VI. SUMMARY
Monocytes are innate immune cells essential for host protection against malaria. Upon activation, monocytes function to help reduce parasite burden through phagocytosis, cytokine production, and antigen presentation. However, monocytes have also been implicated in the pathogenesis of severe disease through production of damaging inflammatory cytokines resulting in systemic inflammation and vascular dysfunction. Understanding the molecular pathways influencing the balance between protection and pathology is critical. In this review, we discuss recent data regarding the role of monocytes in human malaria, including studies of innate sensing of the parasite, immunometabolism, and innate immune training. Knowledge gained from these studies may guide rational development of novel antimalarial therapies and inform vaccine development.
Keywords: monocyte, macrophage, malaria, plasmodium, innate, training
1. INTRODUCTION
The two major Plasmodium species infecting humans around the world are Plasmodium falciparum (Pf) and Plasmodium vivax (Pv), with Pf causing the majority of deaths and Pv accounting for the most cases. Malaria can have a wide spectrum of disease from asymptomatic parasitemia and uncomplicated malaria, to severe disease including cerebral malaria and death.1,2 Naturally acquired immunity to Pf develops slowly over time with repeated exposures.3,4 Studies have shown that development and maintenance of immunity is mediated through complex interactions and functions of several innate and adaptive immune effectors including monocytes/macrophages, T cells, and B cells.5 While immune-mediated protection is essential to survival, innate immune effectors, including monocytes (MO), have been implicated in the pathogenesis of severe malaria through Pf-induced production of damaging pro-inflammatory cytokines.6,7 MO/macrophages are one of the main sources of cytokines in malaria-infected individuals, and whereas some of these may be of importance for parasite clearance (e.g., IL-12),8 others may be major contributors to disease progression (e.g., TNF).9 MO recognize Pf biological products and Pf-infected erythrocytes directly through pattern recognition receptors (PRR),10–12 as well as complement- or IgG-opsonized infected erythrocytes and parasite components via complement receptors and Fcγ receptors.13,14 Activated MO have several important effector functions in host defense against malaria, including phagocytosis,13–15 cytokine production,16,17 and modulation of adaptive immune responses.18,19
Circulating human MO are a heterogeneous population that are typically classified into three subsets based on levels of expression of CD14 (LPS co-receptor) and CD16 (low affinity Fcγ receptor III [Fcγ-RIII]). The subsets include CD14++CD16– “classical,” CD14++CD16+ “intermediate” or “inflammatory,” and CD14+CD16++ “nonclassical” MO.20 These subsets vary in differentiation properties, migratory capabilities, and cytokine production. The intermediate and nonclassical MO subsets appear to have more activated pro-inflammatory phenotypes with distinct motility and crawling patterns.21–23 These subsets may have enhanced antiparasitic activity,24–26 though there are still significant gaps in our understanding of the functional roles of MO subsets during malaria. In a typical healthy resting state, classical MO are the predominant subset, representing approximately 80% of the total circulating MO population.
In this review, we summarize the human data regarding the role of MO in malaria with an emphasis on conflicting conclusions, severity of disease, and new research directions. This is not an exhaustive review of the literature, but one that represents recent advances in the field with embellishments in immunometabolism and innate immune training. Some murine data is included, but a complete review of the murine MO field (which is extensive) was not performed. Some challenges in pulling meaningful conclusions from a vast array of scientific work arise from variability in experimental methodologies. Confounders include i) use of ex vivo MO vs. adherent cells after peripheral blood mononuclear cells (PBMC) are rested in plastic wells (these are presumably enriched for MO-derived macrophages, but this is rarely validated by flow cytometry); ii) variability in Plasmodium components used in stimulation assays (which might include intact infected erythrocytes from different laboratory-adapted strains, parasite lysate, hemozoin (Hz) derived from parasite culture, or synthetic Hz); iii) MO interrogation using cells from children vs. adults with mild, moderate, or severe malaria; iv) Pf vs. Pv infections (and clinical spectrum of disease); and v) human primary MO vs. THP-1 or other MO-like cell lines. We have included what we think are relevant details that facilitate or hinder direct comparisons of results.
2. CLINICAL MALARIA AND MONOCYTE CHARACTERISTICS
2.1. Systemic inflammation
Malaria is characterized by fever and systemic inflammation during blood stage infection. The innate immune system responds to parasites and parasite components by inducing pro-inflammatory cytokines and chemokines such as IL-1β, IL-6, IL-8, IL-12(p70), IFN-ɣ, and TNF.25,27–29 The cytokines prime phagocytes for parasite uptake and clearance. Pro-inflammatory cytokines are balanced by an increase in regulatory cytokines such as TGF-β and IL-10.30,31 Excessive inflammation has been linked to severe malaria and death. Severe malaria has been associated with higher pro-inflammatory to regulatory cytokine ratios.32 Chemokines help recruit MO and other cells to sites where parasites are sequestered for efficient clearance, and to regional lymph nodes where activation of T cells takes place.33–35 Chemokines such as macrophage inflammatory protein (MIP)-1α/CXCL3, MIP-1β/CCL4, IL-8/CXCL8, and CCL5 have been found to be elevated in multiple studies of acute malaria.27,36–38 Other markers of MO activation can be measured in plasma and include soluble CD14 (sCD14) and sCD163 (hemoglobin-haptoglobin scavenger receptor). Otterdal et al. found that Mozambican adults with malaria had increased plasma levels of sCD14 and sCD163 compared with healthy controls.39 Soluble CD14 also correlated with disease severity and sCD163 correlated with parasitemia. Complex regulatory pathways to maintain homeostasis are explored in subsequent sections.
The presentation of severe malaria varies with age and geographical distribution. In areas of high transmission, severe malaria mainly affects children under 5 years of age.40,41 Severe malaria is a density-dependent disease of Pf-infected erythrocytes sequestered in multiple tissues. Cerebral malaria accounts for roughly half of all malaria deaths and is defined as unarousable coma accompanied by Pf parasitemia with no other identifiable cause for coma.42 Death from cerebral malaria is rapid, and the case fatality rate is 15–20% even among patients receiving appropriate treatment.43 Fatal pediatric cerebral malaria is associated with cerebral intravascular MO accumulation.44 Plasma and/or cerebral spinal fluid concentrations of pro-inflammatory cytokines and chemokines, including TNF, IL-6, IL-1β, and interferon-gamma induced protein 10 (IP-10/CXCL10), frequently correlate with the development of cerebral malaria and, in some cases, the severity of cerebral malaria.45,46
2.2. Frequencies of circulating monocytes
Hematologic changes are common in malaria and typically include lymphopenia, thrombocytopenia, and anemia. In a study of Ghanaian children with a spectrum of clinical Pf malaria, MO frequencies were consistently elevated during disease. The authors found that the ratio of MO to lymphocytes in peripheral blood (M:L ratio) was increased in children with malaria, and the M:L ratio correlated with parasitemia and presumably severity of malaria.47 In re-examining our own data of Kenyan children presenting with uncomplicated Pf malaria and 6 weeks after treatment, we also found an increased M:L ratio in acute malaria that normalized to that of healthy children upon recovery.29 Additionally, Warimwe et al. found elevated M:L ratios in Kenyan children with asymptomatic parasitemia.48 How clinically relevant M:L ratios are remains questionable.
Total MO frequencies in peripheral blood may change with disease state, but so too may MO subset frequencies. We found that Kenyan children with uncomplicated Pf malaria had increased proportions of intermediate MO compared to their own samples collected 6 weeks following treatment. Additionally, acute malaria was associated with distinct activated MO phenotypes with increased surface expression of several markers important in innate and adaptive immunity such as toll-like receptor (TLR)-2, TLR4, and B cell activating factor (BAFF).29 Ogonda et al. examined MO frequencies and the level of CD16 expression in nonclassical and intermediate MO in Kenyan children with severe malarial anemia, cerebral malaria, or uncomplicated Pf malaria at presentation and 4 weeks after treatment.49 They found that the frequency of MO was higher at presentation in children with severe malarial anemia and uncomplicated malaria (but not cerebral malaria) at presentation compared to the recovery time point. Also, children with severe malarial anemia had higher CD16 expression levels at presentation (measured as MFI) in nonclassical MO (CD14+CD16++) compared to recovery. When looking at intermediate MO (CD14++CD16+), CD16 expression was higher at presentation compared to recovery time points for children with severe malarial anemia and some with uncomplicated malaria. The frequency of MO in these groups were higher at presentation, which may contribute to the higher CD16 levels observed. Expansion of the CD14+CD16++ nonclassical MO subset was also observed in adult Thai patients with uncomplicated Pf malaria.24
2.3. Markers of monocyte activation
In a number of studies, surface expression of important receptors and effector proteins is examined. During inflammation, upregulation of adhesion molecule expression serves to enhance cell-to-cell interactions and transmission of signals that direct effector functions. The major adhesion molecule families involved in leukocyte trafficking, activation, and differentiation are the integrins, selectins (L-selectin, E-selectin, and P-selectin) and immunoglobulin superfamily members.50 Integrins incorporating β2 (CD18) are restricted to leukocytes and all subtypes are expressed on MO. CD11a, b, and c all combine with CD18 to form different integrins. They bind a variety of ligands including ICAM-1 and ICAM-2, which are expressed by endothelial cells. HLA-DR and CD86 are expressed on MO and serve as activation markers. While HLA-DR is critical for cognate interactions associated with peptide presentation to CD4+ T cells, CD86 is key for accessory signaling.51
Mandala et al. examined total MO from Malawian children presenting with uncomplicated malaria, severe malarial anemia, and cerebral malaria and upon recovery (4 weeks later) compared to healthy children.52 They specifically examined the surface expression of CD11a, CD11b, CD11c, CD18, HLA-DR, CD86, TLR2, TLR4, and intracellular production of TNF and IL-6 after LPS stimulation, by flow cytometry. During acute malaria (all groups), MO expression of CD11b, CD11c, HLA-DR, CD86 and percentage of TNF- and IL-6-producing MO was lower compared to healthy controls. This was most pronounced in the severe malarial anemia and cerebral malaria groups. Upon recovery, expression levels normalized, with the exception of cytokine-producing MO remaining suppressed in the severe malarial anemia group. TLR2 and TLR4 expression was lower in the severe malarial anemia and cerebral malaria groups compared to healthy controls, and expression of these markers also normalized upon recovery from malaria. The authors conclude that low expression of integrins, TLRs, and activation markers during acute malaria is indicative of an immunosuppressive effect. The researchers of this study examined expression in total MO and did not examine expression differentiated by MO subsets. Their findings contrast to our study where intermediate MO from Kenyan children with acute uncomplicated malaria had elevated surface expression of TLR2 and TLR4 compared to healthy children (no difference in TLR expression was observed in the classical and nonclassical subsets).29 A similarity between the studies was that CD86 was lower in total MO in both studies, but among the Kenyan child MO subsets, CD86 expression was lower in classical and nonclassical MO but elevated in intermediate MO compared to healthy children. Expression of these targets normalized upon recovery (6 weeks).29 Differential expression of TLRs and activation markers may reflect differential functions of the subsets during malaria.
One of many challenges in comparing studies is differential methodologies. The Mandala et al. study reports flow cytometry-acquired expression as “geometric mean fluorescent intensity (GMFI)” which does not take into account the different frequencies of MO in the comparator group. Dobbs et al. presented flow data as “integrated MFI (iMFI)” which is computed by multiplying the relative frequency (percent positive) of cells expressing a particular cytokine with the MFI of that population.53 In a study conducted in Thailand of adults presenting with either severe malaria (n=7) or uncomplicated malaria (n=10), total MO were found to have increased expression (by median MFI) of TLR2 but not TLR4 compared to healthy controls.54 However, there were fewer TLR2+ monocytes in the severe or uncomplicated malaria populations. In rough calculations of iMFI for this study, malaria participants had higher TLR2 than healthy controls, similar to the Dobbs et. al study. Comparing study results can be challenging with variable methodologies, child vs. adult samples, and diversity of clinical presentations.
