Abstract
Objective
Long noncoding RNA small nucleolar RNA host gene 1 (SNHG1) has been reported to be aberrantly expressed and plays an important role in human cancers, including esophageal squamous cell cancer. However, the regulatory mechanism underlying SNHG1 in the progression of esophageal squamous cell cancer is poorly defined.
Materials and Methods
Fifty-three esophageal squamous cell cancer patients were recruited and overall survival was analyzed. EC9706 and KYSE150 cells were cultured for study in vitro. The expression levels of SNHG1, microRNA (miR)-204 and homeobox c8 (HOXC8) were detected by quantitative real-time polymerase chain reaction and Western blot. Cell cycle distribution, apoptosis, migration and invasion were determined by flow cytometry and transwell assays, respectively. The target interaction among SNHG1, miR-204 and HOXC8 was validated by luciferase reporter assay and RNA immunoprecipitation. Xenograft model was established to investigate the role of SNHG1 in vivo.
Results
High expression of SNHG1 was exhibited in esophageal squamous cell cancer and indicated poor outcomes of patients. SNHG1 silence led to cell cycle arrest at G0-G1 phase, inhibition of migration and invasion and increase of apoptosis. miR-204 was validated to sponge by SNHG1 and target HOXC8 in esophageal squamous cell cancer cells. miR-204 knockdown or HOXC8 restoration reversed the inhibitive role of SNHG1 silence in the progression of esophageal squamous cell cancer cells. Furthermore, inhibiting SNHG1 decreased xenograft tumor growth by regulating miR-204 and HOXC8.
Conclusion
SNHG1 knockdown suppresses migration and invasion but induces apoptosis of esophageal squamous cell cancer cells by increasing miR-204 and decreasing HOXC8.
Keywords: esophageal squamous cell cancer, SNHG1, miR-204, HOXC8
Introduction
Esophageal cancer with the sixth cancer deaths consists of esophageal squamous cell cancer and esophageal adenocarcinoma, and esophageal squamous cell cancer predominates worldwide.1 Therefore, this study focuses on esophageal squamous cell cancer. Recently, great advances have been gained for the pathogenesis, diagnosis and treatment of esophageal squamous cell cancer.2 However, the survival of patients remains poor.3 Hence, much hope is placed in understanding the pathogenesis and exploring a novel strategy for the treatment of esophageal squamous cell cancer.
Noncoding RNAs, including long noncoding RNAs (lncRNAs) with more than 200 nucleotides and microRNAs (miRNAs), have been reported to be aberrantly expressed and associated with cancer progression in esophageal squamous cell cancer.4 LncRNAs are suggested to be involved in the development and therapeutics of esophageal squamous cell cancer.5 Moreover, lncRNAs could act as oncogenes or tumor suppressors in esophageal squamous cell cancer through regulating cell processes, such as proliferation, migration, invasion and apoptosis by functioning as competing endogenous RNAs (ceRNAs). For example, Sun et al6 reveal that LINC00657 promotes cell proliferation, migration and radioresistance in esophageal squamous cell cancer by regulating miR-615-3p and JunB. Chu et al7 report that lncRNA motor neuron and pancreas homeobox 1-antisense RNA1 (MNX1-AS1) regulates cell proliferation, migration, invasion, cell cycle and apoptosis by miR-34a/Sirtuin 1 (SIRT1) axis in esophageal squamous cell cancer. Furthermore, phosphoglucomutase 5 antisense RNA 1 (PGM5-AS1) as a lncRNA suppresses cell proliferation, migration and invasion by regulating miR-466/phosphatase and tensin homolog deleted on chromosome 10 (PTEN) axis in esophageal squamous cell cancer.8 Previous study demonstrates that lncRNA small nucleolar RNA host gene 1 (SNHG1) is highly expressed and associated with poor outcomes of patients in multiple cancers.9 What’s more, accruing evidences suggest SNHG1 as oncogenic lncRNA to promote cell proliferation, migration and invasion in gastric cancer and pancreatic cancer.10,11 More importantly, recent works indicate that abnormally expressed SNHG1 is involved in the regulation of esophageal squamous cell cancer progression.12,13 However, the mechanism underlying SNHG1 participating in esophageal squamous cell cancer development remains largely unclear.
Intriguingly, starBase (http://starbase.sysu.edu.cn/) predicts that SNHG1 and homeobox c8 (HOXC8) have and share the potential complementary sequences of miR-204, which stimulates us to assume the ceRNA network of SNHG1/miR-204/HOXC8. In the present study, we measured the expression of SNHG1 in esophageal squamous cell cancer tissues and cells and investigated the effect of SNHG1 on progression of esophageal squamous cell cancer by detecting migration, invasion, cell cycle distribution and apoptosis. Moreover, we explored the regulatory network of SNHG1/miR-204/HOXC8.
Materials and Methods
The Cancer Genome Atlas (TCGA) Assay
TCGA assay was conducted via the starBase tool. The expression levels of SNHG1, miR-204 and HOXC8 in esophageal cancer were analyzed via TCGA. The correlation among SNHG1, miR-204 and HOXC8 in esophageal cancer was also analyzed via TCGA.
Patient Tissues and Cell Culture
We recruited 53 patients with esophageal squamous cell cancer from the Tumor Hospital Affiliated to Zhengzhou University and all patients have signed the informed consent. The esophageal squamous cell cancer tissues and corresponding adjacent normal samples were collected during the surgery and then stored at −80°C. This research was approved by the Ethics Committee of the Tumor Hospital Affiliated to Zhengzhou University.
The human esophageal squamous cell cancer cell lines (EC9706, KYSE450, KYSE150 and Eca109) and normal esophageal epithelium cell Het-1A were purchased from BeNa Culture Collection (Beijing, China) and verified by the company. All cells were cultured in DMEM (Sigma, St. Louis, MO, USA) with 10% fetal bovine serum (FBS) and antibiotics at 37°C with 5% CO2.
