Abstract
Uterine receptivity is critical for establishing and maintaining pregnancy. For the endometrium to become receptive, stromal cells must differentiate into decidual cells capable of secreting factors necessary for embryo survival and placental development. Although there are multiple reports of autophagy induction correlated with endometrial stromal cell (ESC) decidualization, the role of autophagy in decidualization has remained elusive. To determine the role of autophagy in decidualization, we utilized 2 genetic models carrying mutations to the autophagy gene Atg16L1. Although the hypomorphic Atg16L1 mouse was fertile and displayed proper decidualization, conditional knockout in the reproductive tract of female mice reduced fertility by decreasing the implantation rate. In the absence of Atg16L1, ESCs failed to properly decidualize and fewer blastocysts were able to implant. Additionally, small interfering RNA knock down of Atg16L1 was detrimental to the decidualization response of human ESCs. We conclude that Atg16L1 is necessary for decidualization, implantation, and overall fertility in mice. Furthermore, considering its requirement for human endometrial decidualization, these data suggest Atg16L1 may be a potential mediator of implantation success in women.
Keywords: implantation, fertility, pregnancy
For the successful establishment of pregnancy, the human endometrium must undergo a series of defined changes at the molecular and cellular level to allow for a competent blastocyst to implant (1–3). The importance of this uterine preparation is emphasized by the low success rate of assisted reproductive technology (~30% resulting in live birth), where the majority of unsuccessful in vitro fertilization and/or embryo-transfer procedures are attributed not to chromosomal abnormalities or other embryonic defects, but to implantation failure of maternal causes (4, 5). Maternal obesity further decreases implantation and live birth rate following in vitro fertilization, independent of embryo quality (6), suggesting that obesity and metabolic dysfunction are detrimental to uterine receptivity.
A critical precursor to implantation is the morphological and functional transformation of uterine stromal cells into epithelioid-like decidual cells (7). This process of decidualization is tightly regulated by the ovarian steroid hormones, estrogen and progesterone, through the actions of their cognate nuclear receptors in the endometrium (8). Genetically modified mouse models ablating key regulators of decidualization, such as Foxo1, Pgr, or Esr1, result in implantation failure and sub- or infertility, underscoring a key role for decidualization in pregnancy success (9–12). Furthermore, in vitro models of decidualization using primary cell lines with genetic knockdown (mediated via small interfering RNA [siRNA]- or short hairpin RNA [shRNA]) of these key genes (13) provide evidence that similar mechanisms drive decidualization in the human endometrium.
Although the molecular complexity of hormone responsive genes governing decidualization have been intricately examined, a comprehensive understanding of the mechanisms that drive metabolic pathways during decidualization is lacking (1, 14). To meet the high energetic demand of decidualization, endometrial stromal cell (ESCs) increase the expression of glucose transporters (15). However, glucose is preferentially metabolized through the pentose phosphate pathway where it produces less adenosine triphosphate (ATP) per molecule of glucose than if metabolized by glycolysis (16). To address the question of stromal differentiation in energy-depleted states, we investigated the potential role of autophagy in implantation and uterine decidualization.
Autophagy is a conserved catabolic pathway necessary for the maintenance of cellular homeostasis (17, 18) activated by a broad range of stressors including nutrient deprivation (starvation), inflammation, and oxidative stress. Autophagy plays a role in a variety of physiological and pathological processes, and more recently has been implicated in both the cyclic remodeling of the endometrium (19) and implantation (20). Autophagy is upregulated during decidualization in both mouse and human ESCs, but is diminished in obesity models with impaired decidualization (21). Upon activation, the autophagy pathway provides a rapid mechanism for cellular remodeling in a hormonally-responsive manner (22). Since the cellular transformation of fibroblast cells to secretory decidual cells is characterized by a drop in cellular ATP (21) and intracellular remodeling—both hallmarks of autophagy—we hypothesize that autophagy is necessary for decidualization.
In mammals, autophagy is initiated by the formation of a double-membrane vesicle, termed the phagophore, which sequesters cytoplasmic contents for lysosomal degradation (23). Following nucleation of the phagophore by autophagy initiation complexes, ubiquitin-like conjugation systems promote elongation and expansion of the phagophore membrane. One of these critical conjugation systems consists of the autophagy protein autophagy related 16-like 1 (ATG16L1), along with ATG12 and ATG5 (24). This conjugation system is responsible for lipidation of the autophagosome membrane protein, microtubule-associated protein 1A/1B-light-chain 3 (LC3B) to promote elongation (24, 25) before membrane closure and fusion with the lysosome for cargo degradation.
Given the high energy demands of decidualizing cells, and the recent finding that autophagy is upregulated in decidualizing cells of both mice and humans (21), we investigated the role of ATG16L1 in ESC decidualization. Using both in vitro and in vivo models, we demonstrate that loss-of-function for ATG16L1 results in impaired endometrial decidualization.
Methods
Animal care and use
All animal studies were approved by the Animal Care and Use Committee at Washington University School of Medicine. Atg16L1 hypomorphic mice (ATG16L1 HM) were a generous gift from Dr. Herbert Virgin at Washington University School of Medicine and previously described (26). Atg16L1flox/flox mice, in which exons 3 is flanked by loxp sites, were bred to progesterone receptor cre (PRcre/+) mice (27) to generate (Atg16L1flox/flox PRcre/+ mice), hereafter referred to as Atg16L1 cKO. All mice were age-matched and on a C57/BL6 genetic background (The Jackson Laboratory, Bar Harbor, ME). Mice were genotyped using RedTaq Polymerase (Sigma) Mix and the gene specific primers listed in Table 1.
Table 1.
