Abstract
An acidic thermostable xylanase (AT-xynA) which was stable at low pH and high temperature was considered to have great potential in animal feed. For large-scale production, AT-xynA activity was enhanced about 1-fold in Pichia pastoris by constructing a double-copy expression strain in this study. Furthermore, impacts of different AT-xynA levels on growth performance, nutrient digestibility, short-chain fatty acids, and bacterial community in weaned piglets were determined. Compared with the control group, ADFI and ADG were higher for the pigs fed 4,000 or 6,000 U/kg AT-xynA (P < 0.05). AT-xynA supplementation also significantly increased the digestibility of OM, GE, and DM (P < 0.05). AT-xynA supplementation increased the concentrations of acetate in ileal (P < 0.01) and cecal digesta (P < 0.05). Isobutyrate (P < 0.05) and valerate (P < 0.05) concentrations in colonic digesta also significantly increased compared with the control group. AT-xynA supplementation increased the abundance of Lactobacillus in the ileal, cecal, and colonic digesta of weaned piglets (P < 0.05). AT-xynA alleviated anti-nutritional effects of nonstarch polysaccharides (NSP) by preventing the growth of Pateurella and Leptotrichia in the ileum (P < 0.05). AT-xynA increased the abundance of NSP-degrading bacteria, such as Ruminococcaceae, Prevotella in the cecum and colon (P < 0.05). In summary, AT-xynA addition could improve the growth performance of weaned piglets by altering gut microbiota.
Keywords: acidic xylanase, bacterial community, copy number, thermostable xylanase, weaned piglets
Introduction
Wheat is a major feed material in pig diets in many countries due to abundant availability of sources (Kim et al., 2005). However, nonstarch polysaccharides (NSP) contained in wheat limit its application in pig diets due to anti-nutritional effects (Kim et al., 2005). The main component of NSP in wheat is xylan (Saha, 2003). Endo-1,4-β-xylanases (EC 3.2.1.8) degrade xylan in plant cell wall, releasing encapsulated protein and starch from within the nonsoluble cell wall fraction and decreasing the viscosity of digesta caused by the soluble fraction (Amerah, 2015). However, mammals do not produce endogenous xylanases to digest xylan. Exogenous xylanases have been successfully applied in the pig industry and it is well documented that addition of xylanases in feed can increase nutrient digestibility and improve animal growth performance (Barrera et al., 2004; Nortey et al., 2008; Kiarie et al., 2016). However, the application of exogenous xylanases in animal feed can be challenging.
Challenges associated with exogenous xylanase use in animal feed are related, in part, to the acidic nature of the stomach and high temperatures required for pelleting (Inborr et al., 1999; Yang et al., 2017a). For example, exogenous xylanases are easily inactivated at gastric pH levels (i.e., 2.0 to 5.0) common after ingestion of feed (Sams et al., 2016). Therefore, acidic and thermo stability are characteristics of superior xylanases. In our previous study, an acidic xylanase gene (xynA) from Aspergillus sulphureus was successfully expressed in Pichia pastoris (Cao et al., 2006, 2007). Yang et al. (2017b) reported improved thermostability of the xylanase (AT-xynA) by a proline substitution and introduction of a disulfide bond. Although AT-xynA was expressed in P. pastoris, the activity of AT-xynA did not reach sufficient levels for commercial purposes.
High activity is a requirement for the application of xylanases in large-scale production. Heterologous expression is considered an effective method to produce xylanases in high yield and decrease the cost of production (Juturu and Wu, 2012). Pichia pastoris as an excellent host has been widely used for heterologous expression of xylanases (Zhao et al., 2013). Furthermore, increasing the gene dosage can effectively enhance recombinant protein production in P. pastoris. Constructing the plasmid harboring multicopy expression cassettes is a well-established method to increase copy number of the target gene in P. pastoris (Camara et al., 2016). It has been reported that the proteinase K activity of the double-copy expression strain increased 1.68-fold over that of the single-copy expression strain (Yang et al., 2016).
In this study, the enzyme activity of AT-xynA was improved by constructing a double-copy expression strain and the modified xylanase was evaluated based on growth performance, nutrient digestibility, short-chain fatty acid (SCFA) concentrations, and bacterial community of weaned piglets.
MATERIALS AND METHODS
Strains, Vectors, and Chemicals
Escherichia coli Top10 (Tiangen, China) and P. pastoris X-33 (Invitrogen) were used in this experiment. pPIC-xynA-1 containing the coding sequence (xynA) of AT-xynA was preserved in our laboratory. Yeast extract and peptone were purchased from Oxoid (Hampshire, England). T4 DNA ligase, BglII, and BamHI were bought from TaKaRa (Otsu, Japan). Birchwood xylan was obtained from Sigma. All other chemicals used in this study not specifically mentioned were of analytical grade and commercially available.
Construction and Identification of the Recombinant Yeast
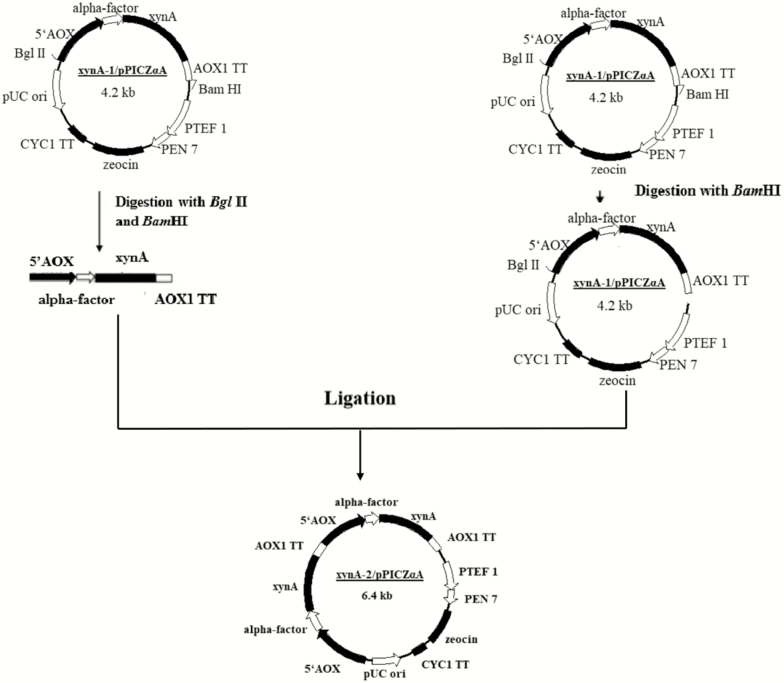
The pPIC-xynA-1 was digested with BglII and BamHI at 37 °C overnight. The gene expression cassette was recovered and purified using Gel Extraction Kit (Tiangen, China). The purified gene cassette was ligated to the pPIC-xynA-1 pre-treated with BamHI. Then, the ligation mixtures were transformed into E. coli Top10 cell. The construction roadmap was shown in Figure 1. The positive colonies were selected and cultured on LB plates (1% tryptone, 0.5% NaCl, 0.5% yeast extract, and 1.5% agar) containing 25 μg/mL of zeocin. The recombinant plasmid, pPIC-xynA-2, was identified by enzyme digestion and sequence analysis.
Figure 1.
A schematic diagram of the construction of the recombinant expression vector.
