Abstract
Upper extremity function is the highest priority of tetraplegics for improving quality of life. We aim to determine the therapeutic potential of transcutaneous electrical spinal cord stimulation for restoration of upper extremity function. We tested the hypothesis that cervical stimulation can facilitate neuroplasticity that results in long-lasting improvement in motor control. A 62-year-old male with C3, incomplete, chronic spinal cord injury (SCI) participated in the study. The intervention comprised three alternating periods: 1) transcutaneous spinal stimulation combined with physical therapy (PT); 2) identical PT only; and 3) a brief combination of stimulation and PT once again. Following four weeks of combined stimulation and physical therapy training, all of the following outcome measurements improved: the Graded Redefined Assessment of Strength, Sensation, and Prehension test score increased 52 points and upper extremity motor score improved 10 points. Pinch strength increased 2- to 7-fold in left and right hands, respectively. Sensation recovered on trunk dermatomes, and overall neurologic level of injury improved from C3 to C4. Most notably, functional gains persisted for over 3 month follow-up without further treatment. These data suggest that noninvasive electrical stimulation of spinal networks can promote neuroplasticity and long-term recovery following SCI.
Keywords: Neuroplasticity, spinal cord injury, transcutaneous electrical spinal cord stimulation, upper extremity function, engineered plasticity
I. Introduction
TRAUMATIC spinal cord injury (SCI) affects the cervical spine in 58% of cases [1]. Ensuing paralysis of the hand and arm imposes significant limitations in most activities of daily living and impairs quality of life. Patients have difficulties feeding, grooming, handwriting or performing other upper extremity motor tasks. In these individuals, restoration of hand and arm function is the highest treatment priority, five times greater than bladder, bowel, sexual or lower extremity function [2].
Given the limited regeneration potential of the spinal cord, reorganization of spared spinal circuits and facilitation of weak or silent descending drive are important targets for restoration of sensory and motor function after SCI. Growing evidence indicates that tonic electrical spinal stimulation can leverage the intrinsic capacity of neural plasticity [3], [4], and can be utilized for restoration of function after SCI [5]. Epidural stimulation can enhance conscious motor control of locomotion in humans with incomplete SCI [6]–[8], and produce initiation of voluntary leg movements and gains in postural control even in cases of clinically-complete SCI [9]–[11]. In addition, direct current spinal cord stimulation via commercially available stimulators was used to activate the posterior spinal cord roots through the skin [12]. Minassian and colleagues reported reduced spasticity and increased activity of lumbosacral central pattern generators in both incomplete [13] and motor complete [14] individuals following spinal cord injury.
Although recent studies of spinal cord stimulation have largely focused on lower extremity function, almost three decades ago Waltz et al. [15] reported improvement in upper extremity motor function, reduced spasticity and improved bladder function in 65% of the 169 patients with SCI treated with cervical epidural stimulation. Recently, Lu et al. [16] demonstrated that even seven or eight sessions of cervical epidural stimulation improved hand strength in two human subjects with chronic, motor complete cervical SCI.
Transcutaneous electrical spinal cord stimulation is a novel, non-invasive strategy to stimulate the spinal cord from the surface of the skin. Utilization of a unique waveform permits high-current electrical stimulation to reach spinal networks without causing discomfort [17]. Application of this type of stimulation to lumbosacral spinal cord improved lower extremity function for several people with spinal cord injury [17], [18]. Recently, Gad et al. [19] reported that after 8 sessions of transcutaneous stimulation, maximum voluntary hand grip forces increased by ~3-fold in the presence of stimulation and ~2-fold without simultaneous stimulation in 6 AIS B and AIS C chronic cervical SCI subjects. The present case study was designed to test the therapeutic potential of transcutaneous spinal cord stimulation on long-term restoration of upper extremity function. We tested the hypothesis that the combination of cervical transcutaneous spinal cord stimulation combined with intensive physical therapy (PT) can modulate spinal networks to create lasting improvements in hand and arm function in chronic, incomplete SCI.
II. Methods
A. Clinical Characteristics of the Subject
A 62-year-old male with cervical SCI participated in the study. Two years prior to beginning the study, this man sustained an incomplete cervical SCI while body surfing. The injury was graded as American Spinal Injury Association (ASIA) Impairment Scale (AIS) [20] category D (C3 AIS D). Acute magnetic resonance imaging of the cervical spine revealed hemorrhage and contusion of the spinal cord at C3/4 in the setting of severe spinal stenosis. Cervical x-rays and CT imaging were obtained in order to rule out bony fracture or instability. The patient was initially treated conservatively. Following modest initial functional recovery, progress came to a halt and repeat cervical MRI four months after injury revealed spinal myelomalacia at C3/4 in the setting of severe cervical spinal stenosis (Fig. 1A). Six months following his injury, he underwent a C3–7 laminectomy and arthrodesis (Fig. 1B).
Fig. 1.
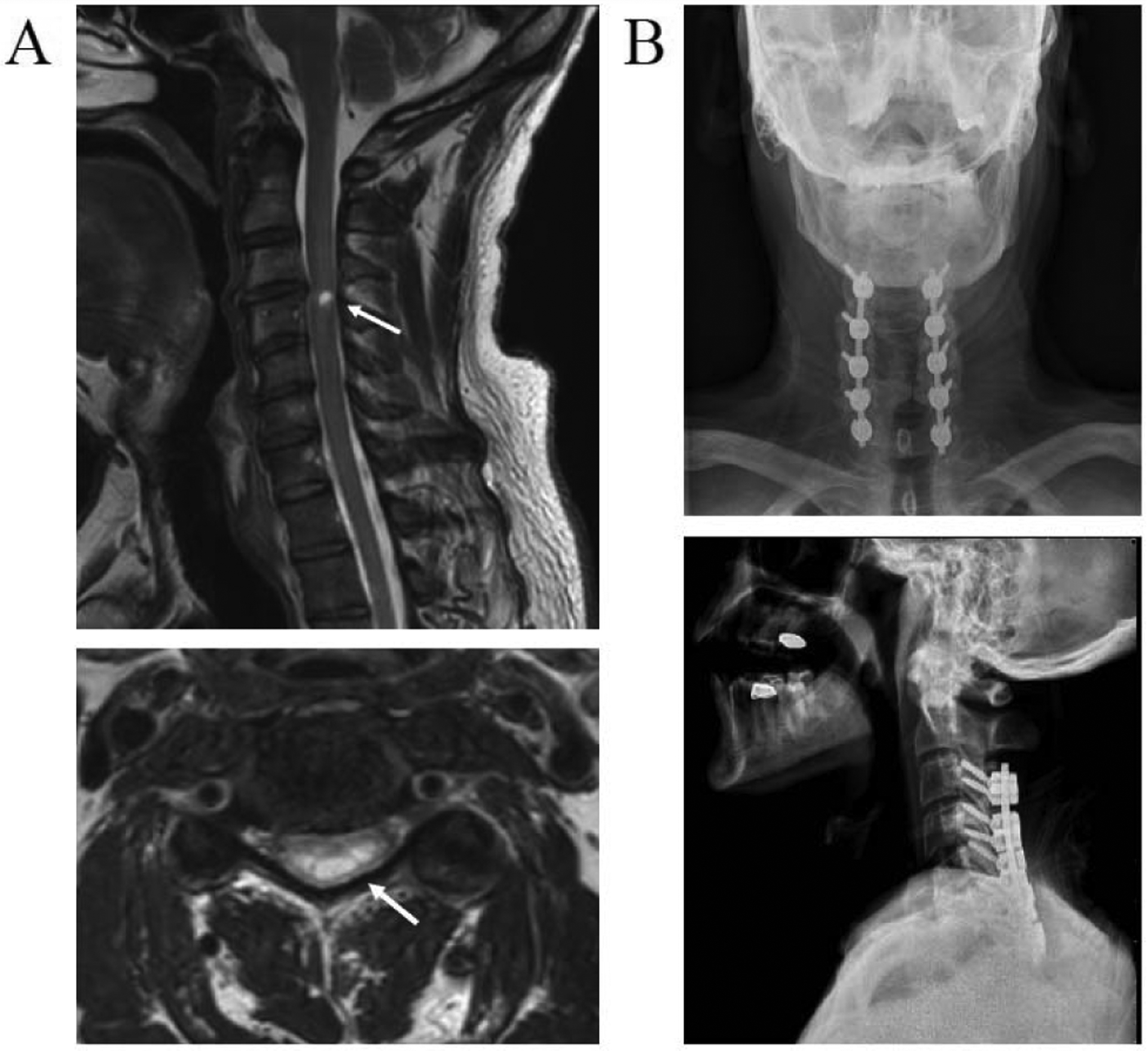
Radiographic images of the injury location and decompression surgery of the cervical spine. (A) T2 weighted sagittal (top) and axial (bottom) magnetic resonance images of the subject’s cervical spine at 6 months post-injury. Arrows shows high intensity T2 signal of myelomalacia and atrophy at C3 and C4 spinal level. (B) Anteroposterior (top) and lateral (bottom) x-ray images of cervical vertebra showing laminectomy and arthrodesis surgery.