List of primers and probes
| Gene name | Species | Application, Chemistry | Company | Sequence/Catalogue number |
|---|---|---|---|---|
| ATG16L1 HM | Mouse | Genotyping | IDT | P1: TGGCTGGAGTGCGATCTTCC P2: CAGACGGCAAACGACTGTCCT P3: CAGGATCCTTCTGCACACATTT P4: CACCTGGTTACATTGGCAAACA |
| PR Cre | Mouse | Genotyping | IDT | P1: ATG TTT AGC TGG CCC AAA TG P2: TAT ACC GAT CTC CCT GGA CG P3: CCC AAA GAG ACA CCA GGA AG |
| Atg16L1 flox | Mouse | Genotyping | IDT | P1: GGAACCACGCTGACATTTGACACTG P2: CAAAGAACAACGAGTGGCAGTAG P3: CATCAGATACACTAGAGCTGG |
| Prp | Mouse | qPCR, Sybr | IDT | F: TCC TGG CCA ATA ATG CTG CCA TTG |
| R: AGC AGC CAT TCT CTC CTG TTT GAC | ||||
| 18S | Mouse | qPCR, Sybr | IDT | F: TTC CTT ACC TGG TTG ATC CTG CCA |
| R: AGC CAT TCG CAG TTT CAC TGT ACC | ||||
| Wnt4 | Mouse | qPCR, Taqman | ABI | Mm01194003_m1 |
| Bmp2 | Mouse | qPCR, Taqman | ABI | Mm01340178_m1 |
| Atg16L1 | Mouse | qPCR, Taqman | ABI | Mm00513084_m1 |
| 18S | Mouse | qPCR, Taqman | ABI | 4318839 |
| IGFBP1 | Human | qPCR, Taqman | ABI | Hs00236877_m1 |
| PRL | Human | qPCR, Taqman | ABI | Hs00168730_m1 |
All primer sequences are written 5’ to 3’
Abbreviations: ABI, Applied Biosystems; IDT, integrated DNA technologies; qPCR, quantitative polymerase chain reaction.
Fertility tests and timed mating
To determine fecundity, control (Atg16L1flox/flox) and Atg16L1 cKO (Atg16L1flox/floxPRcre/+) female mice were mated to wildtype (Wt) C57/BL6 male mice of proven fertility for 6 months. Singleton breeding cages were checked daily for pups, which were promptly removed from the cage. For timed mating experiments, 1-day post coitum (dpc) denotes the morning a copulatory plug was observed. Blastocysts were flushed from the uterine horns with phosphate-buffered saline at 4 dpc (just before implantation) as an indirect measure of ovulation and fertilization. To visualize implantation sites, mice received a tail vein injection of 50 µL of 1% Chicago Sky Blue dye (cat. no. C8679, Sigma-Aldrich) at 6 dpc just prior to sacrifice.
Mouse ESC culture
On 4 dpc, blastocysts were flushed from the uterine horns of pregnant female mice to confirm pregnancy. ESCs from uteri of pregnant females were then isolated as previously described (15) and cultured in Dulbecco's Modified Eagle Medium Nutrient Mixture F-12 without phenol, containing 2% heat-inactivated fetal bovine serum, 1% penicillin/streptomycin (Corning). Cells were treated with 10 nM β-estradiol (E2) (cat. no. E1024, Sigma-Aldrich) and 10 µM progesterone (P4) (cat. no. M1629, Sigma-Aldrich) for 3 days in culture to stimulate decidualization before collection. Un-decidualized control cells received 0.2% ethanol vehicle.
Artificial decidualization
Oil-induced artificial decidualization was performed on 6- to 8-week-old female mice as previously described (16, 28). Briefly, mice were ovariectomized and allowed to recover for 2 weeks. Mice were primed with a hormone regime described in (Fig. 1E). Six hours following the last hormone injection on day 0, a volume of 50 µl of sesame oil (cat. no. S3547, Sigma-Aldrich) was injected into the lumen of the right uterine horn. Mice continued receiving daily progesterone (P4) (cat. no. P0130, Sigma-Aldrich) injections and uterine horns were collected and weighed 5 days after oil stimulation.
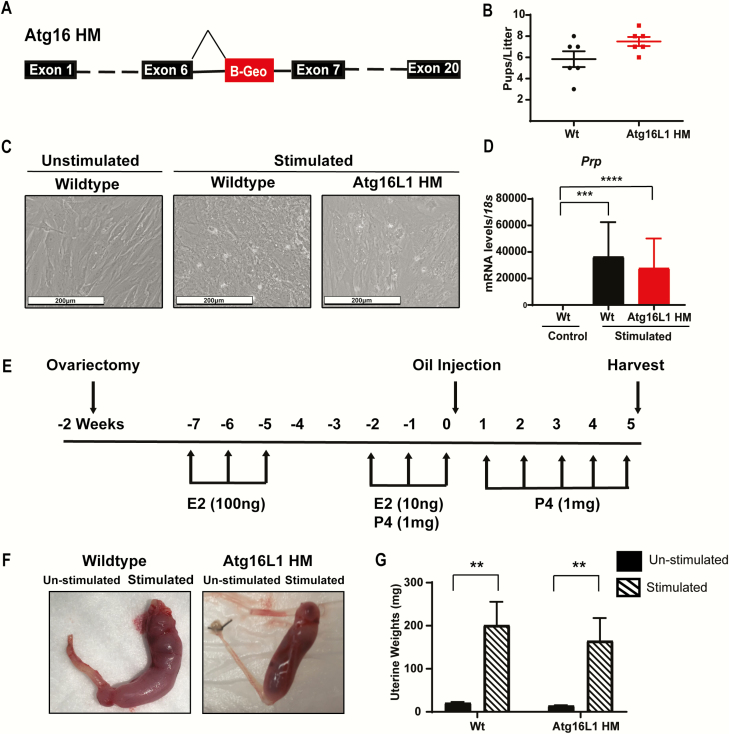
Figure 1.
