPIF) of fluorescein in a series of eight wells across the plate. The emission was iso-lated using a 4–96 filter from Corning.
FIG. 4.
Concentration-dependent two-photon-induced fluorescence of fluorescein in a microwell plate. Experimental conditions are given in the legend to Figure 3.
DNA assays
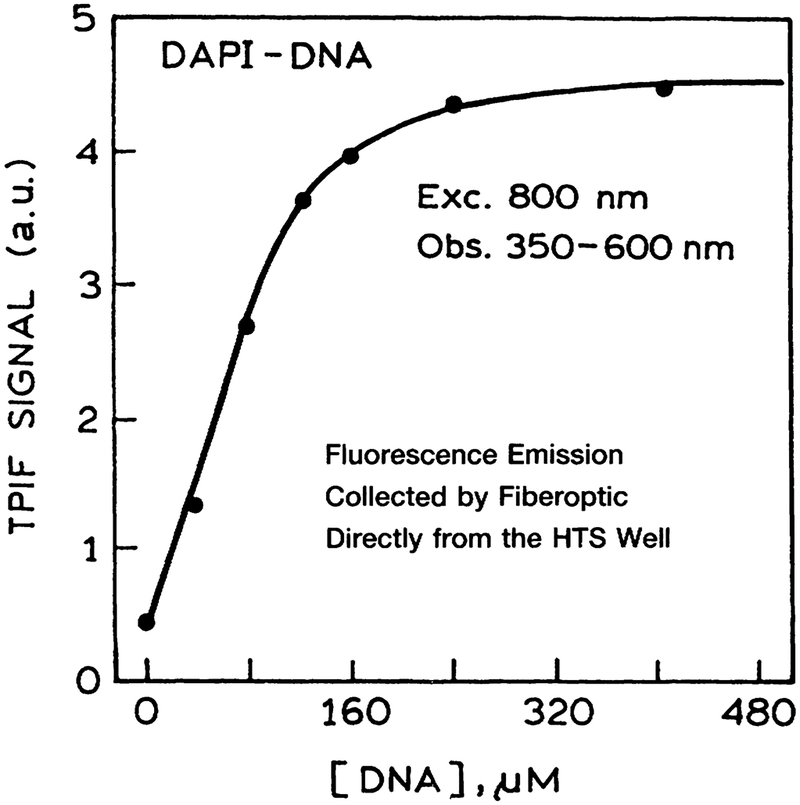
Many HTS assays require measurement of the DNA content of the sample, which is usually accomplished using fluorescent dyes that bind to DNA. DAPI is one of the widely used DNA dyes. We examined the TPIF of DAPI. The DNA concentration was increased in each well across the plate. The emission intensity of DAPI increased until it was completely bound to the DNA (Fig. 5), after which the signal remained constant.
FIG. 5.
Two-photon-induced fluorescence (TPIF) of DAPI in microwell plates as titrated with calf thymus DNA. The emission of DAPI was isolated using a 4–96 filter from Coming.
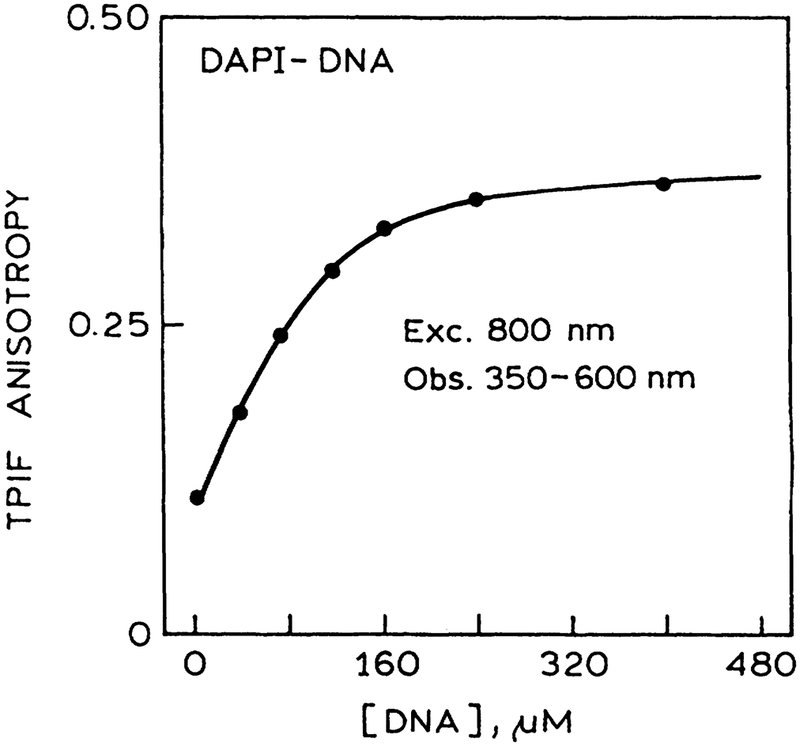
Many HTS assays are now being performed and developed that use polarization or anisotropy measurements. Hence we examined the possibility of anisotropy measurements using the multi well plates. We found the measurements were not possible with our 96-well plate because the excitation was depolarized by the plastic bottom of the plate. Of course, this would not be a problem if the excitation was directed into the sample from the top. Rather than reconfigure the options, we replaced the plastic bottom of the plate with a glass microscope slide. In this case the excitation was not depolarized and the anisotropy could be measured. The anisotropy of DAPI increased as the DNA concentration increased (Fig. 6), and the anisotropy became constant after all the DAPI was bound to the DNA. The results demonstrate that anisotropy or polarization assays can be performed in HTS format with MPE. It should be noted that MPE frequently results in larger polarization values as a result of stronger orientation photoelection.7
FIG. 6.
