Abstract
Objective:
Although studies have shown an association between poor sleep and chronotype with psychiatric problems in young adults, few have focused on identifying multiple concomitant risk factors.
Methods:
We assessed depressive symptoms (Beck Depression Inventory [BDI]), circadian typology (Morningness-Eveningness Questionnaire [MEQ]), sleep quality (Pittsburgh Sleep Quality Index [PSQI]), perceived stress (Perceived Stress Scale [PSS]), social rhythm (Social Rhythm Metrics [SRM]), and salivary cortisol (morning, evening and night, n=37) in 236 men (all 18 years old). Separate analyses were conducted to understand how each PSQI domain was associated with depressive symptoms.
Results:
Depressive symptoms were more prevalent in individuals with higher perceived stress (prevalence ratio [PR] = 6.429, p < 0.001), evening types (PR = 2.58, p < 0.001) and poor sleepers (PR = 1.808, p = 0.046). Multivariate modeling showed that these three variables were independently associated with depressive symptoms (all p < 0.05). The PSQI items subjective sleep quality and sleep disturbances were significantly more prevalent in individuals with depressive symptoms (PR = 2.210, p = 0.009 and PR = 2.198, p = 0.008). Lower levels of morning cortisol were significantly associated with higher depressive scores (r = -0.335; p = 0.043).
Conclusion:
It is important to evaluate multiple factors related to sleep and chronotype in youth depression studies, since this can provide important tools for comprehending and managing mental health problems.
Keywords: Depression, circadian rhythm, circadian typology, cortisol, psychological stress, eveningness
Introduction
The prevalence of mood disorders, such as major depression, is growing in young populations, and the impairment they cause may affect development and functionality.1,2 Such psychiatric disorders are essentially multifactorial, encompassing a set of mood and anxiety symptoms, as well as neurochemical balance, which is also related to altered sleep patterns and impaired circadian rhythm entrainment.3,4
Disrupted sleep increases vulnerability to psychiatric symptoms.5,6 Recent evidence from correlational and experimental studies in healthy adults suggests that a loss of sleep results in poorer emotional response, such as heightened reactivity to negative emotional experiences.7,8 Biological rhythmicity in mammals is generated by complex molecular machinery, and the main environmental cue that synchronizes biological clocks is the light-dark cycle.9 However, social routines also interfere in this mechanism, since they determine light exposure and feeding times, as well as rest-activity patterns.10 Such changes in social routines have been considered risk factors for developing depressive symptoms.11 Furthermore, theoretical models indicate that physiological response to chronic stress may function as a vulnerability factor, linking environmental stress or trauma to the etiology of depression.12 Poorer mental health outcomes, such as depression, are associated with flatter diurnal cortisol rhythm,13,14 lack of control, negative affective reactions, and less ability to deal with stressors, which is measured by perceived stress.15
We hypothesized that subjective factors related to stress, sleep, and circadian rhythm are independently linked to clinically significant depressive symptoms in young men. To test this, we assessed circadian typology, sleep quality, perceived stress, and social rhythm (both the regularity and volume of activities) in a homogenous sample of 18-year-old male recruits in compulsory military service. To further classify sleep quality in this population, we conducted a separate analysis aiming to understand how each domain of the Pittsburgh Sleep Quality Index (PSQI) was associated with depressive symptoms. We also hypothesized that the higher the depressive symptoms scores, the flatter the diurnal cortisol variation. To test this we measured salivary cortisol three times of a day: upon awakening, in the afternoon, and at bedtime.
Material and methods
Subjects and study protocol
The investigation was conducted in accordance with the latest version of the Declaration of Helsinki. The research ethics committee of the Hospital de Clínicas de Porto Alegre (HCPA), Brazil, approved all procedures (case 2015-0263 GPPG/HCPA).
Eligible participants were healthy young conscripts accepted for compulsory military service in the Brazilian Air Force (Canoas Air Force Base/ALA 3, Canoas, Rio Grande do Sul) between June and December 2015. Conscription is considered the period between compulsory enrollment and enlistment for duty, which in Brazil could last up to 12 months. The Regional Air Force Commander and other local authorities endorsed this study. All candidates had already passed the Air Force medical evaluation and physical fitness test. Exclusion criteria for this study were continuous medication, clinical comorbidities, or inability to understand the research instruments.
This study included 236 young male conscripts (all 18 years old) who were undergoing the standard psychological evaluation. Individuals who agreed to participate in the study protocol provided written consent after the nature of the procedures had been fully explained, and were led to a separate room where the research team guided self-completion of the study’s instruments.
Study questionnaires
Beck Depression Inventory (BDI)
The self-reported 21-item BDI refers to symptoms and attitudes related to depression. In this study, individuals who scored >10 were considered to have clinically significant depressive symptoms, following the cutoff of Gomes-Oliveira et al.16 for Portuguese-speaking non-clinical Brazilian populations, which demonstrated excellent internal consistency (Cronbach's alpha = 0.93).