Healthy adult malaria naïve volunteers (n=18) received intradermal sporozoites in a controlled human malaria infection (CHMI) study.55 As soon as parasites were detected by microscopy, participants were treated regardless of symptoms. Longitudinal examination of MO frequency and activation showed that classical MO frequencies peaked on the day of treatment and intermediate MO frequencies peaked at 3 days after treatment (nonclassical MO were not examined). In both classical and intermediate MO, expression of HLA-DR and CD86 (measured by MFI) were increased on the day of treatment and 3 days after, respectively. Intermediate MO showed greater expression of these activation markers than classical MO. Frequencies normalized to baseline values 35 days after infection. Expression of the glycolipid-presenting molecule CD1c on classical and intermediate MO was increased during CHMI at the time of treatment. CD16 expression as measured by MFI was also increased in both classical and intermediate MO at the time of treatment. The CHMI model provides interesting insights into the chronologic changes in MO subsets and activation with several similarities to observations made in field studies of uncomplicated Pf malaria.
2.4. Plasmodium vivax malaria
Pv malaria, while associated with less morbidity compared to Pf malaria, also results in similar hematological changes. An important caveat to be considered is whether Pv malaria is a primary infection or recrudescence. Chaves et al. found in Brazilian adults that peripheral MO frequencies were lower during Pv malaria, whether primary infection or recrudescence.56 MO subsets were not examined. In contrast, Antonelli et al. found that Brazilian adults with Pv had higher MO frequencies compared to recovery time points.25 Adults with Pv had increased surface expression on total MO of adhesion molecules CD106 (VCAM-1), CD54 (ICAM-1), and the chemokine receptor CX3CR1 (measured by MFI) compared to their recovery MO. In contrast, significantly lower levels of HLA-DR, adhesion molecule CD31, and chemokine receptor CCR7 were found on MO during acute Pv infection compared to recovery. When these markers were examined for each MO subset, in general they followed a similar pattern. The investigators showed both the classical (CD14++CD16−) and intermediate (CD14++CD16+) MO were important sources of cytokines during acute Pv infection. The intermediate MO showed the highest levels of TNF expression upon exposure to Pv-infected reticulocytes, indicating an activated status of these cells. Additionally, intermediate MO were more efficient at phagocytizing Pv-infected reticulocytes in vitro compared to the other MO subsets. Finally, intermediate MO displayed the highest mitochondria content and activity.
3. MALARIA IN PREGNANCY AND MONOCYTES
Pf in pregnancy can have severe consequences such as severe anemia of the mother, miscarriage, stillbirth, preterm delivery, intrauterine growth restriction (IUGR), and low birth weight, leading to more than 100,000 infant deaths annually in sub-Saharan Africa.57 There are several recent reviews on malaria in pregnancy,58,59 with one in particular by Seitz et al. emphasizing the molecular principles of IUGR in Pf infection.60
In histological samples of placentas from patients with Pf infection, erythrocytes in the intervillous space can be inundated with parasites. This leads to local placental inflammation that recruits maternal macrophages, MO, and lymphocytes, leading to their activation. Local maternal immune cells express elevated levels of chemokines (e.g., MIP1, monocyte chemoattractant protein 1 (MCP1), and IP-10/CXCL10, which causes an increased migration of MO.61–64 With earlier in pregnancy infections and in active chronic infections, Hz can be detected in migrated MO or in fibrin deposits by histopathology.65 MO play an important role in elimination of infected erythrocytes in the placenta, but excessive MO infiltration contributes to malaria pathogenesis and correlates in numerous studies with negative consequences such as low birth weight, premature death, and maternal anemia. MO accumulation in placental blood intervillous spaces is associated with poor clinical outcomes.66–68 Interestingly, Chua et al. found that pregnant Malawian women with placental malaria had elevated levels of plasma sCD163, which was inversely correlated with maternal hemoglobin levels.69
4. MONOCYTE PHAGOCYTOSIS
MO function is assessed ex vivo or with in vitro experiments in a number of ways, from quantifying cytokine production, phagocytosis of beads or parasites, reactive oxygen species (ROS) production, or antibody-dependent cellular inhibition. Focusing on phagocytosis, Dobbs et al. found that ex vivo MO from Kenyan children with acute malaria had reduced opsonic phagocytosis compared to their own MO upon recovery 6 weeks later.29 Interestingly, at that recovery time point, those children with asymptomatic parasitemia had MO with reduced capacity to phagocytose infected erythrocytes compared to children with no detectable parasitemia (Pf PCR negative). Intermediate and nonclassical MO had greater phagocytic activity compared to classical MO.
While isolated MO are capable of phagocytosing infected erythrocytes in vitro, Zhou et al. argue that other components in circulating blood may contribute and are overlooked in some assays.26 Using whole blood co-cultured with labeled infected erythrocytes, they found that intermediate MO had greater Pf phagocytic activity than the other MO subsets. Additionally, they found that complement opsonization was required for IgG-mediated phagocytosis, as heat inactivated plasma (which inactivates complement) promoted greatly diminished phagocytic activity. They also found that intermediate MO had the highest levels of complement receptor 4 (CD11c) and activated complement receptor 3 (CD11b) expression, thereby facilitating complement-mediated opsonic phagocytosis of parasites.
MO function can also be affected by antimalarial medications. Cumming et al. used mouse (J774A.1) and human (U937) MO cell lines as well as adult healthy primary PBMC to assess the effects of amodiaquine, artemisinin, chloroquine, doxycycline, primaquine, pyrimethamine, and quinine on MO phagocytosis of synthetic Hz or latex beads in vitro.70 Focusing on results using adherent human cells, they found that phagocytosis was increased in the presence of pyrimethamine and quinine and reduced by chloroquine and amodiaquine (other medications had no effect), suggesting an additional role for these medications other than killing parasites.
5. MACROPHAGE TISSUE REPLENISHMENT BY MONOCYTES
Macrophages are resident in virtually all tissues of the body and have an important role in innate immune defense against pathogens.71–73 Recent studies have shown that the majority of tissue-resident macrophages are established prenatally, maintaining their numbers within the tissues by self-renewal without any major input from circulating MO.74–76 However, with inflammation77 and infection,78,79 resident macrophages can be depleted but refilled by infiltrating MO from the circulation.80 In a murine model of malaria using P. yoelii, investigators studied the dynamics of tissue-resident macrophages in multiple organs and how they were refilled during the course of malaria. They found that the loss of embryonically established resident macrophages occurred prior to the peak of parasitemia, and that circulating inflammatory MO repopulated splenic and hepatic tissues. In contrast, lung alveolar macrophages refilled their niche primarily through self-renewal.81
Interestingly, in another murine model of severe malaria using P. berghei, investigators examined the nature and extent of malaria-associated lung injury. They found that MO from the circulation were recruited to and phagocytized infected erythrocytes sequestered on post-capillary endothelial surfaces, and that alveolar macrophages did not play a significant role in the clearance of infected erythrocytes.82 Thus, circulating MO can be utilized for tissue macrophage replacement and/or for clearance of infected erythrocytes in specific tissues within the murine model. Whether or not this holds true for the human host remains to be determined.
6. INNATE IMMUNE SENSING OF MALARIA IN MONOCYTES
Innate immune cells use PRRs to sense molecules associated with microbial pathogens, termed pathogen-associated molecular patterns (PAMPs). PRRs include the membrane-bound TLRs and C-type lectin receptors; cytosolic RIG-I-like receptors and NOD-like receptors (NLRs); and cytosolic nucleic acid sensors such as absent in melanoma 2 (AIM2) and cyclic GMP-AMP synthase (cGAS). Plasmodium PAMPs include glycosylphosphatidylinositol (GPI) anchors, parasite nucleic acids, and Hz. In addition, certain endogenous factors released during malaria can activate PRRs. These factors are called damage-associated molecular patterns (DAMPs), and heme, uric acid, host nucleic acids, and extracellular vesicles act as DAMPs during malaria. Innate immune sensing of malaria has been thoroughly reviewed by Gazzinelli et al., 201483 and by Gowda and Wu, 2018.84 Here we will highlight recent advancements in our understanding of the molecular mechanisms by which malaria infection is sensed by MO innate immune receptors.
6.1. GPI anchors
GPI anchors of Pf are glycolipids that link several proteins to the surface of the merozoite plasma membrane which are needed for erythrocyte invasion, and thus GPIs are essential to parasite survival.5,85,86 GPI was the first component identified as a malaria PAMP, and GPIs are abundantly expressed on the parasite surface both linked to proteins and in free form.87 In mouse macrophages and adherent human MO, malarial GPIs induce a pro-inflammatory response with increased production of TNF, IL-1β, IL-6, IL-12, and nitric oxide (NO).9,88–90 Additionally, GPI-induced MO production of TNF and IL-1β indirectly increases ICAM-1 adhesion molecule expression on human umbilical vein endothelial cells.88 GPI signaling in MO is mediated mainly through recognition by TLR2 heterodimers, and to a lesser extent by TLR4 homodimers.91 Structural variations of Pf GPIs determine preferential binding by an auxiliary receptor (TLR1 or TLR6) for recognition by TLR2. GPIs with three fatty acyl groups are preferentially recognized by TLR2/TLR1, and GPIs with two fatty acyl groups are preferentially recognized by TLR2/TLR6.91 Downstream signaling involves MyD88-dependent activation of extracellular signal-related kinase (ERK), c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and NF-κB pathways, leading to production of pro-inflammatory cytokines and NO.90–92 In mouse macrophages, the scavenger receptor CD36 was also shown to contribute significantly to GPI-induced production of TNF.93 Recently, Dunst et al. used synthetic GPI affinity chromatography to identify other potential host receptors for Pf GPI and found that Pf GPI interacts with moesin, an intracellular protein that links actin filaments to transmembrane proteins. However, the interaction does not appear to contribute to Pf-induced innate inflammatory responses or to disease pathogenesis in a murine model.45 In addition to inflammatory cytokine production, other mechanisms may allow for host regulation of GPI activity in the innate immune response to infection. In both mouse macrophages and adherent human MO, surface phospholipases degrade Pf GPIs into predominantly inactive species.91 Human C1-inhibitor (C1INH) is a highly glycosylated serine protease inhibitor secreted by the liver into plasma, with a broad role in regulation of coagulation, vascular permeability, and inflammation.94 Mejia et al. showed that C1INH binds to Pf GPI at the merozoite surface and inhibits erythrocyte invasion, and in human MO co-cultures with Pf-infected erythrocytes and schizont extract, C1INH reduces MO production of TNF, IL-1β, and IL-6 in a dose-dependent manner, presumably due to GPI neutralization.95 C1INH was also shown to directly interact with the host receptors CD36 and chondroitin sulfate A, and in an experimental cerebral malaria model, mice given C1INH had reduced parasitemia and improved survival, suggesting potential use of C1INH as a therapeutic antimalarial.95
6.2. DNA
Plasmodial DNA stimulates innate immune cells through recognition by TLR9 in phagolysosomes and by DNA sensors in the cytosol. Parasite DNA enters phagolysosomes of innate immune cells after uptake of merozoites, intact infected erythrocytes, Hz, or DNA-protein complexes.96–100 It has been shown that within the phagolysosome of mouse and human dendritic cells, unmethylated CpG motifs of plasmodial DNA are recognized by TLR9,101 leading to activation of MyD88-NF-κB signaling pathways and production of pro-inflammatory cytokines.96–98,102 It should be noted that in humans, TLR9 is predominantly expressed in B cells and plasmacytoid dendritic cells, and so MO are unlikely to contribute significantly to recognition of parasite DNA via this pathway.17,103,104
If parasite DNA within phagolysosomes is released into the cytosol, it is recognized by cytosolic DNA sensors: AIM2 and cGAS. This can lead to activation of the AIM2 inflammasome and production of mature IL-1β,100 as well as to induction of a type I interferon (IFN) response via the cGAS-stimulator of IFN genes (STING) pathway.105,106 It was recently shown that cGAS is the sensor upstream of STING that recognizes Pf genomic DNA in the cytosol.106 Activated cGAS synthesizes 2’3’-cGAMP, which acts as a second messenger to activate STING, leading to TBK1 and IRF3-IRF7 phosphorylation and production of type I IFNs.105,106
