Abstract
BACKGROUND:
Diffuse astrocytomas constitute the largest group of primary malignant human intracranial tumours. They are classified by the World Health Organization (WHO) into three histological malignancy grades: diffuse astrocytomas (grade II), anaplastic astrocytomas (grade III) and glioblastoma (grade IV) based on histopathological features such as cellular atypia, mitotic activity, necrosis and microvascular proliferation. Epidermal growth factor receptor (EGFR) is a 170-kDa transmembrane tyrosine kinase receptor expressed in a variety of normal and malignant cells regulating critical cellular processes. When activated, epidermal growth factor receptor (EGFR) triggers several signalling cascades leading to increased proliferation and angiogenesis and decreased apoptosis and hence associated with aggressive progression of the tumour. Epidermal growth factor receptor (EGFR) level is known to be a strong indicator associated with the aggressive behaviour of the tumour and acts as a prognostic factor for evaluating the survival rate.
AIM:
To evaluate the expression of epidermal growth factor receptor (EGFR) in different grades of astrocytoma.
MATERIAL AND METHODS:
formalin-fixed paraffin-embedded astrocytic tumours of 44 patients were collected from the archival material of pathology department of Ghazi Al Hariri Teaching Hospital during the period from June to December 2018. Hematoxylin and eosin-stained sections were used to characterise the tumours histologically based on cellularity, nuclear hyperchromasia, polymorphism, mitotic activity, vascular proliferation and necrosis with or without pseudopallisading of tumour cells. Diagnosis and grading of astrocytic tumours in this study were made according to WHO criteria (2016). Using a monoclonal antibody to the epidermal growth factor receptor (EGFR) and immunohistochemical analysis, the expression and distribution of epidermal growth factor receptor in astrocytic tumours were examined.
RESULTS:
The study included 1 case pilocytic astrocytoma (grade I), 20 cases diffuse astrocytoma (grade II), 5 cases anaplastic astrocytoma (grade III) and 18 cases of glioblastoma (grade IV). Expression of EGFR was found in 38.88% of the glioblastoma samples (grade IV). However, none of the astrocytomas of WHO grades I, II and III showed immunoreactivity for EGFR protein. Different patterns of immunoreactive cells and significant intratumor heterogeneity of EGFR expression were observed in glioblastomas.
CONCLUSION:
The immunohistochemical expression of Epidermal growth factor receptor (EGFR) was restricted only to high-grade astrocytic tumours, namely glioblastoma, thus may use to predict glioblastoma.
Keywords: Epidermal growth factor receptor, Glioblastoma, Astrocytoma
Introduction
Primary brain tumours are a heterogeneous group of benign and malignant tumours arising from the brain parenchyma and its surrounding structures. These tumours are an important cause of morbidity and mortality in both adults and children, often generating severe disabilities and producing high burden in both families and health care systems [1], [2].
The world age-standardized incidence rate for all primary brain tumours ranged from 4.3 to 18.6 per 100 000 per year [3].
In the United States, the incidence rate for primary brain and nervous system tumours in adults (aged 20 years or older) is estimated to be 29.9 per 100,000 persons (data from 52 cancer registries, 2011 to 2015) [4]. Approximately one-third of tumours are malignant and the remainder is benign or borderline malignant [4], [5].
Gliomas are tumours of the brain parenchyma that are classified histologically based on their resemblance to different types of glial cells. The major types of glial tumours are astrocytoma, oligodendrogliomas, and ependymomas [6], [7].
The WHO classification and grading of CNS tumours recognise seven sub-types of astrocytic neoplasms grouped into two major categories. These include diffusely invasive astrocytoma (diffuse astrocytoma, anaplastic astrocytoma, glioblastoma) and the relatively more circumscribed tumours (pilocytic and pilomyxoid astrocytoma, pleomorphic xanthoastrocytoma, subependymal giant cell astrocytoma).
The so-called ‘gliomatosis cerebri’, a clinicopathologic and radiologic entity, is also a diffuse glioma, usually of the astrocytic type [8], [9].
Diffuse astrocytomas (WHO grades II to IV) account for roughly 40% of primary intracranial tumours, with an annual incidence of 4 per 100,000 person-years. They occur at all ages, although the median age is 30 to 40 for astrocytoma (grade II), 40 to 50 years for anaplastic astrocytoma (grade III), and 50 to 60 years for glioblastoma (grade IV). Glioblastomas are the most frequent, with low-grade examples being comparatively uncommon, particularly in the elderly [10].
