Abstract
Background:
The liver has unique anatomy in that most blood flow to normal hepatocytes is derived from the portal venous system, while liver tumors obtain their nutrient blood supply exclusively from the hepatic artery. The focused arterial delivery of anticancer agents to liver tumors has been performed for decades; however, preclinical models to standardize drug regimens and examine novel agents have been lacking. The purpose of this study was to establish preclinical hepatic artery infusion (HAI) models in a mouse, and to evaluate the safety and delivery capability of the models.
Material and methods:
Female C57BL/6 and BALB/c mice aged 8–12 wk were used to develop models of HAI via the hepatic artery (HA), superior pancreaticoduodenal artery (SPDA), or lienogastric artery (LGA). Success rates, distribution of perfusion, and associated morbidity and mortality were analyzed between groups.
Results:
All three models were feasible and reproducible in mice, and there was no statistical difference on body weight change between models. The HA model had a 13.3% mortality from acute liver failure, and the SPDA model demonstrated significant duodenal and pancreatic toxicity. SPDA and LGA routes had the highest success rates (96.7% and 91.4%, respectively) with low mortality, better drug delivery, and preserved physiologic liver function compared to the HA model.
Conclusions:
The optimal route of HAI was mouse breed-specific; SPDA access in BALB/c mice, and the LGA access in C57BL/6 mice. The described techniques serve as a reproducible platform for the identification and characterization of therapeutics for diverse metastatic liver tumors.
Keywords: Hepatic artery infusion, Liver metastases, First-pass effect, Regional therapy, Murine model
Introduction
The liver is a frequent site of metastases from a variety of cancers including gastric, breast, melanoma and pancreatic with colorectal cancer being the most common source.1–5 While surgical resection of metastatic lesions has proven effective and improves overall survival, many patients develop extensive or unresectable diseases not amenable to surgery. Regardless of the tumor source, the 5-year survival rate is below 10% in patients with unresectable liver metastases.6–10 Even though systemic chemotherapy may prolong overall survival in patients with unresectable metastatic disease, intravenous chemotherapy and/or radiation therapy does not achieve a cure in these cases and is limited by systemic toxicity and cumulative dose.
Hepatic artery infusion (HAI) chemotherapy for the treatment of liver cancers has been investigated for over four decades.11 The rationale for HAI is that hepatic metastases receive their nutritive blood supply from the hepatic arterial system.12 HAI concentrates chemotherapy agents delivering them directly to the tumor bed. Also, typical drugs used for HAI have a high first-passage hepatic clearance, resulting in less systemic exposure.13 Although, HAI chemotherapy as a regional therapy has been used clinically for decades in the treatment of both primary and metastatic cancers of the liver, significant toxicity remains a concern, and clinical results have been mixed in terms of improved survival outcomes.14–20
What has been lacking in the field is a reproducible and stringent preclinical animal model. Experimental models with pigs, rabbits, or rats have been used previously, but cost, animal size, and a lack of syngeneic tumor lines limit their utility.21–23 A mouse model would be ideal as there are multiple cell lines and techniques to generate hepatic metastases.24 Moreover, as the tumor cell lines are syngeneic with the mice, the immune system remains intact and can be investigated for contributions towards anti-tumor effects of HAI. Several requirements are necessary for an optimal preclinical HAI model including (1) reproducibility, (2) post-procedure liver integrity, and (3) clinical similarity. We sought to design a mouse model of HAI that could serve as a platform for preclinical testing of novel agents and treatment conditions. Herein, three individual methods of HAI delivery in mice are reported and compared for feasibility, morbidity, and toxicity.
Materials and Methods
Mice
Female C57BL/6 and BALB/c mice aged 8–12 wk were purchased from Charles River (Kingston, NY). Mice were fed a standard laboratory diet and housed under standard light and accommodation conditions. All experimental protocols were approved by the Roswell Park Cancer Institutional Animal Care and Use Committee.