Atg16L1 HM mice are fertile and maintain the ability to decidualize. A) Diagram of the Atg16L1 HM mutation. B) Live birth rate of Wildtype (Wt) and Atg16L1 HM mice reveals that Atg16L1 HM mice are fertile. C) In vitro hormonal decidualization of endometrial stromal cells from Wt and Atg16L1 HM mice and D) Expression of the decidualization marker Prp show proper decidualization in Atg16L1 mice (n = 3 Wt, 5 Atg16L1 HM). E) Diagram of in vivo artificial decidualization method. F) Uterine horns of Wt and Atg16L1 HM mice and G) Resulting wet weights demonstrate Atg16L1 HM mice maintain the ability to decidualize in vivo (n = 10 Wt, 12 Atg16L1 HM). E2-estrogen, P4-progesterone; **P < 0.01, ***P < 0.001. ****P < 0.0001.
RNA isolation and real-time quantitative polymerase chain reaction (PCR)
Total RNA was isolated with the Purelink RNA mini kit (Invitrogen) and quantified with a NanoDrop 2000 (Thermo Scientific). Then, 1 µg of RNA was DNase treated and reverse transcribed with the QuantiTect Reverse Transcription Kit (Qiagen). To quantify Atg16L1 expression in Atg16L1 cKO mice, primers specific to the excised exon (Table 1) were used with Fast SYBR green mastermix (Applied Biosystems) on a 7500 Fast Real-time PCR system (Applied Biosciences). Cycling parameters were as follows: 95°C for 20 seconds followed by 40 cycles of 95°C for 3 seconds and 60°C for 30 seconds. Efficiency of all SYBR green primer sets were determined and validated using serial dilutions to generate a 5-point curve. Efficiencies of primer sets were 89% and 91.1% for 18s and Prp respectively. Additionally, the melt curve analysis was used to determine the amplification of a single PCR product. Other genes of interest were amplified with TaqMan 2× master mix (Applied Biosystems/Life Technologies, Grand Island, NY) and gene-specific assays (Table 1). Cycling parameters were as follows: 50°C for 20 seconds and 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. The delta delta cycle threshold method was used to normalize expression to the reference gene 18S (29).
Human ESC isolation, decidualization, and siRNA knockdown
Endometrial biopsies from healthy women of reproductive age were obtained during the proliferative phase (days 8-12) of the menstrual cycle. Volunteers provided written informed consent in accordance with an Institutional Review Board-approved protocol from Washington University School of Medicine and the guidelines of the Declaration of Helsinki (30). Human endometrial stromal cells (hESCs) were isolated as previously described (31), and stimulated to decidualize by treating for 6 days with 100 nM Estradiol (cat. no. E1024, Sigma-Aldrich), 10 µM medroxyprogesterone17-acetate (MPA) (cat. no. M1629, Sigma-Aldrich and 50 µM N6, 2′-O-dibutyryladenosine 3′ 5′–cyclic monophosphate sodium salt (cat. no. D0260, Sigma-Aldrich) (32). For siRNA-mediated knockdown of ATG16L1, hESCs were treated with Lipofectamine RNA iMAX reagent and 60 pmol of either nontargeting siRNA (D-001810-10-05) or siRNAs targeting ATG16L1 (L-021033-01-0005), (GE Healthcare Dharmacon Inc., Lafayette, CO). After 48 hours, decidualization was induced, and medium was changed every 2 days. After 6 days of stimulation, cells were collected, and the total RNA isolation kit (Invitrogen/Life Technologies, Grand Island, NY) was used to isolate RNA. Quantitative PCR analyses were performed with the gene-specific assays described in Table 1, and values were normalized to 18S ribosomal RNA. All experiments were conducted with early-passage hESCs (no more than 4 passages). Each experiment carried out on hESCs derived from 4 different individuals. Results are shown as mean ± standard error (SE) from 3 replicates of 1 representative hESC cell line.
SDS-PAGE and Western blotting
The lysates containing 40 μg of protein were loaded on a 4% to 15% SDS-polyacrylamide gel (Bio-Rad), separated with 1xTris-Glycine Running Buffer (Bio-Rad), and transferred to polyvinylidene difluoride (PVDF) membranes in a wet electroblotting system (Bio-Rad), all according to the manufacturer’s directions. PVDF membranes were blocked for 1 hour in 5% nonfat milk in TBS-T (Bio-Rad), then incubated overnight at 4oC with antibodies to LC3B (cat. no. NB100-2220, Novus Biological) (33), ATG16L1 (cat. no. A7356, Sigma-Aldrich) (34) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (cat. no. #2118S, Cell Signaling Technology) (35) in TBS-T containing 5% bovine serum albumin (BSA). After washing and incubating with Anti-rabbit IgG, HRP-linked Antibody (1:5000, cat. no. #7074 Cell Signaling Technology) (36) in TBS-T plus 5% BSA for 1 hour at room temperature, blots were developed with HRP Chemiluminescent Substrates and imaged on a Bio-Rad ChemiDoc imaging system. The GAPDH was used as an internal loading control.
Statistical analysis
All data are presented as mean ± SE. A 2-tailed paired Student t-test was used to analyze experiments with 2 experimental groups, and a 2-way analysis of variance (ANOVA) with Tukey’s correction for multiple comparisons was used to analyze experiments containing 4 or more groups. Finally, experiments with 3 groups were analyzed using a 1-way ANOVA with Tukey’s correction. If the data were not normally distributed, a log transformation was performed before performing statistical analysis. P < 0.05 was considered significant. GraphPad Prism 8 software was used for all statistical analyses.