The pPIC-xynA-2 was transformed into P. pastoris X-33 competent cell by electroporation (2,000 V, 5 ms). Transformants were cultivated on YPDS plates (1% yeast extract, 2% peptone, 1 mol/L sorbitol, and 2% agar) with 100 μg/mL of zeocin. The recombinant strains (X-33/xynA-2) were picked up and cultivated on YPD plates.
X-33/xynA-2 was cultured in 25 mL of buffered glycerol-complex medium (100 mM potassium phosphate, 0.34% YNB, 4 × 10−5% biotin, 1.00% glycerol, 1.00% yeast extract, and 2.00% peptone; pH 6.0) at 28 °C and 250 rpm for 1 d. The cells were harvested by centrifugation at room temperature and 5,000 rpm for 5 min. The cell pellet was resuspended to an OD600 of 1.0 in buffered methanol-complex medium (100 mM potassium phosphate, 0.34% YNB, 4 × 10−5% biotin, 0.50% methanol, 1.00% yeast extract, and 2.00% peptone; pH 6.0) to induce expression in a flask at 250 rpm under 28 °C. Inductive expression was carried out with the addition of methanol (0.5%, v/v) per 24 h for 72 h.
After collecting supernatant of the culture, the expression of AT-xynA was confirmed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. The enzyme activity was measured as previously reported (Yang et al. 2017b). One unit (U) of enzyme activity was defined as the amount of recombinant xylanase that liberated 1 μmol reducing sugar (xylose) from the substrate solution per minute at conditions of pH 3.0 and 55 °C.
Quantitative real-time PCR was performed to determine xynA gene copy number of X-33/xynA-1 and X-33/xynA-2 on Applied Biosystems 7500 Real-Time PCR System (Life Technologies, CA) according to the method of Yang et al. (2017b).
Production of AT-xynA
High-density fermentation of X-33/xynA-2 was processed according to the method of Lv et al. (2013). The recombinant strain was cultured in 2 L of basic liquid medium (50 g glucose, 5 g KH2PO4, 0.93 g CaSO4, 18.2 g K2SO4, 14.9 g MgSO4, 1.5 g KOH, and 50 g NH4H2PO4/L) for 24 h. Then, 50% (w/v) glycerol was added into the medium after glucose was exhausted. In the process of fermentation, pH of the medium was adjusted to 5.5 using ammonia, the dissolved oxygen concentration was controlled above 20% by adjusting the rotation speed and air flow, and the temperature was controlled at 28 °C. When the biomass reached 256 g/L, we stopped adding glycerol and started to add methanol to induce the expression. The biomass and enzyme activities of the samples that collected every 12 h were measured.
The actual AT-xynA product used in this study was obtained by mixing 66% of the liquid fermentation broth produced above with 34% wheat bran and then air drying for 24 h. This resulted in AT-xynA preparation containing approximately 2,820,000 units (U) of xylanase per kilogram.
Animals Feeding and Dietary Treatments
All animal experimental procedures were approved by China Agricultural University Laboratory Animal Welfare and Animal Experimental Ethical Inspection Committee (Beijing, China). A total of 144 crossbred (Duroc × Landrace × Yorkshire) weaned piglets at 28 d with average initial BW of 8.06 ± 1.31 kg were allotted to treatments. They were randomly allotted by sex and BW to four groups: AT-xynA added at a concentration of 0 U/kg diet (CON), 2,000 U/kg diet (LD), 4,000 U/kg diet (MD), and 6,000 U/kg diet (HD) in wheat–soybean-based diets. Each group consisted of six replicates with six piglets per replicate (equal barrows and gilts/replicate). The diets contained 3,542 kcal/kg of digestible energy, 20.24% of CP (Table 1). The standard ileal digestible lysine, methionine, and threonine of diets all satisfied the National Research Council (NRC, 2012) requirements. 0.3% chromic oxide (Cr2O3) was included in each diet as an indigestible marker to calculate apparent total tract digestibility (ATTD). The experiment lasted 28 d (early phase, days 0 to 14; late phase, days 15 to 28). The piglets were housed under standard conditions. They had access to water and food ad libitum.
Table 1.
Composition and nutrient levels of diets (as fed)
| Item | Diet |
|---|---|
| Ingredient, % | |
| Wheat | 61.55 |
| Soybean meal | 11.51 |
| Extruded soybean | 5.00 |
| Fish oil | 3.00 |
| Whey powder | 10.00 |
| Soybean protein concentrate | 2.00 |
| Soybean oil | 3.25 |
| Dicalcium phosphate | 1.00 |
| Limestone | 0.82 |
| Salt | 0.15 |
| l-lysine HCl | 0.61 |
| Methionine | 0.13 |
| Threonine | 0.18 |
| Chromic oxide | 0.30 |
| Vitamin–mineral premix1 | 0.50 |
| Total | 100.00 |
| Chemical composition (as fed) | |
| Digestible energy, kcal/kg2 | 3,542 |
| SID3 lysine, %2 | 1.35 |
| SID3 methionine, %2 | 0.39 |
| CP, %4 | 20.24 |
| Calcium, %4 | 0.80 |
| Phosphorous, %4 | 0.67 |
1Premix provided the following per kilogram of complete diet: vitamin A, 12,000 IU; vitamin D3, 2,500 IU; vitamin E, 30 IU; vitamin K3, 3 mg; vitamin B1, 0.96 mg; vitamin B2, 5.2 mg; vitamin B6, 2 mg; vitamin B12, 0.012 mg; nicotinic acid, 40 mg; pantothenic acid, 15 mg; folic acid, 0.4 mg; biotin, 0.04 mg; choline chloride, 0.4 g; Fe, 90 mg; Cu, 10 mg; Zn, 80 mg; Mn, 16 mg; I, 0.24 mg; Se, 0.3 mg; NaCl, 4.4 g.
2Values were calculated according to NRC (2012).
3SID: standardized ileal digestible.
4Analyzed values.
Sample Collection and Processing
One piglet from each pen was slaughtered at the end of the experiment. Ileal, cecal, and colonic digesta were collected. The digesta were immediately placed in liquid nitrogen and kept at −80 °C until analysis.
The ADFI, ADG, and G:F were calculated by measuring BW and feed intake of each pen on experimental days 0, 14, and 28. Fecal samples were collected from each pen on the last 3 d of the study to calculate nutrient digestibility. The fecal samples were dried in a forced-air oven for 72 h at 65 °C. Then, the fecal samples of each pen were ground through a 1-mm screen. The nutrient digestibility can be calculated by the following equation: ND (%) = 1 − [(DC × FN)/(FC × DN)] × 100%. In this equation, ND represents ATTD, DC is the content of Cr2O3 in diets (%), FN is the content of the nutrient in feces (%), FC is the content of Cr2O3 in feces (%), DN is the content of the nutrient in diets (%).
Analysis of diets and fecal samples was performed according to methods of the Association of Official Analytical Chemists (AOAC, 2006). GE, CP (Method 990.03), DM (Method 934.01), ether extract (EE), ash and chromium of feed and fecal samples were analyzed. An adiabatic oxygen bomb calorimeter (Parr Instruments, Moline, IL) was used to measure GE. The concentrations of chromium were measured via the Polarized Zeeman Atomic Absorption Spectrometer (Hitachi Z2000, Tokyo, Japan). The concentrations of SCFA in ileal, cecal, colonic digesta were analyzed as previously reported (Porter and Murray, 2001). All samples were run in duplicate and all errors were <5%.