He participated in standard inpatient physical rehabilitation for six months that included occupational therapy and gait training. At discharge, his neurological level of injury and AIS category did not change. Despite adequate muscle strength in both lower and left upper extremities, he was completely dependent for all self-care activities (feeding, bathing, dressing, grooming, bowel and bladder management), and had limited indoor walking with moderate assistance for transfers, standing, balance and stepping. After discharge, he attended an exercise-based therapy center regularly, approximately 2 hours per day, 4–5 times per week until the time of this study. He also participated in lower extremity exercise therapy at home on a regular basis using an elliptical trainer.
B. Procedures
This study is registered with ClinicalTrials.gov, number . The subject signed informed consent for all procedures, which were approved by University of Washington Institutional Review Board. The study consisted of two weeks baseline measurements, nine weeks alternating intervention program and three months follow-up testing with no further therapy.
Baseline evaluation consisted of full physical and neurological examinations including the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) assessment. Upper extremity functional capacity and performance were evaluated by the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) test [21] as the primary outcome measure. Lateral pinch strength was also measured (Jamar Hydraulic Pinch Gauge, Lafayette Instruments, USA). Prior to beginning treatment, the GRASSP test and strength measurements were repeated three times over two weeks to explore the consistency of functional status and to document possible learning effects of the tests. WHO Quality of Life – BREF [22], SF-Qualiveen [23], and the Spinal Cord Independence Measure III (SCIM III) [24] questionnaires were used to address quality of life and subject’s ability to perform activities of daily living.
A three-phase, alternating intervention program delivered: (1) transcutaneous electrical spinal cord stimulation accompanied by activity-based physical therapy (PT) targeting upper extremity functions for the first four weeks, (2) PT only for the next four weeks, and (3) stimulation + PT again for one week. This order of interventions was derived from a randomized two arm cross over design. Participants are randomly assigned to either PT only or stimulation + PT intervention phases (AB or BA). This subject randomized into stimulation + PT intervention first. The rationale for this study design is to control for the after-effect of either PT only and/or stimulation + PT. As the data show, sustained effects of treatment persist for many months. Therefore, it is important to randomize the order of the treatments. For this participant, a final one week of stimulation was delivered in order to assess any additional benefit of stimulation since the results of the initial month with stimulation + PT were quite marked.
During the stimulation phases of the study, non-invasive, transcutaneous electrical stimulation was delivered to the cervical spinal cord surrounding the injury site (NeuroRecovery Technologies Inc., San Juan Capistrano, CA, USA). The stimulation waveform was biphasic, rectangular, 1 ms pulses at a frequency of 30 Hz, filled with a carrier frequency of 10 kHz (Fig. 2) [17]. This permitted stimulation intensities of 80–120 milliamperes (mA) to be delivered to the skin over the cervical spinal cord without discomfort.
Fig. 2.
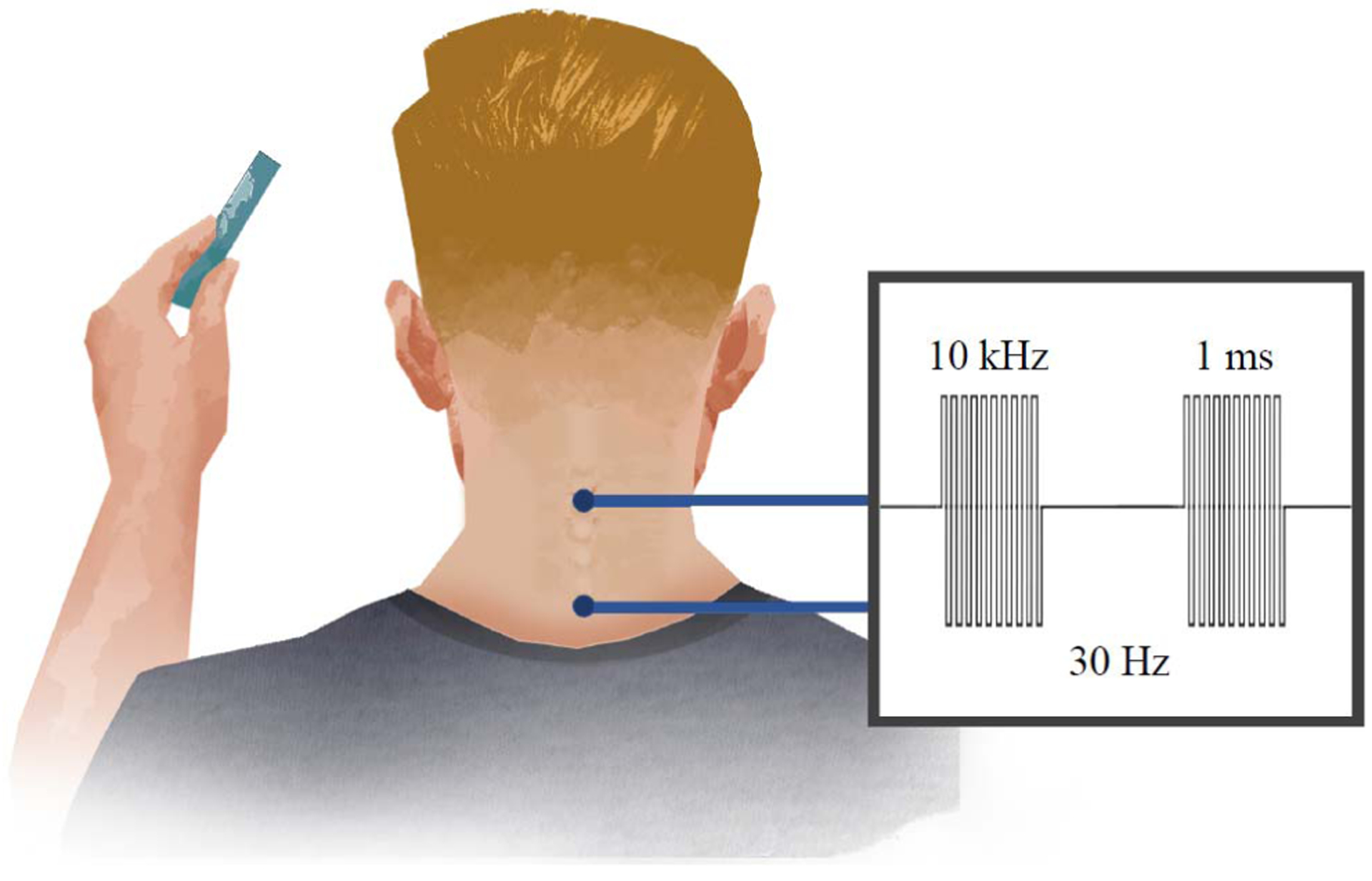
Schematic of the intervention showing electrical cervical spinal stimulation applied to the surface of the skin via electrodes placed midline at C3–4 and C6–7 bony landmarks. (Inset) Biphasic, rectangular, 1 ms pulses are delivered at a frequency of 30 Hz. Each pulse is filled with a carrier frequency of 10 kHz to permit stimulation intensities of 80–120mA to pass through the skin and reach the spinal cord without discomfort.
Stimulation was delivered via two 2.5 cm round electrodes placed midline at C3–4 and C6–7 spinous processes as cathodes and two 5 × 10 cm rectangular plates (Axelgaard Manufacturing Co., Ltd., USA) placed symmetrically over the iliac crests as anodes. A total of 1451 minutes of stimulation was applied over the five weeks (mean duration was 60 ± 20 minutes/session, range 25 – 120 minutes/session).
