Abstract
Decidualization, the process by which fibroblastic human endometrial stromal cells (HESC) differentiate into secretory decidual cells, is a critical event during the establishment of pregnancy. It is dependent on the steroid hormone progesterone acting through the nuclear progesterone receptor (PR). Previously, we identified insulin receptor substrate 2 (IRS2) as a factor that is directly regulated by PR during decidualization. IRS2 is an adaptor protein that functionally links receptor tyrosine kinases, such as insulin receptor (IR) and insulin-like growth factor 1 receptor (IGF1R), and their downstream effectors. IRS2 expression was induced in HESC during in vitro decidualization and siRNA-mediated downregulation of IRS2 transcripts resulted in attenuation of this process. Further use of siRNAs targeted to IR or IGF1R transcripts showed that downregulation of IR, but not IGF1R, led to impaired decidualization. Loss of IRS2 transcripts in HESC suppressed phosphorylation of both ERK1/2 and AKT, downstream effectors of insulin signaling, which mediate gene expression associated with decidualization and regulate glucose uptake. Indeed, downregulation of IRS2 resulted in reduced expression and membrane localization of the glucose transporters GLUT1 and GLUT4, resulting in lowered glucose uptake during stromal decidualization. Collectively, these data suggest that the PR-regulated expression of IRS2 is necessary for proper insulin signaling for controlling gene expression and glucose utilization, which critically support the decidualization process to facilitate pregnancy. This study provides new insight into the mechanisms by which steroid hormone signaling intersects with insulin signaling in the uterus during decidualization, which has important implications for pregnancy complications associated with insulin resistance and infertility.
Keywords: insulin signaling, decidualization, glucose transport, metabolism
Infertility is a common issue faced by couples trying to conceive, and therefore it is critical to understand the events occurring during early pregnancy to help improve assisted reproductive technology. In women, the uterus prepares for pregnancy with each menstrual cycle. During the proliferative phase of the menstrual cycle, estrogen (E) produced by the ovaries drives proliferation of the endometrium. During the secretory phase, rising levels of progesterone (P) produced from the corpus luteum promote differentiation of the stromal cells, a process called decidualization. In humans, decidualization is critical to facilitate embryo implantation in the event of pregnancy, and impaired decidualization can lead to pregnancy loss.
During decidualization, under the direction of E and P, stromal cells undergo a transformation from spindle-shaped fibroblasts to enlarged epithelioid-like decidual cells. Decidual cells produce and secrete factors to modulate the activities of uterine vascular endothelial cells, epithelial cells, and immune cells to help protect and nurture the embryo during early pregnancy (1, 2). Several rodent models have demonstrated that impaired decidualization often correlates with subfertility or infertility (3). Because this process is necessary for successful pregnancy, it is critical to understand what uterine factors are involved in driving decidualization.
Progesterone signaling though the progesterone receptor (PR) is essential for decidualization (4). PR is a member of the nuclear receptor superfamily of transcription factors and regulates gene expression upon hormone binding. To better understand the role of PR signaling in the uterus, our lab has performed ChIP sequencing (ChIP-Seq) to identify regions in the human genome bound by PR during decidualization of primary human endometrial stromal cells (HESC) (5). This study identified insulin receptor substrate 2 (IRS2) as a direct target of PR during HESC decidualization. IRS2 expression is induced in response to progesterone and can be reduced by siRNA-mediated knockdown of endogenous PR (5). The purpose of this study is to further elucidate the role of IRS2 during decidualization.
IRS2 is part of a family of large docking proteins called insulin receptor substrates (IRS) and serves as a cytoplasmic signaling molecule that mediates the downstream signaling of insulin and insulin-like growth factor 1 (IGF1) receptor tyrosine kinases (RTK) (6). Phosphorylation of IRS2 by these RTKs activates 2 main pathways: the mitogen-activated protein kinase (MAPK) cascade to regulate transcriptional activity and the PI3K/AKT pathway to regulate glucose influx, glycogen synthesis, and transcriptional activity (7). Mice lacking IRS2 through global deletion of the Irs2 gene exhibit peripheral insulin resistance and β-cell deficiency, culminating in a diabetic phenotype (8). Irs2-null mice also showed reduced circulating steroid hormones and gonadotropins, ovarian defects, and severely reduced fertility, indicating an important role for IRS2 in reproductive function (9).
Based on insights gained from the rodent model, it is likely that IRS2 plays a critical role during human pregnancy as well, although little is known about the function of IRS2 in the uterus. Because the Irs2-null mouse exhibits symptoms of type 2 diabetes, we hypothesize that the PR target IRS2 facilitates decidualization by mediating local insulin signaling to promote glucose uptake to meet the increasing energy demands of the decidual cell. We found that loss of IRS2 impairs decidualization of HESC, decreases expression of glucose transporters, and reduces glucose uptake during in vitro decidualization. Our results offer insight into insulin receptor/IRS2 signaling in the uterus and implicate dysregulation of IRS2 as a contributing factor to early pregnancy loss.
Material and Methods
Primary human endometrial stromal cell culture
Endometrial samples from the early proliferative stage of the menstrual cycle were obtained by pipelle biopsy at Emory University and Wake Forest Medical Centers from fertile, regularly cycling women under anesthesia before laparoscopy as described previously (10). Donors displayed no signs of endometrial pathological conditions and provided written informed consent. The subjects ranged in age from 28 to 42 years and in parity from 1 to 2. Primary human endometrial stromal cell cultures (HESC) were isolated from the samples as described previously (10). HESC were cultured in DMEM/F-12 medium (Gibco) supplemented with 5% (v/v) fetal bovine serum (Atlanta Biologicals), 50 μg/mL penicillin, and 50 μg/mL streptomycin (Invitrogen). For in vitro decidualization, the cells were treated with a decidualization cocktail (DC) composed of 0.5 mM 8-bromo-adenosine-3′,5′-cyclic monophosphate (8-Br-cAMP) (Sigma-Aldrich), 1 μM progesterone (Sigma-Aldrich), and 10 nM 17β-estradiol (Sigma-Aldrich) in DMEM/F-12 medium (Invitrogen) supplemented with 2% (v/v) charcoal dextran–stripped fetal bovine serum (CD-FBS) (Atlanta Biologicals). In certain experiments, cells were pre-treated for 1 hour with 100nM MK2206 (ApexBio), 10µM U0126 (Promega), or 5µM rosiglitazone (Cayman Chemicals) before being co-treated with the respective compound and DC. The medium/decidualization cocktail/indicated compound was refreshed every 48 hours.
siRNA-mediated knockdown
HESC were transfected with siRNA targeting IRS2, IR, or IGF1R transcripts (Dharmacon) or control scrambled siRNA (Dharmacon) following the manufacturer’s protocol (Transfectin; Bio-Rad Laboratories). In brief, a final concentration of 50 nM siRNA was mixed with Transfectin lipid reagent to transfect the cells. Cells were incubated with siRNA for 24 hours and then the media was replaced with DC to induce decidualization. Cells were stimulated with DC for 24 to 72 hours with media refreshed every 48 hours.
RNA isolation and real time PCR analysis
Total RNA was extracted from cultured HESC using TRIzol (Invitrogen) according to the manufacturer’s instructions. Total RNA was converted to cDNA using the AffinityScript Multiple Temperature Reverse Transcriptase kit (Agilent), following the manufacturer’s instructions. cDNA was subjected to quantitative PCR analysis using Power Sybr Green PCR master mix (Applied Biosystems) and gene-specific primers (IDT Integrated DNA Technologies). 36B4 was used as the reference gene. For a given sample, threshold cycle (Ct) and SD were calculated as an average of individual Ct values from 3 replicates. The normalized mean Ct (ΔCt) was calculated by subtracting the mean Ct of the reference gene 36B4 from the mean Ct of a target gene. The ΔΔCt was then calculated for each gene as a difference in ΔCt values between the control and the experimental sample. Fold change in gene expression was then computed as 2−ΔΔCt. The mean fold induction and SEM were calculated from 3 to 5 independent experiments.