Results
Since global knock out Atg16L1 is embryonic lethal, we first used a previously described hypomorphic mouse model of Atg16L1 (Atg16L1 HM) which was created using a gene trap vector to introduce a false splice site within the intron following exon 6 (Fig. 1A) (26). When mated to Wt males of proven fertility, live birth rates were similar between Wt and Atg16L1 HM female mice (Fig. 1B). To determine whether the autophagy protein ATG16L1 played a role in endometrial decidualization, we cultured endometrial cells from both Wt and Atg16L1 HM mice and hormonally stimulated them to decidualize. When stimulated, cells from both Wt and Atg16L1 HM mice became rounded decidual cells and increased expression of the decidualization marker Prp (Fig. 1B and 1C). To determine if the Atg16L1 HM mice were able to decidualize in vivo, we performed oil-induced artificial decidualization as depicted (Fig. 1E). The stimulated horns of both Wt and Atg16L1 HM mice had increased uterine weight, indicative of decidualization (Fig. 1F and 1G). Together these data show that Atg16L1 HM mice are fertile and do not have a decidualization defect.
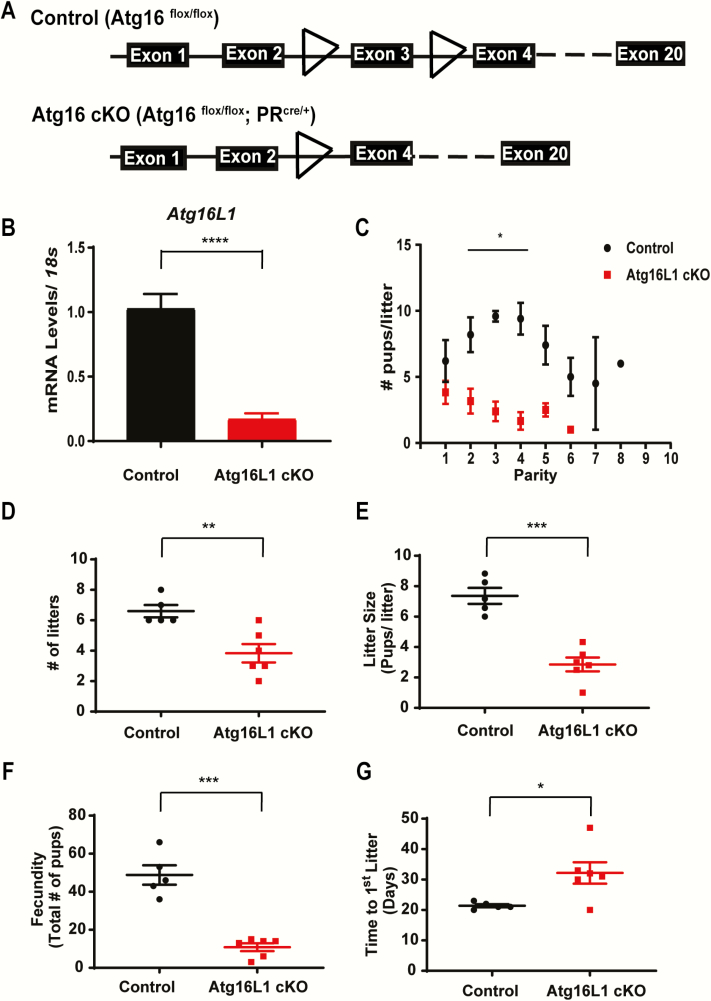
We next sought to eliminate Atg16L1 expression in the female reproductive tract using the progesterone receptor cre mouse model (27). Atg16L1flox/flox mice were mated with PRcre/+ mice to create Atg16L1flox/ flox PRcre/+ (Atg16L1 cKO) female mice in which exon 3 of the Atg16L1 gene was excised in the reproductive tract (Fig. 2A). Compared with control (Atg16L1flox/flox), Atg16L1 cKO mice had decreased expression of Atg16L1 in the uterus (Fig. 2B). When mated with Wt males of proven fertility, Atg16L1 cKO females produced on average 2 fewer pups than controls during their first parity (Fig. 2C). Although this difference was not statistically different (P = 0.2) in the first parity, Atg16L1 cKO females produced fewer pups than control in subsequent parities (Fig. 2C). Over a course of 6 months of continuous breeding, female Atg16L1 cKO mice had fewer litters (Fig. 2D), smaller litter size (Fig. 2E), and reduced fecundity (Fig. 2F). During the natural mating experiments, we also observed the time to delivery of the first parity, as measured from the morning of a visible plug to the day of birth, was longer in Atg16L1 cKO females (Fig. 2G), suggesting many Atg16L1 cKO mice failed to get pregnant after their first mating.
Figure 2.
Uterine specific knock out of Atg16L1 impairs fertility. A) Diagram of the Atg16L1 cKO mutation. B) Expression of Atg16L1 is decreased in the uterus of Atg16L1 cKO virgin mice (n = 6 Control, 7 Atg16L1 cKO). C) Live birth rate, D) Number of litters, E) Litter size, and F) Total number of pups produced by control and Atg16L1 cKO mice over a 6-month period reveals compromised fertility in Atg16L1 mice (n = 5 Controls, 6 Atg16L1 cKO). G) Time to first litter as measured by plug date to delivery date is extended in Atg16L1 cKO mice; *P < 0.05, **P < 0.01, ***P < 0.001. ****P < 0.0001.