HAI procedures
HAI was performed by a single investigator (MK) with three different approaches that included the hepatic artery (HA) directly, the HA via the superior pancreatico-duodenal artery (SPDA) or via the lieno-gastric artery (LGA) (Fig. 1). Non-tumor bearing mice received inhaled anesthesia with isoflurane (induction with 4%, maintenance 1.5%) to an appropriate level. Mice were placed on a warming platform to maintain normothermia throughout the procedure under a dissecting microscope (magnification ×6.5 ~ ×45) (VWR, West Chester, PA). The abdomen of the mouse was prepped with alcohol and betadine, and a 3-cm longitudinal midline incision was made using scissors, and then the xiphoid process was resected to obtain a wide working field. The abdomen was opened using two 5–0 silk sutures as anchors, the viscera were gently displaced to the left side of the abdominal cavity, and the portal vein (PV), abdominal inferior vena cava, bile duct, and celiac artery (CA) were exposed. The CA gives off the HA directly in most cases (Fig. 2A–D). For the direct HA approach, a 6–0 silk tie was placed around the proximal and distal parts of the HA respectively (Fig. 2E). The proximal 6–0 silk tie was then tied (Fig. 2F). An arteriotomy was created using microscissors (Roboz, Gaitherburg, MD) in the HA between the silks, which allowed for cannulation with a sterile microcatheter. The cannula consisted of low-density polyethylene with an inner diameter of 0.127 mm and outer diameter of 0.254 mm (Scientific Commodities, Lake Havasu City, AZ). Under direct visualization, the microcatheter was advanced into the HA and secured with the distal 6–0 silk ties (Fig. 2G). The microcatheter was then attached to a peristaltic infusion pump. At the completion of the infusion, the cannula was removed, and then the silk tie was used to fully occlude the HA cannulation site in order to prevent bleeding (Fig. 2H). For the SPDA approach, the SPDA as a branch of the CA, and the HA were identified (Fig. 2A). The SPDA was anchored with 6–0 silk first (Fig. 2I), and then a vascular clip was applied to the CA (Fig. 2J). A small arteriotomy was made by microscissors in the distal portion of the SPDA, and a microcatheter was placed to the opening of the HA without blocking of the CA (Fig. 2K). The microcatheter was connected to a pump after removing the vascular clip. The ischemia time was less than 5 min. The microcatheter was removed after infusion, and then the SPDA was ligated with 6–0 silk to control bleeding (Fig. 2L). For the LGA access, a view of the CA, HA and LGA were obtained under microscopy (Fig. 2D). A 6–0 silk tie was placed around the distal LGA (Fig. 2M), and then a vascular clip was applied to the CA (Fig. 2N). An arteriotomy was created using microscissors on the LGA, and the microcatheter was advanced near the opening of the HA without flow disturbance of the CA (Fig. 2O). The vascular clip was released after the microcatheter was secured with a silk. The procedural ischemia time for the celiac artery was less than 5 min. The silk tie was used to fully ligate the proximal portion of the LGA to prevent bleeding after infusion (Fig. 2P). After all cannulations, the abdominal wall and skin were re-approximated with 5–0 vicryl (Ethicon Inc., New Brunswick, NJ) and Vetbond adhesive (3M, Two Harbors, MN), respectively. All mice were recovered on a warming blanket and injected subcutaneously with buprenorphine (0.2 mg/kg body weight) for pain control and 0.5 ml of normal saline for hydration. The success of each procedure was defined as completion of 15-min infusion without any leakage, and recovery from anesthesia.
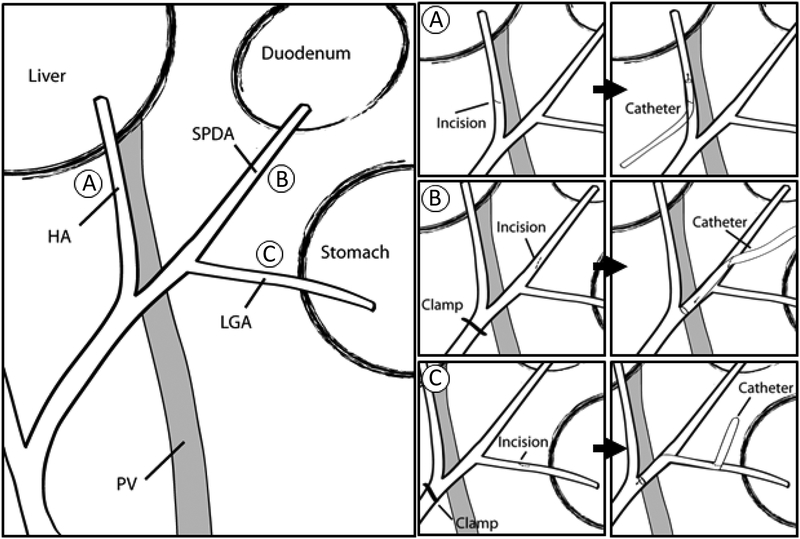
Fig. 1.