The physical therapy program included standard stretching, active assistive range of motion exercises, and intensive gross and fine motor skill trainings, which resemble most of the daily upper extremity motor tasks [25]. The total dosage of physical therapy was 58.5 hours over nine intervention weeks, approximately 90 minutes/session. Exactly the same PT activities were repeated during each phase of the study.
The subject participated in 2-hour sessions, 4–5 days/week, over the 9 weeks of intervention. Blood pressure and heart rate were monitored throughout all sessions. Pinch strength measurements were performed weekly, and reported values represent the average of three consecutive maximal force contractions. GRASSP tests were repeated in the first, second and fourth weeks of stimulation + PT and PT only interventions, and once at the end of the second stimulation + PT phase. During stimulation + PT sessions, tests were repeated both with and without stimulation on successive days in order to avoid fatigue.
Spinal motor evoked potentials from stimulation delivered both at and below the level of injury were recorded at the end of each week of stimulation + PT sessions. The stimulator was set to monophasic, rectangular, 1 ms single pulses at a frequency of 1 Hz [17], [26], [27]. Stimulation intensity was increased in 10 mA intervals from 10 to 120 mA. Motor responses were collected via surface electrodes from eight muscles in each arm (deltoid, triceps, biceps, brachioradialis, extensor digitorum, flexor digitorum, abductor digiti minimi and thenar muscle groups). A 16 channel Bagnoli electromyography (EMG) system (Delsys, Boston, MA, USA) was used to filter (20–450 Hz) and amplify EMG signals 1000 times. Both the stimulation and EMG signals were digitized at 1 kHz and recorded simultaneously using PowerLab (AD Instruments, Milford, MA, USA). Signals were then rectified, and stimulus triggered averages were subsequently compiled using MATLAB (Matworks Inc., Natick, MA, USA).
During the three-month follow-up period, GRASSP and pinch strengths were retested once every two weeks. ISNCSCI assessment, WHO Quality of Life - BREF, SF-Qualiveen, and SCIM III scores were re-evaluated at the end of each intervention period, and at study completion.
III. Results
A. Baseline Outcome Measurements
Initial ISNCSCI assessment revealed an AIS category D injury, with a central cord syndrome pattern. Intact light touch sensation was present to C3 and pinprick to C4 dermatomes, bilaterally. The subject had increased muscle tone in all extremities, recorded as 1 – 2 points on the modified Ashworth Scale and experienced infrequent spasms with moderate severity described in Penn Spasm Frequency Scale. On the right side, muscle tone was higher (especially in right biceps and pectoralis muscles) and muscle strength was weaker compared to the left side.
B. Effect of Stimulation on Hand and Arm Function
Cervical transcutaneous electrical spinal cord stimulation + PT resulted in both dramatic and durable improvements in hand and arm function on all motor tasks measured. Upper extremity muscle strength nearly doubled over the course of treatment and stabilized at 75% stronger than baseline for three months without further treatment (Fig. 3). Composite scores of ten key muscles of the GRASSP test increased from 41/100 to 78/100 with stimulation treatment and stabilized above 70/100 during the entire follow-up period.
Fig. 3.
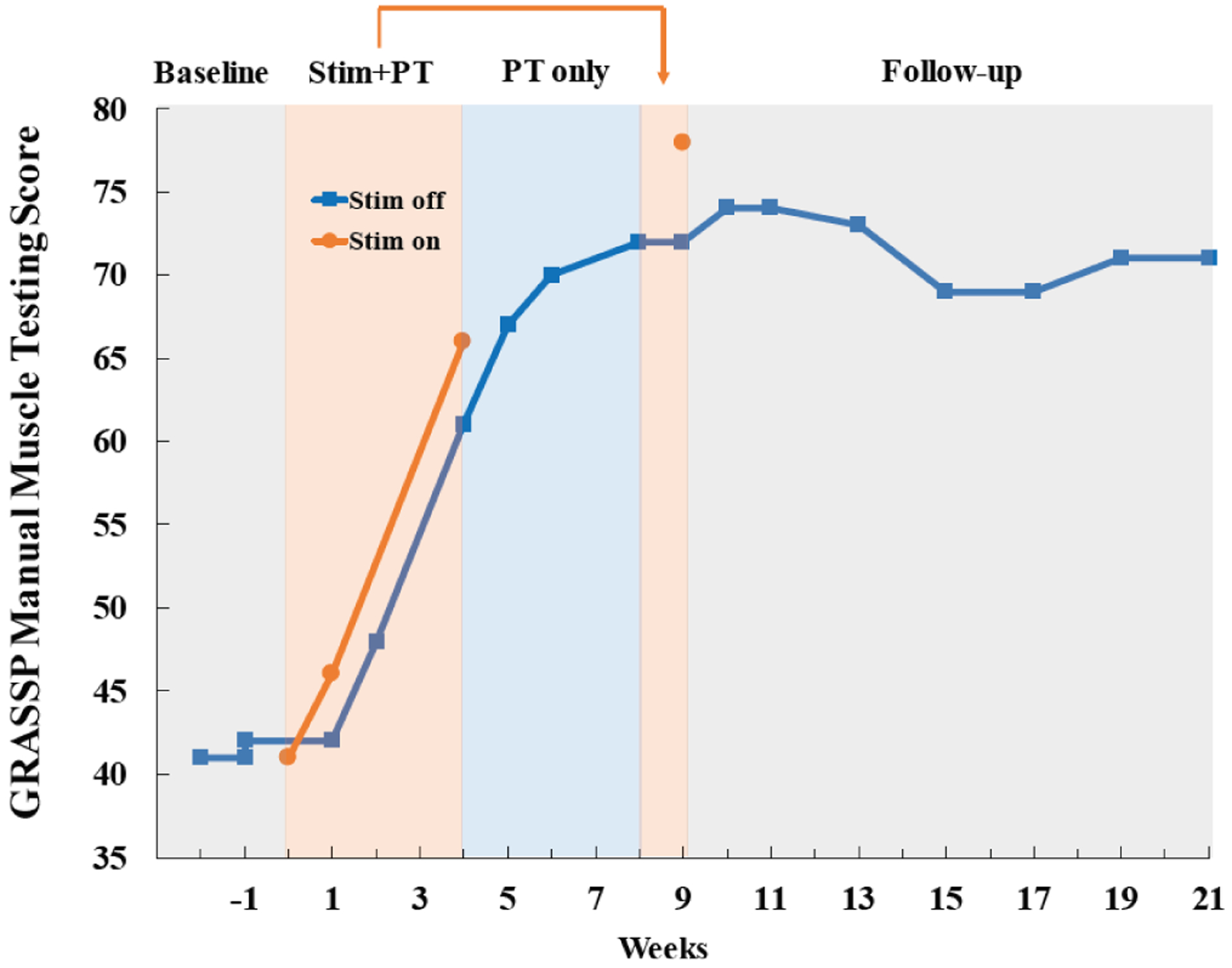
Bilateral manual muscle testing scores derived from Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) test throughout the study. Motor score is comprised of 10 muscles tested bilaterally (deltoid, triceps, biceps, wrist extensors, finger flexors, finger abductors, extensor digitorum, opponens pollicis, flexor pollicis longus, and first dorsal interossei). Strength was stable during baseline testing, increased 37 points during stimulation combined with physical therapy through week 9 (Stim + PT), and was maintained throughout three months of follow-up with no further treatment.
Gains were also observed in all motor function measures of the GRASSP test reflecting restoration of strength, dexterity and prehension. Total GRASSP score improved 56% during the four-week stimulation + PT period (Fig. 4). Although stimulation was initially required to achieve such high performance, functional gains were maintained even without stimulation during the entire follow-up period. This 52-point improvement on the total GRASSP score far exceeded the minimal detectable difference of 4–7 points for all sub-scores of the test except fingertip sensation (Fig. 5) [28].
Fig. 4.