Gut Microbial Diversity Analysis
In order to analyze intestinal microbial diversity, DNA of ileal, cecal, and colonic digesta samples from four treatments were extracted using the E.Z.N.A. stool DNA kit (Omega Biotek, Norcross, GA). The V3–V4 region of the 16S rRNA gene was amplified by PCR with primers 338F (5′barcodeACTCCTACGGG AGGCAGCAG3′) and 806R (5′GGACTACHVGGGTWTCTAAT3′). The PCR reaction procedure was 95 °C for 3 min, 27 cycles (95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s), finally 72 °C for 10 min. The PCR products were examined by electrophoresis with 2% agarose gel and purified using the AxyPrep DNA Gel Extraction kit (Axygen Biosciences, Union City, CA). The purified PCR products were quantified using QuantiFluorST (Promega, Madison, WI) and then sequenced on the Illmumina MiSeq platform as described previously.
Trimmomatic (version 3.29) was used to quality-filter raw sequences. The analysis of raw sequences was conducted in accordance with the following criteria: (i) the read data were truncated on any site with an average quality score <20 over a sliding window of 50 bp; (ii) only gene sequences with overlap longer than 10 bp were merged. (iii) The maximum allowable mismatch ratio for merged overlap sequence was 0.2. Operational taxonomic units (OTUs) with 97% similarity were clustered by using UPARSE (version 7.0 http://drive5. com/uparse/). During the clustering process, chimeras were removed and representative OTUs sequences were obtained. The 16S rRNA gene sequence was classified and analyzed by RDP Classifier (version 2.2 http://sourceforge.net/projects/rdp-classifier/) against the Silva (SSU123) 16S rRNA database. The raw data were deposited in the Sequence Read Archive NCBI database (Accession Number: SUB5390933).
Statistical Analysis
The data of growth performance, nutrient digestibility, and SCFA concentrations were analyzed by the procedure of SAS (version 9.2, 2008). Pen was considered as an experimental unit. Statistical differences among different treatments were analyzed using the one-way ANOVA. Linear and quadratic comparisons were also applied for evaluating effects of different levels of AT-xynA supplementation. The Kruskal–Wallis method was used to analyze the bacterial community in the ileal, cecal, and colonic digesta samples from weaned piglets at the phylum, family, and genus level. The Welch’s test was used to analyze the bacterial community between two treatments. P < 0.05 was considered as a significant difference.
RESULTS
Construction of Recombinant Yeast and AT-xynA Production
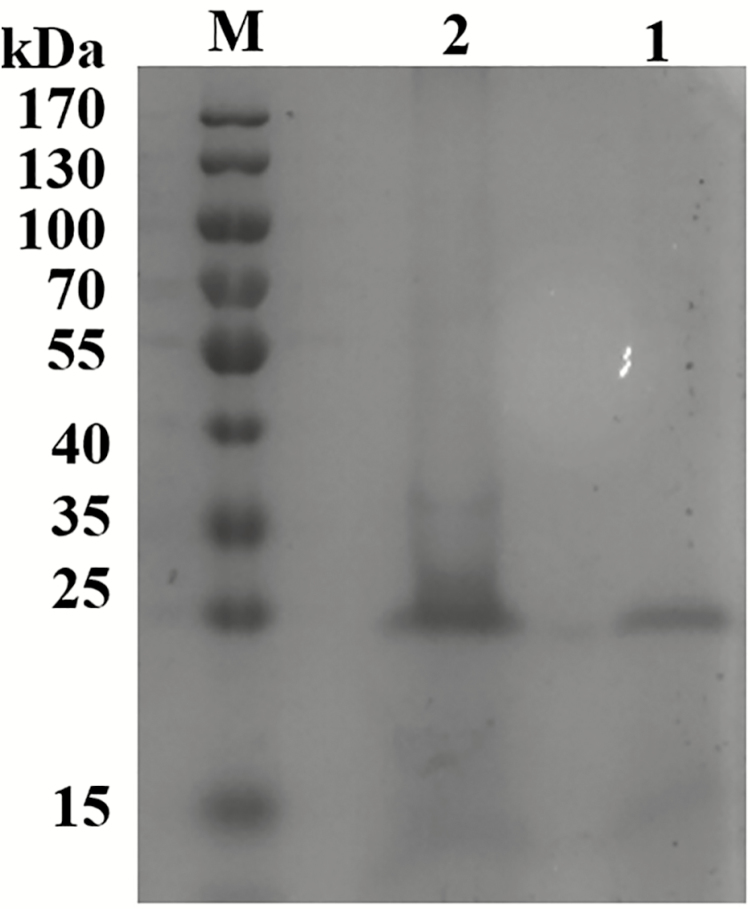
The recombinant strains (X-33/xynA-2 and X-33/xynA-1) were cultured in the medium for enzyme induction, respectively. After induction by methanol for 72 h, the activity in the supernatant of X-33/xynA-2 culture was 2.2 times of that in X-33/xynA-1 (Supplementary Table S1). The single protein bands of AT-xynA were observed on a 12% SDS-PAGE (Figure 2). SDS-PAGE analysis showed that the molecular weight of AT-xynA was ~25 kDa. The copy number of xynA gene was determined by real-time qPCR. As expected, results indicated that X-33/xynA-1 was single-copy insertion and X-33/xynA-2 was double-copy insertion (Table 2).
Figure 2.
SDS-PAGE analysis of recombinant X-33/xynA-1 and X-33/xynA-2 proteins. Lane M, protein molecular weight marker; Lane 1, recombinant X-33/xynA-1 protein in the culture supernatant; Lane 2, recombinant X-33/xynA-2 protein in the culture supernatant.
Table 2.
The copy number of X-33/xynA-1 and X-33/xynA-2 in P. pastoris genome
| Strain | xynA CT1 | GAP CT1 | ΔCT (Avg.xynA CT-Avg.GAP CT)1 | ΔΔCT(ΔCT-ΔCTxynA2)1 | Normalized amount |
|---|---|---|---|---|---|
| xynA-1 | 12.95 ± 0.06 | 13.62 ± 0.05 | -0.67 ± 0.05 | 1.43 ± 0.05 | 0.37 (0.36–0.38) |
| xynA-2 | 10.22 ± 0.17 | 13.31 ± 0.21 | -2.10 ± 0.30 | 0.00 ± 0.30 | 1.00 (0.80–1.20) |
1Values are means ± SD (n = 3).
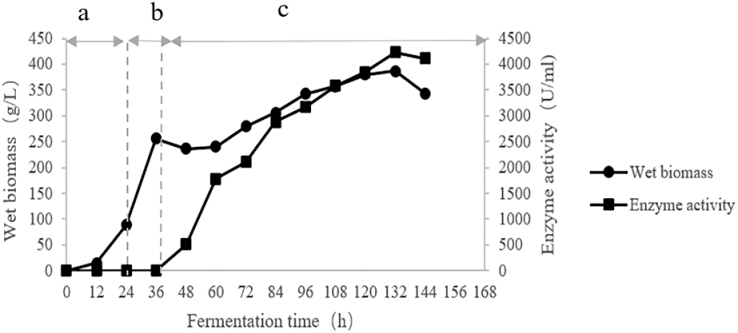
The expression level of heterologous genes is controlled by engineering strains and appropriate fermentation conditions. In this study, X-33/xynA-2 was cultured in a 5-L fermentor. When the biomass reached 88.5 g/L, glycerol was fed continuously and cell growth continued. When the biomass reached 256 g/L, methanol was added and cell growth slowed. After 144 h fermentation time, the expression level of AT-xynA was high, reaching 4,115.2 IU/mL, the biomass was 343.6 g/L (Figure 3).