Western blot analysis
Primary HESC were subjected to different treatment conditions as described in the figure legends. Whole-cell extracts were prepared using RIPA lysis buffer containing Complete Protease Inhibitor Cocktail (Roche) and HALT Phosphatase Inhibitor Cocktail (Thermo Fisher). Membrane-associated proteins were isolated using Mem-PER Plus Membrane Protein Extraction kit (Pierce) per manufacturer’s instruction. Equal amounts of proteins were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membrane (Amersham) and the specific proteins were detected by Western blotting using antibodies against IRS2 (Abcam; RRID:AB_2810948) (11), calnexin (Santa Cruz; RRID:AB_2243890) (12), IGFBP1 (Santa Cruz; RRID:AB_2123081) (13), BMP2 (Proteintech; RRID:AB_10641999) (14), WNT4 (Proteintech; RRID:AB_2215428) (15), HAND2 (Millipore; RRID:AB_2810947) (16), AKT (Santa Cruz; RRID:AB_671714) (17), phosphorylated AKT (Santa Cruz; RRID:AB_2225021) (18), ERK1/2 (Santa Cruz; RRID:AB_2650548) (19), phosphorylated ERK1/2 (Santa Cruz; RRID:AB_2141287) (20), GLUT1 (Abcam; RRID:AB_10903230) (21), GLUT4 (EMD Millipore; RRID:AB_1587080) (22), Integrin β3 (Santa Cruz; RRID:AB_647490) (23), HSP90 (Santa Cruz; RRID:AB_2121235) (24). Membranes were incubated overnight at 4°C with primary antibodies diluted in 5% milk buffer. On the next day, membranes were washed with tris-buffered saline with Tween 20 (Thermo Fisher Scientific) (TBST) and incubated for 1hour at room temperature with an appropriate IgG HRP-linked secondary antibody (Cell Signaling Technology; RRID:AB_330924; RRID:AB_2099233) (25,26) diluted in 5% milk buffer. Membranes were washed, incubated with SuperSignal® West Femto Maximum Sensitivity Substrate (Pierce), and imaged using the iBright chemiluminescent imaging system (Thermo Fisher Scientific). Image J software (NIH) was used to perform densitometry analysis.
Immunocytochemistry
HESC were plated in chamber slides (Cell Treat) and treated as indicated in the figure legend. Cells were fixed with 3.7% formaldehyde and permeabilized with 0.1% to 0.25% Triton-X in PBS. Following blocking with 5% normal donkey serum (Jackson Laboratories) and 1% bovine serum albumin (BSA) (Jackson Laboratories) in PBS, cells were incubated at 4°C overnight with primary antibodies targeting Ki67 (BD Pharmingen; RRID:AB_393778) (27), phosphorylated AKT (18), phosphorylated ERK1/2 (20), GLUT1 (21), and GLUT4 (22). On the next day, cells were washed with PBS and then incubated with the appropriate Cy3 conjugated secondary antibody (Jackson Laboratories; AB_2315777; RRID:AB_2307443) (28,29). For some experiments, F-actin filaments were stained using Alexa Fluor 488 Phalloidin (Thermo Fisher; RRID:AB_2315147) (30). Cells were mounted with Prolong GOLD mounting medium (Promega) containing 4′,6-diamidino-2-phenylindole nuclear counterstain. Imaging was performed using an Olympus BX51 microscope fitted with an Olympus DP71 camera (Olympus). ImageJ software was used to perform post-imaging analysis.
Glucose uptake assay
HESC were subjected to siRNA knockdown, as indicated above. These cells were stimulated for 72 hours with vehicle (ethanol) or DC, with media replacement every 48h. Cells were then washed 3 times with PBS and incubated in fresh glucose-free DMEM supplemented with 2% (CD-FBS) containing 50µM of a fluorescently-labeled glucose analog, 2-NBDG (Cayman Chemicals), and DC for 1 hour. Cells were then washed 3 times with PBS. Uptake of 2-NBDG was detected with fluorescent filters designed to detect fluorescein (excitation/emission = 485/535 nm). Cells were then lysed and protein concentration was measured by bicinchoninic acid (BCA) assay (Pierce). Uptake of 2-NBDG was normalized to total protein and expressed relative to vehicle-treated control siRNA–treated samples.
Statistical analysis
Statistical analysis was performed using 3 to 5 independent samples; each experiment was performed using HESC isolated from an individual donor. Statistical significance was determined using the Student t test. Asterisks denotes significance with a P value of < 0.05.
Results
Insulin receptor substrate 2 is required for human stromal cell decidualization
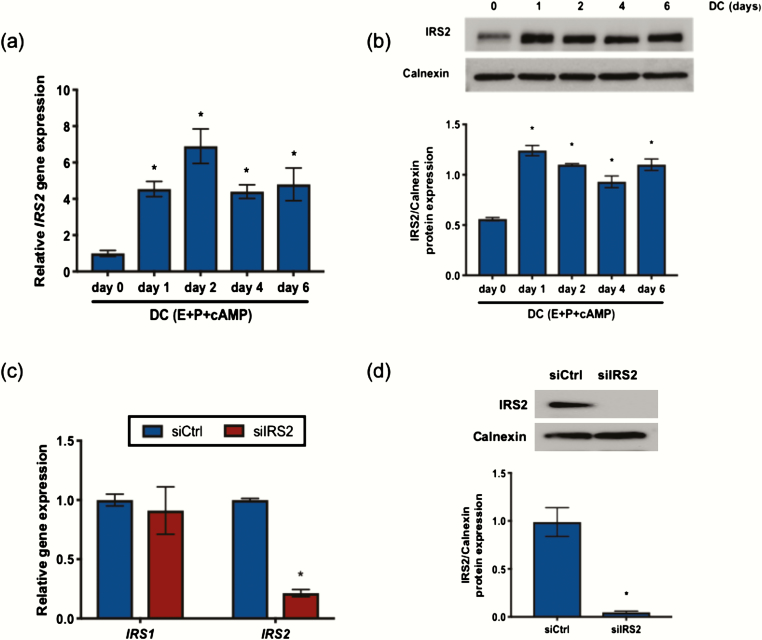
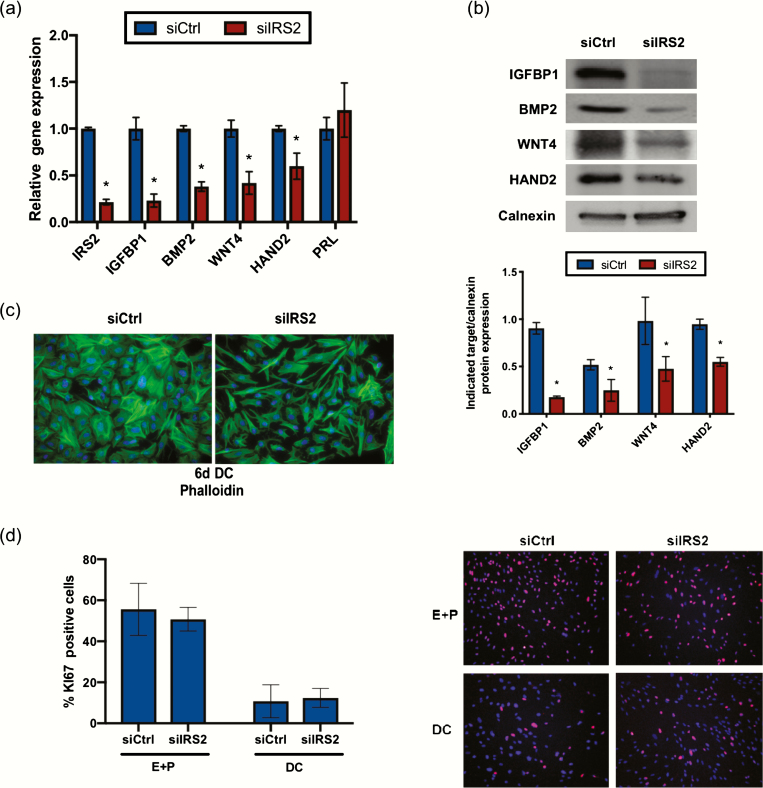
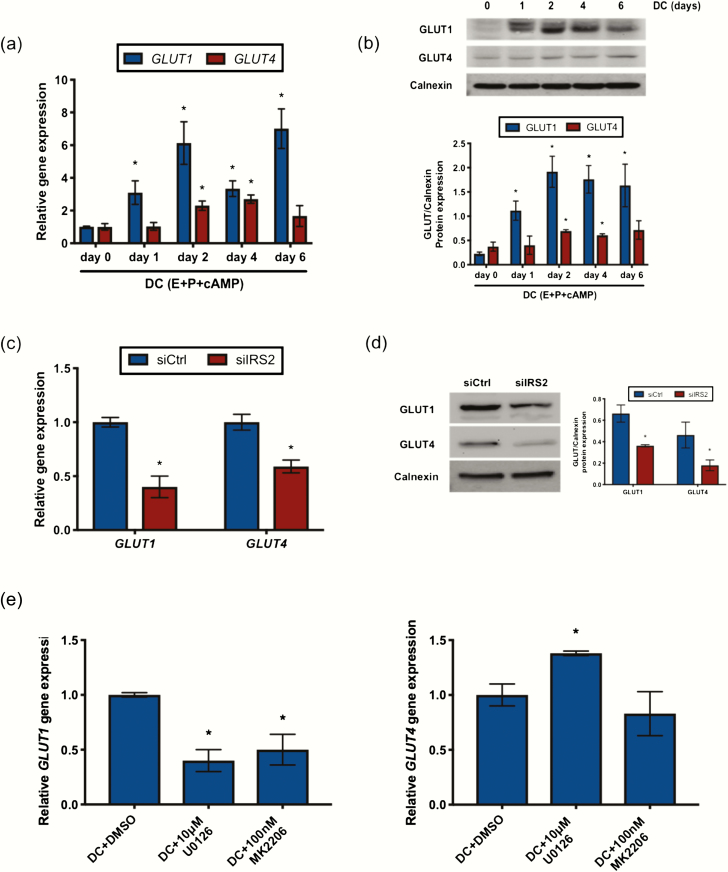