Perceived Stress Scale (PSS)
This 10-item self-report questionnaire includes six items assessing lack of control and negative affective reactions and four positively stated items representing the ability to deal with stressors. The total score is obtained by reversing the responses to the four positively stated items (e.g., 0 becomes 4, etc.) and then summing all items. In this population, the 75th percentile of the total score was used to separate lower from higher perceived stress. The Brazilian Portuguese version of the PSS was validated by Luft et al.,17 showing good reliability (Cronbach's alpha = 0.83).
Morningness-Eveningness Questionnaire (MEQ)
This self-report questionnaire includes 19 questions related to individual preferences in the temporal organization of activities throughout the day based on an optimal sleep-wake cycle.18 The global score is used to classify circadian typology, i.e., individuals are classified as morning-type (66-86), evening-type (16-44), and intermediate-type (45-65). These cutoffs were established for Brazilian populations by Benedito-Silva et al.19
Pittsburgh Sleep Quality Index (PSQI)
This self-report questionnaire was developed to assess sleep quality and disorders over the past month. The PSQI includes seven components: (C1) subjective sleep quality; (C2) sleep latency; (C3) sleep duration; (C4) habitual sleep efficiency; (C5) sleep disturbances; (C6) use of sleeping medication; (C7) daytime dysfunction.
The total score, i.e., the sum of all seven components, describes a continuum of impaired sleep. Individuals scoring > 5 were considered to have poor global sleep quality. To analyze individual PSQI components, the first two (i.e., 0 and 1) and the last two (i.e., 2 and 3) scores were grouped to create separate cutoffs (described below). The Brazilian Portuguese version of the PSQI, which was validated by Bertolazi et al., presented good reliability (Cronbach's alpha = 0.82).20
Social Rhythm Metric (SRM)
SRM assesses social cues as a means of regularity in social routines and social activity level. The short version used in this study (SRM-6) consists of a list of six activities used to quantify an individual’s daily social rhythms.21 The recruits completed the scale one week prior to reporting for military training. Those who were enlisted delivered it to the researchers in person on enlistment day, while those were discharged from service delivered it by mail.
Two parameters are obtained from the SRM: a “hit” score, which reflects the regularity of activities, and the Activity Level Index (ALI), which refers to the volume of different activities in that period. A total of 178 participants completed the SRM-6. The Portuguese version of the SRM-6 was developed by Schimitt & Hidalgo.21
Salivary cortisol measurement
After the questionnaire assessment, the research team provided three Eppendorf flasks with a 1.5 mL sampling capacity for saliva collection at three times of day: morning (upon awakening), afternoon (between 4:00 p.m. and 5:00 p.m.) and night (just before going to bed). A random sample of 37 conscripts provided all three salivary samples. The participants were instructed to avoid eating and to perform brief oral hygiene with water before sample collection. They were also instructed to refrigerate the samples overnight before returning them between 6:30 a.m. and 7 a.m. the next morning. All samples were returned on the same day to the HCPA and were stored at -80 °C until further analysis.
Biochemical analysis began by homogenizing and centrifuging the salivary samples at 3,000 g for 10 minutes to remove debris. The samples and reagents were handled at room temperature, and the assay was conducted according to manufacturer instructions. Each sample was analyzed in duplicate and the salivary cortisol levels (ng/mL) (DBC-Diagnostics Biochem Canada Inc., London, Canada) were determined by enzyme-linked immunosorbent assay (ELISA). The sensitivity of the ELISA kit was 1.0 ng/mL, with an inter-assay variability of 8% and an intra-assay variability of 8.7%. The presented results are the mean of the duplicates of each sample. We used a computerized method (My Assays; https://www.myassays.com/home.aspx) with a four-parameter curve fit to obtain the results.
Statistical analysis
The Shapiro-Wilk test was used to assess the normality of data distribution. For parametric variables, groups were compared using Student’s t-test for independent samples. ANOVA with Tukey post-hoc analysis was used to assess differences in mean cortisol levels between the three sampling times. These results are expressed as mean and 95% confidence interval (95%CI). Data with non-Gaussian distributions (i.e., ALI scores) were compared using the Mann-Whitney U test and are expressed as median ± interquartile range (IQR).
Univariate analysis with robust Poisson regression was performed to obtain prevalence ratios (PR) for continuous variables (as covariates) and categorical variables (as cofactors). Multivariate modeling with robust Poisson regression was also performed. Variables reaching a significance level of 0.2 in the univariate models were entered the multivariate analysis. The same significance levels were used for all steps of the multivariate models. Parametric tests were used to compare cortisol levels with BDI scores and obtain Pearson’s correlation coefficient. A quadratic regression model was used to test for non-linear effects of the correlation between cortisol and BDI scores.
