Abstract
Background
Bronchial thermoplasty is a procedure that consists of the delivery of controlled radiofrequency‐generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. It has been suggested that bronchial thermoplasty works by reducing airway smooth muscle, thereby reducing the ability of the smooth muscle to bronchoconstrict. This treatment could then reduce asthma symptoms and exacerbations, resulting in improved asthma control and quality of life.
Objectives
To determine the efficacy and safety of bronchial thermoplasty in adults with bronchial asthma.
Search methods
We searched the Cochrane Airways Group Specialised Register of Trials (CAGR) up to January 2014.
Selection criteria
We included randomised controlled clinical trials that compared bronchial thermoplasty versus any active control in adults with moderate or severe persistent asthma. Our primary outcomes were quality of life, asthma exacerbations and adverse events.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias.
Main results
We included three trials (429 participants) with differences regarding their design (two trials compared bronchial thermoplasty vs medical management and the other compared bronchial thermoplasty vs a sham intervention) and participant characteristics; one of the studies included participants with more symptomatic asthma compared with the others.
The pooled analysis showed improvement in quality of life at 12 months in participants who received bronchial thermoplasty that did not reach the threshold for clinical significance (3 trials, 429 participants; mean difference (MD) in Asthma Quality of Life Questionnaire (AQLQ) scores 0.28, 95% confidence interval (CI) 0.07 to 0.50; moderate‐quality evidence). Measures of symptom control showed no significant differences (3 trials, 429 participants; MD in Asthma Control Questionnaire (ACQ) scores ‐0.15, 95% CI ‐0.40 to 0.10; moderate‐quality evidence). The risk of bias for these outcomes was high because two of the studies did not have a sham intervention for the control group.
The results from two trials showed a lower rate of exacerbation after 12 months of treatment for participants who underwent bronchial thermoplasty. The trial with sham intervention showed a significant reduction in the proportion of participants visiting the emergency department for respiratory symptoms, from 15.3% on sham treatment to 8.4% over 12 months following thermoplasty. The trials showed no significant improvement in pulmonary function parameters (with the exception of a greater increase in morning peak expiratory flow (PEF) in one trial). Treated participants who underwent bronchial thermoplasty had a greater risk of hospitalisation for respiratory adverse events during the treatment period (3 trials, 429 participants; risk ratio 3.50, 95% CI 1.26 to 9.68; high‐quality evidence), which represents an absolute increase from 2% to 8% (95% CI 3% to 23%) over the treatment period. This means that six of 100 participants treated with thermoplasty (95% CI 1 to 21) would require an additional hospitalisation over the treatment period. No significant difference in the risk of hospitalisation was noted at the end of the treatment period.
Bronchial thermoplasty was associated with an increase in respiratory adverse events, mainly during the treatment period. Most of these events were mild or moderate, appeared in the 24‐hour post‐treatment period, and were resolved within a week.
Authors' conclusions
Bronchial thermoplasty for patients with moderate to severe asthma provides a modest clinical benefit in quality of life and lower rates of asthma exacerbation, but no significant difference in asthma control scores. The quality of life findings are at risk of bias, as the main benefits were seen in the two studies that did not include a sham treatment arm. This procedure increases the risk of adverse events during treatment but has a reasonable safety profile after completion of the bronchoscopies. The overall quality of evidence regarding this procedure is moderate. For clinical practice, it would be advisable to collect data from patients systematically in independent clinical registries. Further research should provide better understanding of the mechanisms of action of bronchial thermoplasty, as well as its effect in different asthma phenotypes or in patients with worse lung function.
Plain language summary
Bronchial thermoplasty for people with asthma
Review question
We reviewed the effects of bronchial thermoplasty in people with asthma.
Background
Asthma is a chronic condition in which people experience symptoms of breathlessness, wheezing, coughing and chest tightness due to airway inflammation and airway muscle contraction. With inhaled treatments, including bronchodilators (drugs that relax airway muscle and so open up the airways) and steroids (which treat underlying inflammation in the lungs), symptoms usually can be controlled. However, for some people, asthma cannot be adequately controlled with these drugs, either because they are truly resistant or because they do not take them.
The muscle in the airways of the lungs is thicker in people with asthma than in people who do not have asthma. During asthma attacks, these muscles tighten, making it hard to breathe.
Bronchial thermoplasty is a relatively new procedure that reduces the amount of muscle bulk in the airways of the lungs. A long flexible tube, called a bronchoscope, is passed down into the lung under direct observation, and the walls of specific areas of the lungs are heated to 65 degrees Celsius. This causes some of the muscle to break up, making it harder for the muscles to tighten.
Generally, three sessions of treatment are given.
Study characteristics
We found three trials comparing groups of adults treated with bronchial thermoplasty versus adults who received standard medical treatment or a "sham" (simulated) bronchial thermoplasty treatment.
Key results
These studies showed moderate improvement only in quality of life of patients treated with bronchial thermoplasty and in the number of asthma attacks (exacerbations) that they experienced. In addition, patients treated with this procedure had more respiratory problems than patients who received the alternative intervention during the period when they were undergoing treatment, resulting in increased risk of hospitalisation due to a respiratory symptom during this phase, but not afterward.
Quality of evidence
Confidence in the results of this review is moderate because two of the studies had no sham intervention and there were differences regarding the characteristics of patients and the comparisons performed. More studies should be conducted to determine whether the observed effect and safety of bronchial thermoplasty are durable over the long term, and to identify whether particular patients can be identified who could benefit most.
This plain language summary is current as of January 2014.
Summary of findings
Summary of findings for the main comparison. Bronchial thermoplasty compared with any active control for persistent asthma in adults.
| Bronchial thermoplasty compared with any active control for persistent asthma in adults | ||||||
| Patient or population: adult patients with asthma Settings: hospital Intervention: bronchial thermoplasty Comparison: any active control (medical management or sham intervention) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk5 | Corresponding risk | |||||
| Any active control | Bronchial thermoplasty | |||||
| Quality of life (AQLQ) AQLQ scores1. Scale from 1 to 7 Follow‐up: mean 12 months | Mean quality of life (AQLQ) ranged across control groups from 5.1 to 5.7 | Mean quality of life (AQLQ) in the intervention groups was 0.28 higher (0.07 to 0.50 higher) | 429 (3 studies) | ⊕⊕⊕⊝ moderate2,3 | ||
| Asthma control (ACQ) ACQ scores. Scale from 0 to 36 Follow‐up: mean 12 months | Mean change in asthma control measure (ACQ) ranged across control groups from ‐0.55 to ‐0.01 | Mean change in asthma control measure (ACQ) in the intervention groups was 0.15 lower (0.40 lower to 0.10 higher) | 429 (3 studies) | ⊕⊕⊕⊝ moderate2,3 | ||
|
Number of exacerbations Follow up: 12 months |
See comment | See comment | See comment | 409 (2 studies) |
Two trials reported on exacerbations, but we did not pool the data. AIR showed no significant differences in the data on number of severe exacerbations per participant per week, with participants in both groups experiencing a decrease at 12 months. AIR 2 reported that the rate of severe exacerbations per participant per year was significantly lower in participants who received bronchial thermoplasty compared with controls (0.48 ± 0.067 vs 0.70 ± 0.122 exacerbations per patient‐year, respectively). In the bronchial thermoplasty group, a significantly lower proportion of participants experienced severe exacerbations compared with controls (26% of participants vs 40%, respectively) |
|
| Participants admitted to hospital because of respiratory adverse events (treatment period) Follow‐up: mean 12 weeks | Two per 100 | Eight per 100 (three to 23) | RR 3.5 (1.26 to 9.68) | 429 (3 studies) | ⊕⊕⊕⊕ high |
|
| Participants admitted to hospital because of respiratory adverse events (post‐treatment period) Follow‐up: mean 12 months | Four per 100 |
Five per 100 (two to 12) |
RR 1.12 (0.44 to 2.85) | 429 (3 studies) | ⊕⊕⊕⊝ moderate4 | |
| Use of rescue medication Short‐acting bronchodilator puffs per week Follow‐up: mean 12 months | Mean use of rescue medication ranged across control groups from ‐9.99 to ‐0.10 puffs per week | Mean use of rescue medication in the intervention groups was 0.68 lower (3.63 lower to 2.28 higher) | 429 (3 studies) | ⊕⊕⊝⊝ low3,4 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes5. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1A change in the score of 0.5 points is considered the threshold of clinical relevance (Juniper 1994). 2Two of the included trials (AIR and RISA) were open. 3The imbalance in the RISA trial could bias the effect estimates of the pooled analysis (see Quality of the evidence section in the Discussion). 4Wide confidence interval.
5Median baseline risk in the included studies.
Background
Description of the condition
Bronchial smooth muscle hypertrophy, hyperplasia and contraction play a central role in bronchial obstruction in patients with asthma. In fact, the first treatment step in such patients, particularly when they are symptomatic, is the use of bronchodilators (beta2‐adrenergic agonists) to relax bronchial smooth muscle (GINA 2011). Over the past 20 years, significant advances have been made in our understanding of the pathophysiology of bronchial smooth muscle, as well as in the development of treatments such as inhaled corticosteroids and long‐acting bronchodilators. Both treatments require daily maintenance doses. Application of these advances, in combination with adherence to diagnostic and treatment guidelines, has had a major positive impact on morbidity, mortality and quality of life in people with asthma (Rodriguez‐Trigo 2008).
Despite these efforts, however, asthma remains a poorly controlled disease in a small but important minority of patients, even when they are compliant; as such, it is still a common reason for emergency department visits. Furthermore, between 3% and 6% of patients with asthma respond poorly to treatment (Torrego 2010), including oral corticosteroid therapy, and continue to experience symptoms and a diminished quality of life. Care for patients with more severe disease is a major public health burden, which makes it clear that new treatments are required to improve the prognosis of this group of patients (Moore 2006).
Description of the intervention
Bronchial thermoplasty is an innovative procedure that consists of the delivery of controlled radiofrequency‐generated heat via a catheter inserted into the bronchial tree through a flexible bronchoscope. Once the bronchoscope has been placed into the airway, the catheter is advanced to a bronchial segment and is expanded so that it is in contact with bronchial mucosa (Mayse 2007). Then radiofrequency energy is applied through the catheter, across the contact points. Treatments are applied consecutively so that the entire bronchopulmonary segment is treated. Three separate bronchoscopic treatment sessions are required to treat all of the airways (except for the right middle lobe).
How the intervention might work
The mechanism of action of bronchial thermoplasty is not definitively established, but experimental evidence in animal models (Brown 2005; Danek 2004) suggests that it might work by reducing airway smooth muscle bulk, thereby reducing the effect of smooth muscle bronchoconstriction.
As a consequence of this mechanism of action, this treatment could reduce asthma symptoms and exacerbations, resulting in improved asthma control and quality of life.
