Abstract
Background
Episodic viral wheeze (EVW) associated with viral respiratory tract infections is a common reason for pre‐school children to utilise health care resources and for carers to take time away from employment. About a third of children experience a wheezing episode before the age of five years. EVW therefore represents a significant public health problem. Many pre‐school children only wheeze in association with viral infections and in such cases EVW appears to be a separate entity from atopic asthma. Some trials have explored the effectiveness of leukotriene receptor antagonists (LTRAs) as regular (maintenance) or episodic (intermittent) treatment in this context.
Objectives
To evaluate the evidence for the efficacy and safety of maintenance and intermittent LTRAs in the management of EVW in children aged one to six years.
Search methods
We searched the Cochrane Airways Group register of trials with pre‐specified terms. We performed additional searches by consulting the authors of identified trials, online trial registries of manufacturers' web sites, and reference lists of identified primary papers and reviews. Search results are current to June 2015.
Selection criteria
We included randomised controlled trials with a parallel‐group or cross‐over (for intermittent LTRA only) design. Maintenance was considered as treatment for more than two months and intermittent as less than 14 days. EVW was defined as a history of at least one previous episode of wheezing in association with a viral respiratory tract infection in the absence of symptoms between episodes. As far as possible, relevant specific data were obtained from authors of studies that included children of a wider age group or phenotype.
Data collection and analysis
Two authors independently assessed studies for inclusion in the review and assessed risk of bias. The primary outcome was number of children with one or more viral‐induced episodes requiring one or more treatments with rescue oral corticosteroids. We analysed combined continuous data outcomes with the mean difference and dichotomous data outcomes with an odds ratio (OR).
Main results
We identified five studies eligible for inclusion in the review (one investigated maintenance treatment, three intermittent therapy and one had both maintenance and intermittent treatment arms) these included 3741 participants. Each study involved oral montelukast and was of good methodological quality, but differed in choice of outcome measures thus limiting our ability to aggregate data across studies. Only primary outcome and adverse event data are reported in this abstract.
For maintenance treatment, specific data obtained from a single study, pertaining to children with only an EVW phenotype, showed no statistically significant group reduction in the number of episodes requiring rescue oral corticosteroids associated with daily montelukast versus placebo (OR 1.20, 95% CI 0.70 to 2.06, moderate quality evidence).
For intermittent LTRA, pooled data showed no statistically significant reduction in the number of episodes requiring rescue oral steroids in children treated with LTRA versus placebo (OR 0.77, 95% CI 0.48 to 1.25, moderate quality evidence). Specific data for children with an EVW phenotype obtained from a single study of intermittent montelukast treatment showed a small, but statistically significant reduction in unscheduled medical attendances due to wheeze (RR 0.83, 95% CI 0.71 to 0.98).
For maintenance compared to intermittent LTRA treatment no data relating to the primary outcome of the review were identified.
There were no other significant group differences identified in other secondary efficacy outcomes for maintenance or intermittent LTRA treatment versus placebo, or maintenance versus intermittent LTRA treatment. We collected descriptive data on adverse events as reported by four of the five included studies, and rates were similar between treatment and placebo groups.
Potential heterogeneity in the phenotype of participants within and across trials is a limitation of the evidence.
Authors' conclusions
In pre‐school children with EVW, there is no evidence of benefit associated with maintenance or intermittent LTRA treatment, compared to placebo, for reducing the number of children with one or more viral‐induced episodes requiring rescue oral corticosteroids, and little evidence of significant clinical benefit for other secondary outcomes. Therefore until further data are available, LTRA should be used with caution in individual children. When used, we suggest a therapeutic trial is undertaken, during which efficacy should be carefully monitored. It is likely that children with an apparent EVW phenotype are not a homogeneous group and that subgroups may respond to LTRA treatment depending on the exact patho‐physiological mechanisms involved.
Plain language summary
What is the evidence for benefit from leukotriene receptor antagonists in pre‐school children who wheeze when they have a cold?
Background to the review
Acute episodes of wheezing in pre‐school aged children are common. Many children in this age group seem to wheeze only when they have a common cold‐type virus with no ongoing symptoms between episodes, unlike older children with allergic‐type asthma. Acute episodes of wheezing cause the child to breathe more quickly than normal and they may require supportive treatment such as the use of rescue inhalers; in moderate or severe episodes they may also need a short course of oral steroids and not uncommonly may require treatment in hospital and supplemental oxygen. Their carers may need to take time off work to look after children who are unwell. Although episodic wheezing with viruses is a common problem, there is controversy about the best way to prevent or shorten episodes.
Leukotriene receptor antagonists (LTRAs) are drugs that are taken by mouth and work by reducing inflammation and allergic reactions in the airways.
Aim of the review
In this review, we have combined the evidence from the different studies comparing the maintenance (regular) or intermittent (just during episodes of wheeze with viruses) use of LTRAs with placebo in episodic viral wheezing in pre‐school children.
What did we find?
We identified five eligible studies that varied in their choice of outcomes and therefore limited our ability to combine the findings between different studies. We failed to find any evidence of benefit of maintenance or intermittent LTRA treatment over placebo for preventing acute episodes of wheezing requiring use of rescue oral steroids, and little evidence of clinical benefit in other outcomes.
Summary of findings
Summary of findings for the main comparison. Maintenance LTRA compared to placebo for episodic viral wheeze in children.
| Maintenance LTRA compared to placebo for episodic viral wheeze in children | ||||||
| Patient or population: episodic viral wheeze in children Settings: community and hospitals Intervention: maintenance LTRA Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | Maintenance LTRA | |||||
| Number of participants experiencing one or more episode requiring treatment with oral steroids | 173 per 1000 | 201 per 1000 (128 to 302) | OR 1.20 (0.70 to 2.06) | 347 (1 study) | ⊕⊕⊕⊝ moderate1,2 |
|
| Number of participants experiencing one or more episodes requiring ED visit | 179 per 1000 | 126 per 1000 (75 to 208) | OR 0.66 (0.37 to 1.20) | 347 (1 study) | ⊕⊕⊕⊝ moderate1,2 |
|
| Number of participants experiencing one or more episodes requiring hospital admission | 52 per 1000 | 34 per 1000 (12 to 93) | OR 0.65 (0.23 to 1.87) | 347 (1 study) | ⊕⊕⊕⊝ moderate1,2 |
|
| Withdrawals ‐ Total number of withdrawals | 154 per 1000 | 137 per 1000 (107 to 171) | OR 0.87 (0.66 to 1.13) | 1729 (2 studies) | ⊕⊕⊕⊝ moderate1,2 |
|
| Serious adverse events | see comment | see comment | see comment | see comment | None reported, so not possible to assess the risks of serious adverse events. | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Confidence interval cannot rule out important benefit or harm.
2Note potential heterogeneity in the phenotype of participants within and across studies.
Summary of findings 2. Intermittent LTRA compared to placebo for episodic viral wheeze in children.
| Intermittent LTRA compared to placebo for episodic viral wheeze in children | ||||||
| Patient or population: episodic viral wheeze in children Settings: community and hospitals Intervention: intermittent LTRA Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | Intermittent LTRA | |||||
| Number of participants experiencing one or more episode requiring treatment with oral steroids | 336 per 1000 | 285 per 1000 (215 to 382) | RR 0.85 (0.64 to 1.14) | 343 (2 studies) | ⊕⊕⊕⊝ moderate1,2 |
|
| Number of participants experiencing one or more episodes requiring ED visit | 553 per 1000 | 553 per 1000 (378 to 714) | OR 1.00 (0.49 to 2.02) | 141 (1 study) | ⊕⊕⊕⊝ moderate1,2 |
|
| Number of participants experiencing one or more episodes requiring hospital admission | 85 per 1000 | 64 per 1000 (18 to 203) | OR 0.73 (0.20 to 2.73) | 141 (1 study) | ⊕⊕⊕⊝ moderate1,2 |
|
| Withdrawals ‐ Total number of withdrawals | 183 per 1000 | 188 per 1000 (151 to 232) | OR 1.03 (0.79 to 1.35) | 1402 (2 studies) | ⊕⊕⊕⊝ moderate1,2 |
|
| Serious adverse events | see comment | see comment | see comment | see comment | None reported, so not possible to assess the risks of serious adverse events. | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Confidence interval cannot rule out important benefit or harm.
2Note potential heterogeneity in the phenotype of participants within and across studies.
Background
Description of the condition
Around a third of children experience at least one episode of wheeze before the age of five years (Kuehni 2001). Evidence suggests that in pre‐school aged children recurrent wheezing that occurs exclusively in association with viral infection of the respiratory tract may represent a separate entity from atopic asthma (Silverman 1993; Spycher 2008). This phenomenon is known as episodic viral wheezing (EVW).
A considerable number of children require emergency treatment or admission to hospital, or both for EVW placing a significant burden on healthcare resources and society as a whole (Anderson 1992; Lougheed 2006, Davies 2008, Ducharme 2014). The evidence base for managing children with EVW is generally thin and acute treatments are largely supportive such as oxygen therapy and inhaled bronchodilators (Bush 2009; McKean 2000). A double‐blind, placebo‐controlled, randomised trial found no clinical benefit from treatment with oral corticosteroids in children presenting to hospital with acute wheeze in association with a viral infection (Panickar 2009). In another study high‐dose inhaled fluticasone initiated at the start of a viral respiratory tract infection was associated with some reduction in the duration of symptoms, but also a reduced increase in height. (Ducharme 2009).
EVW is largely a disease of pre‐school children with the majority of children affected aged one to three years (Ducharme 2012, Martinez 1995; Spycher 2008; Stein 1997; Wilson 1994). The natural history and progression of pre‐school children who wheeze is complex. Several cohort studies suggest different trajectories, including 'transient wheezers' who experience EVW as young children but whose symptoms resolve by school age, 'persistent wheezers' with multiple risk factors who evolve from an EVW phenotype as young children to an atopic asthma, multi‐trigger wheeze, phenotype as school‐aged children, in addition to 'late‐onset wheezers' who first experience symptoms of atopic asthma after the age of three years (Martinez 1995; Savenije 2011; Spycher 2008). An overall paradigm encompassing these studies is that EVW is intermittent in nature, occurs predominantly in pre‐school children and represents a separate entity from atopic asthma, where wheeze is associated with multiple environmental triggers and mediated by allergic mechanisms.
Description of the intervention
In this systematic review we evaluate the available evidence for the efficacy of maintenance (regular as preventive treatment) and intermittent (during symptomatic episodes) leukotriene receptor antagonists (LTRAs) in the management of children aged between one and six years seemingly presenting with EVW.
How the intervention might work
Leukotrienes are pro‐inflammatory mediators that are produced by a range of cells, including eosinophils, mast cells and alveolar macrophages, in response to stimuli in the airway. Leukotrienes are derived from arachidonic acid by way of the 5‐lipoxygenase pathway. The cysteinyl leukotrienes (C4, D4 and E4) bind to highly selective receptors and induce bronchoconstriction, eosinophil chemotaxis, tissue oedema and increased mucus secretion (Drazen 1999). The most commonly prescribed LTRA is montelukast, which blocks cysteinyl leukotriene receptors and, when given regularly, is of proven benefit in pre‐school children with persistent wheeze, albeit less effective than low doses of inhaled corticosteroids (BTS 2014; GINA 2012; Knorr 2001). Oomen and Grigg measured urinary leukotriene E4 and serum total immunoglobulin E (IgE) in preschool children with EVW during an acute attack (Oommen 2003). They found heterogeneity in urinary leukotriene E4 excretion. Elevated urinary leukotriene E4 levels occurred in 23 of the 44 children studied with the highest IgE levels. Increased cysteinyl leukotriene production may therefore be clinically relevant to this subgroup of children; alternatively however it is possible that they may have identified a group of children with multi‐trigger atopic asthma, incorrectly perceived to have EVW.