Previously, we showed that IRS2 is a direct target of PR during stromal cell decidualization (5). To establish the pattern of IRS2 expression, HESC were treated with decidualization cocktail (E + P + cAMP; DC). IRS2 expression was induced within 24 hours of treatment with DC both at the mRNA and protein level and remained elevated through the 6-day treatment period (Fig. 1A and 1B). To determine the role of IRS2 during decidualization, we performed siRNA-mediated knockdown as indicated in the Materials and Methods, followed by 72-hour stimulation with DC. Using this knockdown strategy, we observed approximately 80% reduction of IRS2 (siIRS2) at the mRNA level and reduced levels of IRS2 protein to an undetectable concentration, relative to a scrambled siRNA control (siCtrl) (Fig. 1C and 1D). We observed no appreciable change in IRS1 expression with loss of IRS2 (Fig. 1C). We next looked at expression of factors that are induced during decidualization. Loss of IRS2 led to significantly reduced expression of decidualization markers IGFBP1, BMP2, WNT4, and HAND2 in HESC following 72 hours of stimulation with DC (Fig. 2A). PRL expression was not significantly altered from control with IRS2 knockdown. Moreover, we observed reduced protein expression of IGFBP1, BMP2, WNT4, and HAND2 in whole cell lysates, with loss of IRS2 compared with control cells, as indicated by Western blot (Fig. 2B).
Figure 1.
IRS2 is expressed in HESC during decidualization. IRS2 mRNA (A) and protein (B) expression was observed in HESC treated with DC (E+P+cAMP) for 0 to 6 days. (A) Gene expression analysis was performed using primers specific for IRS2. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to day 0. (B) Whole cell lysates were analyzed by Western blot and probed with antibody specific to IRS2. Calnexin is provided as a loading control. Densitometry analysis is provided (below). (C) Following siRNA-mediated knockdown of IRS2 and stimulation with DC for 72 hours, gene expression analysis was performed using primers specific to IRS1 and IRS2. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to siCtrl. (D) Following knockdown and stimulation with DC for 72 hours, IRS2 expression was determined by Western blot. Calnexin is provided as a loading control. Densitometry analysis is provided (below).
Figure 2.
IRS2 is required for HESC decidualization. (A) Gene expression analysis was performed following IRS2 knockdown and 72-hour stimulation with DC using primers specific for IGFBP1, BMP2, WNT4, HAND2, and PRL. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to siCtrl. (B) Following knockdown and stimulation with DC for 72h, IGFBP1, BMP2, WNT4, and HAND2 expression was determined by Western blot. Calnexin is provided as a loading control. Densitometry analysis is provided (below). (C) IRS2 knockdown was performed and cells were stimulated for 6 days with DC and stained with phalloidin to bind actin filaments. DAPI was used as a nuclear stain. Images provided are 40× magnification. (D) IRS2 knockdown was performed and cells were exposed for 24 hours of proliferative (E+P) and decidualization (DC; E+P+cAMP) conditions. Immunofluorescent staining of Ki67 was performed and DAPI was used as a nuclear stain. Ki67 positive cells were counted using ImageJ and expressed as a percentage of total cells. Representative panels are shown; 20× magnification.
Successful stromal decidualization results in changes in cell morphology, shifting from a spindly fibroblast cell to a rounded decidual cell. We examined the impact of IRS2 knockdown on cell morphology by immunocytochemistry, using phalloidin and DAPI to stain actin filaments and nuclei, respectively. Upon stimulation with DC, control cells underwent transformation to rounded decidual cells, while cells lacking IRS2 retained much of their fibroblastic shape, indicating that decidualization was impaired (Fig. 2C).
Another key characteristic of in vitro decidualization is the cessation of stromal proliferation with the introduction of cAMP, a key component of our DC treatment. Following siRNA knockdown, we treated cells with either E and P or the DC containing cAMP for 24 hours. Proliferation was assessed using an antibody targeting Ki67, a proliferative marker, and Ki67-positive cells were quantified by ImageJ analysis. No difference in proliferation was detected between the siCtrl and siIRS2 cells in either the proliferative (E and P) or decidualization (DC) culture conditions (Fig. 2D), suggesting that IRS2 does not play a critical role in regulating HESC proliferation. Taken together, these data demonstrate that loss of IRS2 attenuates the gene expression and morphological changes associated with decidualization. These results establish IRS2 as a critical mediator of progesterone action during endometrial stromal cell decidualization.
IRS2 acts downstream of the insulin receptor to regulate decidualization
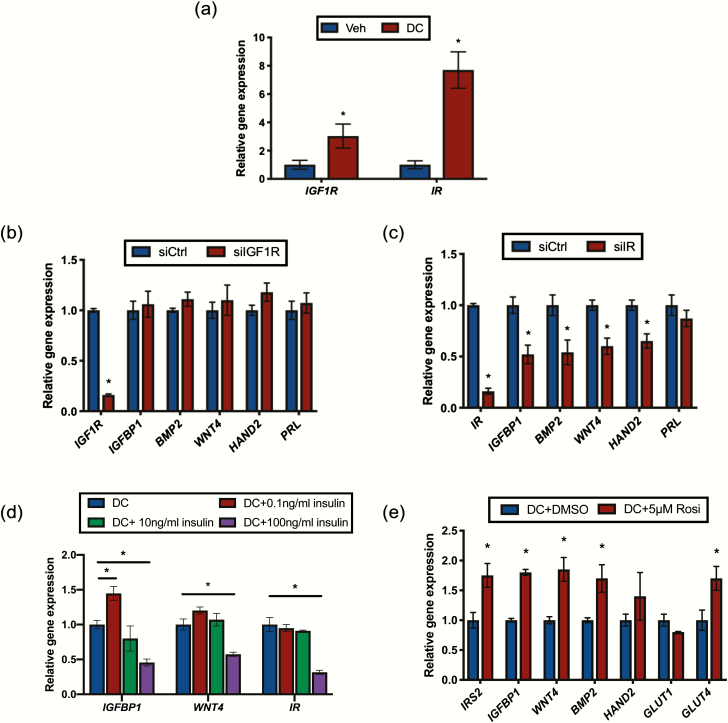
In various cell types, IRS2 is reported to act as an adaptor molecule for both the insulin receptor (IR) and IGF1 receptor (IGF1R). Expression of both of these tyrosine kinase receptors was induced in HESC in response to DC (Fig. 3A). To elucidate the role of IGF1R/IRS2 and IR/IRS2 signaling during decidualization, we performed siRNA-mediated knockdown using siRNA targeting IGF1R or IR transcripts. Loss of IGF1R did not affect the expression of decidualization markers IGFBP1, BMP2, WNT4, HAND2, or PRL (Fig. 3B). siRNA-mediated loss of IR, however, significantly reduced expression of IGFBP1, BMP2, WNT4, and HAND2, but not PRL (Fig. 3C). This pattern of gene expression is similar to that observed with knockdown of IRS2 (Fig. 2A).
