Abstract
Tumor suppressors are cellular proteins typically expressed in normal (non-cancer) cells that not only regulate such cellular functions as proliferation, migration and adhesion, but can also be secreted into extracellular space and serve as biomarkers for pathological conditions or tumor progression. KISS1, a precursor for several shorter peptides, known as metastin (Kisspeptin-54), Kisspeptin-14, Kisspeptin-13 and Kisspeptin-10, is one of those metastasis suppressor proteins, whose expression is commonly downregulated in the metastatic tumors of various origins. The commonly accepted role of KISS1 in metastatic tumor progression mechanism is the ability of this protein to suppress colonization of disseminated cancer cells in distant organs critical for the formation of the secondary tumor foci. Besides, recent evidence suggests involvement of KISS 1 in the mechanisms of tumor angiogenesis, autophagy and apoptosis regulation, suggesting a possible role in both restricting and promoting cancer cell invasion. Here, we discuss the role of KISS 1 in regulating metastases, the link between KISS1 expression and the autophagy-related biology of cancer cells and the perspectives of using KISS 1 as a potential diagnostic marker for cancer progression as well as a new anti-cancer therapeutics.
Keywords: KISS1 tumor suppressor, Autophagy, Breast cancer metastases, Brain tumor
1. Introduction
Cancer progression is a “cascade” of biological events leading to the disease spread that is developed in its early stage and generally may not associate with the development of its clinical symptoms until later stages of progression. Metastatic development has been long recognized as a complex process of cancer propagation controlled by elaborate regulatory mechanisms of cell behavior. The onset of metastasis commonly involves an essential stage of organ-associated colonization of circulating tumor cells (CTC) shed from primary tumors into the blood stream and lymphatic system, although more direct mechanisms of metastatic cancer spread via body cavities, not involving circulation, have been noted (e.g. ovarian cancer) [1]. However, the mechanisms, whereby colonization of CTCs, their survival and formation of new cancer foci occur, remain largely unknown. This is particularly important, since most small molecule-based anti-cancer drugs function by suppressing some of the cancer-promoting programs, but not necessarily the metastatic process itself or clinical symptoms of cancer spreading, and therefore are unable to effectively cure the disease [2]. Besides, metastatic cancer dissemination is extremely difficult to diagnose in patients due to a poorly defined pathogenesis [3], which renders understanding the biology of cancer progression extremely important from the clinical standpoint.
Development of metastases requires a multistep process, where each step is essential for the survival of cancer cells at distant foci [4]. Regardless of their organ distribution patterns, all types of metastases fall under the so-called “seed and soil” concept. In order for disseminating cells to progress into distant foci (brain, bones, lymph nodes and other remote sites), they need to be proficient in all capabilities associated with the metastatic cascade, i.e. invasion, intravasation, survival in circulation, penetration through the blood-brain-barrier(BBB) [5], extravasation, metastatic niche formation, adaptation support [6] and colony progression. In addition, disseminated cancer cells must be able to survive at the sites of spread [7], which greatly depends on their interactions with the new environment that promote seeding. Since heterogeneity of most tumors alone cannot explain priming of metastatic cancer cells for specific sites and organs, accumulation of genetic mutations, altering expression of certain genes [8], could greatly affect distribution of metastatic cells in cancer patients.
The focus of this review is regulatory mechanisms of cancer progression and the role of regulatory (metastasis suppressor) factors, particularly KISS1, in breast cancer metastatic spread to the brain, which represents the classical example of disseminated cancer with the deadliest outcome. KISS1 was initially identified as a human melanoma metastasis suppressor gene by using a subtractive cDNA hybridization approach [9, 10]. In line with that discovery, transfection of KISS1 into metastatic human melanoma cell lines suppressed metastasis in athymic nude mice by 50–95% [9]. Besides, KISS1 has been mapped to chromosome 1q32–q41, which is frequently deleted in late stage human breast carcinomas, while its regulators are encoded by chromosome 6, lost in more than 50% of melanoma metastases [11].
Under physiological conditions, a 145 amino acid KISS1 precursor peptide undergoes post-translational modification and then is processed into shorter products: among them, kisspeptins 10, 13, 14 and 54 [12], which signal through the G protein-coupled receptor GRP54, also known as the KISS1 receptor (KISS1R), implicated in regulating neurosecretory activity of gonadotropin-releasing hormone (GnRH) [13] and neurotransmitters [14]. Besides the long-known involvement of kisspeptins in the onset of puberty, sexual maturity, and pregnancy (through direct regulation of gonadotropin release by the hypothalamus) the peptide has also some other important regulatory roles. Those include modulation of doxorubicin resistance in ERα-negative and triple negative breast cancer (TNBC) cells as well as multiple roles, proposed particularly for KISS1/KISS1R complex, in both tumor and metastasis development mechanisms. Although the latter functions of KISS1 are currently quite controversial, here we discuss the emerging evidence implicating KISS1 in regulation of the process of metastatic breast cancer survival and colonization in the brain environment.
The complexity of KISS1 interactions with important cancer cell survival-related signaling pathways has been extensively reviewed in the literature. Some of the most recent review papers also discuss the role of KISS1 in cancer progression [15–17]. In some excellent reviews leading authorities discuss signaling pathways, believed to be activated upon KISS1 interaction with its cognate KISS1R receptor, and the relevance of that interaction to expression of tumor-associated markers. In contrast to previous review articles on KISS1, a main goal of our review is to discuss the emerging evidence for the relationship between KISS1 expression and induction of apoptosis or autophagy. In addition, here we overview the latest evidence linking KISS 1 protein with activation of its downstream targets viatranscription-dependent or transcription-independent mechanisms. Finally, we discuss whether KISS1 expression can be artificially modulated and whether KISS1 can be used as a clinical biomarker for assessment of tumor progression or even as a potential anti-cancer therapeutic, specifically suppressing metastatic progression in cancer patients.
2. Autophagy and metastases
Autophagy is a network of catabolic pathways that support cellular homeostasis by digesting cytoplasmic components and damaged cellular organelles inside the cytoplasmic vesicles, called lysosomes. Autophagy can play both cancer-promoting and cancer-suppressing roles, depending on the stage of cancer progression. In many types of cancer, especially in the early stage of the disease, autophagy plays a cytoprotective role by clearing organelles and misfolded proteins from unhealthy cells as well as preventing cells from DNA damage and genomic instability. In line with that notion, Gu et al. [18] carried out bioinformatics analysis of several datasets (GSE21653, GSE3494 and GSE7390) from the Gene Expression Omnibus (GEO), database for autophagy-related gene expression in breast cancer tissue specimens in order to define autophagy prognostic signature. This study demonstrated association of expression of several autophagy markers (BCL2, BIRC5, EIF4EBP1, ERO1L, FOS, GAPDH, ITPR1 and VEGFA) with steroid receptor levels and metastasis-free survival of breast cancer patients. Consistent with the observation that reduction in autophagy levels promotes oxidative stress and stimulates cancer progression from precancerous to malignant state, the level of autophagy marker Beclin-1 mRNA was much higher in the normal breast tissue than in the tissue, undergoing malignant transformation. Interestingly, no correlation between expression of autophagy siganture and estrogen receptor (ER), progesterone receptor (PR) or HER2 was observed in that study, suggesting regulation of those genes by independent pathways.
On the other hand, cancer progression is associated with upregulation of autophagy-related genes [19], improving the survival capacity of cancer cells under stress conditions due to activation of recycling mechanisms and utilization of endogenous resources. Sivridis et al. analyzed expression of LC3A autophagy marker protein implicated in autophagosome formation during autophagy in breast cancer specimens and demonstrated a direct association between elevated levels of the cytoplasmic form of LC3A and expression of both estrogen (ER) and progesterone (PR) receptors [20], as well as its correlation with metastatic state of the cells, indicating that an excessive autophagic response is associated with high-grade tumors and poor disease prognosis. In line with that, later Kim et al. showed that expression of Beclin-1 and P62 was greater in the androgen-rich(AR) subtype of triple-negative breast cancer (TNBC) (p = 0.008), which suggested high autophagic activity in those cancer cells [22]. While in this study expression of LC3A and LC3B showed no difference between the molecular subtypes of TNBC, overall high expression of autophagy adaptor p62 [21] in certain TNBC subtypes was significantly associated with longer disease-free and overall survival of the breast cancer patients [22]. Similar data were obtained by Choi et al., who also analyzed expression of autophagy proteins in various subtypes of breast cancer. They found that cytoplasmic Beclin-1 as well as p62, LC3A and LC3B exhibited the highest expression levels in TNBC tumors and the lowest levels in stroma. Moreover, direct correlations between cytoplasmic levels of p62, ER, PR and HER2 receptor, expression levels of LC3B marker and ER or PR and, finally, cytoplasmic level of Beclin-1 and that of ER were found in breast cancer tumors, in contrast to benign neoplasms [23]. Finally, a study by Zhao et al. showed a direct correlation between expression of LC3B autophagy marker and the development of TNBC breast cancer metastases [24]. Whether autophagy induction is a risk factor for the development of metastases is yet to be clarified. However, considering that breast cancer cells express various tumor suppressors, the recently discovered relationship between KISS1 expression and autophagy downregulation in breast cancer brain metastatic cells [25] deserves a special attention and may suggest a unique role for this regulatory gene in brain metastases suppression mechanism(s).
