Abstract
Objectives:
Over the last decades, novel echocardiographic measures have constantly emerged. It is still unclear which echocardiographic measures have the most significant prognostic value in the general population. Accordingly, we compared the prognostic value of a large panel of echocardiographic measures to identify the most promising measures.
Methods.
We evaluated 1497 Framingham Study participants (mean age 65 years, 55.4% women) who underwent echocardiographic measurements of left ventricular ejection fraction, left ventricular mass index, global longitudinal strain, global circumferential strain, mitral annular plane systolic excursion, mitral E/e’, maximum and minimum left atrial volume index (LAVImax and LAVImin), left atrial emptying fraction (LAEF), and LV longitudinal synchrony. We related these measures to the incidence of two composite outcomes: CVD or death; atrial fibrillation (AF) or congestive heart failure (CHF).
Results.
On follow-up (mean 8.3 years), there were 241 CVD events/death, and 139 AF/CHF events. In multivariable-adjusted Cox models, higher LAEF was associated with a lower risk (Hazard Ratio [HR] per SD, 0.80, and 0.70 for CVD/death and AF/CHF, respectively, P≤0.001for both) and higher LAVImin (HR per SD 1.32 and 1.70 for CVD/death and AF/CHF, respectively, P≤0.001 for both) and LAVI max (HR per SD 1.26, and 1.54 for CVD/death and AF/CHF, respectively, P≤0.002 for both) were associated with a higher risk of both composite outcomes.
Conclusions.
In our community-based sample, LA volumes and function were the best echocardiographic predictors of clinical outcomes. Therefore, these values should be considered for inclusion in standard echocardiographic assessments for the purpose of risk stratification.
Keywords: epidemiology, risk stratification, outcome, mortality, echocardiography, cardiovascular disease, strain, diastolic dysfunction, left atrium, synchrony, mitral annulus, atrial fibrillation, heart failure
Introduction
It is challenging to capture the complex cardiac chamber movements during the cardiac cycle as well as other related physiological events with a single image-based measure of cardiac function.1 LV systolic function as assessed by the LV ejection fraction (LVEF) has been the gold standard for decades,2 although it is widely accepted that it largely reflects LV pump function rather than intrinsic myocardial function.1,3 Myocardial function can additionally be characterized by mitral annular plane systolic excursion (MAPSE)4, global longitudinal strain (GLS)1 and global circumferential strain (GCS)5. Changes in the relative timing of regional ventricular electrical activation and/or mechanical contraction can be used to evaluate LV longitudinal segmental synchrony (LSS).3 LV diastolic function can be assessed by the ratio of the early peak transmitral and tissue Doppler velocities (E/e’).6 The size of the left atrium can be assessed by maximum and minimum left atrial volume index (LAVImax and LAVImin) and global LA function can be measured by left atrial emptying fraction (LAEF).7
Several of the aforementioned echocardiographic indices have been associated individually with the risk of coronary artery disease, stroke, congestive heart failure (CHF), atrial fibrillation (AF), and death, both in samples with and without prevalent cardiovascular disease.7–21 Yet, to our knowledge, no prior study has performed a direct comparison of a panel of these echocardiographic measures against one another in a community-based sample. Aim of our study was to investigate if there is one or if there are several echocardiographic measures which outperform the others in risk stratification in the general population.
Accordingly, we evaluated the relative prognostic significance of both novel and traditional echocardiographic indices for cardiovascular disease (CVD), AF, CHF, and death, modeled both as composite outcomes (primary analyses) and individually (secondary analyses). We hypothesized that select echocardiographic measures outperform the others: LVMI is the best predictor of CVD and death; E/e’, GLS and GCS are associated with increased risk of CHF; and LAVImin, LAVImax and LAEF are associated with risk of AF. We tested these hypotheses in a large middle-aged community-based sample.
Material and Methods
Study Sample
The design and selection criteria for the Framingham Heart Study (FHS) Offspring Study and the companion minority Omni Cohorts have been published previously.22–24 The primary study sample for the present investigation comprised participants from the FHS Offspring/Omni cohort who attended their eighth/third examination cycle (N= 3319; 2005–2008). The Institutional Review Board of Boston University Medical Center approved the study protocol. All study participants provided written informed consent.
Overall, of 3319 eligible participants across the cohort at the examination, we excluded 1822 individuals for various reasons shown in Supplementary Figure 1, resulting in a sample size of 1497 participants for the present analyses.
Participants excluded due to missing echocardiographic variables were generally older, had higher body mass index, and faster heart rates (Supplementary Table 1).
Measurements of Covariates
At the FHS examinations, participants provided a detailed medical history, and underwent phlebotomy for assessment of CVD risk factors (including a fasting lipid panel and serum creatinine), anthropometry, blood pressure measurements, standard 12-lead electrocardiogram, and routine echocardiography (see below). Hypertension was defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg or use of antihypertensive medications. Diabetes was defined if the participant had fasting glucose concentration ≥126 mg/dL or used hypoglycemic medications. Current smoking status was determined based on whether or not the participant reported smoking regularly during the year antedating the FHS examination. Methods and criteria of measurement of all covariates have been described previously.25
Measurement of Echocardiographic Variables
For the present investigation, we used the following echocardiographic variables: LV mass [LVM], LVM index by body surface area [LVMI in g/m2], LVEF, MAPSE, GLS, GCS, LSS, E/e’, LV septum wall, LV posterior wall, LV wall thickness, LV end-diastolic dimension, LV end-systolic dimension, left atrial end-systolic dimension, left atrial end-diastolic dimension (LADD). All attendees underwent routine transthoracic echocardiography using a HP Sonos 5500 ultrasound machine (Phillips Medical Systems, Andover, MA) and a standardized protocol. Images were digitized and measured offline per recommendations of the American Society of Echocardiography. Using a leading-edge-to-leading-edge technique, digital M-mode tracings were used to measure the end-diastolic thicknesses of the LV septum and LV posterior wall (summated to yield LV wall thickness [LVWT]), LV end-diastolic (LVDD) and end-systolic (LVSD) dimensions, left atrial end-systolic dimension (LASD), and left atrial end-diastolic dimension (LADD).26
We calculated LV mass (LVM) using the method by Devereux et al as equal to (0.8*1.04*((LVDD + septal wall thickness + posterior wall thickness)3 −LVDD3)+0.6),27 and indexed it to body surface area to estimate LVM index (LVMI in g/m2). We used the Teichholz formula28 to calculate LVEF, which closely approximates that estimated by quantitative two-dimensional methods in our laboratory.29 MAPSE was measured, as recommended,30 at the lateral side of the mitral valve annulus in the apical four-chamber view by measuring the systolic excursion of the annulus from its lowest point at end-diastole to its highest point at the time of aortic valve closure.