6.3. RNA
Microbial RNA can be detected in endosomes by TLR3, TLR7, and TLR8, or in the cytosol by nucleic acid sensors such as RIG-I and the RIG-I-like receptor melanoma differentiation-associated protein 5 (MDA5).17,103 Results from a recent study by Coch et al. suggest that endosomal TLR8 in human MO recognizes Pf RNA, leading to the production of IL-12p70 and IL-18, which in turn induce IFN-ɣ release by NK cells.107 These experiments were conducted using healthy donor human PBMC and illustrate potential limitations of mouse models for human malaria, most notably that while TLR7 was shown to play an important role in the early innate immune response to malaria in a mouse model,108 Coch et al. found that IFN-ɣ release from human PBMC in response to stimulation with Pf-infected erythrocytes and Pf RNA was elicited solely by TLR8.107 This may be explained in part by important differences between murine and human innate immune sensing of nucleic acids within the endosome. While murine TLR7 is expressed in many myeloid cell types, human TLR7 is largely restricted to B cells and plasmacytoid dendritic cells.17 In human MO, TLR8 is preferentially expressed and its detection of single-stranded RNA induces release of IL-12p70.109–111
6.4. Hemozoin
Hz is a crystalline pigment formed in the food vacuole of the Plasmodium parasite during blood stage infection. As the parasite digests host hemoglobin as an essential nutrient source, heme is generated, which is highly toxic. The parasite survives by polymerizing heme into insoluble Hz crystals.112 During merozoite egress, Hz is released into the circulation, where it is phagocytosed by innate immune cells.113 The amount of Hz accumulation within MO and neutrophils reflects parasite burden and disease severity.114–116 Ingested Hz persists, apparently unmodified, within macrophages for long periods of time.112,117,118 The precise mechanisms for its persistence are unclear and may involve the lack of induction by Hz of lysosomal heme oxygenase, which is needed to catalyze the degradation of heme.118
Several studies have examined the in vitro immunomodulatory effects of Hz on MO and other innate immune cells. A key consideration in evaluating the results of such studies is the Hz preparation protocol used. Synthetic Hz (sHz) crystals (β-hematin) can be prepared in vitro from hematin at varying levels of purity.96,119 Native Hz (nHz) prepared from Pf cultures has the same crystal size and structure as sHz,112,120 but is also bound to host and parasite proteins, lipids, and nucleic acids.96,121,122 Synthetic Hz can activate the NLRP3 inflammasome in THP-1 cells and in murine macrophages, leading to secretion of IL-1β.123–125 In a murine macrophage cell line, sHz induced ROS generation and transcription of the chemokines CCL2, CCL3, CCL4, and CXCL2 via ERK1/2 phosphorylation and NF-κB activation.126 It has been shown that nHz, bound to parasite DNA, activates the NLRP3 and AIM2 inflammasomes in murine macrophages.100 It has also been proposed that nHz acts as a vehicle to deliver parasite DNA to the cytosol, where AT-rich stem-loop motifs can induce type I IFN production via the cGAS-STING pathway.105,106
Proteins complexed to Hz likely play an important role in the immunomodulatory effects of nHz. Barrera et al. showed that host fibrinogen bound to Hz induced human MO to produce ROS, TNF, and CCL2 via interaction with TLR4 and integrin CD11b/CD18.122 In addition to direct stimulatory effects, proteins might act as a bridge between the Hz crystal and DNA. Kalantari et al. showed that sHz interacted weakly with parasite DNA, but if sHz was first coated with fetal bovine serum, it bound parasite DNA very strongly. In addition, proteinase K treatment of nHz resulted in loss of its DNA coating.100
Little is known about the receptors that recognize Hz at the cell surface. Binding of Hz may be largely independent of specific cell surface receptors, as has been shown for uric acid crystals, which directly engage cholesterol components in the cell membrane.127 Consistent with this, Hz internalization and subsequent IL-1β induction was shown to be dependent on the integrity of lipid rafts.112,123 Recently, Raulf et al. demonstrated that the cell surface C-type lectin receptor CLEC12A recognizes Hz, and its engagement leads to impaired effector functions in murine dendritic cells.128 Human MO express CLEC12A,129 though the potential role of CLEC12A in Hz recognition and signaling in MO has not yet been studied.
6.5. Extracellular vesicles
An additional mechanism whereby innate immune receptors are engaged is via MO uptake of parasite antigens contained in extracellular vesicles (EVs). These small vesicles contain cytoplasm within an outer lipid bilayer and are involved in intercellular communication in several physiological and pathological processes, including the immune response to infection.130,131 EVs derive from many different cell types and are generally classified, according to size and biogenesis, into microvesicles (MVs) (50–1000 nm, form by direct budding from plasma membrane, also referred to as microparticles); exosomes (40–120 nm, derive from endosomes in intracellular multi-vesicular bodies); and apoptotic bodies (500–2000 nm, form by outward blebbing of plasma membrane during apoptosis).132,133 While the different types of EVs have distinct biogenesis pathways, it is often difficult to fully differentiate between MVs and exosomes given that many EV markers are not exclusively specific, and there exists substantial variation in EV purification protocols. Thus, the EVs included in many studies may contain a mixture of MVs and exosomes. Here, we will refer to both MVs and exosomes as EVs. EV cargo consists of proteins, lipids, and nucleic acids, the composition of which reflects the parent cell type and, in the case of infection, pathogen components.130–133 Importantly, these molecules are functional and can act to deliver signals to recipient cells. There is increasing evidence that EVs play a role in malaria pathogenesis, including the inflammatory response to infection as well as parasite evasion of host immunity (reviewed in Mantel and Marti, 2014,134 Sampaio et al., 2017,135 and Debs et al., 2018136).
Several human field studies have demonstrated elevated levels of EVs in plasma during malaria. Endothelial cell-derived EVs were increased in plasma of Malawian children with cerebral malaria compared to healthy children, children with uncomplicated malaria, and severe malarial anemia without cerebral involvement.137 Similarly, a study conducted in Cameroon showed that children with cerebral malaria had higher plasma levels of EVs derived from platelets, erythrocytes, leukocytes, and endothelial cells compared to healthy children, children with uncomplicated malaria, and severe malarial anemia without cerebral involvement.138 Erythrocyte-derived EVs were elevated in plasma of individuals from Bangkok with Pf, Pv, and P. malariae infection, with highest levels observed in patients with severe Pf malaria.139 Brazilian adults with uncomplicated Pv malaria had increased platelet-, erythrocyte-, and leukocyte-derived EVs compared to healthy controls.140 In all of the above studies, plasma levels of EVs decreased following treatment and clinical recovery.137–140
Pro-inflammatory properties of erythrocyte-derived EVs were first demonstrated in a murine model of severe malaria, in which EVs purified from plasma of P. berghei ANKA (PbA) infected C57BL/6 mice induced strong in vitro activation of bone marrow-derived macrophages, assessed by CD40 expression and TNF production; this activation was TLR4 and MyD88 dependent.141 Phenotypic characterization of EVs derived from PbA infection showed that a large proportion of these EVs were erythrocyte-derived and contained PbA-specific antigens.141
Several recent studies have shown that EVs derived from in vitro culture of Pf-infected erythrocytes have immunomodulatory effects on human MO. Mantel et al. characterized EVs purified from Pf culture supernatant at all stages of the parasite cycle, and found that these vesicles are enriched in host proteins associated with erythrocyte lipid rafts as well as parasite proteins associated with erythrocyte membranes and those needed for erythrocyte invasion.142 To study the effects of these EVs on the host immune response, they incubated EVs derived from uninfected vs. infected erythrocytes with PBMC from healthy malaria-naïve donors and evaluated surface activation markers of T cells, B cells, and MO by flow cytometry. In these experiments, CD14+ MO were the main targets of EVs from infected erythrocytes, which induced upregulation of activation markers CD40, CD54, and CD86 and downregulation of CD163.142 Monocyte-derived macrophages (MDM) obtained from healthy donor PBMC were stimulated with EVs from infected erythrocytes, which led to increased transcript levels of IL-6, TNF, and IL-10 as well as increased secretion of TNF and IL-10 into culture supernatants. Phagocytosis of EVs by MDM was demonstrated by microscopy, and inhibition of phagocytosis by cytochalasin D abrogated EV-induced expression of IL-6, TNF, and IL-10 mRNA.142
Others have evaluated the innate immune effects of EVs derived specifically from ring-stage infected erythrocytes.143,144 Sampaio et al. showed that EVs from ring-stage infected erythrocytes induce a transcriptional response in primary MO, with enrichment in genes related to transcription and translation, cellular metabolism, antigen presentation, and interferon response genes.143 Though transcriptional changes were observed in MO, EVs did not induce a cytokine response from primary MO in this study.143 In addition, the protein cargo of infected erythrocyte-derived EVs were characterized, and the key virulence factor Pf erythrocyte membrane protein 1 (PfEMP1) was detected in EVs from early ring-stage infected erythrocytes but absent in EVs from later stages. While a previous study suggested that PfEMP1 expressed on intact infected erythrocytes might dampen the inflammatory response by MO,145 there were no significant differences in transcriptional changes in MO stimulated with EVs derived from PfEMP1 knockout strains compared to wild type parasites.143 Further work is needed to determine the effects of PfEMP1 and potentially other parasite protein cargo in EVs on MO functions.
In another study of EVs derived specifically from ring-stage infected erythrocytes, Sisquella et al. demonstrated that these vesicles contain human and Pf small RNA, intact Pf mRNA species, and Pf genomic DNA, including mitochondrial and apicoplast DNA.144 Notably, human microRNA (miRNA) species present in infected erythrocyte-derived EVs were enriched for miRNA targets involved in cell adhesion regulation, including genes encoding CD36, P-selectin, ICAM-1, and VCAM-1. In addition, the authors showed that Pf DNA was present only in EVs from ring-stage infected erythrocytes and absent from EVs produced at later stages.144 DNA, RNA, and lipid cargo of ring-stage infected erythrocyte EVs were efficiently taken up by the THP-1 MO-like cell line and by primary CD14+ human MO. After uptake, DNA cargo progressively dispersed through the cells, suggesting that its destination is the cytosol. To evaluate the innate immune response to EV-DNA cargo, cells were incubated with EVs from ring-stage infected erythrocytes (containing Pf DNA) and trophozoite-stage infected erythrocytes (lacking Pf DNA). THP-1 cells and primary MO had increased mRNA levels of type I IFNs IFNA and IFNB, as well as IFN-regulated genes CCL5, IFIT1, and CXCL10, after incubation with EVs from ring-stage infected erythrocytes but not EVs from later stage infected erythrocytes. Similar strong induction of these genes was observed in THP-1 cells transfected with Pf gDNA, which was not seen in THP-1 cells treated with naked Pf gDNA. The type I IFN response in THP-1 cells to ring-stage EVs was dependent on cytosolic DNA sensing by STING and involved downstream activation and phosphorylation of TBK1 and IRF3.144 While this study demonstrated that uptake of EV-DNA activated the type I IFN response via cytosolic DNA sensing, additional mechanisms might contribute to the MO response to infected erythrocyte-derived EVs, such as sensing of EV-RNA via TLR8107 or the RIG-I-like receptor MDA5.146
In addition to direct effects of EVs on MO, EV-induced vascular dysfunction likely further contributes to MO activation during malaria. Infected erythrocyte-derived EVs were shown to contain human miRNA bound to Argonaute 2 (Ago2), forming a functional translational repression complex. Uptake of these EVs by endothelial cells led to altered expression of known miRNA targets; increased transcription of IL1, IL6, and VCAM1; and disruption of the integrity of the endothelial barrier.147 This would be expected to lead to recruitment of activated MO and other cells, triggering further vascular dysfunction and disease pathogenesis. Similarly, activation of other innate cell types by EVs could trigger further MO activation. Ye et al. showed that NK cells become activated and release IFN-γ after sensing Pf RNA from infected erythrocytes and infected erythrocyte-derived EVs via MDA5/TBK1/IKKε signaling.146 As mentioned above, whether MO are also activated by EV-RNA remains to be determined.