Cell Transfection
The overexpression vectors of SNHG1 and HOXC8 were generated by inserting their full-length sequences into pcDNA3.1 (Thermo Fisher Scientific, Wilmington, DE, USA), with pcDNA3.1 empty vector (pcDNA) as a corresponding control. siRNA against SNHG1 (si-SNHG1-1, 5ʹ-CUUAAAGUGUUAGCAGACATT-3ʹ; si-SNHG1-2, 5ʹ-AUUCCAUUUUUUAUACACCUU-3ʹ; si-SNHG1-3, 5ʹ- UGUAUCUAAAAAACAAAAGGG-3ʹ;), si-HOXC8 (5ʹ-AGGAUUAAAGAGAAACUCCUU-3ʹ), siRNA negative control (si-NC) (5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ), miR-204 mimic (miR-204) (5ʹ-UUCCCUUUGUCAUCCUAUGCCU-3ʹ), miRNA negative control (miR-NC) (5ʹ-CGAUCGCAUCAGCAUCGAUUGC-3ʹ), miR-204 inhibitor (anti-miR-204) (5ʹ-AGGCAUAGGAUGACAAAGGAA-3ʹ) and inhibitor negative control (anti-miR-NC) (5ʹ-CUAACGCAUGCACAGUCGUACG-3ʹ) were generated by GenePharm (Shanghai, China). The transfection of oligonucleotides with 20 nM concentration in EC9706 and KYSE150 cells was received by using Lipofectamine 2000 (Thermo Fisher Scientific). Blank is the non-transfected group. After 24 h, transfected EC9706 and KYSE150 cells were collected and used for further experiments.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
After the extraction using Trizol reagent (Thermo Fisher Scientific), 1 μg RNA was reversely transcribed to cDNA by using the special RT-PCR Kit (Thermo Fisher Scientific) following the manufacturer’s instructions. The qRT-PCR was performed on ABI 7500 Real-time PCR system (Bio-Rad, Hercules, CA, USA) using the diluted cDNA along with SYBR Green mix (Thermo Fisher Scientific) and primers generated by Sangon Biotech (Shanghai, China): SNHG1 (Forward, 5ʹ-TAACCTGCTTGGCTCAAAGGG-3ʹ; Reverse, 5ʹ-CAGCCTGGAGTGAACACAGA-3ʹ); HOXC8 (Forward, 5ʹ-CACGTCCAAGACTTCTTCCACCACGGC-3ʹ; Reverse, 5ʹ-CACTTCATCCTTCGATTCTGGAACC-3ʹ); miR-204 (Forward, 5ʹ-CTGTCACTCGAGCTGCTGGAATG-3ʹ; Reverse, 5ʹ-ACCGTGTCGTGGAGTCGGCAATT-3ʹ). GAPDH (Forward, 5ʹ-CATGAGAAGTATGACAACAGCCT-3ʹ; Reverse, 5ʹ-AGTCCTTCCACGATACCAAAGT-3ʹ) and U6 (Forward, 5ʹ-CTCGCTTCGGCAGCACA-3ʹ; Reverse, 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ) were regarded as internal controls, respectively. The relative expression levels of SNHG1, miR-204 and HOXC8 were analyzed by 2−ΔΔCt method.14
Flow Cytometry
Flow cytometry was used for analysis of cell cycle distribution and apoptosis. For measurement of cell cycle distribution, EC9706 and KYSE150 cells were cultured for 48 h and then fixed with 75% ethanol (Sigma), followed by incubated with RNase A and PI solution for 30 min at 37°C. For apoptosis analysis, after culture for 48 h, the cells were resuspended in binding buffer and then stained with Annexin V-FITC and PI (Beyotime, Shanghai, China) in the dark. The distribution of cells at different phases and apoptosis were analyzed by a flow cytometer. The apoptotic rate of cells was expressed as the percentage of cells stained with Annexin V-FITC positive and PI negative or positive.
Transwell Assay
The motility of cells was investigated by migration an invasion using transwell assay. For migration assay, EC9706 and KYSE150 cells (1 × 104/well) were suspended in DMEM without serum and added to the upper chambers of the transwell. For invasion assay, 1 × 104 cells were seeded into Matrigel-coated chambers. The lower chambers were added with 500 μL DMEM with 10% FBS. After the incubation for 24 h, the cells on lower chambers were stained with 0.1% crystal violet. The number of migrated or invasive cells was counted under a 200× magnification microscope with three random fields.
Western Blot
Equal amounts (20 μg) of protein lysates from cells or tumors were subjected to SDS-PAGE and electro-transfer with PVDF membranes (Millipore, Billerica, MA, USA). The membranes were blocked with 5% non-fat milk for 2 h, incubated with specific primary antibodies overnight and secondary antibody (ab97051, 1:20,000 dilution) for 2 h. The antibody against HOXC8 (ab86236, 1:1000 dilution) was purchased from Abcam (Cambridge, MA, USA), with β-actin (ab227387, 1:10,000 dilution) as a loading control. After the incubation of enhanced chemiluminescence solution (Beyotime), the targeted protein signaling was normalized to β-actin in each sample.
Luciferase Reporter Assay and RNA Immunoprecipitation (RIP)
The sequences of SNHG1 or HOXC8 3ʹUTR containing miR-204 binding sites were inserted into a pmirGLO luciferase reporter vector (Promega, Madison, WI, USA) to generate the wild-type luciferase reporter vectors (SNHG1-WT and HOXC8-WT). Meanwhile, the corresponding mutants (SNHG1-MUT and HOXC8-MUT) were generated via mutating the binding sites. Co-transfection of the constructed luciferase reporter vectors together with miR-204 or miR-NC was performed into EC9706 and KYSE150 cells. The luciferase reporter system (Promega) was used for luciferase activity analysis at 24 h post-transfection.