We performed speckle-tracking analyses of LV function using an offline analysis program (2D Cardiac Performance Analysis version 1.1, TomTec Imaging Systems GmbH, Unterschleißheim, Germany) according to a standardized protocol as previously published.31 GLS was assessed using two apical views (apical two and apical four chamber view), whereas GCS was assessed in the short axis view.32,33 Frame rates for all analyses were >=30 frames/sec and not >70 frames/sec for all views, as detailed previously.34
We also measured LSS, calculated as the standard deviation of time-to-peak strain as previously published.35
For the measurement of LV diastolic function, we derived the ratio of early transmitral flow velocity (E) and the early systolic mitral annulus velocity (e’) to calculate E/e’. E’ was measured using tissue Doppler imaging at the lateral mitral annulus.36
We assessed the prevalence of abnormalities of the different echocardiographic measures of cardiac function using cutpoints based on the published literature for their reference ranges. 6,12,30,35,37–40
We performed offline analysis of digital images to obtain maximum and minimum volumetric measurements of the LA (LAmax and LAmin) using the recommended Simpson’s biplane summation of disks method on Digisonics DigiView System Software (ver. 3.7.9.3, Digisonics, Houston, TX) from apical two and four-chamber views. LAVI was indexed by body surface area.
LAEF was calculated as ([LAmax - LAmin]/LAmax)*100 as previously described.37,41
Follow-Up
All FHS participants are under continuous surveillance for the development of CVD and death through 2015. An endpoints review committee consisting of 3 physicians reviews all available medical records (from office visits and hospitalizations) to adjudicate events including CVD, CHF, and AF based on previously published criteria.25,42,43 The presence of CVD was defined as the presence of coronary heart disease (acute coronary syndrome, myocardial infarction, and angina), cerebrovascular disease (stroke or transient ischemic attack), intermittent claudication, or CHF.44 A diagnosis of AF was adjudicated by 2 or more FHS cardiologists who reviewed all FHS or available clinically obtained electrocardiograms, telemetry, or Holter monitor recordings with suspected AF, and hospital records documenting AF diagnoses made by treating physicians.45
For the present investigation, we evaluated two primary composite endpoints defined a priori to maximize our statistical power to detect associations of echocardiographic variables with clinical outcomes, and to reduce multiple statistical testing. The first primary composite endpoint included incidence of CVD or all-cause mortality. The second primary endpoint was a composite of incidence of CHF or AF, and was chosen because these two outcomes are more directly related to cardiac remodeling and to the echocardiographic indices evaluated (as compared to other events such as cerebrovascular or peripheral arterial disease, which may be viewed as somewhat more distal events). In secondary analyses, we related each echocardiographic index to the incidence of CVD, CHF, AF, and all-cause mortality (separate model for each outcome) to gain additional insights.
Statistical Analyses
We natural-logarithmically transformed E/e’, LSS, LAVImax and LAVImin to normalize their skewed distributions. Due to differences in variances of longitudinal synchrony, LAVImax, and LAVImin between the two FHS cohorts, these variables were standardized within cohorts.
We calculated Pearson correlation coefficients among the echocardiographic variables, adjusting for age and sex to avoid modeling highly correlated echocardiographic variables together in our multivariable models. We estimated multivariable-adjusted Cox proportional hazards regression models relating the echocardiographic variables (modeled individually) to the two primary composite outcomes (separate models for each) after confirming that the assumption of proportionality of hazards was met. In all analyses, the echocardiographic indices were modeled as continuous variables to maximize statistical power and individuals with prevalent disease (based on outcome of interest) were excluded. We used the robust sandwich estimator for the covariance matrix to account for familial correlations. Models were adjusted for age, sex, cohort type, BMI, current smoking, diabetes, antihypertensive treatment, systolic and diastolic blood pressure, heart rate, serum creatinine, high-density and low-density lipoprotein cholesterol, and triglycerides. To account for multiple hypothesis testing (10 echocardiographic variables [LVEF, LVMI, GLS, GCS, MAPSE, E/e’, LAVImax, LAVImin, LAEF, Synchrony], 2 endpoints [incidence of CVD or all-cause mortality, incidence of CHF or AF]) a Bonferroni-corrected P-value of <0.0025 (=0.05/20) was considered statistically significant for our primary analyses. For the statistically significant echocardiographic measures, we constructed cumulative incidence curves (using the Kaplan-Meier method) for the composite endpoints stratified by tertiles of the echocardiographic measure (within sex and 5-year age groups), and used the log rank test to compare the tertile groups. We tested for nonlinearity of associations using multivariable-adjusted splines. Additionally, we tested for effect modification by sex by entering appropriate interaction terms into the statistical models.