Importantly, KISS1 represents a kind of metastasis suppressor that is capable of modulating behavior of some cancer cells by suppressing their proliferation probably via induction of autophagy and apoptosis [26]. On the other hand, its expression in some tumors is elevated relative to normal cells [27–29]. Under stress conditions KISS1 expression has been reported to be a subject for various modes of regulation [30, 31], suggesting multiple mechanisms of cellular adaption to hypoxia. As a tumor suppressor, KISS1 mainly plays an inhibitory role in metastatic cancer spreading through downregulation of NFkB signaling [32], pro-invasive factor MMP9 [25] and its other downstream targets. By using animal models, it has been shown that neurons activate KISS1 expression in response to various internal stress-promoting stimuli [33], suggesting a new role for KISS1 in cell stress management. It is yet to be determined how KISS1 controls physiological stress, but in healthy cells under in vitro- or in vivo-simulated stress conditions, KISS1 has been shown to mediate suppression of both cell proliferation [34, 35] and invasion [31]. Although some data indicate that KISS1 protein may exert no effect on cell proliferation [37] or invasion [38], to date, suppression of the above cellular activities is commonly accepted as the main function of KISS1 in cancer control.
Tumor dormancy and cell adaptation [36] represent yet another stage in the process of metastatic focus formation by spreading tumor cells/CTCs. The above states in disseminating tumor cell behavior are usually pending significant changes that occur in distal (organ) compartments and may provide favorable conditions for successful cell seeding and colonization. Specifically, interaction of cancer cells with organ parenchyma could induce profound physiological changes affecting survival rate of CTCs/spreading tumor cells. As first demonstrated by Kim S.J. et al. [39] and later by Kaverina N. et al. [30], interaction of tumor cells with astrocytes in the brain compartment can promote brain metastatic transformation of CTCs. It appears that astrocyte-CTC interaction is required to overcome growth arrest in cancer cells and boost their survival at the distant foci via inhibition of KISS1 expression. Yet another example was provided by an earlier study performed by Nash et al. [40], where the Kisspeptin-54 peptide expressing melanoma cells demonstrated strong propensity to skin while remaining dormant in the lung. Furthermore, physiological changes taking place in cancer cells after their interaction with the organ environment are beneficial for their subsequent survival, biodistribution and seeding in the tissue (Table 1).
Table 1.
Involvement of KISS1 suppressor in cancer progression
| Tumor progression/Metastatic event | Cancer type | KISS1 peptide responsibility | Reference | |
|---|---|---|---|---|
| 1 | Tumor proliferation | Hepatocelluar carcinoma, renal cell carcinoma | No cell proliferation | [37, 41] |
| Colorectal carcinoma | Inhibit cell proliferation | [32] | ||
| 2 | Early dissemination Tumor adhesion | Hepatoclluar carcinoma | Suppress adhesion | [37, 41, 42] |
| 3 | Tumor Invasion | Gastric cancer, Renal cell carcinoma, Esophageal squamous carcinoma, Breast adenocarcinoma, Colorectal cancer, | Suppress invasion | [41, 43–45] |
| 4 | Intravasation | |||
| 5 | CTC circulation/survival | |||
| 6 | Extravasation | |||
| 7 | Dormancy/support adaptation/Micrometastasis | Breast cancer | Regulates autophagy; Maintain dormancy | [30, 46] |
| 8 | Colonization | Melanoma, breast cancer, ovarian cancer | Block colonization | [9, 10, 40, 47, 48] |
3. KISS1 interplays with cellular signaling beyond migration: in silico evidence
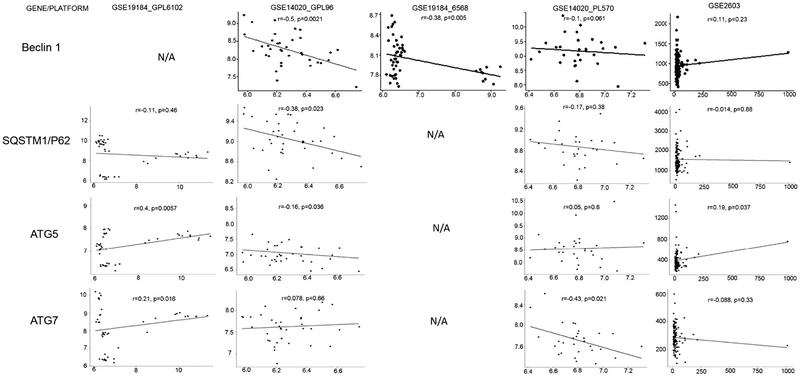
A global gene expression analysis can offer an important resource for systematization of genetic interactions and reveal new interplays between cancer signaling and expression of tumor suppressors, such as KISS1. In this regard, recently our laboratory undertook a computational analysis of cell survival-affecting signaling pathways that are regulated by ATG5, ATG7, p62/SQSTM1 and BECN1 genes, assuming that metastatic cells have the ability to survive at the sites of dissemination prior to initiating their proliferation and invasion into the brain tissue. We analyzed KISS1 expression by using eight GEO-deposited arrays obtained from metastatic tissues resected from primary specimens that represented metastatic cells grown in their native environment. In the array described by Zhang et al. the authors, using lung, brain and bone metastases developed from primary breast cancer tumor (GSE14020/GPL570), observed no changes in KISS1 expression between the groups (ANOVA, p value <0.05) as well as no correlation (p < 0.05) between expression of ATG5, ATG7, P62 or BECN1 and that of KISS1. Given the complexity of KISS1 protein’s functional interactions, we also attempted to validate our earlier findings regarding the KISS1-regulated autophagy and apoptosis by analyzing GEO-deposited arrays. An inverse correlation was observed between the averaged IDs for KISS1 and SQSTM1/p62 mRNAs (GSE14020_GPL96, r = −0.38, p = 0.023) or KISS1 and ATG7 mRNA (GSE14020_PL570, r= −0.43, p = 0.021) or KISS1 and Beclin1 mRNA (GSE14020_GPL96, r = −0.5, p = 0.0021 and GSE19184_6568, r = −0.38, p = 0.005) Fig. 1). While a direct correlation between ATG5 and KISS1 mRNAs levels was observed in two-gene array datasets GSE2603 (r = 0.19, p = 0.037) and GSE19184_GPL6102 (r = 0.4, p = 0.0057), no significant correlation (p < 0.05) was noted in GSE14020_PL570, GSE12276 (not shown), GSE2034 (not shown), GSE3141 (not shown), GSE19184_GPL6102, and GSE19184_GPL6568 datasets (Fig. 1). Some of those correlations have been further validated in a subsequent follow-up study [49]. Although the clustering analysis of gene signatures associated with high and low levels of KISS1 gene expression (GSE14020_PL570) suggests a role for KISS1 in protein synthesis and metabolic stress response (Fig. 2A and B), it remains to be investigated how overexpression of KISS1 in CTCs allows maintaining their metastatic phenotype and viability without killing the cells [30] [50]. Taking into account that KISS1 is implicated in all stages required for the development and maintenance of a cancer metastatic phenotype, including primary tumor growth and its subsequent invasion of secondary foci, disseminated cancer cell dormancy and ultimate colonization, we believe that KISS1 might be involved in modulating primary and metastatic tumor cell survival through an autophagy-mediated signaling. Below, we will briefly discuss the most recent evidence for the role of tumor suppressor KISS1 in regulating autophagy- and apoptosis-related gene expression [30] in metastasizing cancer cells (Figure 3).
Fig. 1. Comparative correlation of autophagy-related genes with KISS1 using GEO-deposed mRNA arrays.
