Abstract
An experiment was conducted to test the hypothesis that inclusion of the direct fed microbial Clostridium butyricum in diets for weanling pigs will improve growth performance, systemic immune function, microbiota composition, and gut morphology in weaned pigs. A total of 275 newly weaned pigs (20 ± 2 d of age) with an average initial BW of 6.4 ± 0.8 kg were allotted to a randomized complete block design with 11 pens per treatment. Diets included a positive control diet containing Carbadox, a negative control diet without Carbadox, and three treatment diets in which 1,250 × 108 cfu/kg, 2,500 × 108 cfu/kg, or 3,500 × 108 cfu/kg of C. butyricum was added to the negative control diet. A two-phase feeding program was used (phase 1, 14 d; phase 2, 21 d). At the conclusion of the experiment (day 35), a blood sample was collected from one pig per pen (11 pigs per treatment) and this pig was then euthanized and digesta and tissues samples were collected. Results indicated that for the overall phase, pigs fed the positive control diet had greater (P < 0.05) ADG and ADFI and tended (P = 0.064) to have greater final BW than pigs fed the negative control diet. The ADG and G:F increased and then decreased as increasing doses of C. butyricum were included in the diet (quadratic, P < 0.05). The concentration of tumor necrosis factor-α was less (P < 0.05) in pigs fed the positive control diet compared with pigs fed the negative control diet or diets containing C. butyricum. Crypt depth tended (P = 0.08) to be less in pigs fed the negative control diet compared with pigs fed the positive control diet and villus height tended to increase as the doses of C. butyricum increased in the diets (quadratic, P = 0.08). Villus height also tended (P = 0.084) to be greater in pigs fed diets containing C. butyricum compared with pigs fed the positive control diet. Crypt depth increased as the dose of C. butyricum increased (quadratic, P < 0.05) and villus width at the bottom tended to increase (linear, P = 0.072) as the dose of C. butyricum increased in the diet. Alpha and beta diversity indices of ileal and colonic microbiota were not affected by diet. In conclusion, addition of 1,250 × 108 cfu/kg of C. butyricum, but not greater levels, to diets fed to weanling pigs increased growth performance and tended to increase villus height and crypt depth, but changes in the abundance of intestinal microbiota were not observed.
Keywords: Clostridium butyricum, direct-fed microbials, growth performance, intestinal morphology, microbiota, pigs
Introduction
The utilization of antimicrobial growth promoters is being reduced in the United States, but removal of antimicrobial growth promoters may reduce intestinal health of pigs and result in reduced growth performance (Liu et al., 2018). Direct-fed microbials are one of the many feed additives that are used to improve health and growth performance of pigs and is, therefore, one of the additives that may be used in diets for weanling pigs instead of antimicrobial growth promoters (Giang et al., 2010; Liu et al., 2018). Direct-fed microbials improved the microbial balance and intestinal environment (Jensen, 1998; Giang et al., 2010; Liao and Nyachoti, 2017) and are believed to enhance the immune function and benefit intestinal morphology (Yang et al., 2012).
Clostridium butyricum is a spore forming bacterium that is present in soil and in the intestinal tract of healthy animals. It is able to survive at a low pH (<3) and relatively high bile concentrations (Kong et al., 2011). Clostridium butyricum produces butyric acid, which is an important source of energy for intestinal cells, and positive effects of C. butyricum on intestinal health have been reported in patients with inflammatory bowel disease (Kong et al., 2011). Clostridium butyricum may also have positive effects on immune function and is associated with increased population of Lactobacillus and Bifidobacterium and reduced concentration of pathogenic bacteria in the intestinal tract of mice, humans, and broiler chickens (Yang et al., 2012). A mixture of spray-dried spores of Bacillus subtilis and C. butyricum caused increased ADG and G:F in growing finishing pigs (Meng et al., 2010), but there is limited information about the effects of C. butyricum on growth performance, intestinal morphology, and immune function in weanling pigs. Therefore, the objective of this experiment was to test the hypothesis that inclusion of increasing doses of C. butyricum in diets for weanling pigs results in increased growth performance, improved systemic immune function, increased concentration of Lactobacillus and Bifidobacterium in ileal and colon digesta, and improved intestinal morphology. The second hypothesis was that pigs fed diets containing C. butyricum have growth performance that is not different from that obtained by pigs fed a diet containing an antibiotic growth promotor.
Materials and Methods
The protocol for the experiment was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Illinois prior to initiation of the animal work. Pigs used in the experiment were the offspring of L 359 boars mated to Camborough females (Pig Improvement Company, Hendersonville, TN). The experiment was conducted at the Swine Research Center at the University of Illinois in a farrow to finish unit. The herd is a high health status herd that is free of pseudorabies, brucellosis, atrophic rhinitis, swine dysentery, lice, mange, Porcine Epidemic Diarrhea Virus, Transmissible gastro-enteritis, salmonella, and Acintobacillus pleuropneumoniae. The herd is vaccinated for the following diseases; Lawsonia intracellularis, six strains of Lepto, parvo virus, and circovirus.
Animals, Experimental Design, and Housing
A total of 275 pigs were weaned at 20 ± 2 d of age. There were 110 barrows and 165 gilts and pigs had an average initial BW of 6.4 ± 0.8 kg. Pigs were divided in 2 blocks according to weaning date and allotted to treatments using a randomized complete block design. Pigs were allotted to 5 treatments with 25 pens in block 1 and 30 pens in block 2. Thus, there were 5 and 6 replicate pens per diet in blocks 1 and 2, for a total of 11 replicate pens per diet in the experiment. There were 5 pigs per pen (3 gilts and 2 barrows). Pigs were housed in pens (1.2 × 1.4 m) with fully slatted floors. Each pen was equipped with a feeder and a nipple drinker and the room temperature was 28 °C in phase 1, and then reduced to 27, 26, and 25 °C in the following 3 wk.
Diets and Feeding
There were 5 dietary treatments and a 2-phase feeding program was used; therefore, a total of 10 diets were formulated (Tables 1, 2, and 3). Dietary treatments included the positive control diet containing an antibiotic growth promoter (Mecadox, Phibro Animal Health, Ridgefield Park, NJ), the negative control diet without antibiotic growth promoter, and 3 treatment diets in which 1,250 × 108 cfu/kg, 2,500 × 108 cfu/kg, or 3,500 × 108 cfu/kg of C. butyricum (Miya Gold; BASF, Florham Park, NJ) was added to the negative control diet. All diets were formulated to meet or exceed requirements for all nutrients for weanling pigs (NRC, 2012), and Cu and Zn were not included at pharmacological levels. Samples of all mixed diets were collected at the time of diet mixing. Phase 1 diets were mixed in one batch and phase 2 diets were mixed in two batches, and samples were collected from both batches that were manufactured. Pigs were fed experimental diets for 35 d. Phase 1 diets were fed in weeks 1 and 2, and phase 2 diets were fed in weeks 3 to 5. Feed was provided on an ad libitum basis and water was available at all times.
Table 1.
Ingredient composition of experimental diets for phase 1
| Item, % | Positive control | Negative control, (NC1) | NC+ 1,250 cfu | NC + 2,500 cfu | NC + 3,500 cfu |
|---|---|---|---|---|---|
| Corn | 45.35 | 46.35 | 46.25 | 46.15 | 46.07 |
| Whey, dried | 15.00 | 15.00 | 15.00 | 15.00 | 15.00 |
| SBM, 48% | 28.00 | 28.0 | 28.0 | 28.0 | 28.0 |
| Fish meal | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Choice white grease | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Limestone | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Dicalcium phosphate | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| l-Lys HCl | 0.37 | 0.37 | 0.37 | 0.37 | 0.37 |
| dl-Met | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
| l-Thr | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin–mineral premix2 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Mecadox premix3 | 1.00 | — | — | — | — |
| C. butyricum premix4 | — | — | 0.10 | 0.20 | 0.28 |
1NC, negative control.