EC9706 and KYSE150 cells transfected with miR-204 or miR-NC were lysed for RIP assay with a Magna RNA immunoprecipitation kit (Millipore) following the manufacturer’s protocols. The enrichment levels of SNHG1 and HOXC8 in Ago2 or IgG RIP complex were determined by qRT-PCR.
Murine Xenograft Model
The animal experiments were performed in accordance with the guide for care and use of laboratory animals and this study was approved by the Ethics Committee of the Tumor Hospital Affiliated to Zhengzhou University. The BALB/c nude mice (4-week-old, male) purchased from Shanghai Animal Laboratory Center (Shanghai, China) were acclimatized for 1 week and then randomly divided into three groups (n=3) for xenograft model. EC9706 cells (2 × 106 cells) stably transfected with recombinant lentivirus expressing shRNA for SNHG1 (sh-SNHG1), control (sh-NC) or empty lentivirus were used for subcutaneously infection of nude mice. The tumor volume was monitored every week and calculated with a formula: volume (mm3) = length × width2 × 0.5. At 5 weeks after the injection, mice were killed and tumors were collected for weight and molecular analyses.
Statistical Analysis
The data from three independent experiments were processed by GraphPad Prism 7.0 and presented as mean ± standard deviation. Overall survival was generated by Kaplan–the Meier method and analyzed by the Log rank test. The comparison between two or more groups was conducted by student’s t-test or ANOVA with Tukey’s post hoc test. The difference was statistically significant when P<0.05 (*P<0.05, **P<0.01, ***P<0.001).
Results
The Expression of SNHG1 Is Enhanced in Esophageal Squamous Cell Cancer
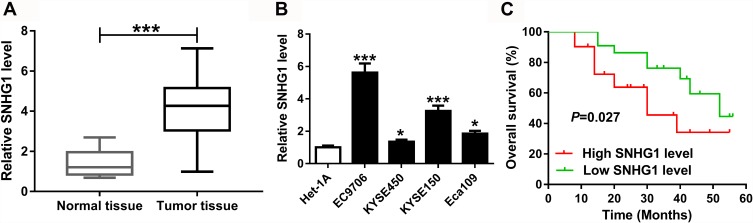
The data of TCGA showed a high expression of SNHG1 in esophageal cancer (Supplementary Figure 1A). To detect the abundance of SNHG1 in esophageal squamous cell cancer, 53 patients were recruited. As shown in Figure 1A, the expression of SNHG1 in esophageal squamous cell cancer tissues (n=53) was increased fourfold compared with that in corresponding normal samples. Meanwhile, when compared to Het-1A cells, esophageal squamous cell cancer cells, especially EC9706 and KYSE150 cells, exhibited higher level of SNHG1 (Figure 1B). Moreover, the patients were divided into high (n=31) or low (n=22) SNHG1 level group according to the mean level of SNHG1 in cancer tissues. The patients with high SNHG1 level displayed poor overall survival compared with those in low expression group (P=0.027) (Figure 1C).
Figure 1.
The expression of SNHG1 in esophageal squamous cell cancer. (A) qRT-PCR assay was performed to detect SNHG1 expression in esophageal squamous cell cancer tissues compared with normal samples (n=53). (B) qRT-PCR assay was carried out to measure SNHG1 level in esophageal squamous cell cancer cells compared with normal esophageal epithelium cell. (C) The overall survival of patients was assessed according to the mean level of SNHG1 in esophageal squamous cell cancer tissues. *P<0.05, ***P<0.001.
SNHG1 Knockdown Inhibits Progression of Esophageal Squamous Cell Cancer Cells
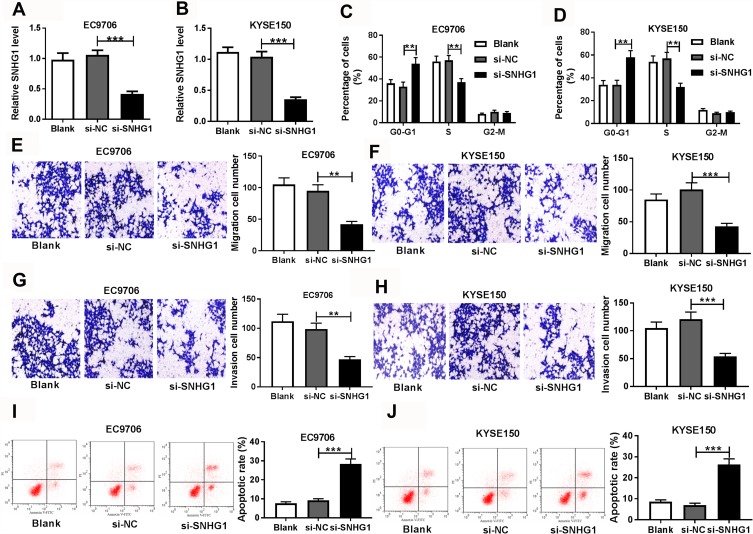
To investigate the role of SNHG1 in esophageal squamous cell cancer development, EC9706 and KYSE150 cells with relatively higher level of SNHG1 (5.6-fold and 3.25-fold, respectively) were chosen for in vitro study. Overexpression of SNHG1 had an oncogenic role in esophageal squamous cell cancer by promoting migration and invasion and inhibiting apoptosis (Supplementary Figure 2). Furthermore, we constructed three siRNAs for SNHG1, and the siRNA with the highest efficacy was chosen for further experiments (Supplementary Figure 3). As shown in Figure 2A and B, the abundance of SNHG1 in EC9706 and KYSE150 cells was effectively decreased 60–65% by transfection of si-SNHG1 in comparison to that in the si-NC group and Blank group. Moreover, knockdown of SNHG1 induced cell cycle arrest at G0-G1 phase, revealed by increasing percentage of cells at G0-G1 phase and reducing distribution at S phase (Figure 2C and D). In addition, the abilities of migration and invasion in EC9706 and KYSE150 cells were significantly decreased by SNHG1 silence (Figure 2E–H). Besides, interference of SNHG1 led to great apoptosis production in EC9706 and KYSE150 cells (Figure 2I and J).