Why it is important to do this review
LTRAs are prescribed by clinicians for children with EVW, however, the evidence‐base supporting this intervention is unclear and the question 'What evidence exists for clinical efficacy of LTRAs in children with EVW either regularly or at the onset of viral respiratory tract infections?' is a pertinent one. This is the first Cochrane Review to address this issue. We focus on young children as this is the age group where EVW is most recognised, causes the most morbidity and is therefore most clinically relevant. In children with EVW, acute episodes only occur in association with viral respiratory tract infections, with no interval symptoms or other triggers (Bacharier 2008; Brand 2008). To distinguish young children with EVW from those with atopic asthma may be challenging in 'real life' however because most children become more symptomatic during viral respiratory tract infections and interval symptoms, as reported by a third party, are not always evident (Ducharme 2012). To try and address this issue we carefully sought to only include studies that involved children with an EVW phenotype in this systematic review. It is also important to note that EVW represents a separate entity from viral bronchiolitis, which is recognised in infants (younger than 12 months of age) and is associated predominantly with crackles on auscultation (Øymar 2014; SIGN 2006). We chose, 'number of children with one or more viral‐induced episodes requiring one or more treatment with rescue oral corticosteroids' as the primary outcome when devising our review protocol. It may be argued that the use of oral corticosteroids in the management of EVW has recently been debated (Beigelman 2014; Bush 2009; Panickar 2009; Tal 1990) however they remain a marker of moderate and severe episodes and a comprehensive range of secondary outcomes were also assessed.
Objectives
To evaluate the evidence for the efficacy and safety of maintenance and intermittent LTRAs in the management of EVW in children aged one to six years.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), parallel‐group and cross‐over (intermittent only) studies were considered for this review.
Types of participants
-
Children aged one to six years with EVW were included. EVW was defined by the following criteria:
history of at least one reported episode of wheezing in association with clinical evidence of a viral respiratory tract infection (confirmation of virus by serology, culture or antigen assay not necessary);
no symptoms between exacerbations.
-
Exclusion criteria were:
children with cystic fibrosis, bronchopulmonary dysplasia or any other chronic lung disease;
children with bacterial pneumonia;
children with viral bronchiolitis (children younger than 12 months were excluded to avoid infants with viral bronchiolitis, i.e. the presence of crackles or the first or second episode of wheeze in a child less than 12 months);
children with atopic or multi‐trigger asthma defined by episodes of wheeze caused by a range of stimuli other than viruses;
children who wheeze with viral respiratory illnesses and have interval symptoms between viral infections.
Types of interventions
Participants must have been randomised to receive a LTRA, within the licensed dose range, as maintenance (treatment for > 2 months) or intermittent (treatment for < 14 days at the onset of a viral‐induced episode) therapy versus placebo.
We assessed the effects of LTRAs in three planned comparisons.
Maintenance (regular) LTRA therapy versus placebo.
Intermittent (episodic) LTRA therapy versus placebo.
Maintenance LTRA therapy versus intermittent LTRA therapy.
Types of outcome measures
The following categories of outcome measures were considered important in establishing whether LTRAs have a role to play in secondary prevention of EVW.
Primary outcomes
Number of children with one or more viral‐induced episodes requiring one or more treatments with rescue oral corticosteroids.
Secondary outcomes
Number of viral‐induced episodes requiring treatment with oral corticosteroids by treatment group (adjusting for the number of viral respiratory tract infections and the intraclass correlation for clustering of infections within individual children, if feasible).
-
Indicators of the severity of episodes.
Number of children with one or more viral‐induced episodes requiring one or more emergency department or emergency doctor visits.
Number of children with one or more viral‐induced episodes requiring hospital admission.
Group mean symptom scores during episodes (adjusting for the number of viral respiratory tract infections and the intraclass correlation for clustering of infections within individual children, if feasible).
Group mean use of rescue bronchodilator during episodes (adjusting for the number of viral respiratory tract infections and the intraclass correlation for clustering of infections within individual children, if feasible).
New prescription or increased dosage of inhaled corticosteroids or other maintenance add‐on therapy.
-
Indicators of duration of episodes by group (adjusting for the number of viral respiratory tract infections and the intraclass correlation for clustering of infections within individual children, if feasible).
Duration of lower respiratory tract symptoms (dyspnoea, cough and wheeze).
Duration of bronchodilator use.
Length of emergency department stay.
Length of hospital stay.
-
Adverse health events.
Patients with any, as well as specific, adverse effects (growth, headache, etc.).
-
Withdrawals.
Patients withdrawn for any reason, because of poor response and because of adverse health effects, respectively.
-
Indicators of frequency ‐ for maintenance treatment (adjusting for the number of viral respiratory tract infections and the intraclass correlation for clustering of infections within individual children, if feasible).
Frequency of EVW episodes.
Frequency of lower respiratory tract symptoms suggestive of asthma (dyspnoea, wheeze, cough).
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details) until the end of June 2015. All records in the CAGR coded as 'asthma' were searched using the following terms:
(wheez* or episodic or viral* or virus* or evw ) AND (leukotriene* or leukotriene* or ltra or anti‐leukotriene* or anti‐leucotriene* or anti‐leuk* or "anti leuk*" or anti‐leuc* or "anti leuc*" or *lukast or lukast* or montelukast* or singulair or zafirlukast* or accolate or pranlukast* or ultair)
We also conducted a search of ClinicalTrials.gov (www.clinicaltrials.gov/). All databases were searched from their inception to June 2015 and there was no restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished studies. We also contacted manufacturers and experts in the field.
Data collection and analysis
Selection of studies
Two review authors (MB and AG) from our team selected studies as being potentially relevant based on a review of the titles and abstracts, if available. The complete text of these studies was retrieved and reviewed independently by the same reviewers. Disagreement as to which papers to include was resolved by consensus. Where there was a lack of consensus, a third reviewer (MCM) determined the final decision. Full reports were obtained for trials appearing to meet the inclusion criteria or for which there was insufficient information in the title and abstract to make a clear decision.
Data extraction and management
Two review authors (MB and AG) independently extracted data using specially designed data extraction forms. Data extraction forms were pilot‐tested to ensure clarity, completeness and ease of use. The characteristics of the trial participants, interventions and outcomes for the included trials were presented in study tables. Authors were contacted for clarification or further information. We accepted data from groups of children who were eligible, as per our protocol, who were included within studies that covered a larger age span or involved patients (such as those with interim symptoms) who would have been excluded as per our protocol.
Assessment of risk of bias in included studies
Two review authors (MB and AG) independently assessed the design of eligible studies in terms of bias protection. Any discordance between the authors was discussed and resolved by consensus. Where there was lack of consensus, a third reviewer (MCM) determined the final decision. Where methodological issues were unclear, we attempted to contact the corresponding author of the original paper for clarification.
We identified and present information pertaining to the following potential sources of bias.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We provided judgements for each of these domains alongside the descriptions of study methodology using The Cochrane Collaboration’s tool for assessing risk of bias (Higgins 2011a).
Measures of treatment effect
Continuous data
Data for parallel group trials were expressed as mean difference (MD) and 95% confidence intervals (CI). We pooled data from crossover studies with generic inverse variance (GIV) (Higgins 2011b). For continuous data variables extracted from crossover studies (intermittent LTRA use only), we used the mean differences (MD) and estimated the standard errors (SE) based upon the published P value, or from 95% CI if available. Where these were not available, we used the published standard deviations (SDs) for the two groups to derive a SE.
A standardised mean difference (SMD) was calculated when studies had measured the same outcome but with different metrics (e.g. for pooling data from different symptom scores). For GIV outcomes reporting SD units, we expressed effect sizes in terms of the pooled standard deviation for each study (e.g. where the effect size for a given study is 0.5, this represented a mean difference between the treatment and control groups of half a pooled standard deviation as reported in that study).
Dichotomous data
For dichotomous variables (e.g. admission to hospital), we calculated relative risks as these are easier to interpret clinically. An odds ratio (OR) was calculated based on the event rate in the studies for the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH). NNTB & NNTH were calculated using an online statistical package (Visual Rx).
Data were pooled using a fixed‐effect model (FE), unless heterogeneity was identified (Deeks 2011).
Unit of analysis issues
The unit of analysis was the patient.
Dealing with missing data
We noticed in previous reviews that some papers do not report the spread of data in a format that allows meta‐analysis, for example, not reporting standard deviations. Where possible we used established techniques for estimating standard deviations or 95% CI in order for us to include all eligible studies in the analysis.
Where only one study presented data in a usable form, we entered the data into Review Manager 5.3 (RevMan 2014), but reported the statistics from the published paper.
Assessment of heterogeneity
We tested heterogeneity using the I2 statistic, which measures the extent of heterogeneity not attributable to the play of chance (Higgins 2003). Where the I2 statistic exceeds 30%, random‐effects modelling was applied in order to determine whether the pooled effect estimate was altered (Deeks 2011).
Assessment of reporting biases
We used funnel plots to assess the presence of publication bias for trials contributing data to the main outcomes (Egger 1997).
Data synthesis
We extracted data for trials and entered them into Review Manager Version 5.3 (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
If possible, we aimed to explore the effect of atopy as a subgroup analysis (atopy versus non atopy ‐ on a basis of two or more positive RAST tests or skin prick tests, > 3 mm wheal above control), and the impact of age groups (age one to three years vs. four to six years). We tested differences between subgroups with a test for interaction (Altman 2003). In addition, we reported the percentage of children with atopy in each trial and performed a sensitivity analysis, whereby trials where atopy exceeded 20% were excluded.
Sensitivity analysis
We performed a sensitivity analysis to examine the effect of methodological quality on the pooled estimate after removing studies that were deemed not to have optimally addressed the sources of bias detailed above. We also removed studies which reported incomplete data for the primary outcome in the review as a sensitivity analysis.
We planned to perform a sensitivity analysis whereby trials where atopy exceeded 20% were excluded.
Results
Description of studies
Please see Characteristics of included studies and Characteristics of excluded studies for further details.
Results of the search
We carried out the search in June 2015 and identified a total of 471 citations. Once duplicates were removed 408 references were screened. From these 23 references were examined in detail (see Characteristics of excluded studies) and five studies (10 references) met the inclusion criteria for the review (Bacharier 2008a; Bisgaard 2005; Nwokoro 2014 (EVW only); Robertson 2007; Valovirta 2011). Figure 1 provides details of the study search and assessment process.
1.
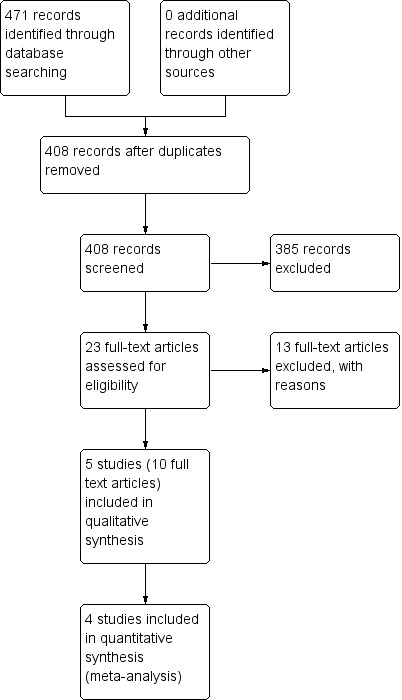
Study flow diagram.