The relative expression levels of KISS1, ATG5, ATG7, BECLIN1 and SQSTM1/P62 mRNAs in brain metastases of breast cancer and their correlation analyzed by using various mRNA platforms and data arrays deposed to GEO
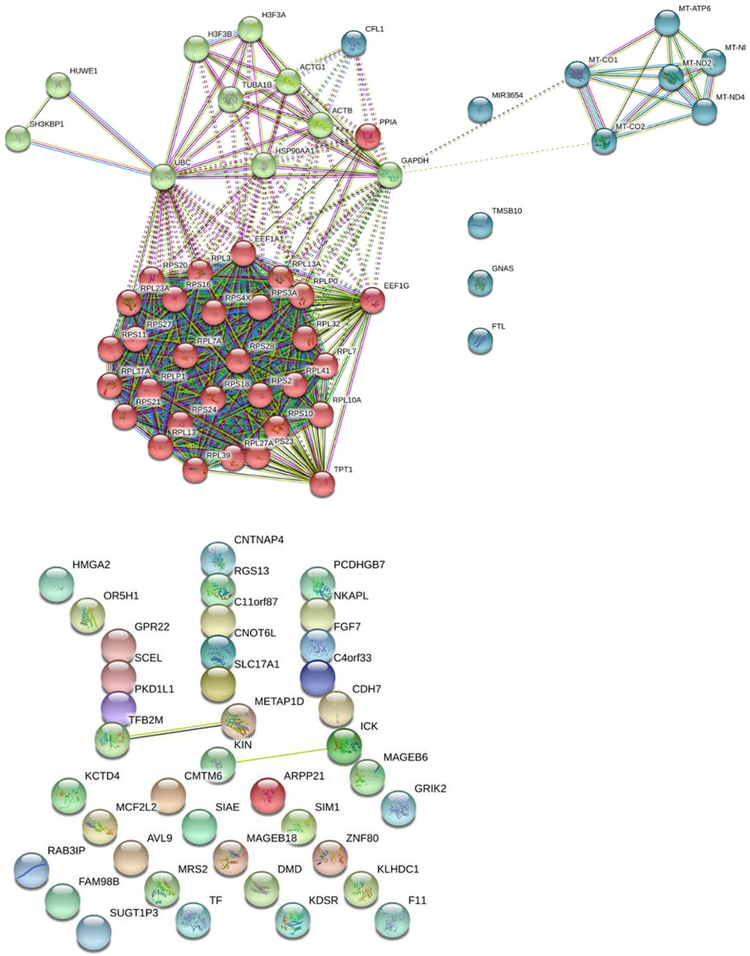
Fig. 2. A map of functional interactions (interactome) of KISS1 with various.
brain metastatic cellular proteins. Previously reported interactions are shown by solid lines, whereas potential or predicted interactions are indicated by dashed lines. Subnetworks of upregulated (top) KISS1-interacting proteins (with expression profiles directly correlating with that of KISS1) are presented by 3 distinct cohorts grouped by color, each representing protein networks implicated in the following cellular functions: mechanisms of protein synthesis (red spheres), cellular metabolism involving mitochondrial proteins (green spheres), and cellular invasion mechanism (blue spheres). Downregulated proteins with expression profiles inversely correlating with that of KISS1 (bottom) do not fall into any distinct functional (color-grouped) cohorts and also exhibit a very few experimentally-evidenced/established functional links.
Fig. 3. Ingenuity Pathway Analysis (IPA) for KISS1-regulated network.
KISS1-regulated network is associated with proteins, ions, RNA molecules and chemical compounds. KISS1 is positioned in the center of the protein network. The interacting proteins are depicted as various geometric shapes, each representing a particular functional class of proteins, whereas their position with regard to two horizontal solid lines reflects their compartmentalization properties/ intracellular localization, i.e. in the nucleus, cytoplasm or extracellular space. Proteins with unknown localization are positioned inside the vertical box on the right (“unknown”). The length and type of each protein-connecting line reflects either predicted (dashed line) or experimentally evidenced/published (solid line) interactions. Data were analyzed through the use of IPA (Ingenuity® Systems, http://www.ingenuity.com).
4. Does KISS1 expression trigger a new signaling?
Previous studies revealed that the KISS1-mediated signaling occurs through KISS1R. While a discrepancy has been noted between KISS1R expression levels and KISS1-mediated metastasis suppression [40], the involvement of autocrine or paracrine mechanisms activated even by low levels of GRP54 receptor has been proposed [10]. In support of that hypothesis, inhibition of intracellular calcium (Ca2+) mobilization, induced by the SDF-1/CXCL12 ligand, was observed in response to treatment of melanoma [40] or cervical carcinoma [51] cells with KP-10. Whether the KISS1-mediated signaling exhibits cell and/or organ specificity still needs to be elucidated. It has been demonstrated that KISS1-mediated signaling activates MAPK, AKT and ERK pathways [52]. Interaction of KISS1 with CXCR4 and gonadotropin-releasing hormone (GnRH) receptors suggest its broad effect on and interconnection with various signaling pathways including the ones modulating autophagy and apoptosis.
Autophagy in metastatic brain tumor cells is activated by a variety of stimuli, such as brain environment and pharmacological stress. Given the crosstalk between apoptotic and autophagy signaling pathways, there is always a fine line between induction of apoptosis and autophagy activation. To elucidate the mechanism whereby suppression of KISS1 promotes autophagy, our laboratory recently identified a number of proteins downregulated in response to induced overexpression of KISS1 [53], which include transcription factors GATA4 and SOX9. As shown in the latter study [54], translocation of nuclear transcription factor SOX9 to the cytoplasm in some breast cancer cell lines is associated with abrogation of growth arrest response in those cancer cells, leading to their uncontrolled proliferation, as well as with the development of metastases [55] and poor survival of breast cancer patients [56]. In contrast to KISS1 mRNA that shows a direct correlation with estrogen receptor ESR2 levels, the level of SOX9 mRNA inversely correlates with ESR2 expression (p < 0.001). This observation implies an inverse relationship between KISS1 and SOX9 on the one hand, and autophagy, on the other. Although the revealed association between KISS1 expression and the intracellular status of the ESR2 receptor may seem highly contradictory [57–59], activation of known downstream targets of KISS1 (such as GATA4, regulating inflammation and senescence [60], or SOX9, known to modulate WNT [61], cAMP [62] and TGFβ [63] signaling pathways) through interaction with its cognate receptor (GRP54/KISS1R), suggests a new potential role for KISS1 beyond suppression of metastases [53].
5. KISS1 as a candidate blood-basedand/or IHC biomarker of cancer progression
KISS1 could potentially serve as a potent biomarker for cancer disease progression, since its expression is detectable not only in hard tissues, but also in the blood [64, 65]. Expression level of KISS1 in brain metastases or other metastatic lesions of melanoma was found to be lower than in primary tumor specimens. We and others have reasoned that the reduced expression levels of KISS1 in metastatic tissues might inversely correlate with accumulation of certain kisspeptin (KP) peptide(s) in the blood [66] and may account for low retention of the tumor suppressor in metastatic cancer tissues. It has indeed been reported that kisspeptin 54 (KP-54) was detectable in plasma of patients with progressed gestational trophoblastic disease [67]. This intriguing clinical study data raises questions about the source of the peptide, considering its abundant expression in healthy placenta as a known source of KISS1 production. In this regard, it should be noted that the KISS1/KISS1R signaling system plays an important role in repressing trophoblast invasion [68], and has been implicated in controlling normal puberty [69] and preeclampsia in humans. However, it turns out that the normal placental level of KISS1 is much lower than that observed during malignant transformation, suggesting that blood level of KISS1 could reflect cancer progression. In support of this notion, KISS1 blood levels were found to be decreasing as a result of chemotherapy treatment [70]. However, further assessment of KISS1 in the plasma during the development of clinical symptoms of non-small lung [71] or prostate [72] cancers revealed no correlation of KISS1 blood levels with cancer progression, although in some (pancreatic and gastric) cancer patients the level of KISS1 was significantly higher as compared to healthy patients [73]. In contrast, colon cancer progression to the lymph nodes exhibited a strong direct correlation with KISS1 levels in the cancer patient’s plasma [74]. Nonetheless, the existing clinical evidence altogether fails to unambiguously support the utility of KISS1 as a potential blood-based biomarker, although in many cases accumulation of KISS1 in cancer tissues could be definitively observed.
Deregulation of ErbB2/Her2 (Her2/neu), PR or ER are the most common cellular signaling abnormalities in metastasizing breast adenocarcinomas. Although, other repertoires of signaling defects may contribute to adenocarcinoma progression, the role of cell signaling through the above receptors in metastatic dissemination of lung and breast carcinomas is well established. Current data regarding expression of the main cancer signaling receptors, including the primary KISS1 receptor GRP54, ER alpha and PR show a positive correlation with KISS1 mRNAs [75] as well as an association between HER2 and the GRP54 expression. Earlier our group observed a direct correlation between KISS1 and PR expression (Spearman correlation, p = 0.023) [58], but the lack of any significant association of KISS1 levels with those of HER2 or ER, indicating that hormone-sensitive cancer cells expressing KISS1 might respond to some endocrine therapy, such as Tamoxifen (ER-independent adjuvant effect) [76]. Furthermore, the relationship between ER, KISS1R and KISS1, investigated by Papaoiconomou et al. [59], indicated no correlation between KISS1R and ER expression levels in breast cancer cells. However, no statistical significance was observed for those findings. Interestingly, a weak correlation between KISS1 expression and the abundance of its receptor KISS1R (P <0.05) on the cell surface was observed as well, suggesting a variable distribution of both markers in different cancers. The discordance found between cell invasiveness and KISS1/GRP54 abundance on the surface of mammalian pancreatic cancer cells [77] and trophoblasts [78] may suggest an alternate biological role for KISS1.