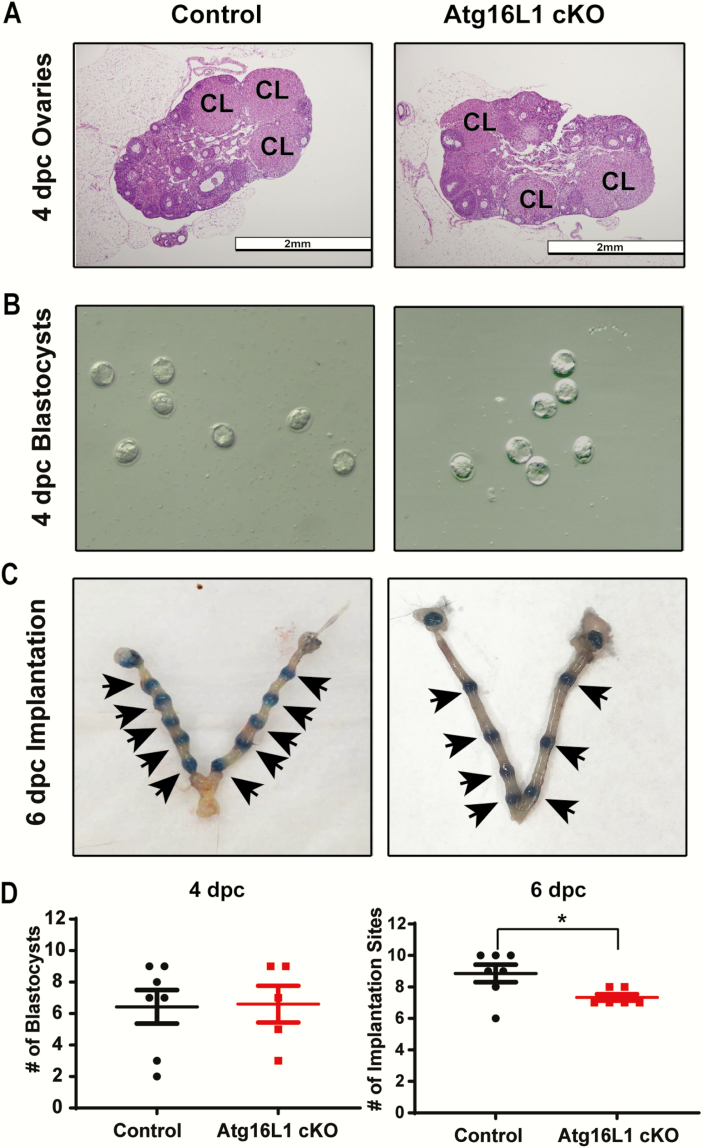
To discern if the observed subfertility was due to an ovulation defect, we histologically evaluated ovaries from control and Atg16L1 cKO mice at 4 dpc of pregnancy. Ovaries of Atg16L1 cKO mice had normal morphology and contained corpora lutea indicating ovulation had occurred (Fig. 3A). Additionally, a similar number of blastocysts were recovered from Atg16L1 cKO and control uteri at 4 dpc, further indicating the subfertility was not caused by impaired ovulation or fertilization (Fig. 3B). However, Atg16L1 cKO females had on average 2 fewer implantation sites than controls at 6 dpc (Fig. 3C and 3D). Together these data indicate that the subfertility of Atg16L1 cKO mice is not caused by an ovarian defect but is due to a decreased implantation rate.
Figure 3.
Atg16L1 cKO mice have an implantation defect. A) Histological sections of 4 dpc ovaries from control and Atg16L1 cKO mice show normal morphology with the presence of corpora lutea (denoted CL) (n = 6 Control, 5 Atg16L1 cKO). B) Blastocysts flushed from the uterine horns of control and Atg16L1 cKO mice on 4 dpc show normal morphology (n = 7 Control, 5 Atg15L1 cKO pregnant dams). C) Implantation cites observed at 6 dpc in control and Atg16L1 cKO mice (n = 7 Control and 6 Atg16L1 cKO pregnant dams). D) Quantification of blastocysts at 4 dpc and implantation cites at 6 dpc reveal 2 fewer blastocysts implant in the Atg16L1 cKO uterus. Arrows indicate implantation sites; *P < 0.05
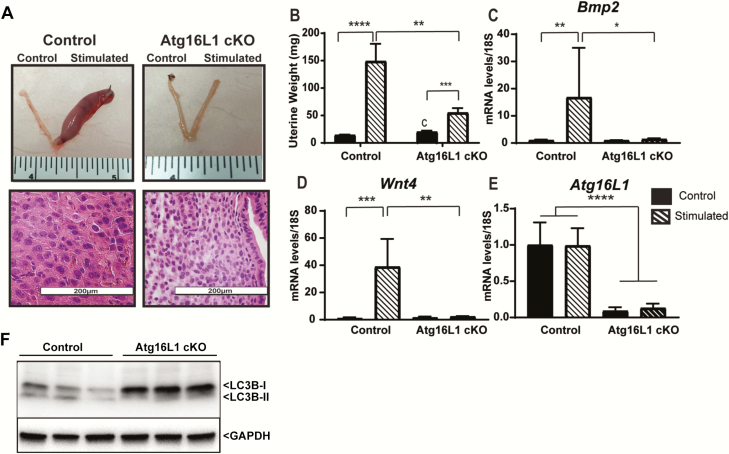
To determine whether the decreased implantation rate of Atg16L1 cKO mice was caused by impaired ESC decidualization, we performed oil-induced artificial decidualization. Stimulated uterine horns of Atg16L1 cKO mice weighed more than unstimulated horns, but were smaller than stimulated horns of the control mice (Fig. 4A and 4B). Whereas the stimulated horns of the control animals contained rounded polynucleated decidual cells, the stromal cells of the Atg16L1 cKO maintained a fibroblast morphology (Fig. 4A). The stimulated horns of the Atg16L1 cKO mouse failed to increase expression of the decidualization markers Bmp2 and Wnt4 (Fig. 4 C and 4D). Finally, expression of Atg16L1 did not change with decidualization but was decreased in the Atg16L1 cKO uterus (Fig. 4E). A hallmark of autophagy induction is a decrease in the autophagosome membrane protein LC3B-I paired with a subsequent increase in LC3B-II. As shown in Fig. 4F, we found LC3B-II protein levels were elevated with the decidualization and ATG16L ablation led to downregulation of these elevated LC3B-II levels. Together these data indicate that although Atg16L1 expression is not increased during decidualization, it is necessary for decidualization.