7. MONOCYTE IMMUNOMETABOLISM
During infection, immune cell activation and modulation of effector functions are linked to changes in cellular metabolism.148 Several studies have demonstrated that recognition of PAMPs by innate immune cells induces a metabolic reprogramming required for the immune response against infection, with differential metabolism of carbon and nitrogen sources leading to differential functional capabilities.148–152 For example, stimulation of murine macrophages and dendritic cells with LPS induces a switch in metabolic programs which favors aerobic glycolysis over oxidative phosphorylation153–156 (similar to the metabolic switch that occurs in rapidly proliferating tumor cells, first described by Otto Warburg in 1927157). This switch is accompanied by decreased TCA cycle activity,153 increased flux through the pentose phosphate pathway,158 and increased production of lactate and ROS.152,158 In activated dendritic cells, this metabolic switch is antagonized by IL-10.153
Few studies have investigated microbial-induced changes in cellular metabolism in human MO. Lachmandas et al. studied the effects of stimulation with different TLR ligands on metabolic programs in primary human MO.159 They showed that while stimulation of TLR4 with LPS led to increased glycolysis and decreased oxygen consumption, stimulation of TLR2 with Pam3CysSK4 (P3C) led to increased glycolysis, increased oxygen consumption, and increased mitochondrial enzyme activity. In MO stimulated with P3C, increased oxidative phosphorylation was required to retain phagocytic capacity and cytokine production. These data strongly suggest that individual stimuli induce complex and specific patterns of metabolic reprogramming in immune cells, which in turn governs functional changes during the host response to infection.159 This notion is supported by a study of the differential effects on human MO of Candida yeast vs. hyphae forms, which showed that MO responses to C. albicans yeast involved increased glycolysis, oxidative phosphorylation, and glutaminolysis, while responses to hyphae primarily involved increased glycolysis.160
While glucose metabolism and oxidative phosphorylation in innate immune cells during malaria have not been well studied to date, numerous studies have examined arginine metabolism and NO bioavailability in the context of severe malaria. Arginine metabolism is closely linked to MO/macrophage functional polarization and occurs via two competing pathways: NO synthase (NOS) or arginase.161 Arginine metabolism by NOS produces NO and citrulline, where NO can be metabolized further into reactive nitrogen species, and citrulline can be reused to drive further NO synthesis. Arginine metabolism by the arginase pathway leads to the production of ornithine and urea and limits arginine availability for NO synthesis. Macrophages with high NOS expression have been classified as M1 polarized, whereas those with high arginase expression have been classified as M2 polarized.162 Other characteristics of M1 polarization include high MHC-II expression and high IL-12 production, while M2 polarization has been characterized by high expression of the mannose receptor CD206 and CD163 and high production of IL-10.163 (The M1/M2 paradigm can be helpful in analyzing MO/macrophage activation during infection, though we recognize that this is a dynamic and complex process in which phenotypes are often mixed, and a full discussion of the M1/M2 paradigm is beyond the scope of this review.162)
In children and adults, severe malaria is associated with low arginine and NO bioavailability, reduced leukocyte NOS2 expression, and increased arginase activity.164–168 Weinberg et al. recently investigated the relationship between low arginine and NO bioavailability and MO activation in a cohort of children from Tanzania.169 They found that, compared to healthy community controls, children with severe and moderate malaria had higher plasma arginase activity and lower plasma arginine levels; higher plasma IL-10, IL-13, IL-4, and sCD163; and higher MO surface expression of CD206 and CD163, indicative of an M2-like polarization of MO during malaria. PBMC (not specifically MO) from children with severe and moderate malaria had higher arginase 1 mRNA and protein along with lower NOS2 mRNA compared to healthy children, again suggestive of M2-like activation during malaria. The authors contend that MO polarization with increased arginase activity during malaria may affect the immune response by altering metabolic substrates available to immune cells, and that IL-10, which is markedly elevated in children with Pf malaria,29,164 likely plays an important role in driving MO polarization.169–172
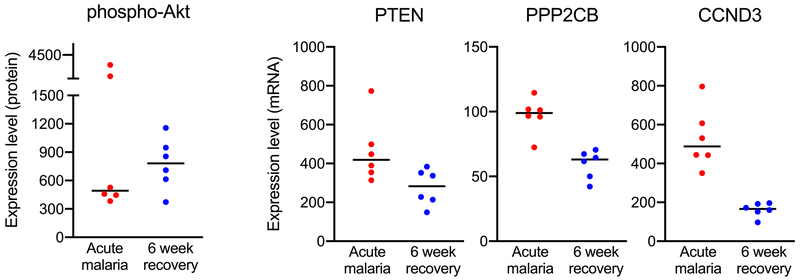
In a series of in vitro experiments, Bobade et al. showed that stimulation of adherent human MO with nHz led to an M2-like polarization, with increased arginase activity, decreased NO and ROS production, increased IL-10 production, and increased expression of CD206.173 Multiple signaling pathways are involved in regulating inflammatory and metabolic responses in MO, and in this study, nHz-induced polarization of MO involved the p38-MAPK, PI3K-Akt, and NF-κB pathways, including increased levels of phospho-p38-MAPK, phospho-Akt, and phospho-p65(NF-κB).173 In small preliminary studies, we found decreased levels of phospho-Akt and altered expression of several genes in the PI3K-Akt pathway in isolated MO from Kenyan children with acute uncomplicated malaria compared to recovery 6 weeks later (Figure 1), suggesting an important role for these pathways in MO activation and functional changes in response to malaria. Discrepancies in the direction of effect in these in vitro vs. ex vivo studies may be explained by multiple factors, including possible down-regulation of these pathways by the time of presentation with clinical disease.
Figure 1.
Changes in the PI3K-Akt pathway in monocytes during acute malaria. Negatively selected monocytes were obtained from Kenyan children at presentation with acute uncomplicated malaria and 6 weeks following treatment (n = 6). RNA and protein were purified from monocyte cell lysates and hybridized to a NanoString CodeSet of genes and proteins important to regulation of several cellular processes such as differentiation, apoptosis, and metabolism (mRNA PanCancer Pathways Panel of 730 target genes and protein Solid Tumor Panel of 25 total and phospho-proteins). PTEN = phosphatase and tensin homolog, PPP2CB = protein phosphatase 2 catalytic subunit beta, CCND3 = cyclin D3.
8. EPIGENETIC CHANGES IN MONOCYTES DURING MALARIA
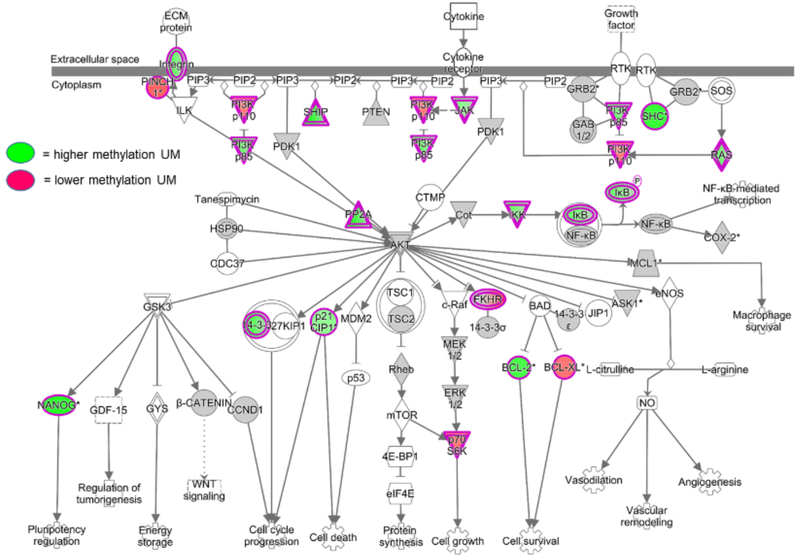
Epigenetic processes tightly regulate cellular functioning in health and disease.174 Modifications to chromatin structure affect gene regulation by repressing or enhancing transcription factor binding through two main mechanisms: DNA methylation and histone modification.175 The most frequent epigenetic modification is DNA methylation, by which a methyl group is introduced into cytosine, with most methylation occurring at CpG sites.176,177 DNA methylation patterns can rapidly change in response to infection. Pacis et al. demonstrated that MO-derived dendritic cells exposed to Mycobacterium tuberculosis for only two hours induced DNA demethylation in enhancer regions of master regulators of innate immune responses such as NF-ĸB, which increased the expression of inflammatory cytokine genes including IL-6, IL-1β, and TNF.178 They found that hypo-methylated CpG sites were associated with increased gene expression, indicating that most active changes in DNA methylation in response to infection were losses rather than gains in methylation.178 In malaria, DNA methylation and histone modifications regulate life cycle progression, host parasite interactions, and parasite adaptation to the host environment.179 As an example, epigenetic memory plays a role in antigenic switching of var genes, important virulence factors unique to Pf and one of the main factors of var-driven immune evasion.180,181 Little is known about epigenetic processes important for host defense against malaria. Al-Quaraishy et al. using DNA methylation arrays found that whole liver tissue in Balb/c mice infected with P. chabaudi exhibited changes in DNA methylation in promoters for genes involved in MO recruitment, parasite removal, and liver regeneration.182 Human epigenetic immune-parasite studies, however, are lacking. Our preliminary studies of isolated MO from Kenyan children with acute uncomplicated malaria compared to recovery 6 weeks later showed important changes in DNA methylation patterns using genome-wide DNA methylation arrays, including differential methylation in the promoter regions for several inflammatory genes such as TNF/LTA, IL1A, IL1R1, IL10, CXCR5, and CCR5, as well as PADI4, an enzyme that citrullinates histones and is known to promote chromatin decondensation during the innate immune response in neutrophils.183 Interestingly, multiple gene promotors in the PI3K-Akt and NF-κB pathways were differentially methylated in acute malaria compared to 6 week-recovery monocytes (Figure 2). Importantly, it has been shown that metabolites such as butyrate and fumarate can act to modulate activities of epigenetic enzymes such as histone deacetylases and demethylases.184,185 Future areas of research include deciphering the mechanisms by which MO metabolic changes and epigenetic modifications affect MO functional polarization during malaria (and in subsequent responses to infection, as discussed below).
Figure 2.
Differentially methylated promoter regions of genes in PI3K-Akt and NF-κB signaling pathways during acute malaria. Negatively selected monocytes were obtained from Kenyan children at presentation with acute uncomplicated malaria and 6 weeks following treatment (n = 8). Monocyte genomic DNA was isolated for DNA methylation analysis using the MethylationEPIC array, which measures methylation at over 850,000 sites across the genome (Illumina). Differential methylation between acute malaria and recovery samples was analyzed for single nucleotide positions, gene and promoter regions, and pathway enrichment using R/Bioconductor and the ChAMP package. UM = uncomplicated malaria.