Figure 2.
The effect of SNHG1 on cell cycle distribution, migration, invasion and apoptosis in esophageal squamous cell cancer cells. (A and B) The level of SNHG1 in EC9706 and KYSE150 cells was detected by qRT-PCR after transfection of si-SNHG1 or si-NC. Cell cycle distribution (C and D), migration (E and F), invasion (G and H) and apoptosis (I and J) of EC9706 and KYSE150 cells transfected with si-SNHG1 or si-NC were determined by flow cytometry and trans-well assays. Blank was non-transfected group. **P<0.01, ***P<0.001.
SNHG1 Is a Decoy of miR-204
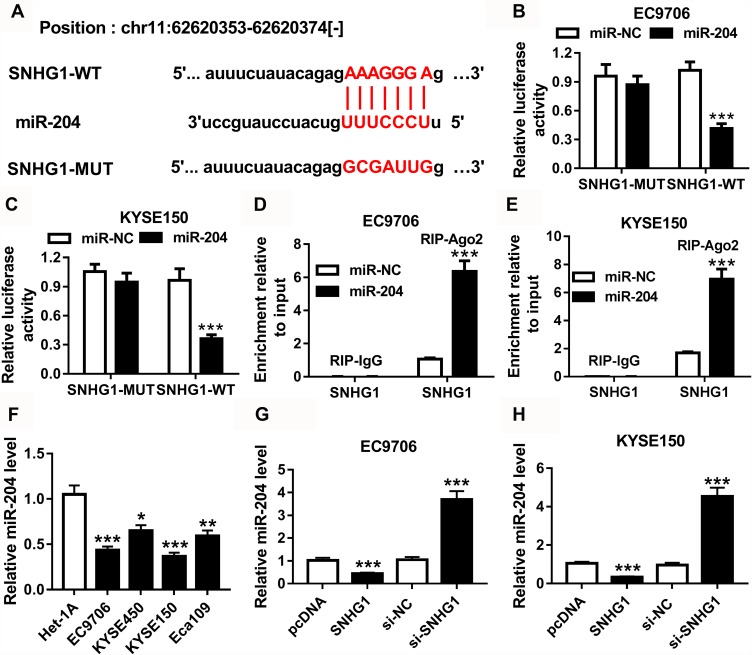
As shown in Figure 3A, starBase database predicted the complementary sequences of SNHG1 and miR-204 at position: chr11: 62620353-62620374. The wild-type and mutant luciferase reporter vectors (SNHG1-WT and SNHG1-MUT) were constructed and transfected into EC9706 and KYSE150 cells. As revealed in Figure 3B and C, overexpression of miR-204 led to more than 60% reduction of luciferase activity in the SNHG1-WT group, while it did not affect the activity in SNHG1-MUT group. Moreover, the addition of miR-204 led to a markedly increased level of SNHG1 enriched by Ago2 RIP in EC9706 and KYSE150 cells (Figure 3D and E). The data of TCGA displayed that miR-204 expression was decreased in esophageal cancer (Supplementary Figure 1B). Additionally, the expression of miR-204 was notably reduced in esophageal squamous cell cancer cells compared with that in Het-1A cells (Figure 3F). Besides, miR-204 level in EC9706 and KYSE150 cells was evidently decreased by overexpressing SNHG1 and increased via silencing SNHG1 (Figure 3G and H). Furthermore, SNHG1 expression was also negatively regulated by miR-204 (Supplementary Figure 4).
Figure 3.
The target association between SNHG1 and miR-204. (A) starBase database predicted the binding sites of SNHG1 and miR-204. (B and C) Luciferase activity was determined in EC9706 and KYSE150 cells co-transfected with SNHG1-WT or SNHG1-MUT and miR-204 or miR-NC. (D and E) qRT-PCR assay was performed to detect SNHG1 abundance in EC9706 and KYSE150 cells transfected with miR-204 or miR-NC after RIP assay. (F) The expression of miR-204 was detected in esophageal squamous cell cancer cells compared with normal esophageal epithelium cell. (G and H) The level of miR-204 was measured in EC9706 and KYSE150 cells transfected with pcDNA, SNHG1, si-NC or si-SNHG1. *P<0.05, **P<0.01, ***P<0.001.
Knockdown of miR-204 Reverses the Effect of SNHG1 Silence on Progression of Esophageal Squamous Cell Cancer Cells
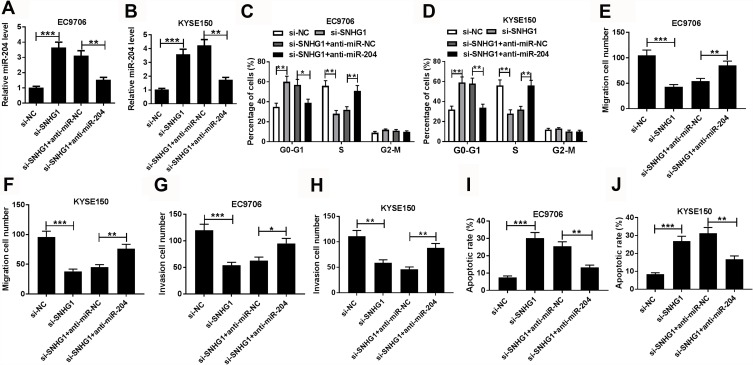
In order to explore whether miR-204 was associated with SNHG1-mediated progression of esophageal squamous cell cancer, EC9706 and KYSE150 cells were transfected with si-NC, si-SNHG1, si-SNHG1 and anti-miR-NC or anti-miR-204. After the transfection, the abundance of miR-204 in EC9706 and KYSE150 cells increased by SNHG1 knockdown was obviously decreased by the transfection of anti-miR-204 (Figure 4A and B). Furthermore, inhibition of miR-204 significantly attenuated cell cycle arrest at G0-G1 phase induced by SNHG1 silence (Figure 4C and D). In addition, the suppressive effect of SNHG1 knockdown on migration and invasion of EC9706 and KYSE150 cells was weakened via miR-204 deficiency (Figure 4E–H). What is more, the apoptosis production induced by silencing SNHG1 was specially mitigated by down-regulation of miR-204 in the two cells (Figure 4I and J). Besides, miR-204 overexpression also reversed the promoting role of SNHG1 in esophageal squamous cell cancer development in vitro (Supplementary Figure 2).