A schematic diagram of three models for HAI. (A) The HA access, (B) The SPDA access, (C) The LGA access. The duodenum was displaced superiorly
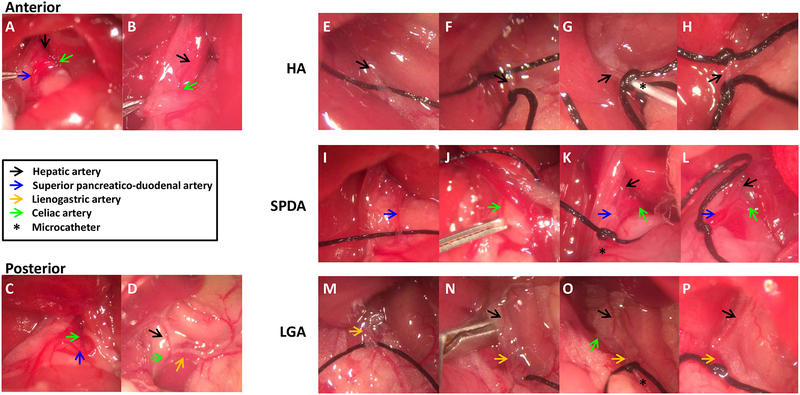
Fig. 2.
HA position relative to the caudate lobe and three approaches of HAI. (A, B) The HA crossed either the anterior or (C, D) the posterior part of the caudated lobe. For the HA access, (E) the HA was anchored with 6–0 black silk. (F) A small arteriotomy was made with microscissors on the HA after applying 6–0 black silk suture. (G) A microcatheter was cannulated through the arteriotomy, and then a microcatheter was secured with a tie. (H) Two black silk ties applied permanently after removing catheter. For the SPDA access, (I) The SPDA was anchored with 6–0 black silk. (J) A vascular clip was applied to the celiac artery. (K) A microcatheter was advanced to near the CA, secured with a tie, and the vascular clip removed. (L) One tie was applied permanently after catheter removal. For the LGA access, (M) the LGA was anchored with 6–0 black silk. (N) A vascular clip was applied to the CA. (O) A microcatheter was advanced to the junction of the HA, and then vascular clip removed. (P) One tie was applied permanently after catheter removal.
Infusion variables
Normal saline was delivered by a peristaltic pump (Cole-Parmer Instrument Co., Vernon Hills, IL) with 30±3 μL/min of a flow rate based upon mouse body weight (500 μL/25g) for 15 min at room temperature.
Anatomy and safety assessment
The mouse anatomy of the celiac artery and branches was evaluated in all mice and recorded. Body weights of mice were measured 1, 2, 3, 4, 7, and 9 d after a HAI procedure with an electric scale (AWS Inc., Saturn Court Norcross, GA). Aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (Tbili), and amylase levels were measured as markers of liver and pancreas toxicity. 50 μL of blood was obtained from each mouse 1, 2, 3 and 9 d after HAI using a superficial temporal vein method.25 All levels of blood chemistry were measured in plasma prepared from the collected blood samples by VITROS 5.1 FS (Ortho Clinical Diagnostics, Inc., Rochester, NY). For fibrosis studies, 9-μm-thick liver sections were obtained 2 wk after HAI. Each section was underwent trichrome staining (Abcam, Cambridge, MA) and examined injury to the hepatic triad (Olympus, Hamburg, Germany) followed by image capture with SPOT RT color camera (Diagnostic instruments Inc, Sterling Heights, MI).
Infusate delivery assessment
A 0.5% solution of Evans Blue dye (EBD) (Sigma Aldrich, St Louis, MO) was infused via either the previously described HA, SPDA, or LGA methods to investigate distribution of the infusate. Five minutes following infusion with EBD, the liver was fixed in OCT compound (Sakura Finetek, Torrance, CA) by liquid nitrogen. Liver sections were prepared and viewed with bright-field light microscopy.