Anisotropy of DAPI bound to DNA with two-photon-induced fluorescence (TPIF). The plastic bottom of the micro well plate was replaced with a microscope slide to avoid depolarization caused by strains in the plastic.
Time-resolved measurements
In recent years there has been increased interest in the use of time-resolved measurements in clinical and biological assays. Lifetime measurements are advantageous because the lifetime measurements are typically independent of the probe concentration. Hence we questioned whether lifetime measurements could be accomplished with MPE in microwell plates.
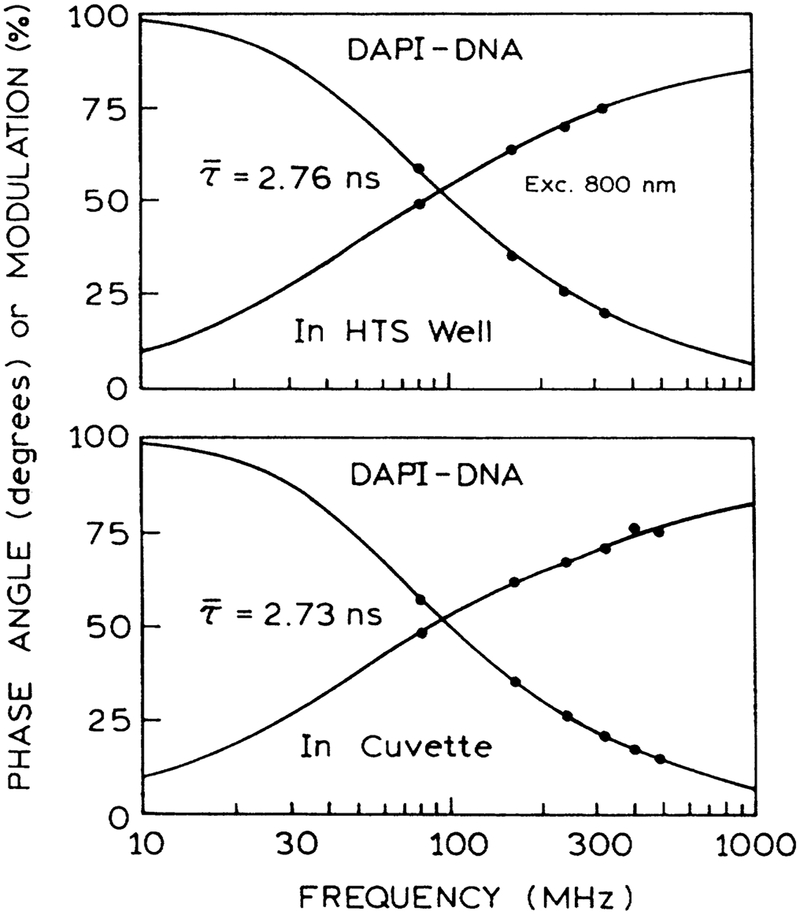
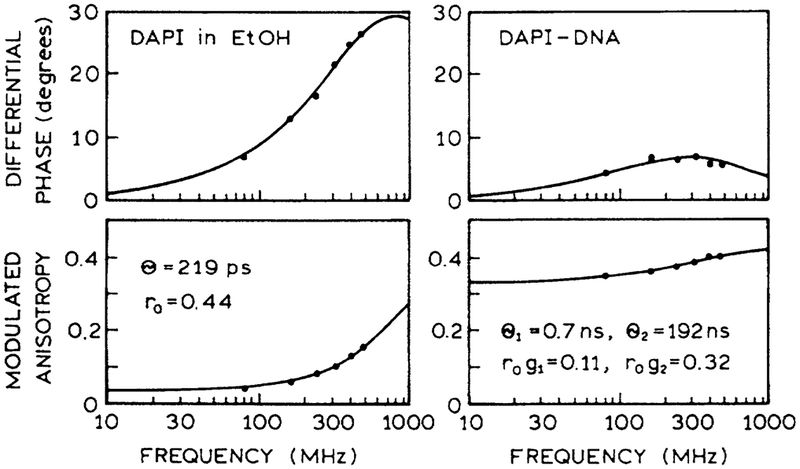
Frequency-domain (FD) intensity decays of DAPI bound to DNA were measured in the micro well plates and in a standard 1-cm2 cuvette. Essential identical FD intensity decay were observed in both geometries (Fig. 7). Similarly, we measured the FD anisotropy decay with DAPI in ethanol and when bound to DNA, both in the 96-well plate (Fig. 8). The correlation times found in the multiwell plate agree with those in numerous reports.22,23 The results depicted in Figures 7 and 8 show that high-resolution time-resolved intensity and anisotropy decays can be measured in a HTS format.