2Provided the following quantities of vitamins and micro-minerals per kilogram of complete diet: Vitamin A as retinyl acetate, 11,136 IU; vitamin D3 as cholecalciferol, 2,208 IU; vitamin E as dl-alpha tocopheryl acetate, 66 IU; vitamin K as menadione dimethylprimidinol bisulfite, 1.42 mg; thiamin as thiamine mononitrate, 0.24 mg; riboflavin, 6.59 mg; pyridoxine as pyridoxine hydrochloride, 0.24 mg; vitamin B12, 0.03 mg; d-pantothenic acid as d-calcium pantothenate, 23.5 mg; niacin, 44.1 mg; folic acid, 1.59 mg; biotin, 0.44 mg; Cu, 20 mg as copper sulfate and copper chloride; Fe, 126 mg as ferrous sulfate; I, 1.26 mg as ethylenediamine dihydriodide; Mn, 60.2 mg as manganese sulfate; Se, 0.3 mg as sodium selenite and selenium yeast; and Zn, 125.1 mg as zinc sulfate.
3The Mecadox premix provided 50 mg/kg of Carbadox to the complete diet.
4The C. butyricum premix was added to provide 1,250 × 108 cfu/kg of complete diet, 2,500 108 cfu/kg of complete diet, or 3,500 × 108 cfu/kg of complete diet.
Table 2.
Ingredient composition of experimental diets for phase 2
| Item, % | Positive control | Negative control (NC1) | NC+ 1,250 cfu | NC + 2,500 cfu | NC + 3,500 cfu |
|---|---|---|---|---|---|
| Corn | 42.58 | 43.58 | 43.48 | 43.38 | 43.30 |
| Wheat middlings | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| SBM, 48% | 27.00 | 27.00 | 27.00 | 27.00 | 27.00 |
| Fish meal | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 |
| Whey, dried | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Choice white grease | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Limestone | 1.22 | 1.22 | 1.22 | 1.22 | 1.22 |
| Dicalcium phosphate | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| l-Lys HCl | 0.36 | 0.36 | 0.36 | 0.36 | 0.36 |
| dl-Met | 0.19 | 0.19 | 0.19 | 0.19 | 0.19 |
| l-Threonine | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin–mineral premix2 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Mecadox premix3 | 1.00 | — | — | — | — |
| C. butyricum premix4 | — | — | 0.10 | 0.20 | 0.28 |
1NC, negative control.
2Provided the following quantities of vitamins and micro-minerals per kilogram of complete diet: Vitamin A as retinyl acetate, 11,136 IU; vitamin D3 as cholecalciferol, 2,208 IU; vitamin E as dl-α tocopheryl acetate, 66 IU; vitamin K as menadione dimethylprimidinol bisulfite, 1.42 mg; thiamin as thiamine mononitrate, 0.24 mg; riboflavin, 6.59 mg; pyridoxine as pyridoxine hydrochloride,0.24 mg; vitamin B12, 0.03 mg; d-pantothenic acid as d-calcium pantothenate, 23.5 mg; niacin, 44.1 mg; folic acid, 1.59 mg; biotin, 0.44 mg; Cu, 20 mg as copper sulfate and copper chloride; Fe, 126 mg as ferrous sulfate; I, 1.26 mg as ethylenediamine dihydriodide; Mn, 60.2 mg as manganese sulfate; Se, 0.3 mg as sodium selenite and selenium yeast; and Zn, 125.1 mg as zinc sulfate.
3The Mecadox premix provided 50 mg/kg of Carbadox to the complete diet.
4The C. butyricum premix was added to provide 1,250 × 108, 2,500 108, or 3,500 × 108 cfu/kg of complete diet.
Table 3.
Analyzed composition of experimental diets1, 2, 3
| Item | Positive control | Negative control (NC) | NC + 1,250 cfu | NC + 2,500 cfu | NC + 3,500 cfu |
|---|---|---|---|---|---|
| Phase 1 | |||||
| DM, % | 88.64 | 88.94 | 88.27 | 88.56 | 88.50 |
| GE, kcal/kg | 4,030 | 4,022 | 3,999 | 4,025 | 4,041 |
| CP, % | 23.24 | 22.47 | 22.94 | 22.83 | 22.37 |
| Ca, % | 1.24 | 1.12 | 1.15 | 1.09 | 1.11 |
| P, % | 0.60 | 0.62 | 0.62 | 0.58 | 0.58 |
| Phase 24 | |||||
| DM, % | 88.20 | 88.08 | 88.26 | 89.15 | 89.21 |
| GE, kcal/kg | 4,015 | 4,027 | 4,030 | 4,047 | 4,025 |
| CP, % | 20.95 | 21.85 | 22.10 | 22.55 | 21.02 |
| Ca, % | 0.95 | 0.86 | 0.94 | 0.82 | 0.94 |
| P, % | 0.62 | 0.61 | 0.62 | 0.59 | 0.61 |
1NC, negative control diet.
2 C. butyricum analysis in phase 1 diets indicated that the positive and negative control diets did not contain C. butyricum. The three diets with added C. butyricum contained 4.94, 5.47, and 5.66 log10 cfu per gram of diet. In phase 2 diets, no C. butyricum was detected in the positive and the negative control diets, but the three diets containing the C. butyricum premix analyzed 4.95, 5.45, and 5.59 log10 cfu per gram of diet.
3Phase 1 diets were formulated to contain 3,430 kcal ME/kg and the following quantities of standardized ileal digestible AA: His, 0.51; Ile, 0.84; Leu, 1.63; Lys, 1.45; Met, 0.47; Met + Cys, 0.79; Thr, 0.83; Trp, 0.24; and Val, 0.90. Phase 2 diets were formulated to contain 3,380 kcal ME/kg and the following quantities of standardized ileal digestible AA: His, 0.56; Ile, 0.79; Leu, 1.54; Lys, 1.35; Met, 0.50; Met + Cys, 0.81; Thr, 0.79; Trp, 0.23, and Val, 0.86.
4Values for analyses are the average of two batches of feed being produced.
Data Recording and Sample Collection
Pig weights were recorded at the start of the experiment and on the last day of phase 1 and at the end of the experiment (day 35). The amount of feed offered to each pen was recorded daily and the amount of feed left in the feeder was recorded on the last day of each phase.
On the last day of phase 2, one pig in each pen was randomly selected and a blood sample was collected from the jugular vein of this pig, using a vacutainer containing EDTA. All blood samples were centrifuged at 1,500 × g at 4 °C for 15 min to collect plasma, which was stored at −20 °C until analyzed. Following blood collection, pigs were euthanized via captive bolt penetration and exsanguination. Jejunum samples of 2 to 3 cm were collected ~2 m from the pylorus. Samples were cut at the mesenteric side and pinned with the serosa side on a piece of cardboard (Nabuurs and Hoogendoorn, 1993). Samples were fixed in 10% neutral buffered formalin. After fixation, each sample was cut in 2 to 3 mm thick cross-sections and embedded in paraffin for slide preparation. From each sample, three to four transverse sections were selected and stained with hematoxylin and eosin. Slides were scanned using a 2.0-HT NanoZoomer (Hamamatsu, Bridgewater, NJ) and 10 villi and associated crypts were measured using NDP.View2 (Hammatsu). Villus height was measured from the villus tip to the crypt mouth, and the crypts were measured from the crypt mouth to the top of the crypt valley. Villus width was measured in the third top of the villus, and villus width at the bottom was measured at the crypt mouth. The width of the lamina propia was measured at the middle of the villus.
