Abstract
Objective:
To describe the early hemodynamic changes after fetal aortic valvuloplasty (FAV) for evolving hypoplastic left heart syndrome due to mid-gestational aortic stenosis and to assess whether these early changes predict biventricular (BiV) circulation at neonatal discharge.
Method:
We retrospectively reviewed all technically successful FAV cases resulting in live birth between 2000 and 2015 (n = 93,45% BiV circulation at neonatal discharge). Paired testing methods were used to compare pre-intervention and post-intervention measures of left ventricular hemodynamics. Logistic regression was used to determine whether these changes were predictive of post-natal outcome.
Results:
Measures of left heart physiology were markedly abnormal pre-FAV and improved significantly post-FAV. No subjects had systolic antegrade transverse aortic arch flow pre-FAV and 65% of subjects had antegrade flow post-FAV. The number of subjects with abnormal left-to-right patent foramen ovale flow decreased, and the number with biphasic mitral valve inflow increased. The median left ventricular ejection fraction improved after intervention. Amongst the pre-post changes, gaining partially or exclusively antegrade systolic arch flow was the most significant independent predictor of BiV circulation (OR 9.80 and 19.83, respectively, both P < 0.001).
Conclusion:
Technically successful FAV is associated with immediate improvements in left heart physiology that are predictive of BiV circulation at neonatal discharge.
1 |. INTRODUCTION
Hypoplastic left heart syndrome (HLHS) comprises a spectrum of cardiac malformations characterized by significant underdevelopment of left heart structures. These small left-sided structures are unable to support systemic circulation, necessitating palliative procedures to allow for survival with univentricular (UV) circulation with associated morbidity and mortality.1 One of the etiologies of HLHS is fetal aortic stenosis (AS). Natural history studies have demonstrated that in mid-gestational fetuses with severe aortic stenosis and a normal-sized or dilated left ventricle (LV), left heart growth arrest can occur leading to HLHS at birth.2–6 Echocardiographic-derived hemodynamic parameters that are moderately predictive of development of HLHS in these fetuses include LV dysfunction, monophasic mitral valve (MV) inflow, retrograde systolic transverse aortic arch flow, and foramen ovale (FO) flow reversal.7,8
Beginning in the early 1990s, fetal aortic valvuloplasty (FAV) has been performed for fetuses with AS and features of evolving HLHS.9 The goal of the intervention is to relieve LV outflow tract obstruction and allow increased flow through the left heart to stimulate continued growth through gestation and to allow the LV to support biventricular (BiV) circulation after birth. FAV has been shown to promote LV growth and function and may improve the likelihood of BiV circulation.10 Pre-FAV echocardiographic data have been used to iteratively refine and adjust selection criteria.11All fetuses who have undergone FAV have had retrograde aortic arch flow. Currently, approximately 50% of subjects who have undergone FAV at our institution achieve BiV circulation at neonatal discharge.12 Pre-intervention factors including LV pressure, MV inflow time, ascending aorta size, and LV long axis-size have been shown to be independently associated with BiV circulation.11
A prior study13 (n = 26) from our institution described changes in left heart hemodynamics from pre-FAV to approximately 2 months post-FAV and demonstrated that technically successful FAV leads to an increased rate of biphasic MV inflow, bidirectional FO flow, and antegrade systolic transverse aortic arch flow. Accurately predicting the post-natal outcome of a fetus that has undergone a FAV for severe AS has been challenging and parents wait until after birth to learn whether their child is a candidate for BiV circulation. It is not known whether hemodynamic changes immediately after FAV predict UV versus BiV outcome. In this study, we evaluate hemodynamic changes 24 hours after FAV in order to understand how fetal LV physiology adjusts acutely to new afterload conditions. Moreover, we aim to determine if acute hemodynamic changes after in-utero intervention are predictive of neonatal circulatory outcome.
2 |. METHODS
We reviewed the records of all fetuses in which FAV was attempted for treatment of fetal AS with evolving HLHS physiology within Boston Children’s Hospital and Brigham and Women’s Hospital between March 2000 and August 2015. Criteria for offering the FAV procedure has evolved over time as our institutional experience has grown and generally include factors such as LV systolic function and dimension, aortic valve patency and dimension, systolic gradient, and retrograde systolic transverse aortic arch flow.11,14 Circulatory status was determined at the time of neonatal discharge. BiV circulation was defined as the LV being the only source of systemic cardiac output with no intracardiac shunt apart from atrial communication. Any patient who required a Stage 1 or hybrid palliation was classified as UV circulation. Of note, the UV group includes subjects who underwent initial neonatal UV palliation and later BiV conversion after neonatal discharge (n = 6).15 The Scientific Review Committee of the Department of Cardiology and the Institutional Review Board at Boston Children’s Hospital approved this study.