It has been demonstrated that the abundance of KISS1R/ GRP54 exhibits a direct correlation with ER alpha (ERα) levels in breast cancers [79]. Furthermore, high levels of KISS1 and KISS1R/GRP54 expression found in estrogen receptor-positive tumors correlate with poor disease prognosis for breast cancer patients. It is well established that in a fraction of cases, one can identify a dramatic difference between primary and metastatic cells with regard to expression of steroid hormone receptors, such ERα, PR, AR or human epidermal growth factor receptor 2 (HER2/neu). This difference often arose from autocrine/paracrine regulatory loop mechanisms and might contribute to cancer progression and induction of hormone therapy resistance. A recent study by Nauroth et al. [80] provided an evidence for such drug therapy-mediated receptor status “conversion” in metastatic breast cancer cells with regard to the loss of ER, PR or HER2 expression levels relative to primary tumors. Given the potential variability in each receptor’s status, dependent upon such factors as cell distribution, tumor heterogeneity and prior drug therapy, it would be of interest to further explore correlation between KISS1 and KISS1R/GRP54 surface marker expression in those cases.
6. Can KISS1 expression be therapeutically modulated?
KISS1 represents an attractive candidate target for cancer therapy applications, since it plays an important role in suppressing cancer metastases by controlling cell apoptosis [26, 81], tumor angiogenesis [82], invasion [77, 83] and colonization [40]. These functions, in particular, dictate potential therapeutic applications of KISS1 based on its ectopic overexpression, induced upregulation or indirect modulation of its activity in tumor cells via a drug therapy treatment.
6.1. Targeting cancer cells by a vector for delivery and ectopic expression of KISS1
It has previously been shown that breast cancer metastatic nodules express lower levels of KISS1 [58] than the parental primary tumor tissues, suggesting that reversing KISS1 down-regulation may suppress the associated induction of cancer cell spreading and seeding. The latter approach has obvious limitation related to the efficacy and specificity of vector-mediated KISS1 delivery. Additionally, some recent data show a positive correlation between KISS1 expression and the aggressiveness of TNBC [84] or hepatocellular carcinoma [85]. These contradictory findings indicate that the kisspeptin/KISS1R signaling function could be tissue- or tumor context-specific and its role in cancer progression requires further investigation before KISS1/ kisspeptins can be implicated for use either as biomarkers or anticancer therapeutics.
McNally et al. were the first to explore the gene therapy approach aimed at suppressing pancreatic cancer metastases by using a plasmid-based delivery and ectopic expression of a truncated form of KISS1 devoid of the leader peptide sequence [86], required for the protein secretion. Delivery of truncated KISS1 significantly reduced lung and liver metastases in S2VP5 pancreatic cancer model in nude mice. Most recently, secretion of bioactive kisspeptin by lactic acid bacteria inhibited proliferation and migration of HT29 human colon cancer cells in vitro [87]. Thus, changes in the cell proliferation activity directly correlated with induction of apoptosis and expression of MMP9, as one of the pro-invasive markers. This was consistent with the data from our group obtained by transducing brain metastatic clones of MDA-MB-231Br breast cancer cells with replication-competent adenoviral vector encoding KISS1 [30]. Given that KISS1 expression induces apoptotic signals, overexpression of KISS1 in the target cells could be a promising approach for treating metastatic breast cancers.
6.2. WASF3-KISS1 interplay as a model for KISS1 upregulation
The crosstalk between KISS1 and anti-apoptotic/survival response signaling pathways indicates that pharmacological induction of KISS1 expression could be beneficial against a variety of cancer types. It is obvious that the mechanisms of cancer cell invasion and proliferation are complex and their targeting with chemical drugs requires high degree of specificity. For instance, the Wiskott-Aldrich Syndrome Protein Family Member 3 (WASF3) [88] and WAVE3 genes, responsible for cell invasion and motility in vitro [89] and in vivo [90], is activated in metastatic breast cancer cells, thereby accounting for their invasive phenotype. It has also been noted that inactivation of WASF3 results in elevated IκBα (NF-κB inhibitor) levels in the cytoplasm and the concomitant inhibition of NF-κBp65/50 subunits in the nucleus. This effect is similar to that of KISS1 overexpression, which is also known to inhibit NF-kB signaling and nuclear accumulation ofp65/50 subunits.
6.3. Dual targeting of cancer cells and cancer cell vicinity
Although KISS1 gene transfer/delivery strategies are conceptually straightforward, in clinical settings KISS1 gene delivery may be associated with a substantial activation of immune response against the delivery vehicle (vector)and/or the restricted permeability of the brain compartment. Considering that brain environment cooperates with invading cancer cells via production of chemokines, cytokines, exosomes etc., suppressing tumor cell-tumor environment communication could potentially be used to augment the anti-cancer effect of therapeutic interventions. Earlier, honokiol derivatives [91] and later honokiol itself [92], a small-molecule biphenol isolated from Magnolia spp. bark that has been shown to be a potential anticancer agent involved in multiple facets of signal transduction, were demonstrated to suppress neuroinflammation via interaction with cannabinoid receptors. Expression of those receptors on the surface of neuroglial cells and astrocytes [93] offers a potential application of honokiol for treatment of metastatic cancers. In line with this notion, simultaneous blocking of cancer cell proliferation, invasion and possibly chemokine production by honokiol demonstrated a strong dose-dependent suppression of cancer invasion and colony formation in 786–0 renal cell carcinoma (RCC) cell line [83]. However, not all invasion-and/ormigration-suppressing anti-metastatic drugs affect expression of metastasis suppressor KISS1. It has been reported by Curtis et al. that hydroxyflutamide, gefitinib or resveratrol do not affect expression of KISS1 upon treatment of LNCaP, DU145 or PC3 prostate cancer cells in vitro [72], despite the well-known ability of those drugs to target tumor cells [94] and tumor environment [95]. Unlike honokiol, hydroxyflutamide, gefitinib and resveratrol interfere with either ER- [96] or androgen receptor (AR) [97] dependent signaling pathways. The latter may be critical for transmission of KISS1 expression-modulating signals and activation of autophagy-mediated cell death [98] that inversely correlates with KISS1 expression [30].
7. Concluding remarks
Cancer metastasis to the distant organs such as brain is based on genetic, metabolic and phenotypic changes in cancer cells that prompt their extravasation to circulation, followed by dissemination, intravasation into the brain parenchyma and subsequent colonization. For each potential therapeutic approach involving metastasis targeting strategy it remains unclear whether increasing “tumor vulnerability” alone is going to be sufficient for achieving therapeutic efficacy in clinical settings, especially considering the nature of the tumor-surrounding environment. The benefit of using KISS1-based cancer therapies lies in the multiple roles this molecule plays in cancer progression including tumor angiogenesis, cancer cell invasion and autophagy-mediated survival of cancer as well as non-cancer cells. The study of Terasaka et al. [99], showing that the expression of kisspeptin-induced gonadotropin-releasing hormone (GnRH) can be suppressed by bone morphogenetic protein (BMPs) and especially by metastasis suppressor BMP-4 [100] via reducing ERK signaling activity, amply illustrates the complexity of KISS1-mediated molecular interactions involved in GnRH regulation. The finding that BMPs suppress expression of kisspeptin receptor KISS1R/GRP54 is not surprising in light of the fact that treatment with BMP4 or other BMPs [101] is known to regulate cell sensitivity to drugs and other biological molecules such as somatostatin. This study also indicates that KISS1-mediated induction of GnRH occurs through KISS1R/GRP54 signaling, which is antagonized by BMPs. Several experimental data point out that the response to KISS1 is dose-dependent with pro-cancerous effect on cells in vitro achieved at concentrations of about 100 nM [102, 103]. In that regard, any cells expressing KISS1 should have developed a molecular “rheostat”-like mechanism to maintain their homeostasis.
KISS1 upregulation-based strategies could be more efficacious in combination with induction of cancer cell vulnerability via suppression of their interaction with cancer cell-reactive astrocyte, mentioned above. To date, attempts to identify or isolate molecular inhibitors of such biological interaction between tumor cells localized to the brain and healthy cells of the brain environment, such as astrocytes, has not yet been successful. Although no clinically proven anti-metastatic treatment is available yet, several potential metastatic inhibitors have been tested in vitro by using cervical [104], small cell lung [105], metastatic renal carcinoma [106] and prostate [107] cancer models. Those include miRNA inhibitors as well as antagonist compounds capable of suppressing C-X-C chemokine receptor type 4 (CXCR4)-mediated signaling involving CXCR4, a cognate receptor for CXCL12/SDF-1 ligand. CXCR4 is known to be overexpressed in and abundant on the surface of brain metastatic cells of renal carcinoma [108] and non-small cell lung carcinoma [109]. While CXCL12-CXCR4 signaling is also implicated in the mechanism of breast cancer metastasis to the brain [110], strategies based on CXCR4 inhibition could be as effective in treatment breast cancer metastases as the ones involving inactivation of miRNAs that target or could potentially target KISS1 mRNA during brain invasion and colonization of circulating breast cancer cells [30].