For echocardiographic measures that were significantly related to the primary composite outcomes, we compared their relative prognostic significance by fitting Cox models (for each primary composite outcome) adjusting for the risk factors listed above and then entering the individual candidate echocardiographic variables. We used Uno’s C-statistic to assess the incremental prognostic value of the echocardiographic variables when added to a baseline model incorporating all risk factors.46
In secondary analyses, we investigated the associations between the echocardiographic variables and the incidence of each component of the composite outcomes, i.e., all-cause mortality, CVD, CHF, and AF modeled separately. Participants with the corresponding prevalent condition were excluded from the analyses corresponding to incidence of each outcome. A less conservative p value threshold of <0.05 was used for these exploratory analyses. All analyses were performed using SAS version 9.4 (SAS, Cary, NC).
Results
Baseline Characteristics
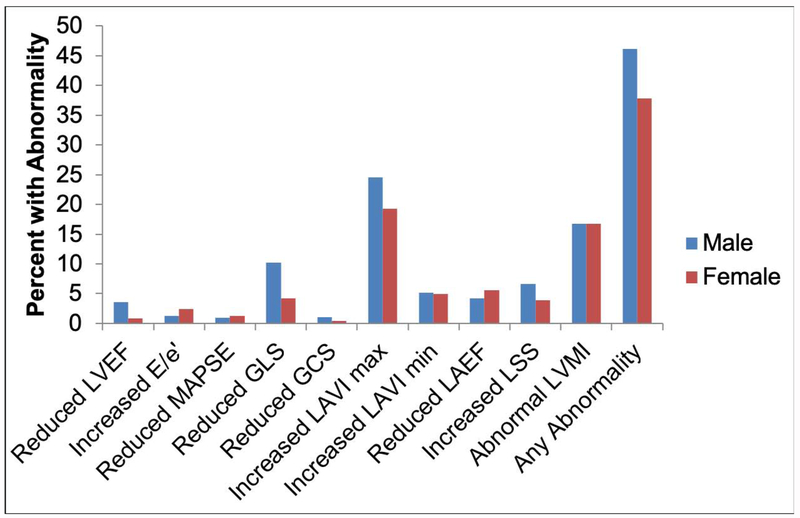
The baseline characteristics of our study sample are presented in Table 1 and the age distribution is shown in Supplementary Figure 2. Overall, our sample included older adults (mean 65 years) and with slightly more women (55.4%) than men. The prevalence of abnormalities for the echocardiographic indices (binary classification based on published reference limits) was overall low (Figure 1), but with a high rate of abnormal LAVImax (22%) and LVMI (17%). The prevalence of abnormal values of GLS and LSS ranged from 5-10%, whereas abnormalities of the other indices were less frequent (<5%).
Table 1.
Baseline characteristics of study sample.
| Men (n=667) | Women (n=830) | |
|---|---|---|
| Cardiovascular Risk Factors | ||
| Age, years | 65 (9) | 65 (9) |
| Height, cm * | 175 (7) | 161 (6) |
| Weight, kg * | 86 (15) | 70 (15) |
| Body mass index, kg/m2 * | 28.2 (4.3) | 27.3 (5.5) |
| Smoking, % | 8 | 9 |
| Systolic Blood Pressure, mm Hg | 128 (16) | 127 (17) |
| Diastolic Blood Pressure, mm Hg * | 75 (10) | 72 (9) |
| Hypertension, % | 56 | 52 |
| Hypertension treatment, % | 47 | 43 |
| Heart Rate, bpm * | 57 (9) | 60 (9) |
| Diabetes, % * | 14 | 8 |
| Serum creatinine, mg/100mL *† | 1.02 (0.31) | 0.81 (0.28) |
| High Density Lipoprotein, mg/100mL * | 51 (15) | 64 (18) |
| Low Density Lipoprotein, mg/100mL * | 102 (30) | 111 (33) |
| Triglycerides, mg/100ml † | 111 (55) | 110 (53) |
| Prevalent CVD, % * | 15 | 10 |
| Echocardiographic Variables | ||
| LV ejection fraction, % * | 66 (7) | 69 (7) |
| E/e’ *† | 6.6 (2.0) | 7.5 (2.2) |
| Mitral Annular Plane Systolic Excursion, cm * | 1.5 (0.2) | 1.5 (0.2) |
| Global longitudinal strain, % * | −19.8 (3.0) | −21.3 (3.1) |
| Global circumferential strain, % * | −30.9 (5.6) | −33.4 (5.8) |
| Left Atrial Emptying Fraction, % | 48 (7) | 47 (8) |
| Minimum Left Atrial Volume Index (mL/m2) † | 15.6 (5.2) | 15.2 (5.3) |
| Maxiumum Left Atrial Volume Index (mL/m2) *† | 29.5 (7.8) | 28.6 (7.8) |
| Longitudinal segmental synchrony, msec *† | 74 (41) | 70 (38) |
| Left Ventricular Mass Index, g/m2 * | 98 (20) | 81 (16) |
Data are shown as means (standard deviation) for continuous variables and as percentage for categorical variables. Prevalent CVD = prevalent cardiovascular disease excluding congestive heart failure (participants suffering from congestive heart failure have been excluded from all analyses)
Significant differences between men and women (p<0.05). Two-tailed, two-sample t-test was used for continuous variables unless otherwise specified. Chi square test was used for binary variables.
Wilcoxon test was used to test differences between men and women.
Figure 1. Prevalence of Abnormal Echocardiographic Variables.
Following cut-off values were used to define abnormal echocardiographic variables: LVEF < 52% in men or < 54% in women;37 E/’E > 13;6 MAPSE < 1.0cm;30 GLS > −16% and GCS > −18%;35 LAVI max > 34 mL/m2;37 LVMI < 49 g/m2 or > 115 g/m2 in men and < 43 g/m2 or > 95 g/m2 in women.37
For LAEF we used the 5th percentile (33%) and for longitudinal segmental synchrony and LAVImin the 95th percentile (151 ms and 25 mL/m2, respectively) of the study sample as cut-off values.