The technical features of the FAV procedure have been described previously.10,16,17 A technically successful FAV was defined as one in which the aortic valve was crossed and a balloon inflated, with clear evidence of increased flow across the valve.
All subjects had a complete fetal echocardiogram zero to 1 day before FAV and a follow-up echocardiogram 1 to 2 days after FAV. The fetal echocardiograms were reviewed on the day of acquisition by a single reader (W.T.) who was blinded to the neonatal circulation outcome of the subject. Outcome-blinded analysis of the echocardiograms by a consistent reader was used to reduce variability in measurements. Not every fetal echocardiogram allowed for measurement of each echocardiographic parameter.
Methods of echocardiographic measurements have been detailed elsewhere13 but include the following key methods. LV volumes were calculated using the 5/6th-area-length method, which allowed for calculation of LV ejection fraction. LV sphericity was calculated as the ratio of short-axis area to long-axis length. Pulsed wave Doppler was used to derive velocities and time intervals, as well as the MV inflow profile. The MV inflow profile was judged to be either biphasic, partially fused, or monophasic. The MV inflow index was calculated by dividing the MV inflow time by the fetal heart rate. Systolic transverse aortic arch flow and the direction of flow across the FO were determined by color Doppler. The direction of systolic transverse aortic arch flow was determined between the brachiocephalic vessels. Exclusively retrograde flow was defined as the transverse arch being supplied solely retrograde from the ductus arteriosus. Bidirectional systolic transverse aortic arch flow, with competing antegrade and retrograde flows, was labeled as partially antegrade flow. Exclusively antegrade arch flow was defined as systolic flow into the transverse arch solely from the LV. Diastolic retrograde flow around the arch was ignored, typically in the setting of aortic regurgitation (AR). LV systolic pressure was calculated by taking the larger value of either the aortic systolic jet gradient plus the gestational age or the mitral regurgitation jet gradient plus 5 mmHg, in keeping with published fetal pressure measurements.18 The aortic jet width index was calculated by dividing the antegrade aortic jet width by the aortic valve annulus diameter. Z-scores for fetal anatomic and hemodynamic measurements were calculated using internally derived normative data based on gestational age using previously published methods.19
Analyses were performed with Stata (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). All continuous variables are reported as median and interquartile range [25th percentile, 75th percentile]. For all analyses, a P-value of <0.05 was considered significant. The Wilcoxon signed-rank test was used to compare pre-FAV and post-FAV continuous and ordered categorical variables. Pre-FAV and post-FAV dichotomous variables were compared using McNemar’s test. Table 1 displays summary statistics based on all available pre-intervention data, whereas Table 2 displays values, changes, and summary statistics based on complete-case analysis (ie, data from subjects with both pre-FAV and post-FAV data available for each variable). Univariate logistic regression was used to analyze the association between hemodynamic changes and neonatal BiV circulation. All variables with a univariate P-value of <0.10 were candidate predictors for the multivariable model. The most significant variable was used initially; then, each additional variable was tested for significance in the multivariable model.
TABLE 1.
Pre‐FAV demographic, anatomic, and hemodynamic characteristics (n = 93, unless otherwise stated)
| Characteristic | Value |
|---|---|
| Gestational age (weeks) | 23.7 [22.6, 26] |
| Malea | 71 (78%) |
| Weightb (grams) | 632 [536, 917] |
| Echo‐to‐FAV (days) | 1 [1,2] |
| FAV‐to‐echo (days) | 1 [1,1] |
| LV end‐diastolic volume (mL) | 2.2 [1.5, 3.7] |
| LV end‐diastolic volume z‐score | 1.8 [0.6, 3.1] |
| Aortic annulus diameter (mm) | 2.9 [2.6, 3.2] |
| Aortic annulus diameter z‐score | −2.7 [−3.4, −2.1] |
| Retrograde systolic transverse aortic arch flow | 93 (100%) |
| FO flowc: Right‐to‐left | 0 (0%) |
| Bidirectional | 10 (11%) |
| Left‐to‐right | 78 (88%) |
| Mitral inflow pattern: Biphasic | 15 (16%) |
| Partial fusion | 11 (12%) |
| Monophasic | 67 (72%) |
| Mitral inflow time z‐score | −3.5 [−4.4, −2.0] |
| Mitral regurgitation (> mild) | 49 (53%) |
| LV ejection fraction (%) | 23 [18, 32] |
| LV systolic pressure (mmHg)d | 42.7 [32.9, 58.2] |
| Antegrade aortic jet width (mm) | 1.3 [1, 1.5] |
| Aortic regurgitation (< mild)d | 0 (0%) |
All values listed as “median, [25th percentile, 75th percentile]” or “number of subjects (% of the total).”
