Abstract
The liver is the largest lymph producing organ. A significant increase in the number of hepatic lymphatic vessels, or lymphangiogenesis, has been reported in various liver diseases, including, but not limited to, cirrhosis, viral hepatitis and hepatocellular carcinoma. Despite its apparent relevance in healthy and diseased livers as these and other observations indicate, the hepatic lymphatic system has been poorly studied. With knowledge of the lymphatic system in other organs and tissues incorporated, this review article addresses the current knowledge of the hepatic lymphatic system and the potential role of lymphatic endothelial cells in the health and the disease of the liver and concludes with a brief description on future directions of the study of the hepatic lymphatic system.
Introduction
The liver is the largest lymph producing organ, accounting for 25%–50% of lymph passing through the thoracic duct.[1] The production of lymph increases up to 30-fold in cirrhotic patients with concomitant increases in the formation of new lymphatic vessels, i.e., lymphangiogenesis.[1,2] Despite its apparent relevance in healthy and diseased livers, little is known about the hepatic lymphatic system.[1,3,4]
The hepatic lymphatic system helps to remove waste products and immune cells derived from the sinusoidal microcirculation as well as hepatocytes and non-parenchymal cells in the form of lymph, by transporting lymph through lymphatic vessels to draining lymph nodes. The production of lymph in the liver is initiated by the filtration of plasma components through fenestrae of liver sinusoidal endothelial cells (LSECs) into the space of Disse, the interstitial space between LSECs and hepatocytes (Figure 1).[5,6] Approximately 80% of lymph in the space of Disse flows through the space of Mall, a space between the stroma of the portal tract and the outermost hepatocytes [7], and drains into lymphatic vessels in the portal tract [1,6] The rest of lymph in the space of Disse diffuses into the interstitium around the central vein or underneath the hepatic capsule. Thus, hepatic lymphatic vessels are mainly observed in the portal tract [1,6]. Hepatic lymphatic vessels are connected to one or more draining lymph nodes outside the liver.[1] Antigen-presenting cells (APCs) including dendritic cells and macrophages in lymph interact with lymphocytes in draining lymph nodes, facilitating adaptive immune responses.[8]
Figure 1. Lymphatic system in the liver.
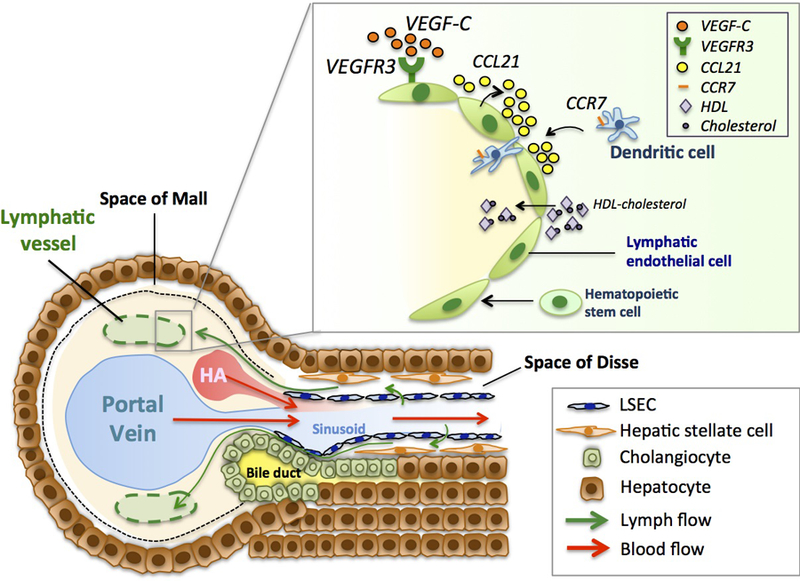
A majority of lymphatic vessels (specifically, lymphatic capillaries due to lack of smooth muscle cell or pericyte coverage) are located in the portal tract, which accommodates the portal vein, the hepatic artery (HA) and bile ducts. Blood in branches of the portal vein and the hepatic artery flows into the sinusoidal microcirculation (red arrows) where these branches merge and ultimately into the central vein (which coalesces to be the hepatic vein). Sinusoids are covered by fenestrated liver sinusoidal endothelial cells (LSECs). Hepatic lymphatic fluid originates mainly from plasma components filtered through fenestrae of LSECs and flows into the space of Disse, the interstitial space between LSECs and hepatocytes (green arrows). Hepatic stellate cells are also located in this space. Lymphatic fluid in the space of Disse primarily flows through the space of Mall, a space between the stroma of the portal tract and the outermost hepatocytes, into the interstitium of the portal tract and then into lymphatic vessels (green allows).