Safety is one of the cornerstones of drug specificity reflected in its selective toxicity towards cancer cells and a lack thereof for normal cells. Therefore, the risk of modulating KISS1 expression may be associated with the risk of cancer outcome since KP-10, but not other kisspeptins, has been identified as a novel paracrine/endocrine regulator with a fine-tuning role in trophoblast invasion [111]. The most recent study demonstrated that blocking KiSS1 with a specific small peptide antagonist (p234) impairs TGFβ-mediated cell invasion and MMP9 induction, thereby establishing the essential role of KiSS1 in mediating the pro-invasive effects of TGFβ as a downstream target of the canonical TGFβ/Smad2 signaling pathway. In this regard, to determine conditions for application of anti-cancer drugs in clinical settings, dose escalation studies for KISS1 and KISS1-inducers, such as TGFβ1, TNFα etc., as well as prolonged treatment with the clinically-relevant doses thereof should be conducted by using both normal and cancer cells in parallel.
Highlights
Development of metastases is a property of malignant tumors characterized by the ability of tumor cells to spread across the hematological barrier and develop secondary nodes in distant organs.
The effectiveness of therapies suppressing or preventing distant metastases depends on the cancer stage at diagnosis. Early detection and resection of a primary tumor sometimes allows preventing its metastatic spread.
Metastatic tumor suppressor protein KISS1 regulates behavior of cancer cells beyond invasion and proliferation.
Restoration of metastasis suppressor KISS1 expression in KISS1-deficient metastatic tissue can either induce or suppress metastatic progression depending on its dose, the presence/abundance of KISS1R, ER or PR on the cell surface and the pressure of the tumor environment. Therefore, it should be taken into account that utilization of KISS1 in gene therapy applications may pose a serious safety concern.
KISS1 tumor suppressor represents an integral part of cell signaling, exerting either direct or indirect effect on cancer cell survival, behavior via autocrine or paracrine regulatory mechanisms.
Funding information
This research study was supported by Russian academic excellence project “5–100”.
Abbreviations
- AR
androgen receptor
- BMP
bone morphogenetic protein
- CXCR4
C-X-C chemokine receptor type 4
- CXCL12 or SDF1
The stromal cell-derived factor 1 (SDF 1), also known as C-X-C motif chemokine 12 (CXCL12)
- ER
estrogen receptor
- GEO
Gene Expression Omnibus (GEO), a database repository
- GFAP
Glial fibrillary acidic protein
- HER2
a member of the human epidermal growth factor receptor (HER/EGFR/ErbB) family
- NFkB
Nuclear Factor Kappa B
- PDGFRb
Platelet Derived Growth Factor Receptor Beta
- PR
Progesterone Receptor
- WASF3
WAS Protein Family Member 3
Footnotes
Conflict of interest Authors declare no conflict of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pranjol MZ, Gutowski N, Hannemann M, & Whatmore J (2015). The potential role of the proteases Cathepsin D and Cathepsin L in the progression and metastasis of epithelial ovarian Cancer. Biomolecules, 5(4), 3260–3279. 10.3390/biom5043260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha R, Sage W, & Watts C (2017). The evolving clinical management of cerebral metastases. European Journal of Surgical Oncology, 43(7), 1173–1185. 10.1016/j.ejso.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada Y, Fouad A, Pickren JW, & Lane WW (1983). Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer, 52(12), 2349–2354. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ (2003). The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nature Reviews. Cancer, 3(6), 453–458. 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 5.Jia W, Lu R, Martin TA, & Jiang WG (2014). The role of claudin-5 in blood-brain barrier (BBB) and brain metastases (review). Molecular Medicine Reports, 9(3), 779–785. 10.3892/mmr.2013.1875. [DOI] [PubMed] [Google Scholar]
- 6.Van Swearingen AE, Siegel MB, & Anders CK (2014). Breast cancer brain metastases: Evidence for neuronal-like adaptation in a ‘breast-to-brain’ transition? Breast Cancer Research, 16(3), 304 10.1186/bcr3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niwinska A, Murawska M, & Pogoda K (2010). Breast cancer brain metastases: Differences in survival depending on biological subtype, RPA RTOG prognostic class and systemic treatment after whole-brain radiotherapy (WBRT). Annals of Oncology, 21(5), 942–948. 10.1093/annonc/mdp407. [DOI] [PubMed] [Google Scholar]
- 8.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. (2009). Genes that mediate breast cancer metastasis to the brain. Nature, 459(7249), 1005–1009. 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, & Welch DR (1996). KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. Journal of the National Cancer Institute, 88(23), 1731–1737. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, & Welch DR (1997). Identification of highly expressed genes in metastasis-suppressed chromosome 6/human malignant melanoma hybrid cells using subtractive hybridization and differential display International Journal of Cancer, 71(6), 1035–1044. [DOI] [PubMed] [Google Scholar]
- 11.Miele ME, Jewett MD, Goldberg SF, Hyatt DL, Morelli C, Gualandi F, Rimessi P, Hicks DJ, Weissman BE, Barbanti-Brodano G, & Welch DR (2000). A human melanoma metastasis-suppressor locus maps to 6q16.3-q23. International Journal of Cancer, 86(4), 524–528. [DOI] [PubMed] [Google Scholar]
- 12.Beck BH, & Welch DR (2010). The KISS1 metastasis suppressor: A good night KISS for disseminated cancer cells. European Journal of Cancer, 46(7), 1283–1289. 10.1016/j.ejca.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avendano MS, Vazquez MJ, & Tena-Sempere M (2017). Disentangling puberty: Novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Human Reproduction Update, 23(6), 737–763. 10.1093/humupd/dmx025. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JP, Guerriero KA, Keen KL, Kenealy BP, Seminara SB, & Terasawa E (2017). Kisspeptin and neurokinin B signaling network underlies the pubertal increase in GnRH release in female rhesus monkeys. Endocrinology, 158(10), 3269–3280. 10.1210/en.2017-00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fratangelo F, Carriero MV, & Motti ML (2018). Controversial role of Kisspeptins/KiSS-1R signaling system in tumor development. Front Endocrinol (Lausanne), 9, 192 10.3389/fendo.2018.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciaramella V, Della Corte CM, Ciardiello F, & Morgillo F (2018). Kisspeptin and Cancer: Molecular interaction, biological functions, and future perspectives. Front Endocrinol (Lausanne), 9, 115 10.3389/fendo.2018.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhu VV, Sakthivel KM, & Guruvayoorappan C (2013). Kisspeptins (KiSS-1): Essential players in suppressing tumor metastasis. Asian Pacific Journal of Cancer Prevention, 14(11), 6215–6220. [DOI] [PubMed] [Google Scholar]
- 18.Gu Y, Li P, Peng F, Zhang M, Zhang Y, Liang H, Zhao W, Qi L, Wang H, Wang C, & Guo Z (2016). Autophagy-related prognostic signature for breast cancer. Molecular Carcinogenesis, 55(3), 292–299. 10.1002/mc.22278. [DOI] [PubMed] [Google Scholar]
- 19.Lorin S, Hamai A, Mehrpour M, & Codogno P (2013). Autophagy regulation and its role in cancer. Seminars in Cancer Biology, 23(5), 361–379. 10.1016/j.semcancer.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Sivridis E, Koukourakis MI, Zois CE, Ledaki I, Ferguson DJ, Harris AL, et al. (2010). LC3A-positive light microscopy detected patterns of autophagy and prognosis in operable breast carcinomas. The American Journal of Pathology, 176(5), 2477–2489. 10.2353/ajpath.2010.090049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei H, Wang C, Croce CM, & Guan JL (2014). p62/SQSTM1 synergizes with autophagy for tumor growth in vivo. Genes & Development, 28(11), 1204–1216. 10.1101/gad.237354.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Jung WH, & Koo JS (2012). Differences in autophagy-related activity by molecular subtype in triple-negative breast cancer. Tumour Biology, 33(5), 1681–1694. 10.1007/s13277-012-0424-1. [DOI] [PubMed] [Google Scholar]