Abbreviations: GCS: Global Circumferential Strain; GLS: Global Longitudinal Strain; LAEF: Left Atrial Emptying Fraction; LAVI: Left Atrial Volume index; LSS: Left Ventricular Longitudinal Segmental Synchrony LVEF: Left Ventricular Ejection Fraction; LVMI: Left Ventricular Mass Index; MAPSE: Mitral Annular Plane Systolic Excursion.
Correlations among the Echocardiographic measures
Generally, age- and sex-adjusted correlations among the echocardiographic variables were weak to moderate (−0.25≤r≤0.25), with the exception of a stronger inverse correlation between LVEF and GCS, and strong pairwise correlations between GLS and GCS, Log E/e’ and MAPSE, and LAEF and LAVImin. The strongest correlation was observed between LAVImin and LAVImax (r=0.9) (Supplementary Table 2).
Associations between Echocardiographic Variables and the Composite Endpoints (Table 2)
Table 2.
Associations between echocardiographic variables (modeled individually) and the composite primary endpoints.
| Composite I (All Cause Mortality + CVD) | Composite II (CHF + AF) | |||
|---|---|---|---|---|
| No. of events/No. At risk | 241/ 1318 | 139/1448 | ||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| LVEF | 0.82 (0.70-0.97) | 0.02 | 0.99 (0.82-1.19) | 0.90 |
| MAPSE | 0.94 (0.83-1.07) | 0.35 | 0.95 (0.81-1.13) | 0.58 |
| GLS | 1.16 (1.01-1.35) | 0.04 | 1.11 (0.95-1.29) | 0.20 |
| GCS | 1.09 (0.94-1.26) | 0.25 | 1.09 (0.90-1.32) | 0.38 |
| Log E/e’ | 1.20 (1.04-1.38) | 0.01 | 1.23 (1.02-1.49) | 0.03 |
| Log LAVI max | 1.26 (1.09-1.46) | 0.002 | 1.54 (1.24-1.92) | <0.0001 |
| Log LAVI min | 1.32 (1.15-1.52) | 0.0001 | 1.70 (1.40-2.08) | <0.0001 |
| LAEF | 0.80 (0.70-0.92) | 0.001 | 0.70 (0.59-0.83) | <0.0001 |
| Log LSS | 1.01 (0.88-1.15) | 0.92 | 1.06 (0.88-1.26) | 0.55 |
| LVMI | 1.21 (1.05-1.40) | 0.008 | 1.20 (1.00-1.45) | 0.05 |
Hazard ratios are for echocardiographic variables modeled individually as continuous variables and are for a SD-deviation increase. P values considered significant after Bonferroni correction (p<0.0025) are bolded.
Models were adjusted for age, sex, cohort, body mass index, current smoking, diabetes, antihypertensive treatment, systolic and diastolic blood pressure, heart rate, creatinine, high-density lipoprotein, low-density lipoprotein, triglycerides.
For composite endpoint I all participants free of CVD were eligible for analysis and for composite endpoint II all participants free of atrial fibrillation. Participants with CHF were excluded from all analyses. LAV min and max were indexed by body surface area.
Abbreviations: AF: atrial fibrillation; CHF: Congestive Heart Failure; CVD: Cardiovascular Disease; GCS: Global Circumferential Strain; GLS: Global Longitudinal Strain; LAEF: Left Atrial Emptying Fraction; LAVI: Left Atrial Volume index; LSS: longitudinal segmental synchrony; LVEF: Left Ventricular Ejection Fraction; LVMI: Left Ventricular Mass Index; MAPSE: Mitral Annular Plane Systolic Excursion.
During follow-up (mean 8.3 years), there were 188 deaths, 129 CVD events, 56 CHF events and 117 AF events. With regard to time to first event analyses, there were 241 events (120 in women) with the composite endpoint of incident CVD or all-cause mortality, and 139 events (63 in women) with the composite endpoint of incident CHF or AF.
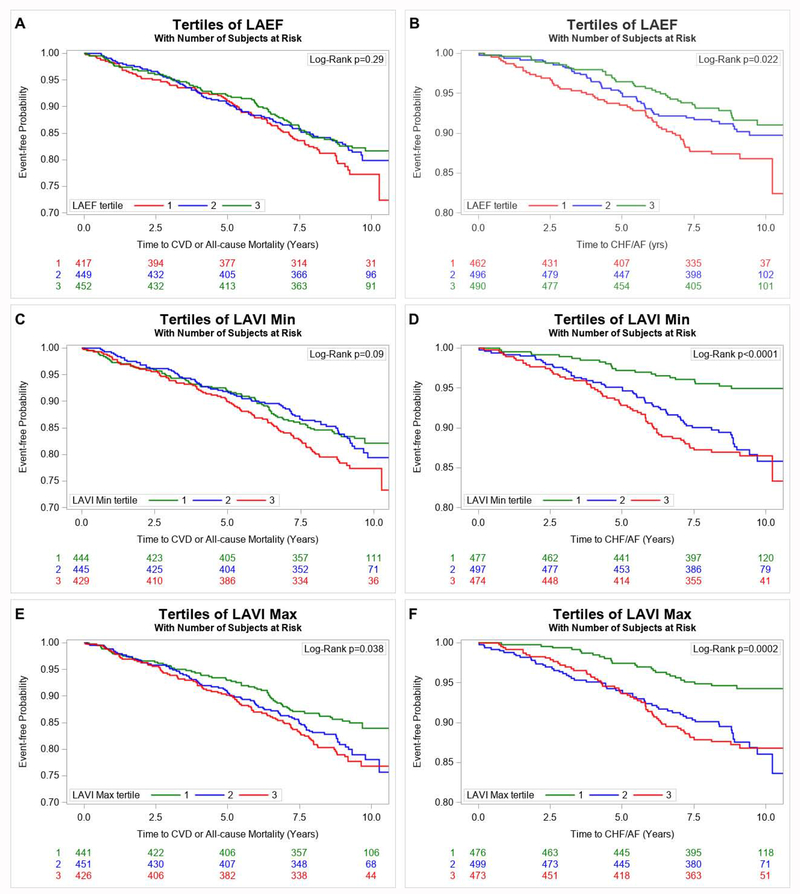
Based on a conservative p value threshold correcting for multiple testing (p<0.0025), higher LAVImin and LAVImax and lower LAEF (each modeled individually) were associated with higher risk of CVD or all-cause mortality as well as with a higher risk of CHF or AF in multivariable-adjusted analyses (Table 2). We did not observe any effect modification by sex. Figure 2 (Panels A–F) displays the cumulative incidence of the composite endpoints, respectively, stratified by tertiles of LAEF, LAVImin and LAVImax. Regression splines confirmed that the aforementioned associations were linear (Supplementary Figures 3–5).