Included studies
Two studies contributed data on maintenance treatment (Bisgaard 2005 (EVW only); Valovirta 2011) and four studies contributed data on intermittent LTRA treatment (Bacharier 2008a; Nwokoro 2014 (EVW only); Robertson 2007; Valovirta 2011). All were published as full text manuscripts.
Design
All of the studies were randomised, placebo‐controlled trials. Bisgaard 2005; Nwokoro 2014 (EVW only) and Robertson 2007 involved placebo and intermittent or maintenance LTRA (oral montelukast) treatment arms respectively. In the Valovirta 2011 study there were three groups; intermittent, maintenance and placebo treatment. The Bacharier 2008a study involved three groups, placebo, intermittent LTRA and intermittent budesonide; only data from the first two groups were included. The studies of intermittent therapy all involved parents or caregivers monitoring for symptoms in children and initiating treatment when an acute episode occurred. In all studies parents or caregivers were responsible for administering study medication to children.
Population
A total of 3741 participants were recruited to the included studies. The review protocol set out to investigate the evidence of benefit from maintenance or intermittent LTRA treatment versus placebo in children aged one to six years presenting with EVW.
One study included children aged from 2 to 14 years, but the study authors sent us outcome data for the number of participants experiencing one or more healthcare resource utilisation for children aged two to five years (Robertson 2007). In addition the Valovirta 2011 study included some infants aged six months to one year, although for several outcomes data were presented for children over two years of age only. The Bisgaard 2005 study included some children with interval symptoms. We contacted Merck and they provided the available specific data for participants exhibiting an EVW phenotype, with only intermittent symptoms (Bisgaard 2005 (EVW only)). In the Nwokoro 2014 study specific data were obtained from the authors for children with an EVW phenotype only (Nwokoro 2014 (EVW only)).
In our protocol we defined atopy as two or more positive radioallergosorbent (RAST) tests or skin prick tests (> 3 mm wheal above control). The included studies were examined for atopic features using a variety of methods. In the Bacharier 2008a study, an Asthma Predictive Index (API) was used to give each child a negative or positive API status to reflect atopic status. Around 60% of children in the study had a positive API. Similarly 55% of children in the Valovirta 2011 study were API positive. In the Bisgaard 2005 study around a third of children had a positive specific‐IgE test for dog dander, cat dander, cockroach or Alternaria alternata. Of note in the Robertson 2007 study, there was a relatively high incidence of participants with at least one allergic condition (seasonal or perennial rhinitis, allergic conjunctivitis or atopic dermatitis) and this differed significantly between LTRA treatment (80.4%) and placebo (65.7%) groups (P = 0.019).
Interventions
All five included studies compared oral montelukast (4 mg once daily under the age of six years, 5 mg once daily over the age of six years) to placebo. Four studies (Bacharier 2008a; Nwokoro 2014 (EVW only); Robertson 2007; Valovirta 2011) investigated intermittent use of montelukast during symptomatic episodes and two studies (Bisgaard 2005, Valovirta 2011) investigated maintenance treatment.
Outcomes
Outcome measures varied between all five studies such that the pooling of data was unfortunately limited (see Characteristics of included studies for more details).
Excluded studies
We excluded 13 studies and provide reasons for exclusion in the Characteristics of excluded studies section. In general studies were excluded because they included children with interval, multi‐trigger, 'asthma' type symptoms (rather than an EVW phenotype) or because they had not published primary data.
Risk of bias in included studies
A summary is provided in Figure 2 along with further details for individual studies in the Characteristics of included studies table.
2.
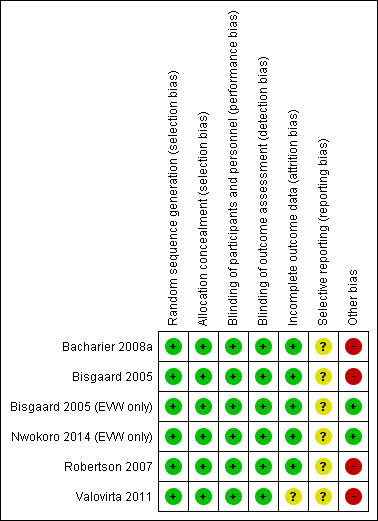
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All five included studies had low risk of allocation bias with adequate random sequence generation and allocation concealment. Notably however in the Robertson 2007 study, there was a statistically significant higher number of children in the montelukast group with rhinitis, eczema or at least one allergic condition than in the placebo group. These data were not available for the group of participants aged two to five years.
Blinding
All five included studies were double‐blind with low risk of performance (blinding of participants and personnel) and detection (blinding of outcome assessment) bias.
Incomplete outcome data
All studies reported loss to follow‐up and withdrawals, and performed an intention‐to‐treat analysis. In Bacharier 2008a there was a statistically significant higher number of dropouts (predominantly due to loss to follow‐up) in the montelukast (12.6%) compared to the placebo (2.1%) group (P = 0.04). In the Bisgaard 2005, Bacharier 2008a and Valovirta 2011 studies 91%, 95% and 83% respectively of children randomised completed the study, with similar proportions in the treatment and placebo groups.
Selective reporting
In all five included studies, there was insufficient information to permit a judgment about selective reporting (reporting bias).
Other potential sources of bias
No other sources of bias were identified.
Effects of interventions
Maintenance LTRA treatment
Primary outcome
Number of children with one or more viral‐induced episodes requiring treatment with oral corticosteroids
It was not possible to pool data for this outcome. The only trial reporting data was from the specific group of children with EVW (Bisgaard 2005 (EVW only)), n = 347, that participated in the Bisgaard 2005 study that was obtained directly from the pharmaceutical company. There was no statistically significant difference between maintenance montelukast versus placebo (OR 1.20, 95% CI 0.70 to 2.06, Analysis 1.1). Similarly there was no statistically significant difference between montelukast and placebo groups found for this outcome for all children (n = 549) randomised in the Bisgaard 2005 study (event rate/year of 0.53, 95% CI 0.40 to 0.70, vs. 0.64, 95% CI 0.47 to 0.88; relative rate reduction 0.82, 95% CI 0.54 to 1.25, rate reduction 17.5%, P = 0.368).
1.1. Analysis.
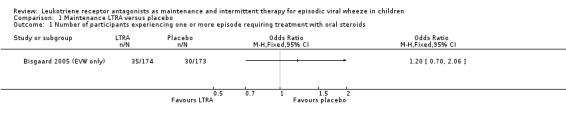
Comparison 1 Maintenance LTRA versus placebo, Outcome 1 Number of participants experiencing one or more episode requiring treatment with oral steroids.
Secondary outcomes
Number of participants experiencing one or more episodes requiring Emergency Department visit
It was not possible to pool data for this outcome. The only trial contributing data was the Bisgaard 2005 (EVW only) group (n = 347) where there was no statistically significant difference between maintenance montelukast treatment and placebo (OR 0.66, 95% CI 0.37 to 1.20, Analysis 1.2). This finding was similar to the conclusion drawn from all children (n = 549) randomised in the Bisgaard 2005 study, where data were not directly comparable.
1.2. Analysis.
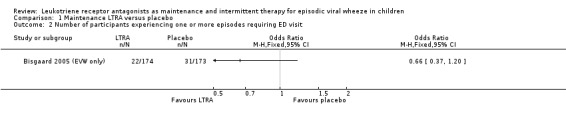
Comparison 1 Maintenance LTRA versus placebo, Outcome 2 Number of participants experiencing one or more episodes requiring ED visit.
Number of participants experiencing one or more episodes requiring hospital admission
It was not possible to pool data for this outcome. The only trial contributing data was the Bisgaard 2005 (EVW only), n = 347, group where there was no statistically significant difference between maintenance montelukast treatment and placebo (OR 0.65, 95% CI 0.23 to 1.87, Analysis 1.3). The rate of hospitalisation during the 12 month period was low across all children (n = 549) randomised in the Bisgaard 2005 study at 4.2% in the montelukast group and 5.8% in the placebo group. This finding was similar to the data, which were not in a directly comparable form, derived from all children randomised in the Bisgaard 2005 study.
1.3. Analysis.
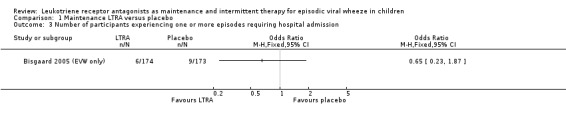
Comparison 1 Maintenance LTRA versus placebo, Outcome 3 Number of participants experiencing one or more episodes requiring hospital admission.
Number of participants experiencing one or more Healthcare Resource Utilisation
It was not possible to pool data for this outcome. Valovirta 2011 (n = 1100) was the only included study to report this outcome where there was no statistically significant difference from placebo (OR 0.85, 95% CI 0.68 to 1.07, Analysis 1.4).
1.4. Analysis.

Comparison 1 Maintenance LTRA versus placebo, Outcome 4 Number of participants experiencing one or more HRU.
Number of participants experiencing one or more episode
It was not possible to pool data for this outcome. The only data identified for this outcome were from the Bisgaard 2005 (EVW only) group, n = 347, that showed no statistically significant difference between maintenance montelukast treatment and placebo (OR 0.68, 95% CI 0.44 to 1.05, Analysis 1.5). This finding was similar to the conclusion drawn from all children (n = 549) randomised in the Bisgaard 2005 study, where data were not directly comparable
1.5. Analysis.

Comparison 1 Maintenance LTRA versus placebo, Outcome 5 Number of participants experiencing one or more asthma exacerbation episodes.
Mean symptom scores during episodes
It was not possible to pool data for this outcome. Valovirta 2011 (n = 1100) reported a composite score derived from the daily average symptom scores for wheeze, difficulty breathing, daytime cough and interference with daily activity during episodes for maintenance LTRA treatment in a post hoc analysis of children aged more than two years with at least one episode. The maximum symptom score possible was 14 and minimum score 0. There was a small improvement in symptom scores in the maintenance (MD ‐0.12, 95% CI ‐0.24 to 0, Analysis 1.6) treatment arm compared to placebo that reached statistical significance (P = 0.045).
1.6. Analysis.

Comparison 1 Maintenance LTRA versus placebo, Outcome 6 Symptoms during episode.
Mean use of rescue bronchodilator during episodes
It was not possible to pool data for this outcome. Valovirta 2011 (n = 1100) reported a small reduction in the least squares mean number of times per day a beta2‐agonist was used during episodes. The reduction was statistically lower for the maintenance (2.14, 95% CI 1.94 to 2.34) montelukast versus placebo (2.42, 95% CI 2.22 to 2.62) groups, (MD ‐0.29, 95% CI ‐0.58 to 0.00, P = 0.048, Analysis 1.7).
1.7. Analysis.
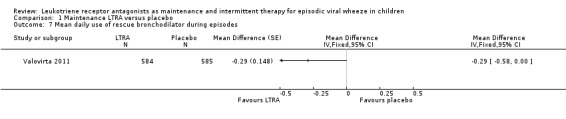
Comparison 1 Maintenance LTRA versus placebo, Outcome 7 Mean daily use of rescue bronchodilator during episodes.
New prescription or increased dose of inhaled corticosteroids or other maintenance add‐on therapy
No data were identified in the included studies relating to this outcome.