Figure 3.
Growth curve and enzyme activity of AT-xynA in 5-L fermentor. (a) Glucose batch phase; (b) glycerol fed-batch phase; (c) methanol induction phase; the samples that collected every 12 h were measured by biomass and enzyme activities.
Growth Performance and Nutrient Digestibility
The growth performance of weaned piglets was presented in Table 3. In the early phase (days 0 to 14 of the experiment), ADG of MD and HD groups increased compared with CON group (P < 0.05). In the late phase (days 14 to 28 of the experiment), ADG of MD group was higher compared with CON group (P < 0.05). In the entire phase (days 0 to 28 of the experiment), ADG of MD and HD groups improved compared to CON group (P < 0.05), ADFI of MD and HD groups was greater compared with pigs fed the control diet (P < 0.05).
Table 3.
Effects of different levels of AT-xynA on performance of weaned piglets1
| AT-xynA, U/kg | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 2,000 | 4,000 | 6,000 | SEM | ANOVA | Linear | Quadratic |
| Days 0 to 14 | ||||||||
| ADG (g) | 323.23b | 353.39ab | 422.37a | 423.65a | 20 | 0.06 | 0.01 | 0.71 |
| ADFI (g) | 463.17 | 533.02 | 546.82 | 575.10 | 18 | 0.08 | 0.02 | 0.48 |
| G:F | 0.70 | 0.74 | 0.74 | 0.78 | 0.05 | 0.32 | 0.56 | 0.74 |
| Days 15 to 28 | ||||||||
| ADG (g) | 342.85b | 396.08ab | 483.02a | 432.35ab | 23 | <0.01 | <0.01 | 0.05 |
| ADFI (g) | 682.15 | 704.82 | 776.89 | 706.30 | 24 | 0.15 | 0.28 | 0.12 |
| G:F | 0.50 | 0.54 | 0.63 | 0.59 | 0.04 | 0.17 | 0.10 | 0.35 |
| Days 0 to 28 | ||||||||
| ADG (g) | 323.41b | 362.86ab | 415.12a | 406.04a | 16 | <0.01 | <0.01 | 0.10 |
| ADFI (g) | 572.66b | 626.86ab | 661.86a | 646.03a | 17 | 0.02 | 0.02 | 0.09 |
| G:F | 0.57 | 0.58 | 0.63 | 0.63 | 0.03 | 0.56 | 0.19 | 0.86 |
1In the same row, values with different small letter superscripts mean significant difference (P < 0.05) G:F, ratio of ADG/ADFI.
The influence of AT-xynA supplementation on nutrient digestibility of weaned piglets was shown in Table 4. Compared with CON group, the digestibility of OM and DM in treatment groups was higher (P < 0.01). The digestibility of GE in LD and MD groups was also significantly higher than that in CON group (P < 0.05). There were no differences on the digestibility of CP and EE among groups (P > 0.05).
Table 4.
Effects of dietary supplementation with different levels of AT-xynA on nutrient digestibility (%) in weaned piglets1
| AT-xynA, U/kg | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 2,000 | 4,000 | 6,000 | SEM | ANOVA | Linear | Quadratic |
| Days 0 to 28 | ||||||||
| DM | 85.44b | 88.03a | 87.34a | 87.73a | 0.37 | <0.01 | <0.01 | <0.01 |
| OM | 88.24b | 91.86a | 91.75a | 90.84a | 0.37 | <0.01 | <0.01 | <0.01 |
| CP | 81.90 | 81.19 | 83.87 | 78.43 | 2.33 | 0.48 | 0.48 | 0.32 |
| GE | 82.43b | 85.56a | 85.25a | 84.20ab | 0.65 | 0.01 | 0.09 | <0.01 |
| EE | 66.51 | 60.57 | 64.41 | 68.99 | 2.05 | 0.30 | 0.35 | 0.11 |
1In the same row, values with different small letter superscripts mean significant difference (P < 0.05). EE, ether extract.
Microbial Metabolites
The results of intestinal SCFA concentrations were shown in Table 5. AT-xynA supplementation increased acetate concentrations in the ileal (P < 0.01) and cecal digesta (P < 0.05). The isobutyrate (P < 0.05) and valerate (P < 0.05) concentrations in the colonic digesta of MD and HD groups also significantly increased compared with CON group.
Table 5.
Effects of dietary supplementation with different levels of AT-xynA on SCFA concentrations (mg/kg) in different intestinal sites of weaned piglets1
| AT-xynA, U/kg | |||||
|---|---|---|---|---|---|
| Item | 0 | 2000 | 4000 | 6000 | P-value |
| Acetate | |||||
| Ileum | 167.6b | 449.4a | 445.6a | 475.4a | <0.01 |
| Cecum | 2874.6b | 4070.0a | 4152.0a | 4036.5a | 0.01 |
| Colon | 3382.0 | 3606.6 | 3901.2 | 3643.7 | 0.25 |
| Propionate | |||||
| Cecum | 2352.2 | 2675.8 | 2384.0 | 2760.8 | 0.50 |
| Colon | 2333.9 | 2625.5 | 2707.6 | 2345.7 | 0.21 |
| Isobutyrate | |||||
| Cecum | 345.2 | 336.1 | 343.2 | 385.2 | 0.70 |
| Colon | 107.3b | 116.1b | 167.6a | 147.6a | 0.02 |
| Butyrate | |||||
| Cecum | 987.7 | 1056.0 | 917.9 | 950.2 | 0.34 |
| Colon | 1562.8 | 1720.4 | 1540.8 | 1593.4 | 0.83 |
| Isovalerate | |||||
| Cecum | 137.5 | 171.5 | 168.4 | 136.1 | 0.30 |
| Colon | 147.0 | 151.0 | 145.8 | 128.9 | 0.40 |
| Valerate | |||||
| Cecum | 62.5 | 142.7 | 87.6 | 131.6 | 0.53 |
| Colon | 137.8b | 157.8b | 218.8a | 238.4a | <0.01 |
1In the same row, values with different small letter superscripts mean significant difference (P < 0.05).
Bacterial Community
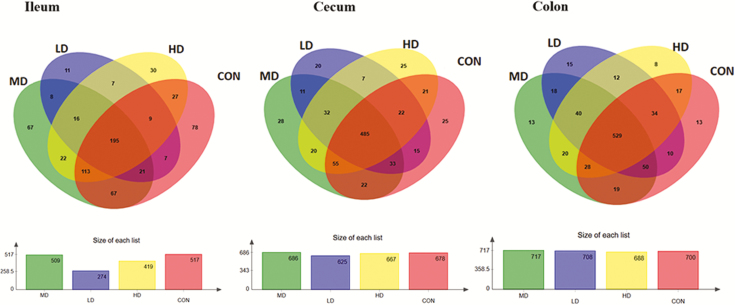
The OTUs of bacteria community were classified from valid sequence at a 97% similarity level. The analysis of OTUs was shown using Venn Diagrams (Figure 4). OTUs and α diversity indices could reflect richness and evenness of bacterial community. The indices of sobs (OTUs), Ace, and Chao represented the richness of bacterial community. The indices of Shannon, Simpson, and Coverage represented the evenness of bacterial community. The α diversity indices of ileal, cecal, and colonic digesta samples were not affected (P > 0.05) by AT-xynA supplementation (Supplementary Tables S2–S4).