Figure 3.
IRS2 acts downstream of the insulin receptor (IR) to regulate decidualization. (A) Gene expression analysis was performed with RNA isolated from HESC treated with vehicle (ethanol) or DC for 72 hours using primers specific to IGF1R and IR. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to vehicle. Following siRNA mediated knockdown of IGF1R(B) or IR(C) and stimulation with DC for 72 hours, gene expression analysis was performed using primers for IGFBP1, BMP2, WNT4, HAND2, and PRL. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to siCtrl. (D) HESC were co-stimulated with DC and the indicated concentration of insulin for 72 hours. Gene expression analysis was performed using primers for IGFBP1, WNT4, and IR. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to DC alone. (E) Gene expression analysis was performed with RNA isolated from HESC treated with DC with or without 5μM rosiglitazone (Rosi) for 72 hours using primers specific to IRS2, IGFBP1, BMP2, WNT4, HAND2, GLUT1, and GLUT4. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to DC alone.
The culture conditions used in our in vitro decidualization experiments contain 0.04 ng/mL of insulin, so we next wanted to see if increasing IR activation through increased concentrations of the ligand in the culture medium can enhance decidualization. At 0.1 ng/mL, the lowest concentration of insulin utilized, IGFBP1 expression was increased compared with DC alone (Fig. 3D). At higher concentrations, we observed reduced expression of IGFBP1 and WNT4 compared with DC alone. Interestingly, we also observed reduced expression of IR itself, suggesting that excess ligand caused downregulation of the receptor and had similar effects on gene expression as those observed with siRNA-mediated knockdown of IR. We next treated cells with rosiglitazone, an antidiabetic drug that enhances peripheral insulin sensitivity and glucose uptake. Addition of rosiglitazone to the culture media significantly increased expression of the decidualization markers WNT4, BMP2, and IGFPB1, as well as the glucose transporter GLUT4 compared with DC alone (Fig. 3E). Rosiglitazone also enhanced the DC stimulated expression of IRS2. Together, these data suggest that IRS2 acts as part of the IR signaling pathway during stromal decidualization and that this pathway is sensitive to hyperinsulinemia.
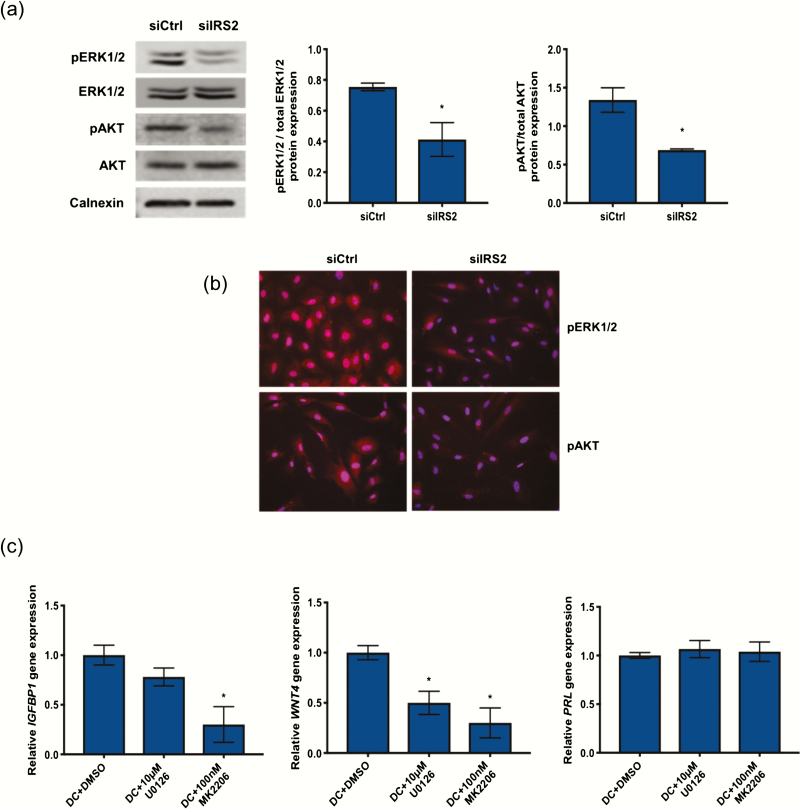
Loss of IRS2 reduces PI3K/AKT and MAPK activation in HESC during decidualization
IR activation in insulin target tissues such as liver, muscle, and fat leads to recruitment and phosphorylation of IRS2, which in turn mediates the downstream activation of MAPK and/or PI3K/AKT to mediate changes in gene expression and regulate glucose transport into the cell (6). Loss of IRS2 in HESC stimulated for 24 hours with DC reduced the phosphorylation of both AKT and ERK1/2, as determined by Western blot (Fig. 4A) and immunofluorescence (Fig. 4B). To determine if PI3K/AKT and/or MAPK activation are required for decidualization, we used pharmacological inhibitors MK2206 and U0126 to inhibit AKT and ERK1/2 phosphorylation, respectively. Similar to what we observed with either IR or IRS2 knockdown, co-treatment with DC and either MK2206 or U0126 had no effect on PRL expression (Fig. 4C). However, IGFBP1 and WNT4 expression were both reduced in response to either MK2206 or U0126. Together, these data suggest that both MAPK and PI3K/AKT mediate the downstream effects of IRS2 activation to control stromal decidualization.
Figure 4.
Loss of IRS2 reduces PI3K/AKT and MAPK activation in HESC during decidualization. Following siRNA mediated knockdown of IRS2 and 24-hour stimulation with DC, protein expression was investigated by Western blot (A) and immunofluorescence (B). (A) Whole cell lysates were analyzed by Western blot and probed with antibodies specific for phosphorylated and total ERK1/2 and phosphorylated and total AKT. Calnexin was used as a loading control. Densitometry analysis is provided (right). (B) Immunofluorescent staining using antibodies specific for phosphorylated ERK1/2 and phosphorylated AKT. DAPI was used as a nuclear stain. Images provided are 40× magnification. (C) Gene expression analysis was performed with RNA isolated from HESC treated with DC and DMSO or either 10μM U0126 or 100nM MK2206 for 72 hours using primers specific for IGFBP1, WNT4, and PRL. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to DC+DMSO.
IRS2 regulates GLUT expression and localization during HESC decidualization
Because IR/IRS2 signaling is important for glucose uptake and utilization, we wanted to explore how these functions are altered with loss of IRS2 during decidualization. We first looked at the RNA and protein expression pattern of glucose transporters in HESC stimulated with DC. GLUT1 mRNA (Fig. 5A) and protein (Fig. 5B) expression increased within 24 hours of DC stimulation and remained elevated throughout the treatment period. GLUT4 mRNA (Fig. 5A) and protein (Fig. 5B) expression was also modestly increased in response to DC. This indicates that glucose transporters are induced during decidualization and likely facilitate glucose transport into the cell to meet the energy demands of decidualization.
Figure 5.
IRS2 regulates GLUT expression during decidualization. GLUT mRNA (A) and protein (B) expression was observed in HESC treated with DC for 0 to 6 days. (A) Gene expression analysis was performed using primers specific for GLUT1 and GLUT4. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to day 0. (B) Whole cell lysates were analyzed by Western blot and probed with antibodies specific to GLUT1 and GLUT4. Calnexin is provided as a loading control. Densitometry analysis is provided (below). GLUT mRNA (C) and protein (D) expression was observed in HESC stimulated for 72 hours with DC following siRNA mediated knockdown of IRS2. (C) Gene expression analysis was performed using primers specific for GLUT1 and GLUT4. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to siCtrl. (D) Whole cell lysates were analyzed by Western blot and probed with antibodies specific for GLUT1 and GLUT4. Calnexin is provided as a loading control. Densitometry analysis is provided (right). (E) Gene expression analysis was performed with RNA isolated from HESC treated with DC and DMSO or either 10μM U0126 or 100nM MK2206 for 72 hours using primers specific for GLUT1 and GLUT4. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to DC+DMSO.