The origin of astrocytic neoplasms may include neural stem cells, progenitor cells, or differentiated glial cells.
Many molecular markers used as prognostic markers in glioma these include IDH mutations, 1p/19q codeletion, MGMT promoter methylation, TERT promoter mutations and EGFR amplification [11].
Isocitrate dehydrogenase (IDH1, IDH2) and TP53 gene mutations are considered to be early events in neoplastic progression. In contrast, allelic loss of chromosome 10 occurs predominantly in glioblastomas. Molecular genetic studies have revealed differences between glioblastomas that evolve over the years from low-grade astrocytoma (secondary) and those that arise de novo (primary). In particular, Epidermal growth factor receptor (EGFR) overexpression is common in primary glioblastoma, while IDH1 mutations are common in secondary glioblastoma [12].
Epidermal growth factor (EGF) and the epidermal growth factor receptor (EGFR) have long been recognised for their role in tumour growth [13]. There are four transmembrane epidermal growth factor receptors: EGFR (also known as human EGF receptor 1 or HER1), HER2, HER3, and HER4 [14].
The EGFR protein contains an extracellular ligand-binding domain, a transmembrane region and an intracellular domain with intrinsic protein-tyrosine kinase activity. Ligand binding of the EGF receptor activates the EGFR tyrosine kinase which phosphorylates proteins in the signal transduction pathway leading to activation of genes that regulate cell proliferation, angiogenesis, motility, and metastasis [15], [16].
In astrocytoma, overexpression of EGFR or ErbB1 (chromosome 7p11-p12) is a late event promoting malignant progression to a glioblastoma, with amplification and often accompanying activating mutations. EGFR amplification varying in ranges of 0-4%, 0-33% and 34%-64% in grade II, III and IV astrocytomas, respectively. This amplification correlated to the histological malignancy grade and lower overall survival [17], [18], [19], [20], [21].
It has been shown that EGFR amplification promotes invasion, proliferation and resistance to radiotherapy and chemotherapy [22], [23], [24], [25].
We aimed to evaluate the expression of epidermal growth factor receptor (EGFR) in different grades of astrocytoma in a sample of Iraqi patients.
Material and Methods
This cross-sectional study enrolled 44 formalin-fixed paraffin-embedded astrocytic tumours, 17 were females and 27 were males diagnosed with different grades of astrocytic tumours of which 1 case was Pilocytic astrocytoma grade I, 20 cases were diffuse astrocytoma grade II (18 cases were diffuse fibrillary astrocytoma and 2 cases were pleomorphic xanthoastrocytoma), 5 cases were anaplastic astrocytoma grade III and 18 cases were glioblastoma grade IV. Graded according to WHO criteria 2016 [26]. These cases were retrieved from the archival material of pathology department of Ghazi Al Hariri Teaching Hospital during the period from June to December 2018.
All the clinical information, including age, gender and location, had been taken from patients archive files.
All biopsies were obtained through open brain biopsy, from each paraffin block, 2 representative (4 micrometres) sections were obtained, one section stained with hematoxylin and eosin stain and characterized on the basis of cellularity, nuclear hyperchromasia, polymorphism, mitotic activity, vascular proliferation and necrosis with or without pseudo pallisading of tumor cells into different grades and the other section was subjected to immunohistochemical testing for Anti- EGFR antibody, clone (EP38Y) manufactured by Abcam dilution (1:100).
Interpretation of the results of IHC staining
Immunoreactivity was scored based on membranous and / or cytoplasmic staining [27].
A positive stain is indicated by a golden brown coloured precipitate at the site of specific cellular antigen localisation.
The positive control for EGFR was obtained from tonsillar tissue sections, which are known to express EGFR in its basilar squamous epithelial cells was used with each run.
Technical negative control was obtained by omission of the primary antibody (EGFR).
Scoring system
Immunohistochemical stains for EGFR were graded as follows: 0 (no cell stained), 1 + (< 5% tumor cells stained), 2 + (5- 50% cells stained), and 3 + (> 50% cells stained). For statistical analysis, a score of 0 and 1 were considered negative and a score of 2 or 3 was considered positive [28].
