Abstract
Glaucoma is a common blinding disease; while there is no cure, effective treatments include medications, laser, and incisional surgery. There is significant interest from patients and doctors to develop safer surgical options throughout the spectrum of disease, to minimize treatment burden in mild glaucoma patients and to minimize risk of complications in patients needing more aggressive treatment. Surgical procedures called Minimally or Micro-Invasive Glaucoma Surgery (MIGS) are growing in popularity. Eighty-seven prospective studies on MIGS were identified and assessed for quality. Most (74%) did not have a control group. Twelve of the highest quality were reviewed. MIGS procedures appeared to have fewer complications, and lowered intraocular pressure, and reduced medication use. Studies were limited by small sample size, narrow spectrum of glaucomatous disease, and/or conflicts of interest. There is a need for high quality, independently funded and performed, comparative studies on the MIGS to help make treatment decisions.
Keywords: MIGS, angle surgery, ab interno, microinvasive, glaucoma treatment
Introduction
Traditional treatments for glaucoma and ocular hypertension include topical and oral anti-ocular hypertensive medications, glaucoma lasers (such as trabeculoplasty or cycloablation), and incisional surgery such as trabeculectomy or insertion of glaucoma tube shunt. Recently, new glaucoma surgical procedures have increased in popularity; these procedures are called Minimally or Micro- Invasive Glaucoma Surgery (MIGS).
There are a variety of MIGS procedures – some performed with an implantable device, some with the use of specialty surgical equipment, and some MIGS do not require special equipment or implant. Common mechanisms of actions for MIGS are: 1) Tissue-sparing cycloablation; 2) Trabecular meshwork bypass; 3) Non-physiologic bypass. Many MIGS offer the potential advantage of a better safety profile than traditional incisional glaucoma surgery.1 Further, the hope is that MIGS may be equal to or more efficacious than topical anti-hypertensives; which may be advantageous in patients who have barriers to medication adherence.2
Since these procedures are relatively new, the long-term safety and efficacy as well as the reproducibility of MIGS procedures are still being determined. There are many clinical scenarios where MIGS may be appropriate for a patient and may offer advantages over traditional glaucoma therapies.3 Decisions on which clinical scenarios to perform MIGS will be aided by a better understanding of the relative safety and efficacy of the MIGS procedures.
At the time of this review, there are many publications surrounding MIGS – spanning from case series to randomized clinical trials. As the literature expands, there is a growing need to aggregate and compare study results to draw conclusions from past MIGS studies. All studies involving MIGS, however, do not have equal merit, thus this review offers a critical appraisal of prospective studies with regards to study design and length of follow-up, as well as the funding sources and potential conflicts of interest of the authors.
Materials and Methods
We systematically searched the published literature using PubMed on November 14, 2018, using the following terms: MIGS OR “minimally invasive glaucoma surgery” OR “micro-invasive glaucoma surgery” OR trabectome OR iStent OR CyPass OR “suprachoroidal shunt” OR Xen OR Hydrus OR ECP OR ECPC OR “endoscopic cyclophotocoaguation” OR GATT OR “gonioscopic-assisted transluminal trabeculotomy” OR trabeculotomy OR goniotomy OR “ab interno trabeculotomy” OR “ab interno canaloplasty” OR “Kahook Dual Blade” OR KDB OR Trab360 OR Visco360 OR ABiC OR Omni; AND glaucoma. This search generated 6825 records. After filtering for English-language (5568 records) and “prospective” studies, the remaining 1015 records were reviewed by title and abstract (JAR) and 877 were excluded (reasons: trabeculectomy only, trabeculoplasty only, pediatric patients, angle-closure glaucomas). For the purposes of this review, MIGS were defined as procedures done for the purpose of treating glaucoma requiring an operating or procedural room, without a conjunctival incision (needling, subconjunctival injections were allowed), and less invasive than traditional trabeculectomy or glaucoma drainage device surgery.
The remaining 138 articles were evaluated by title and abstract to determine eligibility for inclusion. The inclusion criteria were prospective studies on minimally invasive glaucoma surgical outcomes, in adult patients with open-angle glaucomas, and written in English. Studies that were retrospective were excluded. The articles were assessed independently by each author, followed by a discussion where there was initial disagreement (19 references) with consensus reached (8 included, 11 excluded), resulting in a total of 83 articles to be included. A hand search (by JAR) of references of recent relevant review articles1,3–5 generated 8 additional articles, verified for inclusion (by DG), resulting in a total of 91 articles for review and quality assessment.
The quality of each of the articles was assessed based on the criteria in Table 1 (DG and JAR). During the quality assessment, 4 of the articles were excluded due to being retrospective (one) or non-MIGS (three), resulting in 87 articles included in the quality assessment. The articles with quality scores of 4 or greater and a control group were selected for abstraction and review.
Table 1.
Quality Assessment Criteria
| Quality Metric | Point Value |
|---|---|
| Evidence of Transparent Study Design | |
| Registered in clinicaltrials.gov | +1 |
| Use of Control Group | |
| Control treatment arm (e.g. placebo, sham surgery, etc) | +1 |
| Any control group included | 0 |
| No control group (for example, baseline metrics of treatment group used) | −1 |
| Masking | |
| Masking of study participants to treatment group | +1 |
| Masking of study doctors to treatment group | +1 |
| No masking | 0 |
| Duration of Study Follow-Up | |
| Follow-up greater than 2 years | +1 |
| Follow-up 1–2 years | +1 |
| Follow-up 6 months to 1 year | 0 |
| Follow-up less than 6 months | −1 |
| Independence of Funding | |
| Government or independent foundational funding | +1 |
| Funded by the manufacturer or inventor | 0 |
| Conflicts of Interest | |
| Authors without conflicts of interest | +1 |
| Authors with conflicts | 0 |
Results
Of the 87 articles included in the quality assessment (Appendix Table), 36 articles described a MIGS procedure done as a stand-alone intervention; 34 articles, combined with phacoemulsification; and 16 articles, with both patients with the MIGS as a stand-alone and MIGS with phacoemulsification. One study (of quality of life)6 did not indicate whether cataract surgery was done.
Forty-nine of the 87 articles (56%) had a quality score of 1 or lower. Only 22 studies (25%) registered on a national clinical trials database (Table 2). Most (64 articles, 74%) of the prospective studies did not have a control group. Over half of the studies (46 articles, 53%) were funded by the companies making the technology. Over half of the studies (48 articles, 55%) were done by authors with financial conflicts of interest. Most of the studies lasted for at least 1 year, with 26 (30%) lasting for 2 or more years.
Table 2.
