INTRODUCTION
With age, immunologic function changes substantially, resulting in impaired responses to pathogens or vaccines. As a result, older adults are at increased risk for morbidity and mortality from infectious diseases and impaired responses to vaccination 1. Clearly, non-immunologic factors also contribute to these adverse outcomes; for example, age-related changes in chest wall mechanics and lung elasticity may affect respiratory mechanics and medications may affect cough—all potential contributors to respiratory infection risk 2. However, it is evident that immunologic changes influence host defense against infection. The aging immune system is characterized by a variety of alterations that encompass developmental impairment, diminished signaling and the effects of antigen exposure history on chronic inflammation and antigen receptor repertoire diversity —all of which contribute to defects in immune activation in response to pathogens or vaccines. However, the aged innate immune system also shows substantial inflammatory dysregulation with a paradoxical heightened pro-inflammatory environment; this may arise in part from endogenous stimuli linked to cellular damage. Here we provide an overview of age-related changes in human host defense, with an emphasis on consequences for outcomes to pathogens or vaccines in older adults.
CHANGES IN INNATE IMMUNITY WITH AGING
The innate immune system is the first line of defense in mounting a host resistance response to antigens; it is responsible for the earliest responses to pathogens or vaccines 3, 4. Innate immune responses are mediated by a network of cell types that include neutrophils, monocytes/macrophages, dendritic cells, natural killer cells (NK cells), eosinophils, and basophils; endothelial and epithelial cells may also play roles in innate immunity 4. Innate immune responses are closely linked to the activation of inflammatory processes including phagocytosis, intracellular killing, pathogen-induced pro-inflammatory cytokine production, and upregulation of co-stimulatory proteins on antigen presenting cells (APCs) such as dendritic cells, monocytes, or macrophages. Such co-stimulatory protein expression provides additional signals facilitating T cell activation, and thus links innate to adaptive (i.e. mediated by antigen receptors on B and T cells) immune responses.
Age-related Dysregulation of Inflammation
Several lines of evidence indicate that chronic inflammation is a characteristic of the aging immune system in humans. In particular, levels of pro-inflammatory cytokines (particularly IL-6, but also TNF-α, IL-1β and others), acute phase reactants such as C-reactive protein (CRP) and clotting factors (including D-dimer) are generally elevated in older, compared to young adults 5–10. Moreover, such increases in cytokine production have been correlated with all-cause mortality in several studies 11–13. Basal elevation of pro-inflammatory cytokines and other products may affect the ability of the aged immune system to respond to new pathogens or vaccines; in this regard, both pro- and anti-inflammatory cytokine production may be augmented, resulting in more complex patterns of age-related inflammatory dysregulation 14. The etiology underlying this heightened pro-inflammatory state (termed Inflamm-Aging 15, 16) remains incompletely understood, but may in part reflect the consequences of cellular damage and endogenous activators of the innate immune system, as described below.
Neutrophils
Neutrophils are short-lived cells that are among the first to migrate in response to an infectious agent. For example, chemotaxis, describing movement toward a gradient of a stimulus (such as a chemokine or cytokine), appears impaired in neutrophils from older, compared to young adults 17–19. Moreover, phagocytosis of pathogens such as Streptococcus pneumoniae as well as intracellular killing both appeared impaired in neutrophils from older, versus young individuals 20, 21. In addition, the generation of neutrophil extracellular traps (NETs), extracellular scaffolds of extruded chromatin containing antimicrobial peptides and proteases, is also diminished in neutrophils from older adults—further affecting pathogen capture and killing 22. Several age-related signal transduction defects have been reported in neutrophils; for example, diminished accuracy of neutrophil migration with age has been linked to increased Phosphoinositide (PI)-3 kinase neutrophil signal transduction 18. Other alterations may influence neutrophil survival by affecting anti-apoptotic pathways mediated by Granulocyte Macrophage Colony Stimulating Factor (GM-CSF) 23, 24; moreover, TLR-1-dependent cytokine production, induction of activation markers and glucose utilization were all diminished in neutrophils from older, compared to young adults 25. While most of these studies indicate that neutrophil function is diminished with age, it should also be noted that defects in chemotaxis for example may result in both impaired trafficking to sites of infection and inappropriate persistence of neutrophils during the resolution of inflammation. Indeed, such delayed neutrophil egress from the lungs has been found in a murine model of burn injury 26; such persistence, even of neutrophils with impaired function, could contribute to a heightened pro-inflammatory milieu in the context of aging.
Monocytes
Monocytes also contribute to innate immune responses as a source for cytokine and chemokine production. They are particularly adept at migrating from the circulation to sites of infection and inflammation, where they differentiate into macrophages and also to dendritic cells 27. Studies of monocyte function in the context of human aging have revealed evidence for both impaired function and in some cases, a dysregulated, enhanced pro-inflammatory response. For example, studies of Toll-like Receptor (TLR) function, a family of invariant membrane-associated receptors recognizing conserved portions of pathogens (so-called pathogen-associated molecular patterns, or PAMPs), reveal an age-associated decrease in IL-6 and TNF-α production following stimulation of the TLR1/2 heterodimer with triacylated bacterial lipoproteins 28, 29. In addition, a generalized impairment in expression of co-stimulatory proteins (such as CD80) was found in monocytes from older, compared to young adults following in vitro stimulation with a variety of TLR agonists 30; such co-stimulatory proteins interact with ligands found on the T cell surface, and together with the T cell receptor for antigen, facilitate optimal T cell activation and link innate to adaptive immune responses. These alterations in TLR-induced costimulatory protein expression could be expected to adversely affect innate and adaptive immunity, and indeed a significant association was found between TLR-induced costimulatory protein expression and antibody response to influenza vaccination 30.
Other studies of monocyte function in the context of aging reveal further evidence of age-related dysregulation of inflammatory responses. For example, monocytes partially activated following isolation by adherence to plastic in vitro showed an age-associated increase in TLR5-induced cytokine production, using the TLR5 ligand flagellin 31; interestingly, an increase in TLR5-induced cytokine production was also found in macrophages from aged mice 32, suggesting a potential role for monocyte activation in augmenting some innate immune responses (and the possibility that preserved TLR5 function could be utilized for vaccine adjuvants in older adults).