All statistical analyses were performed using SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA). Univariate data were summarised using standard descriptive statistics, tabulation of categorical variables and histograms of numerical variables. Associations between categorical variables were assessed via cross-tabulation and chi-square. T-test and ANOVA were used to compare means of continuous variables.
Spearman correlation was used to measure the association between two continuous variables or when at least one variable was ordered. Exact tests were used to calculate the p value. A p-value of less than 0.05 was accepted as statistically significant.
Results
Frequency distribution of different grades of astrocytoma
Histopathological review of primary brain astrocytic tumours involved in this study revealed the following:
The highest frequency was noticed in low-grade astrocytoma (grade II) which constituted 20 cases (45.45%), (18 cases were diffuse fibrillary astrocytoma and 2 cases were pleomorphic xanthoastrocytoma), followed by glioblastoma (grade IV) which constituted 18 cases (40.9%), anaplastic astrocytoma (grade III) which constituted 5 cases (11.36%) and pilocytic astrocytoma (grade I) with only one case (2.27%) according to WHO criteria 2016 [11].
Among cases with glioblastoma, the majority was primary15 (83.33%), and only 3(16.7%) were secondary (progress from low grade astrocytoma) (Figure 1 and 3).
Figure 1.
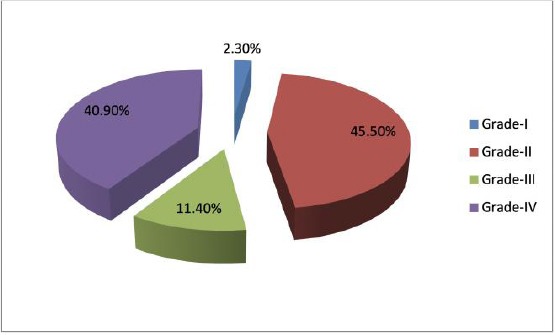
Pie chart showing distribution of cases according to grade of astrocytoma
Figure 3.

a) Pilocytic astrocytoma shows Rosenthal fibres, note homogenous eosinophilic staining (H & E × 10); b) Anaplastic astrocytoma, grade III. This lesion exhibits increased cellularity, the cytological features of a fully malignant neoplasm (H & E × 400); c) Glioblastoma grade IV, H & E, (x 400); d) Diffuse fibrillary astrocytoma, grade II with negative immunohistochemical expression of EGFR IHC (x 400); e) Anaplastic astrocytoma, grade III with negative IHC expression of EGFR (x 400); f) Glioblastoma (grade IV) stained with anti-EGFR showing positive staining for EGFR (complete membranous and cytoplasmic staining) + 3 (× 400)
Age group and sex distribution
Distribution among age groups revealed that the mean age of cases in this study was 37.41 ± 19.02 year.
The mean age of patients with grade IV astrocytoma was 44 ± 17.92 year, grade III 44.4 ± 6.66 year, grade II 30.95 ± 19.56 year and grade I 13 year with no statistically significant difference (p value = 0.073) (Table 1).
Table 1.
Mean age of different grades of astrocytoma in the studied cases
| Tumor Grade | Mean Age (year) | P value |
|---|---|---|
| Grade-I | 13 | 0.073 (N.S) |
| Grade-II | 30.95 ± 19.56 | |
| Grade-III | 44.4 ± 6.66 | |
| Grade-IV | 44 ± 17.92 | |
| TOTAL | 27 (61.4) |
Males constituted 61.4% of total cases. Differences between males and females among different grades of astrocytic tumors showed the following: males constituted 9/18 (50%) of patients with grade IV, 4/5 (80%) of patients with grade III, and 14/20 (70%) of patients with grade II with no statistically significant difference (p value = 0.3) (Table 2).
Table 2.
Distribution of different grades of astrocytoma according to the gender of the studied cases
| Tumor Grade | Males No. (%) | Females No. (%) | P value |
|---|---|---|---|
| Grade-I | 0 (0) | 1(100) | 0.3 (N.S) |
| Grade-II | 14 (70) | 6 (30) | |
| Grade-III | 4 (80) | 1 (20) | |
| Grade-IV | 9 (50) | 9 (50) | |
| Total | 27 (61.4) | 17 (38.6) |
Epidermal growth factor receptor (EGFR) immunohistochemical expression
EGFR expression was positive in 7 (15.9%) of the cases, all of which were of grade IV astrocytoma. All cases of grade I, II, III were negative for EGFR expression 37 (84.1%) of the cases (Figures 2 and 3).