Assessment of tumor responses
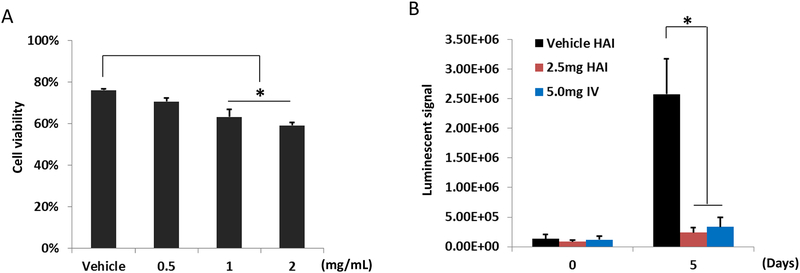
CT26-Luc colon cancer cells were generated for in vitro and in vivo experiments. 5-FU was chosen over the commonly used FUDR (floxuridine) for clinical HAI due to FUDR being a prodrug of 5-FU. The direct use of 5-FU facilitated comparisons between in vitro and in vivo responses. Cells were plated at 5.0 × 104 cells per well in 96-well. 24 hours later, cells were treated with vehicle, 0.5, 1.0, and 2.0 mg/mL of 5FU (Sigma-Aldrich, St. Louis, MO), with each condition being tested in 10 replicate wells. Cell viability was measured 12 hours after the start of treatment using the CytoSelect MTT Cell Proliferation Assay (Cell Biolabs, Inc., San Diego, CA). For in vivo experiments, female BALB/c mice were injected with 3 × 105 CT26-Luc colon cancer cells using the intrasplenic injection technique to implant liver metastases.24 Tumor bearing mice were infused either with vehicle or 2.0 mg/mL of 5FU through the SPDA 7 d after tumor inoculation. Tumor responses were investigated 5 d after HAI with the in vivo luciferase measurement.
Statistical analysis
Comparison between two groups were performed using Student’s t test, and statistical significance was accepted with P < 0.05.
Results
Mouse breed anatomy
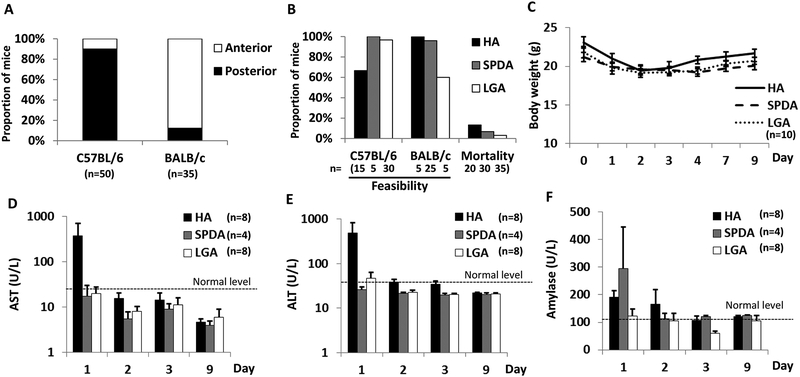
C57BL/6 and BALB/c mice exhibited divergent anatomy with respect to the HA. As a branch of the CA, the HA crossed anterior to the caudate lobe in most BALB/c mice but passed posteriorly in 90% of C57BL/6 mice which influenced accessibility (Fig. 3A).
Fig. 3.
The feasibility and safety of three models with C57BL/6 and BALB/c mice. (A) The HA position relative to the caudate lobe. (B) All models were reproducible with low mortality. (C) Body weight changes did not differ between techniques. (D and E) Abnormal AST/ALT levels were measured 1 d after HAI in the HA model. (F) Normal Tbili levels were measured after HAI in all models. (G) The highest level of amylase was detected 1 d after HAI through the SPDA access. No statistical differences noted. ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Assessment of feasibility and safety
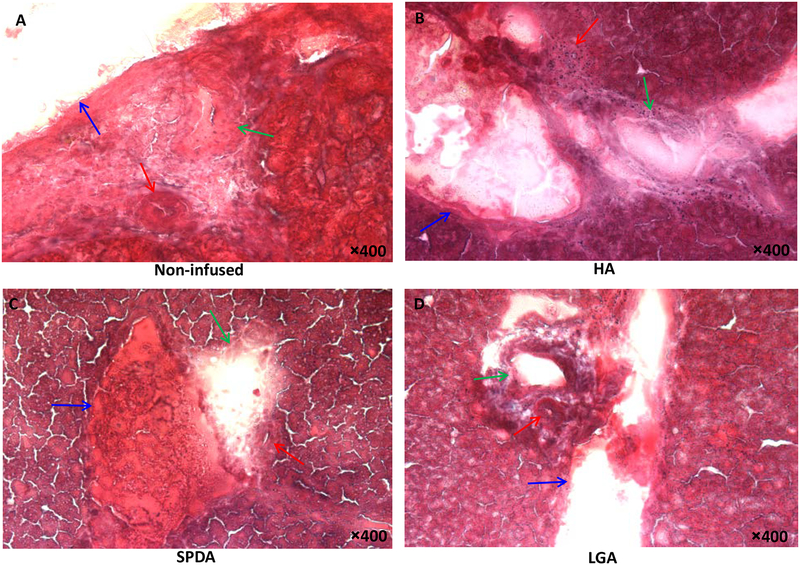
The success rates of HAI with saline were dependent on the technique and mouse strain. Feasibility was defined as the complete delivery of the defined saline volume via HAI and operative survival; achieving these combined end points was deemed “feasible”. The HA (66.6%) and the LGA (60%) models were the most difficult procedure to perform in C57BL/6 and BALB/c mice, respectively, due to anatomy of the vasculature. The SPDA model appeared feasible in both mouse strains (C57BL/6; 100%, BALB/c; 96%), but the procedure was prolonged in C57BL/6 strain compared with BALB/c strain due to a shorter segment of SPDA. Over 90% of successful infusions were made with the SPDA and LGA techniques in C57BL/6 strain, and highly successful infusion rates of the HA (100%) and the SPDA (96%) models were achieved in BALB/c strain. Although all HAI models were well tolerated in most mice, ruffled fur was a common morbidity 1 d after HAI procedure. The HA technique was associated with the highest mortality within 2 d after infusion (13.3% mortality). SPDA infusion resulted in two mortality cases (6.8%) within 2 d as a result of duodenal perforation and pancreatic necrosis. The LGA technique demonstrated