Abbreviations: FAV, fetal aortic valvuloplasty; LV, left ventricle; FO, patent foramen ovale.
n = 91.
Fetal weight estimated by Hadlock formulas.
n = 88.
n = 92.
TABLE 2.
Paired data sets of early fetal hemodynamic changes after FAV
| Variable | Pre‐FAV | Post‐FAV | Change | P‐Value |
|---|---|---|---|---|
| Any antegrade systolic transverse aortic arch flowa (n = 91) | 0 (0%) | 59 (65%) | 65% | <0.001 |
| Bidirectional FO flow (n = 81) | 9 (11%) | 22 (27%) | 16% | 0.002 |
| Change in FO flow (n = 81): | ||||
| Unchanged left‐to‐right flow | ---- | ---- | 59 (73%) | |
| New bidirectional flow | ---- | ---- | 15 (19%) | |
| Unchanged bidirectional flow | ---- | ---- | 7 (9%) | |
| Mitral inflow pattern (n = 82): | ||||
| Biphasic | 15 (18%) | 32 (39%) | 21% | 0.003 |
| Partially fused | 11 (13%) | 15 (18%) | ||
| Monophasic | 56 (68%) | 35 (43%) | ||
| Change in mitral inflow pattern (n = 82): | ||||
| Worsened to monophasic or partially fused, or unchanged monophasic | ---- | ---- | 37 (45%) | |
| Unchanged partially fused or biphasic | ---- | ---- | 12 (15%) | |
| Improved to partially fused or biphasic | ---- | ---- | 33 (40%) | |
| MV inflow time (msec) (n = 75) | 105 [81, 150] | 155 [133, 190] | 32 [8, 78] | <0.001 |
| MV inflow time z‐score (n = 75) | −3.4 [−4.4, −1.8] | −1.5 [−2.5, −0.2] | 1.2 [0.3, 3.0] | <0.001 |
| MV inflow index (msec/bpm) (n = 75) | 0.75 [0.58, 1.06] | 1.06 [0.89, 1.36] | 0.26 [0.03, 0.53] | <0.001 |
| Mitral regurgitation (> mild) (n = 93) | 49 (53%) | 37 (40%) | −13% | 0.017 |
| LV end‐diastolic volume (mL) (n = 93) | 2.23 [1.53, 3.73] | 2.22 [1.35, 3.21] | −0.06 [−0.19, 0.06] | 0.013 |
| LV end‐diastolic volume z‐score (n = 89) | 1.78 [0.72, 3.04] | 1.26 [0.25, 2.94] | −0.26 [−1.3, 0.97] | <0.001 |
| LV sphericity (n = 88) | 0.68 [0.59, 0.75] | 0.66 [0.57, 0.75] | −0.02 [−0.09, 0.05] | 0.16 |
| LV ejection fraction (%) (n = 87) | 23 [18, 32] | 28 [22, 41] | 6.9 [−0.4, 14.7] | <0.001 |
| Change in LV ejection fraction (≥ 5%) (n = 87) | 54 (62%) | |||
| LV systolic pressure (mm Hg) (n = 84) | 45.9 [35, 60.3] | 40.1 [32.1, 55] | −3.6 [−10.9, 3.2] | 0.001 |
| Aortic jet width (mm) (n = 83) | 1.3 [1, 1.5] | 2.8 [2.4, 3.1] | 1.5 [1.1, 1.9] | <0.001 |
| Aortic jet width index (jet width/annulus) (n = 82) | 0.44 [0.35, 0.52] | 0.88 [0.75, 0.97] | 0.41 [0.31, 0.54] | <0.001 |
| Aortic regurgitation (< mild) (n = 92) | 0 (0%) | 31 (34%) | 34% | <0.001 |
All values listed as “median, [25th percentile, 75th percentile]” or “number of subjects (% of the total).” All data displayed in pre‐FAV and post‐FAV columns are limited to data pairs that allow for complete‐case analysis. The Change column displays either the pre‐FAV percentage subtracted from the post‐FAV percentage for categorical variables, or the median of the paired differences for continuous variables.