Frequency of Quality Characteristics
| Quality Characteristic | Frequency (Percentage) |
|---|---|
| Registration with clinicaltrials.gov | 20 (24%) (2 registered in Japan) |
| Control Group | |
| No control group (comparison with baseline) | 64 (74%) |
| No control group (comparison with historical group) | 5 (5.7%) |
| Non-matched control group | 2 (2.3%) |
| Randomized control | 16 (18%) |
| Masking of participants or doctors | 6 (6.9%) |
| Follow-Up Period | |
| 2 years or greater | 26 (30%) |
| 1 to 2 years | 46 (53%) |
| 6 months to 1 year | 12 (14%) |
| Less than 6 months | 3 (3.4%) |
| Funding | |
| Industry-funded | 46 (53%) |
| No outside funding | 22 (25%) |
| Government or foundation grant support | 6 (6.9%) |
| Unknown | 13 (15%) |
| Conflicts of Interest (COI) | |
| At least 1 author with COI | 48 (55%) 16, all of the authors 30, most or all of the authors |
| No authors with COI | 34 (39%) |
| Unknown | 5 (5.7%) |
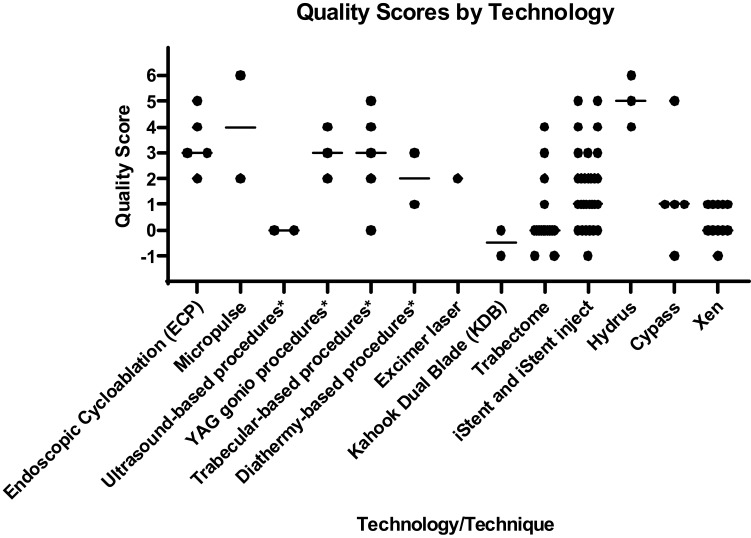
Many of the technologies and surgical techniques were each described by a small number of prospective studies (Figure 1) including endocyclophotocoagulation (ECP),7–11 MicroPulse,12,13 Ultrasound-based procedures,14,15 YAG gonio procedures,16–18 micro trabecular procedures,19–23 Diathermy-based procedures,24,25 Excimer Laser,26 Kahook Dual Blade (KDB),27,28 Hydrus,29–31 and CyPass.32–36 iStent, Trabectome, and Xen had the highest number of prospective studies. There were 29 prospective studies of the iStent (25 iStent,37–61 3 iStent inject,62–64 1 iStent+Supra),65 with quality scores ranging from −1 to 5. There were 16 prospective studies of the Trabectome,66–81 but many described the outcomes of patients who had Trabectome using data from the Trabectome Database; thus, while the data were collected prospectively, the research questions were asked retrospectively, and many of these studies had lower quality scores based on lack of control group. There were 11 prospective studies of the Xen,82–92 but none included a control group.
Figure 1.
Quality Scores by Technology. This scattered column graph displays the quality scores of individual studies of the technologies and surgical techniques. Each manuscript was assessed using the quality scale shown in Table 1. The total score for each manuscript was plotted for each type of MIGS. The line indicates the median, where appropriate. Similar techniques were grouped together (*) for single and few manuscripts. Ultrasound-based procedures included high intensity focused ultrasound (HIFU)15 and ultrasonic circular cyclocoagulation (UC3).14 YAG gonio procedures included Er:YAG gonio puncture16,17 and Er:YAG goniotomy.18 Trabecular-based procedures included ab-interno gonio trabeculotomy,19 ab interno trabeculectomy (with vitrectomy forceps),20 endoscopic trabecular surgery,21 irrigation/aspiration of the trabecular meshwork,22 suture trab 360.23 Diathermy-based procedures were intrastromal diathermal keratostomy (IDK).24,25
Twelve articles (Table 3) were found to be higher quality (scores of 4–6) and are summarized by technology.
Table 3.
Studies Selected for Review
| First Author, Year Type of MIGS | With Cataract Surgery? | Key Findings | Quality Score |
|---|---|---|---|
| Lima 200410 Endoscopic cyclo-photocoagulation (ECP) |
Stand-alone | ECP vs Ahmed (in refractory glaucoma) Success rates were similar between the ECP and Ahmed groups, with fewer complications in the ECP group. Number of patients: 68 (54 at 24 months) |
4 |
| Gayton 199911 Endoscopic cyclo-photocoagulation (ECP) |
With cataract surgery | Phaco/ECP vs phaco/trab The phaco/ECP group had less inflammation but similar IOP-lowering after 1 month and similar rates of IOP control over time. The phaco/trab group had lower IOP early, higher percentage with IOP controlled without medications (42% vs 30%) but more complications. Number of patients: 58 (variable follow-up) |
5 |
| Aquino 201513 Micropulse |
Stand-alone | Micropulse vs continuous wave transscleral diode cyclophotocoagulation (in refractory glaucoma) The micropulse group had more patients with IOP controlled (52% for micropulse, 30% for continuous wave; p=0.13). More complications in the continuous wave group. Number of patients: 48 (46 at 18 months) |
6 |
| Quaranta 199919 Ab-interno gonio trabeculotomy |
Stand-alone | Ab-interno gonio trabeculotomy vs trabeculectomy The trabeculectomy group had lower IOP early, but the groups had similar IOP after 6 months. The rate of complications, especially serious ones, were higher in the trabeculectomy group. Number of patients: 32 (32 at 24 months) |
5 |
| Vold 201658 iStent |
Stand-alone | 2 iStents vs Travatan (in treatment-naïve POAG) IOP was reduced in both groups. Visual field data were similar. More of the patients in the iStent group had IOP<18. Two operative complications the iStent group were hyphema and iridodialysis. Treatment groups were not masked and standardized cataract grading was not done. Number of patients: 101 (100 at 24 months, 73 at 36 months) |
4 |
| Fernandez-Barrientos 201059 iStent |
With cataract surgery | Phaco/iStent vs phaco alone (POAG or ocular hypertension) Trabecular outflow facility increased in both groups, more so in the phaco/iStent group. The phaco/iStent group had greater IOP reduction. Both groups needed fewer medications after surgery, more so in the phaco/iStent group. Number of patients: 33 (all at 12 months) |
4 |
| Fea 201060 iStent |
With cataract surgery | Phaco/iStent vs phaco alone At 15 months, the phaco/iStent group needed fewer medications and had lower washed out IOP than the phaco alone group. Number of patients: 36 (33 at 15 months) |
5 |
| Fea 201561 iStent |
With cataract surgery | Follow-up findings from above, at 4 years The difference in mean IOP was not statistically significant between the groups. The washed out IOP was lower in the phaco/iStent group than in the phaco alone group. Both groups used fewer medications compared with their baseline, and there was no difference between the phaco/iStent and phaco alone groups. Number of patients: 24 of the 36 patients above at 4 years |
5 |
| Fea 201729 Hydrus |
Stand-alone | Hydrus vs SLT (in uncontrolled POAG) At 1 year, both groups had reduced IOP significantly. The Hydrus group were using less medications at 12 months, and more were off of medications, than in the SLT group. This study was not randomized. Number of patients: 56 (55 at 12 months) |
4 |
| Pfeiffer 201530 Hydrus |
With cataract surgery | Phaco/Hydrus vs phaco alone (in POAG) More patients in the phaco/Hydrus group had >20% IOP reduction than the phaco alone group. Washed out IOP was lower in the phaco/Hydrus group and more of those patients were off of medication. One device-related adverse event of peripheral anterior synechiae was reported. Number of patients: 100 (78 at 24 months) |
5 |
| Samuelson 201931 Hydrus |
With cataract surgery | Phaco/Hydrus vs phaco alone (in mild-moderate glaucoma) At 24 months, unmedicated IOP was reduced in more of patients in the phaco/Hydrus group, and by a larger amount. Medications were reduced in both groups, more so in the phaco/Hydrus group. There were more adverse events in the Hydrus group, and most were not serious. Number of patients: 556 patients (95% at 24 months) |
6 |
| Vold 201636 CyPass |
With cataract surgery | CyPass/phaco vs phaco alone 77% of CyPass group had >20% reduction in unmedicated IOP, compared to 60% phaco alone at 24 months. Low rate of complications in both groups and high proportion with good visual acuity. Number of patients: 505 (480 at 24 months) |
5 |
Endocyclophotocoagulation (ECP)
In 1999, Gayton et al11 performed a prospective randomized study comparing cataract surgery with either ECP or trabeculectomy. The ECP was applied to 240–270 degrees using the same incision used for the cataract surgery; the trabeculectomy surgeries (14 with mitomycin C and 15 without) were performed at a separate site from the cataract surgery wound. All patients had cataract surgery with phacoemulsification (phaco) and posterior chamber IOL placement. 58 patients (29 in each group) with uncontrolled glaucoma requiring surgery were enrolled and were followed for an average of 705 days (ECP group) and 817 days (trabeculectomy group). Outcomes measured included anterior chamber inflammation, IOP, complications and failure rates. The phaco/ECP group experienced less inflammation but similar IOP-lowering after 1 month and similar rates of IOP control over time (with and without medications). The phaco/trabeculectomy group had lower mean IOP in the first month, higher percentage with IOP controlled without medications (42% vs 30%) but more complications (hyphema, early hypotony). Treatment failures were similar between the 2 groups (4 in the ECP group, 3 in the trabeculectomy group). Limitations included single site and limited size, unknown glaucoma type and stage, and lack of masking between groups; also, the stated purpose of the study was to assess differences in inflammation not IOP between the treatments. The authors had no financial conflicts of interest.