Figure 4.
The recuse effect of miR-204 on SNHG1-mediated progression of esophageal squamous cell cancer cells. (A and B) The level of miR-204 in EC9706 and KYSE150 cells was detected by qRT-PCR after transfection of si-NC, si-SNHG1, si-SNHG1 and anti-miR-NC or anti-miR-204. Cell cycle distribution (C and D), migration (E and F), invasion (G and H) and apoptosis (I and J) of EC9706 and KYSE150 cells transfected with si-NC, si-SNHG1, si-SNHG1 and anti-miR-NC or anti-miR-204 were analyzed by flow cytometry and trans-well assays. *P<0.05, **P<0.01, ***P<0.001.
HOXC8 Is a Target of miR-204
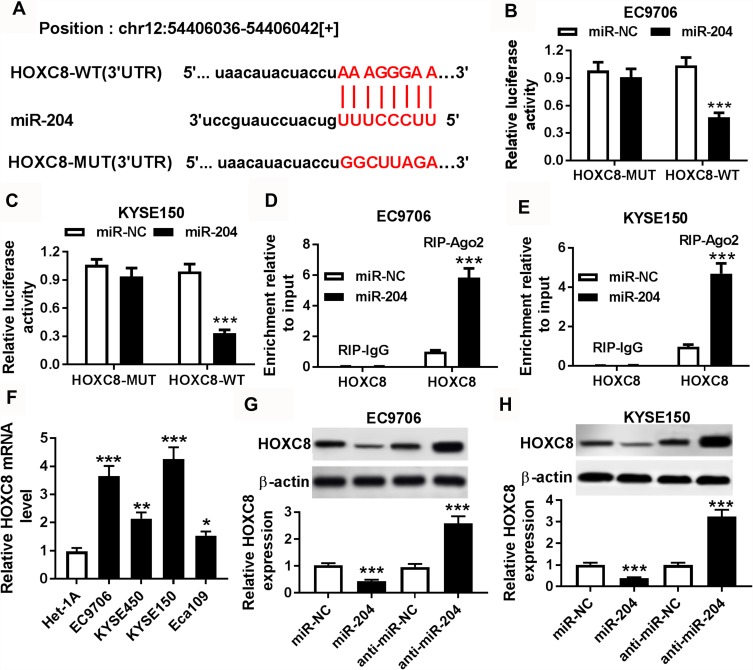
The target of miR-204 was searched by starBase database, which provided the putative binding sites of miR-204 and HOXC8 at position: chr12: 54406036-54406042 (Figure 5A). To validate this association, luciferase reporter assay and RIP assay were performed in EC9706 and KYSE150 cells. As displayed in Figure 5B and C, the luciferase activity was greatly decreased via transfection of miR-204 in the HOXC8-WT group, whereas it was not changed when the seed sites were mutated in HOXC8-MUT group. Meanwhile, the level of HOXC8 enriched by Ago2 RIP but not IgG RIP was significantly increased by miR-204 overexpression (Figure 5D and E). TCGA database showed that HOXC8 level was elevated in esophageal cancer (Supplementary Figure 1C). Furthermore, the mRNA level of HOXC8 in esophageal squamous cell cancer cells was notably higher than that in Het-1A cells (Figure 5F). In addition, the abundance of HOXC8 protein in EC9706 and KYSE150 cells was conspicuously reduced via miR-204 addition and elevated by miR-204 exhaustion (Figure 5G and H). Besides, there was a positive correlation between SNHG1 and HOXC8, and a negative correlation between miR-204 and SNHG1 or HOXC8 in esophageal squamous cell cancer, although the correlation between miR-204 and SNHG1 or HOXC8 was insignificant in esophageal cancer by TCGA (Supplementary Figure 1D–I). We hypothesized that the inconsistent results might be explained by demographic difference or cancer subtype difference. Moreover, HOXC8 expression was promoted by SNHG1 overexpression and weakened by miR-204 (Supplementary Figure 2).
Figure 5.
The target association between miR-204 and HOXC8. (A) The seed sites of miR-204 and HOXC8 were predicted by starBase database. Luciferase reporter assay (B and C) and RIP assay (D and E) were conducted to confirm the bind of miR-204 and HOXC8 in EC9706 and KYSE150 cells. (F) The expression of HOXC8 mRNA was measured in esophageal squamous cell cancer cells compared with normal esophageal epithelium cell. (G and H) The protein level of HOXC8 was examined in EC9706 and KYSE150 cells transfected with miR-NC, miR-204, anti-miR-NC or anti-miR-204. *P<0.05, **P<0.01, ***P<0.001.