[Upper right box] Hepatic lymphatic endothelium.
Vascular endothelial growth factor-C (VEGF-C) is one of the most potent lymphangiogenic factors. Lymphatic endothelial cells (LyECs) express VEGFR3, a receptor for VEGF-C. Hepatic LyECs are also known to express lymphokines, such as C-C-motif chemokine ligand 21 (CCL21) that guides dendritic cells through their expression of C-C chemokine receptor 7 (CCR7). LyECs can take up cholesterols carried by HDL. In normal postnatal liver, it is shown that hematopoietic stem cells can differentiate into LyECs.
The condition of the liver could impact on this series of events, including the production of lymph, lymphangiogenesis, contents of lymph and the subsequent immune response in draining lymph nodes. For example, the level of lymph production is proportional to the hydrostatic pressure within the sinusoidal microcirculation of the liver. In cirrhosis, the sinusoidal hydrostatic pressure increases due to an increase in resistance to sinusoidal blood flow, which subsequently increases plasma components filtrated through sinusoids and thus the formation of lymph.[9] However, the relevance of changes in lymph contents and lymphangiogenesis to the health and the disease of the liver is largely unknown.
In recent years, understanding of the lymphatic system has been advanced significantly in other organs and tissues, such as the skin, and cancer microenvironments, particularly in relation to phenotypic changes of lymphatic endothelial cells (LyECs) and how these phenotypic changes of LyECs relate to disease progression. With such knowledge incorporated, this review article summarizes the current knowledge of the hepatic lymphatic system and the potential role of LyECs in the health and the disease of the liver.
Contents of lymph
Contents of lymph mainly originate from plasma components of blood and contain immune cells and apoptotic cells as well as cellular products, including products of organ and cellular catabolism, proteins, peptides and lipids.[10] Proteins can be derived from intracellulrar sources (endosomes, Golgi, ER, mitochondria and cytoplasm), shed surface receptors, cytokines, chemokines and fragments of extracellular matrix proteins such as collagens.[10] In the liver, contents of lymph are regulated first by hydrostatic pressure in sinusoids and by LSECs. The presence of fenestrae in LSECs allows hepatic lymph to contain a higher protein concentration with approximately 90% of plasma proteins, compared to those of the intestine (approx. 70%) and peripheral tissues (approx. 60%).[11,12] Since substances secreted from hepatic cells such as hepatocytes, hepatic stellate cells, Kupffer cells and LSECs flow into the space of Disse, these substances also likely contribute to lymph contents. LyECs, which constitute lymphatic vessels, are also involved in the selection of lymph contents and can serve as a sort of final gatekeeper for cells and substances to be drained into lymph nodes.
Therefore, contents of lymph, particularly before passing through lymph nodes (i.e., pre-nodal lymph), are local and could reflect properties of its producing organs. Accordingly, analysis of pre-nodal lymph could provide a molecular and cellular signature of organ specific lymph as well as more specific biomaterials related to the conditions of organs and tissues than the circulating blood could provide. Liver specific lymph contents are largely unknown.
Lymphatic endothelial cells in the liver
As mentioned, lymphatic vessels are primarily present in the portal tract area along with the portal vein, the hepatic artery and bile ducts (Figure 1). Specifically speaking, lymphatic vessels in the liver are lymphatic capillaries without smooth muscle cell/pericyte coverage. Therefore, lymphatic vessels can be identified by expression of LyEC markers, such as Lyve-1, Prox1 and podoplanin and by the absence of αSMA-positive cells (i.e., smooth muscle cells/pericytes). However, Lyve-1 is also expressed by LSECs and Prox1 is expressed by hepatocytes [13]. Therefore, the combination of Lyve-1 or Prox1 with podoplanin helps to distinguish hepatic lymphatic vessels clearly from other vessels and hepatocytes.