Figure 2.
Kaplan-Meier Plot Comparing Times to Event (Composite Endpoint of All-Cause Mortality or Incidence of CVD, Panel A, Composite Endpoint of Incidence of CHF or AF, Panel B) in participants stratified by tertiles of Left Atrial Emptying Fraction. Kaplan-Meier Plot Comparing Times to Event (Composite Endpoint of All-Cause Mortality or Incidence of CVD, Panel C, Composite Endpoint of Incidence of CHF or AF, Panel D) in participants stratified by tertiles of minimum left atrial volume index. Kaplan–Meier Plot Comparing Times to Event (Composite Endpoint of All-Cause Mortality or Incidence of CVD, Panel E, Composite Endpoint of Incidence of CHF or AF, Panel F) in participants stratified by tertiles of minimum left atrial volume index. The red line shows the most unfavorable tertile, the green line the most optimal tertile.
We modeled the echocardiographic variables that were statistically significant in primary analyses (LAEF, LAVImax, and LAVImin) conjointly, adjusting for CVD risk factors. LAVImax and LAVImin were not included in the same model because they were highly correlated (r=0.9). When LAEF and LAVImax were modeled conjointly, both measures remained associated with both composite endpoints at p<0.05 (Table 3). When LAEF and LAVImin were modeled conjointly, only LAVImin remained associated with both composite endpoints (Table 3).
Table 3.
Associations between LAEF and LAVI max (modeled conjointly) and LAEF and LAVI min (modeled conjointly) and the composite primary endpoints.
| Composite I (All Cause Mortality + CVD) | Composite II (CHF + AF) | |||
|---|---|---|---|---|
| No. of events/No. At risk | 241 /1318 | 139/1448 | ||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Models with LAEF and LAVI max | ||||
| LAEF | 0.84 (0.73-0.96) | 0.01 | 0.74 (0.61-0.89) | 0.001 |
| Log LAVI max | 1.21 (1.04-1.40) | 0.01 | 1.46 (1.18-1.82) | 0.0007 |
| Models with LAEF and LAVI min | ||||
| LAEF | 0.92 (0.77-1.09) | 0.33 | 0.90 (0.70-1.14) | 0.38 |
| Log LAVI min | 1.25 (1.05-1.49) | 0.01 | 1.59 (1.22-2.07) | 0.0006 |
Hazard ratios are for echocardiographic variables modeled as continuous variables and are for a SD-deviation increase. LAEF and LAVI min or LAVI max were modeled conjointly. P values of interest (p<0.05) are bolded.
All models adjusted for age, sex, cohort, body mass index, current smoking, diabetes, antihypertensive treatment, systolic and diastolic blood pressure, heart rate, creatinine, high-density lipoprotein, low-density lipoprotein, triglycerides. The analysis for the endpoint of all-cause mortality was additionally adjusted for prevalence of CVD.
For composite endpoint I all participants free of CVD were eligible for analysis and for composite endpoint II all participants free of atrial fibrillation. Participants with CHF were excluded from all analyses. LAV min and max were indexed by body surface area.
Abbreviations: AF: Atrial Fibrillation; CHF: Congestive Heart Failure; CVD: Cardiovascular Disease; LAEF: Left Atrial Emptying Fraction; LAVI: Left Atrial Volume index;
In terms of incremental predictive utility over models incorporating CVD risk factors alone (base model), the model C-statistic including LAVImax increased from 0.741 for the base model to 0.746 for CVD or all-cause mortality, and from 0.768 to 0.784 including LAVImax for CHF or AF. Addition of LAEF increased the model C-statistic to 0.752 for CVD or all-cause mortality, and to 0.798 for CHF or AF.
For LAVImin, the model C-statistic increased to 0.751 (from 0.741) for CVD or all-cause mortality and to 0.795 (from 0.768) for CHF or AF. Subsequential addition of LAEF increased the model C-statistic only minimally.
Associations between Echocardiographic Variables and Individual Outcomes (Table 4)
Table 4.