HOXC8 Restoration Weakens the Effect of SNHG1 Knockdown on Progression of Esophageal Squamous Cell Cancer Cells
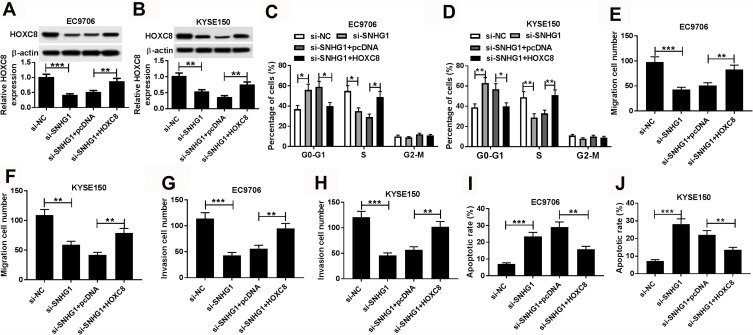
To explore whether SNHG1-regulated progression of esophageal squamous cell cancer was mediated by HOXC8, EC9706 and KYSE150 cells were transfected with si-NC, si-SNHG1, si-SNHG1 and pcDNA or HOXC8. As shown in Figure 6A and B, the protein level of HOXC8 in EC9706 and KYSE150 cells was significantly decreased by silencing SNHG1, which was restored by the introduction of HOXC8 overexpression vector. Moreover, the arrest of cell cycle in EC9706 and KYSE150 cells caused by SNHG1 interference was abrogated by the up-regulation of HOXC8 (Figure 6C and D). Meanwhile, the inhibition of migration and invasion mediated by SNHG1 knockdown was abated by the restoration of HOXC8 in EC9706 and KYSE150 cells (Figure 6E–H). Additionally, the introduction of HOXC8 significantly relieved the apoptosis of EC9706 and KYSE150 cells induced by silencing SNHG1 (Figure 6I and J). Besides, knockdown of HOXC8 also weakened the promoting role of SNHG1 in esophageal squamous cell cancer development in vitro (Supplementary Figure 2).
Figure 6.
The regulatory effect of HOXC8 on SNHG1-mediated progression of esophageal squamous cell cancer cells. (A and B) The protein level of HOXC8 in EC9706 and KYSE150 cells was measured by Western blot after transfection of si-NC, si-SNHG1, si-SNHG1 and pcDNA or HOXC8. Cell cycle distribution (C and D), migration (E and F), invasion (G and H) and apoptosis (I and J) of EC9706 and KYSE150 cells transfected with si-NC, si-SNHG1, si-SNHG1 and pcDNA or HOXC8 were detected by flow cytometry and trans-well assays. *P<0.05, **P<0.01, ***P<0.001.
Silencing SNHG1 Decreases Xenograft Tumor Growth
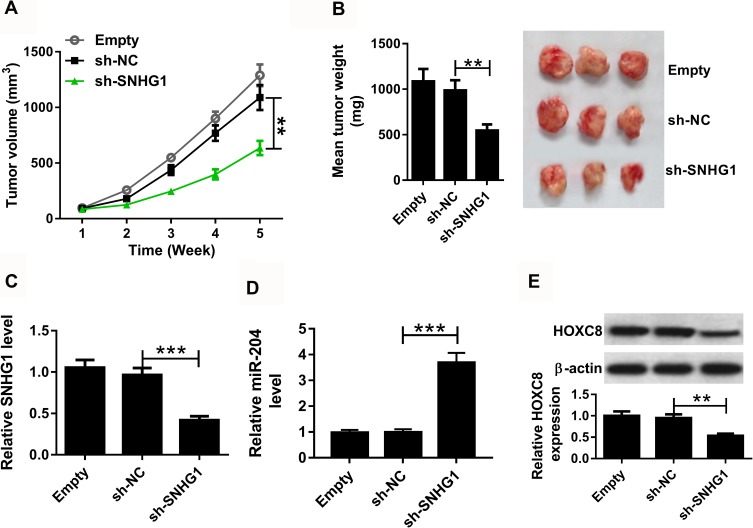
To further evaluate the role of SNHG1 in esophageal squamous cell cancer in vivo, EC9706 cells stably infected with sh-SNHG1 or sh-NC were injected into nude mice. After 5 weeks, the xenograft tumor volume and tumor weight were significantly reduced in the sh-SNHG1 group compared with those in the sh-NC group (Figure 7A and B). Moreover, the analyses of qRT-PCR and Western blot revealed that the expression levels of SNHG1 and HOXC8 protein were remarkably decreased while miR-204 abundance was evidently enhanced in tumor tissues of the sh-SNHG1 group compared with those in the sh-NC group (Figure 7C–E).
Figure 7.
The effect of SNHG1 on xenograft tumor growth. EC9706 cells stably infected with sh-SNHG1 or sh-NC were introduced into nude mice. Empty was infection of empty lentiviral vectors group. Tumor volume (A), weight (B) and the levels of SNHG1 (C), miR-204 (D) and HOXC8 protein level (E) in tumor tissues were detected. **P<0.01, ***P<0.001.
Discussion
Esophageal squamous cell cancer is a common cancer wreaking havoc on the health of people worldwide.15 LncRNAs with ceRNA networks exhibit important roles in regulating esophageal squamous cell cancer malignancy.16 In the current study, we investigated the function of SNHG1 on esophageal squamous cell cancer cell processes and elucidated a novel ceRNA mechanism associated with miR-204/HOXC8 axis.