Abbreviations: FAV, fetal aortic valvuloplasty; LV, left ventricle; MV, mitral valve; FO, patent foramen ovale.
Among the 59 subjects with antegrade systolic arch flow, 36 (61%) had partially antegrade flow and 23 (39%) had exclusively antegrade flow.
3 |. RESULTS
Between March 2000 and August 2015, 123 subjects underwent attempted FAV for fetal AS with evolving HLHS physiology (Figure 1). The majority of procedures (101/123, 78%) were technically successful. Of the technically successful FAV, there were 8 fetal demises (8%). The 93 technically successful interventions that resulted in live birth comprised the analysis cohort for this study. Forty-two of the 93 subjects (45%) were discharged from their neonatal hospitalization with BiV circulation.
FIGURE 1.
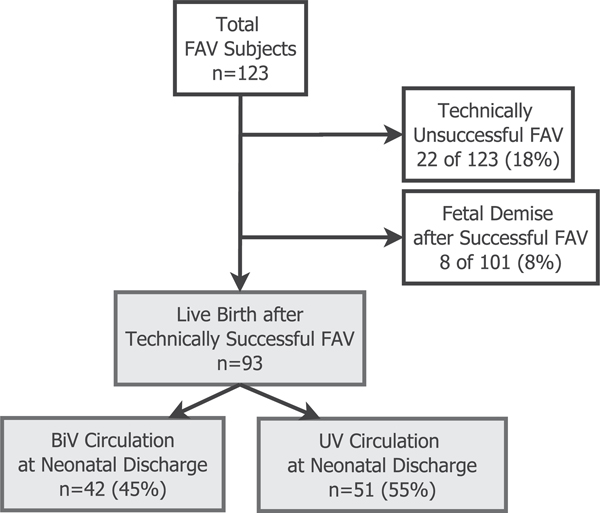
Derivation of analysis cohort. Among all subjects undergoing FAV for fetal aortic stenosis with high-risk features of evolving HLHS, those with technically unsuccessful procedures and/or fetal demise after FAV were excluded. The remaining 93 subjects were live-born after a technically successful FAV. Within this analysis cohort, 42 subjects (45%) achieved BiV circulation at neonatal discharge. Abbreviations: BiV (biventricular), FAV (fetal aortic valvuloplasty), HLHS (hypoplastic left heart syndrome), UV (univentricular)
The pre-FAV demographic, anatomic, and hemodynamic features of all 93 subjects in the analysis cohort are listed in Table 1. The median gestational age at FAV was 23.7 [22.6,26.0] weeks. Per inclusion criteria for performing FAV, all subjects (100%) had abnormal retrograde systolic transverse aortic arch flow prior to FAV. Abnormal exclusively left-to-right FO flow was present in 78 subjects (88%), while 10 subjects (11%) had abnormal bidirectional FO flow. No subjects had normal right-to-left flow at the FO. A majority of subjects had a monophasic MV inflow pattern (72%) and an abnormally short MV inflow time (median z-score −3.5 [−4.4, −2.0]), suggestive of diastolic dysfunction.
The post-FAV fetal echocardiogram was performed 1 to 2 days after FAV, with hemodynamic changes shown in Table 2. The percentage of subjects with any antegrade systolic transverse aortic arch flow increased from 0 subjects (0%) pre-FAV to 59 subjects (65%) post-FAV (P < 0.001). Of the 59 subjects with antegrade systolic arch flow, 36 (61%) had partially antegrade flow, and 23 (39%) had exclusively antegrade flow. The number of total subjects with bidirectional FO flow (versus left-to-right flow) increased from 11% to 27% (P = 0.002).
Diastolic and systolic function indices improved after FAV. The number of subjects with biphasic MV inflow Doppler pattern increased from 15 (18%) to 32 (39%) (P = 0.003). The median MV inflow time improved from 105 msec (z-score −3.4) to 155 msec (z-score −1.5) (P < 0.001). There was a modest increase in LV ejection fraction from a median of 23% to 28%, with a median change of 6.9% (P < 0.001). Within the BiV outcome group, the median LV ejection fraction change was 9.6% [5, 21.7] compared with a median change of 5% [−2.1, 11.1] in the UV group. Sixty-two percent of all subjects experienced a ≥5% increase in LV ejection fraction.