Figure 4.
The bacterial OTU community composition of the ileum, cecum, and colon in weaned piglets. Venn diagrams of the bacterial OTU community among four groups. The results were analyzed by the Kruskal–Wallis H test and showed as the average percentage of different bacteria. OTUs, operational taxonomic units.
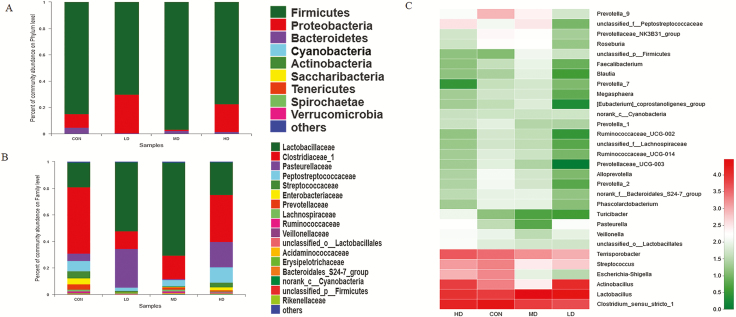
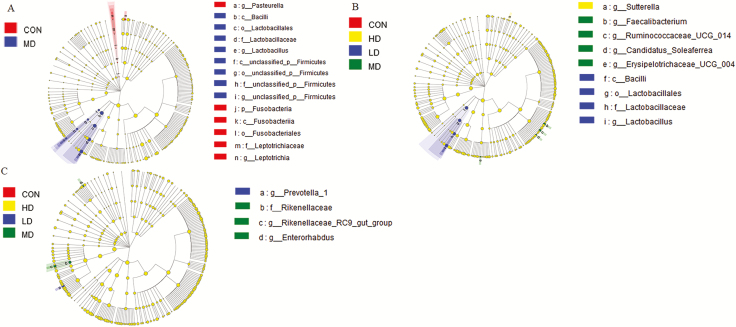
At the phylum level, nine phyla in ileal samples of weaned piglets were shown in Figure 5A. Firmicutes, Proteobacteria, and Bacteroidetes were dominant bacteria. The analysis showed that AT-xynA supplementation did not affect (P > 0.05) the bacterial community at the phylum level in ileal samples (Supplementary Table S5). The effects of different levels of AT-xynA on ileal bacterial community at the family level were shown (Figure 5B; Supplementary Table S6). MD group showed a significantly higher abundance of Lactobacillaceae than CON group in ileal digesta (P < 0.05). The abundance of the top 30 bacterial communities was analyzed using heatmap at the level of genus. The heatmap showed similarities and differences of bacterial community among different samples. The bacterial communities with high abundance were indicated in red regions of heatmap. As shown in Figure 5C, Lactobacillus abundance in CON group of the ileal digesta was lower than that in MD group (P < 0.05). Different bacterial communities were analyzed from phylum to genus levels in the cladogram of LEfSe (Figure 8A). Circles from inner to outer indicated different bacteria from phylum to genus levels. The yellow dots in the circle showed that there were no significant differences among different groups. The abundance of Bacilli and Firmicutes of MD group at the class level were higher compared with CON group (P < 0.05). Pasteurella and Leptotrichia abundance in MD group at the genus level were lower compared with CON group (P < 0.05).
Figure 5.
Effects of AT-xynA supplementation on ileal bacterial community of weaned piglets in phylum, family, and genus level. (A) Microbial community on the phylum level with the relative abundance higher than 0.01%. (B) Microbial community on the family level with the relative abundance higher than 0.1%. (C) Microbial community heatmap of the bacteria on the genus level. The bacterial phylogenetic tree was calculated by the neighbor-joining method. The different colors represented the bacteria abundance in four groups. CON was AT-xynA unsupplemented group; LD was the supplemented group with 2,000 U/kg AT-xynA; MD was the supplemented group with 4,000 U/kg AT-xynA; HD was the supplemented group with 6,000 U/kg AT-xynA.
Figure 8.
The analysis for different bacteria communities from phylum to genus level in the cladogram of LEfSe among four different groups. (A) Different bacteria communities from phylum to genus level in ileal samples between CON group and MD group. (B) Different bacteria communities from phylum to genus level in cecal samples among four groups. (C) Different bacteria communities from phylum to genus level in colonic samples among four groups.
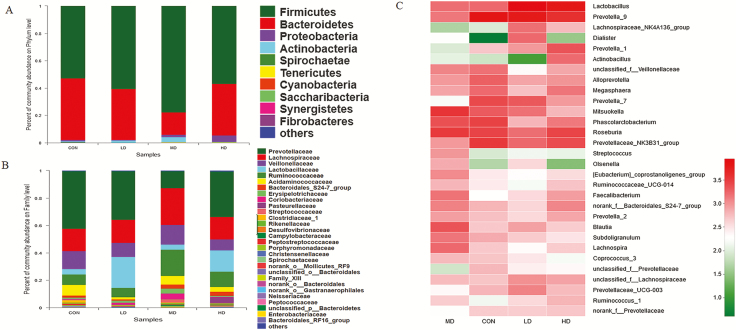
The bacterial community at the phylum level in the cecal digesta of weaned piglets was shown in Figure 6A and Supplementary Table S7. Firmicutes and Bacteroidetes were the most abundant bacteria in four treatments, and total abundance accounted for above 93%. The addition of AT-xynA did not change bacterial community of the cecal digesta at the phylum level (P > 0.05). At the family level, the abundance of bacterial community was shown in Figure 6B and Supplementary Table S8. In the cecal digesta of MD group, Ruminococcaceae abundance increased compared with that in CON group (P < 0.05). The top 30 bacterial communities at the genus level of the cecal digesta were shown in Figure 5C. As shown in Figure 8B, the abundance of Faecalibacterium, Ruminococcaceae_ UCG_014, and Candidatus_Soleaferrea genus belonging to Ruminococcaceae family of MD group was higher than other groups (P < 0.05). The genus Erysipelotrichaceae_UCG_004 abundance in MD group was also higher than that in other groups (P < 0.05). The abundance of Bacilli class including Lactobacillus of LD group also increased (P < 0.05).
Figure 6.
Effects of AT-xynA supplementation on cecal bacterial community of weaned piglets in phylum, family, and genus level. (A) Microbial community on the phylum level with the relative abundance higher than 0.01%. (B) Microbial community on the family level with the relative abundance higher than 0.1%. (C) Microbial community heatmap of the bacteria on the genus level. The bacterial phylogenetic tree was calculated by the neighbor-joining method. The different colors represented the bacteria abundance in four groups. CON was AT-xynA unsupplemented group; LD was the supplemented group with 2,000 U/kg AT-xynA; MD was the supplemented group with 4,000 U/kg AT-xynA; HD was the supplemented group with 6,000 U/kg AT-xynA.