Secondary Analysis: Associations between echocardiographic variables and incidence of individual outcomes of all-cause CVD, CHF and AF.
| All-Cause Mortality | CVD | CHF | Atrial Fibrillation | |||||
|---|---|---|---|---|---|---|---|---|
| No. of events/No. At risk | 188/1497 | 129/1318 | 56/1497 | 117/1448 | ||||
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| LVEF | 0.91 (0.78-1.06) | 0.22 | 0.79 (0.65-0.97) | 0.02 | 0.74 (0.57-0.96) | 0.03 | 1.06 (0.87-1.29) | 0.56 |
| MAPSE | 0.87 (0.75-1.00) | 0.0496 | 0.99 (0.81-1.20) | 0.89 | 0.79 (0.58-1.07) | 0.12 | 0.94 (0.79-1.13) | 0.51 |
| GLS | 1.16 (0.99-1.35) | 0.07 | 1.17 (0.95-1.43) | 0.13 | 1.29 (0.97-1.71) | 0.08 | 1.15 (0.98-1.36) | 0.09 |
| GCS | 1.04 (0.90-1.21) | 0.58 | 1.07 (0.86-1.33) | 0.54 | 1.17 (0.85-1.62) | 0.33 | 1.10 (0.90-1.35) | 0.36 |
| Log E/e’ | 1.35 (1.19-1.54) | <0.0001 | 1.16 (0.93-1.43) | 0.19 | 1.64 (1.19-2.26) | 0.003 | 1.30 (1.08-1.55) | 0.005 |
| Log LAVI max | 1.41 (1.17-1.69) | 0.0003 | 1.20 (0.98-1.48) | 0.08 | 2.11 (1.54-2.89) | <0.0001 | 1.48 (1.16-1.88 | 0.001 |
| Log LAVI min | 1.51 (1.28-1.77) | <0.0001 | 1.21 (0.99-1.49) | 0.06 | 2.23 (1.69-2.95) | <0.0001 | 1.64 (1.31-2.05) | <0.0001 |
| LAEF | 0.73 (0.64-0.83) | <0.0001 | 0.88 (0.72-1.09) | 0.23 | 0.62 (0.50-0.76) | <0.0001 | 0.70 (0.57-0.85) | 0.0003 |
| Log LSS | 1.00 (0.86-1.17) | 0.98 | 1.03 (0.88-1.22) | 0.69 | 1.17 (0.94-1.47) | 0.17 | 1.08 (0.89-1.31) | 0.45 |
| LVMI | 1.21 (1.04-1.41) | 0.02 | 1.29 (1.07-1.55) | 0.009 | 1.59 (1.17-2.17) | 0.003 | 1.23 (0.99-1.51) | 0.06 |
Echocardiographic variables modeled individually and continuously per SD-deviation increase. P values of interest (p<0.05) are bolded.
All models adjusted for age, sex, cohort, body mass index, current smoking, diabetes, antihypertensive treatment, systolic and diastolic blood pressure, heart rate, creatinine, high-density lipoprotein, low-density lipoprotein, triglycerides. The analysis for the endpoint of all-cause mortality was additionally adjusted for prevalence of CVD. LAV min and max were indexed by body surface area.
For the endpoint of CVD all participants free of CVD were eligible for analysis and for atrial fibrillation all participants free of atrial fibrillation. Participants with CHF were excluded from all analyses.
Abbreviations: CHF: Congestive Heart Failure; CVD: Cardiovascular Disease; GCS: Global Circumferential Strain; GLS: Global Longitudinal Strain; LAEF: Left Atrial Emptying Fraction; LAVI: Left Atrial Volume index; LSS: Longitudinal Segmental Synchrony; LVEF: Left Ventricular Ejection Fraction; LVMI: Left Ventricular Mass Index; MAPSE: Mitral Annular Plane Systolic Excursion.
In secondary analyses relating the echocardiographic variables (modeled individually) to each of the outcomes evaluated (all-cause mortality, CVD, CHF, and AF), higher E/e’ was associated with a greater risk of all-cause mortality, CHF, and AF whereas higher LVMI was associated with a greater risk of all-cause mortality, CHF, and CVD. Lower LAEF and higher LAVImin and LAVImax were all associated with a higher risk of all-cause mortality, CHF and AF. A higher LVEF was associated with a lower risk of CVD and CHF whereas higher MAPSE was associated with a lower risk for all-cause mortality. GLS, GCS and LSS were not associated with any of the outcomes evaluated.
Discussion
Principal findings
We evaluated the prognostic significance of a panel of novel and conventional echocardiographic indices of cardiac remodeling and function in a large community-based sample. Our principal findings are four-fold. First, the prevalence of asymptomatic LV systolic and diastolic dysfunction, and left atrial dysfunction was low (<10%) while there was a higher prevalence of abnormal LAVImax (22%) and left ventricular hypertrophy (17%) in our older, relatively healthy community-based sample. Second, markers of left atrial size and function (LAVImin, LAVImax and LAEF) emerged as the most prognostically significant variables, being directly (LAVImin and LAVImax) or inversely (LAEF) associated with both the composite outcomes. Third, in secondary analyses, we observed several interesting associations (see below) between the different echocardiographic indices and individual outcomes. Especially E/e’ and LVMI were associated with the hard endpoint of death and, therefore, are important echocardiographic measures in risk stratification (Table 4). Fourth, addition of LAVImin, LAVImax and LAEF to a base model incorporating CVD risk factors resulted in a minimal increase in the C-statistic suggesting that their incremental predictive utility over usual CVD risk factors was limited. It is critical to note that the base model with standard CVD risk factors yielded a high C-statistic for both outcomes, a scenario in which increments contributed by additional variables typically tend to be very modest. It is also important to mention that it was not our intent to build a CVD prediction model, but rather to compare the relative prognostic utility of several echocardiographic measures.