Duration of episodes
No data were identified in the included studies relating to this outcome.
Adverse health events
Included studies varied in the reporting of adverse effects and we chose to report these in a descriptive fashion (Table 3). No serious adverse effects were identified.
1. Reported adverse health events.
| Study | Reported adverse health events |
| Bacharier 2008a | Not specified. |
| Bisgaard 2005 | Proportion of drug‐related adverse experiences was similar in each group: 5% of patients in the montelukast group and 4.1% in the placebo group experienced at least one adverse event. There was one accidental overdose of montelukast that resulted in vomiting, after which the patient fully recovered. No patients in montelukast group discontinued because of an adverse experience considered by an investigator to be drug‐related. |
| Nwokoro 2014 (EVW only) | Data only available for study as a whole (Nwokoro 2014). Only one serious adverse event was recorded, a skin reaction in the placebo group. Proportion of adverse events was similar between montelukast and placebo groups. |
| Robertson 2007 | Proportion of possibly/probably drug‐related adverse experiences was similar in each group: 12.4% in the montelukast group and 11.4% in the placebo group. No serious drug‐related adverse experiences were reported. |
| Valovirta 2011 | Proportion of drug‐related adverse experiences was similar in each group and not felt to be "clinically meaningful": 0.5% of patients in the maintenance montelukast group; 1% in the intermittent montelukast group; and 0.9% in the placebo group experienced at least one adverse event. Three serious adverse effects were considered by the investigator to be drug‐related: pneumonia and asthma in a child receiving placebo and somnolence associated with overdose in a child receiving montelukast. |
Withdrawals
There was no statistically significant difference in the number of withdrawals between the placebo and intervention (maintenance LTRA) groups (Bisgaard 2005, Valovirta 2011) (OR 0.87, 95% CI 0.66 to 1.13, n = 1729, Analysis 1.8).
1.8. Analysis.

Comparison 1 Maintenance LTRA versus placebo, Outcome 8 Withdrawals.
Intermittent LTRA treatment
Primary outcome
Number of children with one or more viral‐induced episodes requiring treatment with oral corticosteroids
Pooling of data from two studies (Bacharier 2008a, Robertson 2007), showed no statistically significant reduction in the number of episodes requiring rescue oral steroids between intermittent montelukast and placebo (RR 0.85, 95% CI 0.64 to 1.14, n = 343, Analysis 2.1).
2.1. Analysis.
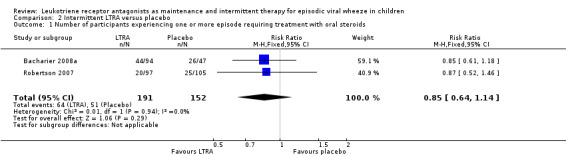
Comparison 2 Intermittent LTRA versus placebo, Outcome 1 Number of participants experiencing one or more episode requiring treatment with oral steroids.
Secondary outcomes
Number of participants experiencing one or more episodes requiring Emergency Department visit
It was not possible to pool data for this outcome. The individual study by Bacharier 2008a (n = 238) found no apparent beneficial effect (OR 1.00, 95% CI 0.49 to 2.02, Analysis 2.2). A similar outcome was reported by Robertson 2007 (n = 220) in terms of the number of treated episodes with at least one Healthcare Resouce Utilisation for asthma requiring Emergency Department visit (Analysis 2.3).
2.2. Analysis.
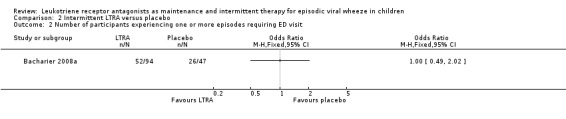
Comparison 2 Intermittent LTRA versus placebo, Outcome 2 Number of participants experiencing one or more episodes requiring ED visit.
2.3. Analysis.
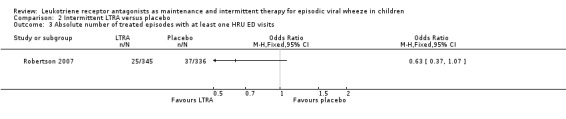
Comparison 2 Intermittent LTRA versus placebo, Outcome 3 Absolute number of treated episodes with at least one HRU ED visits.
Number of participants experiencing one or more episodes requiring hospital admission
It was not possible to pool data for this outcome. In the individual Bacharier 2008a (n = 238) study there was no statistically significant reduction in admissions in the intermittent LTRA compared to placebo group (OR 0.73, 95% CI 0.20 to 2.73, Analysis 2.4). A similar outcome was reported by Robertson 2007, n = 220, in terms of the number of treated episodes with at least one Healthcare Resource Utilisation for asthma requiring hospital admission (OR 0.74, 95% CI 0.32 to 1.72, Analysis 2.5).
2.4. Analysis.
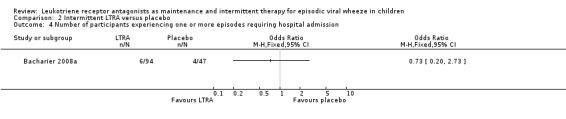
Comparison 2 Intermittent LTRA versus placebo, Outcome 4 Number of participants experiencing one or more episodes requiring hospital admission.
2.5. Analysis.

Comparison 2 Intermittent LTRA versus placebo, Outcome 5 Absolute number of treated episodes with at least one HRU hospital admissions.
Number of participants experiencing one or more Healthcare Resource Utilisation
It was not possible to pool data for this outcome. Valovirta 2011 (n = 1102) was the only included study to report this outcome where there was a non‐significant difference from placebo (OR 0.96, 95% CI 0.76 to 1.21, Analysis 2.6).
2.6. Analysis.

Comparison 2 Intermittent LTRA versus placebo, Outcome 6 Number of participants experiencing one or more HRU.
Data for a similar outcome were obtained from the authors of Robertson 2007 for children aged two to five years, n = 176, in terms of number of episodes treated with study medication requiring healthcare resource utilisation that showed a significant benefit with intermittent montelukast versus placebo (OR 0.62, 95% CI 0.44 to 0.88).
Rate of unscheduled medical attendances with wheeze
It was not possible to pool data for this outcome. In specific group data for children with an EVW phenotype in the Nwokoro 2014 (EVW only) study, n = 964, there was a statistically significant reduction in the risk ratio comparing intermittent montelukast to placebo (RR 0.83, 95% CI 0.71 to 0.98, P = 0.03, Analysis 2.8).
2.8. Analysis.
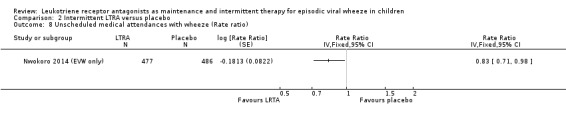
Comparison 2 Intermittent LTRA versus placebo, Outcome 8 Unscheduled medical attendances with wheeze (Rate ratio).
Mean symptom scores during episodes
It was not possible to pool data for this outcome. Valovirta 2011 (n = 1,100) reported a composite score derived from the daily average symptom scores for wheeze, difficulty breathing, daytime cough and interference with daily activity during episodes for intermittent LTRA treatment in a post hoc analysis of children aged more than two years with at least one episode. Maximum symptom score possible was 14 with minimum being 0. There was a small statistically significant improvement in symptom scores in the intermittent LTRA arm compared to placebo (MD ‐0.11, 95% CI ‐0.23 to 0, P = 0.061, Analysis 2.7).
2.7. Analysis.
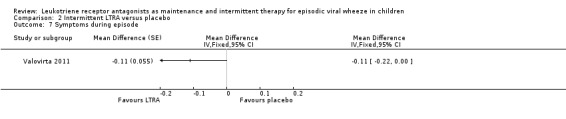
Comparison 2 Intermittent LTRA versus placebo, Outcome 7 Symptoms during episode.
In a broader age group Robertson 2007, n = 220, reported median symptom scores from diary cards for montelukast treatment during episodes compared to placebo and found a modest but statistically significant reduction in the median total symptom score in the montelukast group (37, interquartile range, IQR, 19 to 62) compared to placebo (43, IQR 22 to 73), P = 0.049.
The inability to obtain mean score and standard deviation prevented pooling of data of Robertson 2007 with that of Valovirta 2011.
Mean use of rescue bronchodilator during episodes
It was not possible to pool data for this outcome. Valovirta 2011 (n = 1100) found a small reduction in terms of the mean number of times per day a beta2‐agonist was used during episodes that was statistically significant for intermittent montelukast vs. placebo (MD ‐0.31, 95% CI ‐0.59 to ‐0.03, P = 0.028, Analysis 2.9). In the Robertson 2007, n = 220, study, there was no difference in the cumulative number of puffs of beta2‐agonist used per episode (median of 36 puffs for montelukast and placebo groups).
2.9. Analysis.

Comparison 2 Intermittent LTRA versus placebo, Outcome 9 Mean daily use of rescue bronchodilator during episodes.
New prescription or increased dose of inhaled corticosteroids or other maintenance add‐on therapy
No data were identified in the included studies relating to this outcome.
Duration of episodes
No significant group difference was observed in median duration of episodes by Robertson 2007, n = 220, for intermittent montelukast (6.5 days, IQR 4 to 10) versus placebo (7 days, IQR 4 to 10), P = 0.3.
Adverse health events
The included studies varied in reporting of adverse effects and we chose to report these in a descriptive fashion (Table 3). No serious adverse effects were identified.
Withdrawals
There was no significant difference between the intermittent LTRA and placebo groups (OR 1.03, 95% CI 0.79 to 1.35, n = 1402, Analysis 2.12). While it was not possible to add the data for the Bacharier 2008a study of intermittent LTRA vs. placebo due to differences in reporting, there was no statistically significant difference between the placebo and montelukast group for drop outs (OR 6.65, 95% CI 0.84 to 52.79) or treatment failures (OR 0.99, 95% CI 0.37 to 2.64) in that study (n = 238). There were also no data available for the specific EVW phenotype group in the Nwokoro 2014 (EVW only) study, however withdrawals were similar between montelukast and placebo across the whole study.
2.12. Analysis.
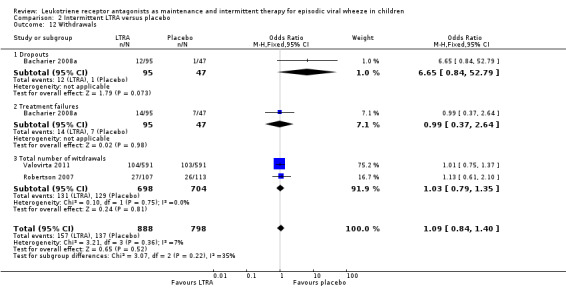
Comparison 2 Intermittent LTRA versus placebo, Outcome 12 Withdrawals.
Maintenance vs. intermittent LTRA treatment
It was only possible to include data from the Valovirta 2011 (n = 1,102) study for this comparison.
Primary outcome
Number of children with one or more viral‐induced episodes requiring treatment with oral corticosteroids
No data were available relating to this outcome.
Secondary outcomes
Number of participants experiencing one or more episodes requiring Emergency Department visit
No data were available relating to this outcome.
Number of participants experiencing one or more episodes requiring hospital admission
No data were available relating to this outcome.
Number of participants experiencing one or more Healthcare Resource Utilisation
There was no significant difference between maintenance and intermittent treatment groups for this outcome (OR 0.88, 95% CI 0.70 to 1.11, Analysis 3.1), n = 1102.
3.1. Analysis.

Comparison 3 Maintenance LTRA vs. intermittent LTRA, Outcome 1 Number of participants experiencing one or more HRU.