9. INNATE IMMUNE TOLERANCE VS. TRAINING
Exposure of MO and other innate cells to pathogens can result in a memory-like phenotype with functional adaptation to subsequent stimuli. Impaired responsiveness to subsequent stimuli has been described as “tolerance”, whereas enhanced responsiveness has been termed “training.” Shalova et al. showed that peripheral MO from adult patients with gram negative sepsis displayed a pro-inflammatory gene expression profile but exhibited endotoxin tolerance manifest as blunted inflammatory cytokine production when subsequently challenged with LPS ex vivo.186 However, the capacity to phagocytize bacteria, express antimicrobial activity, and perform tissue repair functions were increased in these same MO, indicating that key functions are reprogrammed rather than globally suppressed.186 In contrast to tolerance, in trained immunity, priming of MO by an initial trigger results in an enhanced response to a second challenge.187 It was observed that BCG vaccination in healthy volunteers increased the production of IFN-ɣ, TNF, and IL-1β in response to unrelated pathogens; enhanced function of MO persisted for at least 3 months and was accompanied by increased histone modifications in promotor regions of TNF and IL-6 genes.188 In addition, β-glucans from C. albicans also induce training by enhancing the production of pro-inflammatory cytokines through histone modifications in inflammatory promoter regions.189 The nature of the pathogen is thought to play a role in these opposing responses, but recently, “dose” of the pathogen is thought to be important, with low doses potentially promoting training and high doses promoting tolerance.190 In typical training experiments, investigators use a “two hit” procedure. Cells are first treated with a pathogen component such as β-glucan for a short period of time (~24 hours), allowed to rest for 5–7 days, and then re-challenged with the TLR4 agonist LPS, which induces an exaggerated burst of inflammatory cytokines compared to cells initially treated with LPS. Epigenetic modifications imposed by the first hit, with resulting changes in gene transcription, were revealed as the basis for the development of this memory effect in innate immune cells. Bauer et al.190 propose that the balance and interaction of training signaling from low-dose pathogens through the mTOR pathway (central mediator of anabolic pathways),191 and tolerance signaling from high-dose pathogens through the AMPK pathway (important for catabolic and cellular maintenance pathways),192 represents the link between pathogen dose and widespread reactions of innate immune responses.
Initial evidence that malaria infection could induce trained immune responses came from a CHMI study of humans infected with Pf, which showed enhanced TLR responses in whole blood. Additional in vitro experiments showed that PBMC from malaria-naïve donors which were first primed with Pf lysate had more robust TNF production in response to several different TLR agonists.193 A later study of Brazilian adults with Pf malaria found that PBMC from malaria patients stimulated with TLR agonists produced more TNF, IL-12p40, and IL-1β compared to PBMC from healthy controls, and that MO from malaria cases had elevated surface expression of TLR2 and TLR4.194 Another study in Brazil showed that purified MO from adult Pv patients stimulated with TLR2 or TLR4 agonists had elevated TNF, IL-1β, and IL-6 production.195 Dobbs et al. showed that ex vivo isolated MO from children with uncomplicated Pf malaria and 6 weeks after treatment were both highly responsive when cultured overnight with TLR2 or TLR4 agonists, with much greater cytokine production (TNF, IL-1β, IL-6, IL-8, and IL-12p40) compared to healthy malaria-naïve North American adults.29 These observations were indicators that malaria infection could induce MO to a trained immune state. Of note, immune responses elicited by these studies utilized cells from individuals with uncomplicated malaria. How MO from individuals with severe malaria would respond remains to be determined.
Using an in vitro model of trained immunity, Schrum et al.196 demonstrated that cells stimulated with Pf-infected erythrocytes or with nHz for 24 hours, followed by a rest period in media for 3 days, produced highly elevated levels of TNF and IL-6 in response to TLR2 re-stimulation, compared to cells initially exposed to uninfected erythrocytes or media alone. They demonstrated that Pf-trained cells underwent chromatin remodeling, as the cells had elevated H3K4me3 modifications on the promoters of pro-inflammatory genes TNF and IL6 as well as metabolic genes MTOR and GAPDH. The enrichment of H3K4me3 was also demonstrated by ChIP for TNF, IL6, MTOR, and GAPDH in purified MO from Kenyan children with uncomplicated Pf malaria compared to malaria-naïve North American donors. The elevated TNF and IL-6 production induced by nHz training could be blocked by addition of a methyl-transferase (MTA) inhibitor, indicating that chromatin remodeling was necessary to mediate the trained phenotype. The MTA inhibitor treatment did not block trained immune responses in cells trained with infected erythrocytes, however. The addition of the mTOR inhibitor rapamycin was able to block hyper-production of IL-6 in both infected erythrocyte- and nHz-trained cells, indicating that there is a requirement for metabolic reprogramming of cells in order to induce training. Altogether, this work showed that malaria could induce the key hallmarks of innate immune training, but several important questions remain.
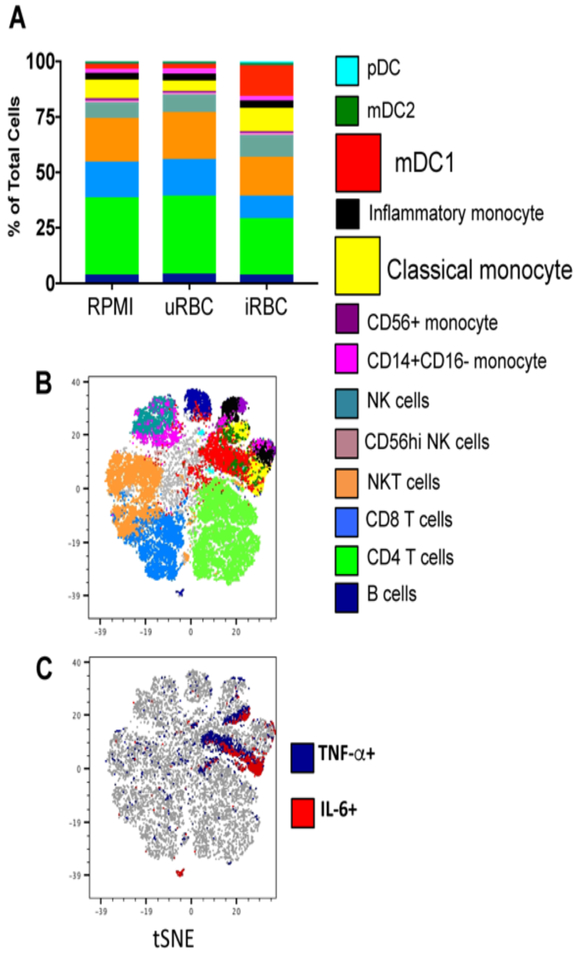
An important consideration for innate immune training studies is the protocol used to purify innate cells. For example, the use of adherent PBMC is a commonly employed method to enrich for MO by adhering PBMC to a tissue culture plate for several hours followed by washes with PBS to remove the non-adherent lymphocytes.196 It is possible that this purification method leaves lymphocyte contaminants, which could be major contributors to the phenotypes presented in the data. Any contaminating T or B cells could act as major contributors to TNF or IL-6 production. Even in the case of malaria naïve donors, who would presumably have no memory T or B cells to malarial antigens, de novo activation of naïve lymphocytes could potentially happen over the 4–5 day training experiments. It is therefore important to confirm the cellular source of pro-inflammatory cytokines in trained immunity to malaria. We have found that in PBMC trained with Pf-infected erythrocytes, the major cellular sources of TNF and IL-6 are in fact MO and dendritic cells (Figure 3), indicating that even in the presence of lymphocytes, it is largely the myeloid lineage that are undergoing a trained immune response to malaria.
Figure 3.
CyTOF of trained PBMC indicates myeloid cells produce TNF and IL-6. PBMC were trained with media, uninfected erythrocytes, or Pf-infected erythrocytes for 24 hours, and rested in media for 3 days, then re-stimulated with Pam3CSK4 for 6 hours in the presence of brefeldin A. Cells were stained for surface markers and intracellular cytokines and analyzed by mass cytometry (CyTOF). A) Cell types determined by surface phenotyping; frequency of each cell type shown for each trained condition. B) tSNE plot of combined samples, with cell types overlaid using colors indicated in legend. C) tSNE indicating total TNF+ cells (blue) and IL-6+ cells (red). Data shown is from 1 donor representative of 3 donors. PBMC = peripheral blood mononuclear cells, CyTOF = cytometry by time of flight, tSNE = t-distributed stochastic neighbor embedding.
The argument has been made that trained immune responses are a result of chronic stimulation.197 It is possible that persistence of Hz after an episode of malaria provides chronic stimulation that leads to development of trained immunity. One study tracked the clearance kinetics of Hz-containing MO after treatment for severe malaria and found that it took ten days for Hz-containing MO to clear from the peripheral blood, and it took 12–13 days for them to clear from the dermis.198 Clinical symptoms of severe malaria persisted after parasite clearance for 88% of these patients, and only subsided after Hz-containing MO had cleared,198 indicating that the persistence of Hz may be an important contributor to disease. Autopsies of children who died from malaria showed that Hz accumulates to very high levels in the spleen and bone marrow, and in cases of cerebral malaria, extra-erythrocytic Hz can be found in the capillaries of the brain.199,200 Hz accumulates in the spleen due to the splenic reticular mesh, which filters out mature schizonts from the peripheral blood circulation.201 In the bone marrow, Hz-containing macrophages populate the extravascular space.199 Human studies of Hz accumulation rely on autopsies, and therefore it is difficult to ascertain the clearance rate of Hz from these organs after parasite clearance. A study of the kinetics of Hz clearance in mouse P. berghei infection showed that Hz accumulated to very high levels in the spleen, bone marrow, and liver, and that Hz persisted in these organs up to 196 days after completion of chloroquine treatment.202 In a study of the effects of murine Plasmodium infection on bone loss, femurs of infected mice developed a dark black discoloration consistent with high accumulation of Hz at 30 and 90 days post-infection, after all parasites had been cleared. The authors demonstrated that Hz also induced production of IL-1α, IL-1β, IL-6, and TNF by osteoclast and osteoblast precursors in the bone marrow.203 Hz accumulation and persistence in tissues may serve as an important reservoir of PAMPs that induce pro-inflammatory cytokine production.
Examining ex vivo innate responses from individuals with malaria at several time points will help to determine whether and how long malaria-induced training of MO persists. With BCG vaccination, evidence for innate training lasted at least 3 months, and could potentially persist longer.188 There is currently limited evidence with regards to malaria and durability of trained innate responses. In future studies, it will be important to consider several contributing factors, such as Plasmodium species, local malaria transmission intensity, age, ancestry and genetic background,204 co-infections, vaccination schedules, and other environmental factors. A key question is whether changes in innate immune responses after uncomplicated malaria and CHMI are similar with severe disease, including cerebral malaria, severe malarial anemia, and malaria in pregnancy (including possible effects on innate responses in both mother and fetus). The clinical significance of potential malaria-induced trained immunity is unknown. Modulation of innate immune functions during and after malaria might have a number of clinical implications, including increased susceptibility to concurrent bacterial infections,205 modified disease severity in subsequent malaria episodes, and altered responses to vaccination.
10. CONCLUDING REMARKS
Recent studies show that MO play important roles in both host protection and pathogenesis during malaria. MO comprise a heterogeneous population with significant differences in phenotypes and functions among the subsets. During malaria, MO exhibit a high amount of functional plasticity that is likely further affected by changes in the local environment and by co-morbidities common in malaria endemic areas, such as HIV and intestinal helminth infections. Priorities for future research include examining how MO are differentially activated according to disease severity and by different Plasmodium species (particularly Pv); further elucidating the signaling mechanisms involved in innate recognition of malaria and downstream effects on cell function; and deciphering the links among cellular metabolism, epigenetic modifications, and functional adaptation. Knowledge gained from these studies could be employed in the rational development of novel therapeutic strategies and inform vaccine development for this devastating infection.
V. ACKNOWLEDGMENTS
This work was supported by NIH grants K23AI132644 (KRD), 5T32CA130807-09 (JNC), RO1-AI079293 (JNC), and 1R01AI130131-01 (AED).
Footnotes
CONFLICT OF INTEREST: The authors have no conflict of interest to declare.