Statistical analysis was performed using SPSS version 19 for Windows and all graphs were generated using GraphPad Prism version 7.0 for Windows. Values of p < 0.05 were considered statistically significant.
Results
The mean BDI score in this population was 6.93±6.79. Using the cutoff established for non-clinical populations (i.e., BDI > 10), 44 (18.6%) individuals had clinically significant depressive symptoms and 192 (81.4%) did not.
Associations between depressive symptoms and perceived stress, chronotype, and sleep quality
Table 1 shows the descriptive statistics of questionnaire data for the total sample, as well as the distribution comparisons between these groups. Table 2 shows estimated PR of depressive symptoms based on the questionnaire data for each BDI group.
Table 1. Questionnaire data, descriptive statistics, and distribution comparisons between BDI groups.
| Variable | n | Total sample | n | BDI ≤ 10 | n | BDI > 10 | p-value |
|---|---|---|---|---|---|---|---|
| Perceived stress (PSS) | 236 | 14.78 (13.98-15.58) | 192 | 13.4 (12.25-14.05) | 44 | 20.7 (18.46-22.91) | < 0.001* |
| Lower | 177 | 0.75 | 163 | 84.9 | 14 | 31.8 | < 0.001* |
| Higher | 59 | 0.25 | 29 | 15.1 | 30 | 68.2 | |
| Chronotype (MEQ) | 236 | 51.18 (50.09-52.27) | 192 | 51.9 (51.46-54.06) | 44 | 47.9 (43.07-50.37) | 0.004 † |
| Morning-types | 6 | 2.5 | 5 | 2.6 | 1 | 2.3 | 0.002 † |
| Intermediate-types | 176 | 74.6 | 152 | 79.2 | 24 | 54.5 | |
| Evening-types | 54 | 22.9 | 35 | 18.2 | 19 | 43.2 | |
| Sleep quality (PSQI) | 236 | 6.35 (5.98-6.72) | 192 | 5.9 (5.4-6.23) | 44 | 8.1 (6.51-8.93) | < 0.001* |
| Good (≤ 5) | 108 | 45.8 | 94 | 49.0 | 14 | 31.8 | 0.040 † |
| Poor (> 5) | 128 | 54.2 | 98 | 51.0 | 30 | 68.2 | |
| Social rhythm (SRM-6) | - | - | - | - | - | - | - |
| “Hit” | 178 | 3.5 (3.29-3.7) | 146 | 3.54 (3.32-3.37) | 32 | 3.27 (2.78-3.75) | 0.28 |
| ALI‡ | 178 | 36 (33-40) | 146 | 36.0 (34.0-40.0) | 32 | 35.5 (32.5-39.0) | 0.37 |
Data presented as mean (95% confidence interval) or percentage, unless otherwise specified.
ALI = Activity Level Index; BDI = Beck Depression Inventory; MEQ = Morningness-Eveningness Questionnaire; PSQI = Pittsburgh Sleep Quality Index; PSS = Perceived Stress Scale; SRM-6 = Social Rhythm Metrics.
Data for continuous variables (i.e., questionnaire scores) are described as mean (95% confidence interval) if they presented Gaussian distribution; distributions were compared with Student's t-test; non-Gaussian distributions were compared with the Mann-Whitney U test. Data for categorical variables are presented as percentage of the total population and were compared with chi-square tests.
p < 0.001;
p < 0.05.
ALI scores were described as median (interquartile range).
Table 2. Prevalence ratio and multivariate regression for predicting depressive symptoms (BDI > 10) based on questionnaire scores.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| PR | 95%CI | p-value | PR | 95%CI | p-value | |
| Perceived stress (PSS) | 1.136 | 1.099-1.173 | < 0.001* | 1.115 | 1.078-1.154 | < 0.001* |
| Lower | 1 | - | - | |||
| Higher | 6.429 | 3.665-11.275 | < 0.001* | |||
| Chronotype (MEQ) | 0.958 | 0.930-0.986 | 0.004 † | 0.964 | 0.934-0.995 | 0.024 † |
| Morning types | 1.222 | 0.197-7.6 | 0.83 | |||
| Intermediate types | 1 | - | - | |||
| Evening types | 2.58 | 1.536-4.335 | < 0.001* | |||
| Sleep quality (PSQI) | 1.187 | 1.113-1.266 | < 0.001* | 1.085 | 1.007-1.169 | 0.033 † |
| Good (≤ 5) | 1 | - | - | |||
| Bad (> 5) | 1.808 | 1.012-3.230 | 0.046 † | |||
| Social Rhythm (SRM-6) | ||||||
| “Hit” | 0.877 | 0.688-1.119 | 0.29 | |||
| ALI | 0.984 | 0.930-1.040 | 0.55 | |||
95%CI = 95% confidence interval; ALI = Activity Level Index; BDI = Beck Depression Inventory; MEQ = Morningness-Eveningness Questionnaire; PR = prevalence ratio; PSQI = Pittsburgh Sleep Quality Index; PR = PSS = Perceived Stress Scale; SRM-6 = Social Rhythm Metrics.