Mean symptom scores during episodes
There was no significant difference between maintenance and intermittent treatment groups for this outcome (MD ‐0.01, 95% CI ‐0.12 to 0.1, P = 0.86, Analysis 3.2), n = 1102.
3.2. Analysis.

Comparison 3 Maintenance LTRA vs. intermittent LTRA, Outcome 2 Symptoms during episode.
Mean use of rescue bronchodilator during episodes
There was no significant difference between maintenance and intermittent treatment groups for this outcome (MD 0.03, 95% CI ‐0.24 to 0.30, P = 0.83, Analysis 3.3), n = 1102.
3.3. Analysis.

Comparison 3 Maintenance LTRA vs. intermittent LTRA, Outcome 3 Mean daily use of rescue bronchodilator during episodes.
New prescription or increased dose of inhaled corticosteroids or other maintenance add‐on therapy
No data were available relating to this outcome.
Duration of episodes
No data were available relating to this outcome.
Adverse health events
No data were available relating to this outcome.
Withdrawals
There was no significant difference between maintenance and intermittent treatment groups for this outcome (OR 0.95, 95% CI 0.70 to 1.28, Analysis 3.4), n = 1102.
3.4. Analysis.

Comparison 3 Maintenance LTRA vs. intermittent LTRA, Outcome 4 Total number of withdrawals.
Discussion
Summary of main results
This systematic review examined the available evidence for the effectiveness of maintenance or intermittent LTRA treatment in children aged one to six years with EVW published up to June 2015. We included five studies in the review with a total of 3741 participants. Two studies contributed data on maintenance treatment (Bisgaard 2005 (EVW only), Valovirta 2011) and four studies contributed data on intermittent LTRA treatment (Bacharier 2008a, Nwokoro 2014 (EVW only), Robertson 2007 and Valovirta 2011).
For maintenance LTRA, we failed to find evidence of clinical benefit for the primary outcome of the review, that is, a reduction in the number of children with one or more viral‐induced episodes requiring treatment with oral corticosteroids. The only data identified for this outcome were from the group of children with EVW (Bisgaard 2005 (EVW only)) that were included in the Bisgaard 2005 study; however findings on the specific group analysis are concordant with that of all randomised children, irrespective of phenotype. Furthermore, no other statistically significant differences were identified between maintenance treatment and placebo groups for any of the secondary outcomes examined. It is important to note that, with only two trials contributing data, the power was limited, and because of differences in outcome selection and reporting between studies, pooling was seldom possible. In the study by Valovirta 2011, maintenance montelukast treatment was associated with a statistically significant, yet clinically modest, reduction in the severity of symptoms derived from a composite score (daily average of wheeze, difficulty breathing, daytime cough and interference with daily living score; maximum score 14) during wheezing episodes compared to placebo. In the Bisgaard 2005 study, there was a statistically significant reduction in the rate of exacerbations (all severity) in the maintenance montelukast versus placebo groups.
We also found no evidence of clinical benefit for intermittent LTRA treatment for the primary outcome of the review, a reduction in the number of children with one or more viral‐induced episodes requiring treatment with oral corticosteroids. This was based on pooled data from two studies (Bacharier 2008a, Robertson 2007). Specific group data obtained from the authors of the Nwokoro 2014 study for children with an EVW phenotype (Nwokoro 2014 (EVW only)) showed a small but statistically significant reduction in the rate ratio of unscheduled medical attendances with wheeze in the intermittent montelukast versus placebo group. There were no statistically significant differences found between intermittent LTRA treatment and placebo for any other secondary outcomes; yet, with the exception of withdrawals, only one trial contributed to each outcome; as our ability to pool data from two or more trials was limited by differences in outcome selection and reporting between studies. In two trials (Bacharier 2008a and Robertson 2007) there was a statistically significant reduction in the severity of symptoms during episodes favouring intermittent LTRA over placebo, but the data could not be pooled due to reporting differences; yet each reduction was quite modest and of questionable clinical importance (Bacharier 2008a: trouble breathing score, area under curve, for 14 days after initiation of therapy and interference with activity score and in Robertson 2007 total diary card symptom score).
The comparison between maintenance and intermittent LTRA treatment was limited by data being contributed from only one study (Valovirta 2011). No data were identified for the primary outcome of the review. We found no evidence of any statistically significant differences between maintenance and intermittent LTRA treatment for the following secondary outcomes of this review: participants experiencing one or more healthcare resource utilisation, symptoms during episodes, mean daily use of bronchodilator during episodes or total number of withdrawals.
The included studies varied in the reporting of adverse effects and we chose to report these in a descriptive fashion. No serious adverse effects were noted, however. In keeping with the wider literature and clinical experience, LTRA treatment was generally well tolerated compared to placebo in included studies, but firm conclusions cannot be drawn due to the paucity of data.
Overall completeness and applicability of evidence
Each study included in the review involved montelukast but differed in terms of the choice and reporting of outcomes, which limited our ability to pool data between two or more studies, including for the main outcome in all three comparisons.
The biggest threat to the validity of the results arises from the possibility that some studies may have included children with multi‐trigger wheeze rather than children with only isolated EVW. The broad phenotypes of children who wheeze (EVW versus multi‐trigger atopic asthma) may be particularly challenging to differentiate in the pre‐school age group, furthermore distinctions are not fixed and phenotypes may vary over time (Bush 2009, Ducharme 2012). We tried to avoid this by careful inclusion of only studies focusing on EVW involving children free of symptoms between episodes. However this did not eliminate the presence of children with other atopic conditions in the studies and it is possible that such children were more likely to have had unrecognised multi‐trigger atopic asthma. For example in the Robertson 2007 study, 73% of participants had at least one allergic condition (seasonal or perennial rhinitis, allergic conjunctivitis or atopic dermatitis). For the Bisgaard 2005 study, we obtained unpublished group data specifically relating to carefully characterised children with only intermittent symptoms (Bisgaard 2005 (EVW only)). These data were not available for all of the outcomes reported in the primary paper, however where comparisons were possible, results were similar between all of the children randomised in the Bisgaard 2005 study and those just with apparent EVW (Bisgaard 2005 (EVW only)). Similarly we obtained group data for children with a specific EVW phenotype in the Nwokoro 2014 (EVW only) study of intermittent montelukast, however this trial contributed to one outcome, namely the rate of unscheduled medical attendance with wheeze.
In our protocol, we set out to include only studies involving children with EVW aged one to six years. The Valovirta 2011 study included some infants aged between six months and one year and the Robertson 2007 study included some older children, although we did obtain data from the authors for children aged two to five years old for the number of participants experiencing one or more healthcare resource utilisation.
In summary, limitations of the evidence as it stands presently include the relatively small number of studies, potential heterogeneity in the phenotype of participants within and across trials, and differences in selection and reporting of outcomes between studies that seriously limited our ability to pool data for most outcomes. Overall however, the included studies arguably involved the sort of pre‐school children who wheeze with colds that clinicians encounter on a daily basis in their clinics and emergency departments.
In the review protocol, we set out to perform subgroup analyses of atopic children (defined as two or more positive RAST tests or skin prick tests > 3 mm wheal above control) and also children aged one to three years and four to six years respectively. Due to incomplete reporting, these subgroup analyses were not performed. Instead, several studies reported the Asthma Predictive Index (API) of their patients; the API includes atopy as one of its criteria but a child may have a positive API index without meeting our protocol definition of atopy. In the Bacharier 2008a study, which enrolled children with a negative or positive API status, greater symptomatic benefit from episodic montelukast use was observed in children with a positive API status in a subgroup analysis. In the Bisgaard 2005 study of maintenance montelukast treatment, no significant subgroup interaction was found for children with atopic features (atopic dermatitis, allergic rhinitis, raised eosinophil count or > 1 positive RAST) on the primary outcome of exacerbation rate; these data were not available for the Bisgaard 2005 (EVW only) group. In the study by Valovirta 2011 children were also categorised for API status at entry, but outcome data was not provided by API subgroups. Robertson 2007 found no significant differences in their primary end‐point (healthcare resource utilisation) for a number of pre‐defined subgroup analyses relating to atopic status (history of rhinitis, family history of asthma or IgE > 130 units/mL) in their study of intermittent montelukast versus placebo. These data were not available for children aged two to five in the Robertson 2007 study. In summary, the current data would suggest that markers of atopy (including API) did not appear to modify the magnitude of effect of LTRA (which was minimal or absent in the study group).
We were also unable to identify any reported data on a number of secondary outcomes of clinical interest, for example increase in inhaled corticosteroid dose or other maintenance add‐on therapy.
Quality of the evidence
The five included studies were of good methodological quality, in that they were all randomised, double‐blind, placebo‐controlled trials with a low risk of bias in terms of random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), and outcome assessment (detection bias). The percentage of children randomised who completed the studies ranged from 83% to 95% and an intention‐to‐treat analysis was performed in all studies. In all five studies, there was insufficient information to permit a judgment about selective reporting (reporting bias).
Data from specific groups of participants from three studies were selected in the review, which may have limited the benefits of randomisation.
As outlined above in the Overall completeness and applicability of evidence section, there are other potential sources of bias due to the characteristics of the participants in some of the included studies. This included an increased proportion of males in the montelukast group in the Bacharier 2008a study, increased proportion of children with an atopic condition in the montelukast compared to the placebo groups in the Robertson 2007 study, and the inclusion of some children with interval symptoms (37% of participants) in the Bisgaard 2005 study. In addition, the Valovirta 2011 study included some infants (16% of participants) aged six to 12 months and the Robertson 2007 study some children (20% of participants) aged six to 14 years. In the case of Bisgaard 2005, we obtained specific group data as far as was available for the 63% of children that participated with an apparently pure EVW phenotype (Bisgaard 2005 (EVW only)) and the authors of Robertson 2007 also provided us with the number of participants experiencing one or more healthcare resource utilisation for the subset of children aged two to five years representing 80% of their randomised participants. The authors of the Nwokoro 2014 (EVW only) study provided us with specific group data for children with an EVW phenotype representing 71% of their randomised children. We aggregated data in children aged one to six years with seemingly intermittent wheeze induced by viral respiratory tract infections, but we cannot firmly confirm whether there might still be contamination with children with multi‐trigger wheeze with or without atopic symptoms, in part due to evolving definitions of persistent asthma over the past decade when the trials were conducted.
In other words, the quality of the data was good, although the strength of the evidence was limited by the paucity of trials comparing maintenance (or intermittent) LTRA to placebo with only one small trial with head‐to‐head comparison between maintenance and intermittent LTRA. Moreover, in two of these three comparisons, there was no, or only one, trial reporting our a priori primary outcome, thus preventing aggregation. Overall where comparisons were possible the GRADE classification (Schünemann 2011) was judged to be moderate (see Table 1 and Table 2).
Potential biases in the review process
This systematic review addressed a focused research question and used pre‐specified inclusion criteria and methodology to search, select, appraise and analyse data from eligible studies. We attempted, throughout the review process, to minimise any potential biases, but as with all systematic reviews, the possibility of publication bias should be considered as a potential threat to validity. We followed the review process recommended by the Cochrane Airway Group strictly, however and this included a rigorous trial search strategy and a check of reference lists of all primary studies and review articles for any additional references. We also contacted authors of identified trials and manufacturers and experts in the field, to ask them to identify other published and unpublished studies, hence we believe that the risk of such a bias affecting the results has been minimised. Multiple individuals were involved at each stage of the process with a consensus being reached where necessary, further limiting subjectivity and errors.