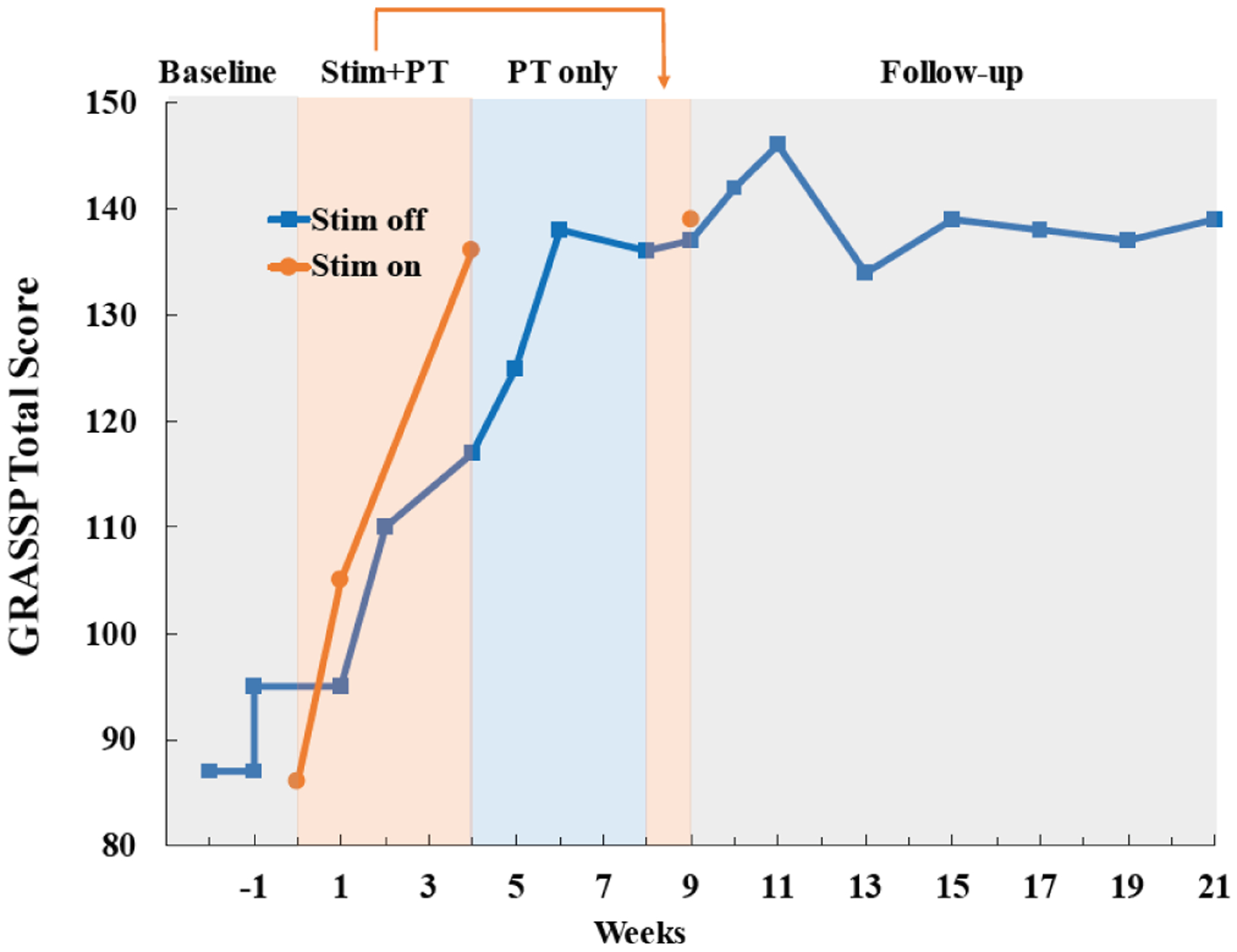
Total GRASSP test scores improve markedly during treatment with stimulation and physical therapy (Stim + PT). The total score combines all domains of the test including strength, sensation, qualitative and quantitative prehension. Improvements were sustained throughout three months of follow-up with no further treatment. Please see Fig. 5 for results from individual test domains.
Fig. 5.
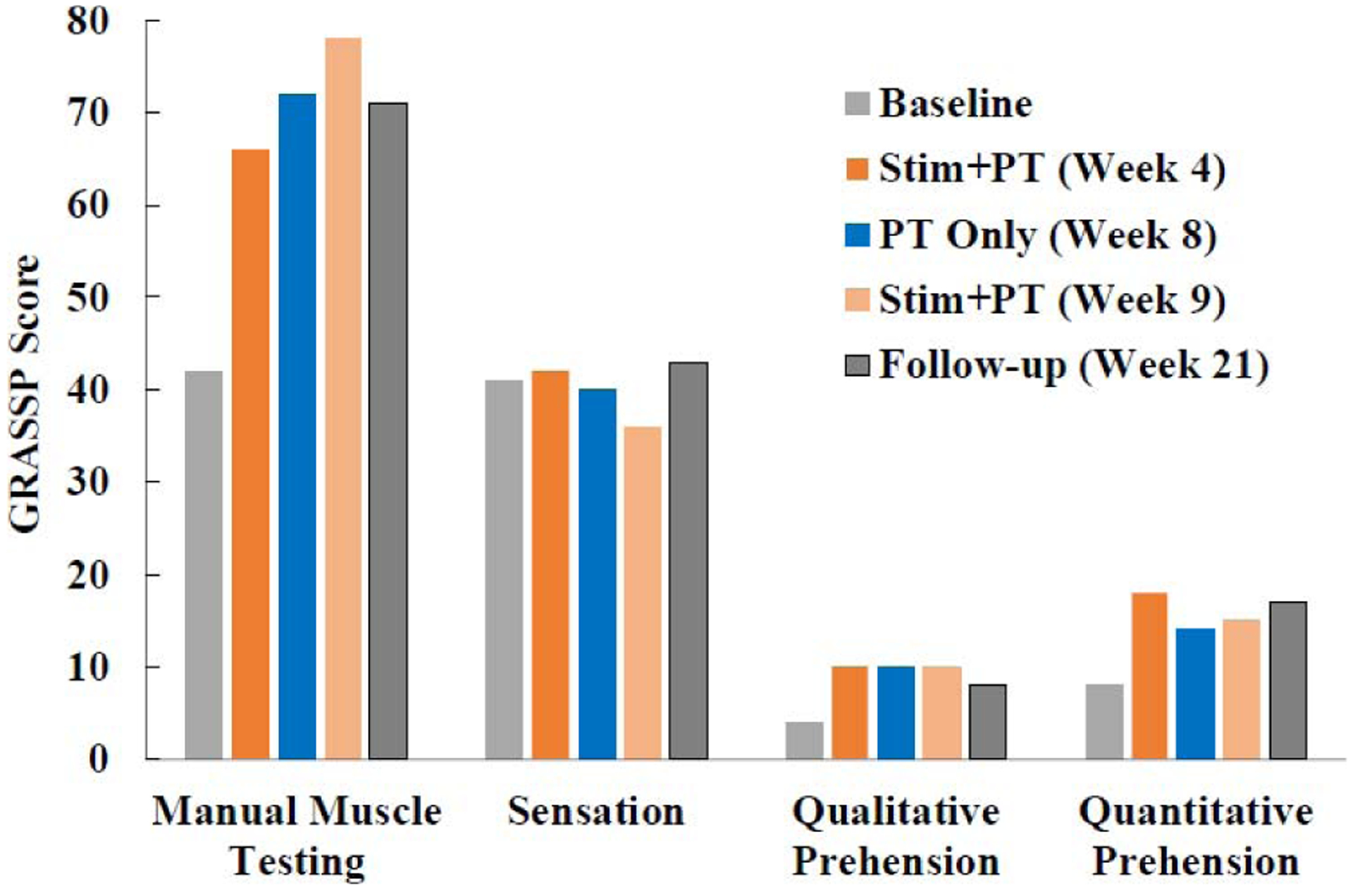
Subscores of the Graded Redefined Assessment of Strength, Sensation and Prehension (GRASSP) test reported at the conclusion of each phase of the study. Improvement (Δ) during stimulation combined with physical therapy (stim + PT) exceeded the minimal detectable difference (MDD) for all subscores of the GRASSP test except fingertip sensation (strength: Δ37 vs. MDD 7; sensation: Δ−2 vs. MDD 4; qualitative prehension: Δ6 vs. MDD 5; and quantitative prehension: Δ11 vs. MDD 6.
Improvements in dexterity and pace of prehension were observed in functional tasks, such as water pouring (cylindrical grasp) and 9-hole peg transfer (tip to tip and three-point pinch). Example videos illustrate the improvements that resulted from treatment with cervical transcutaneous spinal cord stimulation combined with physical therapy (supplementary videos 1 &2).