Several studies suggest that the proportion of so-called inflammatory monocytes expressing high levels of both CD14 and CD16 on the cell surface (as compared to classical monocytes which are CD14+ CD16− and non-classical CD14lo CD16hi monocytes) is increased in older adults. Such inflammatory monocytes have been reported to be increased in adults with HIV, sepsis, myocardial infarction and other conditions associated with increased inflammation27; CD14+ CD16+ monocytes from older adults showed evidence of senescence and increased cytokine production in response to LPS stimulation of TLR4 ex vivo 28, 33–35. The effects of aging on monocyte function, however, may be more complex, as studies of cytokine production in monocytes (using intracellular cytokine staining) following influenza vaccination in the absence of ex vivo stimulation revealed diminished production of IL-6 and TNF-α in both inflammatory (which were induced post-vaccination) and classical monocytes from older, compared to young adults. By contrast, basal levels of the anti-inflammatory cytokine IL-10 were markedly elevated in monocyte populations in older, but not young adults; an age-associated alteration in activation of a negative regulator of IL-10, Dual Specificity Phosphatase (DUSP)-1 suggested a potential basis for impaired cytokine responses following vaccination or exposure to pathogens 36. Interestingly, some studies have utilized CD14+CD16+ monocytes as an indicator of age-related inflammation, and have demonstrated that resistance exercise training in older adults resulted in diminished levels of such monocytes and decreased LPS-induced pro-inflammatory cytokine production 37, 38; these findings suggest that some changes associated with age-related inflammation may be reversible.
Dendritic Cells
Dendritic cells (DCs) are professional antigen presenting cells that also undergo age related changes contributing to the impaired host resistance. These cells may be broadly divided into myeloid DCs (mDCs), which express a variety of TLRs and are critical for production of IL-12 in Th1 responses; and plasmacytoid DCs (pDCs), which express a more narrow range of TLRs (such as TLR7 and TLR9) and are particularly adept at producing type I interferons in response to viral infections. TLR-induced cytokine production (including pro-inflammatory cytokines such as TNF-α, IL-6 and IL-12 in mDCs and type I interferons in pDCs) in both mDCs and pDCs appears diminished in cells from older, compared to young adults in response to ex vivo activation of a broad range of TLRs, and the extent of TLR-induced cytokine production was strongly correlated with influenza vaccine antibody response 39. Several additional studies indicate that pDCs and monocyte-derived DCs (which may be generated in vitro using growth factor stimulation and likely resemble DCs generated under inflammatory conditions) are defective in type I interferon production in response to stimulation by viruses such as West Nile and influenza virus 40, 41, and indeed, gene expression microarray analyses of monocyte-derived DCs have revealed decreased expression of interferon-stimulated genes 42. At the same time, there is evidence for inflammatory dysregulation in DC populations as well. For example, LPS or self DNA-induced cytokine production was increased in monocyte-derived DCs from older, compared to young adults, juxtaposed with in vitro impairment in migration (as assessed in vitro using a chemotaxis assay) 42–44. In addition, basal elevation in cytokine production has been found in pDCs, mDCs, and monocyte-derived DCs from older, but not young adults in the absence of ex vivo stimulation 39, 45. The findings of impaired type I interferon production but increased TLR-induced cytokine production in monocyte-derived DCs may reflect differences in signal transduction pathways mediating pro-inflammatory cytokine vs. interferon production; alternatively, innate immune signaling pathways in addition to TLRs were likely induced in these monocyte-derived DC studies, which employed viral stimulation that would also engage cytoplasmic innate immune receptors recognizing viral nucleic acids such as Retinoic Acid Inducible Gene-I (RIG-I), Melanoma Differentiation Associated Gene-5 (MDA-5), and others. Nonetheless, taken together, these studies provide evidence that DCs may reflect the aging-associated pro-inflammatory environment.
Natural Killer Cells
NK Cells are the predominant class of innate immune lymphocytes 46, and function in cytokine production (identified as CD56bright CD16- NK cells) and cytotoxicity, particularly of virus-infected or cancer cells (CD56dim CD16+ cells). Cytotoxicity functions are regulated by a balance between activating receptors and receptors such as the Killer Immunoglobulin-like Receptors (KIRs) that are inhibited by Major Histocompatibility Complex (MHC) Class I engagement. In general, NK cells in older adults show an increase in the CD56dim CD16+ cytotoxic population, which also represents a more differentiated population compared to CD56bright NK cells 47–52. Expression of activating cytotoxicity receptors is also diminished with age 53, 54, with diminished cytotoxic function in older adults that has been linked to decreased mobilization of perforin to the NK cell immunologic synapse 55. The consequences of age-related NK cell dysfunction remain incompletely understood, but it is worth noting that NK function has been associated with infection risk in at least one study of nursing home residents 56.
Origins of Age-Related Inflammation
The etiology of so-called Inflamm-aging remains incompletely understood, but is likely to reflect a combination of factors, not all of which originate in the immune system. For example, hormonal changes in the context of aging likely contribute; the loss of estrogen and testosterone production with age is associated with increased inflammatory markers in humans 57, 58. In addition, there is decreased production of dihydroepiandosterone (DHEA) in older adults, a corticosteroid with immune enhancing properties (such as promoting Th1 cytokine production and decreasing LPS-induced TNF-α production) 59. Cells other than those typically associated with the immune system may also contribute to age-related inflammation, and indeed, a recent study of gene expression contributors to the age-related increase in IL-6 found a relatively limited contribution of elevated cytokine transcripts from leukocytes 60. In this regard, evidence from animal models suggests that macrophages infiltrating adipose tissue shifts to a pro-inflammatory profile with age 61. Moreover, small studies of human adipose tissue revealed an age-related accumulation of fat cell progenitors that may contribute to age-related inflammation62, particularly in the context of the so-called senescence-associated secretory phenotype (SASP)63. The SASP refers to the secretome of senescent cells (induced by replicative senescence or DNA damage for example), and in adipocytes could be inhibited by treatment with Jun-activated Kinase (JAK) inhibitors 62. Recent studies in model systems have also identified the mammalian Target of Rapamycin (mTOR, which has pleiotropic effects in regulating the balance between anabolic and catabolic metabolism64) as another modulator of the SASP, where mTOR inhibition with rapamycin (clinically employed in higher doses for transplant immunosuppression) also inhibited SASP pro-inflammatory cytokine production 65, 66. mTOR inhibition using rapamycin (clinically employed for transplant immunosuppression in higher doses) has previously been shown to extend lifespan in mice and ameliorate inflammation associated with cerebral ischemia or heart failure 67–69. Notably, low-dose rapamycin given prior to influenza vaccination in older adults resulted in a 20% increase in antibody titers compared to older adults given placebo 70. As a result, there is considerable interest in developing mTOR inhibitors that can modulate immunosenscence-associated inflammation.