the lowest mortality (3.1%) noted 5 d after infusion (Fig. 3B). There was no statistical difference in body weight change between techniques as mice regained weight 4 d after HAI infusion (Fig. 3C). Representative of acute liver toxicity, the HA model showed higher aspartate aminotransferase/alanine aminotransferase levels 1 d after infusion compared with the SPDA and LGA routes, but differences were not statistically significant. With each technique, markers of acute liver toxicity returned to the normal range after 2 d (Fig. 3D, E). As a marker of biliary toxicity, the levels of total bilirubin were normal in all mice with HAI (data not shown). Regarding pancreatic toxicity, SPDA access displayed the highest amylase levels 1 d after infusion but was not statistically significant compared with the other techniques (Fig. 3F). Trichrome staining of liver sections obtained 2 wk after HAI demonstrated that the HA route had the greatest degree of damage to the hepatic triad (Fig. 4). The hepatic arterioles appeared to be either obliterated or completely collapsed consistent with the occlusion of the hepatic artery that occurred after completion of the HA technique. In addition, a mild inflammatory reaction was seen in the HA-treated mice. To the contrary, the LGA and SPDA techniques had minimal changes and were indicative of continued and adequate blood flow via the hepatic arterioles.
Fig. 4.
Trichrome stains 2 wk after HAI with normal saline. (A) Normal portal triad with hepatic arteriole (red arrow), biliary duct (green arrow), and PV branch (blue arrow). (B) Fibrotic change and inflammatory reaction were detected around the arteriole and biliary duct in the HA model. (C) SPDA, and (D) LGA model did not demonstrate inflammatory changes or arteriole damage.
Assessment of delivery
EBD was present in the HA and the PV with blue stained-vasculature in the right, median, and left lobes after infusion through the HA, and the intensity of EBD was similar between lobes. Otherwise, mice infused through the SPDA, showed no evidence of EBD in portal triad. And, a less intense blue stained-vasculature was detected in the median and lateral lobes via the LGA access compared to the HA access (Fig. 5). Overall these findings suggest that distribution throughout the entire liver was achieved, but in the case of the HA technique, less EBD was “washed out” due to occlusion of the hepatic artery at the end of the procedure.
Fig. 5.
EBD distribution following HAI. Higher intensity of EBD was detected in both the hepatic arteries (red arrow) and PV (blue arrow) in the right (RL), median (ML), and left lobes (LL) after HAI via the HA as compared to the SPDA and LGA routes.
Assessment of tumor responses
Consistent with its known clinical efficacy, 5-FU treatment of CT26-Luc colon cancer cells for 12 h at clinically equivalent doses led to significant reduction of tumor cell viability in vitro (Fig. 6A). Allowing 7 d for tumor establishment in the liver, mice were treated with 5-FU via HAI and assessed for response. Because CT26 cells are syngeneic with BALB/c mice, the SPDA approach was used due to favorable anatomy. A significant decrease of luminescent signals was detected 5 d after HAI and intravenous 5-FU compared with vehicle controls (Fig. 6B). It should be noted that the dosing of 5-FU for HAI was only half of the intravenous dose, yet antitumor effects were similar.