Regarding the origin of LyECs, in the embryo, the lymphatic endothelium arises from existing venous endothelial cells.[14] In normal postnatal liver, hematopoietic stem cells (HSCs) were shown to contribute to LyECs.[15] In this study, GFP-labeled hematopoietic stem cells (GFP-HSCs) were transplanted to irradiated recipient mice. These donor-derived GFP-HSCs were identified for 2.4 +/− 0.8% and 3.2 +/− 1.4% of the total LyECs in the liver 1 month and >12 months after transplantation, respectively. This study also performed a parabiotic experiment, in which GFP mice and WT mice were surgically connected for 12 weeks, and found GFP-positive LyECs in hepatic lymphatic vessels of WT mice, suggesting that circulating HSCs can be a source of LyECs in the liver. Although some studies showed tissue macrophages to transdifferentiate into LyECs in inflammatory [16] and wound healing [17] settings, this study [15] did not find evidence of macrophage contribution to LyECs in the liver. It is unknown whether HSC-derived LyECs play a role in the development of hepatic lymphatic vessels observed in liver diseases such as cirrhosis [2,18,19], hepatocellular carcinoma [20] and cholangiocarcinoma [21,22].
Functions of lymphatic endothelial cells and liver diseases
LyECs are not just lining components of lymphatic vessels. Although still limited, their functions have been related to liver diseases. First, LyECs guide mobilization of immune cells and regulate their functions (Figure 1). They modulate these processes through secretion of growth factors, cytokines and chemokines, which are collectively termed “lymphangiocrine” factors.[23] In the inflamed skin, LyECs promote mobilization of dendritic cells (DC) and T-cells to lymphatic vessels with production of C-C-motif chemokine ligand 21 (CCL21).[24,25] In the skin, inflamed LyECs also express intracellular adhesion molecule 1 (ICAM-1) [26] as well as C-X3-C motif chemokine ligand 1 (CX3CL1) and CXC motif chemokine ligand 12 (CXCL12) to guide T-cells, DC and other innate immune cells to lymphatic vessels.[27] In lymph nodes, LyECs secrete sphingosine-1 phosphate (S1P) and promote activated T-cells to egress.[28] Besides regulation of immune cell egress, LyECs also work as an immunosuppressant through secretion of nitric oxide in lymph nodes [29] as well as expression of PD-L1 in tumor-associated lymphatic vessels. [30] In lymph nodes, LyECs induce CD8 T-cell deletion through LAG-3/MHC-II signaling with expression of MHCII type self-antigens as well as through PD-1/PD-L1 signaling.[31] The regulation of immune cells by LyECs is largely unexplored in liver disease. However, given the important role that immune cells play in liver disease, it is likely that LyECs have implications in liver disease through their regulation of immune cells.
Second, LyECs are known to take up cholesterol carried by HDL through expression of scavenger receptor class B type I.[32,33] Dysfunctional LyECs were shown to lead to the development of fatty liver.[34] Thus, the normal function of LyECs seems to be required for the homeostasis of fat metabolism in the liver. It is interesting to investigate to what extent LyECs contribute to the development of hepatic steatosis and the progression to hepatic steatohepatitis, compared with other such factors.
Third, LyECs are also known to facilitate tumor growth and metastasis. LyECs facilitate homing of melanoma cells by producing CCL21, which binds CCR7 expressed in melanoma cells.[35] In B16 melanoma, VEGF-C-activated LyECs, which are often observed in tumor microenvironments, can present tumor antigens and ultimately inhibit activation of CD8+ T-cells. [36] Furthermore, tumor-induced lymphangiogenesis is associated with decreased infiltration of immune cells and thus decreased tumoricidal activity by these immune cells, promoting tumor growth and tumor metastasis of melanoma cells.[36,37] Recently, lymphangiogenesis was also shown to increase hepatocellular carcinoma (HCC) metastasis.[38] Heparanase-1 expressed in HCC cleaves heparin sulfate chains of syndican-1 (SDC-1) in HCC, allowing the release of VEGF-C from the complex of VEGF-C and SDC-1 on the surface of HCC. This release of VEGF-C from the HCC surface stimulates LyEC proliferation, or lymphangiogenesis, facilitating HCC metastasis.[38] Collectively, these studies indicate that in tumor microenvironments, LyECs enhance tumor growth and metastasis and that VEGF-C plays a critical role in these processes by promoting lymphangiogenesis and immunosuppression.[35–37]