Ileal digesta contents were collected by cutting the ileum 5 cm before the ileocecal junction, and colonic contents were collected from the spiral colon ~50 cm from the cecum. Samples were frozen and stored at −80 °C immediately after collection.
Chemical Analysis
Diets were ground through a 1-mm screen in a Wiley mill (model 4; Thomas Scientific, Swedesboro, NJ) before analyses. Diets were analyzed for DM (method 930.15; AOAC, 2007) and for GE on an isoperibol bomb calorimeter (Model 6300, Parr Instruments, Moline, IL) using benzoic acid as the standard for calibration (Table 3). CP was analyzed by combustion (method 990.03; AOAC, 2007) using an Elementar Rapid N-cub Protein/Nitrogen apparatus (Elementar Americas Inc., Mt Laurel, NJ). Diet samples were also analyzed for Ca and P using inductively coupled plasma spectroscopy (method 985.01 A, B, and C; AOAC, 2007).
Tumor necrosis factor-α (TNF-α), and IgG were measured in plasma samples using ELISA kits according to the recommendation of the manufacturer (R&D Systems, Inc., Minneapolis, MN, and Bethyl Laboratories, Inc., Montgomery, TX, respectively). All samples were analyzed in duplicate.
Microbial Evaluation
Total DNA from ileal and colonic digesta was extracted using Mo-Bio PowerSoil kits (MO BIO Laboratories, Inc., Carlsbad, CA). Concentration of extracted DNA was quantified using a Qubit 3.0 Fluorometer (Life Technologies, Grand Island, NY). 16S rRNA gene amplicons were generated using a Fluidigm Access Array (Fluidigm Corporation, South San Francisco, CA) in combination with a Roche High Fidelity Fast Start Kit (Roche, Indianapolis, IN). The primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) that target a 252-bp fragment of the V4 region were used for amplification (primers synthesized by IDT Corp., Coralville, IA; Caporaso et al., 2012). The CS1 forward tag and CS2 reverse tag were added according to the Fluidigm protocol. Quality of the amplicons was assessed using a Fragment Analyzer (Advanced Analytics, Ames, IA) to confirm amplicon regions and sizes. A DNA pool was generated by combining equimolar amounts of the amplicons from each sample. The pooled samples were then size selected on a 2% agarose E-gel (Life Technologies) and extracted using a Qiagen gel purification kit (Qiagen, Valencia, CA). Cleaned size-selected pooled products were run on an Agilent Bioanalyzer to confirm appropriate profile and average size. Illumina sequencing was performed on a MiSeq using v3 reagents (Illumina Inc., San Diego, CA) at the W. M. Keck Center for Biotechnology at the University of Illinois.
Sequencing reads were analyzed to assess the bacterial composition of the intestinal microbial communities. Forward reads were trimmed using the FASTX-Toolkit (version 0.0.13), and QIIME 1.9.1 (Caporaso et al., 2010) was used to process the resulting sequence data. Briefly, high-quality (quality value ≥ 20) sequence data derived from the sequencing process were demultiplexed. Sequences then were clustered into operational taxonomic units (OTUs) using UCLUST (Edgar, 2010) through a closed-reference OTU picking strategy against the Greengenes 13_8 reference database (DeSantis et al., 2006) with a 97% similarity threshold. Singletons (OTUs that were observed fewer than two times) and OTUs that had <0.01% of the total observation were discarded. Taxonomic identity to each OTU was then assigned using UCLUST. A total of 1,714,248 16S rRNA-based amplicon sequences were obtained, with an average of 28,570 reads (range = 10,266 to 50,158) per sample. An even sampling depth (sequences per sample) of 10,266, 17,918, and 10,797 sequences per sample was used for assessing α- and β-diversity measures for datasets containing all samples, ileal samples, or colonic samples, respectively. β-Diversity was calculated using weighted and unweighted UniFrac (Lozupone and Knight, 2005) distance measures.
Calculations and Analysis of Data
At the conclusion of the experiment, data were summarized to calculate ADG, ADFI, and G:F. Normality of data was verified using the UNIVARIATE procedure (SAS Inst. Inc., Cary, NC). Normality was considered when the Shapiro-Wilk’s test reached P > 0.05. Outliers were identified and removed using the PROC BOXPLOT option of SAS. Outliers were considered as values that deviated from the treatment mean by more than three times the interquartile range. Data were analyzed as a randomized complete block design, using the MIXED procedure of SAS (SAS Inst. Inc.). The model included treatment as the main effect and block and pen within block as random effects:
where Yijk is the observed values for the variable, μ is the overall mean, τi is the main effect of the ith treatment (i = 1 to 5), Pj is the effect of the jth block (j = 1 to 2,), Ak is the effect of the kth pen (k = 1 to 8), and eijkl is the random error associated with Yijkl, assuming ∼N(0, σ2). Mean values were calculated using the LSMeans statement. Contrast statements were used to compare the negative and positive control diets, the positive control and the average of the diets containing C. butyricum, and coefficients for unequally spaced linear and quadratic contrasts were derived using the IML procedure in SAS, to analyze the linear and quadratic effects of increasing doses of C. butyricum. Data for the microbiota analysis were analyzed with the lme4 package of R using a mixed model approach with Tukey–Kramer multiple comparison tests. All tests were corrected for multiple inferences using the Benjamini–Hochberg method to control for false discovery rate. Quantitative polymerase chain reaction was used to measure the abundance of specific bacteria as previously described (Malinen et al., 2005; Rossi et al., 2014). The pen was the experimental unit for all analyses of growth performance and the pig was the experimental unit for all other measures. An α value of 0.05 was used to assess significance among means; tendencies were considered at P < 0.05 and 0.05 ≤ P < 0.10, respectively.
Results and Discussion
Growth Performance
Four pigs died in the experiment; two of those pigs were fed the diet with 2,500 cfu C. butyricum, one pig was fed the positive control diet, and one pig was fed the negative control diet. In addition, two pigs fed the negative control diet or the diet containing 3,500 cfu C. butyricum were removed from the trial due to poor body condition. Thus, a total of six pigs (2.18%) did not complete the experiment. In phase 1, no difference in ADFI was observed among treatments, but ADG was greater (P < 0.05) and G:F tended (P = 0.086) to be greater in the positive control diet compared with the negative control diet (Table 4). In phase 2, ADG and ADFI were greater (P < 0.05), and final BW tended (P = 0.064) to be greater, for pigs fed the positive control diet compared with pigs fed the negative control diet. Increasing the dose of C. butyricum from 0 to 1,250 × 108 cfu/kg, tended to increase final BW (quadratic: P = 0.051) and increased ADG (quadratic, P < 0.05). No difference in final BW between pigs fed the positive control diet and pigs fed diets containing C. butyricum was observed. The ADFI tended to be greater (P = 0.093) for pigs fed diets containing C. butyricum compared with pigs fed the negative control diet, and G:F increased (quadratic, P < 0.05) as increasing doses of C. butyricum were included in the diet.
Table 4.