FIG. 7.
Frequency-domain intensity decays of DAPI bound to DNA as seen in the usual 1-cm2 curvette (bottom) and in the microwell plate (top). Excitation was at 800 nm, and the emission was observed using magic angle detection and a 450-nm interference filter.
FIG. 8.
Frequency-domain anisotropy decays with two-photon-induced fluorescence of DAPI and ethanol (left) and DAPI bound to DNA (right). The excitation wavelength was 800 nm, and the emission was observed through a 450-nm interference filter.
Fluorescence sensing
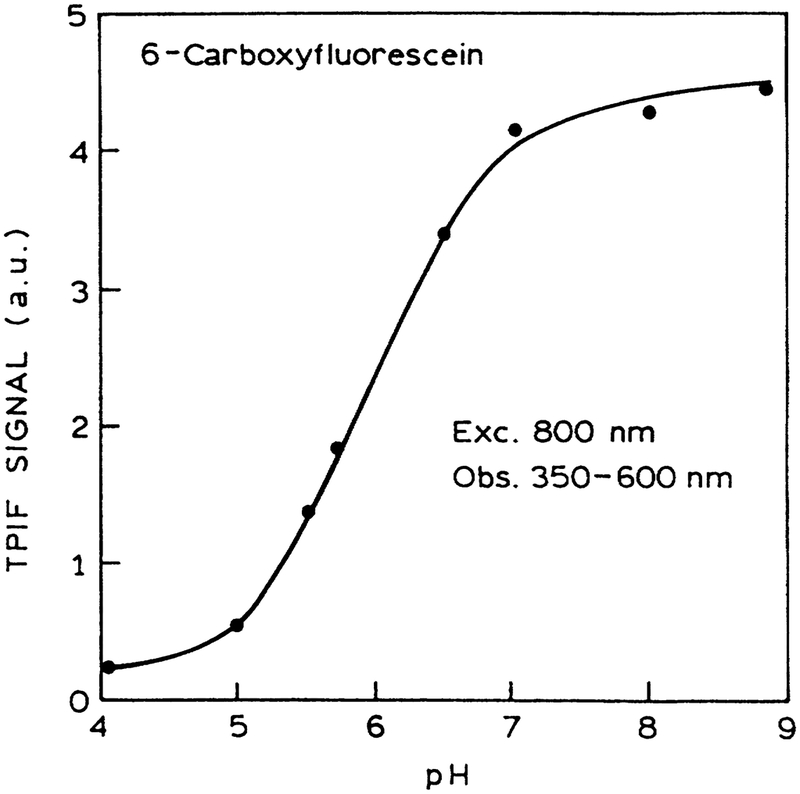
A wide variety of analyte-sensitive probes are known for electrolytes and sugars.24–26 We demonstrated the possibility of fluorescence sensing with MPE by examining the pH-de-pendent intensities of 6-CF. The intensity of 6-CF with MPE increased as expected over the pH range from 5 to 7 (Fig. 9). We note that the relative cross section for one-photon and two-photon absorption need not be similar. In addition to demonstrating the possibility of sensing with MPE, these results one-and two-photon excitation.
FIG. 9.
pH sensing using 6-carboxy fluorescein and two-photon-induced fluorescence (TPIF) in a microwell plate. The emission of DAPI was isolated using a 4–96 filter from Coming.