Finally, lymphangiogenesis is also implicated in organ transportation. In corneal transplantation, lymphangiogenesis is thought to be detrimental and increase the prevalence of chronic graft rejection.[39] Further, the blockade of lymphangiogenic factors, including VEGF-C [40], angiopoietin-2 [41] and galectin-8 [42], improves the survival rate of corneal transplants. Unlike corneal transplantation, a rat model of liver transplantation [43] showed post-transplant lymphangiogenesis in grafts to be associated with long-term survival of recipients for more than 90 days. In addition, lymphatic vessels disappeared from severely rejected areas of the rejected grafts within 11 days due to acute cellular rejection and antibody-mediated rejection. These findings suggest that lymphatic vessels play a role in the early stage of liver transplantation by ameliorating inflammation.[43]
These differences in terms of lymphangiogenesis between corneal and liver transplantation remain to be explained. However, liver’s unique immunologic properties may be involved. Despite its continuous exposure to nutrients, microbial antigens from the gut and chemicals, the liver equips with specialized mechanisms of immune tolerance that avoid overactivation of immune responses.[44,45] The liver resembles lymphoid organs.[46,47] It harbors resident or migratory immune cells responsible for the innate and adaptive immune systems. Similar to peripheral lymphoid organs, the liver is a site for naïve T-cell activation.[46] In addition, various hepatic cell populations, including LSECs, hepatic stellate cells and hepatocytes, are capable of antigen presentation. This unique immuno-tolerant property of the liver is considered to be beneficial when it comes to liver transplantation. In fact, liver transplantation entails less rejection, because human leukocyte antigen (HLA) mismatch is not a problem and the immunosuppressant requirement is less in human patients.[48–50] Thus, the different observations between corneal and liver transplantation may be another implication of the close relation between immunological responses and lymphangiogenesis.
Conclusion and future directions
A significant increase in the number of lymphatic vessels (i.e., lymphangiogenesis) has been reported in various pathological conditions of the liver, including cirrhosis [2,18,19,51], viral hepatitis [3], lymphedema cholestasis syndrome[52,53], idiopathic portal hypertension [54], primary biliary cholangitis [55,56], and hepatocellular carcinoma.[20,38] Despite these observations, the role of lymphangiogenesis in the progression of liver disease is yet to be determined.[4] Indeed, the hepatic lymphatic system has been poorly studied. Even its fundamental aspects such as anatomy remain to be fully elucidated. The development of imaging techniques, in vivo and in vitro experimental models are urgently needed to advance our understanding of the hepatic lymphatic system, which may potentially open a new avenue for prevention and treatment of liver disease.
Acknowledgement
We would like to thank Dr. Teruo Utsumi for his valuable comments. This work was supported by NIH grants (R01AA025342, R21AA023599, 1R21AA023607 and DK34989) to YI, and a research fellowship of The Uehara Memorial Foundation and grants-in-aid of The International Research Fund for Subsidy of Kyushu University School of Medicine Alumni to MT.
Abbreviation used in this paper
- LSEC
liver sinusoidal endothelial cell
- APC
antigen-presenting cell
- LyEC
lymphatic endothelial cell
- HSC
hematopoietic stem cell
- DC
dendritic cell
- CCL21
C-C-motif chemokine ligand 21
- ICAM-1
intracellular adhesion molecule 1
- CX3CL1
C-X3-C motif chemokine ligand 1
- CXCL12
CXC motif chemokine ligand 12
- S1P
sphingosine-1 phosphate
- HCC
hepatocellular carcinoma
- SDC-1
syndican-1
- HLA
human leukocyte antigen
- VEGF-C
vascular endothelial growth factor-C
- CCR7
C-C chemokine receptor 7
Footnotes
Disclosure
The authors disclose no conflicts.
References:
- 1.Tanaka M, Iwakiri Y: The Hepatic Lymphatic Vascular System: Structure, Function, Markers, and Lymphangiogenesis. Cell Mol Gastroenterol Hepatol 2016, 2:733–749.•• This review article summarizes the current knowledge of the lymphatic system in the liver comprehensively.