In 2004, Lima et al10 performed a prospective comparative study (initial patient was randomly assigned, then with consecutively alternating assignments afterwards) comparing ECP with Ahmed tube shunt implantation. The ECP was applied to 210 degrees nasally and included treatment of the anterior pars plana and entire ciliary process using scleral depression. 68 patients who were pseudophakic with refractory glaucoma (elevated IOP>35 mmHg and at least 1 prior trabeculectomy) were included in the study (34 in each group; power calculation of 98.2%) and were followed for an average of 20 months (Ahmed group) and 21 months (ECP group). Outcomes measured included IOP, survival (IOP >6 and <21, with or without medications), and complications. The success rates were similar between the ECP and Ahmed groups, with fewer complications (e.g. choroidal detachment, shallow chamber, hyphema) in the ECP group than the Ahmed group. The ECP group had more eyes with preserved visual acuity. Limitations include single site, non-randomized (but similar) groups, unknown stage of glaucoma, and lack of masking of data collection; most patients had secondary glaucoma, so it is unclear how these data might apply to patients with primary open-angle glaucoma. Financial conflicts of interest of the authors were not included.
MicroPulse
In 2015, Aquino et al13 performed a prospective randomized study comparing MicroPulse to continuous wave transscleral diode cyclophotocoagulation, in patients with refractory glaucoma (IOP>21 mmHg, poor candidates for filtration surgery, and best-corrected vision worse than 6/60). 48 patients (24 patients in each group; post hoc estimated power of 0.97) were enrolled and received either MicroPulse (2 Watts for 100 s treatment time, total 62.6 Joules) or continuous wave (1.5–2 Watts, 2 s, 20–28 spots, total 60–112 Joules) laser treatment; they were followed for 18 months. Patients and doctors measuring IOP were masked to the treatment group. The primary outcome was IOP between 6 and 21 mmHg and at least 30% reduction with or without medications. The secondary outcomes included number of repeat treatments, number medications at 18 months, and complications. The MicroPulse group had a greater percentage of patients with IOP between 6 and 18 mmHg and at least 30% IOP reduction (52% for MicroPulse, 30% for continuous wave; p=0.13). About half of the patients in each group required 2 or more treatments. Both groups had a reduction in number of medications. There were more complications (prolonged inflammation, hypotony, phthisis bulbi) in the continuous wave group, but there were also more patients with neovascular glaucoma in that group. Limitations include single site and limited size (considering diversity of ocular co-morbidities); all patients had advanced glaucoma and poor vision. Financial conflicts of interest of the authors were not included.
Ab-Interno Gonio Trabeculotomy
In 1999, Quaranta et al19 performed a prospective randomized study comparing ab interno goniotrabeculotomy to trabeculectomy with mitomycin C, in patients with medically uncontrolled primary open-angle glaucoma. All patients were white and had a medicated IOP>/=22 mmHg and no prior bulbar surgery. 32 patients (16 in each group, power >0.78) were randomized to either standardized trabeculectomy with mitomycin C (0.3mg/mL for 3 mins) or ab-interno goniotrabeculotomy. This procedure was done under direct gonioscopic view using a Swan’s goniotome creating a 45-degree incision of the iridocorneal angle (deep enough to incise Schlemm’s Canal) in the superonasal quadrant. The patients were followed for 24 months. Outcomes included IOP, diurnal IOP measurements, and post-operative complications. The trabeculectomy group achieved lower mean IOP in the first 1–3 months, but the groups had similar mean IOP after 6 months. The rate of complications, especially serious complications, was higher in the trabeculectomy group (hypotony maculopathy in 1 patient, blebitis in 2 patients). Limitations include single site and single surgeon, limited size, lack of masking of data collection, unknown stage of glaucoma, and all patients were white. The authors had no financial conflicts of interest.
iStent
In 2010, Fernandez-Barrientos et al59 performed a prospective randomized study in patients with open-angle glaucoma or ocular hypertension who were undergoing cataract surgery. They assessed aqueous humor dynamics (using fluorophotometry), safety, and efficacy of the iStent. They included 33 eyes from 33 patients with POAG or OHT, a medicated IOP >17 and <31 mmHg, and an IOP >21 and < 35 mmHg after appropriate washout. Eyes were randomized to receive either: 1) 2 iStent device with cataract surgery or 2) cataract surgery alone. After surgery, there were no significant changes in aqueous flow. Trabecular outflow facility increased in both groups: at one year 0.45 ± 0.27 uL/min/mm Hg in the group receiving phaco/iStent and 0.19 ± 0.05 uL/min/mm Hg in the cataract surgery alone group (p = 0.02). IOP reduction in the phaco/iStent group was 6.6 ± 3.0 mmHg vs 3.9 ± 2.7 mm Hg in the cataract surgery alone group (p =0.002). Medications use was 0.0 vs 0.7 ± 1.0, respectively; p = 0.007); both groups had a reduction in number of medications from baseline. Six (18%) of the iStents were in malposition, including one that fell out the trabecular meshwork and remained in the base of the iris. No complications were reported in either group. Limitations include small sample size and short follow-up. This study was part of a clinical trial listed on ClinicalTrials.gov (NCT00326066) and was funded by the sponsor (Glaukos). Multiple authors received funding from the sponsor.
Also in 2010, Fea et al60 performed a prospective double-masked randomized clinical trial, on patients in Italy with primary open-angle glaucoma who had phacoemulsification or phacoemulsification plus iStent implantation. This study included 36 patients/eyes: 24 in the control group (phaco alone) and 12 in the phaco/iStent group. There was one capsular rupture and one death in the control group. At 15 months, medication used was lower in the phaco/iStent group (0.4 ± 0.7 vs 1.3 ± 1.0; p= 0.007). At 16 months, after medication washout, IOP was 16.6 ± 3.1mmHg in the phaco/iStent group compared with 19.2 ± 3.5mmHg for the phaco alone group (p = 0.042); these IOP measurements were not significantly lower than baseline. Two iStents were in malposition, but no safety events were reported in the iStent group. As in the preceding study, limitations included small sample size and limited follow-up. The authors disclosed no financial conflicts of interest.