Due to the limited number of studies included in the systematic review, few outcomes aggregated the data from two or more trials, thus limiting the power to detect a clinically important group difference.
The choice of the primary outcome of this systematic review (the number of children with one or more episodes requiring treatment with rescue oral corticosteroids) may be challenged. Several studies have questioned this therapy in children with EVW, although the reasons for apparent non‐effectiveness may be due to misclassification in the diagnosis (e.g. inclusion of bronchiolitis) or studies exploring this approach as preventative use of oral steroids at the onset of a cold rather than the therapeutic use by a physician for a moderate or severe exacerbation (Guilbert 2011). However, perhaps pre‐school viral wheeze may be less responsive to oral steroids than in older children, this is currently being examined in a large cohort study of children with moderate and severe asthma (NCT: 02013076). Future findings may or may not eventually challenge this practice in the acute management of children with moderate or severe exacerbations (Panickar 2009, Bush 2009 and Guilbert 2011). Nevertheless, the intention to use rescue oral steroids remains a recognised marker of a moderate or severe exacerbation targeted by our primary outcome. The findings derived from our primary outcome are supported by the secondary outcomes of the review largely showing no evidence of treatment benefit.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review of LTRA in the management of EVW.
Although the findings of included studies of LTRAs in EVW are not easy to compare because of differences in their selection of the main outcome, they are nonetheless broadly consistent with the conclusions of this systematic review. Bisgaard 2005 in the PREvention of Viral Induced Asthma (PREVIA) study found that daily administered montelukast over 12 months reduced the primary endpoint of their trial namely, the number of asthma exacerbations of any severity, by approximately 32% when compared with placebo; however, it did not reduce the need for rescue oral steroids. Montelukast was also associated with a significantly longer time to first exacerbation (any severity) and reduced the overall rate of rescue ICS usage, suggesting some efficacy with regard to mild exacerbations in this mixed population of children with persistent and EVW. Robertson 2007 concluded that episode‐driven montelukast for seven days or until 48 hours after the resolution of symptoms resulted in a significant reduction in healthcare resource utilisation (primary care visits and emergency department visits), missed school or work days, and improved symptom scores. However, there was no significant effect on bronchodilator or rescue oral prednisolone use, again suggesting a benefit for children with mild exacerbations in this mixed population of preschool and school‐aged children with persistent and EVW. Bacharier 2008a in the Acute Intervention Management Strategies (AIMS) study, showed that episodic montelukast was not significantly better than placebo when added to rescue salbutamol for their primary outcome of episode‐free days or for rescue oral steroids over a one‐year study period. However, montelukast significantly improved symptoms and activity scores during exacerbations. Interestingly, children with positive API scores appeared to experience a greater clinical benefit from intermittent montelukast than those with negative API scores, but this is derived from a subgroup analysis and must thus be interpreted with caution. Valovirta 2011 found that neither daily nor intermittent montelukast reduced the number of episodes culminating in an asthma attack (primary outcome). Maintenance montelukast treatment was associated with a reduction in symptoms over the 12‐day treatment period during episodes. Both intermittent and daily treatment was associated with reduced beta‐agonist use. In the Valovirta 2011 study, children with a positive API responded better to montelukast. In summary, as discussed above, the presence of children with a positive API in many of the included studies suggests possible misclassification of children as EVW who actually had undiagnosed multi‐trigger wheeze or atopic asthma. In the Nwokoro 2014 (WAIT) study of intermittent montelukast, there was no significant difference in unscheduled medical attendances for wheezing episodes between montelukast and placebo groups (mean 2.0 [SD 2.6] vs. 2.3 [2.7], incident rate ratio 0.88 [95% CI 0.77 to 1.01], P = 0.06) overall. Interestingly there was a statistically significant benefit detected in the sub‐stratum of children with 5/5 ALOX5 promoter genotype suggesting that these children may represent a montelukast‐responsive group. We have also identified a statistically significant reduction in the specific group of children with an EVW phenotype. These reviews and independent trials may suggest that daily or intermittent montelukast may be modestly effective for the prevention of mild, but less so for moderate or severe, exacerbations.
Authors' conclusions
Implications for practice.
Acute episodes of EVW in pre‐school children are common, are associated with significant morbidity, and place a substantial burden on healthcare resources and society as a whole. Despite well designed trials, there is little available evidence of significant clinical benefit from either maintenance or intermittent LTRA therapy compared to placebo in pre‐school children with EVW. Therefore until further data are available, LTRA should be used with caution in individual children with EVW. When used, a therapeutic trial is suggested during which efficacy should be carefully monitored.
Implications for research.
It is likely that children with an apparent EVW phenotype are not a homogeneous group and that subgroups could respond to specific therapies dependent on the exact patho‐physiological mechanisms involved. In light of the results of this systematic review, a number of unanswered research questions remain that should be addressed by future high‐quality studies.
Careful phenotyping or genotyping or both of children, using consistent and agreed criteria, entering future studies is essential.
Participants in studies should be followed up prospectively for a longer period (12 months or more) to document response to therapy and ascertain the evolution of different wheezing phenotypes.
Head‐to‐head comparisons between intermittent and maintenance LTRA and between intermittent LTRA versus intermittent inhaled corticosteroids should be studied. Stratified randomisation and reporting on those with moderate and severe episodes (previously requiring rescue oral steroids or hospital admission) versus those with mild wheezing episodes (not previously requiring rescue oral steroids or hospital admission) should be considered to clarify the impact in children with different severity of episodes.
Consideration should be given to what are the most clinically relevant outcome measures in trials of interventions for children with EVW depending on their prior severity. For example, it is arguable that the use of rescue oral corticosteroids may not be the most appropriate primary outcome measure given current debate about their efficacy in EVW. Other measures such as use of bronchodilators, symptom scores, healthcare resource utilisation or time off school or nursery for children or off work for parents or caregivers should be considered.
Identification of subgroups of children who are most likely to respond to LTRA therapy. Cluster analysis of large, routinely collected data sets may also help to identify characteristics of children who respond to LTRA treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 26 June 2019 | Amended | This review was not compliant with the conflict of interest policy at the time of publication. An explanation has been added to Published notes. |
Notes
This review was not compliant with the conflict of interest policy at the time of publication. The review was not compliant because three out of six authors (CER‐M, JAC‐R, FMD) received funds for speaking/lecturing and travel/accomodation from a pharmaceutical company who manufactured a drug included in the review or a competing drug.
We have no current plans to update this review at present (June 2019). If this review is prioritised for updating, Cochrane Airways will seek a team that is compliant with policy.
Acknowledgements
Thanks to Toby Lasserson, Emma Welsh and Chris Cates for invaluable help with the review process. Thank you to Merck, Hans Bisgaard, Colin Robertson, David Price and Jonathan Grigg who facilitated and provided access to unpublished specific group data.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | weekly |
| EMBASE (Ovid) | weekly |
| CENTRAL (the Cochrane Library) | Quarterly (4 issues per year) |
| PSYCHINFO (Ovid) | Monthly |
| CINAHL (Ebsco) | Monthly |
| AMED (Ebsco) | Monthly |
Hand‐searches: Core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE Search strategy used to identify trials for the CAGR
Condition search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
17. exp Aspergillosis, Allergic Bronchopulmonary/
18. lung diseases, fungal/
19. aspergillosis/
20. 18 and 19
21. (bronchopulmonar$ adj3 aspergillosis).mp.
22. 17 or 20 or 21
23. 16 or 22
24. Lung Diseases, Obstructive/
25. exp Pulmonary Disease, Chronic Obstructive/
26. emphysema$.mp.
27. (chronic$ adj3 bronchiti$).mp.
28. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
29. COPD.mp.
30. COAD.mp.
31. COBD.mp.
32. AECB.mp.
33. or/24‐32
34. exp Bronchiectasis/
35. bronchiect$.mp.
36. bronchoect$.mp.
37. kartagener$.mp.
38. (ciliary adj3 dyskinesia).mp.
39. (bronchial$ adj3 dilat$).mp.
40. or/34‐39
41. exp Sleep Apnea Syndromes/
42. (sleep$ adj3 (apnoea$ or apnoea$)).mp.
43. (hypopnea$ or hypopnoea$).mp.
44. OSA.mp.
45. SHS.mp.
46. OSAHS.mp.
47. or/41‐46
48. Lung Diseases, Interstitial/
49. Pulmonary Fibrosis/
50. Sarcoidosis, Pulmonary/
51. (interstitial$ adj3 (lung$ or disease$ or pneumon$)).mp.
52. ((pulmonary$ or lung$ or alveoli$) adj3 (fibros$ or fibrot$)).mp.
53. ((pulmonary$ or lung$) adj3 (sarcoid$ or granulom$)).mp.
54. or/48‐53
55. 23 or 33 or 40 or 47 or 54
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Data and analyses
Comparison 1. Maintenance LTRA versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants experiencing one or more episode requiring treatment with oral steroids | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Number of participants experiencing one or more episodes requiring ED visit | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Number of participants experiencing one or more episodes requiring hospital admission | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Number of participants experiencing one or more HRU | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Number of participants experiencing one or more asthma exacerbation episodes | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Symptoms during episode | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Mean daily use of rescue bronchodilator during episodes | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8 Withdrawals | 2 | 1729 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.66, 1.13] |
Comparison 2. Intermittent LTRA versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants experiencing one or more episode requiring treatment with oral steroids | 2 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.64, 1.14] |
| 2 Number of participants experiencing one or more episodes requiring ED visit | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Absolute number of treated episodes with at least one HRU ED visits | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Number of participants experiencing one or more episodes requiring hospital admission | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Absolute number of treated episodes with at least one HRU hospital admissions | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Number of participants experiencing one or more HRU | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Symptoms during episode | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8 Unscheduled medical attendances with wheeze (Rate ratio) | 1 | Rate Ratio (Fixed, 95% CI) | Totals not selected | |
| 9 Mean daily use of rescue bronchodilator during episodes | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 10 Days of OCS use per participant | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Absolute number of treated episodes with at least one HRU GP visits | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Withdrawals | 3 | 1686 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.84, 1.40] |
| 12.1 Dropouts | 1 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.65 [0.84, 52.79] |
| 12.2 Treatment failures | 1 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.37, 2.64] |
| 12.3 Total number of witdrawals | 2 | 1402 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
2.10. Analysis.
Comparison 2 Intermittent LTRA versus placebo, Outcome 10 Days of OCS use per participant.
2.11. Analysis.

Comparison 2 Intermittent LTRA versus placebo, Outcome 11 Absolute number of treated episodes with at least one HRU GP visits.