In univariate regression, several early post-FAV hemodynamic changes were predictive of BiV circulation at neonatal discharge (Table 3). Subjects who gained either partially antegrade or exclusively antegrade systolic transverse aortic arch flow were more likely to have BiV circulation at neonatal discharge compared with those with exclusively retrograde systolic arch flow. A majority of subjects in these 2 antegrade systolic transverse aortic arch flow categories achieved BiV circulation (58% and 74%, respectively) compared with 13% of the 32 subjects with retrograde systolic arch flow post-FAV. Subjects with a change in FO flow direction from left-to-right to bidirectional, ie, new bidirectional FO flow, were more likely to have BiV circulation than those who had unchanged FO flow either left-to-right or bidirectional. The percentage of subjects with bidirectional FO flow in the post-natal BiV group increased from 10% to 38%, while the UV group only increased from 12% to 19%. Improvements in MV inflow pattern and LV ejection fraction (≥5%) were both associated with BiV circulation. Notably, although significant changes in aortic jet width and degree of AR occurred after technically successful FAV, those changes were not predictive of BiV circulation.
TABLE 3.
Univariate models for BiV circulation as a function of early post‐FAV hemodynamic changes
| Variable | Observations | OR | 95% CI | P‐Value |
|---|---|---|---|---|
| Post‐FAV systolic transverse aortic arch flow: | <0.001 | |||
| Exclusively retrograde | 32/91 | Ref | ---- | ---- |
| Partially antegrade | 36/91 | 9.80 | 2.84–34.85 | <0.001 |
| Exclusively antegrade | 23/91 | 19.83 | 4.88–80.54 | <0.001 |
| New bidirectional FO flow | 15/81 | 5.14 | 1.47–17.97 | 0.010 |
| Change in mitral inflow pattern: | <0.001 | |||
| Unchanged monophasic or worsened | 37/82 | Ref | ---- | ---- |
| Unchanged fused or biphasic | 12/82 | 4.17 | 1.04–16.62 | 0.043 |
| Improved to fused or biphasic | 33/82 | 3.21 | 1.20–8.54 | 0.020 |
| Change in MV inflow time (msec) | 75 | 1.00 | 0.99–1.00 | 0.32 |
| Change in MV inflow time z‐score | 75 | 0.89 | 0.73–1.09 | 0.27 |
| Change in MV inflow index (msec/bpm) | 74 | 0.48 | 0.17–1.37 | 0.17 |
| Change in mitral regurgitation (> mild) | 93 | 1.33 | 0.55–3.20 | 0.53 |
| Change in LV end‐diastolic volume (mL) | 93 | 0.95 | 0.64–1.42 | 0.82 |
| Change in LV end‐diastolic volume z‐score | 93 | 1.01 | 0.77–1.32 | 0.95 |
| Change in LV sphericity | 88 | 3.49 | 0.20–60.66 | 0.39 |
| Change in LV ejection fraction (%) | 87 | 1.05 | 1.01–1.10 | 0.016 |
| Improved LV ejection fraction (≥ 5%) | 54/87 | 3.10 | 1.24–7.76 | 0.016 |
| Change in LV systolic pressure (mm Hg) | 84 | 0.97 | 0.93–1.01 | 0.11 |
| Change in aortic jet width (per 1‐mm increase) | 83 | 1.17 | 0.60–2.28 | 0.64 |
| Change in aortic jet width index (jet width/annulus) | 82 | 0.87 | 0.11–6.94 | 0.90 |
| Increasing AR from ≤ mild to ≥ moderate | 31/91 | 0.77 | 0.32–1.85 | 0.56 |
“Ref” signifies the reference group for the estimated odds ratios. “Change” is defined as post‐FAV minus pre‐FAV.
Abbreviations: AR, aortic regurgitation; FAV, fetal aortic valvuloplasty; LV, left ventricle; OR, odds ratio; FO, patent foramen ovale.
To generate a multivariable model (Table 4), the most predictive variable in univariate modeling, post-FAV systolic transverse aortic arch flow, was used to start. Additional variables with a univariate P-value of <0.10 were added individually to this model. Only new bidirectional FO flow (improved from left-to-right pre-FAV) came close to significance (OR 3.75, 0.89–15.80, P = 0.07) when placed in the model with post-FAV systolic transverse aortic arch flow (partially antegrade: OR 9.01, 2.49–32.57, P = 0.001 and exclusively antegrade: 14.63,2.98–72.15, P = 0.001). The additive effects of improvements in systolic aortic arch flow and FO flow direction can be seen in Figure 2. Among subjects with retrograde systolic arch flow and unchanged FO flow direction (either bidirectional or left-to-right) after FAV (n = 28), only 11% achieved BiV circulation. Among the 3 subjects with retrograde systolic arch flow and new bidirectional FO flow after FAV, only one achieved BiV circulation. In subjects who acquired any antegrade systolic arch flow after FAV, but did not experience a change in FO flow direction (n = 36), 56% achieved BiV circulation. In subjects who acquired both antegrade systolic arch flow and new bidirectional FO flow (n = 12), the percentage with BiV circulation was 83%.