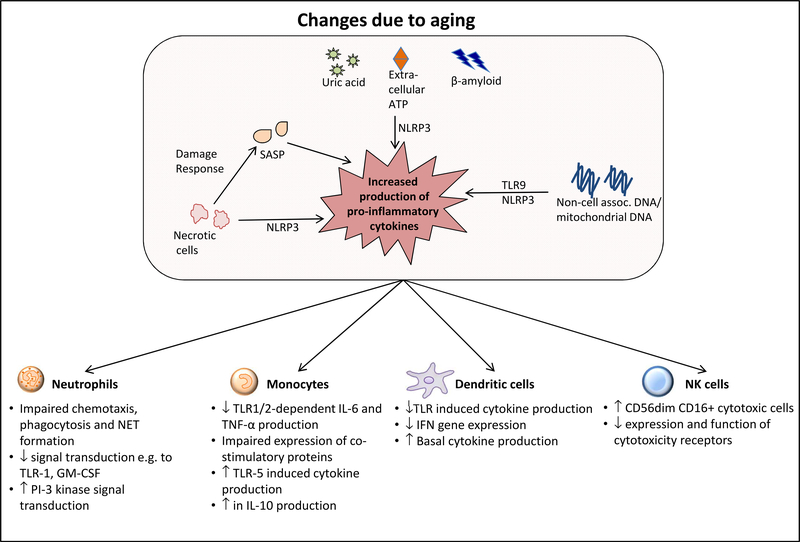
In addition to the SASP, the origins of chronic inflammation in older adults could reflect the presence of endogenous damage-associated molecular patterns (DAMPs) that activate innate immune pattern recognition receptors. For example, levels of non-cell-associated DNA, presumably released from damaged or dying cells, are elevated in older adults 71–73; mitochondrial DNA (mtDNA) in particular is increased in older, compared to young adults, and correlates with increased levels of pro-inflammatory cytokines at baseline and in trauma patients 74,75. Notably, human monocytes treated with mtDNA develop endotoxin tolerance, in which they are refractory to subsequent TLR4 stimulation with LPS—a potential mechanism contributing to impaired innate immune responses in the setting of chronic inflammation 76. The mechanisms by which mtDNA activates the innate immune system likely involve TLR9 recognition of DNA; consistent with this, mtDNA induced NET formation in human neutrophils (with impaired NET formation in older adults) was reduced with TLR9 inhibition 74. Oxidized mtDNA generated in the context of mitochondrial dysfunction and apoptosis activates the NOD-like Receptor NLRP3, a cytoplasmic innate immune pattern recognition receptor 77. NLRP3 activation results in the formation of the NLRP3 inflammasome, a multi-protein scaffold containing NLRP3 and the adaptor protein Apoptosis Inducing Speck-like Protein Containing a CARD Domain (ASC) 78. When assembled, the NLRP3 inflammasome mediates the Caspase 1-dependent processing of pro-IL-18 and pro-IL-1β to their activated, cleaved forms. In addition to oxidized mtDNA, NLRP3 is also engaged by necrotic cell damage 79–81; in fact, the wide range of endogenous (uric acid, extracellular ATP, oxidized mtDNA, ceramide, beta-amyloid), exogenous (silica, asbestos, alum), and infectious (influenza virus, bacterial pore forming toxins, fungi) NLRP3 activators suggests that this receptor could contribute substantially to age-related inflammatory dysregulation 78. Indeed, studies in NLRP3-deficient mice revealed a decrease in age-associated inflammation and improvements in bone loss and cognitive function 82; in addition, recent findings in mice indicate that ASC aggregates from activated inflammasomes can be released from dying cells into the extracellular space, where they may be taken up by other cells to activate new inflammasomes and amplify a pro-inflammatory stimulus 83, 84. Taken together, these findings suggest NLRP3 as a potential target for therapeutic intervention to modulate inflammation in older adults. Changes in the innate immune system with age are depicted in Figure 1.
Figure 1.
Age-associated alterations in the innate immune system. Contributing factors to a heighted basal pro-inflammatory state, and dysregulated responses in individual cell lineages are summarized. Abbreviations: TLR Toll-like Receptor; NLRP3 NOD-like Receptor Protein 3; SASP Senescence-Associated Secretory Phenotype; NET neutrophil extracellular traps; TNF-α tumor necrosis factor-α; IL interleukin. See text for details.
CHANGES IN THE ADAPTIVE IMMUNE SYSTEM WITH AGING
Activation of the innate immune system facilitates the T and B cell mediated adaptive immune response through the production of pro-inflammatory cytokines and expression of costimulatory proteins on monocyte/macrophages or dendritic cells. Such costimulatory proteins (e.g. CD80, CD86 and others) interact with ligands on T cells (such as CD28) to provide critical second signals for optimal T cell activation, in conjunction with T cell receptor (TCR) recognition of a peptide from a pathogen or vaccine bound to a major histocompatibility antigen protein on the APC cell surface. As previously discussed, this innate immune activation is dysregulated and generally impaired with aging; however, the function of the adaptive immune system is also disrupted in older adults, reflecting the effects of chronic antigenic stimulation and exposures and intrinsic alterations in B and T cell development and function. Here, we provide a brief update, emphasizing recent findings regarding adaptive immunosenescence in humans.
T cell aging
Bone marrow progenitor cells migrate to the thymus, where T cell development, or thymopoiesis, occurs. Thymopoiesis is notable for stages of proliferative expansion, and for positive selection (where T cells expressing a TCR that can recognize host MHC proteins on APCs are selected) and negative selection (where T cells recognizing autoreactive, or “self” antigens are deleted) processes. The thymus begins to involute during childhood, and continues to involute at a rate of approximately 3% per year in adults 85. Not surprisingly, thymic involution is accompanied by a decline in generation of new (naïve) T cells. While some studies have suggested that some degree of naïve T cell generation continues in adulthood, one study using metabolic labeling and detection of T cell Receptor Excision Circles (TRECs) (the nonreplicating products of VDJ recombination at TCR loci that are associated with naïve T cells) has concluded that the vast majority (approximately 90% of CD4 T cells) of T cells in older adults are generated not from thymic activity, but from division of cells in the existing T lymphocyte pool 86. Consequently, in older adults most T cells appear to be antigen-experienced memory T cells. The effects of thymic involution on T cell development were demonstrated in a study of young adults who had underwent thymectomy in the first month of life in the context of surgery for congenital heart disease. Numbers of CD4 and CD8 T cells were reduced in such adults, compared to young adults who had not undergone thymectomy, with diminished proportions of naïve T cells. Notably, a subset of young adults in the thymectomy group had T cell parameters that were comparable to aged adults 75 years of age or older, including levels of non-malignant oligoclonal T cell expansions frequently found in older adults 87. The occurrence of an aged T cell profile in thymectomized young adults was strongly associated with seropositivity to cytomegalovirus (CMV), consistent with a substantial body of literature supporting the notion that control of CMV has a profound effect on the T cell compartment in the context of aging 88–92. CMV may reactivate throughout life without end-organ damage, such as in the setting of the stress of medical or surgical illness 93. Notably, a recent study evaluating CMV seronegative and seropositive adults concluded that CMV appears to be the dominant factor in driving the increased proportion of effector memory T cells and likely also non-malignant oligoclonal T cell expansion seen in older adults 94. Indeed, significant levels of CMV-specific, dysfunctional T cells can be detected in older adults 91, 95–98, and functional outcomes such as mental status testing or ability to carry out activities of daily living have been associated with CMV seropositivity or the presence of CMV-specific CD4 T cells 99.