To examine how IRS2 affects GLUT expression, we performed siRNA-mediated knockdown of IRS2 and stimulated the cells with DC. Both GLUT1 and GLUT4 mRNA and protein levels were decreased with loss of IRS2 compared with control cells, as indicated by gene expression analysis (Fig. 5C) and Western blot using whole cell lysates (Fig. 5D), respectively. We next used the pharmacological inhibitors U0126 and MK2206 to elucidate the role of MAPK and PI3K/AKT signaling, respectively, in mediating IRS2 regulation of GLUT expression. GLUT1 levels were reduced with inhibition of either MAPK or PI3K/AKT signaling (Fig. 5E). Interestingly, GLUT4 expression was maintained despite inhibitor treatment, suggesting a mechanism of regulation distinct from GLUT1.
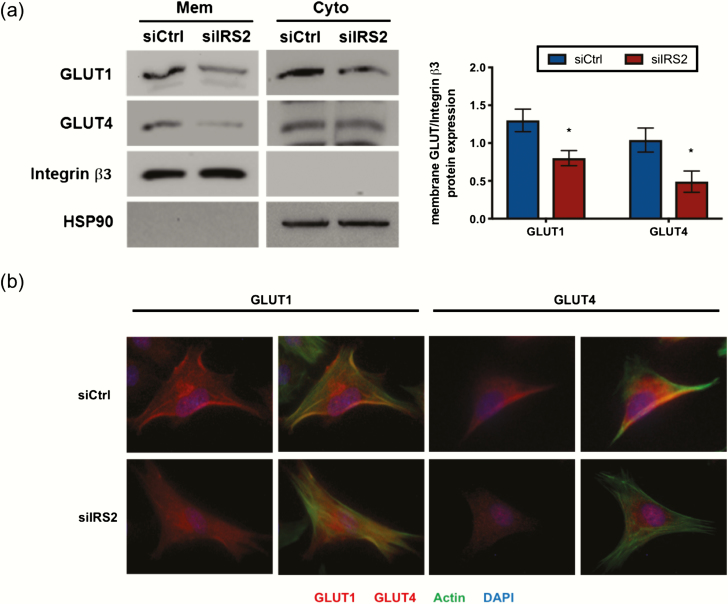
To observe how GLUT localization is influenced by IRS2, we isolated membrane and cytoplasmic protein fractions and measured expression by Western blot. With loss of IRS2, we observed reduced expression of GLUT1 in the cytoplasmic fraction (Fig. 6A). Interestingly, we also saw reduced expression of GLUT1 and GLUT4 in the membrane protein fraction (Fig. 6A). Similar observations were made using immunofluorescence. Following knockdown and treatment with DC, we stained cells for either GLUT1 or GLUT4 (red) and used phalloidin to stain F-actin filaments to visualize the outer boundaries of the cells (green). We observed membrane expression of GLUT1 and GLUT4 in our control cells following stimulation with DC (Fig. 6B), as indicated by the yellow color representing overlap between the respective GLUT and F-actin; this was less apparent with loss of IRS2. Taken together, these data demonstrate that IRS2 is necessary for proper GLUT expression and membrane localization during decidualization.
Figure 6.
IRS2 promotes membrane localization of GLUTs during decidualization. HESC were treated for 72 hours with DC following siRNA-mediated knockdown of IRS2. Membrane-associated GLUT expression was observed using Western blot (A) and immunofluorescence (B). (A) Membrane associated and cytoplasmic protein fractions were isolated as described in Materials and Methods. These fractions were subjected to Western blot and probed with antibodies for GLUT1 and GLUT4. Integrin β3 and HSP90 were used as fraction-specific controls. Densitometry of membrane fraction is provided (right). (B) Immunofluorescent staining using antibodies specific for GLUT1 and GLUT4. DAPI was used as a nuclear stain and phalloidin was used to stain actin filaments.
Signaling via IRS2 controls glucose uptake during HESC decidualization
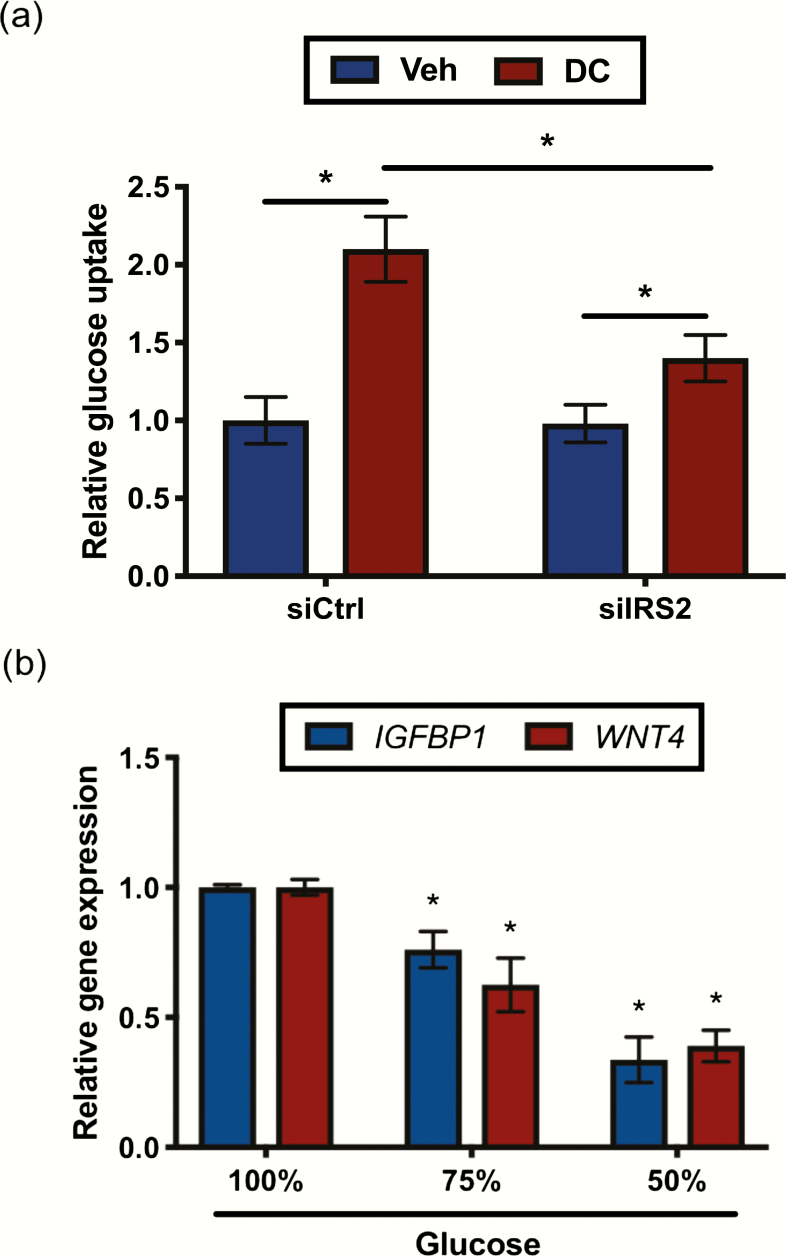
Since glucose movement into the cell is controlled by glucose transporters, we next investigated changes in glucose uptake with loss of IRS2. Following knockdown, cells were treated for 72 hours with DC or ethanol as vehicle and then exposed to the fluorescently-labeled glucose analog 2-NBDG, as described in the Materials and Methods. In our control siRNA conditions, differentiated HESC (DC-treated) exhibited increased 2-NBDG uptake compared with undifferentiated HESC (vehicle-treated) (Fig. 7A) indicating glucose uptake increases during decidualization. With IRS2 knockdown, we still observed an increase in 2-NBDG uptake in differentiated versus undifferentiated treatments conditions, but the magnitude of this increase was reduced (approximately 30%) compared with our control siRNA conditions. To better understand the effects of glucose availability on decidualization, we stimulated HESCs with DC in the presence of reduced concentrations of glucose in the media. We observed a decreased expression of IGFBP1 and WNT4 with reduced glucose concentrations compared with our normal treatment media (Fig. 7B), suggesting that reduced glucose availability impairs decidualization.