- 23.Choi J, Jung W, & Koo JS (2013). Expression of autophagy-related markers beclin-1, light chain 3A, light chain 3B and p62 according to the molecular subtype of breast cancer. Histopathology, 62(2), 275–286. 10.1111/his.12002. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Yang M, Zhao J, Wang J, Zhang Y, & Zhang Q (2013). High expression of LC3B is associated with progression and poor outcome in triple-negative breast cancer. Medical Oncology, 30(1), 475 10.1007/s12032-013-0475-1. [DOI] [PubMed] [Google Scholar]
- 25.Yan C, Wang H, & Boyd DD (2001). KiSS-1 represses 92-kDa type IV collagenase expression by down-regulating NF-kappa B binding to the promoter as a consequence of Ikappa Balpha -induced block of p65/p50 nuclear translocation. The Journal of Biological Chemistry, 276(2), 1164–1172. 10.1074/jbc.M008681200. [DOI] [PubMed] [Google Scholar]
- 26.Yin Y, Tang L, & Shi L (2017). The metastasis suppressor gene KISS-1 regulates osteosarcoma apoptosis and autophagy processes. Molecular Medicine Reports, 15(3), 1286–1290. 10.3892/mmr.2017.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolioulis I, Zafrakas M, Grimbizis G, Miliaras D, Timologou A, Bontis JN, & Tarlatzis BC (2017). Immunohistochemical expression pattern of metastasis suppressor KISS-1 protein in adenomyosis lesions and normal endometrium. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 210, 64–68. 10.1016/j.ejogrb.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Song WW, Gui AP, Li W, Chen HS, & Li JM (2017). Expressions of HIF-1alpha and KISS-1 in patients with liver cancer and correlation analysis. European Review for Medical and Pharmacological Sciences, 21(18), 4058–4063. [PubMed] [Google Scholar]
- 29.Yu L, Zhu B, Wu S, Zhou L, Song W, Gong X, & Wang D (2017). Evaluation of the correlation of vasculogenic mimicry, ALDH1, KiSS-1, and MACC1 in the prediction of metastasis and prognosis in ovarian carcinoma. Diagnostic Pathology, 12(1), 23 10.1186/s13000-017-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaverina N, Borovjagin AV, Kadagidze Z, Baryshnikov A, Baryshnikova M, Malin D, Ghosh D, Shah N, Welch DR, Gabikian P, Karseladze A, Cobbs C, & Ulasov IV (2017). Astrocytes promote progression of breast cancer metastases to the brain via a KISS1-mediated autophagy. Autophagy, 13(11), 1905–1923. 10.1080/15548627.2017.1360466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TH, & Cho SG (2017). Melatonin-induced KiSS1 expression inhibits triple-negative breast cancer cell invasiveness. Oncology Letters, 14(2), 2511–2516. 10.3892/ol.2017.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Chen W, Zhang X, Lin S, & Chen Z (2016). Overexpression of KiSS-1 reduces colorectal cancer cell invasion by downregulating MMP-9 via blocking PI3K/Akt/NF-kappaB signal pathway. International Journal of Oncology, 48(4), 1391–1398. 10.3892/ijo.2016.3368. [DOI] [PubMed] [Google Scholar]
- 33.Yang JA, Song CI, Hughes JK, Kreisman MJ, Parra RA, Haisenleder DJ, Kauffman AS, & Breen KM (2017). Acute psychosocial stress inhibits LH Pulsatility and Kiss1 neuronal activation in female mice. Endocrinology, 158(11), 3716–3723. 10.1210/en.2017-00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruszynska-Oszmalek E, Kolodziejski PA, Sassek M, & Sliwowska JH (2017). Kisspeptin-10 inhibits proliferation and regulates lipolysis and lipogenesis processes in 3T3-L1 cells and isolated rat adipocytes. Endocrine, 56(1), 54–64. 10.1007/s12020-017-1248-y. [DOI] [PubMed] [Google Scholar]
- 35.Manley SJ, Liu W, & Welch DR (2017). The KISS1 metastasis suppressor appears to reverse the Warburg effect by shifting from glycolysis to mitochondrial beta-oxidation. Journal of Molecular Medicine (Berlin, Germany), 95(9), 951–963. 10.1007/s00109-017-1552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maiuri MC, Zalckvar E, Kimchi A, & Kroemer G (2007). Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nature Reviews. Molecular Cell Biology, 8(9), 741–752. 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 37.Xu MF, Zang SB, Liu JF, Gao LY, Gao MQ, Yang YH, & Huang AM (2012). An in vitro study of the relationship between KiSS-1 expression and hepatoma carcinoma cell proliferation, adhesion, and invasion. Zhonghua Gan Zang Bing Za Zhi, 20(12), 925–929. 10.3760/cma.j.issn.1007-3418.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Ikeguchi M, Yamaguchi K, & Kaibara N (2004). Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clinical Cancer Research, 10(4), 1379–1383. [DOI] [PubMed] [Google Scholar]
- 39.Kim SJ, Kim JS, Park ES, Lee JS, Lin Q, Langley RR, Maya M, He J, Kim SW, Weihua Z, Balasubramanian K, Fan D, Mills GB, Hung MC, & Fidler IJ (2011). Astrocytes upregulate survival genes in tumor cells and induce protection from chemotherapy. Neoplasia, 13(3), 286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nash KT, Phadke PA, Navenot JM, Hurst DR, Accavitti-Loper MA, Sztul E, Vaidya KS, Frost AR, Kappes JC, Peiper SC, & Welch DR (2007). Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. Journal of the National Cancer Institute, 99(4), 309–321. 10.1093/jnci/djk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoji S, Tang XY, Umemura S, Itoh J, Takekoshi S, Shima M, Usui Y, Nagata Y, Uchida T, Osamura RY, & Terachi T (2009). Metastin inhibits migration and invasion of renal cell carcinoma with overexpression of metastin receptor. European Urology, 55, 441–449. [DOI] [PubMed] [Google Scholar]
- 42.Yoshioka K, Ohno Y, Horiguchi Y, Ozu C, Namiki K, & Tachibana M (2008). Effects of a KiSS-1 peptide, a metastasis suppressor gene, on the invasive ability of renal cell carcinoma cells through a modulation of a matrix metalloproteinase 2 expression. Life Sciences, 83, 332–338. [DOI] [PubMed] [Google Scholar]
- 43.Dhar DK, Naora H, Kubota H, Maruyama R, Yoshimura H, Tonomoto Y, Tachibana M, Ono T, Otani H, & Nagasue N (2004). Downregulation of KiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. International Journal of Cancer, 111, 868–872. [DOI] [PubMed] [Google Scholar]
- 44.Li N, Li SS, Zhang HY, Xuan XY, Zheng XZ, Wang F, & Yan AH (2009). Effect of KISS-1 on invasive potential and proliferation of esophageal squamous carcinoma cell line EC-1. Zhonghua Bing Li Xue Za Zhi, 38, 263–267. [PubMed] [Google Scholar]
- 45.Olbrich T, Ziegler E, Turk G, Schubert A, Emons G, & Grundker C (2010). Kisspeptin-10 inhibits bone-directed migration of GPR54-positive breast cancer cells: Evidence for a dose-window effect. Gynecologic Oncology, 119, 571–578. [DOI] [PubMed] [Google Scholar]
- 46.Ji K, Ye L, Ruge F, Hargest R, Mason MD, & Jiang WG (2014). Implication of metastasis suppressor gene, Kiss-1 and its receptor kiss-1R in colorectal cancer. BMC Cancer, 14, 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JH, & Welch DR (1997). Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Research, 57, 2384–2387. [PubMed] [Google Scholar]
- 48.Jiang Y, Berk M, Singh LS, Tan H, Yin L, Powell CT, & Xu Y (2005). KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clinical & Experimental Metastasis, 22, 369–376. [DOI] [PubMed] [Google Scholar]
- 49.Xu J, Acharya S, Sahin O, Zhang Q, Saito Y, Yao J, Wang H, Li P, Zhang L, Lowery FJ, Kuo WL, Xiao Y, Ensor J, Sahin AA, Zhang XHF, Hung MC, Zhang JD, & Yu D (2015). 14-3-3zeta turns TGF-beta’s function from tumor suppressor to metastasis promoter in breast cancer by contextual changes of Smad partners from p53 to Gli2. Cancer Cell, 27(2), 177–192. 10.1016/j.ccell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh R, Bhatt ML, Singh SP, Kumar V, Goel MM, Mishra DP, et al. (2016). Evaluation of KiSS1 as a prognostic biomarker in north Indian breast Cancer cases. Asian Pacific Journal of Cancer Prevention, 17(4), 1789–1795. [DOI] [PubMed] [Google Scholar]
- 51.Navenot JM, Wang Z, Chopin M, Fujii N, & Peiper SC (2005). Kisspeptin-10-induced signaling of GPR54 negatively regulates chemotactic responses mediated by CXCR4: A potential mechanism for the metastasis suppressor activity of kisspeptins. Cancer Research, 65(22), 10450–10456. 10.1158/0008-5472.CAN-05-1757. [DOI] [PubMed] [Google Scholar]
- 52.Castano JP, Martinez-Fuentes AJ, Gutierrez-Pascual E, Vaudry H, Tena-Sempere M, & Malagon MM (2009). Intracellular signaling pathways activated by kisspeptins through GPR54: Do multiple signals underlie function diversity? Peptides, 30(1), 10–15. 10.1016/j.peptides.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 53.Platonov ME, Borovjagin AV, Kaverina N, Xiao T, Kadagidze Z, Lesniak M, Baryshnikova M, & Ulasov IV (2018). KISS1 tumor suppressor restricts angiogenesis of breast cancer brain metastases and sensitizes them to oncolytic virotherapy in vitro. Cancer Letters, 417, 75–88. 10.1016/j.canlet.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 54.Chakravarty G, Rider B, & Mondal D (2011). Cytoplasmic compartmentalization of SOX9 abrogates the growth arrest response of breast cancer cells that can be rescued by trichostatin a treatment. Cancer Biology & Therapy, 11(1), 71–83. [DOI] [PubMed] [Google Scholar]