Figure 2.

Pie chart showing the distribution of the cases according to the immunohistochemical expression of EGFR
Immunohistochemical expression of EGFR was seen only among those with glioblastoma, 7/18 cases showed EGFR expression (38.88%) as shown in Table 3, four were females (57.7%), and three were males (42.85%) with a mean age of 46.71 years.
Table 3.
EGFR immunohistochemical expression according to the grade of astrocytoma
| Tumour | No. of positive cases | EGFR expression % |
|---|---|---|
| Pilocyte astrocytoma grade I | 0 / 1 | 0% |
| Diffuse astrocytoma grade II | 0 / 20 | 0% |
| Anaplastic astrocytoma III | 0 / 5 | 0% |
| Glioblastoma IV | 7 / 18 | 38.88% |
| Total | 44 |
Immunohistochemical expression of EGFR according to the age of the studied cases
The mean age of those with immunohistochemical expression of EGFR was 46.71 years, whereas the mean age of negative cases was 35.64 years. The difference was statistically significant (P-value = 0.04). Within grade IV astrocytoma, the mean age of negative cases was 42.27 years compared to 46.71 years for the positive cases. The difference was statistically insignificant (P-value = 0.06) (Table 4).
Table 4.
Association between immunohistochemical expression of EGFR and Age
| Tumour | No. | Mean age (year) | P-value |
|---|---|---|---|
| Astrcytomas with positive expression of EGFR (all grades) | 7 | 46.71 | 0.04 |
| Astrocytomas with negative expression of EGFR (all grades) | 37 | 35.64 | |
| Glioblastomas with positive expression of EGFR | 7 | 46.71 | 0.06 |
| Glioblastomas with negative expression of EGFR | 11 | 42.27 |
Within grade IV astrocytoma, males constituted 3 cases (42.85%) of EGFR-positive cases and 6 cases (54.54%) of EGFR-negative cases.
The difference in sex regarding EGFR positivity was statistically insignificant (P-value = 0.45) (Table 5).
Table 5.
Immunohistochemical expression of EGFR according to the gender of the patient with glioblastoma
| EGFR expression in glioblastoma | Gender | Total | P-value | |
|---|---|---|---|---|
| Male | Female | 0.45 NS | ||
| Positive | 3 | 4 | 7 | |
| Negative | 6 | 5 | 11 | |
| Total | 9 | 9 | 18 | |
Discussion
Epidermal growth factor and its receptor (EGFR) constitute an important and well-characterized mitogenic system in various ectodermal tissues, including glial cells.
Over-expression of the EGFR due to gene amplification has been reported in primary brain tumours of glial origin [29]. This amplification promotes invasion, proliferation and resistance to radiotherapy and chemotherapy thus correlated to the histological malignancy grade and lower overall survival [17], [18], [19], [20], [21], [22], [23], [24], [25]. Based on these features, this marker chooses in this study to predict high-grade astrocytoma.
Astrocytic tumours begin as early as in the first decade of life. Young adults are typically affected by low-grade astrocytoma while glioblastoma shows a peak incidence in the sixth decade. Anaplastic astrocytoma occupies an intermediate position [30]. Glioblastoma is among the most malignant human neoplasms with a mean duration of survival for less than one year.
Extensive research works on the molecular pathogenesis of glioblastoma may facilitate molecular classification of this tumour and predict prognostic markers.
Expression of EGFR is important in molecular classification and is considered as a new prognostic parameter for astrocytic tumors [20], [31], [32], [33].
The current study showed that astrocytic tumours are more common in males than in females, 61.4% compared to 38.6%. The mean age of patients was (37.41 ± 19.02) year. The mean age of patients with grade IV and grade III astrocytoma was higher than that of patients with grade I and II astrocytoma; However, these findings were of no statistical significance.
Several previous studies found similar findings regarding the male predominance and mean of age; in Chaloob et al., study the mean age for cases of astrocytoma was 35.98 ± 2.67 years, they also found slight male predominance (53%) compared to females (47%) in astrocytoma [30].
Maiti et al., in their study, found that males constituted 58% of cases with a mean age of 42 ± 13 years [31]. Kordek et al. showed that males constituted 56% of cases with a mean age of 45 ± 11 years [32]. In Agosti et al., study, males constituted 64% of cases and the mean age was 34 ± 14 years [29].