Prognostic Significance of Echocardiographic Indices: Left atrial size and function as key predictors of outcomes
In our investigation, echocardiographic markers of the left atrium emerged as the best predictors of primary outcomes in a head-to-head comparison with other indices. LAEF is a comprehensive measure of global LA function as it summates volumetric changes from end-systole to end-diastole, while LAVImin and LAVImax reflect left atrial size.7 It has been suggested that impairment of LAEF reflects more advanced LA remodeling (relative to LA enlargement) and also reflects the presence of underlying LV diastolic dysfunction.20,47,48 Alterations in LA function can also represent acute changes in load, in addition to reflecting intrinsic LV or LA disease. Recent investigations have underscored the prognostic importance of LA size as well as LA dysfunction, including variations in LAEF and the closely correlated LA functional index, to a range of outcomes, i.e., death, CVD, HF, and AF, in the general population and in other referral samples.13,20,47,49 However, in our investigation LAEF did not add incremental prognostic information over LAVImin (Table 3); the addition of LAEF to a model that already included LAVImin did not lead to a further increase in the model C-statistic. Recent studies have suggested that LA minimal volume may be a more sensitive marker of LV diastolic dysfunction50 and cardiac hemodynamic load as gauged by brain natriuretic peptide elevations.19,51 Our investigation underscored the prognostic importance of left atrial size but we did not observe any incremental prognostic significance for LAEF.
In secondary analyses we observed that LAVImin, LAVImax, and LAEF were not associated with CVD, but with all-cause mortality, CHF, and AF. The endpoint of CVD includes coronary heart disease, cerebrovascular disease, peripheral artery disease, and CHF. Changes in LA function and size might be more related to left ventricular filling pressure or blood pressure changes. This might explain why LA function and size were not associated with atherosclerotic CVD outcomes but were associated with those outcomes that track more closely with elevated LV filling pressures.
Prior studies have neither compared the prognostic significance between LAEF, LAVImin and LAVImax nor compared their prognostic significance with a panel of other echocardiographic measures. It is noteworthy that a recent Framingham publication was more focused on clinical correlates of left atrial function and another one investigated the associations between left atrial function and CVD and AF.41,52In the present investigation, we offer a comprehensive comparative analysis of the prognostic significance of left atrial size and function and a panel of standard echocardiographic measures.
Prognostic Significance of other Echocardiographic Indices
Apart from LAVImin, LAVImax and LAEF, we observed associations between E/e’ and LVMI and components of the composite outcomes (E/e’ with all-cause mortality, CHF, and AF; LVMI with all-cause mortality, CVD, and CHF). If we would have used a more liberal significance level, that did not account for multiple testing, LVMI was associated with the combined endpoint of death and CVD. The association of LVMI with mortality and CVD outcomes, including CHF is well known and has been described in several prior publications.53
LV diastolic dysfunction, as assessed by the E/e’ ratio, has been associated with incident CHF and death.54 However, in two reports from the Copenhagen City Study, E/e’ was not associated with all-cause mortality.9,10 In another study of a Flemish general population-based sample, a borderline statistically significant association of E/e’ with cardiac events was observed that was rendered statistically non-significant upon adjustment for LVMI.11 These prior reports did not compare the prognostic significance of the E/e’ ratio with other echocardiographic indices, especially with markers of left atrial size and function.
We did not observe any association between LVEF and the incidence of primary outcomes but did demonstrate an association with the incidence of CHF and CVD in secondary analyses. This is in line with some previous publications evaluating associations between LVEF and incidence of CHF29 and CVD.55 We did not observe an association of LVEF with all-cause mortality in our sample paralleling findings from the Rotterdam Study.56 Other reports have linked LVEF to mortality but only when it is diminished, i.e., in the presence of LV systolic dysfunction.14,55 The prevalence of LV systolic dysfunction was very low in our sample, consistent with a recent report of declining prevalence of the condition in recent decades.57
Neither GLS nor GCS was associated with either of the primary composite outcomes or with the secondary outcomes in our sample. In contrast to our findings, a higher (less negative) GLS has been associated with a greater incidence of CVD18 and AF12 in other reports; the study samples of these prior investigations were smaller and older.12,18
We did not observe any associations of MAPSE or LSS with any of the primary or secondary outcomes evaluated based on the threshold chosen to indicate statistical signficance. Investigations relating these two echocardiographic measures to outcomes in the community are limited. Diminished MAPSE has been linked to higher mortality risk in individuals with prevalent disease such as myocardial infarction58 or CHF.59 It is conceivable that MAPSE is a predictor of risk only in the presence of preexistent cardiac disease with subtle or greater degrees of impairment of LV systolic function (typically when MAPSE values are 0.8 cm or less).58,59 To our knowledge, only one prior investigation has related LSS to cardiovascular outcomes in a moderate-sized community-based sample (Multi-ethnic Study of Atherosclerosis, MESA),21 and reported an association of LV dyssynchrony (assessed with cardiac magnetic resonance) with an increased risk of CVD events in women (in whom LVMI was not predictive of outcomes) but not in men (in whom LVMI was associated with increased CVD risk). It is conceivable that these different results may be related to the imaging modality used to assess synchrony (echocardiography vs. cardiac magnetic resonance). It is important to note that longitudinal synchrony is a predictor of mortality risk in patients with severe heart failure undergoing cardiac resynchronization therapy.60 It is likely that LSS may be a predictor of outcomes in samples with a greater burden of LV dysfunction.