- 55.Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, Itzkovitz S, Noske A, Zürrer-Härdi U, Bell G, Tam WL, Mani SA, van Oudenaarden A, & Weinberg RA (2012). Slug and Sox9 cooperatively determine the mammary stem cell state. Cell, 148(5), 1015–1028. 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chakravarty G, Moroz K, Makridakis NM, Lloyd SA, Galvez SE, Canavello PR, Lacey MR, Agrawal K, & Mondal D (2011). Prognostic significance of cytoplasmic SOX9 in invasive ductal carcinoma and metastatic breast cancer. Experimental Biology and Medicine (Maywood, N.J.), 236(2), 145–155. 10.1258/ebm.2010.010086. [DOI] [PubMed] [Google Scholar]
- 57.Jarzabek K, Koda M, Kozlowski L, Milewski R, & Wolczynski S (2015). Immunohistochemical study of KiSS1 and KiSS1R expression in human primary breast cancer: Association with breast cancer receptor status, proliferation markers and clinicopathological features. Histology and Histopathology, 30(6), 715–723. 10.14670/HH-30.715. [DOI] [PubMed] [Google Scholar]
- 58.Ulasov IV, Kaverina NV, Pytel P, Thaci B, Liu F, Hurst DR, Welch DR, Sattar HA, Olopade OI, Baryshnikov AY, Kadagidze ZG, & Lesniak MS (2012). Clinical significance of KISS1 protein expression for brain invasion and metastasis. Cancer, 118(8), 2096–2105. 10.1002/cncr.26525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papaoiconomou E, Lymperi M, Petraki C, Philippou A, Msaouel P, Michalopoulou F, et al. (2014). Kiss-1/GPR54 protein expression in breast cancer. Anticancer Research, 34(3), 1401–1407. [PubMed] [Google Scholar]
- 60.Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, Lu T, Yankner BA, Campisi J, & Elledge SJ (2015). The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science, 349(6255), aaa5612 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, He L, Ma F, Regan MM, Balk SP, Richardson AL, & Yuan X (2013). SOX9 regulates low density lipoprotein receptor-related protein 6 (LRP6) and T-cell factor 4 (TCF4) expression and Wnt/beta-catenin activation in breast cancer. The Journal of Biological Chemistry, 288(9), 6478–6487. 10.1074/jbc.M112.419184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Mejean C, et al. (2005). Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. The EMBO Journal, 24(10), 1798–1809. 10.1038/sj.emboj.7600660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chavez RD, Coricor G, Perez J, Seo HS, & Serra R (2017). SOX9 protein is stabilized by TGF-beta and regulates PAPSS2 mRNA expression in chondrocytes. Osteoarthritis and Cartilage, 25(2), 332–340. 10.1016/j.joca.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurnaz E, Sen Y, & Aydin S (2017). Plasma kisspeptin and ghrelin levels in puberty variant cases. Journal of Pediatric Endocrinology & Metabolism, 30(5), 569–573. 10.1515/jpem-2016-0127. [DOI] [PubMed] [Google Scholar]
- 65.Celik F, Belviranli M, & Okudan N (2016). Circulating levels of leptin, nesfatin-1 and kisspeptin in postmenopausal obese women. Archives of Physiology and Biochemistry, 122(4), 195–199. 10.3109/13813455.2016.1171365. [DOI] [PubMed] [Google Scholar]
- 66.Jayasena CN, Comninos AN, Januszewski A, Gabra H, Taylor A, Harvey RA, Ghatei MA, Bloom SR, & Dhillo WS (2012). Plasma kisspeptin: A potential biomarker of tumor metastasis in patients with ovarian carcinoma. Clinical Chemistry, 58(6), 1061–1063. 10.1373/clinchem.2011.177667. [DOI] [PubMed] [Google Scholar]
- 67.Dhillo WS, Savage P, Murphy KG, Chaudhri OB, Patterson M, Nijher GM, Foggo VM, Dancey GS, Mitchell H, Seckl MJ, Ghatei MA, & Bloom SR (2006). Plasma kisspeptin is raised in patients with gestational trophoblastic neoplasia and falls during treatment. American Journal of Physiology: Endocrinology and Metabolism, 291 (5), E878–E884. 10.1152/ajpendo.00555.2005. [DOI] [PubMed] [Google Scholar]
- 68.Martino NA, Rizzo A, Pizzi F, Dell’Aquila ME, & Sciorsci RL (2015). Effects of kisspeptin-10 on in vitro proliferation and kisspeptin receptor expression in primary epithelial cell cultures isolated from bovine placental cotyledons of fetuses at the first trimester of pregnancy. Theriogenology, 83(6), 978–987 e971. 10.1016/j.theriogenology.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 69.Song W, Li K, Sun C, & Xue J (2017). Kisspeptin permits the sexual development of female rats with normal and precocious puberty but is not a trigger for it. Neuro Endocrinology Letters, 38(6), 422–428. [PubMed] [Google Scholar]
- 70.Dhillo WS, Murphy KG, & Bloom SR (2007). The neuroendocrine physiology of kisspeptin in the human. Reviews in Endocrine & Metabolic Disorders, 8(1), 41–46. 10.1007/s11154-007-9029-1. [DOI] [PubMed] [Google Scholar]
- 71.Karapanagiotou EM, Dilana KD, Gkiozos I, Gratsias I, Tsimpoukis S, Polyzos A, & Syrigos KN (2011). Metastin is not involved in metastatic potential of non-small cell lung cancer. Medical Oncology, 28(2), 559–564. 10.1007/s12032-010-9466-7. [DOI] [PubMed] [Google Scholar]
- 72.Curtis AE, Murphy KG, Chaudhri OB, Ramachandran R, Young AM, Waxman J, Nijher GM, Bewick GA, Ghatei MA, Bloom SR, & Dhillo WS (2010). Kisspeptin is released from human prostate cancer cell lines but plasma kisspeptin is not elevated in patients with prostate cancer. Oncology Reports, 23(6), 1729–1734. [DOI] [PubMed] [Google Scholar]
- 73.Katagiri F, Nagai K, Kida A, Tomita K, Oishi S, Takeyama M, Doi R, & Fujii N (2009). Clinical significance of plasma metastin level in pancreatic cancer patients. Oncology Reports, 21(3), 815–819. [PubMed] [Google Scholar]
- 74.Ergen A, Canbay E, Bugra D, Zeybek U, Yamaner S, & Bulut T (2012). Plasma Kisspeptin-54 levels in gastric cancer patients. International Journal of Surgery, 10(9), 551–554. 10.1016/j.ijsu.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 75.Jarzabek K, Kozlowski L, Milewski R, & Wolczynski S (2012). KiSS1/GPR54 and estrogen-related gene expression profiles in primary breast cancer. Oncology Letters, 3(4), 930–934. https://doi.org/103892/ol.2012.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Degenhardt T, Wuerstlein R, Eggersmann T, & Harbeck N (2018). The safety of palbociclib for the treatment of advanced breast cancer. Expert Opinion on Drug Safety, 17(3), 325–330. 10.1080/14740338.2018.1429402. [DOI] [PubMed] [Google Scholar]
- 77.Wang CH, Qiao C, Wang RC, & Zhou WP (2016). KiSS1mediated suppression of the invasive ability of human pancreatic carcinoma cells is not dependent on the level of KiSS1 receptor GPR54. Molecular Medicine Reports, 13(1), 123–129. 10.3892/mmr.2015.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schafer-Somi S, Ay SS, Kaya D, Sozmen M, Beceriklisoy HB, Agaoglu AR, et al. (2017). Kisspeptin-10 and the G protein-coupled receptor 54 are differentially expressed in the canine pregnant uterus and trophoblast cells. Reproduction in Domestic Animals, 52(Suppl 2), 123–129. 10.1111/rda.12818. [DOI] [PubMed] [Google Scholar]
- 79.Clarke RB, Anderson E, & Howell A (2004). Steroid receptors in human breast cancer. Trends in Endocrinology and Metabolism, 15(7), 316–323. 10.1016/j.tem.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Nauroth A, Kalder M, Rossler M, Wichmann G, Dietz A, & Wiegand S (2017). Conversion of hormone andHER-2 receptor in metachronous neck metastases from breast carcinoma. Journal of Cancer Research and Clinical Oncology, 143(9), 1811–1814. 10.1007/s00432-017-2426-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen SQ, Tu MM, Dai QB, Lin SY, & Ke CL (2012). Kiss-1 gene expression after radiation and its association with proliferation and apoptosis in colorectal cancer cells. Zhonghua Wei Chang Wai Ke Za Zhi, 15(5), 508–511. [PubMed] [Google Scholar]
- 82.Ramaesh T, Logie JJ, Roseweir AK, Millar RP, Walker BR, Hadoke PW, et al. (2010). Kisspeptin-10 inhibits angiogenesis in human placental vessels ex vivo and endothelial cells in vitro. Endocrinology, 151(12), 5927–5934. 10.1210/en.2010-0565. [DOI] [PubMed] [Google Scholar]