Why it is important to do this review
Although several recent reviews have discussed the efficacy of bronchial thermoplasty (Cayetano 2011; Wahidi 2012), this review will provide a more rigorous summary of available evidence regarding the efficacy of this intervention, focusing on the trade‐off between relevant patient outcomes and safety, in the short term and over the long term (Thomson 2011).
Objectives
To determine the efficacy and safety of bronchial thermoplasty in adults with bronchial asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials that compared bronchial thermoplasty versus conventional treatment, with or without the use of a placebo, as treatment for adult participants with moderate or severe persistent asthma.
Types of participants
We included adult participants with moderate or severe persistent asthma according to the Global Initiative for Asthma (GINA) criteria (GINA 2011).
Types of interventions
We included trials assessing bronchoscopically delivered thermoplasty adjuvant to conventional treatment for participants with moderate to severe persistent asthma.
Eligible trials included a control group of participants with moderate to severe persistent asthma who received conventional treatment or preferably a control sham bronchoscopy.
Types of outcome measures
Primary outcomes
-
Health‐related quality of life (HRQOL) evaluated through asthma‐specific or generic questionnaires.
Asthma‐specific (e.g. Asthma Quality of Life Questionnaire (AQLQ; Juniper 1994)); considering a change in the score of 0.5 points the threshold of clinical relevance).
Respiratory‐specific (e.g. St. George's Respiratory Questionnaire; considering a change in the score of four points the threshold of clinical relevance (Jones 2002)).
Generic (e.g. Short Form (SF)‐36 Questionnaire on health status; Nottingham Health Profile (NHP); World Health Organization (WHO) instrument on health‐related quality of life (WHOQOL‐100)).
Asthma control evaluated through validated questionnaires (e.g. Asthma Control Questionnaire (ACQ; Juniper 1999)); considering a change in the score of 0.5 points the threshold of clinical relevance.
-
Number of exacerbations, defined as any of the following.
Exacerbations requiring hospital or intensive care unit admissions.
Exacerbations requiring emergency department visits or unscheduled healthcare visits.
Exacerbations resulting in a course of oral or systemic corticosteroids, or an increase in the regular required doses.
-
Serious adverse events, defined as any of the following.
Fatal events.
Hospital admission.
Risk of death at the time of event.
A permanent or significant disability.
Secondary outcomes
-
Lung function tests.
Bronchial hyperresponsiveness evaluated through non‐specific (direct muscle contraction) bronchial stimulation tests.
Peak expiratory flow (PEF).
Forced expiratory flow during the first second (FEV1).
Regular medication needs for asthma control with inhaled corticosteroids +/‐ long‐acting beta2‐agonist, according to recommendations in international guidelines (GINA 2011).
-
Use of rescue medication with:
the addition of a short‐acting beta2‐agonist relief medication to the combination of long‐acting beta2‐agonist and inhaled corticosteroids; or
the as‐needed use of combined budesonide plus formoterol.
Asthma symptom‐free days.
Days missed from work or school.
Adverse events
Search methods for identification of studies
Electronic searches
The Cochrane Airways Group Trials Search Co‐ordinator searched the Group's Specialised Register of Trials (CAGR) up to January 2014. This Register of Trials is derived from systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and from handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details).
We searched records in the CAGR coded as asthma, using the following terms:
thermoplast* or bronchoscop* or ((surger* or surgical) and bronchi*) or thermal* or catheter or Alair or Asthmatx
We also conducted a search of ClinicalTrials.gov. We searched all databases from their inception to January 2014, with no restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references.
Data collection and analysis
Selection of studies
Two independent evaluators (AT and IS) screened the titles and abstracts identified through the electronic searches to identify studies to include in the review. We discussed disagreements and consulted with a third review author (VP or JJY‐N).
Data extraction and management
Two independent evaluators (AT and JJY‐N) read all reports in detail and summarised the pertinent details on a standard data extraction sheet (which included study design methodology; number and description of participants; characteristics of intervention; type and method of outcome measurement; and evaluation of methodology). We discussed disagreements and reached agreement by consensus with a third review author, who checked the data extraction for accuracy (IS).
Assessment of risk of bias in included studies
Two review authors (IS and AT) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We solved disagreements by discussion, or by involving a third assessor. We assessed risk of bias according to the following domains.
Sequence generation (selection bias): For each included study, we described in detail the methodology used to generate the allocation sequence, and we evaluated the methodology to determine whether it can produce comparable groups. We assessed sequence generation as low risk of bias (any truly random process, e.g. random number table, computer random number generator); high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or unclear risk of bias.
Allocation concealment (selection bias): For each included study, we described in detail the methodology used to conceal the allocation sequence, and we evaluated the methodology to determine whether intervention allocation could have been foreseen in advance, noted during recruitment or changed after assignment. We evaluated allocation concealment as low risk of bias (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes); high risk of bias (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or unclear risk of bias.
Blinding (performance bias): For each included study, we described the methodology used, if any, to blind study participants and personnel from knowing the intervention that a participant received. We collected from the included studies information on whether the intended blinding was effective. When blinding was not possible, we assessed whether lack of blinding was likely to have introduced bias. We assessed blinding for participants and for outcome assessors separately. We evaluated blinding as low risk of bias, high risk of bias or unclear risk of bias for participants; and as low risk of bias, high risk of bias or unclear risk of bias for outcome evaluators.
Incomplete outcome data (attrition bias through withdrawals, dropouts or protocol deviations): For each included study, we provided a description of completeness of data, including attrition and exclusions from analysis, as well as an assessment of the reasons for attrition or data exclusion (if available). We extracted from trials the numbers lost through attrition and exclusion, as well as the numbers of participants included in the analysis at each stage (compared with the total number of randomly assigned participants).
Selective outcome reporting: We assessed selective outcome reporting for each included study. We evaluated selective outcome reporting as low risk of bias (when it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported); high risk of bias (when not all of the study's prespecified outcomes have been reported, one or more reported primary outcome(s) were not prespecified, outcomes of interest are reported incompletely and so cannot be used or study fails to include results of a key outcome that would have been expected to have been reported); or unclear risk of bias.
Overall risk of bias: We classified all studies according to the following criteria: low risk of bias (all individual items were at 'low risk of bias'); moderate risk of bias (one or more individual item(s) was at 'unclear risk of bias', and the remaining were at 'low risk of bias'); or high risk of bias (one or more individual item(s) was at 'high risk of bias').
Measures of treatment effect
When possible, we assessed treatment effect through mean differences (MDs) or standardised mean differences (SMDs) for continuous outcomes, and through risk ratios (RRs) for dichotomous outcomes. We present all measures with 95% confidence intervals (CIs). We performed all statistical analyses using Review Manager 5.1 (RevMan 2011).
We extracted data at the end of follow‐up for continuous outcomes. When trials reported data in terms of changes from baseline, we converted these into final scores. When standard deviations (SDs) at the end of follow‐up were not reported, we imputed these from baseline data.
Unit of analysis issues
For most outcomes, the unit of analysis was the participant (i.e. for dichotomous outcomes such as hospitalisations, we used the number of participants who had an admission to the hospital while experiencing one or more event(s) to avoid double counting of participants). We could not analyse data using this approach for some outcomes that reported data as rates (e.g. for outcomes such as exacerbations, trials reported data as the number of exacerbations per participant per year).
Dealing with missing data
We based our data extraction and main analysis on the available data analysis in each of the papers. If a paper presented both intention‐to‐treat and per‐protocol data, we used the former in the analyses.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the trials in terms of participant characteristics, interventions, controls and definitions of results. We evaluated statistical heterogeneity through the I2 statistic, and we used a cut‐off point of I2 > 50% to indicate relevant statistical heterogeneity. We tried to determine causes of heterogeneity through sensitivity analyses and analyses of subgroups.
Assessment of reporting biases
In future updates of the review, we will explore publication bias using a funnel plot (Egger test; Egger 1997) when 10 or more studies are available (Higgins 2011).
Data synthesis
We performed meta‐analyses using a random‐effects model and using the inverse variance method. We present forest plots for each result when we were able to extract data. We did not perform pooled analyses for outcomes reported as rates.
Subgroup analysis and investigation of heterogeneity
The characteristics of included trials did not allow us to carry out the planned subgroup analysis according to severity of asthma among participants classified as having severe asthma versus moderate asthma. In future updates of the review, we plan to use the classification reported in the included trials and, when possible, to classify severity on the basis of the intensity of treatment required to achieve good asthma control (GINA 2011; Taylor 2008).
Sensitivity analysis
The characteristics of included trials did not allow us to carry out the planned sensitivity analysis. In future updates of the review, we will try to perform sensitivity analyses to determine the effects of the risk of bias from included studies and the meta‐analysis model on treatment effect estimates.
Results
Description of studies
Results of the search
The search of the Cochrane Airways Group Specialised Register of Trials (CAGR) yielded 206 records. Revision of titles and abstracts led to the exclusion of 183 references. We reviewed in detail 23 publications, resulting in the inclusion of five reports and 16 abstract communications with results related to just three randomised clinical trials (AIR; AIR 2; RISA).
The two excluded references discussed in scientific meetings clinical issues related to design and results of the included trials and did not include relevant data for the review (references summarised in the Characteristics of excluded studies table). Figure 1 shows a flowchart of study selection following PRISMA guidance.
1.
Study flow diagram. (CAGR: Cochrane Airways Group Specialised Register of Trials).
Included studies
We included three randomised controlled trials in this review. Two compared bronchial thermoplasty versus usual care (AIR; RISA), and the other compared bronchial thermoplasty versus a sham bronchoscopic procedure (AIR 2). For details of the included studies, see Characteristics of included studies.
Participants
The AIR trial (NCT00214526) recruited 112 participants with moderate to severe asthma (pre‐dose FEV1 65% predicted) who were treated before enrolment with inhaled corticosteroids plus long‐acting beta2‐adrenergic agonists (LABA) and in whom symptom control worsened when the LABA was withdrawn. At baseline, the main participant characteristics were balanced between compared groups.
The RISA trial (NCT00214539) limited its sample to 34 participants with symptomatic, severe asthma (pre‐dose FEV1 74% predicted) receiving a dose of inhaled corticosteroid greater than 750 μg per day of fluticasone or oral prednisolone. After randomisation, participant characteristics were not well balanced, for example baseline symptom score, which was higher among participants allocated to the bronchial thermoplasty group (5.6 ± 3.0 vs 3.4 ± 1.8; P value 0.02). This score comprised the daily record of six asthma symptom measurements with a maximum score of 18 (lower scores shows better asthma control).
Finally, the AIR 2 trial (NCT00231114) randomly assigned 288 participants with symptomatic, persistent asthma (pre‐dose FEV1 78% predicted), treated with high doses of inhaled corticosteroids plus LABA. Baseline characteristics were similar for both groups in this study.