Univariate models were used to define the PR of all variables. A significance level (p-value) of 0.2 in the univariate analyses was the cutoff for including a variable in the multivariate model.
p < 0.001;
p < 0.05.
Perceived stress was significantly different (t = -7.828, p < 0.001) between groups. In the BDI > 10 group, 68.2% of the participants were considered to be suffering from higher stress (χ2 = 53.785, p < 0.001), a PR of 6.429 compared to the lower perceived stress group (95%CI 3.665-11.275, p < 0.001). Crosstab analysis also showed significant between-group differences for chronotype (χ2 = 12.666, p = 0.002). Although 22.9% of the total sample could be classified as evening types, 43.2% of the BDI > 10 group fall into this category. No differences were found between morning types and intermediate types. In addition, the total sleep quality score differed significantly between the BDI groups (t = -3.828, p < 0.001). Social rhythm (both “hit” and ALI scores) did not differ between the BDI groups.
Table 2 shows the multivariate model developed to determine the interactions between BDI scores ≥ 10 and perceived stress, circadian typology, and sleep quality.
Associations between Pittsburgh Sleep Quality Index (PSQI) components and depressive symptoms
A separate univariate analysis of variance was conducted to identify to what extent each PSQI component (PSQI-C) is associated with depressive symptomatology (Table 3). Five components were entered into the multivariate model. At the final step, subjective sleep quality (PSQI-C1; PR = 2.210, 95%CI 1.214-4.021, p = 0.009) and sleep disturbances (PSQI-C5; PR = 2.198, 95%CI 1.234-3.916, p = 0.008) were significantly associated with depressive symptoms.
Table 3. Prevalence ratio (PR) and multivariate regression for predicting depressive symptoms (BDI > 10) with PSQI components.
| PSQI component | n (%) | Univariate | Multivariate step 1 | Multivariate step 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PR | 95%CI | p-value | PR | 95%CI | p-value | PR | 95%CI | p-value | ||
| 1. Subjective sleep quality | ||||||||||
| Very good or fairly good | 192 (81.4) | 1 | - | - | 1 | - | - | 1 | - | - |
| Fairly poor or very poor | 44 (18.6) | 3.316 | 2.014-5.46 | < 0.001* | 2.171 | 1.196-3.941 | 0.011† | 2.210 | 1.214-4.021 | 0.009 † |
| 2. Sleep latency‡ | ||||||||||
| Up to 30 min | 99 (41.9) | 1 | - | - | 1 | - | - | |||
| More than 30 min | 137 (58.1) | 1.584 | 0.868-2.764 | 0.13 | 1.099 | 0.614-1.965 | 0.75 | |||
| 3. Sleep duration | ||||||||||
| Up to 6 hours | 204 (86.4) | 1 | - | - | ||||||
| Less than 6 hours | 32 (13.6) | 1.417 | 0.725-2.767 | 0.3 | ||||||
| 4. Habitual sleep efficiency | ||||||||||
| Up to 75% | 166 (70.3) | 1 | - | - | 1 | - | - | |||
| Less than 74% | 70 (29.7) | 1.493 | 0.871-2.559 | 0.14 | 1.027 | 0.602-1.750 | 0.92 | |||
| 5. Sleep disturbance§ | ||||||||||
| Less troubled sleep | 195 (82.6) | 1 | - | - | 1 | - | - | 1 | - | - |
| More troubled sleep | 41 (17.4) | 3.293 | 2.002-5.417 | < 0.001* | 2.166 | 1.203-3.901 | 0.010† | 2.198 | 1.234-3.916 | 0.008 † |
| 6. Use of sleeping medication | ||||||||||
| Less than once a week | 218 (92.4) | 1 | - | - | ||||||
| Once a week or more | 18 (7.6) | 1.553 | 0.700-3.446 | 0.27 | ||||||
| 7. Daytime dysfunction|| | ||||||||||
| Less dysfunction | 206 (87.3) | 1 | - | - | 1 | - | - | 1 | - | - |
| More dysfunction | 30 (12.7) | 2.575 | 1.498-4.426 | 0.001 | 1.485 | 0.828-2.664 | 0.18 | 1.507 | 0.859-2.644 | 0.15 |
95%CI = 95% confidence interval; BDI = Beck Depression Inventory; PR = prevalence ratio; PSQI = Pittsburgh Sleep Quality Index.