Fig.6.
CT26-Luc colon cancer cells were sensitive to 5-FU in vitro and in vivo. (A) After 12-h exposure in vitro, 5-FU treatment appeared dose dependent on CT26-Luc colon cancer cells (n = 10). (B) Luminescent signal of liver metastases 5 d after 5-FU given by HAI or systemically demonstrated a significant antitumor response, with equivalent responses for HAI using only half of the 5-FU dose given systemically (n = 4), *P < 0.05.
Discussion
HAI chemotherapy is a locoregional therapy that has been used for decades in the treatment of both primary and metastatic cancers of the liver. However, despite a strong body of evidence of clinically meaningful activity for delivering drugs via the HA, which results in higher drug concentrations in the liver and lower systemic toxicity due to first-pass effect clearance, the benefit of HAI in prolonging overall survival is debatable. A potential reason for mixed results may be due to the difficulty of generating large clinical trials of HAI to test novel agents or combinations.15,26 In this study, we described successful methods of HAI in mice as a platform to dissect the mechanisms and optimal conditions for application to clinical HAI.
We performed HAI via three different access routes: the HA, SPDA, and LGA. The HA route did not require a vascular clip during the procedure, so the entire procedure time was shorter than other models in BALB/c mice. However, the HA of most C57BL/6 mice passed posterior to the caudate lobe, so the HA was located in a deep position. For this reason, there was no time advantage with the HA access in C57BL/6 mice as it required increased time for exposure. Furthermore, the HA had to be sacrificed to avoid bleeding after HAI, as it isn’t feasible to repair the HA in mice due to diminutive size. Consequently, the HA model showed higher levels of liver enzymes 1 d after infusion, and over 10% of mice presenting increased liver enzymes died within 2 d after infusion. EBD was retained in the hepatic arteries and the PV after HAI with the HA access, indicating no post-procedural arterial flow and a lack of washout of the dye. The biliary epithelium is more susceptible to ischemic injury than hepatocytes, and the bile ducts are supplied only arterially.27 Our results demonstrated the vulnerability of the biliary system after the HA ligation, as the hepatic arterioles appeared obliterated and an inflammatory reaction was noted around the hepatic triad. A model that demands occlusion of the HA after HAI leaving the tumor ischemic would confound any results from an experimental agent and would not represent an accurate clinical model. For these reasons, the direct HA route was prohibitive in mice and is not a useful approach for preclinical testing.
The SPDA access showed the highest success rate of HAI. Even though a vascular clip was applied during arteriotomy, the ischemic time was less than 5 min, and an increase in liver enzymes was not detected. In addition, residual EBD was minimal in the liver indicating good post-procedure arterial perfusion. However, amylase levels were higher at day 1 post-procedure compared to the other models. Consistent with this finding, there were also two early mortalities from duodenal perforation and pancreas necrosis in the SPDA model. The length of the SPDA was longer in BALB/c mice compared to C57BL/6 mice, so the SPDA model appeared more feasible in BALB/c mice.
The LGA model had a high success rate of HAI in C57BL/6 mice. Liver enzymes were normal after infusion, and the levels of amylase were similar to the HA model and less than the SPDA model. The size of the LGA was smaller in BALB/c mice compared to C57BL/6 mice, so the LGA access with BALB/c mice had the lowest success rate. Therefore, the optimal technique and route of HAI in mice appeared to be species specific which is an important consideration based on preferences of syngeneic cell lines to model liver tumors.
Importantly, given that the LGA and SPDA techniques culminate in the ligation of each artery, respectively, permanent ischemia to downstream tissues may occur and manifest well beyond the temporary procedural ischemia associated with HA occlusion and should be a consideration of the approach and planning for appropriate study numbers (e.g., duodenal perforation or pancreatic necrosis). However, as there is redundancy in the local circulation and no chronic or late toxicities noted in the LGA and SPDA groups, any subsequent ischemia beyond 72 h should be minimal. As this model system is designed for directed infusion, retrograde flow was not observed and would be anticipated to be absent due to the outer diameter of the infusion catheter (0.254 mm) compared with the vessel lumen, the low pressure setting of the pump, and visualization of EBD antegrade distribution seen during the procedure. Because of the transient nature of EBD, future studies could incorporate radioactive tracers to fully discern distribution to the liver and liver metastases.