VIII. REFERENCES
- 1.Reyburn H, Mbatia R, Drakeley C, et al. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. JAMA. 2005;293(12):1461–1470. [DOI] [PubMed] [Google Scholar]
- 2.Kendjo E, Agbenyega T, Bojang K, et al. Mortality patterns and site heterogeneity of severe malaria in African children. PLoS One. 2013;8(3):e58686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22(1):13–36, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28(1–2):51–60. [DOI] [PubMed] [Google Scholar]
- 5.Gilson PR, Nebl T, Vukcevic D, et al. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. Mol Cell Proteomics. 2006;5(7):1286–1299. [DOI] [PubMed] [Google Scholar]
- 6.Chua CL, Brown G, Hamilton JA, Rogerson S, Boeuf P. Monocytes and macrophages in malaria: protection or pathology? Trends Parasitol. 2013;29(1):26–34. [DOI] [PubMed] [Google Scholar]
- 7.Stanisic DI, Cutts J, Eriksson E, et al. gammadelta T cells and CD14+ monocytes are predominant cellular sources of cytokines and chemokines associated with severe malaria. J Infect Dis. 2014;210(2):295–305. [DOI] [PubMed] [Google Scholar]
- 8.Malaguarnera L, Pignatelli S, Musumeci M, Simpore J, Musumeci S. Plasma levels of interleukin-18 and interleukin-12 in Plasmodium falciparum malaria. Parasite Immunol. 2002;24(9–10):489–492. [DOI] [PubMed] [Google Scholar]
- 9.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177(1):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol. 2006;6(12):895–906. [DOI] [PubMed] [Google Scholar]
- 11.McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood. 2000;96(9):3231–3240. [PubMed] [Google Scholar]
- 12.Ruangjirachuporn W, Afzelius BA, Helmby H, et al. Ultrastructural analysis of fresh Plasmodium falciparum-infected erythrocytes and their cytoadherence to human leukocytes. Am J Trop Med Hyg. 1992;46(5):511–519. [DOI] [PubMed] [Google Scholar]
- 13.Silver KL, Higgins SJ, McDonald CR, Kain KC. Complement driven innate immune response to malaria: fuelling severe malarial diseases. Cell Microbiol. 2010;12(8):1036–1045. [DOI] [PubMed] [Google Scholar]
- 14.Tebo AE, Kremsner PG, Luty AJ. Fcgamma receptor-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes in vitro. Clin Exp Immunol. 2002;130(2):300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayi K, Patel SN, Serghides L, Smith TG, Kain KC. Nonopsonic phagocytosis of erythrocytes infected with ring-stage Plasmodium falciparum. Infect Immun. 2005;73(4):2559–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gowda DC. TLR-mediated cell signaling by malaria GPIs. Trends Parasitol. 2007;23(12):596–604. [DOI] [PubMed] [Google Scholar]
- 17.Barchet W, Wimmenauer V, Schlee M, Hartmann G. Accessing the therapeutic potential of immunostimulatory nucleic acids. Curr Opin Immunol. 2008;20(4):389–395. [DOI] [PubMed] [Google Scholar]
- 18.Butler NS, Moebius J, Pewe LL, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholzen A, Teirlinck AC, Bijker EM, et al. BAFF and BAFF receptor levels correlate with B cell subset activation and redistribution in controlled human malaria infection. J Immunol. 2014;192(8):3719–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell AJ, Roediger B, Weninger W. Monocyte homeostasis and the plasticity of inflammatory monocytes. Cellular immunology. 2014;291(1–2):22–31. [DOI] [PubMed] [Google Scholar]
- 21.Sugimoto C, Hasegawa A, Saito Y, et al. Differentiation Kinetics of Blood Monocytes and Dendritic Cells in Macaques: Insights to Understanding Human Myeloid Cell Development. J Immunol. 2015;195(4):1774–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82(2):244–252. [DOI] [PubMed] [Google Scholar]
- 23.Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53(1–3):41–57. [DOI] [PubMed] [Google Scholar]
- 24.Chimma P, Roussilhon C, Sratongno P, et al. A distinct peripheral blood monocyte phenotype is associated with parasite inhibitory activity in acute uncomplicated Plasmodium falciparum malaria. PLoS Pathog. 2009;5(10):e1000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonelli LR, Leoratti FM, Costa PA, et al. The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria. PLoS Pathog. 2014;10(9):e1004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou J, Feng G, Beeson J, et al. CD14(hi)CD16+ monocytes phagocytose antibody-opsonised Plasmodium falciparum infected erythrocytes more efficiently than other monocyte subsets, and require CD16 and complement to do so. BMC Med. 2015;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyke KE, Burges R, Cissoko Y, et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72(10):5630–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walther M, Woodruff J, Edele F, et al. Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol. 2006;177(8):5736–5745. [DOI] [PubMed] [Google Scholar]
- 29.Dobbs KR, Embury P, Vulule J, et al. Monocyte dysregulation and systemic inflammation during pediatric falciparum malaria. JCI Insight. 2017;2(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omer FM, Riley EM. Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J Exp Med. 1998;188(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 1999;67(9):4435–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis. 2002;185(7):971–979. [DOI] [PubMed] [Google Scholar]
- 33.Sponaas AM, Freitas do Rosario AP, Voisine C, et al. Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood. 2009;114(27):5522–5531. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Cui L, Gonsiorek W, et al. CCR2 and CXCR4 regulate peripheral blood monocyte pharmacodynamics and link to efficacy in experimental autoimmune encephalomyelitis. J Inflamm (Lond). 2009;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–670. [DOI] [PubMed] [Google Scholar]
- 36.Burgmann H, Hollenstein U, Wenisch C, Thalhammer F, Looareesuwan S, Graninger W. Serum concentrations of MIP-1 alpha and interleukin-8 in patients suffering from acute Plasmodium falciparum malaria. Clin Immunol Immunopathol. 1995;76(1 Pt 1):32–36. [DOI] [PubMed] [Google Scholar]
- 37.Friedland JS, Ho M, Remick DG, Bunnag D, White NJ, Griffin GE. Interleukin-8 and Plasmodium falciparum malaria in Thailand. Trans R Soc Trop Med Hyg. 1993;87(1):54–55. [DOI] [PubMed] [Google Scholar]
- 38.Ochiel DO, Awandare GA, Keller CC, et al. Differential regulation of beta-chemokines in children with Plasmodium falciparum malaria. Infect Immun. 2005;73(7):4190–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otterdal K, Berg A, Michelsen AE, et al. Soluble markers of neutrophil, T-cell and monocyte activation are associated with disease severity and parasitemia in falciparum malaria. BMC infectious diseases. 2018;18(1):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trampuz A, Jereb M, Muzlovic I, Prabhu RM. Clinical review: Severe malaria. Crit Care. 2003;7(4):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Njuguna P, Newton C. Management of severe falciparum malaria. J Postgrad Med. 2004;50(1):45–50. [PubMed] [Google Scholar]
- 42.Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94 Suppl 1:S1–90. [PubMed] [Google Scholar]
- 43.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. The Quarterly journal of medicine. 1989;71(265):441–459. [PubMed] [Google Scholar]
- 44.Hochman SE, Madaline TF, Wassmer SC, et al. Fatal Pediatric Cerebral Malaria Is Associated with Intravascular Monocytes and Platelets That Are Increased with HIV Coinfection. Mbio. 2015;6(5):e01390–01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunst J, Azzouz N, Liu X, Tsukita S, Seeberger PH, Kamena F. Interaction between Plasmodium Glycosylphosphatidylinositol and the Host Protein Moesin Has No Implication in Malaria Pathology. Front Cell Infect Microbiol. 2017;7:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins SJ, Kain KC, Liles WC. Immunopathogenesis of falciparum malaria: implications for adjunctive therapy in the management of severe and cerebral malaria. Expert Rev Anti Infect Ther. 2011;9(9):803–819. [DOI] [PubMed] [Google Scholar]
- 47.Antwi-Baffour S, Kyeremeh R, Buabeng D, et al. Correlation of malaria parasitaemia with peripheral blood monocyte to lymphocyte ratio as indicator of susceptibility to severe malaria in Ghanaian children. Malar J. 2018;17(1):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warimwe GM, Fletcher HA, Olotu A, et al. Peripheral blood monocyte-to-lymphocyte ratio at study enrollment predicts efficacy of the RTS,S malaria vaccine: analysis of pooled phase II clinical trial data. BMC Med. 2013;11:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogonda LA, Orago AS, Otieno MF, Adhiambo C, Otieno W, Stoute JA. The levels of CD16/Fc gamma receptor IIIA on CD14+ CD16+ monocytes are higher in children with severe Plasmodium falciparum anemia than in children with cerebral or uncomplicated malaria. Infect Immun. 2010;78(5):2173–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith CW. 3. Adhesion molecules and receptors. J Allergy Clin Immunol. 2008;121(2 Suppl):S375–379; quiz S414. [DOI] [PubMed] [Google Scholar]
- 51.Van Gool SW, Vandenberghe P, de Boer M, Ceuppens JL. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev. 1996;153:47–83. [DOI] [PubMed] [Google Scholar]
- 52.Mandala WL, Msefula CL, Gondwe EN, Drayson MT, Molyneux ME, MacLennan CA. Monocyte activation and cytokine production in Malawian children presenting with P. falciparum malaria. Parasite Immunol. 2016;38(5):317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shooshtari P, Fortuno ES 3rd, Blimkie D, et al. Correlation analysis of intracellular and secreted cytokines via the generalized integrated mean fluorescence intensity. Cytometry A. 2010;77(9):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loharungsikul S, Troye-Blomberg M, Amoudruz P, et al. Expression of toll-like receptors on antigen-presenting cells in patients with falciparum malaria. Acta Trop. 2008;105(1):10–15. [DOI] [PubMed] [Google Scholar]
- 55.Teirlinck AC, Roestenberg M, Bijker EM, Hoffman SL, Sauerwein RW, Scholzen A. Plasmodium falciparum Infection of Human Volunteers Activates Monocytes and CD16+ Dendritic Cells and Induces Upregulation of CD16 and CD1c Expression. Infect Immun. 2015;83(9):3732–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaves YO, da Costa AG, Pereira ML, et al. Immune response pattern in recurrent Plasmodium vivax malaria. Malar J. 2016;15(1):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7(2):93–104. [DOI] [PubMed] [Google Scholar]
- 58.Feeney ME. The immune response to malaria in utero. Immunol Rev. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bauserman M, Conroy AL, North K, Patterson J, Bose C, Meshnick S. An overview of malaria in pregnancy. Semin Perinatol. 2019;43(5):282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seitz J, Morales-Prieto DM, Favaro RR, Schneider H, Markert UR. Molecular Principles of Intrauterine Growth Restriction in Plasmodium Falciparum Infection. Front Endocrinol (Lausanne). 2019;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abrams ET, Brown H, Chensue SW, et al. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J Immunol. 2003;170(5):2759–2764. [DOI] [PubMed] [Google Scholar]
- 62.Chaisavaneeyakorn S, Moore JM, Mirel L, et al. Levels of macrophage inflammatory protein 1 alpha (MIP-1 alpha) and MIP-1 beta in intervillous blood plasma samples from women with placental malaria and human immunodeficiency virus infection. Clin Diagn Lab Immunol. 2003;10(4):631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucchi NW, Peterson DS, Moore JM. Immunologic activation of human syncytiotrophoblast by Plasmodium falciparum. Malar J. 2008;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suguitan AL Jr., Leke RG, Fouda G, et al. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J Infect Dis. 2003;188(7):1074–1082. [DOI] [PubMed] [Google Scholar]
- 65.Ismail MR, Ordi J, Menendez C, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31(1):85–93. [DOI] [PubMed] [Google Scholar]
- 66.Ordi J, Ismail MR, Ventura PJ, et al. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22(8):1006–1011. [DOI] [PubMed] [Google Scholar]
- 67.Menendez C, Ordi J, Ismail MR, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181(5):1740–1745. [DOI] [PubMed] [Google Scholar]
- 68.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68(1):115–119. [PubMed] [Google Scholar]
- 69.Chua CL, Brown GV, Hamilton JA, Molyneux ME, Rogerson SJ, Boeuf P. Soluble CD163, a product of monocyte/macrophage activation, is inversely associated with haemoglobin levels in placental malaria. PLoS One. 2013;8(5):e64127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cumming BM, Goldring JPD. Monocyte phagocytosis of malaria beta-haematin in the presence of artemisinin, amodiaquine, chloroquine, doxycycline, primaquine, pyrimethamine and quinine. Exp Parasitol. 2019;197:93–102. [DOI] [PubMed] [Google Scholar]