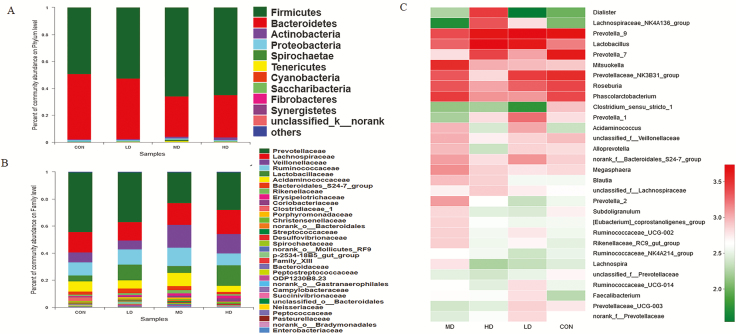
The abundance of colonic bacteria at the phylum level in four dietary treatments was also analyzed (Figure 7A; Supplementary Table S9). Firmicutes and Bacteroidetes were the most abundant bacteria. MD group and HD group had significantly higher abundance of Firmicutes (P < 0.05) and lower abundance of Bacteroidetes (P < 0.05) than CON group. At the family level, the bacterial community was shown in Figure 7B and Supplementary Table S10. Lactobacillaceae abundance in HD group increased compared that in CON group (P < 0.05). The top 30 bacterial communities at the genus level of colonic digesta were shown in Figure 7C. There was also a significant increase of Lactobacillus abundance in HD group compared with that in CON group (P < 0.05). As shown in Figure 8C, compared with other groups, genus Prevotella abundance of LD group increased (P < 0.05) and the abundance of Enterorhabdus and Rikenellaceae_RC9_gut_group genus also increased in MD group (P < 0.05).
Figure 7.
Effects of AT-xynA supplementation on colonic bacterial community of weaned piglets in phylum, family, and genus level. (A) Microbial community on the phylum level with the relative abundance higher than 0.01%. (B) Microbial community on the family level with the relative abundance higher than 0.1%. (C) Microbial community heatmap of the bacteria on the genus level. The bacterial phylogenetic tree was calculated by the neighbor-joining method. The different colors represented the bacteria abundance in four groups. CON was AT-xynA unsupplemented group; LD was the supplemented group with 2,000 U/kg AT-xynA; MD was the supplemented group with 4,000 U/kg AT-xynA; HD was the supplemented group with 6,000 U/kg AT-xynA.
Discussion
Endo-β-1,4-xylanases can effectively catalyze the hydrolysis of xylan to produce a mixture of xylo-oligosaccharides. Although a lot of microorganisms can secrete xylanases, the total activities of xylanases produced from original strains are usually low. P. pastoris is a heterologous expression system which has been utilized to effectively improve expression level of exogenous genes. Furthermore, increasing the copy number of heterologous genes can effectively enhance heterologous protein yield in P. pastoris. Jiao et al. (2018) found that expression level of six-copy transformant was 83% higher than a single-copy transformant. The activities of recombinants carrying one, two, and three copies were lower than the recombinant carrying four copies of GalA gene in P. pastoris (Han et al., 2019). The aim of our experiment was to improve the expression level of AT-xynA by increasing the copy number of xynA. As expected, the activity of AT-xynA increased 2.2 times. The screened strain was cultured in a 5-L fermenter, reaching final activity of 4,115.2 IU/mL.
The application of xylanases is influenced by the animal gastrointestinal environment in animal feed. The pH in the stomach rarely rises above 3.0. The low pH may be detrimental to enzyme activities. However, AT-xynA showed optimal activity at pH 3.0. AT-xynA also maintained acid resistance. Furthermore, AT-xynA displayed higher enzyme activity compared with other acidic xylanases expressed in P. pastoris (Wu et al., 2017; Li et al., 2018). The thermal stability of AT-xynA may also effectively reduce the loss of enzyme activity during feed processing. Thus, AT-xynA with high activity at low pH and high temperature was suitable for large-scale production in animal feed. In this study, we are committed to exploring the application of AT-xynA in animal feed.
Xylan is the main component of NSP in wheat. The addition of NSP to piglet diets reduces the digestion and absorption of nutrients (Taylor et al., 2018). Xylanases destroy the complex cross-linking structure of NSP by degrading the xylan in the plant cell wall. Compared with CON group, the addition of AT-xynA improved ADG and ADFI of weaned piglets, especially AT-xynA at a concentration of 4,000 and 6,000 U/kg diet. Dong et al. (2018) also found that the xylanase supplementation to wheat-based diets significantly increased ADG of weaned piglets. Our results showed that adding AT-xynA to wheat-based diets, ATTD of GE, DM, and OM all increased. Sterk et al. (2007) also observed that the xylanase supplementation improved apparent digestibility of nutrients in piglets. It is expected the improved digestibility with xylanase supplementation was due to degradation of xylan resulting in nutrient release. Xylan was degraded into small molecules, which correspondingly reduced chyme viscosity and increased the contact between digestive enzymes and nutrients (Kim et al., 2005; Gonzalez-Ortiz et al., 2016). Our results suggested AT-xynA supplementation to wheat-based diets alleviated negative effects of NSP in weaned piglets. AT-xynA improved digestibility of nutrients and growth performance in weaned piglets.
After weaning, the environment of gastrointestinal tract in piglets is altered because of weaning stress (Lalles et al., 2007). Alterations of nutrients availability and gut environment affect gut microbiota. SCFA are metabolites of intestinal microorganisms. The results showed that AT-xynA supplementation increased the concentration of acetate in the ileal digesta. Diebold et al. (2004) also reported addition of xylanases increased the concentration of acetate in the ileal digesta of weaned piglets. Xylanases in the small intestine degrade xylan into xylo-oligosaccharides, which stimulate fermentation of intestinal microorganisms to produce SCFA (Diebold et al., 2004). Compared with CON group, the addition of AT-xynA also increased concentrations of acetate in the cecal digesta, isobutyrate, and valerate in the colonic digesta. A lot of microorganisms in the large intestine of piglets can ferment carbohydrates and produce a variety of SCFA. These acids play an important role in the health of hindgut of piglets and provide necessary nutrients for the body. Acetate could enter the peripheral circulation and then participate in cholesterol synthesis in the liver (Wolever et al., 1989). The increase of isobutyrate production may be associated with the lactate utilization (Shota et al., 2018). Therefore, AT-xynA supplementation was considered beneficial to the health of weaned piglets.
The addition of AT-xynA had certain effects on the composition of intestinal flora in piglets. Compared with CON group, the abundance of Leptotrichia, a genus belonging to the phylum Fusobacteira, in MD group of the ileal digesta was significantly lower. Recent studies have proposed that Fusobacteria could lead to some diseases of the gut. Diarrhea in newborn piglets was also related to the increase of Fusobacteira abundance (Cheng et al., 2018). Leptotrichia has been reported to play a key role in the pathogenesis of intestinal diseases (Liu et al., 2015). Leptotrichia was considered to play a potential critical role in the transition from health to disease in piglets. Proteobacteria is known to be associated with different diseases. The higher abundance of Pasteurella included in the Proteobacteria phylum in CON group may be considered a sign of dysbiosis (Shin et al., 2015). The enriched proportion of Lactobacillus in MD group may be because xylo-oligosaccharides are one of the preferred substrates for Lactobacillus (Zhang et al., 2018).