Growth performance of weanling pigs fed the positive control diet (PC), the negative control diet (NC), or diets containing different doses of C. butyricum (CB)1,2
| Treatment | P-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | PC | NC | NC+ 1,250 cfu | NC + 2,500 cfu | NC + 3,500 cfu | SEM | Linear | Quad3 | NC vs. CB | PC vs. CB | PC vs. NC |
| Days 0 to 14 (phase 1) | |||||||||||
| Initial weight, kg | 6.44 | 6.42 | 6.43 | 6.41 | 6.44 | 0.24 | 0.978 | 0.972 | 0.986 | 0.977 | 0.969 |
| ADG, g | 134 | 105 | 120 | 127 | 112 | 10.59 | 0.479 | 0.137 | 0.197 | 0.217 | 0.045 |
| ADFI, g | 217 | 193 | 217 | 207 | 204 | 23.37 | 0.648 | 0.210 | 0.219 | 0.593 | 0.163 |
| G:F | 0.619 | 0.538 | 0.548 | 0.614 | 0.551 | 0.06 | 0.404 | 0.306 | 0.360 | 0.221 | 0.086 |
| Final weight, kg | 8.21 | 7.89 | 8.10 | 8.19 | 8.00 | 0.31 | 0.721 | 0.500 | 0.530 | 0.739 | 0.434 |
| Days 14 to 35 (phase 2) | |||||||||||
| ADG, g | 595 | 554 | 608 | 570 | 562 | 19.86 | 0.904 | 0.027 | 0.122 | 0.375 | 0.049 |
| ADFI, g | 910 | 845 | 901 | 865 | 901 | 27.38 | 0.196 | 0.609 | 0.093 | 0.422 | 0.045 |
| G:F | 0.655 | 0.656 | 0.674 | 0.660 | 0.627 | 0.01 | 0.080 | 0.031 | 0.865 | 0.906 | 0.966 |
| Final weight, kg | 20.69 | 19.50 | 20.85 | 20.14 | 19.79 | 0.44 | 0.886 | 0.051 | 0.145 | 0.406 | 0.064 |
| Days 0 to 35 (overall phase) | |||||||||||
| ADG, g | 408 | 374 | 412 | 393 | 382 | 10.92 | 0.849 | 0.006 | 0.040 | 0.243 | 0.010 |
| ADFI, g | 633 | 584 | 628 | 602 | 622 | 15.67 | 0.213 | 0.421 | 0.073 | 0.403 | 0.033 |
| G:F | 0.646 | 0.641 | 0.657 | 0.653 | 0.617 | 0.01 | 0.133 | 0.013 | 0.910 | 0.780 | 0.749 |
1Each least squares mean represents 11 observations.
2 C. butyricum was added to provide 1,250 × 108, 2,500 × 108, or 3,500 × 108 cfu/kg of complete diet.
3Quad, quadratic effect.
For the overall nursery phase, pigs fed the positive control diet had greater (P < 0.05) ADG and ADFI than pigs fed the negative control diet. The ADG and G:F increased and then decreased as increasing doses of C. butyricum were included in the diet (quadratic, P < 0.05). Likewise, the ADG and ADFI of pigs fed diets containing C. butyricum were greater (P < 0.05) than for pigs fed the negative control diet, but no differences were observed for ADG, ADFI, and G:F among pigs fed the positive control diet and pigs fed diets containing C. butyricum.
Finishing pigs and broiler chickens fed diets containing C. butyricum had increased ADG and G:F (Meng et al., 2010; Yang et al., 2012). The quadratic effects on ADG and G:F that were observed by inclusion of C. butyricum in the diets in the present experiment indicated that high doses of C. butyricum may result in microbial consumption of nutrients that would otherwise be available for the pigs. However, more research is needed to elucidate this effect. Nevertheless, the present results that demonstrated increased growth performance of pigs fed diets containing C. butyricum compared with pigs fed the negative control diet indicated that C. butyricum may be used to partly or fully restore the growth performance that may be lost if antibiotic growth promoters are removed from diets for weanling pigs. The observation that no differences in growth performance between pigs fed the positive control diet and pigs fed diets containing C. butyricum were observed further demonstrated the potential of C. butyricum to improve growth performance of weanling pigs.
Concentrations of TNF-α and IgG
The concentration of TNF-α was reduced (P < 0.05) in pigs fed the positive control diet compared with pigs fed the negative control diet or diets containing C. butyricum, but no effects of increased doses of C. butyricum on the concentration of TNF-α were observed (Table 5). Tumor necrosis factor-α is a pro-inflammatory cytokine released after exposure to pathogens and is responsible for many metabolic responses such as shifts in use of nutrients to support the immune response (Rakhshandeh and de Lange, 2012). It is possible that addition of an antibiotic growth promoter to the positive control diet resulted in a reduction in systemic antigens and reduced concentration of TNF-α compared with the negative control diet or diets containing C. butyricum (Adewole et al., 2016). Thus, reduced inflammation may have contributed to the tendency for increased ADFI that was observed for pigs fed the diets C. butyricum.
Table 5.
Concentration of tumor necrosis factor α (TNF-α) and IgG in weanling pigs fed the positive control diet (PC), the negative control diet (NC), or diets containing different doses of C. butyricum (CB)1,2
| Treatment | P-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | PC | NC | NC+ 1,250 cfu | NC + 2,500 cfu | NC + 3,500 cfu | SEM | Linear | Quad3 | NC vs. CB | PC vs. CB | PC vs. NC |
| TNF-α, pg/mL | 75.1 | 117.8 | 115.0 | 111.6 | 120.6 | 10.58 | 0.939 | 0.526 | 0.838 | <0.001 | 0.003 |
| IgG, mg/mL | 4.3 | 3.5 | 4.5 | 5.1 | 3.7 | 0.66 | 0.604 | 0.096 | 0.240 | 0.840 | 0.415 |
1Each least squares mean represents 11 observations.
2 C. butyricum was added to provide 1,250 × 108, 2,500 × 108, or 3,500 × 108 cfu/kg of complete diet.
3Quad, quadratic effect.
The concentration of IgG in plasma was not different between the positive control diet and the negative control diet, but tended (quadratic, P = 0.096) to increase as C. butyricum increased in the diets. However, no differences were observed between the positive control diets and diets containing C. butyricum. Similar responses for the concentration of IgG and other antibodies in serum were observed in broiler chickens (Yang et al., 2012). It thus appears that the immunomodulatory effects of C. butyricum are minor, and under the conditions of this experiment with usage of high-health pigs, concentrations of IgG were not different between pigs fed an antibiotic growth promoter and pigs fed diets containing C. butyricum. As a consequence, the quadratic increase in ADG and G:F that was observed as C. butyricum was added to the diet likely is not a result of increased immune status of the pigs.
Intestinal morphology
Pigs fed the positive control diet tended (P = 0.080) to have deeper crypts in the jejunum than pigs fed the negative control diet and the villus height to crypt depth ratio tended to be greater (P = 0.068) in pigs fed the negative control diet compared with pigs fed the positive control diet (Table 6). Villus height tended (quadratic P = 0.080) to increase as C. butyricum increased in the diets. The villus height also tended (P = 0.084) to be greater in pigs fed diets containing C. butyricum compared with pigs fed the positive control diet, and crypt depth increased as C. butyricum increased (quadratic, P < 0.05). Villus width at the bottom tended to increase linearly (P = 0.072) as C. butyricum increased in the diet, but the villus width at the top and in the lamina propria were not affected by dietary treatments.
Table 6.
Morphology of proximal jejunum of weanling pigs fed the positive control diet (PC), the negative control diet (NC), or diets containing different doses of C. butyricum (CB)1,2
| Treatment | P-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | PC | NC | NC + 1,250 cfu | NC + 2,500 cfu | NC + 3,500 cfu | SEM | Linear | Quad3 | NC vs. CB | PC vs. CB | PC vs. NC |
| Villus height, µm | 442.5 | 488.6 | 491.4 | 528.3 | 446.4 | 27.78 | 0.448 | 0.080 | 0.997 | 0.084 | 0.156 |
| Crypt depth, µm | 234.6 | 200.7 | 228.7 | 236.8 | 199.5 | 54.96 | 0.831 | 0.022 | 0.177 | 0.424 | 0.080 |
| VH:CD4 | 2.37 | 2.87 | 2.66 | 2.63 | 2.63 | 0.740 | 0.378 | 0.599 | 0.295 | 0.228 | 0.068 |
| Villus width at top, µm | 118.6 | 115.9 | 111.9 | 125.3 | 121.9 | 3.430 | 0.130 | 0.964 | 0.474 | 0.969 | 0.578 |
| Villus width at bottom, µm | 138.9 | 135.7 | 135.0 | 151.7 | 143.8 | 5.190 | 0.072 | 0.649 | 0.199 | 0.450 | 0.659 |
| Lamina propia, µm | 48.5 | 46.4 | 43.9 | 48.1 | 49.7 | 2.957 | 0.299 | 0.430 | 0.809 | 0.720 | 0.624 |
1Each least squares mean represents 11 observations.