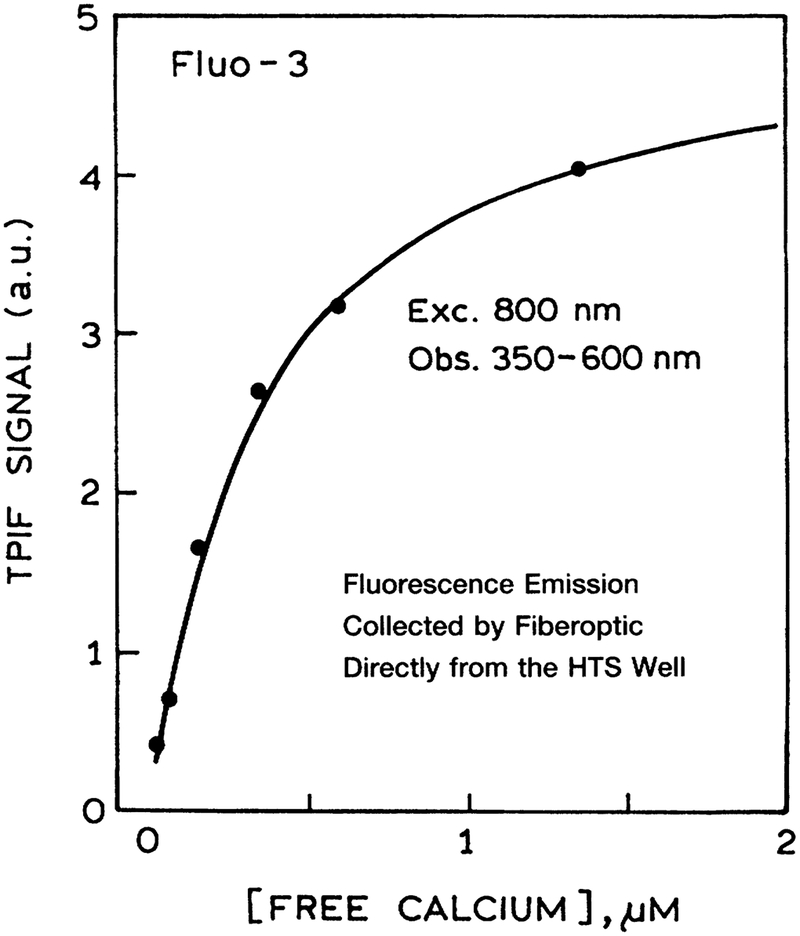
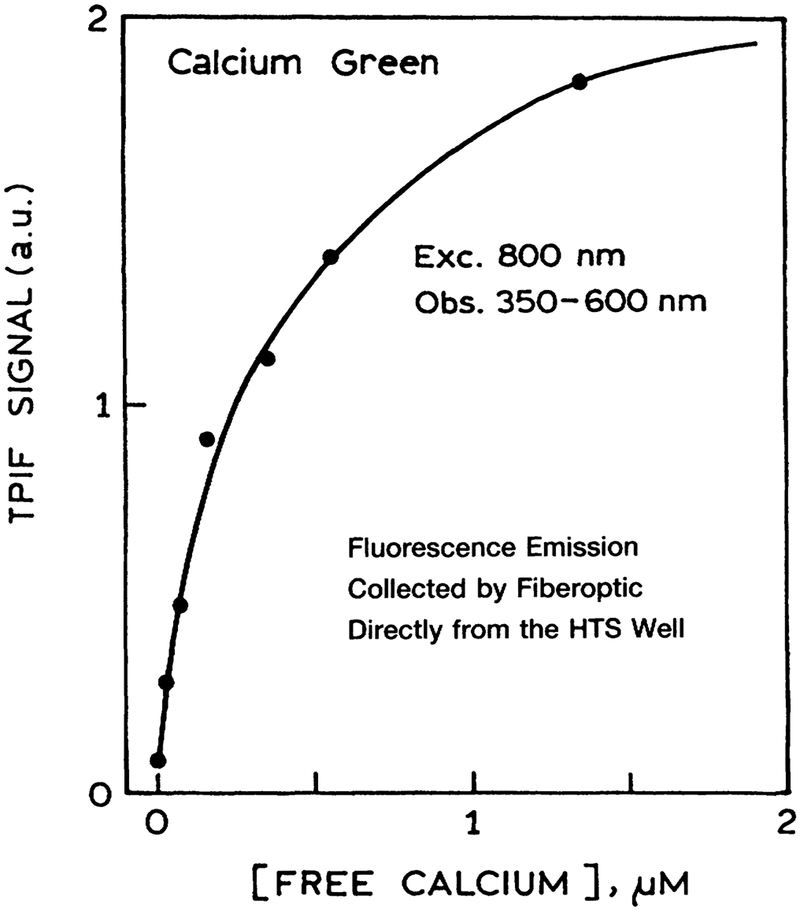
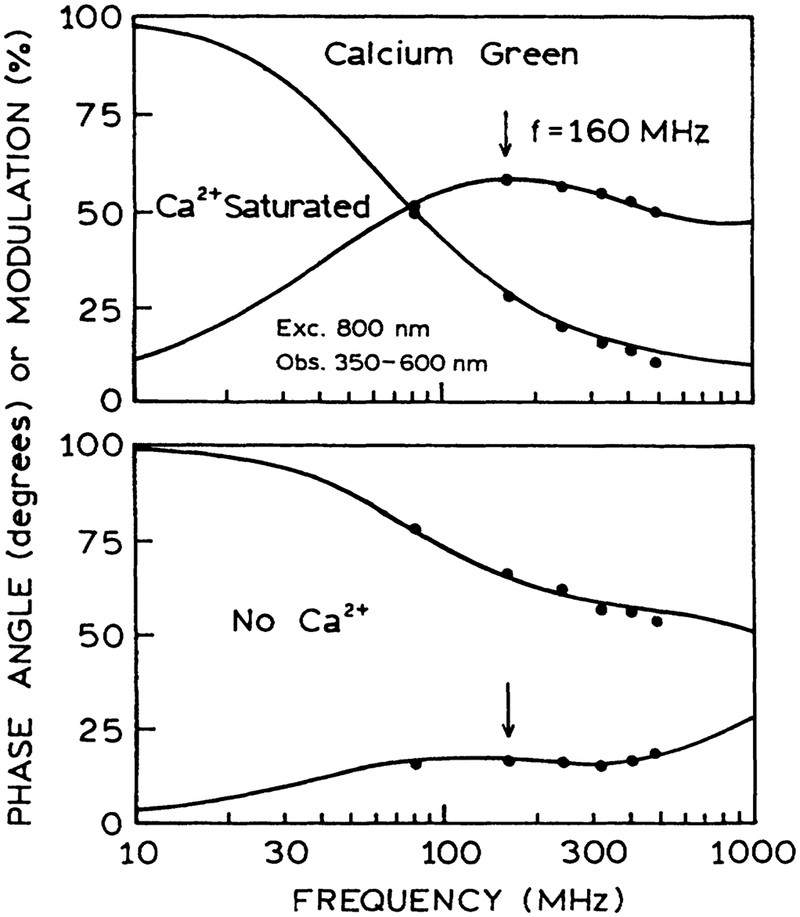
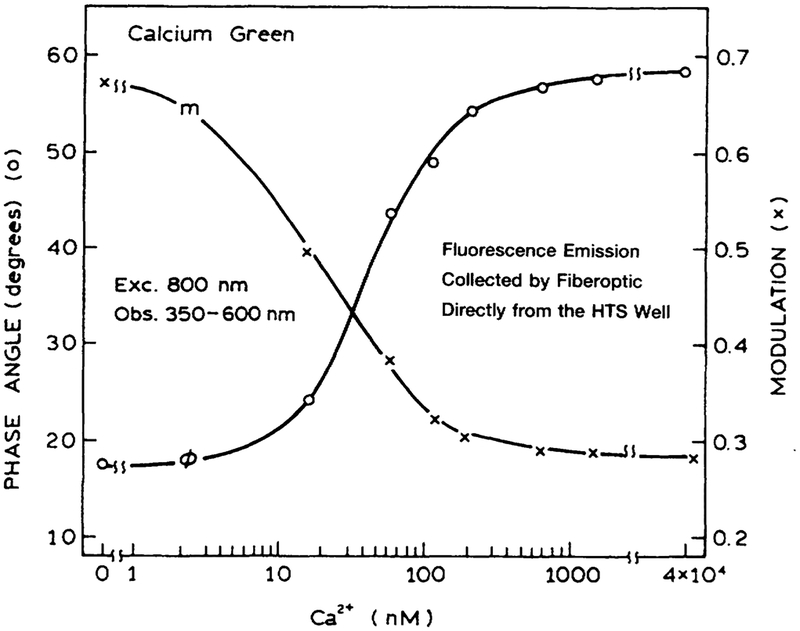
As a final test, we examined calcium sensing with two-photon excitation in a HTS format. Figure 10 shows the data for Fluo-3, which displayed increased intensity as the calcium concentration increased from 0 to 1.5 μM. A similar result was found for Calcium Green in the presence and absence of calcium (Fig. 11). The intensity decay of Calcium Green is complex in both forms (Fig. 12). However, this not a problem because analyte calibration courses for lifetime-based sensing can be determined at a single light modulation frequency.27–28 The calcium calibration curve of 160 MHz (Fig. 13) demonstrates the possibility of lifetime-based sensing with MPE in a high throughput format.