Consistent with previous findings, Firmicutes and Bacteroidetes were the most abundant phyla in the cecal digesta of weaned pigs (Tan et al., 2017). Faecalibacterium, Ruminococcaceae_UCG_014, and Candidatus_Soleaferrea contained in Ruminococcaceae family were significantly higher in MD group than in CON group. The increase of Ruminococcaceae abundance may be a reason for degradation of polysaccharides and fibers (Shang et al., 2016). Many glycoside hydrolase genes contained in Ruminococcaceae could degrade xylan (Zhang et al., 2019). The increase of Ruminococcaceae abundance in MD group may be one reason why the addition of AT-xynA effectively improved digestibility of nutrients. Faecalibacterium is the main bacterium to produce SCFA (Li et al., 2019). The increase of Faecalibacterium may explain the higher concentration of acetate in cecal digesta of AT-xynA supplemented groups.
We speculated that the enrichment of Lactobacillaceae in the colonic digesta of HD group was also associated with AT-xynA supplementation. Lactobacillaceae were related to levels of β-xylosidase in the intestine. Lactobacillaceae could promote the utilization rate of nutrients by fermentation (Le Sciellour et al., 2018). The higher abundance of Prevotella in LD group might be related to digestion and utilization of carbohydrates in the intestine. Prevotella could help to ferment complex polysaccharides (Li et al., 2019).
In conclusion, the results suggested that increasing xynA copy number could effectively improve AT-xynA activity. Adding AT-xynA to wheat-based diets improved growth performance and nutrient digestibility of weaned piglets. AT-xynA supplementation increased the presence of NSP-degrading bacteria, such as Ruminococcaceae, Prevotella in the intestines of weaned piglets. The increase of Lactobacillus was also observed in the ileal, cecal, and colonic digesta of AT-xynA supplemented groups. AT-xynA was considered to effectively alleviate negative effects of NSP in the intestine. The acidic thermostable xylanase, AT-xynA has broad application prospects in weaned piglets. AT-xynA at a concentration of 4,000 U/kg diet was considered an optimal inclusion level in wheat-based diets.
Funding
This project was supported by the National Science and Technology Support Program (2013BAD10B01).
Disclosures
The authors declare no conflict of interest.
Supplementary Material
LITERATURE CITED
- Amerah A. M. 2015. Interactions between wheat characteristics and feed enzyme supplementation in broiler diets. Anim. Feed. Sci. Technol. 199:1–9. doi:10.1016/j.anifeedsci.2014.09.012 [Google Scholar]
- AOAC 2006. Official methods of analysis. 18th ed.Arlington, VA:AOAC International. [Google Scholar]
- Barrera M., Cervantes M., Sauer W. C., Araiza A. B., Torrentera N., and Cervantes M.. . 2004. Ileal amino acid digestibility and performance of growing pigs fed wheat-based diets supplemented with xylanase. J. Anim. Sci. 82:1997–2003. doi:10.2527/2004.8271997x [DOI] [PubMed] [Google Scholar]
- Camara E., Albiol J., and Ferrer P.. . 2016. Droplet digital PCR-aided screening and characterization of Pichia pastoris multiple gene copy strains. Biotechnol. Bioeng. 113:1542–1551. doi:10.1002/bit.25916 [DOI] [PubMed] [Google Scholar]
- Cao Y., Chen X., He P., and Lu W.. . 2006. Cloning, expression and enzyme characterization analysis of Aspergillus sulphureus xylanase gene xynA. Lett. Biotechnol. 17:858–861. [Google Scholar]
- Cao Y., Qiao J., Li Y., and Lu W.. . 2007. De novo synthesis, constitutive expression of Aspergillus sulphureus beta-xylanase gene in Pichia pastoris and partial enzymic characterization. Appl. Microbiol. Biotechnol. 76:579–585. doi:10.1007/s00253-007-0978-9 [DOI] [PubMed] [Google Scholar]
- Cheng C., Wei H., Yu H., Xu C., Jiang S., and Peng J.. . 2018. Metabolic syndrome during perinatal period in sows and the link with gut microbiota and metabolites. Front. Microbiol. 9:1989. doi:10.3389/fmicb.2018.01989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold G., Mosenthin R., Piepho H. P., and Sauer W. C.. . 2004. Effect of supplementation of xylanase and phospholipase to a wheat-based diet for weanling pigs on nutrient digestibility and concentrations of microbial metabolites in ileal digesta and feces. J. Anim. Sci. 82:2647–2656. doi:10.2527/2004.8292647x [DOI] [PubMed] [Google Scholar]
- Dong B., Liu S., Wang C., and Cao Y.. . 2018. Effects of xylanase supplementation to wheat-based diets on growth performance, nutrient digestibility and gut microbes in weanling pigs. Asian-Australas. J. Anim. Sci. 31:1491–1499. doi:10.5713/ajas.17.0867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ortiz G., Olukosi O., and Bedford M. R.. . 2016. Evaluation of the effect of different wheats and xylanase supplementation on performance, nutrient and energy utilisation in broiler chicks. Anim. Nutr. 2:173–179. doi:10.1016/j.aninu.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z. G., Zhang J. W., Jiang X. F., and Yang J. K.. . 2019. Gene dosage and coexpression with endoplasmic reticulum secretion-associated factors improved the secretory expression of α-galactosidase. Protein Expr. Purif. 153:83–91. doi:10.1016/j.pep.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Inborr J., Puhakka J., Bakker J. G., and Van der Meulen J.. . 1999. Beta-glucanase and xylanase activities in stomach and ileum of growing pigs fed wheat bran based diets with and without enzyme treatment. Arch. Tierernahr. 52:263–274. doi:10.1080/17450399909386166 [DOI] [PubMed] [Google Scholar]
- Jiao L., Zhou Q., Su Z., Xu L., and Yan Y.. . 2018. High-level extracellular production of Rhizopus oryzae lipase in Pichia pastoris via a strategy combining optimization of gene-copy number with co-expression of ERAD-related proteins. Protein Expr. Purif. 147:1–12. doi:10.1016/j.pep.2018.02.005 [DOI] [PubMed] [Google Scholar]
- Juturu V., and Wu J. C.. . 2012. Microbial xylanases: engineering, production and industrial applications. Biotechnol. Adv. 30:1219–1227. doi:10.1016/j.biotechadv.2011.11.006 [DOI] [PubMed] [Google Scholar]
- Kiarie E., Walsh M. C., Romero L. F., and Baidoo S. K.. . 2016. Digestibility responses of growing pigs fed corn plus corn distiller grains or wheat plus wheat coproduct-based diets without or with supplemental xylanase. J. Anim. Sci. 94:211–214. doi:10.2527/jas.2015–9736 [Google Scholar]
- Kim J. C., Simmins P. H., Mullan B. P., and Pluske J. R.. . 2005. The digestible energy value of wheat for pigs, with special reference to the post-weaned animal [Review]. Anim. Feed. Sci. Technol. 122:257–287. doi:10.1016/j.anifeedsci.2005.02.022 [Google Scholar]
- Lallès J. P., Bosi P., Smidt H., and Stokes C. R.. . 2007. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 66:260–268. doi:10.1017/S0029665107005484 [DOI] [PubMed] [Google Scholar]