Analyses of TCR repertoire using high throughput sequencing methods and PBMCs as starting material revealed decreased diversity with age 100; another analysis of purified naïve and memory T cell populations revealed a more modest 3–5 fold decrease in diversity that may still be sufficient for adequate adaptive immune responses 101. This last study was notable for analyzing multiple replicate libraries from purified T cell subsets and employed nonparametric statistical methods designed to address some of the challenges in measuring the immense potential range of T cell diversity from relatively limited sample amounts 102. It should be noted that CMV status of analyzed participants was unknown in the study showing a greater contraction in repertoire 100 and that CMV-seropositive subjects were excluded from the second study 101. It seems likely that the effects of oligoclonal CMV-specific T cell expansion, if present, would result in substantial contraction in TCR diversity.
In addition to age-related decreases in T cell generation, developmental and signaling alterations are found in cells from older adults which contribute to functional deficits. For example, one of the most reliable age-related findings in the human T cell compartment is the loss of CD28 expression on CD8 T cells 103. CD28 interacts with cell-associated costimulatory protein ligands such as CD80 and CD86 on APCs to provide a crucial second signal for T cell activation (in conjunction with TCR recognition by peptide bound to host MHC proteins on APCs); the loss of CD28 expression in CD8 T cells is associated with alterations in T cell activation in older individuals, and early studies indicated that reconstitution of CD28 expression in CD8+ CD28− human T cells restored IL-2 production 104. In addition to loss of CD28, CD8 T cells express other markers of exhaustion, replicative senescence, or terminal differentiation in the context of aging (such as PD-1, CD57, or KLRG1) that limit the response to pathogens or vaccines 105–107.
In contrast to aged mice, fewer studies have addressed signaling deficits in human T cells. However, in CD4 T cells from older, compared to young adults, alterations in signaling have been found in naïve cells, where an increase in Dual Specific Phosphatase (DUSP)-6 protein expression was linked to a decline in expression of a specific micro RNA (miR-181a)—resulting in decreased phosphorylation of the Extracellular Signal Regulated Kinase (ERK), a member of the Mitogen Activated Protein (MAP) Kinase family transducing TCR signals 108. In memory CD4 T cells from older adults, increased gene expression of another MAP Kinase phosphatase, DUSP4, also resulted in impaired ERK signaling 109. In addition to alterations in signal transduction resulting in impaired TCR-dependent activation, recent studies have reported that the senescence phenotype (characterized by features such as inhibited telomerase expression and decreased proliferation and TCR signaling) in both CD8 effector memory and CD4 T cells (which lack CD28 expression) is strongly associated with aberrant signaling via the p38 MAP kinase; notably, p38 inhibition appeared to reverse the senescence phenotype 110, 111. Taken together, these findings provide evidence for both diminished activation (as in the ERK pathway) and inappropriate dysregulation (for p38) of signal transduction in T cells from older adults—mirroring the dysregulation and decreased responsiveness in the aged innate immune system.
B Cell Aging
Like T cells, the B cell lineage generates a highly diverse repertoire of rearranged antigen receptor genes, and there is evidence from analyses of Complementarity Determining Region 3 (CDR3) in the Ig heavy chain variable region that diversity in bulk populations of B cells is substantially reduced with age and with changes in functional status such as the geriatric syndrome of frailty 112; chronic infection with EBV (which specifically infects B cells) or CMV influences B cell repertoire, which may also be altered by the presence of non-malignant oligoclonal B cell expansion 112, 113. It should be noted, however, that some studies have shown relative preservation of diversity in tonsillar B cells 114. Current use of next generation sequencing in purified B cell populations has also revealed evidence for age-related repertoire changes, although challenges remain in incorporating subject heterogeneity and variation into the analyses of the enormous amount of sequence information from studies of relatively few individuals 115.
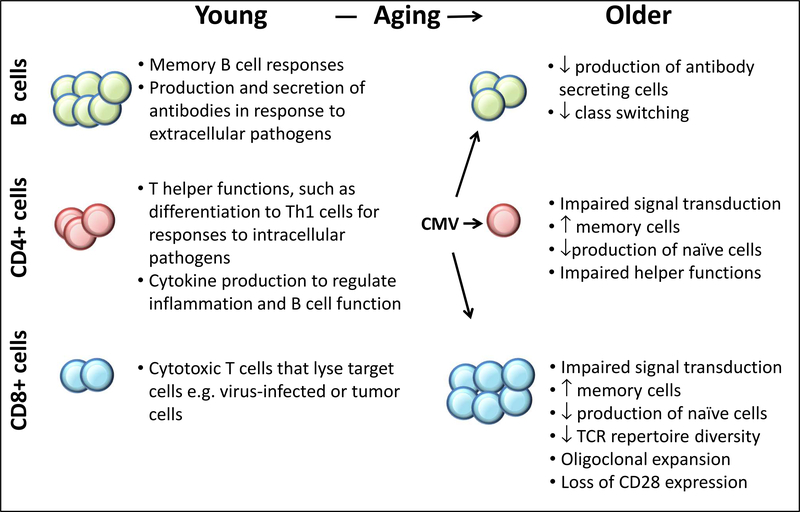
Mature B cells express a rearranged immunoglobulin antigen receptor on their cell surface, and may undergo differentiation to plasma cells secreting immunoglobulin of the same specificity in defense against extracellular pathogens. Because many B cell functions are dependent on T cell help, the effects of aging on the B cell lineage reflect both B cell intrinsic changes and those resulting from altered T-B cell interactions; an example of this would be impaired antibody response to influenza vaccine linked to impaired induction of antibody-producing plasmablasts 116. However, intrinsic B cell defects have been found in expression of activation induced cytidine deaminase (AID), a protein that is essential for heavy chain class switching, in which the constant region exon (denoting the isotype and correlated with function, such as a μ constant region exon for IgM, γ1 constant region for IgG1and so on) encoding an expressed immunoglobulin (Ig) heavy chain “switches” to a different exon, with deletion of the original exon and intervening DNA. AID is also required for another B cell-specific process: somatic hypermutation, in which the variable region of an expressed Ig gene in mature B cells found in germinal centers of secondary lymphoid organs such as lymph nodes undergoes further mutation to enhance its affinity for a given antigen. The age-related impairment of AID expression was associated with a decreased proportion of B cells that had undergone class switching and with impaired influenza vaccine antibody responses 117, 118. Decreased AID expression was linked to decreased levels of the E47 transcription factor that regulates AID, and to upregulation in expression of specific microRNA species 118, 119. Notably, memory B cells from older adults were found to have increased gene expression of TNF-α, and the extent of basal TNF-α mRNA was negatively correlated with proliferative responses 120. Finally, a history of CMV infection may also influence B cell function; individuals with a positive CMV serology had increased intracellular levels of TNF-α in B cells and diminished AID gene expression and switched memory B cell levels 121. These findings in B cells reflect the dysregulated inflammatory responses found in cells of the innate immune system discussed above, as well as the effects of CMV on T cell function in older adults. A summary of age-associated alterations in B and T cell function is depicted in Figure 2.