Intervention and comparison groups
The AIR trial randomly assigned participants to receive bronchial thermoplasty plus medical management with inhaled corticosteroids and LABA (N = 56) or medical management alone (control group; N = 56). Before treatment with the intervention of interest was provided, participants received maintenance therapy for four weeks, with inhaled corticosteroids and LABA for the first two weeks and with LABA withheld for two additional weeks. Maintenance therapy was resumed during the treatment period. Participants allocated to bronchial thermoplasty had three bronchoscopy procedures performed at least three weeks apart with the Alair system (Asthmatx). Participants were followed up at six weeks and at three, six and 12 months.
The RISA trial also randomly assigned participants to receive bronchial thermoplasty plus medical management with inhaled corticosteroids and LABA (N = 17) or medical management alone (N = 17). Before treatment with bronchial thermoplasty, participants were entered into a two‐week run‐in period. During the treatment period, participants allocated to bronchial thermoplasty had three bronchoscopy procedures performed with the Alair system at three‐weekly intervals. During these initial phases, all participants maintained their asthma medications. After recovery, participants entered into a 16‐week steroid stable phase (weeks six to 22), followed by a 14‐week steroid wean phase (weeks 22 to 36) and a final period of 16 weeks with reduced steroid treatment (weeks 36 to 52).
The AIR 2 trial allocated participants in a 2:1 ratio to receive bronchial thermoplasty (N = 190) or a sham intervention (N = 98) to ensure blinding of participants and outcome assessors. Both groups of participants continued their conventional medical treatment with inhaled corticosteroids and LABA. As in the other included trials, participants underwent three bronchoscopy procedures performed with the Alair system at three‐weekly intervals. Participants in the control group underwent three sham bronchoscopies at the same time intervals as those treated with bronchial thermoplasty. After the last bronchoscopy, participants were evaluated after six weeks after the last procedure, which was defined as the end of the treatment period. The post‐treatment period extended from six to 52 weeks after the last procedure, with completion of assessments at three, six, nine and 12 months.
Outcomes of interest
The primary outcome in the AIR trial was the difference between compared groups in the change in rates of mild asthma exacerbations between baseline and follow‐up. The authors analysed only exacerbations recorded in daily diaries that occurred during the two‐week period of abstinence from LABA, before treatment and then at 3, 6 and 12 months. This outcome measurement therefore was different from that in the other two studies. Secondary outcomes were changes in PEF, use of rescue medication, number of symptom‐free days ascertained from a symptom score and changes on ACQ and AQLQ questionnaires. Adverse events were registered from telephone interviews with participants and from data in their diaries and were classified as respiratory or non‐respiratory.
Participants who completed the 12 months of follow‐up in this trial were invited to participate in an extension study to evaluate the long‐term safety of the procedure over five years (NCT00448812). More participants who had received bronchial thermoplasty during the study agreed to participate in this post‐treatment study (45/52; 87%), although fewer did so in the control arm, and data were available for only three years (24/49; 49%) (Thomson 2011).
The RISA trial was designed to assess the safety of bronchial thermoplasty, with respiratory adverse events per participant as the primary outcome of interest. Although the main publication of the trial (Pavord 2007) did not specify primary or secondary outcomes, the protocol of the trial nominated the following secondary outcomes: use of maintenance medications, use of rescue medications, asthma symptom score, symptom‐free days, pre‐ and post‐bronchodilator FEV1 and ACQ and AQLQ scores.
The AIR 2 trial was designed to assess the safety and effectiveness of the procedure and established the difference between study groups in the change in AQLQ scores from baseline as the primary outcome of interest. Secondary outcomes included changes in ACQ scores, symptom‐free days, numbers and days of rescue medication used and pulmonary function (FEV1 and PEF). These outcomes were collected in electronic diaries. This trial used a Bayesian approach to analysis of its results and established a target posterior probability of superiority (PPS) of 96.4% for the primary outcome (changes in AQLQ scores) and of 95% for the remaining outcomes.
Excluded studies
We excluded two scientific meeting abstracts that discussed issues related to the three included trials (Hales 2010; Wechsler 2009). We have included the reasons for exclusion of these abstracts in the Characteristics of excluded studies table.
Risk of bias in included studies
Overall, the included trials had no major limitations in design or execution; with the exception of blinding, assessed domains were judged to be at low risk of bias (see Figure 2).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The AIR and RISA trials had a low risk of selection bias, with a centrally generated computer randomisation sequence in blocks of four participants provided in sealed envelopes to each participating centre.
The AIR 2 trial randomly assigned participants in a 2:1 ratio and stratified them by AQLQ score, percentage of symptom‐free days and site, according to a sequence that was computer generated using minimisation. The publication of trial results did not describe the efforts made to ensure concealment of the randomisation sequence.
Blinding
Both AIR and RISA trials had an open design; therefore both investigators and participants were aware of whether they had received bronchial thermoplasty. Outcomes in the AIR trial were collected by participants in diaries monitored by the research staff at meetings, consultations or telephone interviews. The RISA trial followed the same procedure to assess outcomes. This resulted in high risks of performance and detection bias, particularly for the subjective outcomes of quality of life and asthma control.
The AIR 2 trial ensured blinding of participants by comparing bronchial thermoplasty versus a sham procedure in which participants allocated to the control group received three bronchoscopies that involved sedation and a sham procedure that mimicked the active‐arm procedure. A catheter was deployed into the airways through a bronchoscope, an electrode array was expanded and a radiofrequency controller was activated, simulating indistinguishable audio and visual signals, but no radiofrequency energy was delivered. Duration and time intervals for treatments and assessments in active and control arms were the same. Participants in both groups were unable to guess their treatment assignment after the first bronchoscopy, but in the second procedure, a larger proportion of participants in the bronchial thermoplasty group guessed their assigned treatment. Outcome assessment was performed by a blinded assessment team. The staff that performed bronchoscopies was unblinded to treatment assignment.
Incomplete outcome data
The AIR trial was designed to perform an intention‐to‐treat analysis with no imputation of missing data. Researchers randomly assigned 112 participants (56 to each group, with baseline data reported for 109 participants), and at first follow‐up contact at three months, data on four participants in the bronchial thermoplasty group and eight in the control group were lost (three and four participants, respectively, withdrew consent; one and four participants, respectively, were lost to follow‐up). At 12 months, complete assessment data were available for 52 participants in the bronchial thermoplasty group and for 49 in the medical management group.
The RISA trial did not provide details about the plan to perform analysis of results but reported clearly the numbers of participants randomly assigned and withdrawn from the study. Researchers randomly assigned 34 participants (17 to each group), but two participants in the bronchial thermoplasty group withdrew from the study before they received treatment, in keeping with a recommendation from the study Data and Safety Monitoring Board.
The AIR 2 trial described a minimum recruitment goal of 225 participants (150 in the bronchial thermoplasty group and 75 in the sham group) and planned to analyse its results on an intention‐to‐treat basis, defined as participants randomly assigned who received at least a single bronchoscopy. Missing data for secondary outcomes were imputed using the last observation carried forward approach. The trial reached the randomisation target of 288 participants (190 in the bronchial thermoplasty group and 98 in the sham group), and at 12 months of follow‐up, only nine active participants and one control participant were lost to follow‐up, but all were included in the final analysis.
Selective reporting
The AIR trial described data for all outcomes described in the trial protocol (NCT00214526). Although most results were reported in graphical form, making it difficult to extract data for analysis, accurate data were available from electronic appendices. In the RISA and AIR 2 trials, all outcomes described in the study protocol (NCT00214539, NCT00231114) were reported.
Effects of interventions
See: Table 1
We outline the main results of the review and the quality of evidence in Table 1.
Primary outcomes
Health‐related quality of life
A pooled analysis showed a clinically small but statistically significant increase in AQLQ scores at 12 months of follow‐up among participants who received bronchial thermoplasty (three trials, 429 participants; MD 0.28, 95% CI 0.07 to 0.50; Analysis 1.1). This difference was driven by effect estimates from the two trials that compared bronchial thermoplasty versus medical management, as the trial that compared bronchial thermoplasty versus a sham intervention did not achieve a statistically significant difference in final AQLQ scores.
1.1. Analysis.
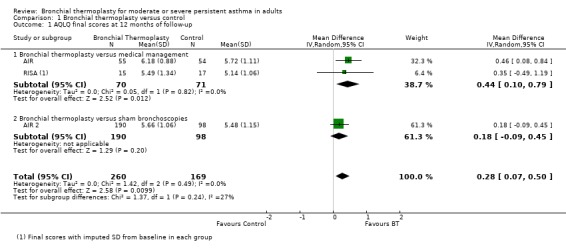
Comparison 1 Bronchial thermoplasty versus control, Outcome 1 AQLQ final scores at 12 months of follow‐up.
The AIR trial reported an improvement at 12 months in the quality of life of participants treated with bronchial thermoplasty. This was explained for a statistically significant improvement in AQLQ score in the bronchial thermoplasty group from baseline to follow‐up (from 4.91 ± 1.23 to 6.18 ± 0.88) compared with the moderate change seen in the control group (from 5.15 ± 1.19 to 5.72 ± 1.11). The difference between groups at 12 months of follow‐up in final AQLQ scores was 0.46 points.
The RISA did not report final scores for quality of life measures. At the end of the steroid stable phase (22 weeks of follow‐up), the trial reported statistically significant improvement in the quality of life of participants who received bronchial thermoplasty compared with those who received medical treatment. The improvement from baseline in AQLQ scores was greater in the bronchial thermoplasty group (1.21 ± 1.05) than in the control group (0.15 ± 0.75). A greater proportion of participants receiving bronchial thermoplasty experienced improvement greater than the minimum clinically important increase in scores of 0.5 (77% vs 35%), and fewer experienced deterioration of 0.5 or more points (8% vs 18%).
Results from AIR 2 suggest a large placebo effect in the studies without a sham intervention. The 12‐month follow‐up of the AIR 2 trial reported similar changes in AQLQ scores both for participants who received bronchial thermoplasty (from 4.30 ± 1.17 to 5.66 ± 1.06) and for participants treated with sham bronchoscopies (from 4.32 ± 1.21 to 5.48 ± 1.15). The improvement from baseline on the AQLQ was slightly greater in the bronchial thermoplasty group (1.35 ± 1.10 compared with 1.16 ± 1.23) but did not reach the posterior probability of superiority (PPS) planned (96.0% observed vs 96.4% planned). Significantly more participants in the bronchial thermoplasty group showed an improvement of 0.5 or greater in AQLQ scores compared with the sham group (78.9% vs 64.3%; PPS = 0.996).
Asthma control
No significant difference in symptomatic control as measured by the ACQ was reported (three trials, 429 participants; MD ‐0.15, 95% CI ‐0.40 to 0.10; Analysis 1.2).