Univariate models were used to define PR of all variables. A significance level (p-value) of 0.2 in the univariate analyses was used to determine which variables would be included in step 1 of the multivariate model. The same criteria were applied in step 2. C1, C5 and C7 were entered in the second multivariate model (step 2).
p < 0.001;
p < 0.05.
Sleep latency encompasses two PSQI questions; one is related to the time (in minutes) it takes to fall asleep, while the other is related to the weekly frequency of occasions when the respondent cannot fall asleep within 30 min.
Sleep disturbances include weekly frequency of troubled sleep due to various factors.
Daytime dysfunction includes PSQI questions related to trouble staying awake and problems keeping the necessary energy level to accomplish normal tasks.
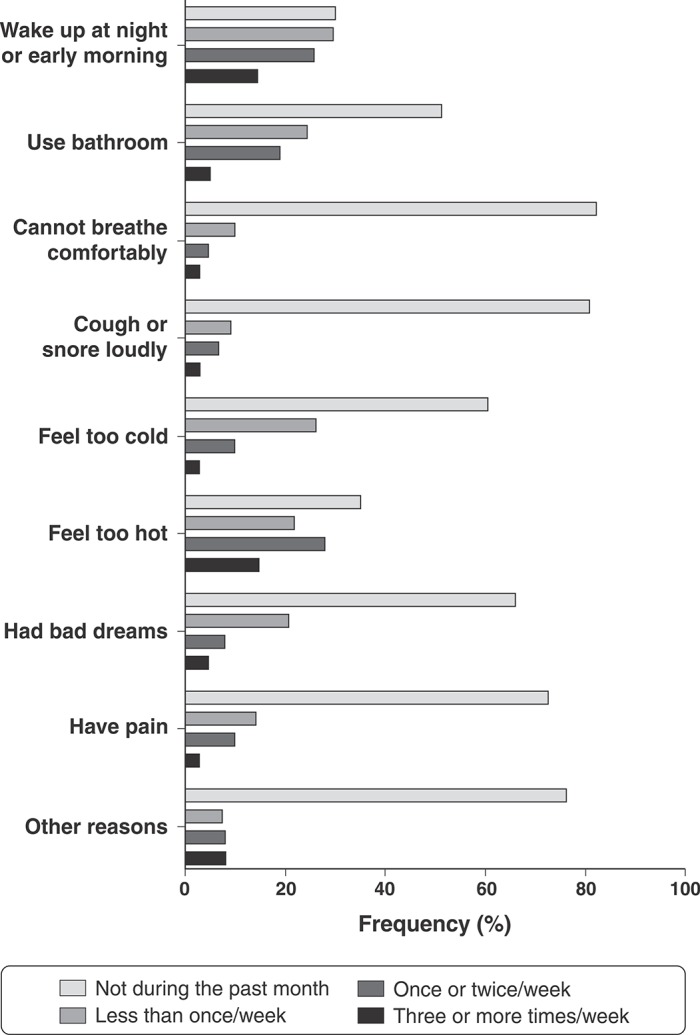
Figure 1 shows all PSQI-C5 sleep disturbance questions. The questions that most directly contributed to higher PSQI-C5 scores were: “Wake up in the middle of the night or early morning,” “Have to get up to use the bathroom,” and “Feel too hot.” Positive responses to the other questions were less frequent in this population.
Figure 1. Questions from component 5 (sleep disturbances) of the Pittsburgh Sleep Quality Index (PSQI).
Correlation between Beck Depression Inventory (BDI) score and salivary cortisol level
Figure 2 shows salivary cortisol distribution (panel A) and Pearson’s correlations of BDI score with morning (B), afternoon (C), and night (D) cortisol levels. Mean cortisol levels were significantly higher (F = 12.94, degree of freedom = 2, p = < 0.001) in the morning (mean = 15.06, 95%CI 11.9-18.2) than the afternoon (mean = 10.27, 95%CI 8.19-12.34) or night (mean = 6.66, 95%CI 5.03-8.29). The higher the BDI score, the lower the morning cortisol level (r = -0.335; p = 0.043). No significant correlations were found between BDI score and afternoon or night cortisol level. The quadratic regression model (which included non-linear effects) was not significant (p = 0.911).
Figure 2. Salivary cortisol distribution (A) and Pearson’s correlations of Beck Depression Inventory (BDI) scores with morning (B), afternoon (C) and night (C) cortisol levels.