Figure 7.
Loss of IRS2 reduces glucose uptake by HESC undergoing decidualization. (A) HESC were treated for 72h with vehicle (Veh; ethanol) or DC following siRNA mediated knockdown of IRS2. Cells were washed with PBS and incubated with 50μM of 2-NBDG in glucose-free media for 1 hour. Cells were then washed and 2-NBDG incorporation was measured and normalized to total protein. Data are represented as the mean fold glucose uptake ± SEM. *P < 0.05 relative to siCtrl treated with vehicle. (B) HESC were stimulated with DC in the presence of full (100%; 17.5mM) or reduced (75% and 50%; 13 and 8.75mM, respectively) glucose concentrations in the culture media for 72 hours. Gene expression analysis was performed using primers specific for IGFBP1 and WNT4. 36B4 was used for normalization. Data are represented as the mean fold induction ± SEM. *P < 0.05 relative to DC in full glucose concentration of the culture media.
Collectively, these data suggest that insulin signaling via IRS2 plays a critical role in decidualization by regulating glucose transporter expression and membrane localization to promote glucose uptake by endometrial stromal cells to meet the energy demands of the decidualization process.
Discussion
Little is known about the function of insulin signaling in the uterus. The aim of this study was to better understand how IRS2, a mediator of insulin signaling, impacts decidualization, the process of endometrial stromal differentiation that is required for maintenance of pregnancy. Previously, we identified IRS2 as a direct target of PR signaling in primary HESC and showed PR occupancy at 2 regions (−88kb and −307Kb) upstream of the IRS2 transcription start site in HESC during decidualization (5). Indeed, we see that IRS2 is induced in HESC with the introduction of a decidualization cocktail containing estrogen, progesterone, and cAMP (Fig. 1A and 1B). Stromal cells undergo several changes during their transformation to decidual cells, including cessation of proliferation, changes in gene expression (including induction of decidual markers IGFPB1, PRL, and WNT4), and a morphological shift from a fibroblastic cell type to an epithelioid cell type. We found that loss of IRS2 via siRNA-mediated knockdown blunted the expression of decidual markers (Fig. 2A and 2B) and led to retention of a fibroblastic cell shape characteristic of undifferentiated HESC (Fig. 2C), indicating that IRS2 function is essential for decidualization.
IRS2 functions as an adaptor molecule bridging receptor tyrosine kinases such as insulin receptor (IR) to downstream signaling proteins. In the uterus, IR expression has been detected in the stroma of human endometrial tissue biopsies taken in the secretory phase of the menstrual cycle and stromal cells decidualized in vitro, although its role is unclear (31–33). Findings from previous in vitro studies are somewhat contradictory as to whether insulin signaling promotes or impairs decidualization (34–36). Our present study establishes a clear requirement for IR expression in stromal decidualization (Fig. 3C). Consistent with this finding, stimulation of HESC with increasing concentrations of insulin, which resulted in reduced IR expression, impaired decidualization (Fig. 3D). IR downregulation in response to hyperinsulinemic conditions has been demonstrated both in vitro and in vivo and is a contributing factor of insulin resistance (6). Moreover, epidemiological studies suggest a link between insulin resistance and an increased risk of spontaneous miscarriage and recurrent pregnancy loss (37, 38). In light of these observations, it appears that insulin signaling is tightly regulated in the uterus, and its dysregulation during early pregnancy may be a contributing factor towards infertility in women presenting with peripheral insulin resistance.
Signaling through IR/IRS2 leads to downstream activation of PI3K/AKT and MAPK pathways to regulate glucose influx, glycogen synthesis, and gene expression to control cell growth, proliferation, and differentiation in target tissues (6). In the present study, loss of IRS2 during decidualization led to reduced MAPK and PI3K/AKT activation (Fig. 4A and 4B). Our studies using pharmacological inhibition of ERK activation confirms earlier observations indicating a role for MAPK signaling in mediating decidualization (Fig. 4C) (39). The role of PI3K/AKT activity during decidualization is less clear. Immunohistochemical analysis of human tissue samples indicated that phosphorylated AKT in the stroma is low in the proliferative and early secretory phase of the uterine cycle, but increases in late secretory phase and in decidual tissues (40). Other groups have demonstrated that AKT phosphorylation decreases during in vitro stromal decidualization (41, 42). Reduced PI3K/AKT activity is necessary for increased nuclear accumulation and transcriptional activity of FOXO1, which can regulate gene expression of decidual markers (43). However, AKT also plays a critical role in glucose metabolism through its regulation and localization of glucose transporters and increasing glycogen synthesis through inhibition of glycogen synthase kinase (44). Increased glucose uptake and formation of intracellular glycogen stores occur during decidualization, suggesting that some level of AKT activation is required to mediate these functions. Our studies indicated that phosphorylation-dependent AKT activation is impaired in the absence of IRS2 function, leading to inhibition of at least certain of the decidualization-associated factors, such as WNT4 (Fig. 4A–C). In light of these observations, AKT activity during decidualization is more nuanced and may involve a fine balance in phosphorylation/activation to support both its downstream transcriptional and metabolic effects.
One of the major functions of insulin signaling is to promote movement of glucose from the blood into the cell through integral membrane transporters called glucose transporters (GLUT). The facilitative GLUT family comprises 14 GLUT protein family members with varying kinetics, regulatory properties, and tissue expression patterns (45). The presence of several GLUT family members was reported in the endometrium, but the most abundant is GLUT1 (46). GLUT1 is robustly expressed in the stroma during the secretory phase of the uterine cycle and in the decidua (32, 47). We observed an increased stromal GLUT1 expression induced by progesterone, estrogen, and cAMP, which reflects its pattern of expression during the secretory phase (Fig. 5A and 5B). Reduced stromal expression of GLUT1 has been observed in women suffering from idiopathic infertility compared with those whose infertility is independent of endometrial function, such as tubal occlusion or male infertility (47). Our study finds that IRS2 is necessary for GLUT1 expression during decidualization (Fig. 5C and 5D), and this expression is mediated in part through activation of PI3K/AKT and MAPK (Fig. 5E). While the insertion of GLUT1 into the cell membrane is not considered to be an insulin-dependent process, insulin, as well as MAPK and PI3K/AKT activity, has been shown to stimulate GLUT1 expression in muscle, liver, fat, and mesangial cells (48–52). Disruption of IR signaling through loss of IRS2 impairs GLUT1 production and decreases the reserve of GLUT1 leading to its decreased presence at the membrane (Fig. 6A and 6B).
In contrast to GLUT1, less is known about uterine GLUT4. Tissue analysis studies report that GLUT4 is weakly expressed in uterine stromal cells (53). Similar to GLUT1, our data suggest that IRS2 is an upstream effector for GLUT4 expression (Fig. 5C and 5D), although this appears to be in a manner somewhat distinct from that of GLUT1 (Fig. 5E). Our observation that rosiglitazone increased GLUT4 expression (Fig. 3E) suggests that PPARγ may be a candidate for GLUT4 regulation in the uterus and is congruous with observations made in adipose tissue following rosiglitazone treatment, although further research is needed to investigate this interaction (54, 55). GLUT4 is considered insulin dependent in that insulin stimulates GLUT4 insertion at the cell membrane. Consistent with this, loss of IRS2 reduced the accumulation of GLUT4 in the cell membrane (Fig. 6A and 6B). Although its expression during decidualization is less robust than that of GLUT1, perturbations in the temporal distribution of GLUT4 likely contribute to the overall reduction of glucose uptake observed with loss of IRS2.
The increase in stromal GLUT expression suggests a functional relevance of increased glucose usage during decidualization. Indeed, previous reports indicated that glucose uptake is increased in endometrial stromal cells undergoing in vitro decidualization (35, 46). As shown in Fig. 7B, culturing HESC in reduced glucose impaired decidualization. IRS2 clearly supports increased glucose flux into HESC (Fig. 7A), although other signaling mediators are likely to be involved. Once in the stromal cell, glucose is shuttled through several metabolic pathways, including glycolysis to provide energy in the form of ATP and the pentose phosphate pathway to aid in cholesterol, fatty acid, and nucleotide synthesis (56, 57). Future work is needed to establish how IRS2 acts in concert with other insulin receptor signaling components to impact the shuttling of glucose during decidualization of HESC.