In 2015, Fea et al61 reported 4-year data on their original cohort of patients.60 This study included 24 patients/eyes (14 control; 10 iStent). At 4 years, post-operative mean IOP was 15.9 ± 2.3 mmHg in the phaco/iStent group and 17 ± 2.5 mmHg in the phaco group (p= NS). After washout, IOPs were 17.5 ± 2.3mmHg and 20.4 ± 3.2mmHg, respectively. In the phaco group, there was an increase in IOP after washout compared to before washout (p= 0.04). There was no significant reduction in IOP from baseline in either group. Both groups had a reduction of medication from baseline (0.5 ± 0.8, p= 0.005 in the phaco/iStent group; and 0.9 ± 1.0, p= 0.01 in the phaco group), but no difference between the groups. No long-term adverse events were reported in either group. This study was limited by its small sample size. This study was part of a clinical trial listed on ClinicalTrials.gov (NCT00847158). The authors declared that they have no conflict of interests.
In 2016, Vold et al58 prospectively studied the efficacy and safety of iStent in phakic patients with POAG (IOP ≥21 and <40) that were naive to treatment. This was an open-label trial conducted in Armenia and patients were randomized to travoprost or to 2 iStent devices. One hundred and one subjects were randomized (54 iStent; 47 travoprost) and 3-year results were reported. At 3 years, IOP was 14.6 (baseline 25.5) mmHg in the iStent group and 15.5 (baseline 25.1) mmHg in the travoprost group. The group reports 91% of patients had IOP <18 and are on no medications in the iStent group; compared with 79% in the travoprost group. Two operative complications in the iStent group were hyphema and iridodialysis, with no long-term ocular sequelae reported. Visual field data were reported as similar between the two groups at the 3-year time point. Limitations include lack of masking, and the authors acknowledge that standardized grading of cataract was not used. This study was conducted in Armenia and may not be generalizable to other populations. The trial was registered on ClinicalTrials.gov (NCT01443988) and funded by the sponsor (Glaukos). All authors disclosed financial support from the sponsor.
Hydrus
In 2015, Pfeiffer et al30 reported the results of a prospective multicenter randomized controlled trial of patients with POAG. Patients were randomized the Hydrus Microstent with cataract surgery or cataract surgery alone. Enrolled patients had a medication IOP ≤24 and a washed out diurnal IOP of 21 to 36. One hundred eyes from 100 patients were enrolled and followed for 2 years, medication washouts were repeated at 12 and 24 months. The main outcome was a 20% reduction in washed-out diurnal IOP. In the phaco/Hydrus group, 80% of patients met the main outcome compared to 46% in the cataract surgery alone group (p = 0.0008). At 24 months, washed-out diurnal IOP were 16.9 ± 3.3 mmHg vs 19.2 ±4.7 mmHg (p = 0.0093), respectively. Also at 24 months, 73% were off medication in the phaco/Hydrus group compared to 38% in the cataract surgery alone group (p = 0.0008). One device-related adverse event of peripheral anterior synechiae was reported. A limitation of this study was it was performed in a 98% white population in Europe and may not be generalizable to other populations. The trial was registered on ClinicalTrials.gov (NCT01818115) and funded by the sponsor (Ivantis). Multiple authors disclosed conflicts of interest and received funding from the sponsor.
Samuelson et al31 published on the HORIZON study, a prospective multicenter, single-masked, randomized-controlled trial. Patients with mild-to-moderate open-angle glaucoma (mean deviation on HVF of −12 or better) were randomized to Hydrus Microstent with cataract surgery compared to cataract surgery alone. Patients had a medicated IOP <21 and a washed-out modified diurnal IOP between 22 and 34 mmHg. The main outcome was 20% reduction in unmedicated diurnal IOP at 2 years. This study was conducted at 26 US sites and 12 International sites. Enrolled patients were randomized 2:1, resulting in 369 eyes in the phaco/Hydrus group and 187 phaco alone. There were 6 cases of Hydrus malposition and 10 reported complications (4 hyphema, 1 cyclodialysis, 1 device in iris root, 1 iridodialysis, 2 corneal abrasion, 1 Descemet’s Membrane detachment). At 24 months, unmedicated diurnal IOP was reduced by >20% in 77.3% of phaco/Hydrus group and 57.8% of the phaco group (p < 0.001). Unmedicated IOP was reduced by 7.6 ± 4.1 mmHg in the phaco/Hydrus group and 5.3 ± 3.9 mmHg in the phaco group (P < 0.001). Medications were reduced 1.7 ± 0.9 at baseline in both groups to 0.3 ± 0.8 in the phaco/Hydrus group and 0.7 ± 0.9 phaco group (P < 0.001). There were more adverse events in the Hydrus group with the most common being non-obstructive focal PAS (14.9% vs 2.1%), conjunctivitis (5.7% vs 7.0%), and uveitis (5.6% vs 3.7%). Limitations of this study include that it was performed in a predominately white population and surgeons had limited experience with device. The trial was registered on ClinicalTrials.gov (NCT01539239) and funded by the sponsor (Ivantis). Multiple authors disclosed conflicts of interest and received funding from the sponsor.
Fea et al29 prospectively compared IOP and medication reduction of SLT versus the Hydrus Microstent in 56 patients/eyes with uncontrolled POAG. At 12 months, IOP reduced in the SLT group from 23.2 ± 2.15 mmHg to 15.9 ±2.49 mmHg IOP and in the Hydrus group from 23.1 ± 5.08 mmHg to 16.5 ±2.6 mmHg. The Hydrus group had a greater reduction of medication use at 12 months (−1.4 ± 0.97 vs −0.5 ± 1.05, p=0.001). 47% of patients were medication-free at 12 months in the Hydrus group and 4% in the SLT group. Two patients had IOP spikes and three patients had decreased visual acuity in the early post-operative phase in the Hydrus group. Limitations of this paper include that it was nonrandomized, small sample size, non-generalizable population (study conducted in Italy), and limited follow-up. One author had a conflict of interest (consultant for Ivantis).
CyPass
In 2016, Vold et al36 performed a prospective randomized study comparing cataract surgery with CyPass supraciliary microstent to cataract surgery alone, in patients with mild to moderate primary open-angle glaucoma. 505 patients were included (131 in the control group, 374 in the CyPass group; appropriately powered) and followed for 24 months. Patients and technicians were masked to treatment group. The primary outcome measured was proportion of eyes with unmedicated diurnal IOP reduction >/=20% at 24 months compared with baseline. Secondary outcomes were mean unmedicated IOP reduction at 24 months and the proportion of eyes with unmedicated IOP between 6 and 18 mmHg. Additional outcomes included number of medications, adverse events, and proportion with best-corrected visual acuity of 20/40 or better. 77% of patients in the phaco/CyPass group had >20% reduction in unmedicated IOP, compared to phaco alone (60%), at 24 months. The phaco/CyPass group had greater unmedicated IOP reduction at 24 months (−7.4 with CyPass, −5.4 for phaco alone); and this group also had greater proportion with unmedicated IOP between 6 and 18 mmHg (65% with CyPass, 44% for phaco alone). Both groups had fewer medications after surgery, with a greater effect seen in the CyPass group. There was a low rate of complications in both groups and high proportion with good visual acuity. Limitations include predominantly white population (84%) and that a majority of the authors have financial conflicts of interest; the study population was limited to mild-to-moderate primary open-angle glaucoma and the majority of outcomes were unmedicated measurements which may limit how these data might apply to others. Note that the CyPass is currently voluntarily withdrawn from the market (https://www.alcon.com/media-release/alcon-announces-voluntary-global-market-withdrawal-cypass-micro-stent-surgical).