To apply one of the models for preclinical in vivo study, CT26-Luc colon cancer liver metastases bearing BALB/c mice were treated with 5-FU either via HAI or intravenously. After HAI using the SPDA model, tumor growth was significantly delayed after 5 d in both 5-FU groups compared with the vehicle group. Equivalent tumor growth inhibition was noted when 5-FU was given via HAI at a dose that was half of the intravenous dose suggesting a potential benefit to the HAI approach. These results may underestimate the HAI approach though, since 5-FU was used to allow for in vitro testing and selection of an optimal dose for antitumor efficacy which would not be possible with the most commonly used HAI prodrug FUDR. Historically, FUDR has markedly different in vivo and in vitro activities28 and would likely be superior to 5-FU clinically. These results suggest that the model mimics clinical conditions and can be leveraged to test novel agents in vivo and as these models are immunocompetent, novel combinatorial strategies with emerging immune agents can be evaluated.
In summary, although all three models for HAI were feasible and reproducible in mice, the optimal model was the SPDA access in BALB/c mice, and the LGA access in C57BL/6 mice. Successful HAI therapy is contingent on the ability to place a catheter in an appropriate place and limit morbidity after perfusion. The described mouse models succeed in this regard and may serve as a platform for identifying novel drugs and optimal conditions for the treatment of an assortment of primary and metastatic liver tumors. Indeed, further study using our novel mouse models by our group and others may lead to new translational investigations to optimize future clinical trials.
Acknowledgements
This research was partially supported by Institutional Research Grant #126771-IRG-14-194-11-IRG from the American Cancer Society and P30 CA016056 from Cancer Center Support Grant.
Footnotes
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in the article.
REFERENCES
- 1.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol; 2012. 4:283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qiu JL, Deng MG, Li W, Zou RH, Li BK, Zheng Y, Lao XM, Zhou K, Yuan YF. Hepatic resection for synchronous hepatic metastasis from gastric cancer. Eur J Surg Oncol 2013; 39:694–700. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz A, Wicherts D, Adam R, Siesling S, Linn S, Hillegersberg R. [Resection of liver metastases in breast cancer]. Ned Tijdschr Geneeskd 2015; 159:A8453. [PubMed] [Google Scholar]
- 4.Kandolf-Sekulovic L, Babovic N, Jokic N, Nikolin B, Nikolic D, Janjic Z, Mijugkovic Z, Rajovic M, Novakovic M, Pejcic I, Kovaevic P, Mihajlovic D, Roganovic T, Ferenc V, Nikolic J, Marinkovic M, Bizetic Z. Clinicopathological characteristics, diagnosis and treatment of melanoma in Serbia--the Melanoma Focus Study. Vojnosanit Pregl 2015; 72:312–316. [DOI] [PubMed] [Google Scholar]
- 5.Ray K Pancreatic cancer: Pancreatic cancer exosomes prime the liver for metastasis. Nat Rev Gastroenterol Hepatol 2015; 12:371. [DOI] [PubMed] [Google Scholar]
- 6.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, Hess K, Curley SA. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004; 239:818–825; discussion 825–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Power DG, Healey-Bird BR, Kemeny NE. Regional chemotherapy for liver-limited metastatic colorectal cancer. Clin Colorectal Cancer 2008; 7:247–259. [DOI] [PubMed] [Google Scholar]
- 8.DeWitt J, Yu M, Al-Haddad MA, Sherman S, McHenry L, Leblanc JK. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by EUS-FNA. Gastrointest Endosc 2010; 71:260–265. [DOI] [PubMed] [Google Scholar]
- 9.Jerraya H, Saidani A, Khalfallah M, Bouasker I, Nouira R, Dziri C. Management of liver metastases from gastric carcinoma: where is the evidence? Tunis Med 2013; 91:1–5. [PubMed] [Google Scholar]