TABLE 4.
Multivariate model for BiV circulation as a function of early post‐FAV hemodynamic changes (n = 79; 34 events (BiV circulation); c‐statistic = 0.791)
| Variable | OR | 95% CI | P‐Value |
|---|---|---|---|
| Systolic transverse aortic arch flow: | <0.001 | ||
| Exclusively retrograde | Ref | ---- | ---- |
| Partially antegrade | 9.01 | 2.49–32.57 | 0.001 |
| Exclusively antegrade | 14.63 | 2.98–72.15 | 0.001 |
| New bidirectional FO flow | 3.75 | 0.89–15.80 | 0.07 |
“Ref” signifies the reference group for the estimated odds ratios.
Abbreviations: FAV, fetal aortic valvuloplasty; OR, odds ratio; FO, patent foramen ovale.
FIGURE 2.
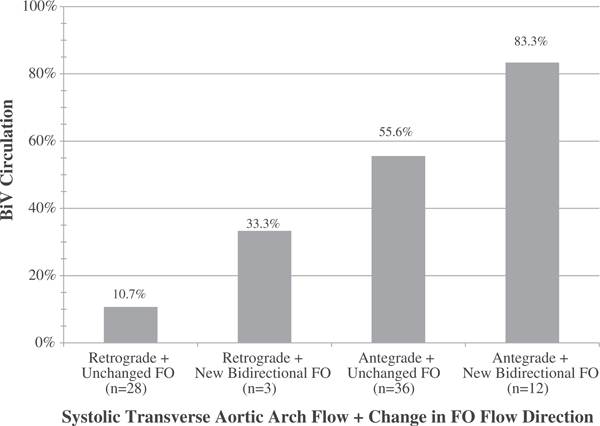
Post-FAV hemodynamic changes predict circulation status at neonatal discharge. After FAV for fetal aortic valve stenosis, there are several acute hemodynamic changes that signify increased forward flow through the left ventricle. Key changes are increasing degree of antegrade systolic flow across the transverse aortic arch and improving direction of flow across the FO, both of which play a role in predicting the chance of BiV circulation after birth. This bar chart depicts the percentage of subjects with BiV circulation at neonatal discharge (n = 34/79) by systolic transverse aortic arch flow direction after FAV (ie, exclusively retrograde versus any amount of antegrade systolic flow) and by the presence of new post-FAV bidirectional FO flow versus unchanged flow direction (either left-to-right or bidirectional). Abbreviations: BiV (biventricular), FAV (fetal aortic valvuloplasty), FO (patent foramen ovale)
Among 54 subjects with antegrade systolic transverse aortic arch flow in the immediate post-FAV period, 39 (72%) maintained antegrade arch flow in late gestation (median gestational age 36 weeks). Thirty of those 39 subjects (77%) achieved BiV circulation. Alternatively, among the 15 subjects who had antegrade arch flow immediately after FAV but developed retrograde flow in late gestation (28% of the 54 subjects), only 5 (33%) achieved BiV circulation. Among 29 subjects with retrograde flow immediately after FAV, 23 (79%) continued to have retrograde flow in late gestation and only one (4% of 23) achieved BiV circulation at neonatal discharge.
4 |. DISCUSSION
In this study, we demonstrate that FAV leads to acute changes in the fetal heart, including alterations in left heart hemodynamics, improved diastolic function, and modest increase in LV systolic function. Improvements in left heart hemodynamics, namely gaining antegrade systolic transverse aortic arch flow and bidirectional FO flow, are predictive of BiV circulation at neonatal discharge.
Our results provide insight into the physiologic effects of FAV and add value to previously published descriptions of pre-FAV factors that predict BiV circulation.11 The goal of FAV is to improve antegrade aortic flow, reduce the pressure load on the LV, and increase preload, thereby preventing evolution of HLHS. Unfortunately, Doppler-based techniques for estimating aortic flow are not valid in the setting of flow acceleration, and it is not feasible to directly measure aortic flow before and after FAV. In this study, we present indirect evidence of increased antegrade flow through the left heart and across the aortic valve acutely after FAV. Specifically, almost 2/3rds of subjects develop new antegrade aortic arch flow after technically successful FAV, which suggests a significant increase in LV output in most subjects. Additionally, a smaller percentage of subjects had evidence of less left-to-right FO flow, suggestive of higher LV preload after FAV. This increased flow may allow the LV to recover and remodel to support BiV circulation after birth. Technically successful FAV has previously been shown to prevent growth arrest of left heart structures.14 Presumably, this effect is at least partially related to the hypothesis that more flow through the left heart promotes ongoing growth of the left heart structures through gestation. The increased rates of BiV circulation seen in our cohort among subjects with persistent antegrade flow in the transverse aortic arch at late-gestational follow-up supports this hypothesis.