Several endocrine conditions associated with insulin resistance have been linked to pregnancy complications. Polycystic ovarian syndrome (PCOS) is a condition characterized by hyperandrogenism and hyperinsulinemia that can reflect insulin resistance in peripheral tissues. A retrospective study performed among PCOS patients suggests that those with accompanying insulin resistance have an increased risk of miscarriage than patients who do not exhibit insulin resistance (58). Metformin is a common treatment for PCOS and other manifestations of type 2 diabetes to improve insulin sensitivity and alleviate hyperinsulinemia. PCOS patients who continued taking metformin during their pregnancy showed a reduced rate of early pregnancy loss compared with PCOS patients who stopped taking the drug upon determination of pregnancy (59, 60). Gestational diabetes mellitus (GDM), a condition manifesting towards the end of the second trimester, is also characterized by maternal insulin resistance and insufficient production of insulin to overcome this resistance. Analysis of postpartum placental tissues from women who presented with GDM showed reduced expression of glucose transporters and components of the insulin signal transduction pathway compared with control tissue from healthy pregnant women (61). Interestingly, Ayaz et al (2014) observed a link between the IRS2 G1057D polymorphism, which has diminished capacity to associate with downstream signaling molecules, and increased risk for developing GDM, suggesting that impaired signaling through IRS2 may contribute to the manifestation of GDM (62, 63).
Conclusion
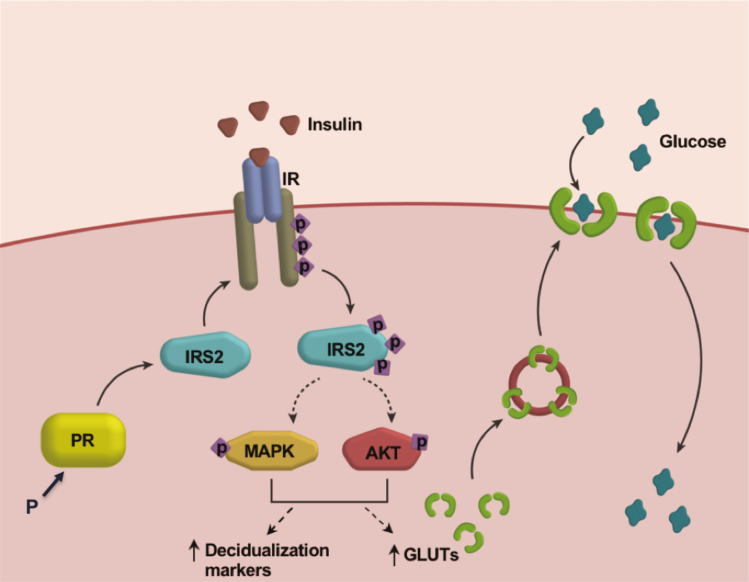
Our study establishes a critical role for the PR target IRS2 in mediating IR signaling during HESC decidualization (Fig. 8). IRS2 acts downstream of the IR and is required for MAPK activation that is critical for decidualization-specific gene expression. IRS2 also controls activation of AKT and expression of glucose transporters, thereby modulating cellular glucose uptake that meets the energy demands of the decidualization process. This study provides insight into the regulation of glucose metabolism in the uterus by the concerted actions of steroid hormone progesterone and insulin, and it increases our understanding of the underlying mechanisms of infertility and pregnancy complications associated with insulin resistance.
Figure 8.
Potential mechanism by which the PR target IRS2 promotes decidualization. During decidualization, PR induces IRS2 expression. IRS2 acts as an adaptor to insulin receptor, leading to downstream activation of MAPK and PI3K/AKT pathways, and increased expression of decidualization markers and glucose transporters. These GLUTs transport glucose into the cell to meet the increased metabolic demands of HESC decidualization, a critical step during pregnancy.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health grant R21 HD078983. Alison Neff was supported by National Institutes of Health grant T32ES007326. We thank Shreya Singh for her assistance in data collection and Jason Neff for creating the figures.
Glossary
Abbreviations
- BMP2
bone morphogenetic protein 2
- cAMP
8-bromo-adenosine-3′,5′-cyclic monophosphate
- E
estrogen
- ERK
extracellular-signal regulated kinase
- DC
decidualization
- GDM
gestational diabetes mellitus
- GLUT
glucose transporter
- HAND2
heart and neural crest derivatives expressed 2
- HESC
human endometrial stromal cells
- IGF1
insulin-like growth factor 1
- IGF1R
insulin-like growth factor 1 receptor
- IGFBP1
insulin-like growth factor binding protein 1
- IR
insulin receptor
- IRS2
insulin receptor substrate 2
- MAPK
mitogen-activated protein kinase
- P
progesterone
- PCOS
polycystic ovarian syndrome
- PI3K/AKT
phosphoinositide 3-kinase/AKT
- PR
progesterone receptor
- PRL
prolactin
- RTK
receptor tyrosine kinase
- siCtrl
scrambled siRNA control
- WNT4
wingless-type MMTV integration site family, member 4
Additional Information
Disclosure Summary: The author reports no conflicts of interest in this work.
References
- 1. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu H, Hou CC, Luo LF, Hu YJ, Yang WX. Endometrial stromal cells and decidualized stromal cells: origins, transformation and functions. Gene. 2014;551(1):1–14. [DOI] [PubMed] [Google Scholar]
- 3. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 5. Kaya HS, Hantak AM, Stubbs LJ, Taylor RN, Bagchi IC, Bagchi MK. Roles of progesterone receptor A and B isoforms during human endometrial decidualization. Mol Endocrinol. 2015;29(6):882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Meyts P. The insulin receptor and its signal transduction network. Updated April 27, 2016. In: Feingold KR, Anawalt B, Boyce A, et al eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; 2000-. Accessed January 11, 2018. [PubMed] [Google Scholar]
- 7. White MF. IRS2 integrates insulin/IGF1 signalling with metabolism, neurodegeneration and longevity. Diabetes Obes Metab. 2014;16 (Suppl 1):4–15. [DOI] [PubMed] [Google Scholar]
- 8. Withers DJ, Gutierrez JS, Towery H, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391(6670):900–904. [DOI] [PubMed] [Google Scholar]
- 9. Burks DJ, Font de Mora J, Schubert M, et al. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407(6802):377–382. [DOI] [PubMed] [Google Scholar]
- 10. Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994;78(3):642–649. [DOI] [PubMed] [Google Scholar]
- 11. RRID:AB_2810948, https://scicrunch.org/resolver/RRID:AB_2810948
- 12. RRID:AB_2243890, https://scicrunch.RRID:AB_2243890.
- 13. RRID:AB_2123081, https://scicrunch.org/resolver/RRID:AB_2123081.
- 14. RRID:AB_10641999, https://scicrunch.org/resolver/RRID:AB_10641999.
- 15. RRID:AB_2215428, https://scicrunch.org/resolver/RRID:AB_2215428.
- 16. RRID:AB_2810947, https://scicrunch.org/resolver/RRID:AB_2810947
- 17. RRID:AB_671714, https://scicrunch.org/resolver/RRID:AB_671714.
- 18. RRID:AB_2225021, https://scicrunch.org/resolver/RRID:AB_2225021.
- 19. RRID:AB_2650548, https://scicrunch.org/resolver/RRID:AB_2650548.
- 20. RRID:AB_2141287, https://scicrunch.org/resolver/RRID:AB_2141287.
- 21. RRID:AB_10903230, https://scicrunch.org/resolver/RRID:AB_10903230.
- 22. RRID:AB_1587080, https://scicrunch.org/resolver/RRID:AB_1587080.
- 23. RRID:AB_647490, https://scicrunch.org/resolver/RRID:AB_647490.
- 24. RRID:AB_2121235, https://scicrunch.org/resolver/RRID:AB_2121235.
- 25. RRID:AB_330924, https://scicrunch.org/resolver/RRID:AB_330924.
- 26. RRID:AB_2099233, https://scicrunch.org/resolver/RRID:AB_2099233.
- 27. RRID:AB_393778, https://scicrunch.org/resolver/RRID:AB_393778.
- 28. RRID:AB_2315777, https://scicrunch.org/resolver/RRID:AB_2315777.