Discussion
This study found important associations between clinically significant depressive symptoms and perceived stress, circadian typology, and sleep quality in a homogenous non-clinical sample of young men. Our results provide further evidence about the multiple concomitant factors associated with depressive symptomatology in youth. We found a clinically significant depressive symptom prevalence of 18% in this sample, which is similar to the rate found in a previous population-based study of Brazilian adolescents.22
Perceived stress as a strong indicator of depression
Perceived stress was significantly higher in individuals suffering from depressive symptoms. Univariate analysis showed a considerable PR of 6.42, which remained significant in the multivariate model. This indicates that young men suffering from clinically significant depressive symptoms are prone to reporting less control and more negative affective reactions, as well as with less ability to deal with stressors. Evidence from previous studies suggests that there is a consistent relationship between perceived stress and depression, i.e., that pro-inflammatory response to an acute stressful event is increased by factors such as loneliness, subclinical depression, and major depression.23 Furthermore, life stressors are associated with the recurrence of depression.24 Our results strongly indicate that mental health professionals should further investigate depressive mood in youth who report high perceived stress.
Correlation between low morning cortisol and depressive symptoms
Cortisol distribution across times of day occurred as expected,25 since average levels were significantly higher in the morning and lower at night. Our results demonstrate a significant negative correlation between BDI score and morning salivary cortisol level. Internalizing problems, including depression, anxiety, and somatic complaints, were associated with gradual declines in cortisol production among adolescents.26 A previous study with a sample of healthy medical students found that the area under the curve of daily cortisol secretion was significantly lower in an acute stress phase than in a control situation.27
Sleep quality, sleep timing, and circadian typology
Sleep quality was strongly associated with depressive symptoms and stress.28,29 The literature suggests that, in young adults, poor sleep quality is associated with altered cortisol response following an acute stressor.30 Moreover, misperception and poor insight regarding sleep problems are prevalent in clinical depression.31 The high prevalence of poor sleep quality in our sample agrees with the findings of a previous large population study of older adolescents and young adults in which more than 60% had poor sleep quality according to the PSQI.32
In our sample, two components of the PSQI score seemed to play a major role in clinically significant depressive symptoms: subjective sleep quality and sleep disturbances. Among factors included in the sleep disturbances component, “wake up in the middle of the night or early morning,” “have to get up to use the bathroom,” and “feel too hot” occurred with significant frequency in our population. These are common factors modifiable through sleep hygiene awareness, in contrast with respiratory problems, pain or nightmares (Figure 1). Thus, sleep hygiene advice is an efficient behavioral intervention for improving sleep health and depressive symptoms in young populations.33
Moreover, there are different physiological predispositions regarding sleep timing over a 24-hour period.34 In our sample, evening types were more prone to report depressive symptoms. However, recent studies indicate that the cognitive and affective impairment found in these individuals are not intrinsic to circadian typology itself but to chronodisruption. That is, due to the constant mismatch between their biological and social times, evening-types suffer more from sleep-related disturbances, including sleep deprivation, hypersomnia, and social jetlag.35 Nevertheless, our results cannot corroborate this hypothesis, since we did not evaluate variables related to chronodisruption. No differences could be found for morning types. Nevertheless, we point out that these individuals correspond only to 2.5% of the sample, which hinders the statistical inference.
The relevance of social rhythm in mood dysfunctions
Previous studies have reported important relationships between social rhythm and depression. Stetler et al.36 evaluated 50 depressed individuals and matched controls, showing intersections of activity regularity (“hit” score) and circadian variations in cortisol. The controls in their study presented significant correlations between “hit” scores and cortisol decline throughout the day, reflected in greater cortisol secretion. Another study from our group found that the regular activity of work days correlates with fewer minor psychiatric symptoms.37 The results of the present study do not support an association between disrupted social rhythm and depressive symptoms. Certain methodological limitations might explain why we could not reproduce the previous findings. First, the data collection time was different from that of the previous study. In our sample, SRM data was collected during one week, although some studies have recommend a minimum of three weeks to provide a more accurate score.38 Nevertheless, other studies have reported using data from a single week.37,39 Furthermore, despite the homogeneity of our population, no records of work activity were recorded. Therefore, we could not determine the influence of social routines as a social zeitgeber (time regulator). If some of the presented issues had been addressed as recommended by previous studies, the social rhythm variables might have been associated in our population.
Strengths and limitations
A recent review article by Pemberton & Fuller-Tyszkiewicz4 describes a series of factors related to depressive mood states, which include poor sleep and stressful negative events. The authors point out that the evaluation of multiple factors in a single study is rare. Furthermore, we chose to examine cortisol at three different times of day, although most studies involve only one. Our study provides supportive evidence that multifactorial relationships are related to clinically significant depressive symptoms in non-clinical populations. It is important to point out that we studied a very homogenous sample (i.e., otherwise healthy 18-year-old men not on continuous medication), which provides internal consistency to our findings. We also emphasize that, even though we found strong associations, our study cannot determine causal relationships due to its cross-sectional design. Future studies could contribute to this topic by assessing similar multifactorial relationships before and after acute stressors in similar vulnerable populations. Moreover, our study was primarily based on self-reported measurements, and no diagnostic interview was performed.