Discussion
This survey of the ophthalmic literature on prospectively performed studies of MIGS revealed a wide range of quality, from case series with no control group to masked randomized controlled studies. While this is an active area of research with a large number of studies (87 identified that met our criteria), there were few of the highest quality. Notable, even some identified of highest quality had significant limitations, particularly in sample size and conflicts of interest. These studies also focus on narrow subsets of glaucoma, for example mild glaucoma or refractory glaucoma, which limits their generalizability.
The most common deficiency among the studies assessed was a lack of a control group. Most of the studies identified used the patients’ baseline metrics for comparison for subsequent effect of the treatment and all patients included received the treatment (no control group); this study design can give the false impression of a positive effect from the treatment because of the phenomenon of regression to the mean.93 This phenomenon happens when a nonrandom group (for example, patients with uncontrolled glaucoma) are selected for a treatment (such as glaucoma surgery) and two clinical variables are measured (such as IOP before and after the surgery). When that first measurement is extreme (for example high IOP), by chance alone, the second is going to be lower, giving the potentially false impression that the treatment has been effective.94 A randomized controlled study design will differentiate between a real treatment effect and one that is due to chance or regression to the mean.
Also of note was the degree to which much of this literature is based on studies designed and funded by the companies making the MIGS technology. This partnership between surgeons and industry is essential for the innovation needed to continue to drive advances for the care of glaucoma patients, but clinicians cannot base decision-making solely on potentially biased data. Additional studies funded by governmental and foundational sources, such as those done for medical and surgical care of glaucoma previously (for example: EMGTS,95 TVT)96 are needed to confirm results.
There have been other systematic reviews and studies that have pooled the data for MIGS studies from the literature. As we found in our review, a consistent finding was that higher quality of evidence and study design are needed to more fully evaluate MIGS.
Lavia et al97 used the Preferred Items for Systematic Reviews and Meta-Analyses Guidelines in their review of MIGS procedures/devices, but cyclodestructive procedures were not included. Thirty-three studies were included in their study, nine of which were randomized controlled trials (RCT). They concluded that MIGS studies showed reduction of IOP, reduction of glaucoma medication, and a good safety profile, but much of these data came from comparative case studies, identifying a need for more RCTs of MIGS. Similarly, in the systemic review by Agrawal and Bradshaw,5 nine RCTs were included and some MIGS procedures/devices were found to have no RCTs. They suggested that more “real world studies” and economic studies are needed to evaluate MIGS.
In a recent review of MIGS by Mathew et al,98 they applied the World Glaucoma Association Guidelines for surgical trials to the MIGS literature. Pooling the data of the 25 studies in their analysis (10 of which were RCTs), there was only 45.6% compliance of the 53 WGA guidelines they selected. Similar to our review, they found that 64% of the studies they considered had industry association. Study methodology, definition of success, follow-up time, and economic evaluation were also found to be lacking. Future MIGS studies may consider WGA guidelines when designing their trial or reporting results.
Ultimately the goal of higher quality evidence is to improve clinical decision-making. At present, there are not enough high-quality data regarding MIGS for evidence-based practice guidelines. Michaelov99 found that only three international clinical practice guidelines mentioned MIGS, and none provided specifics for their uses. Just as MIGS offered an unmet need in glaucoma care, there remains an unmet need in proper evaluation of and guidelines related to MIGS use.
Conclusions
While there is significant interest and scholarly activity regarding MIGS, with many prospective studies on their use for glaucoma care, there are few higher quality studies and only a handful of randomized controlled studies. Of the higher quality studies, many are limited by small sample size, narrow spectrum of glaucomatous disease, and/or conflicts of interest. There is a need for high quality, independently funded and performed, comparative studies on the MIGS to help glaucoma doctors and patients make treatment decisions.
Funding Statement
No funding source for this study.
Disclosure
JAR and DG are employed by Duke University School of Medicine. The authors declare that we have no other conflicts of interest.
References
- 1.Schehlein EM, Kaleem MA, Swamy R, Saeedi OJ. Microinvasive glaucoma surgery: an evidence-based assessment. Expert Rev Ophthalmol. 2017;12(4):331–343. doi: 10.1080/17469899.2017.1335597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015;9:785–793. doi: 10.2147/OPTH.S78918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinod K, Gedde SJ. Ab interno trabeculectomy: patient selection and perspectives. Clin Ophthalmol. 2016;10:1557–1564. doi: 10.2147/OPTH.S99746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malvankar-Mehta MS, Iordanous Y, Chen YN, et al. iStent with phacoemulsification versus phacoemulsification alone for patients with glaucoma and cataract: a meta-analysis. PLoS One. 2015;10(7):e0131770. doi: 10.1371/journal.pone.0131770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal P, Bradshaw SE. Systematic literature review of clinical and economic outcomes of Micro-Invasive Glaucoma Surgery (MIGS) in primary open-angle glaucoma. Ophthalmol Ther. 2018;7(1):49–73. doi: 10.1007/s40123-018-0131-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pahlitzsch M, Klamann MK, Pahlitzsch ML, Gonnermann J, Torun N, Bertelmann E. Is there a change in the quality of life comparing the micro-invasive glaucoma surgery (MIGS) and the filtration technique trabeculectomy in glaucoma patients? Graefes Arch Clin Exp Ophthalmol. 2017;255(2):351–357. doi: 10.1007/s00417-016-3550-4 [DOI] [PubMed] [Google Scholar]
- 7.Murthy GJ, Murthy PR, Murthy KR, Kulkarni VV, Murthy KR. A study of the efficacy of endoscopic cyclophotocoagulation for the treatment of refractory glaucomas. Indian J Ophthalmol. 2009;57(2):127–132. doi: 10.4103/0301-4738.45502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis BA, Kawji AS, Vo NT, Dustin L, Chopra V. Endoscopic cyclophotocoagulation (ECP) in the management of uncontrolled glaucoma with prior aqueous tube shunt. J Glaucoma. 2011;20(8):523–527. doi: 10.1097/IJG.0b013e3181f46337 [DOI] [PubMed] [Google Scholar]
- 9.Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014;40(8):1313–1321. doi: 10.1016/j.jcrs.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 10.Lima FE, Magacho L, Carvalho DM, Susanna R Jr., Avila MP. A prospective, comparative study between endoscopic cyclophotocoagulation and the Ahmed drainage implant in refractory glaucoma. J Glaucoma. 2004;13(3):233–237. doi: 10.1097/00061198-200406000-00011 [DOI] [PubMed] [Google Scholar]