Figure 4.
Atg16L1 mice have impaired decidualization. A) Dissected uteri, H&E histology, and B) Wet weights of control and Atg16L1 cKO mice that have been stimulated to decidualize (n = 13 Control and n = 11 Atg16L1 cKO). Expression of the decidualization markers C) Bmp2 and D) Wnt4 reveal impaired decidualization in Atg16L1 cKO mice. E) Expression of Atg16L1 in control and Atg16L1 cKO mice following artificial decidualization, (n = 6 Control, 5 Atg16L1 cKO). F) Immunoblotting of LC3B protein on the protein lysate collected from stimulated horn of Control and Atg16L1 cKO mice uteri (n = 3) mice. The GAPDH is used as an internal loading control; *P < 0.05, **P < 0.01, ***P < 0.001. ****P < 0.0001.
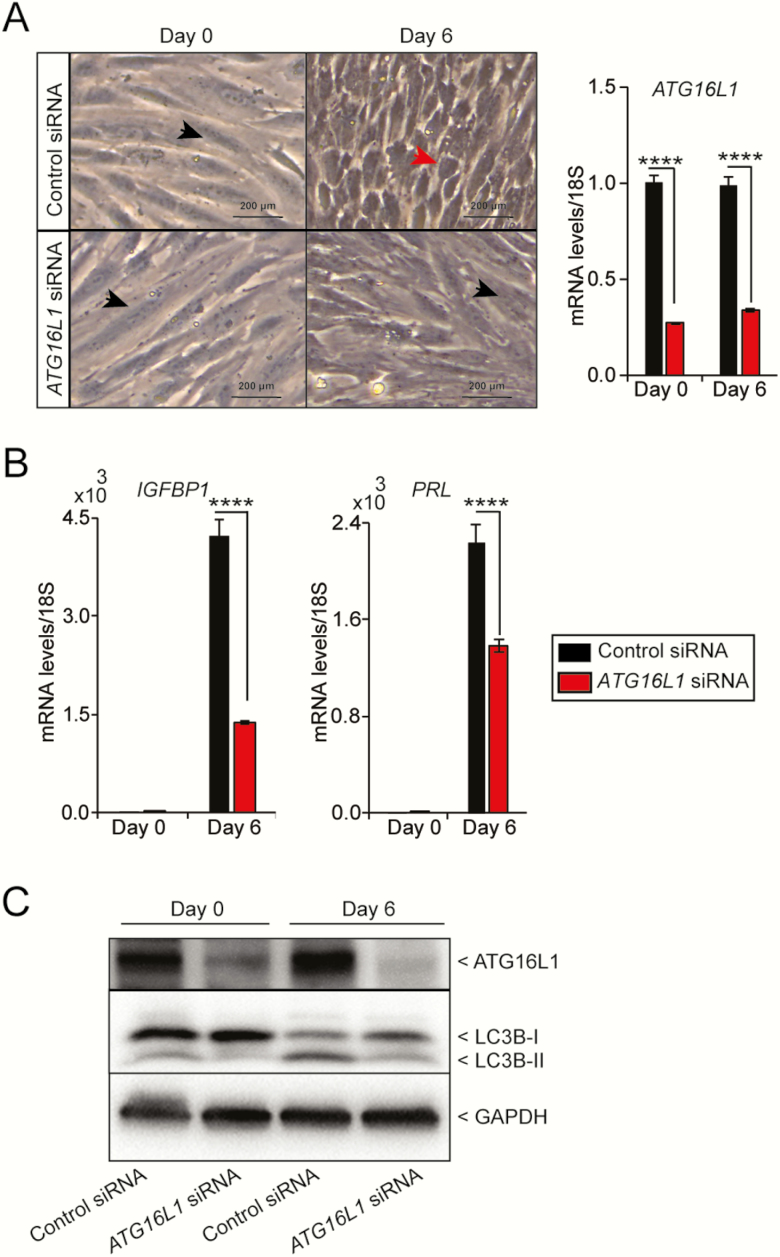
Since Atg16L1 knock out caused impaired decidualization in mice, we next sought to determine whether ATG16L1 also plays a role in human ESC decidualization. We knocked down ATG16L1 expression using an siRNA in cultured hESCs and hormonally-stimulated decidualization. When the control cells were stimulated to decidualize, the cellular morphology changed from long fibroblast-like stromal cells to rounded decidual cells (Fig. 5A) and was accompanied by increased expression of the decidualization marker IGFBP1 and prolactin (PRL) transcript (Fig. 5B). However, when ATG16L1 was knocked down, the stimulated cells maintained a fibroblast appearance and failed to increase expression of IGFBP1 to the same levels as the stimulated control cells. (Fig. 5A and 5B). Similar to the IGFBP1, the PRL transcript levels were significantly reduced with the knockdown of ATG16L (Fig. 5B). When the control cells were stimulated with hormones, the LC3B protein levels were elevated with the decidualization and ATG16L knockdown led to downregulation of this elevated LC3B-II levels (Fig. 5C). Together these data suggest that ATG16L1 is necessary for decidualization of hESCs.
Figure 5.