FIG. 10.
Calcium sensing using Fluo-3 and two-photon-induced fluorescence (TPIF). Each of the microwell plates contained the same concentration of Fluo-3 and different concentrations of calcium. Emission was observed using a Coming 4–96 filter.
FIG. 11.
Calcium sensing in a micro well plate using Calcium Green and two-photon-induced fluorescence (TPIF). Each of the wells contained the same concentration of Calcium Green and a different concentration of calcium. Emission was isolated using a Coming 4–96 filter.
FIG. 12.
Lifetime-based sensing using Calcium Green in a 96-well plate with two-photon-induced fluorescence.
FIG. 13.
Phase (O) and modulation (X) of Calcium Green in a 96-well plate with two-photon-induced fluorescence, at various Ca2+ concentrations. The modulation frequency was 160 MHz.
Three-photon excitation
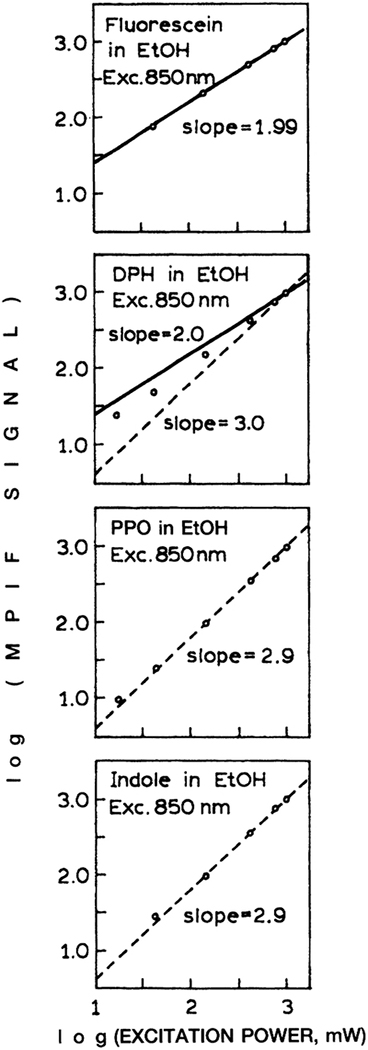
The availability of Ti:sapphire lasers with femtosecond pulse widths has allow MPE with more than two photons. In this laboratory we observed three-photon excitation for a variety of flu-orophores.29–32 Hence we questioned whether three-photon excitation could be accomplished in a HTS format. The number of simultaneously absorbed photons can be determined from the dependence of the emission intensity on the laser power. For such studies one usually uses filters to alternate the laser beam because changing the laser power could distort the pulse width and thus the extent of MPE.
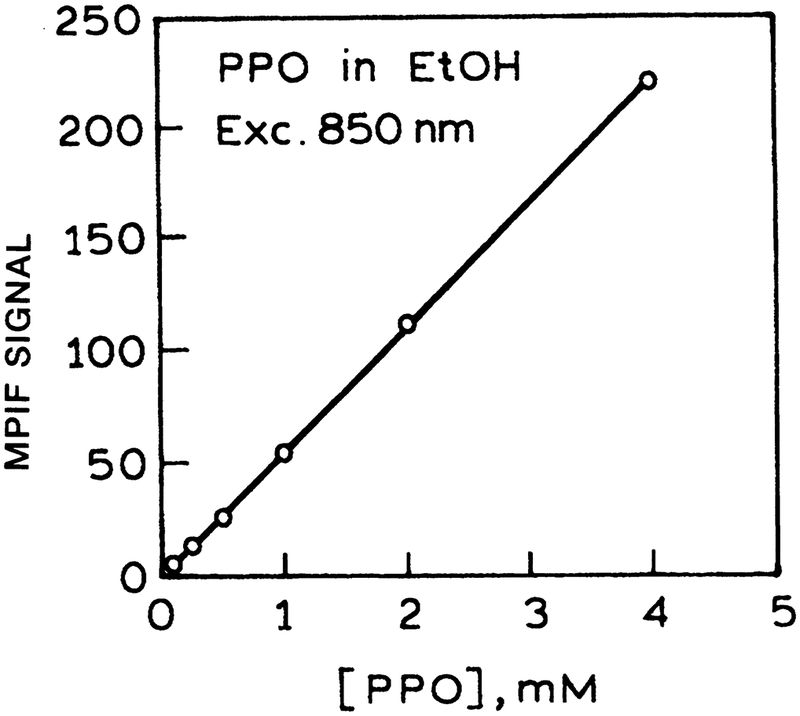
The dependence of the emission intensity on the incident power for four fluorophores is shown in Figure 14. In the case of fluorescein the slope is near 2.0, indicating two-photon excitation of fluorescein at 850 nm. In the case of 1,6-diphenyl-3,4,5-hexatriene, 2,5-diphenyloxazole, and indole, the slope is near 3.0, demonstrating three-photon excitation of these fluorophores at 850 nm. In spite of the cubic dependence on the laser peak intensity, the three-photon-induced fluorescence intensities were still accurately linear with fluorophore concentration (Fig. 15).