- 2.Vollmar B, Wolf B, Siegmund S, Katsen AD, Menger MD: Lymph vessel expansion and function in the development of hepatic fibrosis and cirrhosis. Am J Pathol 1997, 151:169–175. [PMC free article] [PubMed] [Google Scholar]
- 3.Chung C, Iwakiri Y: The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol 2013, 19:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwakiri Y: The lymphatic system: A new frontier in hepatology. Hepatology 2016, 64:706–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trutmann M, Sasse D: The lymphatics of the liver. Anat Embryol (Berl) 1994, 190:201–209. [DOI] [PubMed] [Google Scholar]
- 6.Ohtani O, Ohtani Y: Lymph circulation in the liver. Anat Rec (Hoboken) 2008, 291:643–652. [DOI] [PubMed] [Google Scholar]
- 7.Mall FP: A study of the structural unit of the liver. American Journal of Anatomy 1906, 5:227–308. [Google Scholar]
- 8.Jeltsch M, Tammela T, Alitalo K, Wilting J: Genesis and pathogenesis of lymphatic vessels. Cell Tissue Res 2003, 314:69–84. [DOI] [PubMed] [Google Scholar]
- 9.Dumont AE, Mulholland JH: Flow rate and composition of thoracic-duct lymph in patients with cirrhosis. N Engl J Med 1960, 263:471–474. [DOI] [PubMed] [Google Scholar]
- 10.Clement CC, Rotzschke O, Santambrogio L: The lymph as a pool of self-antigens. Trends Immunol 2011, 32:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Witte MH, Dumont AE, Cole WR, Witte CL, Kintner K: Lymph circulation in hepatic cirrhosis: effect of portacaval shunt. Ann Intern Med 1969, 70:303–310. [DOI] [PubMed] [Google Scholar]
- 12.Aukland K, Kramer GC, Renkin EM: Protein concentration of lymph and interstitial fluid in the rat tail. Am J Physiol 1984, 247:H74–79. [DOI] [PubMed] [Google Scholar]
- 13.Truman LA, Bentley KL, Smith EC, Massaro SA, Gonzalez DG, Haberman AM, Hill M, Jones D, Min W, Krause DS, et al. : ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am J Pathol 2012, 180:1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G: Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 2007, 21:2422–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang S, Bailey AS, Goldman DC, Swain JR, Wong MH, Streeter PR, Fleming WH: Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One 2008, 3:e3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D’Amore PA, Stein-Streilein J, et al. : Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest 2005, 115:2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D’Amore PA: Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 2007, 170:1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamauchi Y, Michitaka K, Onji M: Morphometric analysis of lymphatic and blood vessels in human chronic viral liver diseases. Am J Pathol 1998, 153:1131–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokomori H, Oda M, Kaneko F, Kawachi S, Tanabe M, Yoshimura K, Kitagawa Y, Hibi T: Lymphatic marker podoplanin/D2–40 in human advanced cirrhotic liver--re-evaluations of microlymphatic abnormalities. BMC Gastroenterol 2010, 10:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thelen A, Jonas S, Benckert C, Weichert W, Schott E, Botcher C, Dietz E, Wiedenmann B, Neuhaus P, Scholz A: Tumor-associated lymphangiogenesis correlates with prognosis after resection of human hepatocellular carcinoma. Ann Surg Oncol 2009, 16:1222–1230. [DOI] [PubMed] [Google Scholar]
- 21.Aishima S, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, Maehara Y, Tsuneyoshi M: Lymphatic spread is related to VEGF-C expression and D2–40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol 2008, 21:256–264. [DOI] [PubMed] [Google Scholar]
- 22.Thelen A, Scholz A, Weichert W, Wiedenmann B, Neuhaus P, Gessner R, Benckert C, Jonas S: Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol 2010, 105:1123–1132. [DOI] [PubMed] [Google Scholar]
- 23.Petrova TV, Koh GY: Organ-specific lymphatic vasculature: From development to pathophysiology. J Exp Med 2018, 215:35–49.• This review article provides most up-to-date information of lymphatic endothelial cells and lymphangiogenesis in health and disease.
- 24.Russo E, Teijeira A, Vaahtomeri K, Willrodt AH, Bloch JS, Nitschke M, Santambrogio L, Kerjaschki D, Sixt M, Halin C: Intralymphatic CCL21 Promotes Tissue Egress of Dendritic Cells through Afferent Lymphatic Vessels. Cell Rep 2016, 14:1723–1734.• This study showed that expression of CCL21 in lymphatic vessels and CCL21/CCR signaling are necessary for dendritic cells to drian to lymph nodes in murine skin using time-lapse imaging.