1.2. Analysis.
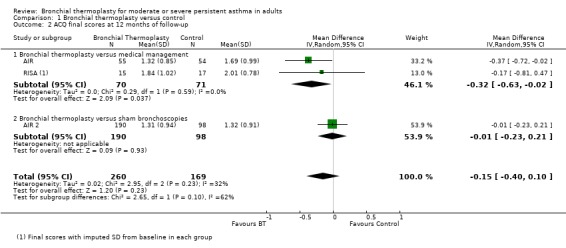
Comparison 1 Bronchial thermoplasty versus control, Outcome 2 ACQ final scores at 12 months of follow‐up.
The AIR trial reported improvement in ACQ at 12 months, with a significantly greater reduction in the scores of participants who received bronchial thermoplasty (from 2.50 ± 0.92 to 1.32 ± 0.85) compared with the reduction between participants in the control group (from 2.16 ± 0.86 to 1.69 ± 0.99). However, it should also be noted that the subjective changes experienced by participants were clinically relevant irrespective of the group to which they were allocated (Juniper 1994), suggesting a strong Hawthorne effect.
The RISA reflected an improvement in symptom‐based ACQ scores with a mean decrease of ‐1.04 ± 1.03 in bronchial thermoplasty compared with a decrease of ‐0.13 ± 1.00 in controls. At the end of the reduced steroid phase (52 weeks of follow‐up), bronchial thermoplasty participants also showed statistically significant improvement from baseline compared with controls in both AQLQ (1.53 ± 0.79 vs 0.42 ± 0.82) and ACQ scores (‐0.99 ± 0.83 vs ‐0.22 ± 0.78). However the baseline difference between the groups was ‐0.76 for the ACQ.
Changes from baseline observed in AIR 2 in ACQ scores (bronchial thermoplasty from 2.13 ± 0.87 to 1.31 ± 0.94 vs sham 2.09 ± 0.90 to 1.32 ± 0.91) were not statistically significantly different between groups (change of ‐0.82 ± 0.95 vs ‐0.77 ± 1.08; PPS = 0.638).
Number of exacerbations
The AIR trial reported the number of mild exacerbations per participant per week, but the exacerbations were counted only from two‐week periods in which LABA was withdrawn from both groups at three, six and 12 months. The change in the mean frequency of exacerbations per participant/wk was statistically significantly different between groups at 12 months of follow‐up (bronchial thermoplasty ‐0.16 ± 0.37 vs control 0.04 ± 0.29). Participants in the bronchial thermoplasty group showed a decrease from 0.35 ± 0.32 exacerbations per participant/wk at baseline to 0.18 ± 0.31 exacerbations per participant/wk at 12 months of follow‐up, compared with an increase in the control group from baseline 0.28 ± 0.31 exacerbations per participant/wk to 0.31 ± 0.46 exacerbations per participant/wk at 12 months of follow‐up. This difference between groups was statistically significant. The trial authors reported that the difference between groups was statistically significant also at three months of follow‐up, but these data were reported graphically only and so do not allow performance of an accurate extraction of the rates for each group. In contrast, the trial showed no significant differences in data on the number of severe exacerbations per participant per week, with participants in both groups experiencing a decrease at 12 months (baseline bronchial thermoplasty 0.07 ± 0.18 vs control 0.09 ± 0.31, and at 12 months bronchial thermoplasty 0.01 ± 0.08 vs control 0.06 ± 0.24).
The rate of severe exacerbations per participant per year was significantly lower among participants who received bronchial thermoplasty compared with controls after 12 months of follow‐up in the AIR 2 trial (0.48 ± 0.067 vs 0.70 ± 0.122 exacerbations per patient‐year, respectively). In the bronchial thermoplasty group, a significantly lower proportion of participants experienced severe exacerbations compared with controls (26.3% of participants (50/190) vs 39.8% (39/98), respectively).
Serious adverse events
Data from included trials showed a significant increase in risk of hospitalisation due to respiratory adverse events among participants treated with thermoplasty during the treatment period (three trials, 429 participants; RR 3.50, 95% CI 1.26 to 9.68; I2 = 0%; Analysis 1.3). This would result in 6% of 100 participants treated with bronchial thermoplasty requiring hospitalisation because of a respiratory adverse event (95% CI 1 to 21; see Table 1). After the treatment period, the risk of hospitalisation was similar between groups (3 trials, 429 participants; RR 1.12, 95% CI 0.44 to 2.85, I2 = 0%; Analysis 1.4).
1.3. Analysis.
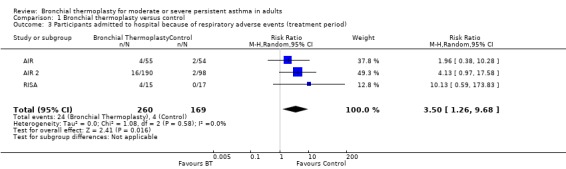
Comparison 1 Bronchial thermoplasty versus control, Outcome 3 Participants admitted to hospital because of respiratory adverse events (treatment period).
1.4. Analysis.
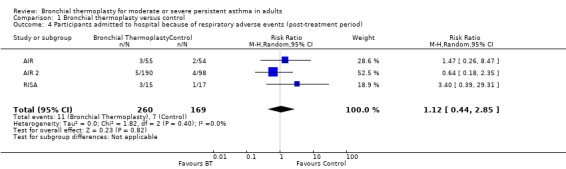
Comparison 1 Bronchial thermoplasty versus control, Outcome 4 Participants admitted to hospital because of respiratory adverse events (post‐treatment period).
During the treatment period in the AIR trial, hospitalisations for adverse respiratory events were more frequent among participants who received bronchial thermoplasty (4 participants with a total of 6 hospitalisations; 4 related to an asthma exacerbation) compared with participants who received medical treatment alone (2 participants with 2 hospitalisations). Hospitalisations in the follow‐up period were similar between groups (3 admissions per group). The trial did not register any deaths during the study period. It is interesting to note that over two to three years of follow‐up, during the extension period of the trial, more participants who had received bronchial thermoplasty required hospitalisation for respiratory symptoms than participants in the control group, but the difference was not statistically significant. The trialists reported that over five years of follow‐up, the number of participants who received bronchial thermoplasty and required hospitalisation and the number of hospitalisations required for a respiratory adverse event did not get worse compared with the first 12 months of follow‐up within the formal trial (Thomson 2011).
The RISA trial reported no hospitalisations due to respiratory adverse events among participants receiving medical management, although a quarter of participants from the bronchial thermoplasty group were hospitalised. Four participants accounted for a total of seven hospital admissions, five of which related to an asthma exacerbation. During the follow‐up period, the groups did not differ in the numbers of participants who required hospitalisation (one participant in the control group had four admissions, and three participants treated with bronchial thermoplasty had five admissions).
In the AIR 2 trial, 16 participants in the bronchial thermoplasty group required a total of 19 hospitalisations for respiratory symptoms during the treatment period, with 12 admissions in 10 participants related to worsening of asthma. In the sham group, only two participants had two hospital admissions—all related to worsening of asthma. In contrast, in the post‐treatment period, five participants in the thermoplasty group had six admissions to hospital compared with 12 hospitalisations among four participants who received sham bronchoscopies. However, the rate of visits to the emergency department was significantly and markedly higher among participants in the sham group (0.43 visits/y vs 0.07 visits/y). Over the study period, the proportion of participants with a respiratory‐related visit to the emergency department was 15.3% of those receiving sham treatment and 8.4% of those treated with thermoplasty, representing an absolute reduction of 7%.
Secondary outcomes
Lung function tests
During the first 22 weeks, participants treated with bronchial thermoplasty in the RISA trial reported greater improvement in change from baseline in pre‐bronchodilator FEV1 (% predicted) in comparison with the control group (14.9 ± 17.4% vs ‐0.9 ± 22.3%). However, no statistically significant differences in FEV1 between the groups were found after one year of follow‐up.
In the AIR trial, participants treated with bronchial thermoplasty experienced significant improvement from baseline in morning PEF (369.4 ± 97.9 to 397.4 ± 100.7 L/min) compared with the control group (394.0 ± 98.2 to 395.4 ± 88.6 L/min) at one‐year follow‐up, but of course, they ended up remarkably similar in this. The study authors reported no statistically significant differences in pre‐bronchodilator FEV1 (% predicted) among participants treated with bronchial thermoplasty compared with the control group.
A report on long‐term follow‐up results for the AIR trial showed stability in FEV1 values after five years (Thomson 2011). Methacholine PC20 (concentration of methacholine needed to produce a 20% fall in FEV1 from baseline) improved in the bronchial thermoplasty group in years two and three, but not in year one. However, lack of follow‐up for the control group in years four and five precludes interpretation of the relevance of this finding.
The AIR 2 trial found no statistical differences in morning PEF nor in FEV1 improvements when the bronchial thermoplasty group was compared with the sham procedure group after one year of follow‐up.
Doses of regular medication for asthma control
In the RISA trial, no statistically significant differences were noted between groups in the numbers of participants who had been receiving oral corticosteroids and were able to completely wean off oral corticosteroids at 52 weeks of follow‐up (four of eight participants in the bronchial thermoplasty group vs one of seven participants in the control group). The mean percentage reduction in oral steroid doses was large but was not significant between active and control groups (63.5 ± 45.4% vs 26.21 ± 40.7%); it was neither marked nor significant for inhaled corticosteroids (28.6 ± 30.4% vs 20.0 ± 32.9%, respectively).
Use of rescue medication
A pooled analysis of the results from included trials showed no significant difference between bronchial thermoplasty and control groups in reduction in the use of rescue medication over 12 months (3 trials, 429 participants; MD ‐0.68 puffs/wk, 95% CI ‐3.63 to 2.28; Analysis 1.5).
1.5. Analysis.
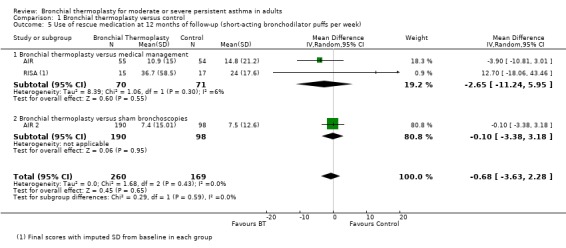
Comparison 1 Bronchial thermoplasty versus control, Outcome 5 Use of rescue medication at 12 months of follow‐up (short‐acting bronchodilator puffs per week).
Participants who received bronchial thermoplasty in the AIR trial did experience a significant reduction in needed doses of rescue medication at 12 months of follow‐up. Participants in the bronchial thermoplasty group reduced their short‐acting bronchodilator doses from 19.8 ± 17.2 puffs per week at baseline to 10.9 ± 15.0 puffs per week at 12 months, compared with the reduction in doses in the control group from 16.0 ± 18.8 puffs per week at baseline to 14.8 ± 21.2 puffs per week at 12 months of follow‐up.