2 C. butyricum was added to provide 1,250 × 108, 2,500 108, or 3,500 × 108 cfu/kg of complete diet.
3Quad, quadratic effect.
4VH:CD, villus height:crypt depth.
Intestinal morphology is a criteria to assess functionality of the intestines and intestinal health (Liao and Nyachoti, 2017). Values for villi height in the proximal jejunum observed in this experiment were greater than reported by Gu et al. (2002), but are within the range reported by Pedersen et al. (2016). However, comparing data from different experiments may be difficult because different methodologies are used to assess morphology (Masri et al., 2015). Shorter villi and deeper crypts are usually observed in pigs with diarrhea and is commonly associated with reduction in the digestive and absorptive function (Nabuurs and Hoogendoorn, 1993; Pluske et al., 1996).
The greater villus height and reduced crypt depth, as well as the greater villus height to crypt depth ratio in pigs fed the negative control diet compared with pigs fed other diets indicated a reduced turnover rate of the intestinal cells (Adewole et al., 2016), which may have been associated with reduced absorptive capacity. Thus, the tendencies for increased villus height and crypt depth that were observed as C. butyricum were included in the diets indicated that C. butyricum improved absorptive capacity, which may have contributed to the improved ADFI observed for pigs fed the diets containing C. butyricum.
Microbial evaluation
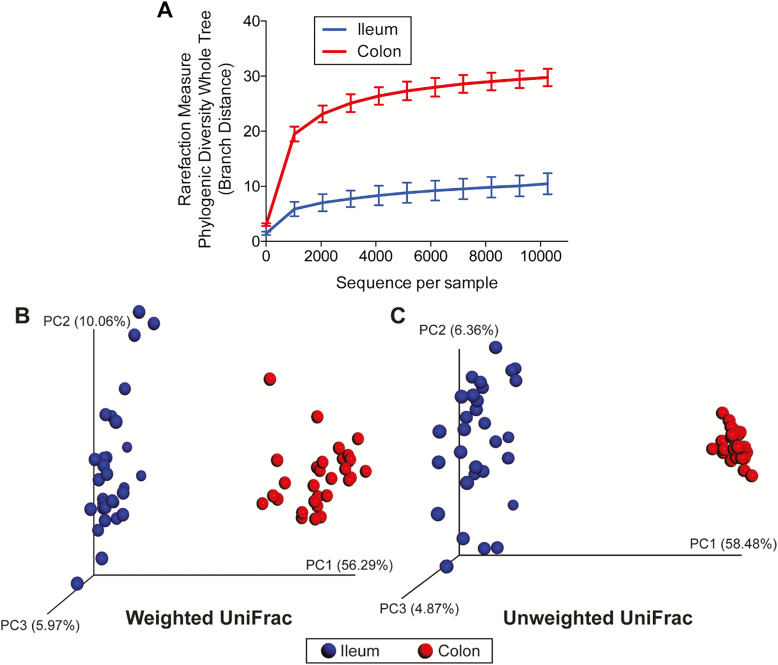
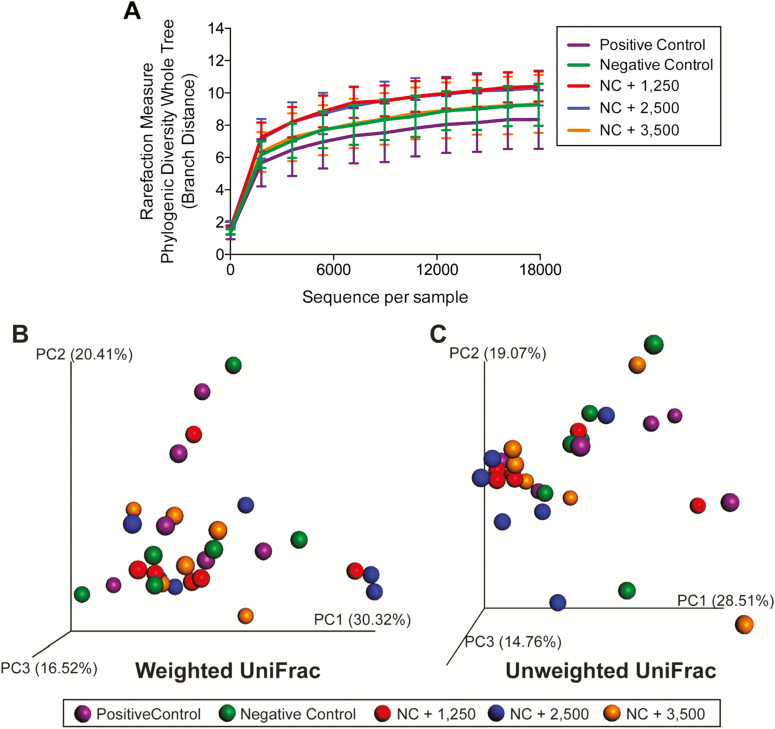
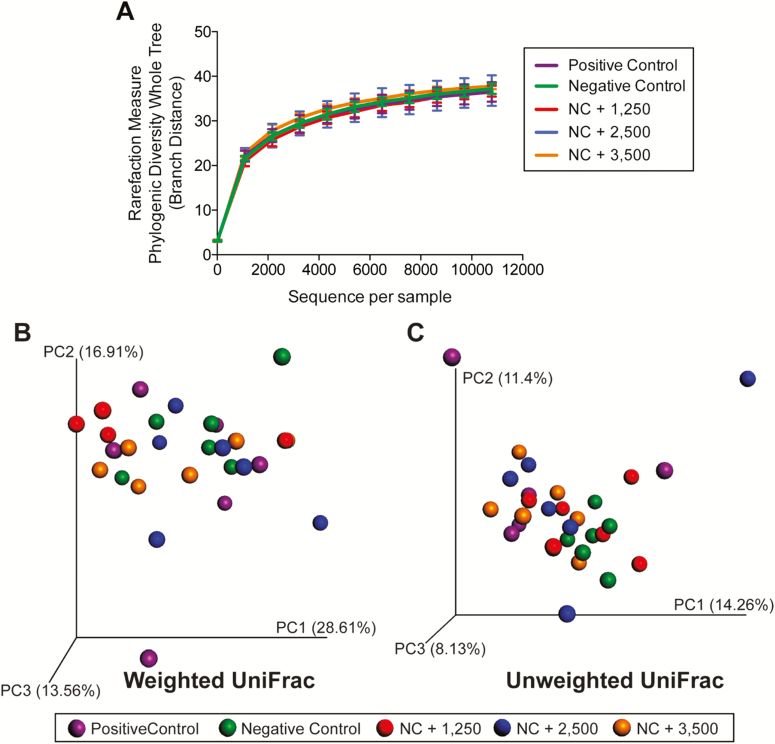
Alpha diversity, which measured species richness within a sample, was lower (P < 0.001) in ileal vs. colonic microbial communities (Figure 1A). Beta diversity, which measured similarity and dissimilarity among samples, is represented by principal coordinates analysis plots. Unweighted UniFrac distances, which measured the presence or absence of microbial taxa, indicated that ileal and colonic microbial communities were different (P < 0.05; Figure 1C). Weighted UniFrac distances, which measured the presence and abundance of microbial taxa, indicated a similar difference between ileal and colonic communities (P < 0.05; Figure 1B). Six phyla and 46 genera were affected (P < 0.05) by intestinal segment (Table 7). Ileal samples had the greatest concentration of Firmicutes, with moderate populations of Actinobacteria and Proteobacteria. Colonic samples were dominated by Firmicutes and Bacteroidetes, with moderate populations of Actinobacteria and Proteobacteria.