The emerging effort summarized the role of SNNHG1 in human cancers, including esophageal squamous cell cancer.17 By detecting the level of SNHG1 in 53 esophageal squamous cell cancer tissues and cell lines, we found that this lncRNA expression was increased compared with matched controls. This indicated the potential oncogenic role of SNHG1 in esophageal squamous cell cancer, which was confirmed by gain-of-function experiments. Furthermore, SNHG1 inhibition induced by si-SNHG1 led to the impaired abilities of migration and invasion and increased apoptosis by arresting cell cycle at G0-G1 phase. These results indicated the potential therapeutic effect of target SNHG1 inhibition on esophageal squamous cell cancer, which is also in agreement with the previous study.13
Former findings suggested that SNHG1 could serve as a ceRNA to be implicated in the development of human cancers.18–20 To figure out whether SNHG1 could play the carcinogenic role in esophageal squamous cell cancer, we searched its targets and validated miR-204 as a target of SNHG1. Intriguingly, there was a negative mutual association between SNHG1 and miR-204. This is also in agreement with the dual-regulation of GAS5/miR-212 and PVT1/miR-143.21,22 We hypothesized that it might be induced by competitively binding to the miRNA response elements.23 As described previously, miR-204 functioned as a tumor suppressor in multiple cancers, including papillary thyroid carcinoma, hepatocellular cancer and lung adenocarcinoma.24–26 Meanwhile, miR-204 played the suppressive role in esophageal cancer by decreasing proliferation, invasion and epithelial-mesenchymal transition.27,28 In our study, we also found the tumor suppressive role of miR-204, revealed by which its knockdown abated silencing SNHG1-mediated inhibition of esophageal squamous cell cancer progression. This also uncovered that SNHG1 mediated esophageal squamous cell cancer development by sponging miR-204.
HOXC8 has been regarded as an oncogene and predicts poor prognosis in multiple cancers, including hepatocellular carcinoma, ovarian cancer, non-small cell lung cancer and cervical cancer.29–32 Moreover, previous studies showed that HOXC8 was highly expressed and associated with poor prognosis and tumor development in esophageal squamous cell cancer.33,34 Using starBase database, we found that HOXC8 and SNHG1 have the similar binding site of miR-204. Hence, we hypothesized that HOXC8 might be required for the ceRNA network addressed by SNHG1. Here we first using luciferase reporter assay and RIP confirmed HOXC8 as a target of miR-204 and found that restoration or knockdown of HOXC8 abolished the effect of SNHG1 silence or overexpression on cancer progression, indicating that SNHG1 regulated esophageal squamous cell cancer development by increasing HOXC8 via competitively sponging miR-204. Tumor xenograft model is responsible for studying the tumorigenesis process in esophageal squamous cell cancer.35 Hence, we also established the murine xenograft model using esophageal squamous cell cancer cells and found the anti-cancer role of SNHG1 knockdown in this cancer in vivo by regulating miR-204/HOXC8 axis.
Conclusion
Our research revealed the therapeutic effect of SNHG1 silence on esophageal squamous cell cancer development by decreasing cell migration and invasion and promoting apoptosis, possibly by regulating HOXC8 via competitively binding miR-204. This study indicates SNHG1 as a potential target for the treatment of esophageal squamous cell cancer and provides a new regulatory mechanism for SNHG1.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Alsop BR, Sharma P. Esophageal cancer. Gastroenterol Clin North Am. 2016;45(3):399–412. doi: 10.1016/j.gtc.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 2.Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149(7):1700–1715. doi: 10.1053/j.gastro.2015.08.054 [DOI] [PubMed] [Google Scholar]
- 3.Pasquali S, Yim G, Vohra RS, et al. Survival after neoadjuvant and adjuvant treatments compared to surgery alone for resectable esophageal carcinoma: a network meta-analysis. Ann Surg. 2017;265(3):481–491. doi: 10.1097/SLA.0000000000001905 [DOI] [PubMed] [Google Scholar]
- 4.Sugihara H, Ishimoto T, Miyake K, et al. Noncoding RNA expression aberration is associated with cancer progression and is a potential biomarker in esophageal squamous cell carcinoma. Int J Mol Sci. 2015;16(11):27824–27834. doi: 10.3390/ijms161126060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen WJ, Zhang F, Zhao X, Xu J. LncRNAs and esophageal squamous cell carcinoma - implications for pathogenesis and drug development. J Cancer. 2016;7(10):1258–1264. doi: 10.7150/jca.14869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Wang J, Pan S, et al. LINC00657 played oncogenic roles in esophageal squamous cell carcinoma by targeting miR-615-3p and JunB. Biomed Pharmacother. 2018;108:316–324. doi: 10.1016/j.biopha.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 7.Chu J, Li H, Xing Y, et al. LncRNA MNX1-AS1 promotes progression of esophageal squamous cell carcinoma by regulating miR-34a/SIRT1 axis. Biomed Pharmacother. 2019;116:109029. doi: 10.1016/j.biopha.2019.109029 [DOI] [PubMed] [Google Scholar]
- 8.Zhihua Z, Weiwei W, Lihua N, Jianying Z, Jiang G. p53-induced long non-coding RNA PGM5-AS1 inhibits the progression of esophageal squamous cell carcinoma through regulating miR-466/PTEN axis. IUBMB Life. 2019;71:1492–1502. doi: 10.1002/iub.2069 [DOI] [PubMed] [Google Scholar]
- 9.Yu Y, Yang J, Yang S, et al. Expression level and clinical significance of SNHG1 in human cancers: a meta-analysis. Onco Targets Ther. 2019;12:3119–3127. doi: 10.2147/OTT.S184803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo W, Huang J, Lei P, Guo L, Li X. LncRNA SNHG1 promoted HGC-27 cell growth and migration via the miR-140/ADAM10 axis. Int J Biol Macromol. 2019;122:817–823. doi: 10.1016/j.ijbiomac.2018.10.214 [DOI] [PubMed] [Google Scholar]