Knock down of ATG16L1 in human endometrial stromal cells impairs decidualization. A) Cellular morphology and B) quantitative PCR analysis of the decidualization marker IGFBP1 and PRL following transfection with control or ATG16L1 siRNA and hormonal stimulation. Data is normalized to 18S and expressed as fold change over Day 0 controls. Results are shown as mean ± standard error (SE) from 3 replicates of 1 patient-derived primary endometrial cell line. The experiment carried out on hESCs derived from 4 different individuals. (n = 4). Black arrowhead indicates fibroblast morphology and red arrowhead indicates decidualizing morphology. C) Immunoblotting of ATG16L1 and LC3B on the protein lysate from the hESCs collected at day 0 and day 6 after transfected with control or ATG16L1 siRNA. The GAPDH is used as an internal loading control; ****P < 0.0001.
Discussion
Here we set out to determine if the autophagy protein ATG16L1 was necessary for ESC decidualization and implantation. Our results reveal conditional knock out of Atg16L1 in the female reproductive tract curtailed female reproductive ability by impairing implantation. We show that Atg16L1 is necessary for the decidualization response in both mouse and human ESCs. Together this work provides genetic evidence that autophagy is necessary for ESC decidualization and suggests it may be a potential mediator of implantation success in human reproduction.
Our study is the first to find that knocking out Atg16L1 in the murine reproductive tract leads to impaired decidualization and implantation. This adds support to other clinical studies suggesting a role for autophagy in decidualization. Recently Citrinovitz and colleagues have shown that siRNA knockdown of ATG7 and ATG5 impaired decidualization of an immortalized hESC line (37). The ATG16L1 protein is part of a complex with ATG5 and ATG12 which is responsible for lipidation of the LC3B to promote phagophore elongation (25). Therefore, our work provides additional support that this autophagy complex is required for decidualization. These observations are in line with other work indicating autophagy is induced during the luteal phase of the menstrual cycle when ESCs are decidualizing (19), and in decidual cells of the placenta (38). Together with our mouse studies, these data suggest the ATG16L1-ATG5-ATG12 complex is not only required for the in vitro decidualization response but is also functionally required for murine decidualization and implantation in vivo.
Autophagy is dynamically activated by signals of cellular stress, nutrient deprivation, or infection as a mechanism to return to cellular homeostasis. Although the exact trigger of autophagy in the remodeling endometrium remains unknown, our data solidify its requirement for proper decidualization. Clues to the autophagy activation stimulus come from clinical and preclinical studies of obesity where energy homeostasis has been disrupted. Maternal obesity is a condition associated with both impaired decidualization and implantation (39, 40) and recent evidence suggests autophagy may be a contributing mechanism (21). In mice, autophagy is stimulated in ESCs during artificial decidualization, but is impaired in mice that consume an obesogenic high-fat diet (21). Similarly, hESCs cultured from endometrial biopsies and treated with palmitic acid to mimic a high-fat diet also display both impaired autophagy as well as impaired decidualization (21). When stimulated to decidualize, cells exposed to palmitic acid fail to sustain the drop in cellular ATP that is necessary to trigger autophagy (21). Taken together these results suggest autophagy may be initiated in ESCs by a drop in cellular energy and required to maintain bioenergetic homeostasis during decidualization. However, future studies are required to elucidate the molecular signaling pathways responsible for activating autophagy in decidualizing ESCs.
Interestingly, a polymorphism in the ATG16L1 gene loci is commonly associated with a risk of developing Crohn’s disease. The Atg16L1 HM mouse model was originally developed to model a Crohn’s population homozygous for the ATG16L1 risk allele (26). In their initial characterization of the mouse model, Cadwell and colleagues described gross abnormalities in the Paneth cells of the intestinal crypt impairing granule exocytosis in Atg16L1 HM mice (26), which effectively models the Crohn’s patient population. We did not observe a reduction in live birth rate or impaired endometrial decidualization in the Atg16L1 HM mice. This finding supports the small number of clinical studies investigating fertility in women with inflammatory bowel disease (IBD) like Crohn’s Disease. These studies report that although women with IBS have fewer children it is thought to be attributed to the decision to remain childless rather than reduced fertility (41, 42).
Here we have shown that knocking out the autophagy protein ATG16L1 impairs ESC decidualization in both mice and women. This study provides genetic evidence for a role of autophagy in decidualization and justifies future studies aimed at defining the direct mechanism by which autophagy regulates decidualization. The requirement of ATG16L1 in the human endometrium suggests that the autophagy pathway is required for early implantation and fertility. Therefore, these data provide a foundation for future studies aimed at understanding the complex regulatory networks that initiate autophagy during decidualization in women with obesity and reoccurring implantation failure.
Acknowledgments
We thank Herbert Virgin for providing the Atg16L1 HM and Atg16L1flox/flox mice. We sincerely thank Alma Johnson, Michaela Reid, and Andrew Cusumano for their technical expertise, and Deborah Frank for her critical editorial review of this manuscript.
Financial Support: This work was supported by the following National Institutes of Health grants: NICHD R01 HD065435 and R01 HD083895 (to K.H.M.), NICHD R01 HD065435 and R00HD080742 (to R.K.), and T32 DK007120 (to A.K.O.).
Glossary
Abbreviations:
- ANOVA
analysis of variance
- ATG16L1
autophagy related 16-like 1
- ATP
adenosine triphosphate
- dpc
day post coitum
- ESC
endometrial stromal cell
- LC3B
microtubule-associated protein 1A/1B light chain 3B
- PCR
polymerase chain reaction
- SE
standard error
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
Additional Information
Disclosure Summary: The authors disclose the US patent 15820886 to K.H.M. and A.K.O. The authors have declared that no conflict of interest exists.