In this study, the majority of cases were of grade II (45.5%) followed by grade IV (40.9%), similarly, Chaloob et al., found in their study that grade II is the most frequent: 7 (13.2%) cases were grade I, 22 (43.1%) cases were grade II, 6 (11.8%) cases were grade III and 16 (31.4%) cases were glioblastomas (grade IV) [30]. On the other hand, other studies showed different findings, Agosti et al. showed that out of 103 cases with astrocytoma, 21 cases were of grade I, 10 cases of grade II, 26 cases of grade III, and the most frequent were of grade IV (46 cases) [29].
Similarly, Maiti et al., in their study that included 40 cases of astrocytoma found that 21 cases were of glioblastoma or Grade IV astrocytoma (52.5%), eight cases of anaplastic astrocytoma or Grade III astrocytoma (20%), six cases of diffuse Grade II astrocytoma (15%) and five cases were grade I (12.5%) [31]. In Kordek et al., study of 56 cases of astrocytoma, 8 cases were of pilocytic (grade I) astrocytoma, 9 cases were of grade (II) fibrillary astrocytoma, 9 cases were of high grade (III) anaplastic astrocytoma and the majority of the cases (30) of glioblastomas (grade IV) [32]. Gaitonde et al. showed in their study that out of 30 cases with astrocytoma, 2 cases were of grade I-II, 11 cases were of the anaplastic type, 13 cases were of glioblastoma type and 4 cases were of other histological types [33].
This difference in grade frequency among different studies may suggest geographical or environmental causes as Iraqi studies show the predominance of grade II while worldwide studies reveal grade IV predominance
EGFR amplification is rare in low-grade gliomas [32], [33], [34], [35], [36] however, many studies have reported EGFR amplification varying in ranges of 0-4%, 0-33% and 34%-64% in grade II, III and IV astrocytomas, respectively [17], [18], [19], [20], [21].
In the recent study, EGFR expression was found in only 7 (38.88%) cases of glioblastomas, while it was negative in all other types, these findings Although other similar studies showed EGFR expression in other grades of astrocytoma, still the expression was higher in higher grades. Kordek et al. showed that 23% of astrocytoma express EGFR and that the immunohistochemical expression of EGFR increased with the grade of malignancy (11.1%, 22.2% and 33.3%) [32]. Gaitonde et al. showed positive EGFR expression in 8 of 30 cases of astrocytoma [33]. Gines et al. found that 53% of primary glioblastoma showed EGFR amplification and 33% of them showed EGFR over-expression [36]. Smith et al., showed that EGFR amplification was present in 17% of anaplastic astrocytoma and 41% of glioblastomas [20].
Stark et al. showed that 64% of glioblastoma showed positive EGFR expression [37].
Maiti et al., and van der Valk et al., in their study, showed that all cases of grade I astrocytoma showed negative immunostaining for EGFR [31], [38]. However, EGFR positivity has been observed in Grades II-IV with increasing expression associated with higher grades of astrocytoma [38]. These results were in agreement with those reported by Smith et al., in their study [20].
Shinojima et al. found that overexpression of EGFR and gene amplification frequently occurs in gliomas and is restricted to high-grade tumours, especially anaplastic astrocytoma and glioblastoma multiforme [19]. In this study, there was a significant association between immunohistochemical expression of EGFR and age of the patients with the mean age of cases with positive expression significantly higher than cases with negative expression; this may be attributable to that the mean age of the patient with glioblastoma is higher where EGFR is over-expressed. Within grade IV astrocytoma, there was no statistically significant association between positive EGFR expression and age of patients, these findings wherein tune with that of Maiti et al., Stark et al., and Bouvier et al., [31], [37], [39].
On the other hand, Kordek et al., showed that higher expression was associated with a younger age group [32]. Smith et al., and Van der Valk et al., showed no significant association of EGFR expression with specific age group [20], [38].
Taking gender of the study sample into consideration, the current study reported no significant association of EGFR expression with the sex of the patients even within those who expressed the EGFR that is in unity with other authors; Maiti et al., and Kordek et al., [31], [32]. On the other hand, Bouvier et al., and Torp et al., showed higher expression of EGFR among female [39], [40], while Smith et al., and Agosti et al., showed more positive EGFR expression in male patients [20], [29]. These differences in relations with age and sex may be due to differences in sample size.