- 71.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta P, Lai SM, Sheng J, et al. Tissue-Resident CD169(+) Macrophages Form a Crucial Front Line against Plasmodium Infection. Cell Rep. 2016;16(6):1749–1761. [DOI] [PubMed] [Google Scholar]
- 73.Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–675. [DOI] [PubMed] [Google Scholar]
- 74.Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44(3):439–449. [DOI] [PubMed] [Google Scholar]
- 75.Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol. 2016;17(1):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sheng J, Ruedl C, Karjalainen K. Most Tissue-Resident Macrophages Except Microglia Are Derived from Fetal Hematopoietic Stem Cells. Immunity. 2015;43(2):382–393. [DOI] [PubMed] [Google Scholar]
- 77.Bain CC, Bravo-Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15(10):929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bleriot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42(1):145–158. [DOI] [PubMed] [Google Scholar]
- 79.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13(10):954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guilliams M, Scott CL. Does niche competition determine the origin of tissue-resident macrophages? Nat Rev Immunol. 2017;17(7):451–460. [DOI] [PubMed] [Google Scholar]
- 81.Lai SM, Sheng J, Gupta P, et al. Organ-Specific Fate, Recruitment, and Refilling Dynamics of Tissue-Resident Macrophages during Blood-Stage Malaria. Cell Rep. 2018;25(11):3099–3109 e3093. [DOI] [PubMed] [Google Scholar]
- 82.Lagasse HA, Anidi IU, Craig JM, et al. Recruited monocytes modulate malaria-induced lung injury through CD36-mediated clearance of sequestered infected erythrocytes. J Leukoc Biol. 2016;99(5):659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gazzinelli RT, Kalantari P, Fitzgerald KA, Golenbock DT. Innate sensing of malaria parasites. Nat Rev Immunol. 2014;14(11):744–757. [DOI] [PubMed] [Google Scholar]
- 84.Gowda DC, Wu X. Parasite Recognition and Signaling Mechanisms in Innate Immune Responses to Malaria. Front Immunol. 2018;9:3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gowda DC, Gupta P, Davidson EA. Glycosylphosphatidylinositol anchors represent the major carbohydrate modification in proteins of intraerythrocytic stage Plasmodium falciparum. J Biol Chem. 1997;272(10):6428–6439. [DOI] [PubMed] [Google Scholar]
- 86.Sanders PR, Gilson PR, Cantin GT, et al. Distinct protein classes including novel merozoite surface antigens in Raft-like membranes of Plasmodium falciparum. J Biol Chem. 2005;280(48):40169–40176. [DOI] [PubMed] [Google Scholar]
- 87.Naik RS, Branch OH, Woods AS, et al. Glycosylphosphatidylinositol anchors of Plasmodium falciparum: molecular characterization and naturally elicited antibody response that may provide immunity to malaria pathogenesis. J Exp Med. 2000;192(11):1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schofield L, Novakovic S, Gerold P, Schwarz RT, McConville MJ, Tachado SD. Glycosylphosphatidylinositol toxin of Plasmodium up-regulates intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin expression in vascular endothelial cells and increases leukocyte and parasite cytoadherence via tyrosine kinase-dependent signal transduction. J Immunol. 1996;156(5):1886–1896. [PubMed] [Google Scholar]
- 89.Tachado SD, Gerold P, McConville MJ, et al. Glycosylphosphatidylinositol toxin of Plasmodium induces nitric oxide synthase expression in macrophages and vascular endothelial cells by a protein tyrosine kinase-dependent and protein kinase C-dependent signaling pathway. J Immunol. 1996;156(5):1897–1907. [PubMed] [Google Scholar]
- 90.Zhu J, Krishnegowda G, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: the requirement of extracellular signal-regulated kinase, p38, c-Jun N-terminal kinase and NF-kappaB pathways for the expression of proinflammatory cytokines and nitric oxide. J Biol Chem. 2005;280(9):8617–8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krishnegowda G, Hajjar AM, Zhu J, et al. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280(9):8606–8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu JZ, Wu XZ, Goel S, et al. MAPK-activated Protein Kinase 2 Differentially Regulates Plasmodium falciparum Glycosylphosphatidylinositol-induced Production of Tumor Necrosis Factor-alpha and Interleukin-12 in Macrophages. Journal of Biological Chemistry. 2009;284(23):15750–15761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patel SN, Lu ZY, Ayi K, Serghides L, Gowda DC, Kain KC. Disruption of CD36 impairs cytokine response to Plasmodium falciparum glycosylphosphatidylinositol and confers susceptibility to severe and fatal malaria in vivo. Journal of Immunology. 2007;178(6):3954–3961. [DOI] [PubMed] [Google Scholar]
- 94.Davis AE 3rd. Biological effects of C1 inhibitor. Drug News Perspect. 2004;17(7):439–446. [DOI] [PubMed] [Google Scholar]
- 95.Mejia P, Diez-Silva M, Kamena F, et al. Human C1-Inhibitor Suppresses Malaria Parasite Invasion and Cytoadhesion via Binding to Parasite Glycosylphosphatidylinositol and Host Cell Receptors. J Infect Dis. 2016;213(1):80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parroche P, Lauw FN, Goutagny N, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104(6):1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu XZ, Gowda NM, Kumar S, Gowda DC. Protein-DNA Complex Is the Exclusive Malaria Parasite Component That Activates Dendritic Cells and Triggers Innate Immune Responses. Journal of Immunology. 2010;184(8):4338–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gowda NM, Wu XZ, Gowda DC. The Nucleosome (Histone-DNA Complex) Is the TLR9-Specific Immunostimulatory Component of Plasmodium falciparum That Activates DCs. Plos One. 2011;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirako IC, Gallego-Marin C, Ataide MA, et al. DNA-Containing Immunocomplexes Promote Inflammasome Assembly and Release of Pyrogenic Cytokines by CD14(+) CD16(+) CD64(high) CD32(low) Inflammatory Monocytes from Malaria Patients. Mbio. 2015;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalantari P, DeOliveira RB, Chan J, et al. Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria. Cell Rep. 2014;6(1):196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lamphier MS, Sirois CM, Verma A, Golenbock DT, Latz E. TLR9 and the recognition of self and non-self nucleic acids. Ann Ny Acad Sci. 2006;1082:31–43. [DOI] [PubMed] [Google Scholar]
- 102.Pichyangkul S, Yongvanitchit K, Kum-Arb U, et al. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a toll-like receptor 9-dependent pathway. Journal of Immunology. 2004;172(8):4926–4933. [DOI] [PubMed] [Google Scholar]
- 103.Schlee M, Hartmann G. Discriminating self from non-self in nucleic acid sensing. Nat Rev Immunol. 2016;16(9):566–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168(9):4531–4537. [DOI] [PubMed] [Google Scholar]
- 105.Sharma S, DeOliveira RB, Kalantari P, et al. Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity. 2011;35(2):194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gallego-Marin C, Schrum JE, Andrade WA, et al. Cyclic GMP-AMP Synthase Is the Cytosolic Sensor of Plasmodium falciparum Genomic DNA and Activates Type I IFN in Malaria. J Immunol. 2018;200(2):768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coch C, Hommertgen B, Zillinger T, et al. Human TLR8 Senses RNA From Plasmodium falciparum-Infected Red Blood Cells Which Is Uniquely Required for the IFN-gamma Response in NK Cells. Front Immunol. 2019;10:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baccarella A, Fontana MF, Chen EC, Kim CC. Toll-like receptor 7 mediates early innate immune responses to malaria. Infect Immun. 2013;81(12):4431–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hartmann G. Nucleic Acid Immunity. Adv Immunol. 2017;133:121–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berger M, Ablasser A, Kim S, et al. TLR8-driven IL-12-dependent reciprocal and synergistic activation of NK cells and monocytes by immunostimulatory RNA. J Immunother. 2009;32(3):262–271. [DOI] [PubMed] [Google Scholar]
- 111.Ablasser A, Poeck H, Anz D, et al. Selection of molecular structure and delivery of RNA oligonucleotides to activate TLR7 versus TLR8 and to induce high amounts of IL-12p70 in primary human monocytes. J Immunol. 2009;182(11):6824–6833. [DOI] [PubMed] [Google Scholar]
- 112.Olivier M, Van Den Ham K, Shio MT, Kassa FA, Fougeray S. Malarial pigment hemozoin and the innate inflammatory response. Front Immunol. 2014;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lawrence C. Laveran remembered: malaria haemozoin in leucocytes. Lancet. 1999;353(9167):1852. [DOI] [PubMed] [Google Scholar]
- 114.Nguyen PH, Day N, Pram TD, Ferguson DJ, White NJ. Intraleucocytic malaria pigment and prognosis in severe malaria. Trans R Soc Trop Med Hyg. 1995;89(2):200–204. [DOI] [PubMed] [Google Scholar]
- 115.Amodu OK, Adeyemo AA, Olumese PE, Gbadegesin RA. Intraleucocytic malaria pigment and clinical severity of malaria in children. Trans R Soc Trop Med Hyg. 1998;92(1):54–56. [DOI] [PubMed] [Google Scholar]
- 116.Newton CR, Taylor TE, Whitten RO. Pathophysiology of fatal falciparum malaria in African children. Am J Trop Med Hyg. 1998;58(5):673–683. [DOI] [PubMed] [Google Scholar]
- 117.Shio MT, Kassa FA, Bellemare MJ, Olivier M. Innate inflammatory response to the malarial pigment hemozoin. Microbes Infect. 2010;12(12–13):889–899. [DOI] [PubMed] [Google Scholar]
- 118.Schwarzer E, De Matteis F, Giribaldi G, Ulliers D, Valente E, Arese P. Hemozoin stability and dormant induction of heme oxygenase in hemozoin-fed human monocytes. Mol Biochem Parasitol. 1999;100(1):61–72. [DOI] [PubMed] [Google Scholar]
- 119.Egan TJ, Ross DC, Adams PA. Quinoline anti-malarial drugs inhibit spontaneous formation of beta-haematin (malaria pigment). FEBS Lett. 1994;352(1):54–57. [DOI] [PubMed] [Google Scholar]
- 120.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment beta-haematin. Nature. 2000;404(6775):307–310. [DOI] [PubMed] [Google Scholar]
- 121.Goldie P, Roth EF Jr., Oppenheim J, Vanderberg JP. Biochemical characterization of Plasmodium falciparum hemozoin. Am J Trop Med Hyg. 1990;43(6):584–596. [DOI] [PubMed] [Google Scholar]
- 122.Barrera V, Skorokhod OA, Baci D, Gremo G, Arese P, Schwarzer E. Host fibrinogen stably bound to hemozoin rapidly activates monocytes via TLR-4 and CD11b/CD18-integrin: a new paradigm of hemozoin action. Blood. 2011;117(21):5674–5682. [DOI] [PubMed] [Google Scholar]
- 123.Shio MT, Eisenbarth SC, Savaria M, et al. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5(8):e1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dostert C, Guarda G, Romero JF, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4(8):e6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Reimer T, Shaw MH, Franchi L, et al. Experimental cerebral malaria progresses independently of the Nlrp3 inflammasome. Eur J Immunol. 2010;40(3):764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jaramillo M, Godbout M, Olivier M. Hemozoin induces macrophage chemokine expression through oxidative stress-dependent and -independent mechanisms. J Immunol. 2005;174(1):475–484. [DOI] [PubMed] [Google Scholar]
- 127.Ng G, Sharma K, Ward SM, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29(5):807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raulf MK, Johannssen T, Matthiesen S, et al. The C-type Lectin Receptor CLEC12A Recognizes Plasmodial Hemozoin and Contributes to Cerebral Malaria Development. Cell Rep. 2019;28(1):30–38 e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lahoud MH, Proietto AI, Ahmet F, et al. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J Immunol. 2009;182(12):7587–7594. [DOI] [PubMed] [Google Scholar]
- 130.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16(1):24–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.S ELA, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. [DOI] [PubMed] [Google Scholar]
- 134.Mantel PY, Marti M. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell Microbiol. 2014;16(3):344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sampaio NG, Cheng L, Eriksson EM. The role of extracellular vesicles in malaria biology and pathogenesis. Malar J. 2017;16(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Debs S, Cohen A, Hosseini-Beheshti E, Chimini G, Hunt NH, Grau GER. Interplay of extracellular vesicles and other players in cerebral malaria pathogenesis. Biochim Biophys Acta Gen Subj. 2019;1863(2):325–331. [DOI] [PubMed] [Google Scholar]