In addition to improved antegrade left heart flow, we demonstrate evidence of improved left ventricular diastolic function, similar to the diastolic recovery and remodeling seen in infants with critical AS after post-natal balloon aortic valvuloplasty.20 This finding is in agreement with recent work that demonstrated marked abnormalities in indices of diastolic function in fetal AS and improvement in those variables 2 to 3 days after FAV.21 Similarly, the modest improvement in LV systolic function found in this study reinforces prior descriptions of improved LV strain profiles after FAV.22 Acute reduction in LV afterload is the most likely explanation for the improvement in diastolic and systolic function. The other contributors to LV dysfunction, which include endocardial and myocardial fibrosis, pathological LV remodeling, and LV hypertrophy, cannot change as quickly.
Our findings will allow improvement in counseling of families after FAV. Prior to the decision to pursue FAV, families are extensively counseled on the risks and possible benefits of the procedure. Patient selection for FAV has 2 primary criteria: (1) the fetus is likely to develop HLHS without FAV and (2) the left ventricle has a chance to recover after successful FAV to support systemic circulation. Retrograde aortic arch flow and FO flow reversal are predictive of development of HLHS in most cases.7 Pre-procedural factors associated with the ability of the LV to recover to support systemic circulation after FAV include LV systolic pressure, diastolic function, long-axis z-score, and ascending aorta dimension all factor into the decision to pursue FAV.11 After the procedure is completed, the post-FAV echocardiogram provides additive information regarding hemodynamic changes in the fetal heart to help the families understand whether BiV circulation is more or less likely. This information may influence a family’s prenatal and postnatal decision-making. Future studies are needed to elucidate how the hemodynamic changes associated with relief of LV outflow tract obstruction allow for LV remodeling and recovery later in gestation. Improved methods to quantify fetal fibrosis and diastolic dysfunction may help improve patient selection for FAV using markers of recoverable fetal myocardium.
Limitations of this study include a retrospective design with several inherent associated limitations. This study reports subtle fetal echocardiographic measurements with known variability. The multivariable analysis was based on interrelated hemodynamic measures of LV function, and with a different model-building approach, different independent predictors might be identified. Additionally, clinical outcome was determined in part by post-natal management strategies that varied by where the patient delivered. As is well described elsewhere, decisions of management of borderline left hearts at birth are complicated and out of the scope of this paper. Achievement of BiV circulation often involves multiple surgeries and suboptimal hemodynamic states, such as LV diastolic dysfunction and pulmonary hypertension. The long-term results of BiV circulation with suboptimal hemodynamics compared with standard UV palliative staging are not known. This study evaluated only short-term post-natal outcomes at the time of neonatal discharge from the hospital.
5 |. CONCLUSION
In summary, FAV produces significant early improvements in left ventricular hemodynamics that are predictive of BiV circulation at neonatal discharge. Further evaluation of this cohort will improve our understanding of the late-gestational hemodynamic effects of FAV and may improve our ability to prevent in-utero progression to HLHS.
What’s already known about this topic?
Mid-gestational aortic stenosis can progress to hypoplastic left heart syndrome, a condition that is associated with high morbidity and mortality.
Fetal aortic valvuloplasty can promote left ventricular growth and function and may prevent progression to hypoplastic left heart syndrome.
What does this study add?
This study describes for the first time the hemodynamic changes that occur in the first 24 to 48 hours after fetal aortic valvuloplasty.
After fetal aortic valvuloplasty, there are significant improvements in fetal hemodynamics, including antegrade systolic transverse aortic arch flow, the direction of FO flow, and left ventricular ejection fraction.
We demonstrate that gaining antegrade systolic transverse aortic arch flow increases the odds of post-natal biventricular circulation.