The requirement for rescue medication was also significantly reduced among participants receiving bronchial thermoplasty compared with controls in the RISA trial. The reduction in short‐acting bronchodilator doses per week was greater among participants in the bronchial thermoplasty group than among those who received only medical management both in the steroid stable phase (up to week 22 of follow‐up; 26.6 ± 40.1 fewer puffs/wk vs 1.5 ± 11.7 fewer puffs/wk) and in the final assessment after 52 weeks of follow‐up (25.6 ± 31.2 fewer puffs/wk vs 6.1 ± 12.4 fewer puffs/wk).
Although after one year of follow‐up, participants in the AIR 2 trial reduced the requirement for rescue medication, no significant difference was noted between compared groups (bronchial thermoplasty: from 13.4 ± 19.17 puffs/wk to 7.4 ± 15.01 puffs/wk vs sham group: from 11.8 ± 11.24 puffs/wk to 7.5 ± 12.60 puffs/wk), nor was a significant difference observed in the number of days that rescue medication was used.
Asthma symptom‐free days
In the AIR trial, participants who received bronchial thermoplasty experienced a significant increase in days that were free of symptoms at 12 months compared with participants who received only medical treatment. The increase in symptom‐free days for participants in the bronchial thermoplasty group was 41% ± 40% at 12 months of follow‐up. For participants in the control group, this increase was more moderate, at 17% ± 40%. However, it should be noted that symptom diaries at 12 months were assessed over a four‐week period, during which LABA had been withdrawn.
Days missed from work or school
The AIR 2 trial reported that the decrease in severe exacerbations experienced by participants in the bronchial thermoplasty group resulted in fewer days lost from work or other activities because of asthma compared with participants who received the sham intervention (1.32 ± 0.36 days/y vs 3.92 ± 1.55 days/y).
Adverse events
During the six weeks that participants were treated with bronchial thermoplasty in the AIR trial, they experienced a greater rate of respiratory adverse events than participants in the control group (407 events vs 106 events), with the most common being dyspnoea, wheezing and cough. Most adverse events were mild (69%), but one‐third of participants experienced moderate adverse events. In the post‐treatment period, the rate of adverse events was similar between groups. During the extension period of the study, when data for control participants were available at two and three years of follow‐up, the rate of respiratory adverse events did not differ significantly between groups (Thomson 2011).
The greater increase in respiratory adverse events with bronchial thermoplasty was also noted in the RISA trial. During the treatment period, the number of respiratory adverse events was 136 in bronchial thermoplasty participants compared with 57 among participants treated with medical management, with again the most frequently observed adverse events being wheezing, cough and, in this case, chest discomfort. Most adverse events were mild (49%) or moderate (41%). Although during the post‐treatment period more than half of participants had wheezing or dyspnoea, no difference between groups was noted.
The AIR 2 trial reported that more participants in the bronchial thermoplasty group experienced respiratory adverse events during the treatment period than those receiving the sham procedure (85% vs 76%), and adverse events were mild to moderate in most cases. More participants in the bronchial thermoplasty group than in the sham group experienced a composite of multiple symptoms related to asthma (52 vs 39); the most common were wheezing, chest discomfort, cough and chest pain.
For all trials included in the review, most adverse events experienced by bronchial thermoplasty participants during the treatment period occurred within one day after the bronchoscopy but were resolved within seven days.
Discussion
At present, bronchial thermoplasty is an add‐on treatment for severe asthma patients who fail to adequately control their disease with conventional pharmacological treatments. This review has identified three trials of thermoplasty that were generally free of major sources of bias and that have reported data on a range of clinically relevant endpoints for asthma, including validated asthma‐related questionnaires on symptoms and quality of life, asthma medication use, lung function measurements, exacerbation rates, use of relief medications, asthma‐free days and hospital attendances/admissions (AIR; AIR 2; RISA).
Summary of main results
Bronchial thermoplasty has shown a modest benefit in quality of life in patients with persistent asthma, with the possibility of a control group 'Hawthorne' effect. The most meaningful improvements with thermoplasty seemed to be seen in a reduction in asthma exacerbation rates and hospital emergency department attendances, although rather limited data were collected on the latter and a lot of variability was noted between studies in the former. The limited quantity of long‐term data suggests that after five years, patients treated with bronchial thermoplasty may maintain their reduction in asthma exacerbations.
Bronchial thermoplasty was associated with a high proportion of participants with respiratory adverse events in all studies, although only during the treatment period, and most of these events were mild or moderate. They appeared in the first 24 hours and resolved by one week post‐treatment. The most common symptoms after these procedures were dyspnoea, wheezing, cough, chest discomfort and mucus production. A proportion of participants required hospitalisation because of these adverse events, including, for a case of haemoptysis, respiratory infection, asthma worsening and atelectasis. Longer‐term follow‐up reports have not shown adverse events related to thermoplasty (Pavord 2011; Thomson 2011). No fatalities or other severe disabilities have been reported after bronchial thermoplasty. Taken together, these results show that despite transient mild to moderate respiratory worsening after the bronchoscopic procedure, bronchial thermoplasty seems to be a generally safe treatment.
Five years of observational follow‐up data from the RISA and AIR 2 trials have been published recently, providing data for participants who received bronchial thermoplasty during the trial period (but not for the control groups). Participants treated with thermoplasty in the RISA trial showed a non–statistically significant decrease in the number of visits to the emergency department during the five‐year follow‐up period (mean of 0.12 visits/participant/y) compared with the year before receiving the intervention (0.36 visits/participant/y). These participants also showed a decrease in the number of hospitalisations during follow‐up (overall rate of 0.23 hospitalisations/participant/y during five years) compared with hospitalisation experienced before the intervention (0.71 hospitalisations/participant/y) (Pavord 2013). At five years of follow‐up, participants treated with bronchial thermoplasty in the AIR 2 trial showed a maintained decrease in the rate of severe exacerbations in comparison with the year before treatment. In this trial, the proportions of participants who had severe exacerbations in each follow‐up year compared with the first year after thermoplasty were not significantly different. The reduction in the proportion of participants experiencing severe exacerbations in the year after thermoplasty (30.9%) compared with the 12 months before thermoplasty (51.6%) was maintained during the follow‐up period (average decrease of 44%) (Wechsler 2013). Results from the US Food and Drug Administration (FDA) post‐approval study, with estimated completion during 2018, should bring new data on the durability of the efficacy and safety of the procedure (NCT01350336).
Overall completeness and applicability of evidence
The proposed mechanism of action for bronchial thermoplasty is a reduction in bronchial smooth muscle mass, which may be expected to be associated especially with improvement in pulmonary function. It is surprising to note that the available trials showed no unequivocal benefit for pulmonary function outcomes. This could be explained by the fact that the procedure is limited to central airways accessible by flexible bronchoscopy. Smaller airways are untreated, and it is known that they are an important contributor to airway resistance in asthma patients, especially those < 2 mm in diameter.
Available evidence does not allow fully firm conclusions about the benefits of bronchial thermoplasty because of the lack of information on some important issues. First of all, no accurate data are available regarding the mechanism of action of bronchial thermoplasty in asthma patients. Smooth muscle reduction, the proposed effect of radiofrequency on the airway wall, was observed in animal models and in a small sample of study participants without asthma in a feasibility study (Miller 2005). Although controlled data about the thermoplasty mechanism of action in humans are lacking, reports on a limited series of participants treated with bronchial thermoplasty have been published with inconclusive results (Doeing 2013; Gordon 2013).
Second, taking into account that severe asthma is a very complex condition with a high health and economic burden, the lack of information on long‐term cost‐effectiveness of the procedure is a limitation to the applicability of the results. Similarly, thermoplasty trials have not provided sufficient information regarding potential asthma medication step‐down after treatment, with only the RISA trial showing a limited number of participants who could reduce their steroid therapy appreciably without losing asthma control. Small case series of patients treated out of clinical trials have reported successful weaning off for other severe asthma‐related treatments after bronchial thermoplasty (Bricknell 2012). One point that is striking about two of the studies that we have reviewed in detail is the improvement seen in control participants, especially in subjective outcomes, perhaps related to better compliance with medication whilst participating in a study. Alternatively, it may be that participants are recruited to such studies whilst especially symptomatic, and subsequent improvements are at least partially a regression back towards the mean.
The included trials have shown a proportion of non‐responder participants to bronchial thermoplasty. The AIR 2 trial showed that 21% of participants treated with thermoplasty did not achieve clinically meaningful improvement in quality of life. Possible explanations for this result could include several factors. Severe asthma applies to a heterogeneous group of patients with different inflammatory disease characteristics. Thus, trial inclusion criteria have used only pragmatic definitions of severe asthma based basically on medication requirements, and no information was obtained about details of asthma phenotype, such as neutrophil, eosinophil or pauci‐cellular predominance in sputum. Moreover, differences in study design, number of radiofrequency activations and bronchoscopic management among centres and variable pharmacological treatments for asthma may help to explain differences in the results of thermoplasty.
Quality of the evidence
The available trials were designed adequately, had an adequate allocation of participants and had a complete follow‐up. The only source of bias is the open design of two of the trials (AIR; RISA), which compared the procedure with medical management only. In these trials, a placebo effect cannot be discarded mainly for subjective outcomes such as quality of life, which is critical for judging the benefits of this intervention. Despite this, the remaining trial ensured blinding of participants by comparing bronchial thermoplasty versus a sham procedure in participants allocated to the control group (AIR 2). Even then, however, a substantial beneficial placebo effect was seen in the control group.
A marked baseline clinical imbalance is evident in the RISA trial, in which participants treated with bronchial thermoplasty showed more symptoms at baseline, which poses an additional source of potential bias and concern for interpretation. This trial reported its results in terms of changes from baseline, omitting the absolute outcome data at the end of follow‐up. For the purposes of our pooled analyses, we converted the data from this trial into final scores and imputed baseline standard deviation into our estimates. As a result of this, our estimates could be biased by the direction of the baseline imbalances.
Despite some differences between the trial design (including different comparators in the AIR 2 trial) and the characteristics of included participants (e.g. 100% participants in the RISA trial had severe persistent asthma compared to 57% and 87% in AIR and AIR 2 respectively), the heterogeneity analyses did not reveal these differences as a source of relevant inconsistency.
Potential biases in the review process
Although we tried to follow rigorous standards in this review and performed an exhaustive search that allowed us to include all trials that have assessed the effects of bronchial thermoplasty to date, no review process is completely free of bias. A potential limitation is that the results of our review rely exclusively on data reported in the trial results publications in biomedical journals and in communications to scientific meetings, and we did not formally contact the trial authors. Interpretation of trial results was not always as transparent as expected, as was the case for the AIR trial, whose main publication reported its main results in graphical form only. In future updates of the review, we will try to obtain additional information directly from trialists to avoid this limitation.