- 137.Combes V, Taylor TE, Juhan-Vague I, et al. Circulating endothelial microparticles in malawian children with severe falciparum malaria complicated with coma. JAMA. 2004;291(21):2542–2544. [DOI] [PubMed] [Google Scholar]
- 138.Pankoui Mfonkeu JB, Gouado I, Fotso Kuate H, et al. Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS One. 2010;5(10):e13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nantakomol D, Dondorp AM, Krudsood S, et al. Circulating red cell-derived microparticles in human malaria. J Infect Dis. 2011;203(5):700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Campos FM, Franklin BS, Teixeira-Carvalho A, et al. Augmented plasma microparticles during acute Plasmodium vivax infection. Malar J. 2010;9:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Couper KN, Barnes T, Hafalla JC, et al. Parasite-derived plasma microparticles contribute significantly to malaria infection-induced inflammation through potent macrophage stimulation. PLoS Pathog. 2010;6(1):e1000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mantel PY, Hoang AN, Goldowitz I, et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe. 2013;13(5):521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sampaio NG, Emery SJ, Garnham AL, et al. Extracellular vesicles from early stage Plasmodium falciparum-infected red blood cells contain PfEMP1 and induce transcriptional changes in human monocytes. Cell Microbiol. 2018;20(5):e12822. [DOI] [PubMed] [Google Scholar]
- 144.Sisquella X, Ofir-Birin Y, Pimentel MA, et al. Malaria parasite DNA-harbouring vesicles activate cytosolic immune sensors. Nat Commun. 2017;8(1):1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sampaio NG, Eriksson EM, Schofield L. Plasmodium falciparum PfEMP1 Modulates Monocyte/Macrophage Transcription Factor Activation and Cytokine and Chemokine Responses. Infect Immun. 2018;86(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ye W, Chew M, Hou J, et al. Microvesicles from malaria-infected red blood cells activate natural killer cells via MDA5 pathway. PLoS Pathog. 2018;14(10):e1007298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mantel PY, Hjelmqvist D, Walch M, et al. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat Commun. 2016;7:12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Arts RJ, Joosten LA, Netea MG. Immunometabolic circuits in trained immunity. Semin Immunol. 2016;28(5):425–430. [DOI] [PubMed] [Google Scholar]
- 150.Arts RJW, Carvalho A, La Rocca C, et al. Immunometabolic Pathways in BCG-Induced Trained Immunity. Cell Rep. 2016;17(10):2562–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lachmandas E, Beigier-Bompadre M, Cheng SC, et al. Rewiring cellular metabolism via the AKT/mTOR pathway contributes to host defence against Mycobacterium tuberculosis in human and murine cells. Eur J Immunol. 2016;46(11):2574–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25(7):771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Krawczyk CM, Holowka T, Sun J, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115(23):4742–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodriguez-Prados JC, Traves PG, Cuenca J, et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185(1):605–614. [DOI] [PubMed] [Google Scholar]
- 155.Everts B, Amiel E, Huang SC, et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat Immunol. 2014;15(4):323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tan Z, Xie N, Banerjee S, et al. The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J Biol Chem. 2015;290(1):46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8(6):519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Freemerman AJ, Johnson AR, Sacks GN, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289(11):7884–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lachmandas E, Boutens L, Ratter JM, et al. Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat Microbiol. 2016;2:16246. [DOI] [PubMed] [Google Scholar]
- 160.Dominguez-Andres J, Arts RJW, Ter Horst R, et al. Rewiring monocyte glucose metabolism via C-type lectin signaling protects against disseminated candidiasis. PLoS Pathog. 2017;13(9):e1006632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front Immunol. 2014;5:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. [DOI] [PubMed] [Google Scholar]
- 164.Anstey NM, Weinberg JB, Hassanali MY, et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184(2):557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lopansri BK, Anstey NM, Weinberg JB, et al. Low plasma arginine concentrations in children with cerebral malaria and decreased nitric oxide production. Lancet. 2003;361(9358):676–678. [DOI] [PubMed] [Google Scholar]
- 166.Weinberg JB, Yeo TW, Mukemba JP, et al. Dimethylarginines: endogenous inhibitors of nitric oxide synthesis in children with falciparum malaria. J Infect Dis. 2014;210(6):913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rubach MP, Mukemba J, Florence S, et al. Impaired systemic tetrahydrobiopterin bioavailability and increased oxidized biopterins in pediatric falciparum malaria: association with disease severity. PLoS Pathog. 2015;11(3):e1004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yeo TW, Lampah DA, Kenangalem E, et al. Impaired systemic tetrahydrobiopterin bioavailability and increased dihydrobiopterin in adult falciparum malaria: association with disease severity, impaired microvascular function and increased endothelial activation. PLoS Pathog. 2015;11(3):e1004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Weinberg JB, Volkheimer AD, Rubach MP, et al. Monocyte polarization in children with falciparum malaria: relationship to nitric oxide insufficiency and disease severity. Sci Rep. 2016;6:29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174(5):1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Niikura M, Inoue S, Kobayashi F. Role of interleukin-10 in malaria: focusing on coinfection with lethal and nonlethal murine malaria parasites. J Biomed Biotechnol. 2011;2011:383962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bogdan C, Paik J, Vodovotz Y, Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992;267(32):23301–23308. [PubMed] [Google Scholar]
- 173.Bobade D, Khandare AV, Deval M, Shastry P, Deshpande P. Hemozoin-induced activation of human monocytes toward M2-like phenotype is partially reversed by antimalarial drugs-chloroquine and artemisinin. Microbiologyopen. 2019;8(3):e00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoeksema MA, de Winther MP. Epigenetic Regulation of Monocyte and Macrophage Function. Antioxidants & redox signaling. 2016;25(14):758–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hennessy C, McKernan DP. Epigenetics and innate immunity: the ‘unTolld’ story. Immunology and cell biology. 2016;94(7):631–639. [DOI] [PubMed] [Google Scholar]
- 176.Cedar H, Bergman Y. Programming of DNA methylation patterns. Annu Rev Biochem. 2012;81:97–117. [DOI] [PubMed] [Google Scholar]
- 177.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature reviews Genetics. 2012;13(7):484–492. [DOI] [PubMed] [Google Scholar]
- 178.Pacis A, Tailleux L, Morin AM, et al. Bacterial infection remodels the DNA methylation landscape of human dendritic cells. Genome Res. 2015;25(12):1801–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gupta AP, Bozdech Z. Epigenetic landscapes underlining global patterns of gene expression in the human malaria parasite, Plasmodium falciparum. International journal for parasitology. 2017;47(7):399–407. [DOI] [PubMed] [Google Scholar]
- 180.Fastman Y, Noble R, Recker M, Dzikowski R. Erasing the epigenetic memory and beginning to switch--the onset of antigenic switching of var genes in Plasmodium falciparum. PLoS One. 2012;7(3):e34168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Merrick CJ, Huttenhower C, Buckee C, et al. Epigenetic dysregulation of virulence gene expression in severe Plasmodium falciparum malaria. J Infect Dis. 2012;205(10):1593–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Al-Quraishy S, Dkhil MA, Abdel-Baki AS, et al. Protective vaccination and blood-stage malaria modify DNA methylation of gene promoters in the liver of Balb/c mice. Parasitology research. 2017;116(5):1463–1477. [DOI] [PubMed] [Google Scholar]
- 183.Berkley JA, Bejon P, Mwangi T, et al. HIV infection, malnutrition, and invasive bacterial infection among children with severe malaria. Clin Infect Dis. 2009;49(3):336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schulthess J, Pandey S, Capitani M, et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity. 2019;50(2):432–445 e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arts RJ, Novakovic B, Ter Horst R, et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016;24(6):807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shalova IN, Lim JY, Chittezhath M, et al. Human monocytes undergo functional reprogramming during sepsis mediated by hypoxia-inducible factor-1alpha. Immunity. 2015;42(3):484–498. [DOI] [PubMed] [Google Scholar]
- 187.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9(5):355–361. [DOI] [PubMed] [Google Scholar]
- 188.Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109(43):17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Saeed S, Quintin J, Kerstens HH, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345(6204):1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bauer M, Weis S, Netea MG, Wetzker R. Remembering Pathogen Dose: Long-Term Adaptation in Innate Immunity. Trends Immunol. 2018;39(6):438–445. [DOI] [PubMed] [Google Scholar]
- 191.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25(48):6373–6383. [DOI] [PubMed] [Google Scholar]
- 192.Jeon SM. Regulation and function of AMPK in physiology and diseases. Experimental & molecular medicine. 2016;48(7):e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McCall MB, Netea MG, Hermsen CC, et al. Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J Immunol. 2007;179(1):162–171. [DOI] [PubMed] [Google Scholar]
- 194.Franklin BS, Parroche P, Ataide MA, et al. Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function. Proc Natl Acad Sci U S A. 2009;106(14):5789–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Leoratti FM, Trevelin SC, Cunha FQ, et al. Neutrophil paralysis in Plasmodium vivax malaria. PLoS Negl Trop Dis. 2012;6(6):e1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schrum JE, Crabtree JN, Dobbs KR, et al. Cutting Edge: Plasmodium falciparum Induces Trained Innate Immunity. J Immunol. 2018;200(4):1243–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Natoli G, Ostuni R. Adaptation and memory in immune responses. Nat Immunol. 2019;20(7):783–792. [DOI] [PubMed] [Google Scholar]
- 198.Day NP, Pham TD, Phan TL, et al. Clearance kinetics of parasites and pigment-containing leukocytes in severe malaria. Blood. 1996;88(12):4694–4700. [PubMed] [Google Scholar]
- 199.Joice R, Nilsson SK, Montgomery J, et al. Plasmodium falciparum transmission stages accumulate in the human bone marrow. Science translational medicine. 2014;6(244):244re245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Milner DA Jr., Valim C, Carr RA, et al. A histological method for quantifying Plasmodium falciparum in the brain in fatal paediatric cerebral malaria. Malar J. 2013;12:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Herricks T, Seydel KB, Molyneux M, Taylor T, Rathod PK. Estimating physical splenic filtration of Plasmodium falciparum-infected red blood cells in malaria patients. Cell Microbiol. 2012;14(12):1880–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Frita R, Carapau D, Mota MM, Hanscheid T. In Vivo Hemozoin Kinetics after Clearance of Plasmodium berghei Infection in Mice. Malar Res Treat. 2012;2012:373086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee MSJ, Maruyama K, Fujita Y, et al. Plasmodium products persist in the bone marrow and promote chronic bone loss. Sci Immunol. 2017;2(12). [DOI] [PubMed] [Google Scholar]
- 204.Nedelec Y, Sanz J, Baharian G, et al. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell. 2016;167(3):657–669 e621. [DOI] [PubMed] [Google Scholar]
- 205.Takem EN, Roca A, Cunnington A. The association between malaria and non-typhoid Salmonella bacteraemia in children in sub-Saharan Africa: a literature review. Malar J. 2014;13:400. [DOI] [PMC free article] [PubMed] [Google Scholar]