In conclusion, the immunohistochemical expression of Epidermal growth factor receptor (EGFR) was restricted only to glioblastoma, thus may use to predict a high-grade glioblastoma.
EGFR expressed in 38.8% of glioblastoma patients which means 38.8% of these patients tend to arise de novo as primary glioblastoma.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Lacy J, Saadati H, Yu J. Complications of brain tumors and their treatment. Hemat Oncol Clin North Am. 2012;26(4):779–796. doi: 10.1016/j.hoc.2012.04.007. https://doi.org/10.1016/j.hoc.2012.04.007 PMid:22794283. [DOI] [PubMed] [Google Scholar]
- 2.Jacques G, Cormac O. Central nervous system tumors. Handb Clin Neurol. 2013;112:931–958. doi: 10.1016/B978-0-444-52910-7.00015-5. https://doi.org/10.1016/B978-0-444-52910-7.00015-5 PMid:23622303. [DOI] [PubMed] [Google Scholar]
- 3.Counsell C, Grant R. Incidence studies of primary and secondary intracranial tumors:a systematic review of their methodology and results. J Neurooncol. 1998;37(3):241–250. doi: 10.1023/a:1005861024679. https://doi.org/10.1023/A:1005861024679 PMid:9524082. [DOI] [PubMed] [Google Scholar]
- 4.Ostrom QT, Gittleman H, Truitt G, et al. CBTRUS Statistical Report:Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20(4):iv1. doi: 10.1093/neuonc/noy131. https://doi.org/10.1093/neuonc/noy131 PMid:30445539 PMCid:PMC6129949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer 1975-2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714. doi: 10.1093/jnci/djr077. https://doi.org/10.1093/jnci/djr077 PMid:21454908 PMCid:PMC3086878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger PC, Scheithauer BW. Tumors of the Central Nervous System. Washington, DC: Armed Forces Institute of Pathology; 2007. Atlas of Tumor Pathology, Series 4, Fascicle 7. [Google Scholar]
- 7.Perry A, Brat DJ. In Practical Surgical Neuropathology:A Diagnostic Approach. Elsevier; 2018. Neuropathology patterns and introduction; pp. 1–17. https://doi.org/10.1016/B978-0-323-44941-0.00001-1. [Google Scholar]
- 8.Beatriz M, Lopes S, VandenBerg S. Tumors of the central nervous system. In: Fletcher C, editor. Diagnostic histopathology of tumors. Fourth edition. Philadelphia: Elsevier Inc; 2013. pp. 1936–55. [Google Scholar]
- 9.Beatriz M, Lopes S, Scheithauer B. Histopathology of brain tumors. In: Andrew H, Edward R, Laws Jr, editors. Brain tumors, an encyclopedic approach. third edition. Saunders, Philadelphia: Elsevier; 2012. pp. 138–87. https://doi.org/10.1016/B978-0-443-06967-3.00009-0. [Google Scholar]
- 10.Perry A, Prayson R. Glial and Glioneuronal Tumors. In: Prayson R, editor. Neuropathology. second edition. Philadelphia: Elsevier; 2012. pp. 765–87. [Google Scholar]
- 11.Aquilanti E, Miller J, Santagata S, et al. Updates in prognostic markers for gliomas. Neuro-Oncology. 2018;20(7):17–26. doi: 10.1093/neuonc/noy158. https://doi.org/10.1093/neuonc/noy158 PMid:30412261, PMCid:PMC6225747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellison D, Love S, Chimelli L, et al. Neuropathology A reference text of CNS pathology. third edition. Italy: Elsevier Inc; 2013. Astrocytic neoplasms; pp. 705–28. [Google Scholar]
- 13.Cohen S. The epidermal growth factor (EGF) Cancer. 1983;51(10):1787–91. doi: 10.1002/1097-0142(19830515)51:10<1787::aid-cncr2820511004>3.0.co;2-a. https://doi.org/10.1002/1097-0142(19830515)51:10<1787::AID-CNCR2820511004>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway:A model for targeted therapy. Clin. Cancer Res. 2006;12(18):5268–72. doi: 10.1158/1078-0432.CCR-05-1554. https://doi.org/10.1158/1078-0432.CCR-05-1554 PMid:17000658. [DOI] [PubMed] [Google Scholar]