Authors' conclusions
Implications for practice.
Bronchial thermoplasty is a novel procedure tested for moderate to severe asthma that provides modest clinical benefit for quality of life but with increased risk of admission to hospital during treatment, which diminishes subsequently. The clearest advantage is related to a reduction in exacerbations and hospitalisations post‐treatment. The benefit in symptoms appeared to be greater in the study that recruited exclusively severe patients on the basis of symptoms, although unfortunately, the main outcome measure was adverse events (RISA), and this study did not include a sham treatment arm. It would be advisable to follow up on the outcomes from participants treated with this procedure by collecting data via independent clinical registries.
Implications for research.
Further research is needed in response to several issues, especially regarding the mechanism of action of thermoplasty in asthma patients, identification of patients and asthma phenotypes that could benefit to the greatest extent, with particular focus on the effect of the intervention in patients with the most severe disease, lower FEV1 and frequent exacerbations. More information is needed on real‐life cost‐effectiveness and long‐term efficacy and safety. Future trials should include sham controls, as in AIR 2, and should assess better the specific airway inflammatory profile in patients with severe asthma.
What's new
| Date | Event | Description |
|---|---|---|
| 28 January 2020 | Amended | The review was amended to include the declarations of interest given at the time of publication via Cochrane's COI forms for all authors. A statement on the declared conflicts and their compliance with Cochrane's policy at the time of publication has been added to Published notes. |
Notes
The first author, AT, received payment from several pharmaceutical companies for lectures and to serve on speakers bureaus. However, as this review does not investigate a pharmaceutical intervention or comparator the Cochrane Airways Editorial team does not believe there to be a genuine or perceived conflict of interest.
Acknowledgements
The authors would like to acknowledge Elizabeth Stovold for designing and updating the search of this review. Also thanks to Emma Welsh and Chris Cates for support and editorial assistance provided during development of the review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL | Quarterly (four issues per year) |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Condition search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
17. exp Aspergillosis, Allergic Bronchopulmonary/
18. lung diseases, fungal/
19. aspergillosis/
20. 18 and 19
21. (bronchopulmonar$ adj3 aspergillosis).mp.
22. 17 or 20 or 21
23. 16 or 22
24. Lung Diseases, Obstructive/
25. exp Pulmonary Disease, Chronic Obstructive/
26. emphysema$.mp.
27. (chronic$ adj3 bronchiti$).mp.
28. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
29. COPD.mp.
30. COAD.mp.
31. COBD.mp.
32. AECB.mp.
33. or/24‐32
34. exp Bronchiectasis/
35. bronchiect$.mp.
36. bronchoect$.mp.
37. kartagener$.mp.
38. (ciliary adj3 dyskinesia).mp.
39. (bronchial$ adj3 dilat$).mp.
40. or/34‐39
41. exp Sleep Apnea Syndromes/
42. (sleep$ adj3 (apnoea$ or apnoea$)).mp.
43. (hypopnoea$ or hypopnoea$).mp.
44. OSA.mp.
45. SHS.mp.
46. OSAHS.mp.
47. or/41‐46
48. Lung Diseases, Interstitial/
49. Pulmonary Fibrosis/
50. Sarcoidosis, Pulmonary/
51. (interstitial$ adj3 (lung$ or disease$ or pneumon$)).mp.
52. ((pulmonary$ or lung$ or alveoli$) adj3 (fibros$ or fibrot$)).mp.
53. ((pulmonary$ or lung$) adj3 (sarcoid$ or granulom$)).mp.
54. or/48‐53
55. 23 or 33 or 40 or 47 or 54
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and the RCT filter are adapted to identify trials in other electronic databases.
Data and analyses
Comparison 1. Bronchial thermoplasty versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 AQLQ final scores at 12 months of follow‐up | 3 | 429 | Mean Difference (IV, Random, 95% CI) | 0.28 [0.07, 0.50] |
| 1.1 Bronchial thermoplasty versus medical management | 2 | 141 | Mean Difference (IV, Random, 95% CI) | 0.44 [0.10, 0.79] |
| 1.2 Bronchial thermoplasty versus sham bronchoscopies | 1 | 288 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.09, 0.45] |
| 2 ACQ final scores at 12 months of follow‐up | 3 | 429 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.40, 0.10] |
| 2.1 Bronchial thermoplasty versus medical management | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.63, ‐0.02] |
| 2.2 Bronchial thermoplasty versus sham bronchoscopies | 1 | 288 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.23, 0.21] |
| 3 Participants admitted to hospital because of respiratory adverse events (treatment period) | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [1.26, 9.68] |
| 4 Participants admitted to hospital because of respiratory adverse events (post‐treatment period) | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.44, 2.85] |
| 5 Use of rescue medication at 12 months of follow‐up (short‐acting bronchodilator puffs per week) | 3 | 429 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐3.63, 2.28] |
| 5.1 Bronchial thermoplasty versus medical management | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐2.65 [‐11.24, 5.95] |
| 5.2 Bronchial thermoplasty versus sham bronchoscopies | 1 | 288 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐3.38, 3.18] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AIR.
| Methods |
Design: randomised controlled trial (NCT00214526) Number of participating centres: 11 from Canada, United Kingdom, Brazil and Denmark |
|
| Participants |
Inclusion criteria: Airflow obstruction (pre‐bronchodilator forced expiratory volume in one second (FEV1) of 60% to 85% of predicted value) Airway hyperresponsiveness (provocative concentration of methacholine required to lower the FEV1 by 20% (PC20) of less than 8 mg/mL) Stable asthma during the six weeks before enrolment (absence of unscheduled physician visits for asthma care, unchanged use of maintenance asthma medication and stable use of rescue medication) Worsening asthma control after abstention from LABA for two weeks (increase in ACQ score ≥ 0.5, or a decline of 5% in PEF during second week) Exclusion criteria: ≥ Three lower respiratory tract infections requiring antibiotics during previous 12 months, or Respiratory tract infection within previous six weeks No. of randomly assigned participants: 112 (56 BT vs 56 control group) Age (mean (SD)): BT group: 39.36 (11.18) versus control group: 41.65 (11.35) Sex (% male): BT group: 44 versus control group: 43 PC20 mg/mL (geometric mean (95% CI)): BT group: 0.25 (0.16 to 0.40) versus control group: 0.35 (0.23 to 0.52) Pre‐bronchodilator FEV1 (% predicted; mean (SD)): BT group: 72.65 (10.41) versus control group: 76.12 (9.28) Dose ICS (µg; mean (SD)): BT group: 1351 (963) versus control group: 1264 (916) Dose LABA (µg; mean (SD)): BT group: 111.3 (35.9) versus control group: 105.8 (30.8) Asthma severity (number of participants with moderate/severe asthma, according to the guidelines of the Global Initiative for Asthma): BT group: 21/26, respectively, versus control group: 34/28, respectively AQLQ score: BT group: 5.58 (1.05) versus control group: 5.72 (0.94) |
|
| Interventions |
Intervention: Bronchial thermoplasty plus medical management. Three bronchial thermoplasty procedures performed three weeks apart Control: medical management |
|
| Outcomes |
Main outcome: Mild exacerbation rate (change from baseline) Secondary outcomes: Severe exacerbation rate Pre‐bronchodilator FEV1 (percentage predicted) Post‐bronchodilator FEV1 (percentage predicted) Peak expiratory flow (morning and evening) (change from baseline) Asthma Control Questionnaire (ACQ) score (change from baseline) Use of rescue medications Use of maintenance medications Asthma Quality of Life Questionnaire (AQLQ) score (change from baseline) Total symptom score (change from baseline) Percent of symptom‐free days (change from baseline) Respiratory adverse events during treatment period Hospitalisations during treatment period for respiratory adverse events (percentage of participants) Hospitalisations during post‐treatment period (number of participants) |
|
| Notes | Funded by Asthmatx Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally generated computer randomisation sequence in blocks of four participants |
| Allocation concealment (selection bias) | Low risk | Randomisations provided in sealed envelopes to each participating centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes collected by participants in diaries monitored by the research staff at medical meetings or in telephone interviews |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis planned with no imputation of missing data 112 participants randomly assigned (56 to each group, but baseline data reported for 109 participants) Participants lost to follow‐up (three‐month visit): four participants in the bronchial thermoplasty group versus eight in the control group (three and four participants, respectively, withdrew consent; one and four participants, respectively, were lost to follow‐up) Participants lost to follow‐up (12‐month visit): complete data for 52 participants in the bronchial thermoplasty group versus 49 in the medical management group |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the study protocol (NCT00214526) reported in the main publication (Cox 2007) Most trial results reported in graphics, but complementary data on electronic appendices allow a more comprehensive analysis of the results |
AIR 2.