Comparison 3. Maintenance LTRA vs. intermittent LTRA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants experiencing one or more HRU | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Symptoms during episode | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 Mean daily use of rescue bronchodilator during episodes | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 Total number of withdrawals | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bacharier 2008a.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | N = 238 randomised (95 montelukast, 47 conventional therapy, 96 budesonide), 351 assessed for eligibility. Children aged 12 to 59 months with moderate‐to‐severe intermittent wheezing. | |
| Interventions | At each respiratory tract illness: 7 days of montelukast 4 mg daily, placebo in addition to conventional treatment or budesonide inhalation suspension 1 mg twice daily. Study duration: 12 months. Two‐week run‐in period of observation with parent‐completed diary cards to assess symptoms and medication use. | |
| Outcomes | Primary outcome: proportion of episode‐free days. Secondary outcomes: severity of lower respiratory tract symptoms (area under the curve for symptom scores); time to initiation of first course of oral corticosteroids; total number of oral corticosteroid courses; number of wheezing episodes; number of days missed from day care and parental work; caregiver quality of life; number of unscheduled visits for acute wheezing episodes (primary care office, urgent care and emergency department/hospitalisation); linear growth. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Clinic staff received a drug kit assignment for eligible participants through an automated, Web‐based randomization application, which maintained the double blinding." |
| Allocation concealment (selection bias) | Low risk | Web‐based randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participant and study staff guesses at treatment assignments revealed no evidence of unblinding." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinding maintained. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three groups were significantly different with respect to dropout rate, intention‐to‐treat analysis performed resulting in minimal effect. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | High risk | Potential bias from significantly increased proportion of males in Montelukast and Budesonide groups. |
Bisgaard 2005.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | N = 549 randomised (278 montelukast and 271 placebo), 768 assessed for eligibility. Children aged 2 to 5 years with a history of intermittent asthma symptoms. | |
| Interventions | Daily montelukast (4 mg aged < 6 years, 5 mg aged > 6 years). Study duration: 48 weeks. Run‐in period: 1 week screening followed by 2 week single blind placebo period. | |
| Outcomes | Primary efficacy endpoint: number of exacerbation episodes. Secondary efficacy endpoints: number of treatment courses of oral and inhaled corticosteroids; duration of exacerbation episodes; percentage of days without asthma; severity of the exacerbation episode; blood eosinophil counts; proportion of patients with an exacerbation episode; time to first exacerbation episode; asthma‐related resource utilisation. | |
| Notes | Specific group data were obtained for children free of interval symptoms (Bisgaard 2005 (EVW only)) due to relaxation of recruitment criteria in the latter part of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocking factor of 4 by the statistical group, which was blinded to the allocation. |
| Allocation concealment (selection bias) | Low risk | Electronically generated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Sponsor, investigator and patient were blinded to the treatment groups." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Sponsor, investigator and patient were blinded to the treatment groups." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details provided, similar percentages included in efficacy analysis from each group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | High risk | Specific group data were obtained for children with an EVW phenotype (Bisgaard 2005 (EVW only)). |
Bisgaard 2005 (EVW only).
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | N = 347 randomised (174 montelukast and 173 placebo), 768 assessed for eligibility. Children aged 2 to 5 years with a history of intermittent asthma symptoms (see notes below). | |
| Interventions | Daily montelukast (4 mg aged < 6 years, 5 mg aged > 6 years). Study duration: 48 weeks. Run‐in period: 1 week screening followed by 2 week single blind placebo period. | |
| Outcomes | Primary efficacy endpoint: number of exacerbation episodes. Secondary efficacy endpoints: number of treatment courses of oral and inhaled corticosteroids; duration of exacerbation episodes; percentage of days without asthma; severity of the exacerbation episode; blood eosinophil counts; proportion of patients with an exacerbation episode; time to first exacerbation episode; asthma‐related resource utilisation. | |
| Notes | Data made available on application from Merck for children with only intermittent symptoms, i.e. EVW phenotype. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocking factor of 4 by the statistical group, which was blinded to the allocation. |
| Allocation concealment (selection bias) | Low risk | Electronically generated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Sponsor, investigator and patient were blinded to the treatment groups." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Sponsor, investigator and patient were blinded to the treatment groups." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details provided, similar percentages included in efficacy analysis from each group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | Low risk | Data made available on application from Merck for children with only intermittent symptoms, i.e. EVW phenotype. |
Nwokoro 2014 (EVW only).
| Methods | Multicentre, parallel‐group, randomised, double‐blind, placebo‐controlled trial. 12 month trial period. | |
| Participants | N = 1346 (963 with EVW), 1883 children screened for eligibility. Children aged 10 months to 5 years with 2 or more previous wheeze episodes. | |
| Interventions | Intermittent montelukast (4 mg) or placebo at the onset of each viral cold or wheezing episode for 10 days. | |
| Outcomes | Primary outcome: number of unscheduled medical attendances for wheezing episodes. Secondary outcomes: duration of hospital admission; number of wheeze episodes; duration of wheeze episodes; number of courses of oral steroids per year; proportion of children receiving oral corticosteroids; use of trial drug; time to first unscheduled medical attendance; time to first unscheduled attendance by site of medical attendance. | |
| Notes | Enrolled children with multi‐trigger and episodic viral wheeze. Specific group data for children with EVW only available for the primary outcome. Participants were allocated according to ALOX5 promoter genotype stratum. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to either ALOX5 genotype stratum, then randomly assigned 1:1 via a permuted block schedule (size 10). |
| Allocation concealment (selection bias) | Low risk | Clinical investigators and parents were masked to treatment group and genotype strata. Placebo and montelukast were packaged as identical granules in identical sachets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinical investigators and parents masked to treatment until final analysis. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinding remained. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details provided, similar proportions included in efficacy data in each group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | Low risk | |
Robertson 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | N = 220 randomised (107 montelukast and 113 placebo), 236 recruited. Children aged 2 to 14 years with a history of intermittent asthma. | |
| Interventions | At the onset of symptoms: montelukast daily for minimum of 7 days or until symptoms resolved up to a maximum of 20 days (4 mg aged 2 to 5 years, 5 mg aged 6 to 14 years). Study duration: 12 months, up to 5 episodes treated. | |
| Outcomes | Primary outcome: total unscheduled acute health care resource utilisation (HRU). Secondary outcomes: individual components included in HRU; duration of episode; total daily symptom score; beta‐agonist use; oral prednisolone use; parent/caregiver days lost from work; number of nights the patient had disturbed sleep; patient days absent from school or childcare. | |
| Notes | The study authors supplied outcome data for number of participants experiencing one or more healthcare resource utilisation for children aged 2 to 5 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stratified by age and in blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Numerical sequence with numbered kits held by Pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding maintained throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding maintained throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details provided, intention‐to‐treat analysis performed. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | High risk | Greater proportion of children with rhinitis and atopic dermatitis in the montelukast group. |
Valovirta 2011.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | N = 1771 randomised (589 daily montelukast, 591 intermittent montelukast and 591 placebo), 1979 screened for eligibility. Children aged 6 months to 5 years with history of intermittent asthma episodes. | |
| Interventions | Three arms: daily montelukast 4 mg plus intermittent episodic placebo, daily placebo plus intermittent episodic montelukast 4 mg daily for 12 days and daily placebo plus intermittent episodic placebo. Run‐in: 2 week placebo period. Study duration: 52 weeks. | |
| Outcomes | Primary efficacy measure: number of asthma episodes culminating in an asthma attack. Secondary endpoints: symptoms in the 3 days before an asthma attack; symptoms during the 12‐day treatment episode; number of asthma attacks; number of asthma episodes; percentage of asthma free days; daily average beta‐agonist use over the 12‐day treatment episode. | |
| Notes | Some infants aged between 6 months and 1 year were included in this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised, computer generated schedule. |
| Allocation concealment (selection bias) | Low risk | Computer generated schedule, numbered packaging used to implement allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All study personnel, including investigators, coordinators, patients, parents/guardians, monitors and central laboratory staff remained blinded throughout the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All study personnel, including investigators, coordinators, patients, parents/guardians, monitors and central laboratory staff remained blinded throughout the study." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details provided, primary analysis based on all patients who received at least one dose of study drug (similar proportion across all groups) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a judgement. |
| Other bias | High risk | Some infants aged between 6 months and 1 year were included in this study. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bacharier 2008b | Review only, no new primary data. |
| Bacharier 2010 | Review only, no new primary data. |
| Goswami 2009 | Commentary only on Bacharier 2008a. |
| Johnston 2007 | Children had chronic asthma symptoms, not EVW. |
| Knorr 2001 | Children had chronic asthma symptoms, not EVW. |
| Kooi 2008 | Children had chronic asthma symptoms, not EVW. |
| Krawiec 2014 | Did not involve children with a specific EVW phenotype. |
| Lilly 2005 | Study did not commence (personal communication with investigators). |
| Micelli 2009 | Review only, no new primary data. |
| Nanulescu 2004 | Children had chronic asthma symptoms, not EVW. |
| Straub 2003 | Infants only. |
| Van Adelsberg 2005 | Children had chronic asthma symptoms, not EVW. |
| Zou 2014 | Excluded because is a post‐RSV bronchiolitis study. |
Differences between protocol and review
The following differences occurred between the protocol and review
It was not feasible to adjust for the number of viral respiratory tract infections and the intraclass correlation for clustering of infections within individual children. Thesedata, which could only be provided by the study authors, was not available.
As stated, some included studies involved children outside of the specified age range of one to six years.
As stated, some included studies involved children with interval symptoms of wheeze other than only during viral respiratory tract infections.
It was not possible to perform subgroup analyses of atopic children as was planned.
Contributions of authors
MB, AG and MCM drafted the protocol that was subsequently reviewed by FMD and CER‐M. MB and AG identified eligible studies and extracted data. MB analysed the data and drafted the review. AG, FMD, MCM, CER‐M and JAC‐R commented on drafts of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Medical Research Council, UK.
MB is a recipient of a MRC Clinician Scientist Fellowship.
Declarations of interest
Malcolm Brodlie has received funding from Pfizer for an investigator‐led pneumonia research study and has been local PI on an asthma study sponsored by Glaxo SmithKline.
Atul Gupta: none known.
Carlos E Rodriguez‐Martinez: in the last three years has received honoraria for lectures from Astra Zeneca, Glaxo SmithKline, Merck Sharp Dohme and Novartis; and travel and accommodation from Glaxo SmithKline.
Jose A Castro‐Rodriguez: has received honoraria from Merck Sharp Dohme for expert testimony about a product of this pharmaceutical company: mometasone furoate and formoterol fumarate dihydrate (Dulera), has received honoraria from Merck Sharp Dohme for lectures about the use of montelukast for asthma treatment in children and has received financial support (travel/accomodation) from Merck Sharp Dohme to attend international meetings about respiratory medicine.
Francine M Ducharme has received travel support, research funds, and fees for speaking from Glaxo SmithKline, Novartis, Nycomed, and/or Merck Frosst Inc.
Michael C McKean: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Bacharier 2008a {published data only}
- Bacharier LB, Phillips BR, Zeiger RS, Szefler SJ, Martinez FD, Lemanske RF, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate‐to‐severe intermittent wheezing. Journal of Allergy and Clinical Immunology 2008;122:1127‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisgaard 2005 {published data only}
- Bisgaard H, Gilles L, Menten J, Tozzi C, Polos P. Montelukast reduces rescue medication use in viral‐induced asthma exacerbations. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004; Vol. A53, issue Poster C72.
- Bisgaard H, Swern AS, Dass SB, Polos PG. Effect of montelukast on asthma exacerbation rates in pediatric patients with or without prior oral steroid use: a post hoc analysis of the PREVIA trial of viral‐associated asthma. American Thoracic Society International Conference, May 18‐23, 2007, San Francisco, California, USA. 2007; Vol. Poster #733.
- Bisgaard H, Zielen S, Garcia‐Garcia ML, Johnston SL, Gilles L, Menten J at el. Montelukast reduces asthma exacerbations in 2‐ to 5‐year‐old children with intermittent asthma. American Journal of Respiratory and Critical Care Medicine 2005;171(4):315‐22. [DOI] [PubMed] [Google Scholar]
- Bisgaard H, Zielen S, Giles L, Menten J, Polos PG. Montelukast and the prevention of viral induced asthma (The PREVIA Study). European Respiratory Journal 2003;22(Suppl 45):Abstract No 3343. [Google Scholar]
Bisgaard 2005 (EVW only) {unpublished data only}
- Rovi AH (MERCK). Unpublished data from MERCK [personal communication]. Email to: M Brodlie 23 September 2012.