Lateral pinch forces improved rapidly in both hands during the stimulation + PT intervention. Lateral pinch force measured during stimulation increased 2- to 7-fold in the left and right hands, respectively (Fig. 6). PT alone did not further improve pinch force, but increases in strength even without the stimulator active were maintained throughout the three-month follow-up period.
Fig. 6.
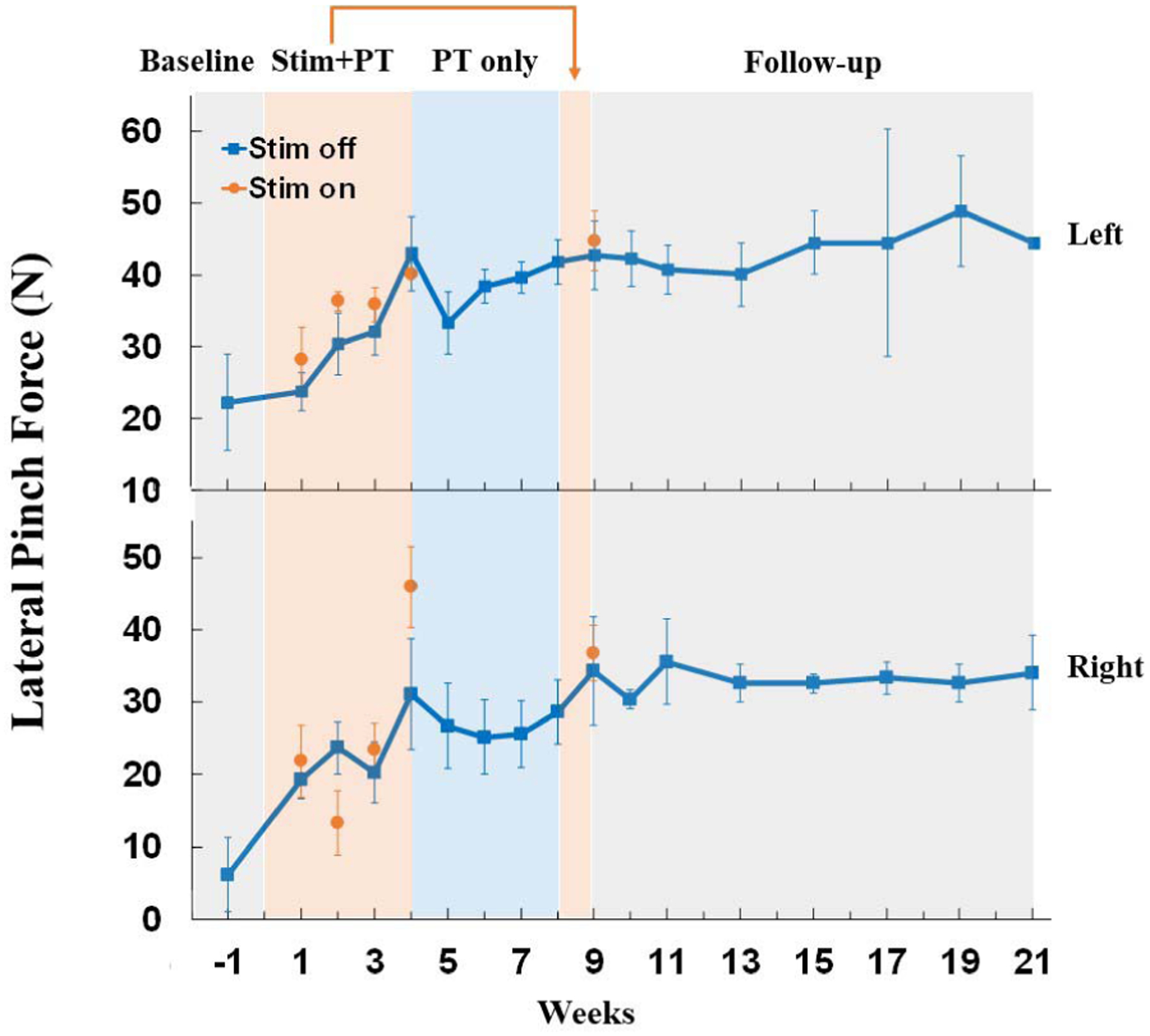
Lateral pinch strength improved in both the right and left hands during stimulation combined with physical therapy. During four weeks of stimulation combined with physical therapy, pinch strength improved 2-to 7-fold in the presence of stimulation for the left and right hand, respectively. Physical therapy alone (PT only) resulted in no further improvement, but all gains were maintained during three months of follow-up. Each data point is the average of three maximal contractions performed on a given day, and error bars are standard deviation.
Following only four weeks of stimulation + PT, overall neurological level of injury improved from C3 to C4 based on the ISNCSCI exam, and was sustained for the duration of the follow-up with no further treatment. This is unusual based on observations that function either reaches a plateau after 1 year post injury [29], or increases only gradually after year 1 post injury [30].
Improved neurological level was driven by a combination of motor and sensory recovery. ISNCSCI Upper Extremity Motor Score (UEMS) increased ten points during the four-week stimulation + PT period and an additional four points during PT only sessions (Table I). This new UEMS of 37 out of 50 points remained unchanged throughout follow-up.
Table I.
International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) Assessments
| Motor Score | Sensory Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Upper Extremity | Lower Extremity | Light Touch | Pin Prick | NLI | |||||
| R | L | R | L | R | L | R | L | ||
| Baseline | 8 | 15 | 18 | 24 | 30 | 30 | 31 | 32 | C3 |
| Stim+PT Week 4 | 12 | 21 | 20 | 24 | 39 | 39 | 40 | 41 | C4 |
| PT only Week 8 | 14 | 23 | 21 | 25 | 34 | 34 | 34 | 34 | C4 |
| Follow-up Week 21 | 14 | 23 | 24 | 25 | 35 | 34 | 35 | 35 | C4 |
Stim = Stimulation; PT = Physical therapy;
NLI = Neurologic Level of Injury.
Surprisingly, the subject reported normal pinprick and light touch sensation descending from C4 all the way to the T10 dermatome bilaterally at the end of four-weeks of stimulation + PT (Fig. 7). This sensory improvement, however, was only partly sustained at the level of the T4 dermatome without continued stimulation.
Fig. 7.
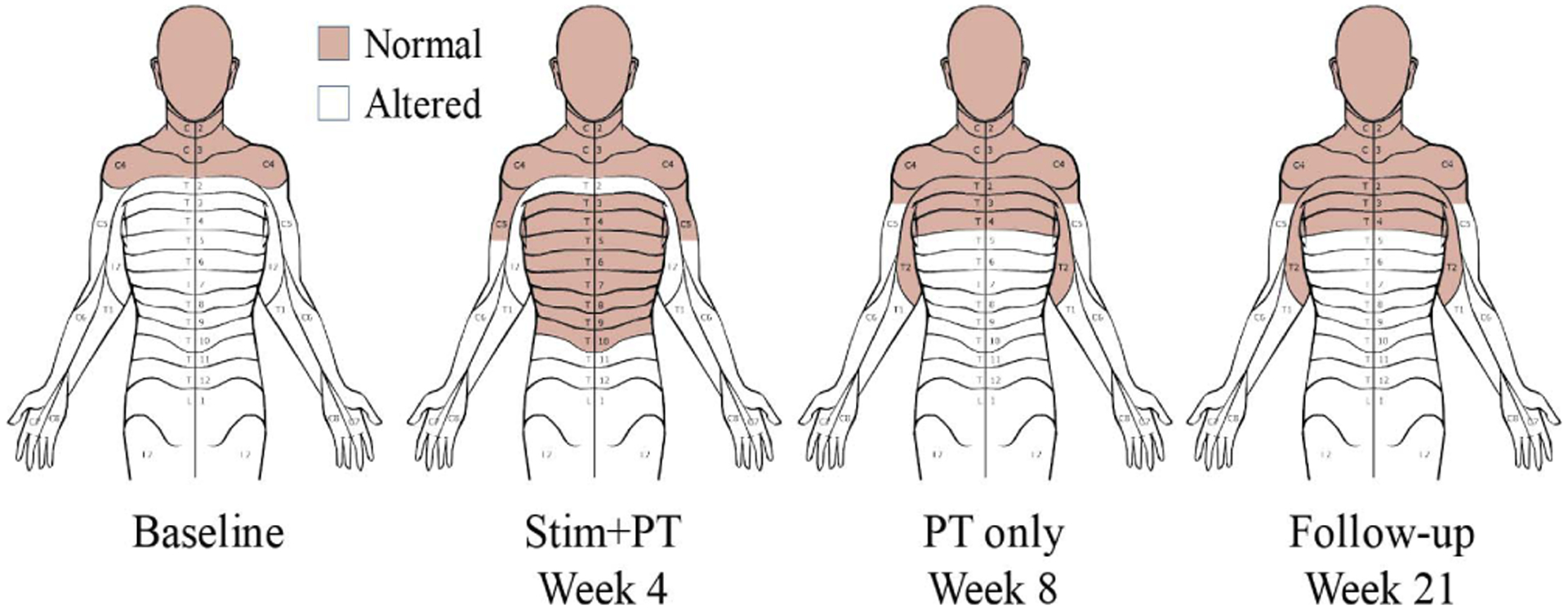
Following four weeks of stimulation combined with physical therapy, normal light touch and pin prick sensations expanded from the C4 to the T10 dermatome. After an additional four weeks of physical therapy only, altered sensation returned below T4, but remained constant at this level throughout the three-month follow-up period.