Figure 2.
Age-associated alterations in the adaptive immune system. Contrasts in function in B and T cells in young and older adults are summarized. Abbreviations: TCR T-cell Receptor; CMV Cytomegalovirus.
SYSTEMS ANALYSIS OF IMMUNE AGING
Several studies have employed global analyses of cytokine production or gene expression to understand age-related alterations in immune response. In general, these studies have shown that immunologic challenge in older adults results in impaired responses when compared to young adults, but with evidence for dysregulated or delayed responses. Such a delayed and diminished gene expression signature of cytokine production in response to TLR4, TLR7/8, and Retinoid acid-Inducible Gene-1 (RIG-I, a cytoplasmic innate immune PRR RNA helicase that senses RNA, particularly in the setting of viral infection) was found in analyses of human PBMCs from older, compared to young adults 122. Transcriptomic analyses of PBMCs before and after influenza vaccination revealed altered kinetics for early induction of interferon-stimulated genes and for a day 7 post-vaccine plasma cell gene expression signature associated with a successful vaccine antibody response 123. These studies also revealed age-related dysregulation of innate immune signaling pathways and a mitochondrial biogenesis gene expression signature that was associated with vaccine response in young and older adults, suggesting an intriguing link between metabolic activity and vaccine response. Other studies of influenza vaccination in older adults showed that pre-vaccine expression of apoptosis pathways was also correlated with vaccine response 124. These studies have excluded neutrophils, which are not found in the PBMC compartment, but a recent study of neutrophil gene expression in patients with hemorrhagic shock revealed an impaired innate immune response in older, compared to young adults 125. Notably, older adults showed persistent gene expression signatures reflecting both inflammation and immunosuppressive states (such as impaired expression of antigen presentation or co-stimulatory proteins) at later timepoints when neutrophils from young adults had trended toward baseline—consistent with an emerging theme of impaired but dysregulated (and frequently delayed) response in the aged immune system.
FUTURE DIRECTIONS
Understanding the biology of altered immune response in the context of aging has obvious clinical impact, and future studies should incorporate the intrinsic heterogeneity in human cohorts (extending beyond gender and race to comorbid medical conditions, medication use, smoking, alcohol use and other factors). In particular, the immunologic basis of alterations in functional status in older adults, such as frailty, remains incompletely understood. Examples of additional complexity include age-associated epigenetic effects, such as changes in methylation status, that strongly correlate with age and mortality 126, 127. Finally, studies of age-related changes in the intestinal microbiome are in early stages 128, but it seems clear that engagement of innate and adaptive immunity by commensal organisms will influence immune responses to pathogens or vaccines. Integrating such complexity and heterogeneity into studies of the aging immune system will be challenging, particularly for large data sets arising from transcriptomic, microbiome, or other analyses, but will be essential to provide increasingly detailed insights for translation to pathways for therapeutic modulation and improvement of health in older adults.
Synopsis.
Aging of the human immune system results in impaired responses to pathogens or vaccines. In the innate immune system, which mediates the earliest pro-inflammatory responses to immunologic challenge, processes ranging from Toll-like Receptor function to Neutrophil Extracellular Trap formation are generally impaired in older adults. However, examples of enhanced inflammation with age, such as in TLR function, are also present, reflecting tissue context or cellular activation state. This inflammatory dysregulation is reflected in the age-associated increase in plasma cytokine, acute phase reactants, and clotting factors, termed “Inflamm-aging”, that likely results from contributions from immunologic and non-immunologic cells, the response to cellular damage, and the presence of endogenous damage-associated ligands activating the innate immune system. In the adaptive immune system, T and B cell subsets and function are altered with age, with examples of both impaired signaling and inappropriate activation, and shifts toward antigen experienced cells at the expense of naïve cells in both lineages. The control of cytomegalovirus infection, particularly in the T lineage, plays a dominant role in the differentiation of the T cell compartment and in the diversity of antigen receptors available to respond to pathogens or vaccines.
Key Points.
Immunosenescence, describing age-associated changes in the immune system, generally results in impaired immune responses, and contributes to the increased morbidity and mortality to infectious diseases and diminished vaccine responses found in older adults.
A heightened pro-inflammatory environment, characterized by increased levels of pro- and anti-inflammatory cytokines, acute phase reactants, and clotting factors, is found in older adults.
Age-related chronic inflammation contributes to dysregulation of innate immune responses, potentially limiting or delaying further activation or contributing to inappropriate persistence of inflammation.
B and T cell signal transduction and function in the adaptive immune system are both impaired in the context of aging. Chronic antigen stimulation throughout life, particularly in the control of cycles of cytomegalovirus reactivation, substantially diminishes the diversity of antigen receptors, particularly in the T cell lineage.