- 15.Sliwkowski M, Lofgren J, Lewis G, et al. Non clinical studies addressing the mechanism of action of Trastuzumab (Herceptin) Semin Oncol. 1999;26(12):60–70. [PubMed] [Google Scholar]
- 16.Harari P, Huang S. Modulation of molecular targets to enhance radiation. Clin Cancer Res. 2000;6:323. [PubMed] [Google Scholar]
- 17.Waha A, Baumann A, Wolf HK, et al. Lack of prognostic relevance of alterations in the epidermal growth factor receptortransforming growth factor-alpha pathway in human astrocytic gliomas. J Neurosurg. 1996;85(4):634–641. doi: 10.3171/jns.1996.85.4.0634. https://doi.org/10.3171/jns.1996.85.4.0634 PMid:8814167. [DOI] [PubMed] [Google Scholar]
- 18.Diedrich U, Lucius J, Baron E, et al. Distribution of epidermal growth factor receptor gene amplification in brain tumors and correlation to prognosis. J Neurol. 1995;242(10):683–688. doi: 10.1007/BF00866920. https://doi.org/10.1007/BF00866920 PMid:8568531. [DOI] [PubMed] [Google Scholar]
- 19.Shinojima N, Tada K, Shiraishi S. Prognostic value to epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–70. [PubMed] [Google Scholar]
- 20.Smith J, Tachibana I, Passe S, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. Journal of the National Cancer Institute. 2001;93(16):1246–56. doi: 10.1093/jnci/93.16.1246. https://doi.org/10.1093/jnci/93.16.1246 PMid:11504770. [DOI] [PubMed] [Google Scholar]
- 21.Järvelä S, Helin H, Haapasalo J, et al. Amplification of the epidermal growth factor receptor in astrocytic tumours by chromogenic in situ hybridization:association with clinicopathological features and patient survival. Neuropathol Appl Neurobiol. 2006;32(4):441–450. doi: 10.1111/j.1365-2990.2006.00758.x. https://doi.org/10.1111/j.1365-2990.2006.00758.x PMid:16866989. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Dutra A, Pak E, et al. EGFRvIII expression and PTEN loss synergistically induce chromosomal instability and glial tumors. Neuro-oncol. 2009;11(1):9–21. doi: 10.1215/15228517-2008-081. https://doi.org/10.1215/15228517-2008-081 PMid:18812521 PMCid:PMC2718963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzoleni S, Politi LS, Pala M, et al. Epidermal growth factor receptor expression identifies functionally and molecularly distinct tumor-initiating cells in human glioblastoma multiforme and is required for gliomagenesis. Cancer Res. 2010;70(19):7500–7513. doi: 10.1158/0008-5472.CAN-10-2353. https://doi.org/10.1158/0008-5472.CAN-10-2353 PMid: 20858720. [DOI] [PubMed] [Google Scholar]
- 24.Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma:signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12(9):675–684. doi: 10.1593/neo.10688. https://doi.org/10.1593/neo.10688 PMid:20824044 PMCid:PMC2933688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chakravarti A, Chakladar A, Delaney MA, Latham DE, Loeffler JS. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Res. 2002;62(15):4307–4315. [PubMed] [Google Scholar]
- 26.Chi Hong, Kim Seung, Hoon Kim, Sonya Youngju Park, Jinyoung Yoo, Sung Kyoung Kim, Hoon Kyo Kim. Identification of EGFR mutations by immunohistochemistry with EGFR mutation-specific antibodies in biopsy and resection specimens from pulmonary adenocarcinoma. Cancer Res Treat. 2015;47(4):653–660. doi: 10.4143/crt.2014.118. https://doi.org/10.4143/crt.2014.118 PMid:25687872 PMCid:PMC4614184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KS, Choe G, Nam KH, Seo AN, Yun S, Kim KJ, Cho HJ, Park SH. Immunohistochemical classification of primary and secondary glioblastomas. Korean journal of pathology. 2013;47(6):541–8. doi: 10.4132/KoreanJPathol.2013.47.6.541. https://doi.org/10.4132/KoreanJPathol.2013.47.6.541 PMid:24421847 PMCid:PMC3887156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis D, Ohgaki H, Wiestler O, Cavenee W. WHO classification of tumors of the central nervous system. revised 4th edition. Geneva: World Health Organization; 2016. [DOI] [PubMed] [Google Scholar]