Acknowledgments
Funding information
Benderson Family Fund; Nomellini Family
Fund; Benderson and Nomellini Family Funds
Footnotes
CONFLICTS OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Siffel C, Riehle-Colarusso T, Oster ME, Correa A. Survival of children with hypoplastic left heart syndrome. Pediatrics. October 1 2015;136(4): e864–e870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan LD, Sharland G, Tynan MJ. The natural history of the hypoplastic left heart syndrome. Int J Cardiol. 1989;25(3):341–343. [DOI] [PubMed] [Google Scholar]
- 3.Danford DA, Cronican P. Hypoplastic left heart syndrome: progression of left ventricular dilation and dysfunction to left ventricular hypoplasia in utero. Am Heart J. June 1992;123(6):1712–1713. [DOI] [PubMed] [Google Scholar]
- 4.Hornberger LK, Sanders SP, Rein AJ, Spevak PJ, Parness IA, Colan SD. Left heart obstructive lesions and left ventricular growth in the midtrimester fetus. A longitudinal study. Circulation. 1995;92: 1531–1538. [DOI] [PubMed] [Google Scholar]
- 5.Simpson JM, Sharland GK. Natural history and outcome of aortic stenosis diagnosed prenatally. Heart. 1997. March;77(3):205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCaffrey FM, Sherman FS. Prenatal diagnosis of severe aortic stenosis. Pediatr Cardiol. 1997;18(4):276–281. [DOI] [PubMed] [Google Scholar]
- 7.Mäkikallio K, McElhinney DB, Levine JC, et al. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation. March 21 2006;113(11): 1401–1405. [DOI] [PubMed] [Google Scholar]
- 8.Gardiner HM, Kovacevic A, Tulzer G, et al. Natural history of 107 cases of fetal aortic stenosis from a European multicenter retrospective study. Ultrasound Obstet Gynecol. September 2016;48(3):373–381. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell D, Allan L, Tynan MJ. Balloon dilatation of the aortic valve in the fetus: a report of two cases. Br Heart J. May 1991;65(5):256–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation. October 12 2004;110(15):2125–2131. [DOI] [PubMed] [Google Scholar]
- 11.Friedman KG, Sleeper LA, Freud LR, et al. Improved technical success, postnatal outcomes and refined predictors of outcome for fetal aortic valvuloplasty. Ultrasound Obstet Gynecol. May 22 2017. [DOI] [PubMed] [Google Scholar]
- 12.Moon-Grady AJ, Morris SA, Belfort M, et al. International fetal cardiac intervention registry. J Am Coll Cardiol. July 28 2015;66(4):388–399. [DOI] [PubMed] [Google Scholar]
- 13.Selamet Tierney ES, Wald RM, McElhinney DB, et al. Changes in left heart hemodynamics after technically successful in-utero aortic valvuloplasty. Ultrasound Obstet Gynecol. Oct 3 2007;30(5):715–720. [DOI] [PubMed] [Google Scholar]
- 14.McElhinney DB, Marshall AC, Wilkins-Haug LE, et al. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. October 13 2009;120(15):1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freud LR, McElhinney DB, Marshall AC, et al. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: postnatal outcomes of the first 100 patients. Circulation. August 18 2014;130(8):638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall AC, Tworetzky W, Bergersen L, et al. Aortic valvuloplasty in the fetus: technical characteristics of successful balloon dilation. J Pediatr. Oct 2005;147(4):535–539. [DOI] [PubMed] [Google Scholar]
- 17.Wilkins-Haug LE, Tworetzky W, Benson CB, Marshall AC, Jennings RW, Lock JE. Factors affecting technical success of fetal aortic valve dilation. Ultrasound Obstet Gynecol. July 2006;28(1):47–52. [DOI] [PubMed] [Google Scholar]
- 18.Johnson P, Maxwell DJ, Tynan MJ, Allan LD. Intracardiac pressures in the human fetus. Heart. Jul 2000;84(1):59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. August 1 2005;99(2):445–457. [DOI] [PubMed] [Google Scholar]
- 20.Friedman KG, McElhinney DB, Colan SD, et al. Left ventricular remodeling and improvement in diastolic function after balloon aortic valvuloplasty for congenital aortic stenosis. Circ Cardiovasc Interv. August 1 2012;5(4):549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wohlmuth C, Wertaschnigg D, Wieser I, Arzt W, Tulzer G. Tissue Doppler imaging in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome before and after fetal aortic valvuloplasty. Ultrasound Obstet Gynecol. May 2016;47(5):608–615. [DOI] [PubMed] [Google Scholar]
- 22.Ishii T, McElhinney DB, Harrild DM, et al. Ventricular strain in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome before and after prenatal aortic Vvalvuloplasty. Fetal Diagn Ther. November 19 2013;35(1):18–26. [DOI] [PubMed] [Google Scholar]