Transcutaneous cervical stimulation + PT also led to improvements in self-care and quality of life. One of the most notable and expeditious functional improvements was observed in self feeding. Within a few minutes of stimulation during the first session, the subject became more smooth and coordinated in both his upper extremity and trunk when performing a self-feeding task compared to the absence of stimulation (supplementary video 3). After 4 weeks of stimulation + PT, the participant was very skilled in self-feeding. The subject began partial self-feeding at home on the second week of the intervention for the first time since his injury and continued this activity even after the intervention. Thus, the SCIM III self-care sub score increased one point, which was derived from the self-feeding activity (Table II).
Table II.
Disability and Quality of Life Related Questionnaires
| Baseline | Stim+PT Week 4 | PT only Week 8 | Follow-up Week 21 | |
|---|---|---|---|---|
| SCIM III* | ||||
| Self-care | 0 | 1 | 1 | 1 |
| Respiration and sphincter management | 21 | 21 | 21 | 21 |
| Mobility | 1 | 1 | 1 | 1 |
| Total score | 22 | 23 | 23 | 23 |
| WHO-QoL-BREF** | ||||
| Physical Health | 31 | 31 | 44 | 38 |
| Psychological wellbeing | 69 | 69 | 69 | 63 |
| Social relationships | 50 | 56 | 56 | 31 |
| Environment | 94 | 88 | 94 | 94 |
| SF-Qualiveen*** | ||||
| Bother with limitations | 2.5 | 1.5 | 1.5 | 1.5 |
| Frequency of limitations | 4 | 3.5 | 3.5 | 3.5 |
| Fears | 0.5 | 0 | 0 | 0 |
| Feeling | 1.5 | 1.5 | 1 | 1.5 |
| Overall score | 2.125 | 1.625 | 1.5 | 1.625 |
Higher scores reflect higher levels of independence
Scores were transferred to 0–100 point scale. Higher scores denote higher quality of life
Lower scores reflect higher levels of bladder functions related quality of life SCIM III = Spinal Cord Independence Measure; WHO-QoL-BREF = World Health Organization Quality of Life questionnaire short version; SF = short form. Other abbreviations as in Table I.
Finally, bladder function improved during treatment. This participant’s residual urine volume decreased from 175–200 ml to 100–125 ml at the end of four-weeks of stimulation. Therefore, bladder function related quality of life (SF-Qualiveen) improved 0.5 points out of 4 at the end of stimulation + PT intervention. Most notably, this and all other functional gains were maintained in the absence of stimulation and persisted for over three months of follow-up with no further treatment.
C. Effect of Stimulation on Self-Reported Functions
Outside of standardized test and measures, the subject and his care giver reported appreciable increases in sensation and locomotion. He reported improvements in proprioception of his lower extremities and a better temperature sensation all over his body especially while showering. On the second week of stimulation, he began walking up and down the stairs with balance assistance using an alternating stepping pattern for this first time since his injury. His step length and balance improved gradually throughout stimulation sessions.
D. Safety and Tolerability of Transcutaneous Spinal Stimulation
No adverse effects were observed throughout the study. Blood pressure and heart rate ranged between 88/58 and 121/85 mmHg and 66–98 beats/minute, respectively. Mild and painless hyperemia was observed under the stimulation electrode site on the neck, which resolved within 5–10 minutes of the completion of stimulation each day. No other skin reaction or irritation occurred. The subject described the stimulation as a continuous and mild tingling sensation on the neck, arms, and the upper trunk without discomfort.
IV. Discussion
Starting from the very first session of stimulation, almost all motor functions of the hand and arm improved in this participant. Isolated muscle strength, lateral pinch force, dexterity and pace of prehension improved progressively over the course of treatment using cervical skin surface stimulation combined with physical therapy. The magnitude of these improvements exceeded previous reports of activity-dependent interventions in individuals with subacute or chronic SCI [25], [31], [32]. The participant also resumed self-feeding for the first time since his injury, resulting in a measurable change in quality of life. Pinprick and light touch sensations returned to the torso, and neurologic level of injury improved from C3 to C4. Most importantly, improved functions persisted throughout the entire three months of follow-up, despite no additional stimulation or physical therapy. This suggests that even a five-week period of transcutaneous spinal cord stimulation and physical therapy can lead to long-term changes in neural circuits and sustained improvements in upper extremity function following spinal cord injury.
Two interrelated mechanism may explain the immediate and sustained improvements in motor and sensory function observed here. The immediate improvements in upper extremity strength and function support the concept that transcutaneous electrical spinal cord stimulation can modulate cervical spinal networks into a physiologic state which enables greater access of supraspinal control to cervical sensory-motor networks. An electrophysiologic study by Hofstoetter et al. [33] recently showed that both epidural and transcutaneous electrical stimulation activates primary afferent fibers within multiple posterior roots. The most likely direct mechanism of stimulation occurs via tonic activation of dorsal root afferent fibers which elevates spinal networks excitability. This in turn brings interneurons and motor neurons closer to motor threshold and thus more likely to respond to limited post-injury descending drive [34]–[36].
It is possible that stimulation of the skin itself also contributes to elevated neural excitability [25], [37], [38]. Hagbarth and Neæss [39] noted cutaneous stimulation of the cat hindlimb increased afferent fiber activity leading to increased motor neuron excitability. To what degree transcutaneous stimulation activated the sensory afferent system in the periphery, at the level of the dorsal roots, and/or via the spinal grey matter is currently unknown. The polysynaptic responses in Figure 8 are consistent with a functional enhancement of interneuronal networks, perhaps via a change in reafferent excitability [33]. We suggest that the more mechanistically important question is not what is directly stimulated, but which components of the spinal networks are being modulated by transcutaneous stimulation. Nonetheless, the benefits for hand function appear to be both immediate and sustained following transcutaneous stimulation of the spinal cord in the present study.
Fig. 8.