- 83.Cheng S, Castillo V, Eliaz I, & Sliva D (2015). Honokiol suppresses metastasis of renal cell carcinoma by targeting KISS1/KISS1R signaling. International Journal of Oncology, 46(6), 2293–2298. 10.3892/ijo.2015.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guzman S, Brackstone M, Radovick S, Babwah AV, & Bhattacharya MM (2018). KISS1/KISS1R in Cancer: Friend or foe? Front Endocrinol (Lausanne), 9, 437 10.3389/fendo.2018.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmid K, Wang X, Haitel A, Sieghart W, Peck-Radosavljevic M, Bodingbauer M, Rasoul-Rockenschaub S, & Wrba F (2007). KiSS-1 overexpression as an independent prognostic marker in hepatocellular carcinoma: An immunohistochemical study. Virchows Archiv, 450(2), 143–149. 10.1007/s00428-006-0352-9. [DOI] [PubMed] [Google Scholar]
- 86.McNally LR, Welch DR, Beck BH, Stafford LJ, Long JW, Sellers JC, Huang ZQ, Grizzle WE, Stockard CR, Nash KT, & Buchsbaum DJ (2010). KISS1 over-expression suppresses metastasis of pancreatic adenocarcinoma in a xenograft mouse model. Clinical and Experimental Metastasis, 27(8), 591–600. 10.1007/s10585-010-9349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang B, Li A, Zuo F, Yu R, Zeng Z, Ma H, & Chen S (2016). Recombinant Lactococcus lactis NZ9000 secretes a bioactive kisspeptin that inhibits proliferation and migration of human colon carcinoma HT-29 cells. Microbial Cell Factories, 15(1), 102 10.1186/s12934-016-0506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teng Y, Liu M, & Cowell JK (2011). Functional interrelationship between the WASF3 and KISS1 metastasis-associated genes in breast cancer cells. International Journal of Cancer, 129(12), 2825–2835. 10.1002/ijc.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sossey-Alaoui K, Ranalli TA, Li X, Bakin AV, & Cowell JK (2005). WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Experimental Cell Research, 308(1), 135–145. 10.1016/j.yexcr.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 90.Sossey-Alaoui K, Safina A, Li X, Vaughan MM, Hicks DG, Bakin AV, & Cowell JK (2007). Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. American Journal of Pathology, 170(6), 2112–2121. 10.2353/ajpath.2007.060975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gertsch J, & Anavi-Goffer S (2012). Methylhonokiol attenuates neuroinflammation: A role for cannabinoid receptors? Journal of Neuroinflammation, 9, 135 10.1186/1742-2094-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang P, Liu X, Zhu Y, Chen S, Zhou D, & Wang Y (2013). Honokiol inhibits the inflammatory reaction during cerebral ischemia reperfusion by suppressing NF-kappaB activation and cytokine production of glial cells. Neuroscience Letters, 534, 123–127. 10.1016/j.neulet.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 93.Rodriguez-Cueto C, Benito C, Fernandez-Ruiz J, Romero J, Hernandez-Galvez M, & Gomez-Ruiz M (2014). Changes in CB(1) and CB(2) receptors in the post-mortem cerebellum of humans affected by spinocerebellar ataxias. British Journal of Pharmacology, 171(6), 1472–1489. 10.1111/bph.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim CW, Hwang KA, & Choi KC (2016). Anti-metastatic potential of resveratrol and its metabolites by the inhibition of epithelial-mesenchymal transition, migration, and invasion of malignant cancer cells. Phytomedicine, 23(14), 1787–1796. 10.1016/j.phymed.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 95.Kawano K, Hattori Y, Iwakura H, Akamizu T, & Maitani Y (2013). Combination therapy with gefitinib and doxorubicin inhibits tumor growth in transgenic mice with adrenal neuroblastoma. Cancer Medicine, 2(3), 286–295. 10.1002/cam4.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nwachukwu JC, Srinivasan S, Bruno NE, Parent AA, Hughes TS, Pollock JA, Gjyshi O, Cavett V, Nowak J, Garcia-Ordonez RD, Houtman R, Griffin PR, Kojetin DJ, Katzenellenbogen JA, Conkright MD, & Nettles KW (2014). Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. Elife, 3, e02057 10.7554/eLife.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poulin R, Baker D, Poirier D, & Labrie F (1991). Multiple actions of synthetic ‘progestins’ on the growth of ZR-75–1 human breast cancer cells: An in vitro model for the simultaneous assay of androgen, progestin, estrogen, and glucocorticoid agonistic and antagonistic activities of steroids. Breast Cancer Research and Treatment, 17(3), 197–210. [DOI] [PubMed] [Google Scholar]
- 98.Selvaraj S, Sun Y, Sukumaran P, & Singh BB (2016). Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway Molecular Carcinogenesis, 55(5), 818–831. 10.1002/mc.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terasaka T, Otsuka F, Tsukamoto N, Nakamura E, Inagaki K, Toma K, Ogura-Ochi K, Glidewell-Kenney C, Lawson MA, & Makino H (2013). Mutual interaction of kisspeptin, estrogen and bone morphogenetic protein-4 activity in GnRH regulation by GT1–7 cells. Molecular and Cellular Endocrinology, 381(1–2), 8–15. 10.1016/j.mce.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnstone CN, Pattison AD, Gorringe KL, Harrison PF, Powell DR, Lock P, Baloyan D, Ernst M, Stewart AG, Beilharz TH, & Anderson RL (2018). Functional and genomic characterisation of a xenograft model system for the study of metastasis in triple-negative breast cancer. Disease Models & Mechanisms, 11(5), dmm032250 10.1242/dmm.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Otsuka F, Tsukamoto N, Miyoshi T, Iwasaki Y, & Makino H (2012). BMP action in the pituitary: Its possible role in modulating somatostatin sensitivity in pituitary tumor cells. Molecular and Cellular Endocrinology, 349(2), 105–110. 10.1016/j.mce.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 102.Golzar F, Javanmard SH, Bahrambeigi V, & Rafiee L (2015). The effect of Kisspeptin-10 on mesenchymal stem cells migration in vitro and in vivo. Adv Biomed Res, 4, 20 10.4103/2277-9175.149851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zajac M, Law J, Cvetkovic DD, Pampillo M, McColl L, Pape C, di Guglielmo GM, Postovit LM, Babwah AV, & Bhattacharya M (2011). GPR54 (KISS1R) transactivates EGFR to promote breast cancer cell invasiveness. PLoS One, 6(6), e21599 10.1371/joumal.pone.0021599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chaudary N, Pintilie M, Jelveh S, Lindsay P, Hill RP, & Milosevic M (2017). Plerixafor improves primary tumor response and reduces metastases in cervical Cancer treated with radio-chemotherapy. Clinical Cancer Research, 23(5), 1242–1249. 10.1158/1078-0432.CCR-16-1730. [DOI] [PubMed] [Google Scholar]
- 105.Taromi S, Kayser G, Catusse J, von Elverfeldt D, Reichardt W, Braun F, Weber WA, Zeiser R, & Burger M (2016). CXCR4 antagonists suppress small cell lung cancer progression. Oncotarget, 7(51), 85185–85195. 10.18632/oncotarget.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hainsworth JD, Reeves JA, Mace JR, Crane EJ, Hamid O, Stille JR, Flynt A, Roberson S, Polzer J, & Arrowsmith ER (2016). A randomized, open-label phase 2 study of the CXCR4 inhibitor LY2510924 in combination with Sunitinib versus Sunitinib alone in patients with metastatic renal cell carcinoma (RCC). Targeted Oncology, 11(5), 643–653. 10.1007/s11523-016-0434-9. [DOI] [PubMed] [Google Scholar]
- 107.Karanika S, Karantanos T, Kurosaka S, Wang J, Hirayama T, Yang G, Park S, Golstov AA, Tanimoto R, Li L, & Thompson TC (2015). GLIPR1-DeltaTM synergizes with docetaxel in cell death and suppresses resistance to docetaxel in prostate cancer cells. Molecular Cancer, 14, 122 10.1186/s12943-015-0395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wyler L, Napoli CU, Ingold B, Sulser T, Heikenwalder M, Schraml P, et al. (2014). Brain metastasis in renal cancer patients: Metastatic pattern, tumour-associated macrophages and chemokine/chemoreceptor expression. British Journal of Cancer, 110(3), 686–694. 10.1038/bjc.2013.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cavallaro S (2013). CXCR4/CXCL12 in non-small-cell lung cancer metastasis to the brain. International Journal of Molecular Sciences, 14(1), 1713–1727. 10.3390/ijms14011713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen Y, & Wang X (2017). Mechanisms of AEG-1 and CXCR4 gene expression regulating the epithelial-mesenchymal transition pathway involved in brain metastases of breast cancer. Journal of BUON, 22(4), 953–957. [PubMed] [Google Scholar]
- 111.Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, et al. (2004). Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. Journal of Cell Science, 117(Pt 8), 1319–1328, doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]