ACKNOWLEDGEMENTS
The authors’ work was supported by the National Institutes of Health (HHS N272201100019C, U19 AI089992, K24 AG042489, and T32 AI007517 ), and was carried out in collaboration with the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342). The authors regret being unable to include many important articles due to space and scope limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Yoshikawa TT. Epidemiology and unique aspects of aging and infectious diseases. Clin Infect Dis 2000;30:931–33. [DOI] [PubMed] [Google Scholar]
- 2.Vaz Fragoso CA and Gill TM. Respiratory impairment and the aging lung: A novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci 2012;67:264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulop T, Le Page A, Fortin C et al. Cellular signaling in the aging immune system. Curr Opin Immunol 2014;29:105–11. [DOI] [PubMed] [Google Scholar]
- 4.Shaw AC, Goldstein DR and Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol 2013;13:875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruunsgaard H, Andersen-Ranberg K, Jeune B et al. A high plasma concentration of tnf-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci 1999;54:M357–64. [DOI] [PubMed] [Google Scholar]
- 6.Fagiolo U, Cossarizza A, Scala E et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol 1993;23:2375–8. [DOI] [PubMed] [Google Scholar]
- 7.Mari D, Mannucci PM, Coppola R et al. Hypercoagulability in centenarians: The paradox of successful aging. Blood 1995;85:3144–9. [PubMed] [Google Scholar]
- 8.Paolisso G, Rizzo MR, Mazziotti G et al. Advancing age and insulin resistance: Role of plasma tumor necrosis factor-alpha. Am J Physiol 1998;275:E294–9. [DOI] [PubMed] [Google Scholar]
- 9.Stowe RP, Peek MK, Cutchin MP et al. Plasma cytokine levels in a population-based study: Relation to age and ethnicity. J Gerontol A Biol Sci Med Sci 2010;65:429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei J, Xu H, Davies JL et al. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci 1992;51:1953–6. [DOI] [PubMed] [Google Scholar]
- 11.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg J et al. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am J Med 2003;115:278–83. [DOI] [PubMed] [Google Scholar]
- 12.Harris TB, Ferrucci L, Tracy RP et al. Associations of elevated interleukin-6 and c-reactive protein levels with mortality in the elderly. Am J Med 1999;106:506–12. [DOI] [PubMed] [Google Scholar]
- 13.Reuben DB, Cheh AI, Harris TB et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc 2002;50:638–44. [DOI] [PubMed] [Google Scholar]
- 14.Morrisette-Thomas V, Cohen AA, Fulop T et al. Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev 2014;139:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franceschi C, Bonafe M, Valensin S et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- 16.Salvioli S, Monti D, Lanzarini C et al. Immune system, cell senescence, aging and longevity--inflamm-aging reappraised. Curr Pharm Des 2013;19:1675–9. [PubMed] [Google Scholar]
- 17.Niwa Y, Kasama T, Miyachi Y et al. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: Cross-sectional and longitudinal studies. Life Sci 1989;44:1655–64. [DOI] [PubMed] [Google Scholar]
- 18.Sapey E, Greenwood H, Walton G et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: Toward targeted treatments for immunosenescence. Blood 2014;123:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenisch C, Patruta S, Daxbock F et al. Effect of age on human neutrophil function. J Leukoc Biol 2000;67:40–5. [DOI] [PubMed] [Google Scholar]
- 20.Butcher SK, Chahal H, Nayak L et al. Senescence in innate immune responses: Reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol 2001;70:881–6. [PubMed] [Google Scholar]
- 21.Simell B, Vuorela A, Ekstrom N et al. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 2011;29:1929–34. [DOI] [PubMed] [Google Scholar]
- 22.Hazeldine J, Harris P, Chapple IL et al. Impaired neutrophil extracellular trap formation: A novel defect in the innate immune system of aged individuals. Aging Cell 2014;13:690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortin CF, Larbi A, Dupuis G et al. GM-CSF activates the JAK/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology 2007;8:173–87. [DOI] [PubMed] [Google Scholar]
- 24.Fortin CF, Larbi A, Lesur O et al. Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J Leukoc Biol 2006;79:1061–72. [DOI] [PubMed] [Google Scholar]
- 25.Qian F, Guo X, Wang X et al. Reduced bioenergetics and Toll-like receptor 1 function in human polymorphonuclear leukocytes in aging. Aging (Albany NY) 2014;6:131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomellini V, Brubaker AL, Mahbub S et al. Dysregulation of neutrophil CXCR2 and pulmonary endothelial ICAM-1 promotes age-related pulmonary inflammation. Aging Dis 2012;3:234–47. [PMC free article] [PubMed] [Google Scholar]
- 27.Wong KL, Yeap WH, Tai JJ et al. The three human monocyte subsets: Implications for health and disease. Immunol Res 2012;53:41–57. [DOI] [PubMed] [Google Scholar]
- 28.Nyugen J, Agrawal S, Gollapudi S et al. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol 2010;30:806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Duin D, Mohanty S, Thomas V et al. Age-associated defect in human TLR-1/2 function. J Immunol 2007;178:970–5. [DOI] [PubMed] [Google Scholar]
- 30.van Duin D, Allore HG, Mohanty S et al. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis 2007;195:1590–7. [DOI] [PubMed] [Google Scholar]
- 31.Qian F, Wang X, Zhang L et al. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell 2012;11:104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim JS, Nguyen KC, Nguyen CT et al. Flagellin-dependent TLR5/Caveolin-1 as a promising immune activator in immunosenescence. Aging Cell 2015;14:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hearps AC, Martin GE, Angelovich TA et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 2012;11:867–75. [DOI] [PubMed] [Google Scholar]
- 34.Merino A, Buendia P, Martin-Malo A et al. Senescent CD14+CD16+ monocytes exhibit proinflammatory and proatherosclerotic activity. J Immunol 2011;186:1809–15. [DOI] [PubMed] [Google Scholar]
- 35.Seidler S, Zimmermann HW, Bartneck M et al. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol 2010;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohanty S, Joshi SR, Ueda I et al. Prolonged proinflammatory cytokine production in monocytes modulated by interleukin 10 after influenza vaccination in older adults. J Infect Dis 2015;211:1174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markofski MM, Flynn MG, Carrillo AE et al. Resistance exercise training-induced decrease in circulating inflammatory CD14+CD16+ monocyte percentage without weight loss in older adults. Eur J Appl Physiol 2014;114:1737–48. [DOI] [PubMed] [Google Scholar]
- 38.Timmerman KL, Flynn MG, Coen PM et al. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: A role in the anti-inflammatory influence of exercise? J Leukoc Biol 2008;84:1271–8. [DOI] [PubMed] [Google Scholar]