Conclusions
There is an evident need in the biomedical literature for multifactorial approaches in the study of mood symptoms. Our study is relevant due to its high internal consistency (given the homogeneity of the sample), as well as for evaluating several concomitant variables. Although causal relationships could not be established, we highlight the strong associations between depressive symptoms and high perceived stress, cortisol secretion disturbances, poor sleep quality and eveningness. Investigating chronotype and sleep quality in adolescents and young adults can provide important tools for understanding and managing mental health problems, considering their significant associations with clinically significant depressive symptoms.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
The authors would like to thank Luciano Santos Pinto Guimarães for statistical support, as well as Daiane Machado for her participation in the cortisol analyses. This study was supported by the Fundo de Incentivo à Pesquisa e Eventos-Hospital de Clínicas de Porto Alegre (FIPE-HCPA). ACT has received grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and MPH has received funding from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
How to cite this article: Tonon AC, Carissimi A, Schimitt RL, de Lima LS, Pereira FS, Hidalgo MP. How do stress, sleep quality, and chronotype associate with clinically significant depressive symptoms? A study of young male military recruits in compulsory service. Braz J Psychiatry. 2020;42:54-62. http://dx.doi.org/10.1590/1516-4446-2018-0286
References
- 1.Hidaka BH. Depression as a disease of modernity: explanations for increasing prevalence. J Affect Disord. 2012;140:205–14. doi: 10.1016/j.jad.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thapar A, Collishaw S, Pine DS, Thapar AK. Depression in adolescence. Lancet. 2012;379:1056–67. doi: 10.1016/S0140-6736(11)60871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaki NF, Spence DW, BaHammam AS, Pandi-Perumal SR, Cardinali DP, Brown GM. Chronobiological theories of mood disorder. Eur Arch Psychiatry Clin Neurosci. 2018;268:107–18. doi: 10.1007/s00406-017-0835-5. [DOI] [PubMed] [Google Scholar]
- 4.Pemberton R, Fuller Tyszkiewicz MD. Factors contributing to depressive mood states in everyday life: a systematic review. J Affect Disord. 2016;200:103–10. doi: 10.1016/j.jad.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Palmer CA, Oosterhoff B, Bower JL, Kaplow JB, Alfano CA. Associations among adolescent sleep problems, emotion regulation, and affective disorders: findings from a nationally representative sample. J Psychiatr Res. 2018;96:1–8. doi: 10.1016/j.jpsychires.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Gregory AM, Sadeh A. Annual research review: sleep problems in childhood psychiatric disorders--a review of the latest science. J Child Psychol Psychiatry. 2016;57:296–317. doi: 10.1111/jcpp.12469. [DOI] [PubMed] [Google Scholar]
- 7.Kahn M, Sheppes G, Sadeh A. Sleep and emotions: bidirectional links and underlying mechanisms. Int J Psychophysiol. 2013;89:218–28. doi: 10.1016/j.ijpsycho.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Palmer CA, Alfano CA. Sleep and emotion regulation: an organizing, integrative review. Sleep Med Rev. 2017;31:6–16. doi: 10.1016/j.smrv.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. J Biol Rhythms. 2012;27:339–52. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- 10.Haynes PL, Gengler D, Kelly M. Social rhythm therapies for mood disorders: an update. Curr Psychiatry Rep. 2016;18:75. doi: 10.1007/s11920-016-0712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlers CL, Frank E, Kupfer DJ. Social zeitgebers and biological rhythms. Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Arch Gen Psychiatry. 1988;45:948–52. doi: 10.1001/archpsyc.1988.01800340076012. [DOI] [PubMed] [Google Scholar]
- 12.Adam EK, Sutton JM, Doane LD, Mineka S. Incorporating hypothalamic-pituitary-adrenal axis measures into preventive interventions for adolescent depression: are we there yet? Dev Psychopathol. 2008;20:975–1001. doi: 10.1017/S0954579408000461. [DOI] [PubMed] [Google Scholar]
- 13.Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2017;83:25–41. doi: 10.1016/j.psyneuen.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, Adam EK. Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Dev Psychopathol. 2013;25:629–42. doi: 10.1017/S0954579413000060. [DOI] [PubMed] [Google Scholar]
- 15.Pereira-Morales AJ, Adan A, Forero DA. Perceived stress as a mediator of the relationship between neuroticism and depression and anxiety symptoms. Curr Psychol. 2019;38:66–74. [Google Scholar]