- 25.Gomez D, Diehl MC, Crosby EJ, Weinkopff T, Debes GF: Effector T Cell Egress via Afferent Lymph Modulates Local Tissue Inflammation. J Immunol 2015, 195:3531–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teijeira A, Hunter MC, Russo E, Proulx ST, Frei T, Debes GF, Coles M, Melero I, Detmar M, Rouzaut A, et al. : T Cell Migration from Inflamed Skin to Draining Lymph Nodes Requires Intralymphatic Crawling Supported by ICAM-1/LFA-1 Interactions. Cell Rep 2017, 18:857–865.• This study showed the mechanism of T-cell migration in the skin through lymphatic vessels, in which intracellular adhesion molecule 1 (ICAM-1) expression in lymphatic endothelial cells faciliates T-cell migration through lymphocyte function- associated antigen 1 (LFA-1) expression of T-cells.
- 27.Johnson LA, Jackson DG: The chemokine CX3CL1 promotes trafficking of dendritic cells through inflamed lymphatics. J Cell Sci 2013, 126:5259–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urso K, Alvarez D, Cremasco V, Tsang K, Grauel A, Lafyatis R, von Andrian UH, Ermann J, Aliprantis AO: IL4RA on lymphatic endothelial cells promotes T cell egress during sclerodermatous graft versus host disease. JCI Insight 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR, Turley SJ: Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol 2011, 12:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dieterich LC, Ikenberg K, Cetintas T, Kapaklikaya K, Hutmacher C, Detmar M: Tumor-Associated Lymphatic Vessels Upregulate PDL1 to Inhibit T-Cell Activation. Front Immunol 2017, 8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouhani SJ, Eccles JD, Riccardi P, Peske JD, Tewalt EF, Cohen JN, Liblau R, Makinen T, Engelhard VH: Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat Commun 2015, 6:6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V: Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab 2013, 17:671–684. [DOI] [PubMed] [Google Scholar]
- 33.Martel C, Randolph GJ: Atherosclerosis and transit of HDL through the lymphatic vasculature. Curr Atheroscler Rep 2013, 15:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu J, Gerhardt H, McDaniel JM, Xia B, Liu X, Ivanciu L, Ny A, Hermans K, Silasi-Mansat R, McGee S, et al. : Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest 2008, 118:3725–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA: Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res 2009, 69:349–357. [DOI] [PubMed] [Google Scholar]
- 36.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA: VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep 2012, 1:191–199. [DOI] [PubMed] [Google Scholar]
- 37.Lund AW, Wagner M, Fankhauser M, Steinskog ES, Broggi MA, Spranger S, Gajewski TF, Alitalo K, Eikesdal HP, Wiig H, et al. : Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest 2016, 126:3389–3402.•• This study showed that lymphangiogenesis impairs anti-tumor immunity and increases tumor metastasis in murine melanoma, using K14-VEGFR3-Ig mice in which VEGFR3-Ig fusion protein can be expressed specifically in the skin and effectively inhibit dermal lymphatic vessel formation.
- 38.Yu S, Lv H, Zhang H, Jiang Y, Hong Y, Xia R, Zhang Q, Ju W, Jiang L, Ou G, et al. : Heparanase-1-induced shedding of heparan sulfate from syndecan-1 in hepatocarcinoma cell facilitates lymphatic endothelial cell proliferation via VEGF-C/ERK pathway. Biochem Biophys Res Commun 2017, 485:432–439.• This study showed that lymphangiogenesis promotes HCC metastasis, in which heparanase-1 expressed in HCC cleaves heparin sulfate chains of syndican-1 in HCC, allowing the release of VEGF-C from the complex of VEGF-C and syndican-1 on the surface of HCC and thus promoting LyEC proliferation (i.e., lymphangiogenesis).