| Methods |
Design: randomised double‐blind controlled trial (NCT00231114) Number of participating centres: 30 from USA, Canada, Brazil, United Kingdom, the Netherlands and Australia |
|
| Participants |
Inclusion criteria: Participants on stable maintenance asthma medications for at least four weeks before entry Baseline Asthma Quality of Life Questionnaire (AQLQ) score 6.25 or lower Pre‐bronchodilator FEV1 > 60% of predicted Airway hyperresponsiveness (methacholine PC20 8 mg/mL) At least two days of asthma symptoms during the four‐week baseline period Being a non‐smoker for at least one year with less than 10 pack‐year smoking history Exclusion criteria: Life‐threatening asthma Chronic sinus disease Respiratory diseases such as emphysema Use of immunosuppressants, beta‐adrenergic blocking agents or anticoagulants History in the previous year of three or more hospitalisations for asthma History in the previous year of three or more lower respiratory tract infections History in the previous year of four or more pulses of OCS use for asthma No. of randomly assigned participants: 297 (196 BT vs 101 placebo) Age (mean (SD)): BT group: 40.7 (11.9) versus control group: 40.6 (11.9) Sex (% men): BT group: 42.6 versus control group: 38.8 PC20 mg/mL (geometric mean (95% CI)): BT group: 0.27 (0.22 to 0.34) versus control group: 0.31 (0.22 to 0.43) Pre‐bronchodilator FEV1 (% predicted: mean (SD)): BT group: 77.8 (15.65) versus control group: 79.7 (15.14) Dose OCS (mg/d): BT group: 6.4 (1.97) versus control group: 5 Dose ICS (µg/d): BT group: 1960.7 versus control group: 1834.8 Dose LABA (µg/d): BT group: 116.8 (34.39) versus control group: 110.3 (26.7) Asthma severity (percentage of participants with severe refractory asthma according to criteria of the American Thoracic Society) BT group: 86% versus control group: 88% AQLQ score: BT group: 4.3 (1.17) versus control group: 4.32 (1.21) |
|
| Interventions |
Intervention: bronchial thermoplasty plus medical management Control: sham bronchoscopies plus medical management |
|
| Outcomes |
Main outcome: Asthma Quality of Life Questionnaire (AQLQ) score (change from baseline) Secondary outcomes: Percentage symptom‐free days (change from baseline) Total symptom score (change from baseline) Number of puffs of rescue medication used (change from baseline) Percentage days rescue medication used (change from baseline) Asthma Control Questionnaire (ACQ) score (change from baseline) Morning peak expiratory flow (change from baseline) Pre‐ and post‐bronchodilator FEV1 (predicted) Number of severe exacerbations (per participant per year) Percentage of participants experiencing severe exacerbations Respiratory‐related unscheduled physician office visits Emergency department (ED) visits (per participant per year) Hospitalisations in the post‐treatment period (percentage of participants) Days lost from work/school or other activities due to asthma (days/y) |
|
| Notes | Funded by Asthmatx Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence computer generated using minimisation |
| Allocation concealment (selection bias) | Unclear risk | The publication of the trial results did not describe the efforts made to ensure concealment of the randomisation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial ensured blinding of participants by comparing bronchial thermoplasty versus a sham procedure. The control group received three bronchoscopies that involved sedation and a mimic procedure to that performed in the bronchial thermoplasty group. A catheter was deployed into the airways through a bronchoscope, an electrode array was expanded and a radiofrequency controller was activated, simulating indistinguishable audio and visual signals, but no radiofrequency energy was delivered. Duration and time intervals between groups coincided Participants in both groups were unable to guess their treatment after the first bronchoscopy, but in the second procedure, a larger proportion of participants in the bronchial thermoplasty group guessed their assigned treatment (the trial publication reported within‐group comparison P values at second bronchoscopy of 0.011 for bronchial thermoplasty group vs 0.342 for sham group) The bronchoscopy team was unblinded to participant assignments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All follow‐up and assessment visits were conducted by a blinded assessment team" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimum recruitment goal of 225 participants (150 in the bronchial thermoplasty group and 75 in the sham group) described Intention‐to‐treat analysis described as participants who were randomly assigned and received at least a bronchoscopy 288 participants randomly assigned (190 in the bronchial thermoplasty group and 98 in the sham group), 278 completed the 12 months of follow‐up visits (93% of the sample) Participants lost to follow‐up: nine participants in the bronchial thermoplasty group versus one participant in the control group No participants were withdrawn from the study for asthma worsening Six participants in the bronchial thermoplasty group were withdrawn by investigators (none in the control group), and two participants in each group were withdrawn for medical reasons |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the study protocol (NCT00231114) were reported in the main publication (Castro 2010) |
RISA.
| Methods |
Design: randomised controlled trial (NCT00214539) Number of participating centres: eight from United Kingdom, Canada and Brazil |
|
| Participants |
Inclusion criteria: Participants with asthma aged 18 to 65 years Requirement of high‐dose inhaled corticosteroid (ICS) and LABA with or without oral prednisone (< 30 mg/d), leukotriene modifiers or theophylline Pre‐bronchodilator FEV1 > 50% of predicted Airway hyperresponsiveness by challenge with methacholine or reversible bronchoconstriction during prior 12 months Uncontrolled symptoms despite taking maintenance medication Abstinence from smoking for at least one year and past smoking history of less than 10 pack‐years Exclusion criteria (not specified in the main publication (Pavord 2007); obtained from clinicaltrials.gov): Participation in another clinical trial involving respiratory intervention that could affect the outcome measures of this study, within six weeks before randomisation. Participants will be disqualified from the study if they enter another study or fail to comply with prescribed asthma medications Use of immunosuppressant therapy (e.g. methotrexate) Current or recent lower respiratory tract infection (resolved within six weeks of enrolment testing) History of recurrent (no more than three in the last three months) lower respiratory tract infections requiring antibiotics Presence of other respiratory diseases including emphysema, cystic fibrosis, vocal cord dysfunction, mechanical upper airway obstruction, obstructive sleep apnoea, Churg‐Strauss syndrome, cardiac dysfunction or allergic bronchopulmonary aspergillosis DLCO (diffusion capacity) < 70% predicted Uncontrolled sinus disease Uncontrolled gastroesophageal reflux disease Use of implanted electronic device such as a pacemaker or internal cardiac defibrillator Use of external pacemaker Significant co‐morbid illness such as cancer, renal failure, liver disease or cerebral vascular disease Post‐bronchodilator FEV1 of less than 55% predicted Known systemic hypersensitivity or contraindication to methacholine chloride or other parasympathomimetic agents Known sensitivity to medications required to perform bronchoscopy, including lidocaine, atropine, benzodiazepines and opioids Use of a systemic beta‐adrenergic blocking agent Other medical criteria. No. of randomly assigned participants: 34 (17 BT vs 17 control group) Age (mean (SD)): BT group: 39.1 (13) versus control group: 42.1 (12.6) Sex (% male): BT group: 40 versus control group: 59 PC20 mg/mL (geometric mean (95% CI)): BT group: 0.19 (0.05 to 0.76) versus control group: 0.31 (0.08 to 1.26) Pre‐bronchodilator FEV1 (% predicted; mean (SD)): BT group: 69.9 (12.2) versus control group: 66.4 (17.8) Dose OCS (mg/d; mean (SD)): BT group: 14.4 (6.2) versus control group: 18.6 (9.8) Dose ICS (µg/d; mean (SD)): BT group: 1166.7 (408.3) versus control group: 1058.9 (336.9) Dose LABA (µg/d; mean (SD)): BT group: 125 (60.5) versus control group: 136.7 (45.5) Asthma severity (percentage of participants with severe persistent asthma according to criteria of the Global Initiative for Asthma (GINA) and the American Thoracic Society (ATS)): BT group: 100 (GINA) versus control group: 100 (GINA) AQLQ score (mean (SD)): BT group: 3.96 (1.34) versus control group: 4.72 (1.06) |
|
| Interventions |
Intervention: bronchial thermoplasty plus medical management Control: medical management |
|
| Outcomes |
Main outcome: Respiratory adverse events per participant Secondary outcomes: Use of maintenance medications Use of rescue medications Total symptom score Morning and evening peak expiratory flow (PEF) Pre‐bronchodilator FEV1 (percentage predicted) Post‐bronchodilator FEV1 (percentage predicted) Asthma Control Questionnaire (ACQ) score Asthma Quality of Life Questionnaire (AQLQ) score Symptom‐free days |
|
| Notes | Funded by Asthmatx Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally generated computer randomisation sequence in blocks of four participants |
| Allocation concealment (selection bias) | Low risk | Randomisation provided to each participating centre in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes collected by participants in diaries monitored by the research staff at medical meetings or in telephone interviews |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial publication did not provide details about the plan needed to perform the analysis of results 34 participants randomly assigned (17 to each group) Participants lost to follow‐up: two participants in the bronchial thermoplasty group withdrawn from the study before receiving treatment in keeping with a recommendation to not treat them from the study Data and Safety Monitoring Board 15 participants in the bronchial thermoplasty group and 17 in the control group included in the final analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the study protocol (NCT00214539) were reported in the main publication (Pavord 2007) |
BT: bronchial thermoplasty.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hales 2010 | Meeting abstract, discussion about patient indication for bronchial thermoplasty |
| Wechsler 2009 | Meeting abstract, discussion about patient indication for bronchial thermoplasty in the allergy setting |
Differences between protocol and review
Although the protocol of the review stated that adverse events would be reported in terms of serious complications derived from bronchial thermoplasty, the included trials reported data about relevant adverse events during the treatment period, and so we decided to include them in that form. The protocol stated that we would contact authors of included trials to ask them about other published or unpublished studies, but we did not contact them formally, as we had stated in the protocol we would do.
Asthma control was added as a primary outcome and adverse events was added as a secondary outcome.
Contributions of authors
AT, IS: eligibility
AT, IS, JJY‐N, AMM: data extraction
AT, IS: risk of bias assessment
IS: summary of findings table
IS, MRF: data analysis
AT, IS, AMM: data interpretation and drafting of the review
All review authors have contributed to the conception of the review and have provided relevant contributions to its discussion and conclusions.
Haydn Walters was the assigned editor for this review. Haydn commented critically and edited the review text.
Sources of support
Internal sources
Iberoamerican Cochrane Centre, Spain.
External sources
-
Programa Enlaza‐Mundos. Alcaldía de Medellín 2011, Colombia.
Postgraduate Training Fellowship
Declarations of interest
AT: Received payment for lectures including service on speakers bureaus GSK, Astra Zeneca, Esteve, Prodesfarma, Pfizer, Chiesi. Furthermore AT is a member of a Scientific Steering Committee of an international registry of patients treated with bronchial thermoplasty. He works at the Respiratory Department of the Hospital de la Santa Creu I Sant Pau, and since October 12 performs bronchial thermoplasty in patients with severe asthma.
IS: None known
AMM: None known
MRiF: None known
JJY‐N: None known
PAC: None known
VP: Recieved payment for lectures including service on speakers bureaus from NOvartis, AstraZeneca and Merck and as a consultant for Almirall. He also received payment for development of educational presentations from AstraZeneca, Chiesi and Amgem and travel/accommodations/meeting expenses unrelated to activities listed from AstraZeneca and Merck.
Edited (no change to conclusions)
References
References to studies included in this review
AIR {published data only}
- Cox G, Laviolette M, Rubin A, Corris P, Niven R, Thomson N, et al. 5‐Year safety of bronchial thermoplasty demonstrated in patients with moderate to severe asthma: Asthma Intervention Research (AIR) trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A6839. [Google Scholar]
- Cox G, Miller J, Rubin A, Corris P, Thomson N, Niven R, et al. Asthma intervention research (AIR) trial evaluating bronchial thermoplasty: early results [Abstract]. American Thoracic Society International Conference; 2006 May 19‐21; San Diego. 2006:A711;Poster A45.
- Cox G, Miller JD, Rubin AS, Corris P, Thomson NC, Niven R, et al. Impact of bronchial thermoplasty on asthma status interim results from the AIR trial [Abstract]. European Respiratory Journal 2006;28(Suppl 50):114s;Abstract 749. [Google Scholar]
- Cox G, Rubin A, Laviolette M, Pavord I, Thomson N. Efficacy of bronchial thermoplasty (BT) in patients with severe asthma: the AIR2 trial [Abstract]. European Respiratory Society 19th Annual Congress; 2009 Sep 12‐15; Vienna. 2009:3044.
- Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, et al. Asthma control during the year after bronchial thermoplasty. The New England Journal of Medicine 2007;356(13):1327‐37. [PUBMED: 17392302] [DOI] [PubMed] [Google Scholar]
- Laviolette M, Thomson N, Niven R, Siersted H, McCormack D, Miller J, et al. Asthma intervention research (AIR) trial: early safety assessment of bronchial thermoplasty [Abstract]. American Thoracic Society 100th international conference; 2004 May 21‐26; Orlando. 2004:B37; Poster G26.
- Niven R, Thomson N, Corris P, Olivenstein R, Siersted H, Pavord I, et al. Bronchial thermoplasty benefits patients following withdrawal of LABA therapy [Abstract]. American Thoracic Society International Conference; 2010 May 14‐19; New Orleans. 2010:P1174.
- Niven RM, Cox G, Miller JD, Laviolette M, Rubin AS, Corris PA, et al. Responder analysis of AQLQ in the Asthma Intervention Research (AIR) trial [Abstract]. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl 1):S6. [Google Scholar]
- Pavord I, Thomson N, Niven R, Shah P, Mansor A, Hacken HT. Persistent effect of bronchial thermoplasty (BT) in severe asthma at two years [Abstract]. European Respiratory Society 20th Annual Congress; 2010 Sep 18‐22; Barcelona. 2010:P2631.
- Pavord ID, Thomson NC, Niven RM, Corris PA, Chung KF, Cox G, et al. for the Research in Severe Asthma Trial Study Group. Safety of bronchial thermoplasty in patients with severe refractory asthma. Annals of Allergy, Asthma & Immunology 2013;111(5):402‐7. [DOI] [PubMed] [Google Scholar]
- Prys‐Picard CO, Niven R, Thomson N, Corris P, Olivenstein R, Siersted H, et al. Bronchial thermoplasty benefits patients following withdrawal of LABA therapy [Abstract]. Annals of Allergy, Asthma and Immunology 2010;105(5 Suppl):Not provided. [Google Scholar]
- Rubin A, Laviolette M, Thomson N, Niven R, Corris P, Siersted H, et al. Bronchial thermoplasty improves asthma status of moderate‐to‐severe persistent asthmatics over and above current standard‐of‐care [Abstract]. Chest 2006;130(4):162s. [Google Scholar]
- Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R, et al. Long‐term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulmonary Medicine 2011;11:8. [PUBMED: 21314924] [DOI] [PMC free article] [PubMed] [Google Scholar]
AIR 2 {published data only}
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G, et al. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Annals of Allergy, Asthma & Immunology 2011;107(1):65‐70. [PUBMED: 21704887] [DOI] [PubMed] [Google Scholar]
- Castro M, Rubin AS, Laviolette M, Fiterman J, Andrade Lima M, Shah PL, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double‐blind, sham‐controlled clinical trial. American Journal of Respiratory and Critical Care Medicine 2010;181(2):116‐24. [PUBMED: 19815809] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro M, Rubin AS, Laviolette M, Hanania NA, Cox G. Two‐year persistence of effect of bronchial thermoplasty (BT) in patients with severe asthma: AIR2 Trial [Abstract]. Chest 2010;138(4):768A. [Google Scholar]
- Castro M, Rubin AS, Laviolette M, Thomson NC. Efficacy of bronchial thermoplasty (BT) in patients with severe asthma: the AIR2 trial [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20 San Diego. 2009:A3644.
- Shah P, Fiterman J, McEvoy C, Erzurum S, AIR2. Safety of bronchial thermoplasty (BT) in patients with severe, symptomatic asthma: positive safety profile in the AIR2 trial [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20 San Diego. 2009:A2814; Poster #J82.
- Wechsler ME, Laviolette M, Rubin AS, Fiterman J, Lapa E, Silva JR, et al. for the Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long‐term safety and effectiveness in patients with severe persistent asthma. Journal of Allergy and Clinical Immunology 2013;132(6):1295‐302. [DOI: 10.1016/j.jaci.2013.08.009; PUBMED: 23998657] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler ME, Shah PL, Niven R, Thomson NC, Rubin A, Fiterman J, Lapa JR, Laviolette M, Olivenstein R, Hales JB, Shifren A, Cox GP, Shargill NS, Armstrong B, Castro M. Benefits of bronchial thermoplasty persist out to 5 years in patients with severe asthma. American journal of respiratory and critical care medicine. 2013; Vol. 187:A6068.
RISA {published data only}
- Pavord I, Laviolette M, Thomson N, Niven R, Cox G, Corris P. 5‐Year Safety Of Bronchial Thermoplasty Demonstrated In Patients With Severe Refractory Asthma: Research In Severe Asthma (RISA) Trial. American Thoracic Society International Conference; 2011 May 13‐18; Denver. 2011; Vol. 183:A6382.
- Pavord ID, Cox G, Thomson NC, Rubin AS, Corris PA, Niven RM, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. American Journal of Respiratory and Critical Care Medicine 2007;176(12):1185‐91. [PUBMED: 17901415] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Hales 2010 {published data only}
- Hales JB, Wechsler ME, Niven R, Prys‐Picard C, Castro M, Cox G. Patient selection for bronchial thermoplasty (BT) following FDA approval: lessons learned from multiple clinical trials [Abstract]. Annals of Allergy, Asthma and Immunology 2010;105(5 Suppl):Not provided. [Google Scholar]
Wechsler 2009 {published data only}
- Wechsler M, Cox G, Niven R, Zoratti E, Hanania N. Where could bronchial thermoplasty (BT) fit into your allergy practice? Lessons learned from multiple BT clinical trials [Abstract]. Annual Scientific Meeting of the American College of Allergy, Asthma and Immunology; 2009 Nov 5‐10; Miami. 2009.
Additional references
Bricknell 2012
- Bicknell S, Chaudhuri R, Shepherd M, Lee N, Pitman N, Spears M, et al. Introducing bronchial thermoplasty treatment into a severe asthma clinical service. Thorax 2012;67:A65‐A66. [DOI: 10.1136/thoraxjnl-2012-202678.146] [DOI] [Google Scholar]
Brown 2005
- Brown RH, Wizeman W, Danek C, Mitzner W. In vivo evaluation of the effectiveness of bronchial thermoplasty with computed tomography. Journal of Applied Physiology 2005;98(5):1603‐6. [PUBMED: 15718404] [DOI] [PubMed] [Google Scholar]
Cayetano 2011
Danek 2004
- Danek CJ, Lombard CM, Dungworth DL, Cox PG, Miller JD, Biggs MJ, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. Journal of Applied Physiology 2004;97(5):1946‐53. [PUBMED: 15258133] [DOI] [PubMed] [Google Scholar]
Doeing 2013
- Doeing DC, Husain AN, Naureckas ET, White SR, Hogarth DK. Bronchial thermoplasty failure in severe persistent asthma: a case report. The Journal of Asthma 2013;50(7):799‐801. [PUBMED: 23651158] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2011
- Global Strategy for Asthma Management, Prevention. Global Initiative for Asthma (GINA) guidelines 2011. www.ginasthma.org/ (accessed 11 April 2012).
Gordon 2013
- Gordon IO, Husain AN, Charbeneau J, Krishnan JA, Hogarth DK. Endobronchial biopsy: a guide for asthma therapy selection in the era of bronchial thermoplasty. The Journal of Asthma 2013;50(6):634‐41. [PUBMED: 23621125] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jones 2002
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. European Respiratory Journal 2002;19(3):398‐404. [PUBMED: 11936514] [DOI] [PubMed] [Google Scholar]
Juniper 1994
- Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease‐specific quality of life questionnaire. Journal of Clinical Epidemiology 1994;47(1):81‐7. [PUBMED: 8283197] [DOI] [PubMed] [Google Scholar]
Juniper 1999
- Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. The European Respiratory Journal 1999;14(4):902‐7. [PUBMED: 10573240] [DOI] [PubMed] [Google Scholar]
Mayse 2007
- Mayse M, Laviolette M, Rubin A, Lampron N, Simoff M, Duhamel D, et al. Clinical pearls for bronchial thermoplasty. How I do it. Journal of Bronchology 2007;14:115‐23. [Google Scholar]
Miller 2005
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005;127(6):1999‐2006. [PUBMED: 15947312] [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore WC, Peters SP. Severe asthma: an overview. Journal of Allergy and Clinical Immunology 2006;117(3):487‐94. [PUBMED: 16522445] [DOI] [PubMed] [Google Scholar]
NCT01350336
- Bronchial Thermoplasty in Severe Persistent Asthma (PAS2). http://clinicaltrials.gov/ct2/show/NCT01350336 (accessed 7 May 2013). [NCT01350336]
Pavord 2011
- Pavord I, Laviolette M, Thomson N, Niven R, Cox G, Corris P, et al. 5‐Year safety of bronchial thermoplasty demonstrated in patients with severe refractory asthma: Research In Severe Asthma (RISA) trial [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A6382. [Google Scholar]
Pavord 2013
- Pavord ID, Thomson NC, Niven RM, Corris PA, Chung KF, Cox G, et al. for the Research in Severe Asthma Trial Study Group. Safety of bronchial thermoplasty in patients with severe refractory asthma. Annals of Allergy, Asthma & Immunology 2013;111(5):402‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rodriguez‐Trigo 2008
- Rodríguez‐Trigo G, Plaza V, Picado C, Sanchis J. Management according to the Global Initiative for Asthma Guidelines with near‐fatal asthma reduces morbidity and mortality. Archivos de Bronconeumologia 2008;44:192‐6. [PubMed] [Google Scholar]
Taylor 2008
- Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, et al. A new perspective on concepts of asthma severity and control. European Respiratory Journal 2008;32(3):545‐54. [PUBMED: 18757695] [DOI] [PubMed] [Google Scholar]
Thomson 2011
- Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R, et al. Long‐term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulmonary Medicine 2011;11:8. [PUBMED: 21314924] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torrego 2010
- Torrego Fernández, A. Bronchial thermoplasty in the treatment of asthma. Archivos de Bronconeumologia 2010;46(2):85‐91. [DOI] [PubMed] [Google Scholar]
Wahidi 2012
- Wahidi MM, Kraft M. Bronchial thermoplasty for severe asthma. American Journal of Respiratory and Critical Care Medicine 2012;185(7):709‐14. [PUBMED: 22077066] [DOI] [PubMed] [Google Scholar]
Wechsler 2013
- Wechsler ME, Laviolette M, Rubin AS, Fiterman J, Lapa E Silva JR, Shah PL, et al. for the Asthma Intervention Research 2 Trial Study Group. Bronchial thermoplasty: long‐term safety and effectiveness in patients with severe persistent asthma. Journal of Allergy and Clinical Immunology. 2013 Aug 30. pii: S0091‐6749(13)01268‐2. [DOI: 10.1016/j.jaci.2013.08.009; PUBMED: 23998657] [DOI] [PMC free article] [PubMed]