- 16.Gomes-Oliveira MH, Gorenstein C, Lotufo F, Neto, Andrade LH, Wang YP. Validation of the Brazilian Portuguese version of the Beck Depression Inventory-II in a community sample. Braz J Psychiatry. 2012;34:389–94. doi: 10.1016/j.rbp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Luft CD, Sanches Sde O, Mazo GZ, Andrade A. [Brazilian version of the Perceived Stress Scale: translation and validation for the elderly]. Rev Saude Publica. 2007;41:606–15. doi: 10.1590/s0034-89102007000400015. [DOI] [PubMed] [Google Scholar]
- 18.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 19.Benedito-Silva AA, Menna-Barreto L, Marques N, Tenreiro S. A self-assessment questionnaire for the determination of morningness-eveningness types in Brazil. Prog Clin Biol Res. 1990;341B:89–98. [PubMed] [Google Scholar]
- 20.Bertolazi AN, Fagondes SC, Hoff LS, Dartora EG, Miozzo IC, de Barba ME, et al. Validation of the Brazilian portuguese version of the Pittsburgh Sleep Quality Index. Sleep Med. 2011;12:70–5. doi: 10.1016/j.sleep.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Schimitt RL, Hidalgo MPL. Desenvolvimento da versão breve da Escala de Ritmo Social. J Bras Psiquiatr. 2012;61:89–95. [Google Scholar]
- 22.Munhoz TN, Santos IS, Matijasevich A. Depression among Brazilian adolescents: a cross-sectional population-based study. J Affect Disord. 2015;175:281–6. doi: 10.1016/j.jad.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Jaremka LM, Lindgren ME, Kiecolt-Glaser JK. Synergistic relationships among stress, depression, and troubled relationships: insights from psychoneuroimmunology. Depress Anxiety. 2013;30:288–96. doi: 10.1002/da.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monroe SM, Harkness KL. Life stress, the ‘kindling’ hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev. 2005;112:417–45. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- 25.Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell-Price J, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94:1548–54. doi: 10.1210/jc.2008-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- 27.Hulme PA, French JA, Agrawal S. Changes in diurnal salivary cortisol levels in response to an acute stressor in healthy young adults. J Am Psychiatr Nurses Assoc. 2011;17:339–49. doi: 10.1177/1078390311419352. [DOI] [PubMed] [Google Scholar]
- 28.Paunio T, Korhonen T, Hublin C, Partinen M, Koskenvuo K, Koskenvuo M, et al. Poor sleep predicts symptoms of depression and disability retirement due to depression. J Affect Disord. 2015;172:381–9. doi: 10.1016/j.jad.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Wallace DD, Boynton MH, Lytle LA. Multilevel analysis exploring the links between stress, depression, and sleep problems among two-year college students. J Am Coll Health. 2017;65:187–96. doi: 10.1080/07448481.2016.1269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassett SM, Lupis SB, Gianferante D, Rohleder N, Wolf JM. Sleep quality but not sleep quantity effects on cortisol responses to acute psychosocial stress. Stress. 2015;18:638–44. doi: 10.3109/10253890.2015.1087503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy M, Peterson MJ. Sleep disturbances in depression. Sleep Med Clin. 2015;10:17–23. doi: 10.1016/j.jsmc.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46:124–32. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Dewald-Kaufmann JF, Oort FJ, Meijer AM. The effects of sleep extension and sleep hygiene advice on sleep and depressive symptoms in adolescents: a randomized controlled trial. J Child Psychol Psychiatry. 2014;55:273–83. doi: 10.1111/jcpp.12157. [DOI] [PubMed] [Google Scholar]
- 34.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 35.Beauvalet JC, Quiles CL, Oliveira MAB, Ilgenfritz CAV, Hidalgo MP, Tonon AC. Social jetlag in health and behavioral research: a systematic review. Chrono Physiol Ther. 2017;7:19–31. [Google Scholar]
- 36.Stetler C, Dickerson SS, Miller GE. Uncoupling of social zeitgebers and diurnal cortisol secretion in clinical depression. Psychoneuroendocrinology. 2004;29:1250–9. doi: 10.1016/j.psyneuen.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Schimitt RL, Zanetti T, Mayer M, Koplin C, Guarienti F, Hidalgo MP. Psychometric properties of social rhythm metric in regular shift employees. Braz J Psychiatry. 2010;32:47–55. doi: 10.1590/s1516-44462010000100010. [DOI] [PubMed] [Google Scholar]
- 38.Monk TH, Kupfer DJ, Frank E, Ritenour AM. The social rhythm metric (SRM): measuring daily social rhythms over 12 weeks. Psychiatry Res. 1991;36:195–207. doi: 10.1016/0165-1781(91)90131-8. [DOI] [PubMed] [Google Scholar]
- 39.van Tienoven TP, Minnen J, Daniels S, Weenas D, Raaijmakers A, Glorieux I. Calculating the social rhythm metric (SRM) and examining its use in interpersonal social rhythm therapy (IPSRT) in a healthy population study. Behav Sci (Basel). 2014;4:265–77. doi: 10.3390/bs4030265. [DOI] [PMC free article] [PubMed] [Google Scholar]