Figure 1.
Rarefaction curves (A) and principal coordinates analysis (PCoA) plots of weighted (B) and unweighted (C) UniFrac distances of ileal and colonic microbial communities from weanling pigs fed diets containing antibiotics or direct-fed microbials. Diversity measures suggested a greater (P < 0.001) species richness in colonic vs. ileal samples. Unweighted and weighted UniFrac distances performed on the 97% OTU abundance matrix revealed that ileal and colonic samples were different from one another (P = 0.01).
Table 7.
Ileal and colonic microbiota populations (% of sequences) of weanling pigs as measured using Illumina sequencing
| Phyla | Genera | Ileum | Colon |
|---|---|---|---|
| Euryarchaeota | 0.00 ± 0.03b | 0.11 ± 0.03a | |
| Methanobrevibacter | 0.00 ± 0.02 | 0.05 ± 0.02 | |
| vadinCA11 | 0.00 ± 0.01b | 0.05 ± 0.01a | |
| Actinobacteria | 5.26 ± 1.01a | 1.08 ± 1.01b | |
| Actinomyces | 0.03 ± 0.00a | 0.00 ± 0.00b | |
| Undefined genus in family Bifidobacteriaceae | 0.38 ± 0.12 | 0.02 ± 0.12 | |
| Bifidobacterium | 4.48 ± 0.85a | 0.68 ± 0.86b | |
| Undefined genus in family Coriobacteriaceae | 0.37 ± 0.16 | 0.24 ± 0.16 | |
| Collinsella | 0.00 ± 0.01b | 0.11 ± 0.01a | |
| Bacteroidetes | 0.01 ± 1.04b | 24.48 ± 1.04a | |
| Undefined genus in order Bacteroidales | 0.00 ± 0.04b | 0.17 ± 0.04a | |
| Parabacteroides | 0.00 ± 0.02 | 0.04 ± 0.02 | |
| Prevotella | 0.01 ± 0.93b | 20.89 ± 0.93a | |
| Undefined genus in family RF16 | 0.00 ± 0.02 | 0.03 ± 0.02 | |
| Undefined genus in family S24-7 | 0.00 ± 0.06b | 0.36 ± 0.06a | |
| Undefined genus in family [Paraprevotellaceae] | 0.00 ± 0.00b | 0.03 ± 0.00a | |
| CF231 | 0.00 ± 0.04b | 0.26 ± 0.04a | |
| [Prevotella] | 0.00 ± 0.30b | 2.69 ± 0.30a | |
| Cyanobacteria | 0.00 ± 0.31b | 2.07 ± 0.31a | |
| Undefined genus in order YS2 | 0.00 ± 0.31b | 2.06 ± 0.31a | |
| Firmicutes | 89.43 ± 2.38a | 68.58 ± 2.38b | |
| Undefined genus in family Planococcaceae | 0.04 ± 0.03 | 0.00 ± 0.03 | |
| Aerococcus | 0.64 ± 0.41 | 0.00 ± 0.41 | |
| Granulicatella | 0.03 ± 0.02 | 0.00 ± 0.02 | |
| Enterococcus | 2.39 ± 1.16 | 0.00 ± 1.16 | |
| Undefined genus in family Lactobacillaceae | 0.32 ± 0.22 | 0.00 ± 0.22 | |
| Lactobacillus | 33.08 ± 3.31a | 9.53 ± 3.32b | |
| Pediococcus | 0.79 ± 0.50 | 0.00 ± 0.50 | |
| Weissella | 3.33 ± 1.46 | 0.00 ± 1.46 | |
| Streptococcus | 29.95 ± 3.13a | 11.71 ± 3.16b | |
| Turicibacter | 0.02 ± 0.01a | 0.01 ± 0.01b | |
| Undefined genus in order Clostridiales | 0.58 ± 0.24b | 2.77 ± 0.24a | |
| Undefined genus in family Clostridiaceae | 10.96 ± 2.00a | 1.05 ± 2.01b | |
| Clostridium | 1.46 ± 0.52 | 0.29 ± 0.52 | |
| SMB53 | 0.07 ± 0.03a | 0.02 ± 0.03b | |
| Undefined genus in family Lachnospiraceae | 0.09 ± 0.29b | 4.57 ± 0.29a | |
| Blautia | 0.01 ± 0.15b | 2.63 ± 0.15a | |
| Coprococcus | 0.00 ± 0.05b | 0.54 ± 0.05a | |
| Dorea | 0.00 ± 0.07b | 0.61 ± 0.07a | |
| Lachnospira | 0.00 ± 0.02b | 0.22 ± 0.02a | |
| Roseburia | 0.00 ± 0.22b | 1.91 ± 0.22a | |
| [Ruminococcus] | 0.00 ± 0.02b | 0.39 ± 0.02a | |
| Peptococcus | 0.00 ± 0.01b | 0.05 ± 0.01a | |
| Undefined genus in family Peptostreptococcaceae | 0.07 ± 0.02 | 0.09 ± 0.02 | |
| Undefined genus in family Ruminococcaceae | 0.02 ± 0.39b | 9.89 ± 0.39a | |
| Faecalibacterium | 0.00 ± 0.38b | 4.85 ± 0.38a | |
| Oscillospira | 0.00 ± 0.01b | 0.07 ± 0.01a | |
| Ruminococcus | 0.00 ± 0.02b | 0.27 ± 0.02a | |
| Undefined genus in family Veillonellaceae | 0.03 ± 0.26b | 2.89 ± 0.26a | |
| Acidaminococcus | 0.01 ± 0.06b | 0.56 ± 0.06a | |
| Anaerovibrio | 0.00 ± 0.07b | 0.34 ± 0.07a | |
| Dialister | 0.03 ± 0.14b | 1.39 ± 0.14a | |
| Megasphaera | 0.40 ± 0.50b | 8.02 ± 0.50a | |
| Mitsuokella | 0.13 ± 0.08b | 0.66 ± 0.08a | |
| Phascolarctobacterium | 0.00 ± 0.03b | 0.32 ± 0.03a | |
| Veillonella | 0.03 ± 0.01a | 0.01 ± 0.01b | |
| Undefined genus in family Erysipelotrichaceae | 0.00 ± 0.02b | 0.06 ± 0.02a | |
| Bulleidia | 0.01 ± 0.03b | 0.26 ± 0.03a | |
| Catenibacterium | 0.07 ± 0.14b | 1.26 ± 0.14a | |
| Sharpea | 0.34 ± 0.13 | 0.17 ± 0.13 | |
| [Eubacterium] | 0.01 ± 0.06b | 0.76 ± 0.06a | |
| Proteobacteria | 5.29 ± 1.92 | 3.41 ± 1.92 | |
| Undefined genus in order RF32 | 0.00 ± 0.02 | 0.06 ± 0.02 | |
| Sutterella | 0.00 ± 0.01b | 0.06 ± 0.01a | |
| Undefined genus in order Tremblayales | 0.00 ± 0.09 | 0.28 ± 0.09 | |
| Desulfovibrio | 0.00 ± 0.04b | 0.18 ± 0.04a | |
| Campylobacter | 0.00 ± 0.17b | 0.85 ± 0.17a | |
| Helicobacter | 0.00 ± 0.01b | 0.04 ± 0.01a | |
| Succinivibrio | 0.00 ± 0.49b | 1.88 ± 0.49a | |
| Undefined genus in family Enterobacteriaceae | 2.78 ± 1.60 | 0.00 ± 1.60 | |
| Trabulsiella | 0.18 ± 0.12 | 0.00 ± 0.12 | |
| Undefined genus in family Pasteurellaceae | 0.05 ± 0.02 | 0.00 ± 0.02 | |
| Actinobacillus | 2.19 ± 0.72 | 0.04 ± 0.72 | |
| Tenericutes | 0.00 ± 0.03b | 0.22 ± 0.03a | |
| Undefined genus in Phylum WPS-2 | 0.00 ± 0.02 | 0.03 ± 0.02 |
abValues within a row not sharing the same superscript letter are different (P < 0.05).