- 10.Ursaru M, Jari I, Naum A, Scripcariu V, Negru D. Causes of Death in Patients with Stage 0-Ii Breast Cancer. Rev Med Chir Soc Med Nat Iasi 2015; 119:374–378. [PubMed] [Google Scholar]
- 11.Sullivan RD, Norcross JW, Watkins E Jr., Chemotherapy of Metastatic Liver Cancer by Prolonged Hepatic-Artery Infusion. N Engl J Med 1964; 270:321–327. [DOI] [PubMed] [Google Scholar]
- 12.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol 1954; 30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr DJ, Los G. Pharmacokinetic principles of locoregional chemotherapy. Cancer Surv 1993; 17:105–122. [PubMed] [Google Scholar]
- 14.Bouchahda M, Levi F, Adam R, Rougier P. Modern insights into hepatic arterial infusion for liver metastases from colorectal cancer. Eur J Cancer 2011; 47:2681–2690. [DOI] [PubMed] [Google Scholar]
- 15.Leal JN, Kingham TP. Hepatic artery infusion chemotherapy for liver malignancy. Surg Oncol Clin N Am 2015; 24:121–148. [DOI] [PubMed] [Google Scholar]
- 16.Shi L, Zhao J, Lu Q, Chen X, Wang H, Jiang Y, Wu J, Ji M, Xu B, Chen L, Jiang J, Wu C. Initial hepatic artery infusion and systemic chemotherapy for asymptomatic colorectal cancer with un-resectable liver metastasis. Int J Clin Exp Med 2015; 8:1000–1008. [PMC free article] [PubMed] [Google Scholar]
- 17.Kemeny NE, Chou JF, Boucher TM, Capanu M, DeMatteo RP, Jarnagin WR, Allen PJ, Fong YC, Cercek A, D’Angelica MI. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol 2016; 113:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konstantinidis IT, Groot Koerkamp B, Do RK, Gonen M, Fong Y, Allen PJ, D’Angelica MI, Kingham TP, DeMatteo RP, Klimstra DS, Kemeny NE, Jarnagin WR. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016; 122:758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemeny N, Huang Y, Cohen AM, Shi W, Conti JA, Brennan MF, Bertino JR, Turnbull AD, Sullivan D, Stockman J, Blumgart LH, Fong Y. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999; 341:2039–2048. [DOI] [PubMed] [Google Scholar]
- 20.Magge D, Choudry HA, Zeh HJ 3rd, Cunningham DE, Steel J, Holtzman MP, Jones, Pingpank JF, Bartlett DL, Zureikat AH. Outcome analysis of a decade-long experience of isolated hepatic perfusion for unresectable liver metastases at a single institution. Ann Surg 2014; 259:953–959. [DOI] [PubMed] [Google Scholar]
- 21.Lilienberg E, Ebeling Barbier C, Nyman R, Hedeland M, Bondesson U, Axen N, Lennernas H. Investigation of hepatobiliary disposition of doxorubicin following intrahepatic delivery of different dosage forms. Mol Pharm 2014; 11:131–144. [DOI] [PubMed] [Google Scholar]
- 22.Cao W, Xu X, Zhang J, Duan Y. Tumor angiogenesis after heated lipiodol infusion via the hepatic artery in a rabbit model of VX2 liver cancer. PLoS One 2013; 8:e61583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pohlen U, Buhr HJ, Berger G, Ritz JP, Holmer C. Hepatic arterial infusion (HAI) with PEGylated liposomes containing 5-FU improves tumor control of liver metastases in a rat model. Invest New Drugs 2012; 30:927–935. [DOI] [PubMed] [Google Scholar]
- 24.Soares KC, Foley K, Olino K, Leubner A, Mayo SC, Jain A, Jaffee E, Schulick RD, Yoshimura K, Edil B, Zheng L. A preclinical murine model of hepatic metastases. J Vis Exp 2014; 51677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes N, Brayton C, Grindle S, Shepherd S, Tyler B, Guarnieri M. Morbidity and mortality rates associated with serial bleeding from the superficial temporal vein in mice. Lab Anim (NY) 2010; 39:236–240. [DOI] [PubMed] [Google Scholar]
- 26.Parks L, Routt M. Hepatic artery infusion pump in the treatment of liver metastases. Clin J Oncol Nurs 2015; 19:316–320. [DOI] [PubMed] [Google Scholar]
- 27.Noack K, Bronk SF, Kato A, Gores GJ. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation 1993; 56:495–500. [DOI] [PubMed] [Google Scholar]
- 28.Schroy PC, Cohen A, Winawer SJ, Friedman EA. Effects of FUdR on primary-cultured colon carcinomas metastatic to the liver. J Surg Oncol 1990; 45:217–223. [DOI] [PubMed] [Google Scholar]