- Le Sciellour M., Labussière E., Zemb O., and Renaudeau D.. . 2018. Effect of dietary fiber content on nutrient digestibility and fecal microbiota composition in growing-finishing pigs. PLoS One 13:e0206159. doi:10.1371/journal.pone.0206159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wu H., Jiang F., Wu J., Xue Y., Gan L., Liu J., and Long M.. . 2018. Heterologous expression and characterization of an acidic GH11 family xylanase from Hypocrea orientalis. Appl. Biochem. Biotechnol. 184:228– 238. doi:10.1007/s12010-017-2532-2 [DOI] [PubMed] [Google Scholar]
- Li H., Li H., Xie P., Li Z., Yin Y., Blachier F., and Kong X.. . 2019. Dietary supplementation with fermented Mao-tai Iees beneficial affects gut microbiota structure and function in pigs. AMB Express. 9:26. doi:10.1186/s13568-019-0747-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Wang W., Zhu X., Sun X., Xiao J., Li D., Cui Y., Wang C., and Shi Y.. . 2018. Response of gut microbiota to dietary fiber and metabolic interaction with SCFAs in piglets. Front. Microbiol. 9:2344. doi:10.3389/fmicb.2018.02344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhao L., Zhai Z., Zhao W., Ding J., Dai R., Sun T., and Meng H.. . 2015. Porcine epidemic diarrhea virus infection induced the unbalance of gut microbiota in piglets. Curr. Microbiol. 71:643–649. doi:10.1007/s00284-015-0895-6 [DOI] [PubMed] [Google Scholar]
- Lv J., Chen Y., Pei H., Yang W., Li Z., Dong B., and Cao Y.. . 2013. Cloning, expression, and characterization of β-mannanase from Bacillus subtilis MAFIC-S11 in Pichia pastoris. Appl. Biochem. Biotechnol. 169:2326–2340. doi:10.1007/s12010-013-0156-8 [DOI] [PubMed] [Google Scholar]
- Nortey T. N., Patience J. F., Sands J. S., Trottier N. L., and Zijlstra R. T.. . 2008. Effects of xylanase supplementation on the apparent digestibility and digestible content of energy, amino acids, phosphorus, and calcium in wheat and wheat by-products from dry milling fed to grower pigs. J. Anim. Sci. 86:3450–3464. doi:10.2527/jas.2007-0472 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 10th rev. ed.Washington, DC:National Academic Press. [Google Scholar]
- Porter M., and Murray R.. . 2001. The volatility of components of grass silage on oven drying and the inter-relationship between dry-matter content estimated by different analytical methods. Grass. Forage. Sci. 56:405− 411. doi:10.1046/j.1365-2494.2001.00292.x [Google Scholar]
- Saha B. C. 2003. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 30:279–291. doi:10.1007/s10295-003-0049-x [DOI] [PubMed] [Google Scholar]
- Sams L., Paume J., Giallo J., and Carrière F.. . 2016. Relevant pH and lipase for in vitro models of gastric digestion. Food Funct. 7:30–45. doi:10.1039/c5fo00930h [DOI] [PubMed] [Google Scholar]
- Shang Q., Shan X., Cai C., Hao J., Li G., and Yu G.. . 2016. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 7:3224–3232. doi:10.1039/c6fo00309e [DOI] [PubMed] [Google Scholar]
- Shin N. R., Whon T. W., and Bae J. W.. . 2015. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33:496–503. doi:10.1016/j.tibtech.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Shota Y., Ryosuke A., Yu K., Toshiyuki I., Hirokuni M., and Hiroaki K.. . 2018. Valerate production by Megasphaera elsdenii isolated from pig feces. J. Biosci. Bioeng. 125:519–524. doi:10.1016/j.jbiosc.2017.12.016 [DOI] [PubMed] [Google Scholar]
- Sterk A., Verdonk J. M. A. J., Mul A. J., Soenen B., Bezencon M. L., Frehner M., and Losa R.. . 2007. Effect of xylanase supplementation to a cereal-based diet on the apparent faecal digestibility in weanling piglets. Livest. Sci. 108:269–271. doi:10.1016/j.livsci.2007.01.077 [Google Scholar]
- Tan Z., Yang T., Wang Y., Xing K., Zhang F., Zhao X., Hong A., Chen S., Liu J., and Wang C.. . 2017. Metagenomic analysis of cecal microbiome identified microbiotaand functional capacities associated with feed efficiency in landrace finishing pigs. Front. Microbiol. 8:1546. doi:10.3389/fmicb.2017.01546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. E., Bedford M. R., and Miller H. M.. . 2018. The effects of xylanase on grower pig performance, concentrations of volatile fatty acids and peptide YY in portal and peripheral blood. Animal 12:2499–2504. doi:10.1017/S1751731118000277 [DOI] [PubMed] [Google Scholar]
- Wolever T. M., Brighenti F., Royall D., Jenkins A. L., and Jenkins D. J.. . 1989. Effect of rectal infusion of short chain fatty acids in human subjects. Am. J. Gastroenterol. 84:1027–1033. PMID 2773895. [PubMed] [Google Scholar]
- Wu H., Li H., Xue Y., Luo G., Gan L., Liu J., Mao L., and Long M.. . 2017. High efficiency co-production of ferulic acid and xylooligosaccharides from wheat bran by recombinant xylanase and feruloyl esterase. Biochem. Eng. J. 120:41–48. doi:10.1016/j.bej.2017.01.001 [Google Scholar]
- Yang Y. Y., Fan Y. F., Cao Y. H., Guo P. P., Dong B., and Ma Y. X.. . 2017a. Effects of exogenous phytase and xylanase, individually or in combination, and pelleting on nutrient digestibility, available energy content of wheat and performance of growing pigs fed wheat-based diets. Asian-Australas. J. Anim. Sci. 30:57–63. doi:10.5713/ajas.15.0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Yang Y., Zhang L., Xu H., Guo X., Yang X., Dong B., and Cao Y.. . 2017b. Improved thermostability of an acidic xylanase from Aspergillus sulphureus by combined disulphide bridge introduction and proline residue substitution. Sci. Rep. 7:1587. doi:10.1038/s41598-017-01758-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Zhai C., Yu X., Li Z., Tang W., Liu Y., Ma X., Zhong X., Li G., Wu D., . et al. 2016. High-level expression of Proteinase K from Tritirachium album Limber in Pichia pastoris using multi-copy expression strains. Protein Expr. Purif. 122:38–44. doi:10.1016/j.pep.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Zhang Y. J., Liu Q., Zhang W. M., Zhang Z. J., Wang W. L., and Zhuang S.. . 2018. Gastrointestinal microbial diversity and short-chain fatty acid production in pigs fed different fibrous diets with or without cell wall-degrading enzyme supplementation. Livest. Sci. 207:105–116. doi:10.1016/j.livsci.2017.11.017 [Google Scholar]
- Zhang F., Zheng W., Xue Y., and Yao W.. . 2019. Suhuai suckling piglet hindgut microbiome-metabolome responses to different dietary copper levels. Appl. Microbiol. Biotechnol. 103:853–868. doi:10.1007/s00253-018-9533-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Meng K., Bai Y., Shi P., Huang H., Luo H., Wang Y., Yang P., Song W., and Yao B.. . 2013. Two family 11 xylanases from Achaetomium sp. Xz-8 with high catalytic efficiency and application potentials in the brewing industry. J. Agric. Food Chem. 61:6880–6889. doi:10.1021/jf4001296 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.