Nwokoro 2014 (EVW only) {published and unpublished data}
- Nwokoro N, Pandya H, Turner S, Eldridge S, Griffiths CJ, Vulliamy T, et al. Intermittent montelukast in children aged 10 months to 5 years with wheeze (WAIT trial): a multicentre, randomised, placebo‐controlled trial. The Lancet Respiratory Medicine 2014;2(10):796‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 2007 {published data only}
- Merck. A study to determine the effect of montelukast sodium as an episode modifier in the treatment of infrequent episodic asthma in children. Clinicaltrials.Gov 2005.
- Robertson CF, Price D, Henry R, Mellis C, Glasgow N, Fitzgerald D, et al. Short‐course montelukast for intermittent asthma in children: a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2007;175(4):323‐9. [DOI] [PubMed] [Google Scholar]
Valovirta 2011 {published data only}
- Valovirta E, Boza M, Robertson C, Verbruggen N, Smugar S, Knorr B, et al. Intermittent and daily montelukast versus placebo for treating episodic asthma in children 6 months to 5 years of age. European Journal of Allergy and Clinical Immunology. 29th Congress of the European Academy of Allergy and Clinical Immunology, EAACI London United Kingdom. 2010.
- Valovirta E, Boza ML, Robertson CF, Verbruggen N, Smugar SS, Nelsen LM, et al. Intermittent or daily montelukast versus placebo for episodic asthma in children. Annals of Allergy, Asthma & Immunology 2011;106(6):518‐26. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bacharier 2008b {published data only}
- Bacharier LB. Management of asthma in preschool children with inhaled corticosteroids and leukotriene receptor antagonists. Current Opinion in Allergy and Clinical Immunology 2008;8(2):158‐62. [DOI] [PubMed] [Google Scholar]
Bacharier 2010 {published data only}
- Bacharier LB. Viral‐induced wheezing episodes in preschool children: approaches to therapy. Current Opinion in Pulmonary Medicine 2010;16(1):31‐5. [DOI] [PubMed] [Google Scholar]
Goswami 2009 {published data only}
- Goswami P, Jones SM. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate‐to‐severe intermittent wheezing. Pediatrics 2009;124(Supplement 2):S147‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2007 {published data only}
- Johnston NW, Mandhane PJ, Dai J, Duncan JM, Greene JM, Lambert K, et al. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics 2007;120(3):e702‐12. [DOI] [PubMed] [Google Scholar]
Knorr 2001 {published data only}
- Knorr B, Franchi LM, Bisgaard H, Vermeulen JH, LeSouef P, Santanello N, et al. Montelukast, a leukotriene receptor antagonist, for the treatment of persistent asthma in children aged 2 to 5 years. Pediatrics 2001;108(3):E48. [DOI] [PubMed] [Google Scholar]
Kooi 2008 {published data only}
- Kooi EM, Schokker S, Marike Boezen H, Vries TW, Vaessen‐Verberne AA, Molen T, et al. Fluticasone or montelukast for preschool children with asthma‐like symptoms: Randomized controlled trial. Pulmonary Pharmacology & Therapeutics 2008;21(5):798‐804. [DOI] [PubMed] [Google Scholar]
Krawiec 2014 {published data only}
- Krawiec M, Strzelak A, Krenke K, Modelska‐Wozniak I, Jaworska J, Kulus M. Fluticasone or montelukast in preschool wheeze: a randomized controlled trial. Clinical Pediatrics 2014;54(3):273‐81. [DOI] [PubMed] [Google Scholar]
Lilly 2005 {published data only}
- Lilly CM. Montelukast for early life wheezing. Clinicaltrials.Gov 2005.
Micelli 2009 {published data only}
- Miceli Sopo S, Onesimo R, Radzik D, Scala G, Cardinale F. Montelukast versus inhaled corticosteroids as monotherapy for prevention of asthma: which one is best?. Allergologia et Immunopathologia 2009;37(1):26‐30. [DOI] [PubMed] [Google Scholar]
Nanulescu 2004 {published data only}
- Nanulescu M, Popescu L, Farcău M, Ichim G. Efficacy of montelukast in recurrent wheezing infants and small children. Pneumologia 2003;52(3‐4):213‐6. [PubMed] [Google Scholar]
Straub 2003 {published data only}
- Straub DA, Minocchieri S, Moller A, Hamacher J, Sennhauser FH, Wildhaber JH. Montelukast improves lung function and airway inflammation in wheezy infants with family history of asthma. Proceedings of the Thoracic Society of Australia & New Zealand, Annual Scientific Meeting, Adelaide, 4‐9 April 2003. 2003; Vol. Abstract number: P167.
Van Adelsberg 2005 {published data only}
- Adelsberg J, Moy J, Wei LX, Tozzi CA, Knorr B, Reiss TF. Safety, tolerability, and exploratory efficacy of montelukast in 6‐ to 24‐month‐old patients with asthma. Current Medical Research and Opinion 2005;21(6):971‐9. [DOI] [PubMed] [Google Scholar]
Zou 2014 {published data only}
- Zou YX, Zhang J, Ma C, Li J, Zai J, Guo YS. Clinical efficacy of montelukast sodium in treating infantile wheezing. European Review for Medical and Pharmacological Sciences 2014;18(6):775‐80. [PubMed] [Google Scholar]
Additional references
Altman 2003
- Altman D, JM Bland. Statistics Notes: Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 1992
- Anderson HR. Epiddmiology of asthma. British Journal of Hospital Medicine 1992;47:99‐104. [PubMed] [Google Scholar]
Bacharier 2008
- Bacharier LB, Boner A, Carlsen KH, Eigenmann PA, Frischer T, Götz M, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy 2008;63(1):5‐34. [DOI] [PubMed] [Google Scholar]
Beigelman 2014
- Beigelman A, Bacharier LB. Infection‐induced wheezing in young children. Journal of Allergy and Clinical Immunology. 2014;133(2):603‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brand 2008
- Brand PL, Baraldi E, Bisgaard H, Boner AL, Castro‐Rodriguez JA, Custovic A, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence‐based approach. European Respiratory Journal 2008;32(4):1096‐1110. [DOI] [PubMed] [Google Scholar]
BTS 2014
- British Thoracic Society and Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. A national clinical guideline. http://www.sign.ac.uk/pdf/SIGN141.pdf 2014.
Bush 2009
- Bush A. Practice imperfect ‐ treatment for wheezing in preschoolers. New England Journal of Medicine 2009;360(4):409‐410. [DOI] [PubMed] [Google Scholar]
Davies 2008
- Davies G, Paton JY, Beaton SJ, Young D, Lenney W. Children admitted with acute wheeze/asthma during November 1998–2005: a national UK audit. Archives of Disease in Childhood 2008;93:952‐958. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Drazen 1999
- Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. New England Journal of Medicine 1999;340:197‐206. [DOI] [PubMed] [Google Scholar]
Ducharme 2009
- Ducharme FM, Lemire C, Noya FJ, Davis GM, Alos N, Leblond H, et al. Preemptive use of high‐dose fluticasone for virus‐induced wheezing in young children. New England Journal of Medicine 2009;360:409‐10. [DOI] [PubMed] [Google Scholar]
Ducharme 2012
- Ducharme FM, Morin J, Davis GM, Gingras J, Noya FJ. High physician adherence to phenotype‐specific asthma guidelines, but large variability in phenotype assessment in children. Current Medical Research and Opinion 2012;28(9):1561‐70. [DOI] [PubMed] [Google Scholar]
Ducharme 2014
- Ducharme FM, Tse SM, Chauhan B. Diagnosis, management, and prognosis of preschool wheeze. Lancet 2014;383:1593‐604. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2012
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. http://www.ginasthma.org/ 2012.
Guilbert 2011
- Guilbert TW, Bacharier LB. Controversies in the treatment of the acutely wheezing infant. American Journal of Respiratory and Critical Care Medicine 2011;183:1284‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kuehni 2001
- Kuehni CE, Davis A, Brooke AM, Silverman M. Are all wheezing disorders in very young (preschool) children increasing in prevalence?. Lancet 2001;358:1821‐1825. [DOI] [PubMed] [Google Scholar]
Lougheed 2006
- Lougheed MD, Garvey N, Chapman KR, Cicutto L, Dales R, Day AG, et al. The Ontario asthma regional variation study: emergency department visit rates and the relation to hospitalization rates. Chest 2006;129(4):909‐17. [DOI] [PubMed] [Google Scholar]
Martinez 1995
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. New England Journal of Medicine 1995;19:133‐8. [DOI] [PubMed] [Google Scholar]
McKean 2000
- McKean M, Ducharme F. Inhaled steroids for episodic viral wheeze of childhood. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001107] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nwokoro 2014
- Nwokoro N, Pandya H, Turner S, Eldridge S, Griffiths CJ, Vulliamy T, et al. Intermittent montelukast in children aged 10 months to 5 years with wheeze (WAIT trial): a multicentre, randomised, placebo‐controlled trial. The Lancet Respiratory Medicine 2014;10(2):796‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oommen 2003
- Oommen A, Grigg J. Urinary leukotriene E4 in preschool children with acute clinical viral wheeze. European Respiratory Journal 2003;21:149‐54. [DOI] [PubMed] [Google Scholar]
Panickar 2009
- Panickar J, Lakhanpaul M, Lambert PC, Kenia P, Stephenson T, Smyth A, et al. Oral prednisolone for preschool children with acute virus‐induced wheezing. New England Journal of Medicine 2009;360(4):329‐38. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Savenije 2011
- Savenije OE, Granell R, Caudri D, Koppelman GH, Smit HA, Wijga A, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. Journal of Allergy and Clinical Immunology 2011;127(6):1505‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
SIGN 2006
- Scottish Intercollegiate Guidelines Network. Bronchiolitis in children. A national clinical guideline. www.sign.ac.uk/pdf/sign91.pdf 2006.
Silverman 1993
- Silverman M. Out of the mouths of babes and sucklings: lessons from early childhood asthma. Thorax 1993;48:1200‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spycher 2008
- Spycher BD, Silverman M, Brooke AM, Minder CE, Kuehni CE. Distinguishing phenotypes of childhood wheeze and cough using latent class analysis. European Respiratory Journal 2008;31(5):974‐81. [DOI] [PubMed] [Google Scholar]
Stein 1997
- Stein RT, Holberg CJ, Morgan WJ, Wright AL, Lombardi E, Taussig L, et al. Peak flow variability, methacholine responsiveness and atopy as markers for detecting different wheezing phenotypes in childhood. Thorax 1997;52(11):946‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tal 1990
- Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics 1990;86(3):350‐6. [PubMed] [Google Scholar]
Visual Rx [Computer program]
- Cates CJ. Visual Rx 2.3. Dr Christopher Cates www.nntonline.net, 2008.
Wilson 1994
- Wilson NM. The significance of early wheezing. Clinical and Experimental Allergy 1994;24(6):522‐9. [DOI] [PubMed] [Google Scholar]
Øymar 2014
- Øymar K, Skjerven HO, Mikalsen IB. Acute bronchiolitis in infants, a review. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2014;22:23. [DOI] [PMC free article] [PubMed] [Google Scholar]