- 11.Gayton JL, Van Der Karr M, Sanders V. Combined cataract and glaucoma surgery: trabeculectomy versus endoscopic laser cycloablation. J Cataract Refract Surg. 1999;25(9):1214–1219. doi: 10.1016/S0886-3350(99)00141-8 [DOI] [PubMed] [Google Scholar]
- 12.Tan AM, Chockalingam M, Aquino MC, Lim ZI, See JL, Chew PT. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38(3):266–272. doi: 10.1111/j.1442-9071.2010.02238.x [DOI] [PubMed] [Google Scholar]
- 13.Aquino MC, Barton K, Tan AM, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol. 2015;43(1):40–46. doi: 10.1111/ceo.12360 [DOI] [PubMed] [Google Scholar]
- 14.Aptel F, Dupuy C, Rouland JF. Treatment of refractory open-angle glaucoma using ultrasonic circular cyclocoagulation: a prospective case series. Curr Med Res Opin. 2014;30(8):1599–1605. doi: 10.1185/03007995.2014.910509 [DOI] [PubMed] [Google Scholar]
- 15.Giannaccare G, Vagge A, Gizzi C, et al. High-intensity focused ultrasound treatment in patients with refractory glaucoma. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):599–605. doi: 10.1007/s00417-016-3563-z [DOI] [PubMed] [Google Scholar]
- 16.Feltgen N, Mueller H, Ott B, Frenz M, Funk J. Endoscopically controlled erbium: yAGgoniopuncture versus trabeculectomy: effect on intraocular pressure in combination with cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2003;241(2):94–100. doi: 10.1007/s00417-002-0557-9 [DOI] [PubMed] [Google Scholar]
- 17.Feltgen N, Mueller H, Ott B, Frenz M, Funk J. Combined endoscopic erbium: YAG laser goniopuncture and cataract surgery. J Cataract Refract Surg. 2003;29(11):2155–2162. doi: 10.1016/S0886-3350(03)00241-4 [DOI] [PubMed] [Google Scholar]
- 18.Philippin H, Wilmsmeyer S, Feltgen N, Ness T, Funk J. Combined cataract and glaucoma surgery: endoscope-controlled erbium: YAG-lasergoniotomy versus trabeculectomy. Graefes Arch Clin Exp Ophthalmol. 2005;243(7):684–688. doi: 10.1007/s00417-004-1004-x [DOI] [PubMed] [Google Scholar]
- 19.Quaranta L, Hitchings RA, Quaranta CA. Ab-interno goniotrabeculotomy versus mitomycin C trabeculectomy for adult open-angle glaucoma: a 2-year randomized clinical trial. Ophthalmology. 1999;106(7):1357–1362. doi: 10.1016/S0161-6420(99)00725-3 [DOI] [PubMed] [Google Scholar]
- 20.Ferrari E, Bandello F, Roman-Pognuz D, Menchini F. Combined clear corneal phacoemulsification and ab interno trabeculectomy: three-year case series. J Cataract Refract Surg. 2005;31(9):1783–1788. doi: 10.1016/j.jcrs.2004.10.050 [DOI] [PubMed] [Google Scholar]
- 21.Jacobi PC, Dietlein TS, Krieglstein GK. Microendoscopic trabecular surgery in glaucoma management. Ophthalmology. 1999;106(3):538–544. doi: 10.1016/S0161-6420(99)90113-6 [DOI] [PubMed] [Google Scholar]
- 22.Jacobi PC, Dietlein TS, Krieglstein GK. Bimanual trabecular aspiration in pseudoexfoliation glaucoma: an alternative in nonfiltering glaucoma surgery. Ophthalmology. 1998;105(5):886–894. doi: 10.1016/S0161-6420(98)95032-1 [DOI] [PubMed] [Google Scholar]
- 23.Sato T, Hirata A, Mizoguchi T. Prospective, noncomparative, nonrandomized case study of short-term outcomes of 360 degrees suture trabeculotomy ab interno in patients with open-angle glaucoma. Clin Ophthalmol. 2015;9:63–68. doi: 10.2147/OPTH.S75739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessing SV, Nissen OI, Thygesen J, Flesner P, Otland N, Riise P. The filtering, clear-cornea diathermal keratostomy: a minor Danish multicenter study. J Glaucoma. 2008;17(4):293–302. doi: 10.1097/IJG.0b013e31815a3455 [DOI] [PubMed] [Google Scholar]
- 25.Bach-Holm D, Storr-Paulsen A, Norregaard JC. A comparative study of trabeculectomy and the new clear-cornea filtering procedure, intrastromal diathermal keratostomy (IDK). Acta Ophthalmol. 2012;90(8):704–708. doi: 10.1111/j.1755-3768.2011.02140.x [DOI] [PubMed] [Google Scholar]
- 26.Toteberg-Harms M, Hanson JV, Funk J. Cataract surgery combined with excimer laser trabeculotomy to lower intraocular pressure: effectiveness dependent on preoperative IOP. BMC Ophthalmol. 2013;13:24. doi: 10.1186/1471-2415-13-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwood MD, Seibold LK, Radcliffe NM, et al. Goniotomy with a single-use dual blade: short-term results. J Cataract Refract Surg. 2017;43(9):1197–1201. doi: 10.1016/j.jcrs.2017.06.046 [DOI] [PubMed] [Google Scholar]
- 28.Dorairaj SK, Seibold LK, Radcliffe NM, et al. 12-Month outcomes of goniotomy performed using the Kahook dual blade combined with cataract surgery in eyes with medically treated glaucoma. Adv Ther. 2018;35(9):1460–1469. doi: 10.1007/s12325-018-0755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fea AM, Ahmed II, Lavia C, et al. Hydrus microstent compared to selective laser trabeculoplasty in primary open angle glaucoma: one year results. Clin Exp Ophthalmol. 2017;45(2):120–127. doi: 10.1111/ceo.12805 [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology. 2015;122(7):1283–1293. doi: 10.1016/j.ophtha.2015.03.031 [DOI] [PubMed] [Google Scholar]
- 31.Samuelson TW, Chang DF, Marquis R, et al. A schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019;126(1):29–37. doi: 10.1016/j.ophtha.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 32.Hoeh H, Ahmed II, Grisanti S, et al. Early postoperative safety and surgical outcomes after implantation of a suprachoroidal micro-stent for the treatment of open-angle glaucoma concomitant with cataract surgery. J Cataract Refract Surg. 2013;39(3):431–437. doi: 10.1016/j.jcrs.2012.10.040 [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Feijoo J, Rau M, Grisanti S, et al. Supraciliary micro-stent implantation for open-angle glaucoma failing topical therapy: 1-Year results of a multicenter study. Am J Ophthalmol. 2015;159(6):1075–1081.e1071. doi: 10.1016/j.ajo.2015.02.018 [DOI] [PubMed] [Google Scholar]
- 34.Hoeh H, Vold SD, Ahmed IK, et al. Initial clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary microstent. J Glaucoma. 2016;25(1):106–112. doi: 10.1097/IJG.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 35.Hoh H, Grisanti S, Grisanti S, Rau M, Ianchulev S. Two-year clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary micro-stent. Klin Monbl Augenheilkd. 2014;231(4):377–381. doi: 10.1055/s-0034-1368214 [DOI] [PubMed] [Google Scholar]
- 36.Vold S, Ahmed II, Craven ER, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123(10):2103–2112. doi: 10.1016/j.ophtha.2016.06.032 [DOI] [PubMed] [Google Scholar]
- 37.Spiegel D, Garcia-Feijoo J, Garcia-Sanchez J, Lamielle H. Coexistent primary open-angle glaucoma and cataract: preliminary analysis of treatment by cataract surgery and the iStent trabecular micro-bypass stent. Adv Ther. 2008;25(5):453–464. doi: 10.1007/s12325-008-0062-6 [DOI] [PubMed] [Google Scholar]
- 38.Spiegel D, Wetzel W, Haffner DS, Hill RA. Initial clinical experience with the trabecular micro-bypass stent in patients with glaucoma. Adv Ther. 2007;24(1):161–170. doi: 10.1007/BF02850004 [DOI] [PubMed] [Google Scholar]