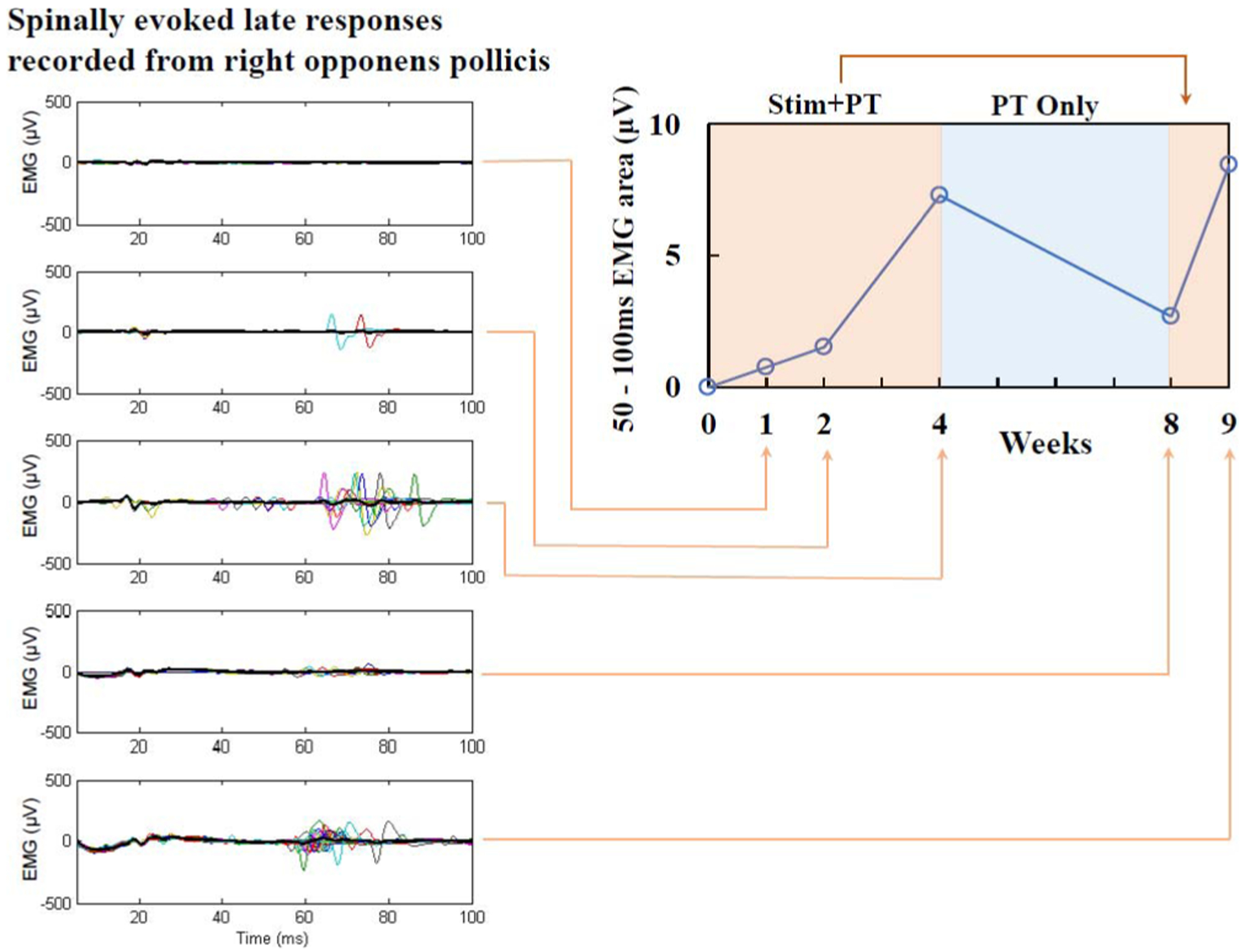
Integrated EMG of stimulus-evoked response recorded from right opponens pollicis muscle (right panel). Spinal evoked potentials were elicited by monophasic, rectangular, 1 ms single pulses filled with a 10 kHz waveform, delivered at 1 Hz. Stimulation intensity was 90 mA applied over the C3–4 spinous processes. The polysynaptic, late EMG responses (left panels) increased gradually over four weeks of stimulation combined with physical therapy, reduced after physical therapy only, but returned with five days of additional stimulation and therapy treatment.
Sustained improvements appear to evolve over time and may be explained by gradual neuroplastic change in the spinal networks surrounding the injury. Observed changes in the evoked potentials of networks projecting to the right thenar muscle provide an example of one mechanism that could have facilitated long-term improvements in pinch force. Monophasic stimulation over C3–4 spinous process revealed changes in delayed, polysynaptic responses in the right thenar muscle. This is one of the muscles contributing to the improvements in right hand strength and function. Compared to pretreatment responses, there was a progressive increase in long-latency, likely polysynaptic responses over the month of stimulation combined with physical therapy (Fig. 8). Interestingly, this response diminished during physical therapy only, but was rapidly restored by just five additional days of stimulation + PT. This example provides some evidence that transcutaneous electrical spinal cord stimulation leads to both rapid and sustained changes in intraspinal networks.
Furthermore, in this study we show that transcutaneous electrical spinal cord stimulation confers both immediate benefits when the stimulator is active, but also durable improvements in hand and arm function which are sustained for over three-months of follow-up without further treatment. One possible mechanism for this long-lasting functional restoration may be reorganization of cervical spinal networks by intensive task-specific exercise combined with transcutaneous spinal cord stimulation. Specifically, stimulation allows weak but remaining voluntarily-controlled descending drive to produce functional muscle contractions, permitting the participant to engage in intensive therapy which subsequently strengthens these neuro-muscular networks [38]. Thus, at the conclusion of treatment, stimulation is no longer required to achieve robust volitional control of hand movements after spinal cord injury.
Similar to long-term improvements in volitional motor control, return of normal sensation below the injury in the present study may be explained by enhanced excitability of sensory networks. This enhanced activity may facilitate initially weak ascending sensory connections passing the injury site to restore partial sensory function even beyond the period of stimulation.
The findings of the current study extend those of Lu et al. [16], who studied the effect of cervical epidural electrical stimulation in two subjects with chronic motor complete (AIS B) tetraplegia. The indication for implantation of epidural stimulator was refractory chronic pain for both subjects. The authors demonstrated improved maximum grip force and volitional motor control both during and shortly after epidural stimulation. Despite the dissimilarities of injury level, severity and outcome measures used, the results of our study are largely comparable with cervical epidural stimulation. Excitingly, transcutaneous spinal stimulation appears to result in similar improvements as epidural stimulation, without the need for implanted electrodes.
Taken together, findings of the current study (1) show that the effect of stimulation is both immediate and long-lasting, (2) provide evidence that electrical neuromodulation of the cervical spinal cord combined with activity based exercise therapy can promote substantial functional recovery of upper extremities in chronic SCI, and (3) demonstrate the therapeutic potential of non-invasive electrical spinal cord stimulation for people with cervical SCI. Future work is needed to explore the exciting potential of transcutaneous spinal stimulation and optimize its ability to restore function following a range of neurological injuries.
Supplementary Material
Acknowledgment
The authors thank Dr. Stephen Burns for his input and edits of the manuscript, and Jan Jimenez for Figure 2 and 7 illustrations. We are especially indebted to our research participant and his family for their motivation, adherence, and dedication that made this study possible.
This work was supported in part by the Center for Sensorimotor Neural Engineering, a National Science Foundation-Engineering Research Center under Grant EEC-1028725, in part by the Christopher and Dana Reeve Foundation, and in part by the Washington State Spinal Cord Injury Consortium.
Footnotes
This paper has supplementary downloadable material available at http://ieeexplore.ieee.org, provided by the author.
Contributor Information
Fatma Inanici, Department of Rehabilitation Medicine, University of Washington, Seattle, WA 98195, USA.
Soshi Samejima, Department of Rehabilitation Medicine, University of Washington, Seattle, WA 98195, USA.
Parag Gad, Department of Integrative Biology and Physiology, UCLA, Los Angeles, CA 90095, USA.