FIG. 14.
Multiphoton-induced fluorescence (MPIF) of fluorescein, l,6-diphenyl-3,4,5-hexatriene (DPH), 2,5-diphenyloxazole (PPO), and indole with 850-nm excitation.
FIG. 15.
Concentration-dependent intensities of 2,5-diphenyloxazole (PPO) with three-photon excitation at 850 nm. (MPIF = multiphoton-induced fluorescence.)
DISCUSSION
MPE is typically accomplished with picosecond or femtosecond laser light sources. Given the current complexity of these lasers, the reader may question the practicality of HTS with MPE. To illustrate the near-term possibility, we note that MPE is now in widespread use in optical microscopy and cellular imaging. This application is much more demanding than HTS and requires spatial resolution not only in the xy plane, but also along the light propagation axis. Additionally, the widespread use of multiphoton imaging microscopes is driving the development of simpler laser sources for MPE. Diode-pumped Ti:sapphire lasers are currently available that are all solid-state devices. One can expect the laser technology to continue to migrate toward less expensive lasers for MPE. These same lasers will also be practical for HTS.
ACKNOWLEDGMENT
This work was supported by Grant RR-08119 from the National Institutes of Health National Center for Research Resources.
REFERENCES
- 1.Giuliano KA, DeBiasio RB, Dunlay RT, Gough A, Volosky JM, Zock J, Pavlakis GN, Taylor DL (1997). High-content screening: A new approach to easing key bottlenecks in the drug discovery process. J. Biomol. Screen 2:249–259. [Google Scholar]
- 2.Nakayama GR (1998). Microplate assays for high throughput screening. Curr. Opin. Drug Discovery Dev 1:85–91. [PubMed] [Google Scholar]
- 3.Fernandes PB (1998). Technological advances in high throughput screening. Curr. Opin. Chem. Biol 2:597–603. [DOI] [PubMed] [Google Scholar]
- 4.Schober A, Gunther R, Tangen U, Goldmann G, Ederhof T, Koltermann A, Wienecke A, Schwienhorst A, Eigen M(1997). High throughput screening by multichannel glass fiber flu-orimetry. Rev. Sci. Instrum 68:2187–2194. [Google Scholar]
- 5.Chiari M, Desperati V, Manera E, Longhi R (1998). Combinatorial synthesis of highly selective cyclohexapeptides for separation of amino acid enantiomers by capillary electrophoresis. Anal. Chem 70:4967–4973. [DOI] [PubMed] [Google Scholar]
- 6.Czamik A, Keene JD (1998). Combinatorial chemistry. Curr. Biol 8:R705–R707. [DOI] [PubMed] [Google Scholar]
- 7.Lakowicz JR (Ed.) (1997). Topics in Fluorescence Spectroscopy, Vol. 5: Nonlinear and Two Photon-Induced Fluorescence, p. 544 Plenum Press, New York. [Google Scholar]
- 8.Callis PR (1997). Two-photon induced fluorescence. Annu. Rev. Phys. Chem 48:271–297. [DOI] [PubMed] [Google Scholar]
- 9.Lakowicz JR, Gryczynski I, Kusba J, Danielsen E (1992). Two photon-induced fluorescence intensity and anisotropy decays of diphenylhexatriene in solvents and lipoid bilayers. J. Fluoresc 2:247–258. [DOI] [PubMed] [Google Scholar]
- 10.Gryczynski I, Malak H, Lakowicz JR, Cheung HC, Robinson J, Umeda PK (1996). Fluorescence spectral properties of Troponin C mutant F22W with one-, two-, and three-photon excitation. Biophys. J 71:3448–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volkmer A, Hatrick DA, Birch DJS (1997). Time-resolved nonlinear fluorescence spectroscopy using femtosecond multiphoton excitation and single-photon timing detection. Meas. Sci. Technol 8:1339–1349. [Google Scholar]
- 12.Watkins AN, Ingbersoll CM, Baker GA, Bright FV (1998). A parallel multiharmonic frequency-domain fluorometer for measuring excited-state decay kinetics following one-, two-, or three-photon excitation. Anal. Chem 70:3384–3396. [DOI] [PubMed] [Google Scholar]