- 39.Ahmed II, Katz LJ, Chang DF, et al. Prospective evaluation of microinvasive glaucoma surgery with trabecular microbypass stents and prostaglandin in open-angle glaucoma. J Cataract Refract Surg. 2014;40(8):1295–1300. doi: 10.1016/j.jcrs.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 40.Shiba D, Hosoda S, Yaguchi S, Ozeki N, Yuki K, Tsubota K. Safety and efficacy of two trabecular micro-bypass stents as the sole procedure in japanese patients with medically uncontrolled primary open-angle glaucoma: a pilot case series. J Ophthalmol. 2017;2017:9605461. doi: 10.1155/2017/9605461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, et al. Mid-term evaluation of the new Glaukos iStent with phacoemulsification in coexistent open-angle glaucoma or ocular hypertension and cataract. Br J Ophthalmol. 2013;97(10):1250–1255. doi: 10.1136/bjophthalmol-2012-302394 [DOI] [PubMed] [Google Scholar]
- 42.Spiegel D, Wetzel W, Neuhann T, et al. Coexistent primary open-angle glaucoma and cataract: interim analysis of a trabecular micro-bypass stent and concurrent cataract surgery. Eur J Ophthalmol. 2009;19(3):393–399. doi: 10.1177/112067210901900311 [DOI] [PubMed] [Google Scholar]
- 43.Vandewalle E, Zeyen T, Stalmans I. The iStent trabecular micro-bypass stent: a case series. Bull Soc Belge Ophtalmol. 2009;311:23–29. [PubMed] [Google Scholar]
- 44.Patel I, de Klerk TA, Au L. Manchester iStent study: early results from a prospective UK case series. Clin Exp Ophthalmol. 2013;41(7):648–652. doi: 10.1111/ceo.12098 [DOI] [PubMed] [Google Scholar]
- 45.Lindstrom R, Lewis R, Hornbeak DM, et al. Outcomes following implantation of two second-generation trabecular micro-bypass stents in patients with open-angle glaucoma on one medication: 18-month follow-up. Adv Ther. 2016;33(11):2082–2090. doi: 10.1007/s12325-016-0420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katz LJ, Erb C, Carceller GA, et al. Prospective, randomized study of one, two, or three trabecular bypass stents in open-angle glaucoma subjects on topical hypotensive medication. Clin Ophthalmol. 2015;9:2313–2320. doi: 10.2147/OPTH.S96695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berdahl J, Voskanyan L, Myers JS, et al. Implantation of two second-generation trabecular micro-bypass stents and topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 18-month follow-up. Clin Exp Ophthalmol. 2017;45(8):797–802. doi: 10.1111/ceo.2017.45.issue-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, Fernandez-Perez C, Garcia-Sanchez J, Garcia-Feijoo J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012;96(5):645–649. doi: 10.1136/bjophthalmol-2011-300218 [DOI] [PubMed] [Google Scholar]
- 49.Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed II. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911–1917. doi: 10.1016/j.jcrs.2012.07.017 [DOI] [PubMed] [Google Scholar]
- 50.Donnenfeld ED, Solomon KD, Voskanyan L, et al. A prospective 3-year follow-up trial of implantation of two trabecular microbypass stents in open-angle glaucoma. Clin Ophthalmol. 2015;9:2057–2065. doi: 10.2147/OPTH.S91732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz LJ, Erb C, Carceller Guillamet A, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018;12:255–262. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang DF, Donnenfeld ED, Katz LJ, et al. Efficacy of two trabecular micro-bypass stents combined with topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 3-year follow-up. Clin Ophthalmol. 2017;11:523–528. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buchacra O, Duch S, Milla E, Stirbu O. One-year analysis of the iStent trabecular microbypass in secondary glaucoma. Clin Ophthalmol. 2011;5:321–326. doi: 10.2147/OPTH.S15025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: long-term results. J Cataract Refract Surg. 2015;41(12):2664–2671. doi: 10.1016/j.jcrs.2015.06.032 [DOI] [PubMed] [Google Scholar]
- 55.Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi: 10.1016/j.ophtha.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 56.Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–1345. doi: 10.1016/j.jcrs.2012.03.025 [DOI] [PubMed] [Google Scholar]
- 57.Tan SZ, Au L. Manchester iStent study: 3-year results and cost analysis. Eye (Lond). 2016;30(10):1365–1370. doi: 10.1038/eye.2016.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vold SD, Voskanyan L, Tetz M, et al. Newly diagnosed primary open-angle glaucoma randomized to 2 trabecular bypass stents or prostaglandin: outcomes through 36 months. Ophthalmol Ther. 2016;5(2):161–172. doi: 10.1007/s40123-016-0065-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez-Barrientos Y, Garcia-Feijoo J, Martinez-de-la-Casa JM, Pablo LE, Fernandez-Perez C, Garcia Sanchez J. Fluorophotometric study of the effect of the glaukos trabecular microbypass stent on aqueous humor dynamics. Invest Ophthalmol Vis Sci. 2010;51(7):3327–3332. doi: 10.1167/iovs.09-3972 [DOI] [PubMed] [Google Scholar]
- 60.Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma: randomized double-masked clinical trial. J Cataract Refract Surg. 2010;36(3):407–412. doi: 10.1016/j.jcrs.2009.10.031 [DOI] [PubMed] [Google Scholar]
- 61.Fea AM, Consolandi G, Zola M, et al. Micro-bypass implantation for primary open-angle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmol. 2015;2015:795357. doi: 10.1155/2015/795357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voskanyan L, Garcia-Feijoo J, Belda JI, Fea A, Junemann A, Baudouin C. Prospective, unmasked evaluation of the iStent(R) inject system for open-angle glaucoma: synergy trial. Adv Ther. 2014;31(2):189–201. doi: 10.1007/s12325-014-0095-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arriola-Villalobos P, Martinez-de-la-Casa JM, Diaz-Valle D, Morales-Fernandez L, Fernandez-Perez C, Garcia-Feijoo J. Glaukos iStent inject(R) trabecular micro-bypass implantation associated with cataract surgery in patients with coexisting cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;2016:1056573. doi: 10.1155/2016/1056573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomized evaluation of the iStent inject ((R)) versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875–882. doi: 10.2147/OPTH.S59932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Myers JS, Masood I, Hornbeak DM, et al. Prospective evaluation of two iStent((R)) trabecular stents, one iStent Supra((R)) suprachoroidal stent, and postoperative prostaglandin in refractory glaucoma: 4-year outcomes. Adv Ther. 2018;35(3):395–407. doi: 10.1007/s12325-018-0666-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Minckler DS, Baerveldt G, Alfaro MR, Francis BA. Clinical results with the Trabectome for treatment of open-angle glaucoma. Ophthalmology. 2005;112(6):962–967. doi: 10.1016/j.ophtha.2004.12.043 [DOI] [PubMed] [Google Scholar]
- 67.Minckler D, Baerveldt G, Ramirez MA, et al. Clinical results with the Trabectome, a novel surgical device for treatment of open-angle glaucoma. Trans Am Ophthalmol Soc. 2006;104:40–50. [PMC free article] [PubMed] [Google Scholar]