References
- 1. Paria BC, Wang H, Dey SK. Endocannabinoid signaling in synchronizing embryo development and uterine receptivity for implantation. Chem Phys Lipids. 2002;121(1-2): 201–210. [DOI] [PubMed] [Google Scholar]
- 2. Psychoyos A. Uterine receptivity for nidation. Ann N Y Acad Sci. 1986;476:36–42. [DOI] [PubMed] [Google Scholar]
- 3. Schlafke S, Enders AC. Cellular basis of interaction between trophoblast and uterus at implantation. Biol Reprod. 1975;12(1):41–65. [DOI] [PubMed] [Google Scholar]
- 4. Lessey BA. Endometrial receptivity and the window of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14(5):775–788. [DOI] [PubMed] [Google Scholar]
- 5. Ola B, Li TC. Implantation failure following in-vitro fertilization. Curr Opin Obstet Gynecol. 2006;18(4):440–445. [DOI] [PubMed] [Google Scholar]
- 6. Bellver J, Ayllón Y, Ferrando M, et al. . Female obesity impairs in vitro fertilization outcome without affecting embryo quality. Fertil Steril. 2010;93(2):447–454. [DOI] [PubMed] [Google Scholar]
- 7. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. [DOI] [PubMed] [Google Scholar]
- 8. Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358(2):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90(23):11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lydon JP, DeMayo FJ, Funk CR, et al. . Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 11. Vasquez YM, Wang X, Wetendorf M, et al. . FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. Plos Genet. 2018;14(11):e1007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–199. [DOI] [PubMed] [Google Scholar]
- 13. Tabanelli S, Tang B, Gurpide E. In vitro decidualization of human endometrial stromal cells. J Steroid Biochem Mol Biol. 1992;42(3-4):337–344. [DOI] [PubMed] [Google Scholar]
- 14. Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100(5):2963–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frolova A, Flessner L, Chi M, Kim ST, Foyouzi-Yousefi N, Moley KH. Facilitative glucose transporter type 1 is differentially regulated by progesterone and estrogen in murine and human endometrial stromal cells. Endocrinology. 2009;150(3):1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frolova AI, O’Neill K, Moley KH. Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol Endocrinol. 2011;25(8):1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22(2):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12(9):814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi J, Jo M, Lee E, Oh YK, Choi D. The role of autophagy in human endometrium. Biol Reprod. 2012;86(3):70. [DOI] [PubMed] [Google Scholar]
- 20. Armant DR. Autophagy’s expanding role in development: implantation is next. Endocrinology. 2011;152(5):1739–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rhee JS, Saben JL, Mayer AL, et al. . Diet-induced obesity impairs endometrial stromal cell decidualization: a potential role for impaired autophagy. Hum Reprod. 2016;31(6):1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi S, Shin H, Song H, Lim HJ. Suppression of autophagic activation in the mouse uterus by estrogen and progesterone. J Endocrinol. 2014;221(1):39–50. [DOI] [PubMed] [Google Scholar]
- 23. Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–2873. [DOI] [PubMed] [Google Scholar]
- 24. Romanov J, Walczak M, Ibiricu I, et al. . Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. Embo J. 2012;31(22):4304–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lystad AH, Carlsson SR, de la Ballina LR, et al. . Distinct functions of ATG16L1 isoforms in membrane binding and LC3B lipidation in autophagy-related processes. Nat Cell Biol. 2019;21(3):372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cadwell K, Liu JY, Brown SL, et al. . A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soyal SM, Mukherjee A, Lee KY, et al. . Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. [DOI] [PubMed] [Google Scholar]
- 28. Tsai JH, Schulte M, O’Neill K, Chi MM, Frolova AI, Moley KH. Glucosamine inhibits decidualization of human endometrial stromal cells and decreases litter sizes in mice. Biol Reprod. 2013;89(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 30.World Medical Association. WMA Declaration of Helsinki Serves as Guide to Physicians. California medicine. 1966;105(2):149–150. [PMC free article] [PubMed] [Google Scholar]
- 31. Kommagani R, Szwarc MM, Kovanci E, et al. . Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. Plos Genet. 2013;9(10):e1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Camden AJ, Szwarc MM, Chadchan SB, et al. . Growth regulation by estrogen in breast cancer 1 (GREB1) is a novel progesterone-responsive gene required for human endometrial stromal decidualization. Mol Hum Reprod. 2017;23(9):646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. RRID:AB_10003146, https://scicrunch.org/resolver/RRID:AB_ 10003146.
- 34. RRID:AB_1840706, https://scicrunch.org/scicrunch/resolver/RRID:AB_1840706.
- 35. RRID:AB_561053, https://scicrunch.org/resolver/RRID:AB_ 561053.
- 36. RRID:AB_2099233, https://scicrunch.org/resolver/AB_2099233.
- 37. Mestre Citrinovitz AC, Strowitzki T, Germeyer A. Decreased autophagy impairs decidualization of human endometrial stromal cells: a role for ATG proteins in endometrial physiology. Int J Mol Sci. 2019;20(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Avagliano L, Terraneo L, Virgili E, et al. . Autophagy in Normal and Abnormal Early Human Pregnancies. Reprod Sci. 2015;22(7):838–844. [DOI] [PubMed] [Google Scholar]
- 39. Brewer CJ, Balen AH. The adverse effects of obesity on conception and implantation. Reproduction. 2010;140(3):347–364. [DOI] [PubMed] [Google Scholar]
- 40. Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R; SART Writing Group Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod. 2011;26(1):245–252. [DOI] [PubMed] [Google Scholar]
- 41. Van Assche G, Dignass A, Reinisch W, et al. ; European Crohn’s and Colitis Organisation (ECCO) The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: special situations. J Crohns Colitis. 2010;4(1):63–101. [DOI] [PubMed] [Google Scholar]
- 42. Hudson M, Flett G, Sinclair TS, Brunt PW, Templeton A, Mowat NA. Fertility and pregnancy in inflammatory bowel disease. Int J Gynaecol Obstet. 1997;58(2):229–237. [DOI] [PubMed] [Google Scholar]