- 39.Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G, Wiegand S, Chen L, Cursiefen C: Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol 2010, 184:535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan H, Yuan J, Peng R, Wang T, Deng J, Li W, Ling S: The Blockade of Vascular Endothelial Growth Factor C Effectively Inhibits Corneal Lymphangiogenesis and Promotes Allograft Survival. J Ocul Pharmacol Ther 2015, 31:546–554. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Li G, Sessa R, Kang GJ, Shi M, Ge S, Gong AJ, Wen Y, Chintharlapalli S, Chen L: Angiopoietin-2 Blockade Promotes Survival of Corneal Transplants. Invest Ophthalmol Vis Sci 2017, 58:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen WS, Cao Z, Sugaya S, Lopez MJ, Sendra VG, Laver N, Leffler H, Nilsson UJ, Fu J, Song J, et al. : Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3. Nat Commun 2016, 7:11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishii E, Shimizu A, Kuwahara N, Arai T, Kataoka M, Wakamatsu K, Ishikawa A, Nagasaka S, Fukuda Y: Lymphangiogenesis associated with acute cellular rejection in rat liver transplantation. Transplant Proc 2010, 42:4282–4285. [DOI] [PubMed] [Google Scholar]
- 44.Calne RY, Sells RA, Pena JR, Davis DR, Millard PR, Herbertson BM, Binns RM, Davies DA: Induction of immunological tolerance by porcine liver allografts. Nature 1969, 223:472–476. [DOI] [PubMed] [Google Scholar]
- 45.Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P: The liver: a special case in transplantation tolerance. Semin Liver Dis 2007, 27:194–213. [DOI] [PubMed] [Google Scholar]
- 46.Crispe IN: The liver as a lymphoid organ. Annu Rev Immunol 2009, 27:147–163. [DOI] [PubMed] [Google Scholar]
- 47.Mackay IR: Hepatoimmunology: a perspective. Immunol Cell Biol 2002, 80:36–44. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Peralvarez M, Germani G, Papastergiou V, Tsochatzis E, Thalassinos E, Luong TV, Rolando N, Dhillon AP, Patch D, O’Beirne J, et al. : Early tacrolimus exposure after liver transplantation: relationship with moderate/severe acute rejection and long-term outcome. J Hepatol 2013, 58:262–270. [DOI] [PubMed] [Google Scholar]
- 49.Whitehouse GP, Hope A, Sanchez-Fueyo A: Regulatory T-cell therapy in liver transplantation. Transpl Int 2017, 30:776–784. [DOI] [PubMed] [Google Scholar]
- 50.Choudhary NS, Saigal S, Bansal RK, Saraf N, Gautam D, Soin AS: Acute and Chronic Rejection After Liver Transplantation: What A Clinician Needs to Know. J Clin Exp Hepatol 2017, 7:358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tugues S, Morales-Ruiz M, Fernandez-Varo G, Ros J, Arteta D, Munoz-Luque J, Arroyo V, Rodes J, Jimenez W: Microarray analysis of endothelial differentially expressed genes in liver of cirrhotic rats. Gastroenterology 2005, 129:1686–1695. [DOI] [PubMed] [Google Scholar]
- 52.Shah S, Conlin LK, Gomez L, Aagenaes O, Eiklid K, Knisely AS, Mennuti MT, Matthews RP, Spinner NB, Bull LN: CCBE1 mutation in two siblings, one manifesting lymphedema-cholestasis syndrome, and the other, fetal hydrops. PLoS One 2013, 8:e75770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viveiros A, Reiterer M, Schaefer B, Finkenstedt A, Schneeberger S, Schwaighofer H, Moser P, Sprenger R, Glodny B, Vogel W, et al. : CCBE1 mutation causing sclerosing cholangitis: Expanding the spectrum of lymphedema-cholestasis syndrome. Hepatology 2017, 66:286–288. [DOI] [PubMed] [Google Scholar]
- 54.Oikawa H, Masuda T, Sato S, Yashima A, Suzuki K, Sato S, Satodate R: Changes in lymph vessels and portal veins in the portal tract of patients with idiopathic portal hypertension: a morphometric study. Hepatology 1998, 27:1607–1610. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi S, Matsui O, Gabata T, Terayama N, Sanada J, Yamashiro M, Minami M, Kozaka K, Harada K, Nakanuma Y: MRI findings of primary biliary cirrhosis: correlation with Scheuer histologic staging. Abdom Imaging 2005, 30:71–76. [DOI] [PubMed] [Google Scholar]
- 56.Yamauchi Y, Ikeda R, Michitaka K, Hiasa Y, Horiike N, Onji M: Morphometric analysis of lymphatic vessels in primary biliary cirrhosis. Hepatol Res 2002, 24:107. [DOI] [PubMed] [Google Scholar]