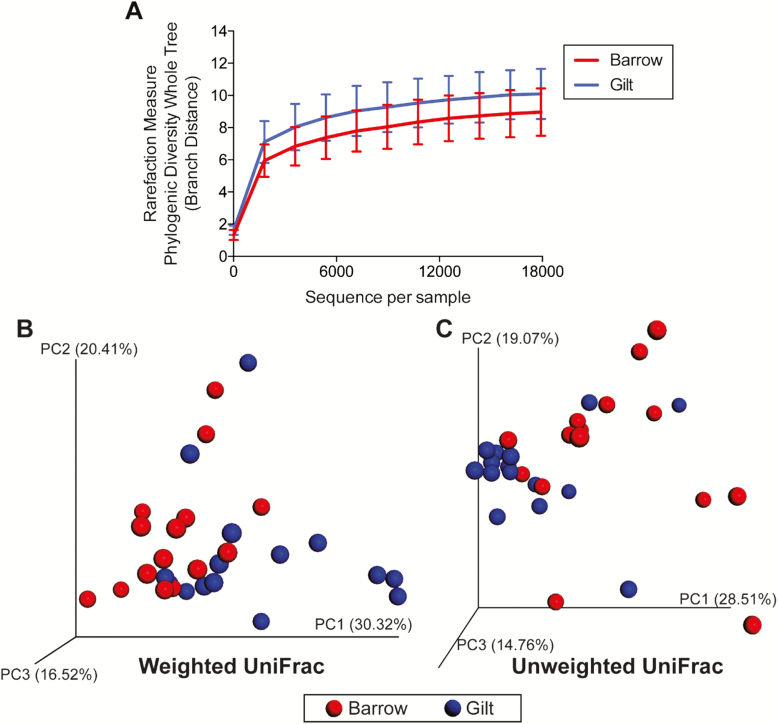
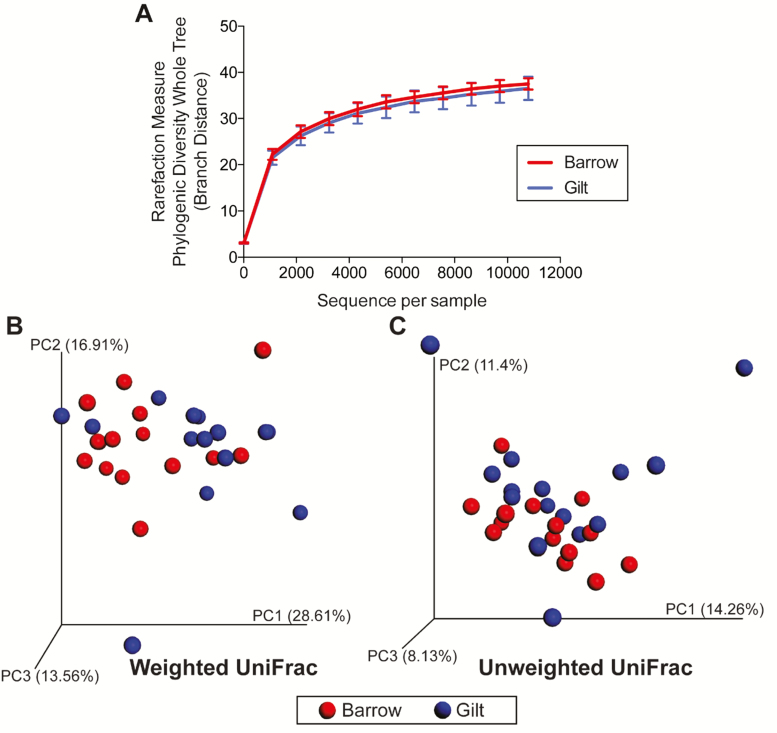
If only ileal digesta samples were considered, α and β diversity indices were not affected by diet (Figure 2A, B, and C). The effect of sex on ileal α diversity indicated a trend (P = 0.061) for greater microbial diversity in barrows vs. gilts when using phylogenetic distance (PD) whole tree (Figure 3A). The effect of sex on α diversity were not different when considering the other measures (observed OTU; Chao1); however, unweighted and weighted UniFrac distances performed on the 97% OTU abundance matrix of ileal samples indicated that sexes tended to be different from one another (unweighted: P < 0.05; weighted: P < 0.05; Figure 3B and C).
Figure 2.
Rarefaction curves (A) and principal coordinates analysis (PCoA) plots of weighted (B) and unweighted (C) UniFrac distances of ileal microbial communities from weanling pigs fed diets containing antibiotics or direct-fed microbials. Diversity measures and unweighted and weighted UniFrac distances performed on the 97% OTU abundance matrix were not different among treatments.
Figure 3.
Rarefaction curves (A) and principal coordinates analysis (PCoA) plots of weighted (B) and unweighted (C) UniFrac distances of ileal microbial communities from weanling pigs fed diets containing antibiotics or direct-fed microbials. While two diversity measures (observed OTU; Chao1) were not different due to sex, phylogenetic distance (PD whole tree) showed a trend (P = 0.061) for greater diversity in barrows vs. gilts. Unweighted and weighted UniFrac distances performed on the 97% OTU abundance matrix showed that sexes tended to be different from one another (unweighted: P = 0.02; weighted: P = 0.01).
If only colonic samples were considered, α and β diversity indices were not affected by diet (Figure 4A, B, and C). The effect of sex on colonic α diversity demonstrated a trend (P = 0.061) for greater microbial diversity in barrows vs. gilts when using observed OTU. The effect of sex (Figure 5A) on α diversity was not different among the other measures (PD whole tree; Chao1), but unweighted and weighted UniFrac distances performed on the 97% OTU abundance matrix indicated that the two sexes tended to be different from one another (unweighted: P < 0.05; weighted: P < 0.05) (Figure 5B and C).
Figure 4.
Rarefaction curves (A) and principal coordinates analysis (PCoA) plots of weighted (B) and unweighted (C) UniFrac distances of colonic microbial communities from weanling pigs fed diets containing antibiotics or direct-fed microbials. Diversity measures and unweighted and weighted UniFrac distances performed on the 97% OTU abundance matrix were not different among treatments.
Figure 5.
Rarefaction curves (A) and principal coordinates analysis (PCoA) plots of weighted (B) and unweighted (C) UniFrac distances of colonic microbial communities from weanling pigs fed diets containing antibiotics or direct-fed microbials. While two diversity measures (PD whole tree; Chao1) were not different due to sex, observed OTU showed a trend (P = 0.061) for greater diversity in barrows vs. gilts. Unweighted and weighted UniFrac distances performed on the 97% OTU abundance matrix showed that sexes tended to be different from one another (unweighted: P = 0.01; weighted: P = 0.02).