- 29.Agosti M, Lethold M, Jullick W, Yasargil M, Wiestler O. Expression of the epidermal growth factor receptor in astrocytic tumours is specifically associated with glioblastoma multiforme. Virchows Archiv A Pathol Anat. 1992;420:321–25. doi: 10.1007/BF01600211. https://doi.org/10.1007/BF01600211 PMid:1314448. [DOI] [PubMed] [Google Scholar]
- 30.Chaloob M, Ali H, Qasim B, Mohammed A. Immunohistochemical expression of Ki-67, PCNA and CD34 in astrocytomas:A clinicopathological study. Oman Med J. 2012;27(5):368–74. doi: 10.5001/omj.2012.93. https://doi.org/10.5001/omj.2012.93 PMid:23074546 PMCid:PMC3472582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maiti A, Ghosh K, Chatterjee U, Chakrobarti S, Chatterjee S, Basu S. Epidermal growth factor receptor and proliferating cell nuclear antigen in astrocytomas. Neurology India. 2008;56:456–62. doi: 10.4103/0028-3886.44827. https://doi.org/10.4103/0028-3886.44827 PMid:19127042. [DOI] [PubMed] [Google Scholar]
- 32.Kordek R, Biernat W, Alwasiak J, Maculewicz R, Yanigihara R, Liberski P. p53 protein and epidermal growth factor receptor expression in human astrocytomas. J Neurooncol. 1995;26(1):11–16. doi: 10.1007/BF01054764. https://doi.org/10.1007/BF01054764 PMid:8583240. [DOI] [PubMed] [Google Scholar]
- 33.Gaitonde P, Jadhav R, Dastur R, Bhagwati S, Nadkarni J. Epidermal growth factor receptor and tenascin expression in astrocytic gliomas. Annals of Neurosciences. 2005;12(4):115–21. https://doi.org/10.5214/ans.0972.7531.2005.120403. [Google Scholar]
- 34.Mottolese M, Natali PG, Coli A, et al. Comparative analysis of proliferating cell nuclear antigen and epidermal growth factor receptor expression in glial tumours:correlation with histological grading. Anticancer Res. 1998;18(3B):1951–1956. [PubMed] [Google Scholar]
- 35.Reifenberger J, Reifenberger G, Ichimura K, Schmidt EE, Wechsler W, Collins VP. Epidermal growth factor receptor expression in oligodendroglial tumors. Am J Pathol. 1996;149(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Gines C, Gil-Benso R, Monleon D, Gonzalez-Darder J, Cerda-Nicolas M. Primary Glioblastoma with different patterns of EGFR Amplification and the relationship with gene expression profile. In Molecular Targets of CNS Tumors. Intech Open. 2011 https://doi.org/10.5772/21697 PMid:22110653 PMCid:PMC3216972. [Google Scholar]
- 37.Stark A, Witzel P, Strege R, Hugo H, Mehdorn H. p53, mdm2, EGFR, and msh2 expression in paired initial and recurrent glioblastoma multiforme. J Neurol Neurosurg Psychiatry. 2003;74:779–83. doi: 10.1136/jnnp.74.6.779. https://doi.org/10.1136/jnnp.74.6.779 PMid:12754350 PMCid:PMC1738476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Valk P, Lindeman J, Kamphorst W. Growth factor profiles of human gliomas:Do non-tumour cells contribute to tumour growth in glioma? Ann Oncol. 1997;8:1023–29. doi: 10.1023/a:1008265905505. https://doi.org/10.1023/A:1008265905505 PMid:9402177. [DOI] [PubMed] [Google Scholar]
- 39.Bouvier-Labit C, Chinot O, Ochi C, Gambarelli D, Dufour H, Figarella-Branger D. Prognostic significance of Ki67, p53 and epidermal growth factor receptor immunostaining in human glioblastomas. Neuropathol Appl Neurobiol. 1998;24:381–88. doi: 10.1046/j.1365-2990.1998.00137.x. https://doi.org/10.1046/j.1365-2990.1998.00137.x PMid:9821169. [DOI] [PubMed] [Google Scholar]
- 40.Torp S, Helseth E, Dalen A, Unsgaard G. Epidermal growth factor receptor expression in human gliomas. Cancer Immunol Immunother. 1991;33:61–64. doi: 10.1007/BF01742530. https://doi.org/10.1007/BF01742530 PMid:2021959. [DOI] [PMC free article] [PubMed] [Google Scholar]