- 68.Bussel II, Kaplowitz K, Schuman JS, Loewen NA. Outcomes of ab interno trabeculectomy with the trabectome by degree of angle opening. Br J Ophthalmol. 2015;99(7):914–919. doi: 10.1136/bjophthalmol-2014-305577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bussel II, Kaplowitz K, Schuman JS, Loewen NA. Outcomes of ab interno trabeculectomy with the trabectome after failed trabeculectomy. Br J Ophthalmol. 2015;99(2):258–262. doi: 10.1136/bjophthalmol-2013-304717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akil H, Chopra V, Huang A, Loewen N, Noguchi J, Francis BA. Clinical results of ab interno trabeculotomy using the Trabectome in patients with pigmentary glaucoma compared to primary open angle glaucoma. Clin Exp Ophthalmol. 2016;44(7):563–569. doi: 10.1111/ceo.2016.44.issue-7 [DOI] [PubMed] [Google Scholar]
- 71.Okeke CO, Miller-Ellis E, Rojas M. Trabectome success factors. Medicine (Baltimore). 2017;96(24):e7061. doi: 10.1097/MD.0000000000007061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nazarali SA, Damji KF. Ab interno trabeculectomy with Trabectome: outcomes in African American versus Caucasian patients. Can J Ophthalmol. 2018;53(4):361–364. doi: 10.1016/j.jcjo.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 73.Tojo N, Abe S, Miyakoshi M, Hayashi A. Comparison of intraocular pressure fluctuations before and after ab interno trabeculectomy in pseudoexfoliation glaucoma patients. Clin Ophthalmol. 2017;11:1667–1675. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arora S, Maeda M, Francis B, et al. Efficacy and safety of ab interno trabeculectomy in juvenile open-angle glaucoma. Can J Ophthalmol. 2018;53(5):482–486. doi: 10.1016/j.jcjo.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 75.Lee JW, Yick DW, Tsang S, Yuen CY, Lai JS. Efficacy and safety of trabectome surgery in Chinese open-angle glaucoma. Medicine (Baltimore). 2016;95(15):e3212. doi: 10.1097/MD.0000000000003212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maeda M, Watanabe M, Ichikawa K. Evaluation of trabectome in open-angle glaucoma. J Glaucoma. 2013;22(3):205–208. doi: 10.1097/IJG.0b013e3182311b92 [DOI] [PubMed] [Google Scholar]
- 77.Francis BA, Minckler D, Dustin L, et al. Combined cataract extraction and trabeculotomy by the internal approach for coexisting cataract and open-angle glaucoma: initial results. J Cataract Refract Surg. 2008;34(7):1096–1103. doi: 10.1016/j.jcrs.2008.03.032 [DOI] [PubMed] [Google Scholar]
- 78.Ting JL, Damji KF, Stiles MC. Ab interno trabeculectomy: outcomes in exfoliation versus primary open-angle glaucoma. J Cataract Refract Surg. 2012;38(2):315–323. doi: 10.1016/j.jcrs.2011.08.043 [DOI] [PubMed] [Google Scholar]
- 79.Jordan JF, Wecker T, van Oterendorp C, et al. Trabectome surgery for primary and secondary open angle glaucomas. Graefes Arch Clin Exp Ophthalmol. 2013;251(12):2753–2760. doi: 10.1007/s00417-013-2500-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mizoguchi T, Nishigaki S, Sato T, Wakiyama H, Ogino N. Clinical results of Trabectome surgery for open-angle glaucoma. Clin Ophthalmol. 2015;9:1889–1894. doi: 10.2147/OPTH [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kinoshita-Nakano E, Nakanishi H, Ohashi-Ikeda H, Morooka S, Akagi T. Comparative outcomes of trabeculotomy ab externo versus trabecular ablation ab interno for open angle glaucoma. Jpn J Ophthalmol. 2018;62(2):201–208. doi: 10.1007/s10384-017-0559-0 [DOI] [PubMed] [Google Scholar]
- 82.Rezkallah A, Mathis T, Denis P, Kodjikian L. XEN gel stent to treat intraocular hypertension after dexamethasone-implant intravitreal injections: 5 cases. J Glaucoma. 2019;28(1):e5–e9. doi: 10.1097/IJG.0000000000001092 [DOI] [PubMed] [Google Scholar]
- 83.Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new Ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36. doi: 10.1016/j.ajo.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 84.Sheybani A, Dick HB, Ahmed II. Early clinical results of a novel Ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25(7):e691–696. doi: 10.1097/IJG.0000000000000352 [DOI] [PubMed] [Google Scholar]
- 85.Sheybani A, Lenzhofer M, Hohensinn M, Reitsamer H, Ahmed II. Phacoemulsification combined with a new ab interno gel stent to treat open-angle glaucoma: pilot study. J Cataract Refract Surg. 2015;41(9):1905–1909. doi: 10.1016/j.jcrs.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 86.Perez-Torregrosa VT, Olate-Perez A, Cerda-Ibanez M, et al. Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions. Arch Soc Esp Oftalmol. 2016;91(9):415–421. doi: 10.1016/j.oftal.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 87.Olate-Perez A, Perez-Torregrosa VT, Gargallo-Benedicto A, et al. Prospective study of filtering blebs after XEN45 surgery. Arch Soc Esp Oftalmol. 2017;92(8):366–371. doi: 10.1016/j.oftal.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 88.Mansouri K, Gillmann K, Rao HL, Guidotti J, Mermoud A. Prospective evaluation of XEN gel implant in eyes with pseudoexfoliative glaucoma. J Glaucoma. 2018;27(10):869–873. doi: 10.1097/IJG.0000000000001045 [DOI] [PubMed] [Google Scholar]
- 89.Mansouri K, Guidotti J, Rao HL, et al. Prospective Evaluation of Standalone XEN Gel Implant and Combined Phacoemulsification-XEN Gel Implant Surgery: 1-Year Results. J Glaucoma. 2018;27(2):140–147. doi: 10.1097/IJG.0000000000000858 [DOI] [PubMed] [Google Scholar]
- 90.Galal A, Bilgic A, Eltanamly R, Osman A. XEN glaucoma implant with mitomycin C 1-year follow-up: result and complications. J Ophthalmol. 2017;2017:5457246. doi: 10.1155/2017/5457246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sng CC, Wang J, Hau S, Htoon HM, Barton K. XEN-45 collagen implant for the treatment of uveitic glaucoma. Clin Exp Ophthalmol. 2018;46(4):339–345. doi: 10.1111/ceo.2018.46.issue-4 [DOI] [PubMed] [Google Scholar]
- 92.De Gregorio A, Pedrotti E, Russo L, Morselli S. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. 2018;38(3):1129–1134. doi: 10.1007/s10792-017-0571-x [DOI] [PubMed] [Google Scholar]
- 93.Morton V, Torgerson DJ. Effect of regression to the mean on decision making in health care. BMJ. 2003;326(7398):1083–1084. doi: 10.1136/bmj.326.7398.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bland JM, Altman DG. Some examples of regression towards the mean. BMJ. 1994;309(6957):780. doi: 10.1136/bmj.309.6957.780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leske MC, Heijl A, Hyman L, Bengtsson B. Early manifest glaucoma trial: design and baseline data. Ophthalmology. 1999;106(11):2144–2153. doi: 10.1016/S0161-6420(99)90497-9 [DOI] [PubMed] [Google Scholar]
- 96.Gedde SJ, Schiffman JC, Feuer WJ, Parrish RK 2nd, Heuer DK, Brandt JD. The tube versus trabeculectomy study: design and baseline characteristics of study patients. Am J Ophthalmol. 2005;140(2):275–287. doi: 10.1016/j.ajo.2005.03.031 [DOI] [PubMed] [Google Scholar]
- 97.Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi: 10.1371/journal.pone.0183142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mathew DJM, Bryon R, Basilious A, Belkin A, Trope GE, Buys YM. Adherence to world glaucoma association guidelines for surgical trials in the era of microinvasive glaucoma surgeries. Ophthalmol Glaucoma. 2019;2(2):78–85. doi: 10.1016/j.ogla.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 99.Michaelov E, Armstrong JJ, Nguyen M, et al. Assessing the methodological quality of glaucoma clinical practice guidelines and their recommendations on microinvasive glaucoma surgery: a systematic review. J Glaucoma. 2018;27(2):e44–e49. doi: 10.1097/IJG.0000000000000820 [DOI] [PubMed] [Google Scholar]