- 29. RRID:AB_2307443, https://scicrunch.org/resolver/RRID:AB_2307443.
- 30. RRID:AB_2315147, https://scicrunch.org/resolver/RRID:AB_2315147.
- 31. Ganeff C, Chatel G, Munaut C, Frankenne F, Foidart JM, Winkler R. The IGF system in in-vitro human decidualization. Mol Hum Reprod. 2009;15(1):27–38. [DOI] [PubMed] [Google Scholar]
- 32. Mioni R, Mozzanega B, Granzotto M, et al. Insulin receptor and glucose transporters mRNA expression throughout the menstrual cycle in human endometrium: a physiological and cyclical condition of tissue insulin resistance. Gynecol Endocrinol. 2012;28(12):1014–1018. [DOI] [PubMed] [Google Scholar]
- 33. Flannery CA, Saleh FL, Choe GH, et al. Differential expression of IR-A, IR-B and IGF-1R in endometrial physiology and distinct signature in adenocarcinoma. J Clin Endocrinol Metab. 2016;101(7):2883–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lathi RB, Hess AP, Tulac S, Nayak NR, Conti M, Giudice LC. Dose-dependent insulin regulation of insulin-like growth factor binding protein-1 in human endometrial stromal cells is mediated by distinct signaling pathways. J Clin Endocrinol Metab. 2005;90(3):1599–1606. [DOI] [PubMed] [Google Scholar]
- 35. Tamura I, Ohkawa Y, Sato T, et al. Genome-wide analysis of histone modifications in human endometrial stromal cells. Mol Endocrinol. 2014;28(10):1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ujvari D, Jakson I, Babayeva S, et al. Dysregulation of in vitro decidualization of human endometrial stromal cells by insulin via transcriptional inhibition of Forkhead Box Protein O1. Plos One. 2017;12(1):e0171004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Craig LB, Ke RW, Kutteh WH. Increased prevalence of insulin resistance in women with a history of recurrent pregnancy loss. Fertil Steril. 2002;78(3):487–490. [DOI] [PubMed] [Google Scholar]
- 38. Tian L, Shen H, Lu Q, Norman RJ, Wang J. Insulin resistance increases the risk of spontaneous abortion after assisted reproduction technology treatment. J Clin Endocrinol Metab. 2007;92(4):1430–1433. [DOI] [PubMed] [Google Scholar]
- 39. Lee CH, Kim TH, Lee JH, et al. Extracellular signal-regulated kinase ½ signaling pathway is required for endometrial decidualization in mice and human. Plos One. 2013;8(9):e75282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toyofuku A, Hara T, Taguchi T, Katsura Y, Ohama K, Kudo Y. Cyclic and characteristic expression of phosphorylated Akt in human endometrium and decidual cells in vivo and in vitro. Hum Reprod. 2006;21(5):1122–1128. [DOI] [PubMed] [Google Scholar]
- 41. Yoshino O, Osuga Y, Hirota Y, et al. Akt as a possible intracellular mediator for decidualization in human endometrial stromal cells. Mol Hum Reprod. 2003;9(5):265–269. [DOI] [PubMed] [Google Scholar]
- 42. Baek MO, Song HI, Han JS, Yoon MS. Differential regulation of mTORC1 and mTORC2 is critical for 8-Br-cAMP-induced decidualization. Exp Mol Med. 2018;50(10):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buzzio OL, Lu Z, Miller CD, Unterman TG, Kim JJ. FOXO1A differentially regulates genes of decidualization. Endocrinology. 2006;147(8):3870–3876. [DOI] [PubMed] [Google Scholar]
- 44. Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin resistant states. Cold Spring Harb Perspect Biol. 2014;6(1). doi: 10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34(2-3):121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frolova AI, Moley KH. Quantitative analysis of glucose transporter mRNAs in endometrial stromal cells reveals critical role of GLUT1 in uterine receptivity. Endocrinology. 2011;152(5):2123–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. von Wolff M, Ursel S, Hahn U, Steldinger R, Strowitzki T. Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2003;88(8):3885–3892. [DOI] [PubMed] [Google Scholar]
- 48. Taha C, Mitsumoto Y, Liu Z, Skolnik EY, Klip A. The insulin-dependent biosynthesis of GLUT1 and GLUT3 glucose transporters in L6 muscle cells is mediated by distinct pathways. Roles of p21ras and pp70 S6 kinase. J Biol Chem. 1995;270(42):24678–24681. [DOI] [PubMed] [Google Scholar]
- 49. Taha C, Liu Z, Jin J, Al-Hasani H, Sonenberg N, Klip A. Opposite translational control of GLUT1 and GLUT4 glucose transporter mRNAs in response to insulin. Role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol 3-kinase in GLUT1 mRNA translation. J Biol Chem. 1999;274(46):33085–33091. [DOI] [PubMed] [Google Scholar]
- 50. Barthel A, Okino ST, Liao J, et al. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J Biol Chem. 1999;274(29):20281–20286. [DOI] [PubMed] [Google Scholar]
- 51. Montessuit C, Thorburn A. Transcriptional activation of the glucose transporter GLUT1 in ventricular cardiac myocytes by hypertrophic agonists. J Biol Chem. 1999;274(13):9006–9012. [DOI] [PubMed] [Google Scholar]
- 52. Nose A, Mori Y, Uchiyama-Tanaka Y, et al. Regulation of glucose transporter (GLUT1) gene expression by angiotensin II in mesangial cells: involvement of HB-EGF and EGF receptor transactivation. Hypertens Res. 2003;26(1):67–73. [DOI] [PubMed] [Google Scholar]
- 53. Zhai J, Liu CX, Tian ZR, Jiang QH, Sun YP. Effects of metformin on the expression of GLUT4 in endometrium of obese women with polycystic ovary syndrome. Biol Reprod. 2012;87(2):29. [DOI] [PubMed] [Google Scholar]
- 54. Armoni M, Harel C, Karnieli E. Transcriptional regulation of the GLUT4 gene: from PPAR-gamma and FOXO1 to FFA and inflammation. Trends Endocrinol Metab. 2007;18(3):100–107. [DOI] [PubMed] [Google Scholar]
- 55. Kolak M, Yki-Järvinen H, Kannisto K, et al. Effects of chronic rosiglitazone therapy on gene expression in human adipose tissue in vivo in patients with type 2 diabetes. J Clin Endocrinol Metab. 2007;92(2):720–724. [DOI] [PubMed] [Google Scholar]
- 56. Frolova AI, O’Neill K, Moley KH. Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells, preventing decidualization and implantation. Mol Endocrinol. 2011;25(8):1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kommagani R, Szwarc MM, Kovanci E, et al. Correction: Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. Plos Genet. 2015;11(9):e1005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chakraborty P, Goswami SK, Rajani S, et al. Recurrent pregnancy loss in polycystic ovary syndrome: role of hyperhomocysteinemia and insulin resistance. Plos One. 2013;8(5):e64446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Roberts KA, Nestler JE. Effects of metformin on early pregnancy loss in the polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(2):524–529. [DOI] [PubMed] [Google Scholar]
- 60. Nawaz FH, Rizvi J. Continuation of metformin reduces early pregnancy loss in obese Pakistani women with polycystic ovarian syndrome. Gynecol Obstet Invest. 2010;69(3):184–189. [DOI] [PubMed] [Google Scholar]
- 61. Zhang B, Jin Z, Sun L, et al. Expression and correlation of sex hormone-binding globulin and insulin signal transduction and glucose transporter proteins in gestational diabetes mellitus placental tissue. Diabetes Res Clin Pract. 2016;119:106–117. [DOI] [PubMed] [Google Scholar]
- 62. Ayaz L, Karakaş Çelik S, Cayan F. The G1057D polymorphism of insulin receptor substrate-2 associated with gestational diabetes mellitus. Gynecol Endocrinol. 2014;30(2):165–168. [DOI] [PubMed] [Google Scholar]
- 63. Bodhini D, Radha V, Deepa R, et al. The G1057D polymorphism of IRS-2 gene and its relationship with obesity in conferring susceptibility to type 2 diabetes in Asian Indians. Int J Obes (Lond). 2007;31(1):97–102. [DOI] [PubMed] [Google Scholar]