V. Reggie Edgerton, Department of Integrative Biology and Physiology, UCLA, Los Angeles, CA 90095, USA.
Christoph P. Hofstetter, Department of Neurological Surgery, University of Washington, Seattle, WA 98195, USA,
Chet T. Moritz, Department of Rehabilitation Medicine, with the Department of Physiology and Biophysics, University of Washington, Seattle, WA 98195, USA,; Department of Electrical Engineering, University of Washington, Seattle, WA 98195, USA, Center for Sensorimotor Neural Engineering (CSNE), Seattle, WA 98105 USA, Washington State Spinal Cord Injury Consortium (WASCIC), Seattle, WA 98195 USA
References
- [1].University Alabama Birmingham. (2016). National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance. [Online]. Available: https://www.nscisc.uab.edu/Public/Facts%202016.pdf [Google Scholar]
- [2].Anderson KD, “Targeting recovery: Priorities of the spinal cord-injured population,” J. Neurotrauma, vol. 21, no. 10, pp. 1371–1383, Oct. 2004. [DOI] [PubMed] [Google Scholar]
- [3].Dietz V and Fouad K, “Restoration of sensorimotor functions after spinal cord injury,” Brain, vol. 137, no. 1, pp. 654–667, Mar. 2014. [DOI] [PubMed] [Google Scholar]
- [4].Edgerton VR and Roy RR, “A new age for rehabilitation,” Eur.J. Phys. Rehabil. Med, vol. 48, no. 1, pp. 99–109, Mar. 2012. [PubMed] [Google Scholar]
- [5].Ievins A and Moritz CT, “Therapeutic stimulation for restoration of function after spinal cord injury,” Physiology, vol. 32, no. 5, pp. 391–398, Sep. 2017. [DOI] [PubMed] [Google Scholar]
- [6].Barolat G, Myklebust JB, and Wenninger W, “Enhancement of voluntary motor function following spinal cord stimulation—Case study,” Appl. Neurophysiol, vol. 49, no. 6, pp. 307–314, 1986. [DOI] [PubMed] [Google Scholar]
- [7].Carhart MR, He J, Herman R, D’Luzansky S, and Willis WT, “Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury,” IEEE Trans. Neural Syst. Rehabil. Eng, vol. 12, no. 1, pp. 32–42, Mar. 2004. [DOI] [PubMed] [Google Scholar]
- [8].Sherwood AM, Sharkey PC, and Dimitrijevic MR, “Biomedical engineering specifications for epidural spinal cord stimulation to augment motor performance,” Int. Rehabil. Med, vol. 2, no. 2, pp. 62–67, 1980. [DOI] [PubMed] [Google Scholar]
- [9].Angeli CA, Edgerton VR, Gerasimenko YP, and Harkema SJ, “Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans,” Brain, vol. 137, no. 1, pp. 1394–1409, May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grahn PJ et al. , “Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia,” Mayo Clin. Proc, vol. 92, no. 4, pp. 544–554, Apr. 2017. [DOI] [PubMed] [Google Scholar]
- [11].Harkema S et al. , “Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study,” Lancet, vol. 377, no. 9781, pp. 1938–1947, Jun. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cogiamanian F et al. , “Transcutaneous spinal direct current stimulation,” Frontiers Psychiatry, vol. 3, p. 63, Jul. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hofstoetter US et al. , “Augmentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor-incomplete spinal cord-injured individuals,” Artif. Organs, vol. 39, no. 10, pp. E176–E186, Oct. 2015. [DOI] [PubMed] [Google Scholar]
- [14].Minassian K et al. , “Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord–injured individuals,” Neurorehabil. Neural Repair, vol. 30, no. 3, pp. 233–243, Mar. 2016. [DOI] [PubMed] [Google Scholar]
- [15].Waltz JM, Andreesen WH, and Hunt DP, “Spinal cord stimulation and motor disorders,” Pacing Clin. Electrophysiol, vol. 10, no. 1, pp. 180–204, Jan. 1987. [DOI] [PubMed] [Google Scholar]
- [16].Lu DC et al. , “Engaging cervical spinal cord networks to reenable volitional control of hand function in tetraplegic patients,” Neurorehabil. Neural Repair, vol. 30, no. 10, pp. 951–962, Nov. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P, and Edgerton VR, “Transcutaneous electrical spinal-cord stimulation in humans,” Ann. Phys. Rehabil. Med, vol. 58, no. 4, pp. 225–231, Sep. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gad P et al. , “Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia,” Frontiers Neurosci, vol. 11, p. 333, Jun. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gad P et al. , “Noninvasive activation of cervical spinal networks after severe paralysis,” (in English), J. Neurotrauma, Apr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kirshblum SC et al. , “International standards for neurological classification of spinal cord injury (revised 2011),” J. Spinal Cord Med, vol. 34, no. 6, pp. 535–546, Nov. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kalsi-Ryan S et al. , “The graded redefined assessment of strength sensibility and prehension: Reliability and validity,” (in English),J. Neurotrauma, vol. 29, no. 5, pp. 905–914, Mar. 2012. [DOI] [PubMed] [Google Scholar]
- [22].Skevington SM, Lotfy M, and O’Connell KA, “The World Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group,” Quality Life Res, vol. 13, no. 2, pp. 299–310, Mar. 2004. [DOI] [PubMed] [Google Scholar]
- [23].Bonniaud V, Bryant D, Parratte B, and Guyatt G, “Qualiveen, a urinary-disorder specific instrument: 0.5 corresponds to the minimal important difference,” J. Clin. Epidemiol, vol. 61, no. 5, pp. 505–510, May 2008. [DOI] [PubMed] [Google Scholar]
- [24].Bluvshtein V et al. , “SCIM III is reliable and valid in a separate analysis for traumatic spinal cord lesions,” Spinal Cord, vol. 49, no. 2, pp. 292–296, Feb. 2011. [DOI] [PubMed] [Google Scholar]
- [25].Beekhuizen KS and Field-Fote EC, “Massed practice versus massed practice with stimulation: Effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury,” Neurorehabil. Neural Repair, vol. 19, no. 1, pp. 33–45, Mar. 2005. [DOI] [PubMed] [Google Scholar]
- [26].Gad P et al. , “Electrophysiological mapping of rat sensorimotor lumbosacral spinal networks after complete paralysis,” (in English), Prog. Brain Res, vol. 218, pp. 199–212, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lavrov I et al. , “Plasticity of spinal cord reflexes after a complete transection in adult rats: Relationship to stepping ability,” J. Neurophysiol, vol. 96, no. 4, pp. 1699–1710, Oct. 2006. [DOI] [PubMed] [Google Scholar]
- [28].Kalsi-Ryan S et al. , “Responsiveness, sensitivity, and minimally detectable difference of the graded and redefined assessment of strength, sensibility, and prehension, version 1.0,” J. Neurotrauma, vol. 33, no. 3, pp. 307–314, 2016. [DOI] [PubMed] [Google Scholar]
- [29].Waters RL, Adkins RH, Yakura JS, and Sie I, “Motor and sensory recovery following complete tetraplegia,” Arch. Phys. Med. Rehabil, vol. 74, no. 3, pp. 242–247, Mar. 1993. [PubMed] [Google Scholar]
- [30].Kirshblum S, Millis S, McKinley W, and Tulsky D, “Late neurologic recovery after traumatic spinal cord injury,” Arch. Phys. Med. Rehabil, vol. 85, no. 11, pp. 1811–1817, Nov. 2004. [DOI] [PubMed] [Google Scholar]
- [31].Larson CA and Dension PM, “Effectiveness of intense, activity-based physical therapy for individuals with spinal cord injury in promoting motor and sensory recovery: Is olfactory mucosa autograft a factor?” J. Spinal Cord Med, vol. 36, no. 1, pp. 44–57, Jan. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zariffa J et al. , “Effect of a robotic rehabilitation device on upper limb function in a sub-acute cervical spinal cord injury population,” in Proc. IEEE Int. Conf. Rehabil. Robot, Jun-Jul 2011, pp. 1–5. [DOI] [PubMed] [Google Scholar]
- [33].Hofstoetter US, Freundl B, Binder H, and Minassian K, “Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes,” PLoS ONE, vol. 13, no. 1, p. e0192013, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gerasimenko YP et al. , “Noninvasive reactivation of motor descending control after paralysis,” J. Neurotrauma, vol. 32, no. 24, pp. 1968–1980, Jun. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gerasimenko YP et al. , “Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats,” J. Neurosci. Methods, vol. 157, no. 2, pp. 253–263, Oct. 2006. [DOI] [PubMed] [Google Scholar]
- [36].Capogrosso M et al. , “A computational model for epidural electrical stimulation of spinal sensorimotor circuits,” J. Neurosci, vol. 33, no. 49, pp. 19326–19340, Dec. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gomes-Osman J, Tibbett JA, Poe BP, and Field-Fote EC, “Priming for improved hand strength in persons with chronic tetraplegia: A comparison of priming-augmented functional task practice, priming alone, and conventional exercise training,” Frontiers Neurol, vol. 7, p. 242, Jan. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gomes-Osman J and Field-Fote EC, “Cortical vs. afferent stimulation as an adjunct to functional task practice training: A randomized, comparative pilot study in people with cervical spinal cord injury,” Clin. Rehabil, vol. 29, no. 8, pp. 771–782, Aug. 2015. [DOI] [PubMed] [Google Scholar]
- [39].Hagbarth K-E and Neæss K, “Reflex effects of tetnnic stimulation of different afferent fibre-systems in the hind limb of the cat,” Acta Physiol. Scand, vol. 21, no. 4, pp. 336–361, Dec. 1950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