- 11.Cui L, Dong Y, Wang X, et al. Downregulation of long noncoding RNA SNHG1 inhibits cell proliferation, metastasis, and invasion by suppressing the Notch-1 signaling pathway in pancreatic cancer. J Cell Biochem. 2019;120(4):6106–6112. doi: 10.1002/jcb.27897 [DOI] [PubMed] [Google Scholar]
- 12.Yan Y, Fan Q, Wang L, Zhou Y, Li J, Zhou K. LncRNA Snhg1, a non-degradable sponge for miR-338, promotes expression of proto-oncogene CST3 in primary esophageal cancer cells. Oncotarget. 2017;8(22):35750–35760. doi: 10.18632/oncotarget.16189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Jin X, Wang Z, Zhang X, Liu S, Liu G. Downregulation of SNHG1 suppresses cell proliferation and invasion by regulating Notch signaling pathway in esophageal squamous cell cancer. Cancer Biomark. 2017;21(1):89–96. doi: 10.3233/CBM-170286 [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 15.Batra R, Malhotra GK, Singh S, Are C. Managing squamous cell esophageal cancer. Surg Clin North Am. 2019;99(3):529–541. doi: 10.1016/j.suc.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 16.Tian W, Jiang C, Huang Z, Xu D, Zheng S. Comprehensive analysis of dysregulated lncRNAs, miRNAs and mRNAs with associated ceRNA network in esophageal squamous cell carcinoma. Gene. 2019;696:206–218. doi: 10.1016/j.gene.2019.02.051 [DOI] [PubMed] [Google Scholar]
- 17.Thin KZ, Tu JC, Raveendran S. Long non-coding SNHG1 in cancer. Clin Chim Acta. 2019;494:38–47. doi: 10.1016/j.cca.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 18.Lan X, Liu X. LncRNA SNHG1 functions as a ceRNA to antagonize the effect of miR-145a-5p on the down-regulation of NUAK1 in nasopharyngeal carcinoma cell. J Cell Mol Med. 2019;23(4):2351–2361. doi: 10.1111/jcmm.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu M, Chen X, Lin K, et al. The long noncoding RNA SNHG1 regulates colorectal cancer cell growth through interactions with EZH2 and miR-154-5p. Mol Cancer. 2018;17(1):141. doi: 10.1186/s12943-018-0894-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng R, Zhang J, Chen J. lncRNA SNHG1 negatively regulates miRNA1013p to enhance the expression of ROCK1 and promote cell proliferation, migration and invasion in osteosarcoma. Int J Mol Med. 2019;43(3):1157–1166. doi: 10.3892/ijmm.2018.4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Zhu Z, Watabe K, et al. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013;20(11):1558–1568. doi: 10.1038/cdd.2013.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Yu Y, Li H, et al. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol Cancer. 2019;18(1):33. doi: 10.1186/s12943-019-0947-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Zhao Z, Sun W, Guo Z, et al. Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life Sci. 2019:116900. doi: 10.1016/j.lfs.2019.116900 [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Li R, Guan L, Jiang T. Knockdown of lncRNA UCA1 inhibits proliferation and invasion of papillary thyroid carcinoma through regulating miR-204/IGFBP5 axis. Onco Targets Ther. 2018;11:7197–7204. doi: 10.2147/OTT.S175467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y, Wang Y, Xiao X, et al. MiR-204 inhibits hepatocellular cancer drug resistance and metastasis through targeting NUAK1. Biochem Cell Biol. 2019;97(5):563–570. doi: 10.1139/bcb-2018-0354 [DOI] [PubMed] [Google Scholar]
- 26.Hu WB, Wang L, Huang XR, Li F. MicroRNA-204 targets SOX4 to inhibit metastasis of lung adenocarcinoma. Eur Rev Med Pharmacol Sci. 2019;23(4):1553–1562. doi: 10.26355/eurrev_201902_17114 [DOI] [PubMed] [Google Scholar]
- 27.Jiao C, Song Z, Chen J, et al. lncRNA-UCA1 enhances cell proliferation through functioning as a ceRNA of Sox4 in esophageal cancer. Oncol Rep. 2016;36(5):2960–2966. doi: 10.3892/or.2016.5121 [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Yu X, Bai Q. miR-204 inhibits invasion and epithelial-mesenchymal transition by targeting FOXM1 in esophageal cancer. Int J Clin Exp Pathol. 2015;8(10):12775–12783. [PMC free article] [PubMed] [Google Scholar]
- 29.Xu P, Zhang X, Ni W, et al. Upregulated HOXC8 expression is associated with poor prognosis and oxaliplatin resistance in hepatocellular carcinoma. Dig Dis Sci. 2015;60(11):3351–3363. doi: 10.1007/s10620-015-3774-x [DOI] [PubMed] [Google Scholar]
- 30.Lu S, Liu R, Su M, et al. Overexpression of HOXC8 is associated with poor prognosis in epithelial ovarian cancer. Reprod Sci. 2016;23(7):944–954. doi: 10.1177/1933719115625845 [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Zhang M, Xu S, et al. HOXC8 promotes proliferation and migration through transcriptional up-regulation of TGFbeta1 in non-small cell lung cancer. Oncogenesis. 2018;7(2):1. doi: 10.1038/s41389-017-0016-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y, Chen L, Guo A. Upregulated expression of HOXC8 is associated with poor prognosis of cervical cancer. Oncol Lett. 2018;15(5):7291–7296. doi: 10.3892/ol.2018.8200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du YB, Dong B, Shen LY, et al. The survival predictive significance of HOXC6 and HOXC8 in esophageal squamous cell carcinoma. J Surg Res. 2014;188(2):442–450. doi: 10.1016/j.jss.2014.01.017 [DOI] [PubMed] [Google Scholar]
- 34.Shen LY, Zhou T, Du YB, Shi Q, Chen KN. Targeting HOX/PBX dimer formation as a potential therapeutic option in esophageal squamous cell carcinoma. Cancer Sci. 2019;110(5):1735–1745. doi: 10.1111/cas.13993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee NP, Chan CM, Tung LN, Wang HK, Law S. Tumor xenograft animal models for esophageal squamous cell carcinoma. J Biomed Sci. 2018;25(1):66. doi: 10.1186/s12929-018-0468-7 [DOI] [PMC free article] [PubMed] [Google Scholar]