- 13.Hell SW, Booth M, Wilms S, Schnetter CM, Kirsch AK, Amdt-Jovin DJ, Jovin TM (1998). Two-photon near-and far-field fluorescence microscopy with continuous-wave excitation. Optics Lett. 23:1238–1240. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Sonek GJ, Liang H, Bems MW, Tromberg BJ(1998). Multiphoton fluorescence excitation in continuous-wave infrared optical traps. Appl. Optics 37:2766–2773. [DOI] [PubMed] [Google Scholar]
- 15.Kirsch AK, Subramaniam V, Striker G, Schnetter C, Amdt-Jovin DJ, Jovin TM (1998). Continuous wave two-photon scanning near-field optical microscopy. Biophys. J 75:1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mertz J (1998). Molecular photodynamics involved in multi-photon excitation fluorescence microscopy. Eur. Phys. J 3:53–66. [Google Scholar]
- 17.Jenei A, Kirsch AK, Subramaniam V, Amdt-Jovin DJ, Jovin TM (1999). Picosecond multiphoton scanning near-field optical microscopy. Biophys. J 76:1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schonle A, Hell SW (1998). Heating by absorption in the focus of an objective lens. Optics Lett. 23(5):325–327. [DOI] [PubMed] [Google Scholar]
- 19.Lakowicz JR, Gratton E, Laczko G, Cherek G, Limkeman M (1984). Analysis of fluorescence decay kinetics from variable-frequency phase shift and modulation data. Biophys. J 46:463–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gratton E, Lakowicz JR, Maliwal B, Cherek H, Laczko G, Limkeman M (1984). Resolution of mixtures of fluorophores using variable-frequency phase and modulation data. Biophys. J 46:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakowicz JR, Cherek H, Kusba J, Gryczynski I, Johnson ML (1993). Review of fluorescence anisotropy decay analysis by frequency-domain fluorescence spectroscopy. J. Fluoresc 3:103–116. [DOI] [PubMed] [Google Scholar]
- 22.Barcellona ML, Cardiel G, Gratton E (1990). Time-resolved fluorescence of DAPI in solution and bound to polydeoxynu-cleotides. Biochem. Biophys. Res. Commun 170:270–280. [DOI] [PubMed] [Google Scholar]
- 23.Barcellona ML, Gratton E (1996). Torsional dynamics and orientation of DNA-DAPI complexes. Biochemistry 35:321–333. [DOI] [PubMed] [Google Scholar]
- 24.Haugland RP (1996). Handbook of Fluorescent Probes and Research Chemicals. Spence MTZ (ed.), pp. 679 Molecular Probes, Inc., Eugene, OR. [Google Scholar]
- 25.Lakowicz JR (ed.). (1994). Topics in Fluorescence Spectroscopy, Vol. 4: Probe Design and Chemical Sensing, p. 501 Plenum Press, New York. [Google Scholar]
- 26.Czamik AW (ed.). (1993). Fluorescent Chemosensors for Ion and Molecule Recognition (ACS Symposium Series), p. 235 American Chemical Society, Washington DC. [Google Scholar]
- 27.Lakowicz JR, Szmacinski H, Johnson ML. (1992). Calcium concentration imaging using fluorescence lifetimes and long-wavelength probes. J. Fluoresc 2:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szmacinski H, Lakowicz JR (1994). Lifetime-based sensing In Topics in Fluorescence Spectroscopy, Vol. 4: Probe Design and Chemical Sensing Lakowicz JR (ed.), pp. 295–334. Plenum Press, New York. [Google Scholar]
- 29.Szmacinski H, Gryczynski I, Lakowicz JR (1996). Three-photon induced fluorescence of the calcium probe Indo-1. Biophys. J 70:547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gryczynski I, Malak H, Lakowicz JR (1996). Multiphoton excitation of the DNA stains DAPI and Hoechst. Bioimaging 4:138–148. [Google Scholar]
- 31.Lakowicz JR, Gryczynski I, Malak H, Schrader M, Engel-hardt P, Kano H, Hell SW (1997). Time-resolved fluorescence spectroscopy and imaging of DNA labeled with DAPI and Hoechst 33342 using three-photon excitation. Biophys. J 72:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gryczynski I, Malak H, Lakowicz JR (1996). Three-photon excitation of a tryptophan derivative using a fs-Ti: sapphire laser. Biospectroscopy 2:9–15. [Google Scholar]