- 39.Panda A, Qian F, Mohanty S et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol 2010;184:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prakash S, Agrawal S, Cao JN et al. Impaired secretion of interferons by dendritic cells from aged subjects to influenza : Role of histone modifications. Age (Dordr) 2013;35:1785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian F, Wang X, Zhang L et al. Impaired interferon signaling in dendritic cells from older donors infected in vitro with West Nile Virus. J Infect Dis 2011;203:1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao JN, Agrawal A, Sharman E et al. Alterations in gene array patterns in dendritic cells from aged humans. PLoS One 2014;9:e106471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agrawal A, Agrawal S, Cao JN et al. Altered innate immune functioning of dendritic cells in elderly humans: A role of phosphoinositide 3-kinase-signaling pathway. J Immunol 2007;178:6912–22. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal A, Tay J, Ton S et al. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol 2009;182:1138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prakash S, Agrawal S, Vahed H et al. Dendritic cells from aged subjects contribute to chronic airway inflammation by activating bronchial epithelial cells under steady state. Mucosal Immunol 2014;7:1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spits H, Artis D, Colonna M et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 2013;13:145–9. [DOI] [PubMed] [Google Scholar]
- 47.Campos C, Pera A, Lopez-Fernandez I et al. Proinflammatory status influences NK cells subsets in the elderly. Immunol Lett 2014;162:298–302. [DOI] [PubMed] [Google Scholar]
- 48.Campos C, Pera A, Sanchez-Correa B et al. Effect of age and CMV on NK cell subpopulations. Exp Gerontol 2014;54:130–7. [DOI] [PubMed] [Google Scholar]
- 49.Borrego F, Alonso MC, Galiani MD et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol 1999;34:253–65. [DOI] [PubMed] [Google Scholar]
- 50.Chidrawar SM, Khan N, Chan YL et al. Ageing is associated with a decline in peripheral blood cd56bright nk cells. Immun Ageing 2006;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayhoe RP, Henson SM, Akbar AN et al. Variation of human natural killer cell phenotypes with age: Identification of a unique KLRG1-negative subset. Hum Immunol 2010;71:676–81. [DOI] [PubMed] [Google Scholar]
- 52.Le Garff-Tavernier M, Beziat V, Decocq J et al. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell 2010;9:527–35. [DOI] [PubMed] [Google Scholar]
- 53.Solana R, Campos C, Pera A et al. Shaping of NK cell subsets by aging. Curr Opin Immunol 2014;29:56–61. [DOI] [PubMed] [Google Scholar]
- 54.Solana R, Tarazona R, Gayoso I et al. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 2012;24:331–41. [DOI] [PubMed] [Google Scholar]
- 55.Hazeldine J, Hampson P and Lord JM. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell 2012;11:751–9. [DOI] [PubMed] [Google Scholar]
- 56.Ogata K, An E, Shioi Y et al. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin Exp Immunol 2001;124:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abu-Taha M, Rius C, Hermenegildo C et al. Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol 2009;183:1393–402. [DOI] [PubMed] [Google Scholar]
- 58.Maggio M, Basaria S, Ble A et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab 2006;91:345–7. [DOI] [PubMed] [Google Scholar]
- 59.Hazeldine J, Arlt W and Lord JM. Dehydroepiandrosterone as a regulator of immune cell function. J Steroid Biochem Mol Biol 2010;In Press. [DOI] [PubMed] [Google Scholar]
- 60.Pilling LC, Joehanes R, Melzer D et al. Gene expression markers of age-related inflammation in two human cohorts. Exp Gerontol 2015;70:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lumeng CN, Liu J, Geletka L et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol 2011;187:6208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu M, Tchkonia T, Ding H et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proceedings of the National Academy of Sciences 2015;112:E6301–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tchkonia T, Zhu Y, van Deursen J et al. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. The Journal of Clinical Investigation 2013;123:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson SC, Rabinovitch PS and Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature 2013;493:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herranz N, Gallage S, Mellone M et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 2015;17:1205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laberge RM, Sun Y, Orjalo AV et al. mTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1a translation. Nat Cell Biol 2015;17:1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flynn JM, O’Leary MN, Zambataro CA et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 2013;12:851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison DE, Strong R, Sharp ZD et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009;460:392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie L, Sun F, Wang J et al. Mtor signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory t cells after focal ischemia. J Immunol 2014;192:6009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mannick JB, Del Giudice G, Lattanzi M et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med 2014;6:268ra179. [DOI] [PubMed] [Google Scholar]
- 71.Jylhava J, Jylha M, Lehtimaki T et al. Circulating cell-free DNA is associated with mortality and inflammatory markers in nonagenarians: The vitality 90+ study. Exp Gerontol 2012;47:372–8. [DOI] [PubMed] [Google Scholar]
- 72.Jylhava J, Kotipelto T, Raitala A et al. Aging is associated with quantitative and qualitative changes in circulating cell-free DNA: The vitality 90+ study. Mech Ageing Dev 2011;132:20–6. [DOI] [PubMed] [Google Scholar]
- 73.Jylhava J, Nevalainen T, Marttila S et al. Characterization of the role of distinct plasma cell-free DNA species in age-associated inflammation and frailty. Aging Cell 2013;12:388–97. [DOI] [PubMed] [Google Scholar]
- 74.Itagaki K, Kaczmarek E, Lee YT et al. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS One 2015;10:e0120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pinti M, Cevenini E, Nasi M et al. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging”. Eur J Immunol 2014;44:1552–62. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez-Ruiz I, Arnalich F, Cubillos-Zapata C et al. Mitochondrial damps induce endotoxin tolerance in human monocytes: An observation in patients with myocardial infarction. PLoS One 2014;9:e95073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimada K, Crother TR, Karlin J et al. Oxidized mitochondrial DNA activates the nlrp3 inflammasome during apoptosis. Immunity 2012;36:401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elliott EI and Sutterwala FS. Initiation and perpetuation of nlrp3 inflammasome activation and assembly. Immunol Rev 2015;265:35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imaeda AB, Watanabe A, Sohail MA et al. Acetaminophen-induced hepatotoxicity in mice is dependent on TLR9 and the NALP3 inflammasome. J Clin Invest 2009;119:305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Iyer SS, Pulskens WP, Sadler JJ et al. Necrotic cells trigger a sterile inflammatory response through the NLRP3 inflammasome. Proc Natl Acad Sci U S A 2009;106:20388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H, Ambade A and Re F. Cutting edge: Necrosis activates the NLRP3 inflammasome. J Immunol 2009;183:1528–32. [DOI] [PubMed] [Google Scholar]
- 82.Youm YH, Grant RW, McCabe LR et al. Canonical NLRP3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab 2013;18:519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baroja-Mazo A, Martin-Sanchez F, Gomez AI et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 2014;15:738–48. [DOI] [PubMed] [Google Scholar]
- 84.Franklin BS, Bossaller L, De Nardo D et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol 2014;15:727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palmer DB. The effect of age on thymic function. Front Immunol 2013;4:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.den Braber I, Mugwagwa T, Vrisekoop N et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 2012;36:288–97. [DOI] [PubMed] [Google Scholar]