Results of the microbiota composition of digesta from the ileum and colon concur with previous data (Gagnon et al., 2007; Roca et al., 2014; Brousseau et al., 2015). Variation in the composition of the microbiota in ileum and colon in a strain-dependent manner has been observed (Brousseau et al., 2015). However, in this experiment, the diversity indices of microbial population in ileum and colon contents were not affected by addition of different doses of C. butyricum. Responses to the supplementation with direct-fed microbials may depend on the age of the pigs, environmental conditions, and health status, and the direct-fed microbial itself (Gagnon et al., 2007). Likewise, the lack of differences in microbial diversity in ileum and colon contents of pigs fed the positive control diet and the negative control diet was also observed in the past (Roca et al., 2014). However, the observation that abundance of microbiota was not altered does not necessarily mean that microbial activity was unchanged, but because microbial activity was not measured, it is not possible to make conclusions about this.
Conclusions
Addition of C. butyricum had no effects on growth performance in phase 1, but ADG, G:F, and final BW at the end of phase 2 and for the overall experiment increased quadratically with increased inclusion of C. butyricum in the diet. There were no differences in growth performance, or in concentrations of TNF-α and IgG among pigs fed the positive control diet and pigs fed diets containing C. butyricum. Addition of C. butyricum to the diets tended to increase villus height and increased crypt depth, which indicates that the absorptive capacity of intestinal cells were increased, which possibly contributed to the improved ADG that was observed for pigs fed diets containing C. butyricum. The abundance of microbial populations in ileal and colon contents were different, but the diversity indices that were determined in this experiment were not affected by dietary treatments.
Funding
Financial support for this research from BASF, Florham Park, NJ, is greatly appreciated.
Conflict of interest statement. None declared.
Literature Cited
- Adewole D. I., Kim I. H., and Nyachoti C. M.. . 2016. Gut health of pigs: challenge models and response criteria with a critical analysis of the effectiveness of selected feed additives – a review. Asian-Australas. J. Anim. Sci. 29:909–924. doi: 10.5713/ajas.15.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC 2007. Official methods of analysis of AOAC international. 18th ed. rev. 2. ed. Gaithersburg, MD:AOAC International. [Google Scholar]
- Brousseau J. P., Talbot G., Beaudoin F., Lauzon K., Roy D., and Lessard M.. . 2015. Effects of probiotics Pediococcus acidilactici strain MA18/5M and Saccharomyces cerevisiae subsp. boulardii strain SB-CNCM I-1079 on fecal and intestinal microbiota of nursing and weanling piglets. J. Anim. Sci. 93:5313–5326. doi: 10.2527/jas.2015-9190. [DOI] [PubMed] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., . et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Lauber C. L., Walters W. A., Berg-Lyons D., Huntley J., Fierer N., Owens S. M., Betley J., Fraser L., Bauer M., . et al. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis T. Z., Hugenholtz P., Larsen N., Rojas M., Brodie E. L., Keller K., Huber T., Dalevi D., Hu P., and Andersen G. L.. . 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Gagnon N., Talbot G., Ward P., Roy D., Dupuis M., Farnworth E., Tompkins T. A., and Lessard M.. . 2007. Evaluation of bacterial diversity in the gut of piglets supplemented with probiotics using ribosomal intergenic spacer analysis. Can. J. Anim. Sci. 87:207–219. doi: 10.4141/A06-065. [DOI] [Google Scholar]
- Giang H. H., Viet T. Q., Ogle B., and Lindberg J. E.. . 2010. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria. Livest. Sci. 129:95–103. doi: 10.1016/j.livsci.2010.01.010 [DOI] [Google Scholar]
- Gu X., Li D., and She R.. . 2002. Effect of weaning on small intestinal structure and function in the piglet. Arch. Tierernahr. 56:275–286. doi: 10.1080/00039420214345. [DOI] [PubMed] [Google Scholar]
- Jensen B. B. 1998. The impact of feed additives on the microbial ecology of the gut in young pigs. J. Anim. Feed Sci. 7:45–64. doi: 10.22358/jafs/69955/1998 [DOI] [Google Scholar]
- Kong Q., He G. Q., Jia J. L., Zhu Q. L., and Ruan H.. . 2011. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice. Curr. Microbiol. 62:512–517. doi: 10.1007/s00284-010-9737-8. [DOI] [PubMed] [Google Scholar]
- Liao S. F., and Nyachoti M.. . 2017. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 3:331–343. doi: 10.1016/j.aninu.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Espinosa C. D., Abelilla J. J., Casas G. A., Lagos L. V., Lee S. A., Kwon W. B., Mathai J. K., Navarro D. M. D. L., Jaworski N. W., . et al. 2018. Non-antibiotic feed additives in diets for pigs: a review. Anim. Nutr. 4:113–125. doi: 10.1016/j.aninu.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C., and Knight R.. . 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinen E., Rinttilä T., Kajander K., Mättö J., Kassinen A., Krogius L., Saarela M., Korpela R., and Palva A.. . 2005. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am. J. Gastroenterol. 100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- Masri S. A., Hunigen H., Aiyan A. A., Rieger J., Zentek J., Richardson K., and Plendl J.. . 2015. Influence of age at weaning and feeding regimes on the postnatal morphology of the porcine small intestine. J. Swine Health Prod. 23:186–203. [Google Scholar]
- Meng Q. W., Yan L., Ao X., Zhou T. X., Wang J. P., Lee J. H., and Kim I. H.. . 2010. Influence of probiotics in different energy and nutrient density diets on growth performance, nutrient digestibility, meat quality, and blood characteristics in growing-finishing pigs. J. Anim. Sci. 88:3320–3326. doi: 10.2527/jas.2009-2308. [DOI] [PubMed] [Google Scholar]
- Nabuurs M. J., Hoogendoorn A., van der Molen E. J., and van Osta A. L.. . 1993. Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in The Netherlands. Res. Vet. Sci. 55:78–84. doi: 10.1016/0034-5288(93)90038-h. [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed Washington, DC:National Academic Press. [Google Scholar]
- Pedersen T. F., Liu Y., and Stein H. H.. . 2016. Effects of diet energy concentration and an exogenous carbohydrase on growth performance of weanling pigs fed diets containing canola meal produced from high protein or conventional canola seeds. J. Anim. Sci. 94:5206–5218. doi: 10.2527/jas.2016-0681. [DOI] [PubMed] [Google Scholar]
- Pluske J., Williams I., and Aherne F.. . 1996. Villous height and crypt depth in piglets in response to increases in the intake of cows’ milk after weaning. Anim. Sci. 62:145–158. doi: 10.1017/S1357729800014429 [DOI] [Google Scholar]
- Rakhshandeh A., and de Lange C. F.. . 2012. Evaluation of chronic immune system stimulation models in growing pigs. Animal 6:305–310. doi: 10.1017/S1751731111001522. [DOI] [PubMed] [Google Scholar]
- Roca M., Nofrarías M., Majó N., Pérez de Rozas A. M., Segalés J., Castillo M., Martín-Orúe S. M., Espinal A., Pujols J., and Badiola I.. . 2014. Changes in bacterial population of gastrointestinal tract of weaned pigs fed with different additives. Biomed Res. Int. 2014:269402. doi: 10.1155/2014/269402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G., Pengo G., Caldin M., Palumbo Piccionello A., Steiner J. M., Cohen N. D., Jergens A. E., and Suchodolski J. S.. . 2014. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS One 9:e94699. doi: 10.1371/journal.pone.0094699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. M., Cao G. T., Ferket P. R., Liu T. T., Zhou L., Zhang L., Xiao Y. P., and Chen A. G.. . 2012. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult. Sci. 91:2121–2129. doi: 10.3382/ps.2011-02131. [DOI] [PubMed] [Google Scholar]