- 87.Sauce D, Larsen M, Fastenackels S et al. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest 2009;119:3070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chidrawar S, Khan N, Wei W et al. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol 2009;155:423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Litjens NH, de Wit EA and Betjes MG. Differential effects of age, cytomegalovirus-seropositivity and end-stage renal disease (ESRD) on circulating T lymphocyte subsets. Immun Ageing 2011;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Looney RJ, Falsey A, Campbell D et al. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol 1999;90:213–9. [DOI] [PubMed] [Google Scholar]
- 91.Ouyang Q, Wagner WM, Voehringer D et al. Age-associated accumulation of CMV− specific CD8+ T cells expressing the inhibitory killer cell lectin-like receptor g1 (KLRG1). Exp Gerontol 2003;38:911–20. [DOI] [PubMed] [Google Scholar]
- 92.Wikby A, Johansson B, Olsson J et al. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: The swedish nona immune study. Exp Gerontol 2002;37:445–53. [DOI] [PubMed] [Google Scholar]
- 93.Limaye AP, Kirby KA, Rubenfeld GD et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 2008;300:413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wertheimer AM, Bennett MS, Park B et al. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating t cell subsets in humans. J Immunol 2014;192:2143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hadrup SR, Strindhall J, Kollgaard T et al. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific t cells in the very elderly. J Immunol 2006;176:2645–53. [DOI] [PubMed] [Google Scholar]
- 96.Khan N, Shariff N, Cobbold M et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol 2002;169:1984–92. [DOI] [PubMed] [Google Scholar]
- 97.Ouyang Q, Wagner WM, Wikby A et al. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J Clin Immunol 2003;23:247–57. [DOI] [PubMed] [Google Scholar]
- 98.Ouyang Q, Wagner WM, Zheng W et al. Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp Gerontol 2004;39:607–13. [DOI] [PubMed] [Google Scholar]
- 99.Vescovini R, Biasini C, Telera AR et al. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J Immunol 2010;184:3242–9. [DOI] [PubMed] [Google Scholar]
- 100.Britanova OV, Putintseva EV, Shugay M et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol 2014;192:2689–98. [DOI] [PubMed] [Google Scholar]
- 101.Qi Q, Liu Y, Cheng Y et al. Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci U S A 2014;111:13139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laydon DJ, Bangham CR and Asquith B. Estimating T-cell repertoire diversity: Limitations of classical estimators and a new approach. Philos Trans R Soc Lond B Biol Sci 2015;370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weng NP, Akbar AN and Goronzy J. CD28(−) T cells: Their role in the age-associated decline of immune function. Trends Immunol 2009;30:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Topp MS, Riddell SR, Akatsuka Y et al. Restoration of CD28 expression in CD28− CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J Exp Med 2003;198:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chou JP and Effros RB. T cell replicative senescence in human aging. Curr Pharm Des 2013;19:1680–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dolfi DV, Mansfield KD, Polley AM et al. Increased t-bet is associated with senescence of influenza virus-specific CD8 T cells in aged humans. J Leukoc Biol 2013;93:825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Henson SM and Akbar AN. KLRG1--more than a marker for T cell senescence. Age (Dordr) 2009;31:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li G, Yu M, Lee WW et al. Decline in mir-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med 2012;18:1518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu M, Li G, Lee WW et al. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc Natl Acad Sci U S A 2012;109:E879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Henson SM, Lanna A, Riddell NE et al. P38 signaling inhibits mTORC1-independent autophagy in senescent human CD8(+) T cells. J Clin Invest 2014;124:4004–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lanna A, Henson SM, Escors D et al. The kinase p38 activated by the metabolic regulator AMPK and scaffold TAB1 drives the senescence of human T cells. Nat Immunol 2014;15:965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gibson KL, Wu YC, Barnett Y et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 2009;8:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang C, Liu Y, Xu LT et al. Effects of aging, cytomegalovirus infection, and EBV infection on human B cell repertoires. J Immunol 2014;192:603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kolar GR, Mehta D, Wilson PC et al. Diversity of the Ig repertoire is maintained with age in spite of reduced germinal centre cells in human tonsil lymphoid tissue. Scand J Immunol 2006;64:314–24. [DOI] [PubMed] [Google Scholar]
- 115.Martin V, Bryan Wu YC, Kipling D et al. Ageing of the B-cell repertoire. Philos Trans R Soc Lond B Biol Sci 2015;370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sasaki S, Sullivan M, Narvaez CF et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest 2011;121:3109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Frasca D, Diaz A, Romero M et al. Intrinsic defects in B cell response to seasonal influenza vaccination in elderly humans. Vaccine 2010;28:8077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frasca D, Landin AM, Lechner SC et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol 2008;180:5283–90. [DOI] [PubMed] [Google Scholar]
- 119.Frasca D, Diaz A, Romero M et al. Micrornas mir-155 and mir-16 decrease AID and E47 in B cells from elderly individuals. J Immunol 2015;195:2134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frasca D, Diaz A, Romero M et al. High TNF-alpha levels in resting B cells negatively correlate with their response. Exp Gerontol 2014;54:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frasca D, Diaz A, Romero M et al. Cytomegalovirus (CMV) seropositivity decreases B cell responses to the influenza vaccine. Vaccine 2015;33:1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Metcalf TU, Cubas RA, Ghneim K et al. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 2015;14:421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thakar J, Mohanty S, West AP et al. Aging-dependent alterations in gene expression and a mitochondrial signature of responsiveness to human influenza vaccination. Aging (Albany NY) 2015;7:38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Furman D, Jojic V, Kidd B et al. Apoptosis and other immune biomarkers predict influenza vaccine responsiveness. Mol Syst Biol 2013;9:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vanzant EL, Hilton RE, Lopez CM et al. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care 2015;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jung M and Pfeifer GP. Aging and DNA methylation. BMC Biol 2015;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marioni RE, Shah S, McRae AF et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol 2015;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zapata HJ and Quagliarello VJ. The microbiota and microbiome in aging: Potential implications in health and age-related diseases. J Am Geriatr Soc 2015;63:776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]