Strength and Limitations
The large community-based sample and the head-to-head comparison of the prognostic significance of a panel of echocardiographic measurements strengthen our investigation. However, several limitations of our approach merit comment. Even though we included the racially diverse minority FHS Omni 1 cohort, our overall sample was comprised predominantly of whites of European ancestry and was a lower risk sample (being community-based). The exclusion of a moderate proportion of the sample because of unavailable echocardiographic measures is an additional limitation. Therefore, validation of our findings in large multiethnic cohorts and samples with higher vascular risk is necessary. Additionally, since our focus was on a comparative analysis of echocardiographic measures, we did not evaluate the impact of including other biomarkers such as N-terminal pro-brain natriuretic peptide or high-sensitivity cardiac troponins in the statistical models. We acknowledge that using the Teicholz method to measure LVEF and M-mode methods to measure LV and LA dimensions may be suboptimal. However, due to the long follow-up period we offer in our study sample (to accrue enough events to permit meaningful analyses), we were constrained to use older echocardiographic data. We were only able to investigate lateral, but not septal, mitral annulus velocities and we did not investigate LV volume measurements diastolic filling indices, and measures of the right heart. We did not adjust the analyses for LV regional wall motion abnormalities. Only 64 participants in our sample (4.3%) had LV regional wall motion abnormalities so we believe that it is unlikely that our analyses are impacted by the low prevalence of regional wall motion abnormalities. We evaluated the prognostic significance of echocardiographic variables in a stable ambulatory cohort – the prognostic signficance of LAVIi in other settings including acute heart failure have been studied by other investigators.7
Because our study was observational, we cannot make causal inferences and cannot exclude residual confounding.
Conclusions
In our large community-based sample, LAVImin, LAVImax and LAEF emerged as the best echocardiographic predictors of clinical outcomes in a comparative analysis of several measures. Their incremental predictive utility over CVD risk factors was marginal, however, presumably due to a high C-statistic of the base model with standard CVD risk factors. Nonetheless, the predominance of LA function and size as indicators of risk incrementally over other echocardiographic measures suggests a possible central and previously underappreciated role of the left atrium in the progression from subclinical cardiac dysfunction to adverse clinical outcomes. Additional studies of larger and multi-ethnic samples are warranted to confirm our findings.
Relationship with industry
GFM is the owner of Cardiovascular Engineering, Inc—a company that develops and manufactures devices to measure vascular stiffness, and serves as a consultant to and receives honoraria from Novartis, Merck, Servier, and Philips. DDM reports grant support from Philips Healthcare, Samsung Electronics, FlexCon, Bristol-Myers Squibb, Pfizer, Biotronik, and serves as a consultant for Bristol-Myers Squibb, Pfizer, and Samsung. He reports equity stake in MobileSense Technologies.
Supplementary Material
Highlights:
The present investigation compared the prognostic values of a large panel of echocardiographic variables in about 1500 participants of the Framingham Heart Study.
Markers of left atrial size and function emerged as the most prognostically significant variables. In secondary analyses, E/e’, mitral annular plane systolic excursion, left ventricular mass index, and left ventricular ejection fraction also were associated with components of the composite outcomes.
Addition of LAVImin, LAVImax and LAEF to a base model incorporating CVD risk factors resulted in a minimal increase in the C-statistic suggesting that their incremental predictive utility over standard CVD risk factors was limited.
Acknowledgments
Funding
This work was partially supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contracts N01-HC-25195, HHSN268201500001I and 75N92019D00031) and by grants HL076784, G028321, HL070100, HL060040, HL080124, HL071039, HL077447, HL107385, HL126136, 2R01HL092577, 1R01HL128914, 1P50HL120163, R01HL131532 (SC), R01HL134168 (SC); and 2-K24-HL04334. DDM was supported by NIH R01HL126911 and NSF-12-512. BVJ was supported by the “German Heart Foundation / German Foundation of Heart Research”. Dr. Vasan is supported by an Evans Scholar award and Jay and Louis Coffman Foundation from the Department of Medicine, Boston University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the other authors have any conflict of interest to disclose.
References
- 1.Cameli M, Mondillo S, Solari M, Righini FM, Andrei V, Contaldi C, et al. Echocardiographic assessment of left ventricular systolic function: from ejection fraction to torsion. Heart Fail Rev. 2016;21(1):77–94. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Kao DP, Breathett KK, Altman NL, Gorcsan J, Gill EA, et al. Structural and Functional Phenotyping of the Failing Heart. JACC Hear Fail. 2017;5(11):772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marwick TH. Methods used for the assessment of LV systolic function: Common currency or tower of Babel? Heart. 2013;99(15):1078–86. [DOI] [PubMed] [Google Scholar]
- 4.Hu K, Liu D, Herrmann S, Niemann M, Gaudron PD, Voelker W, et al. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging. 2013;14(3):205–12. [DOI] [PubMed] [Google Scholar]
- 5.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese society of echocardiography. Eur J Echocardiogr. 2011;12(3): 167–205. [DOI] [PubMed] [Google Scholar]
- 6.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. [DOI] [PubMed] [Google Scholar]
- 7.Hoit BD. Left atrial size and function: Role in prognosis. J Am Coll Cardiol. 2014;63(6):493–505. [DOI] [PubMed] [Google Scholar]
- 8.Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: Longitudinal data from the heart and soul study. J Am Coll Cardiol. 2012;59(7):673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009; 119(20):2679–85. [DOI] [PubMed] [Google Scholar]
- 10.Mogelvang R, Biering-Sørensen T, Jensen JS. Tissue Doppler echocardiography predicts acute myocardial infarction, heart failure, and cardiovascular death in the general population. Eur Heart J Cardiovasc Imaging. 2015;16(12):1331–7. [DOI] [PubMed] [Google Scholar]
- 11.Kuznetsova T, Thijs L, Knez J, Herbots L, Zhang Z, Staessen JA. Prognostic value of left ventricular diastolic dysfunction in a general population. J Am Heart Assoc. 2014;3(3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo C, Jin Z, Sera F, Lee ES, Homma S, Rundek T, et al. Left ventricular systolic dysfunction by longitudinal strain is an independent predictor of incident atrial fibrillation: A community-based cohort study. Circ Cardiovasc Imaging. 2015;8(8):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surkova E, Badano LP, Genovese D, Cavalli G, Lanera C, Bidviene J, et al. Clinical and Prognostic Implications of Methods and Partition Values Used to Assess Left Atrial Volume by Two-Dimensional Echocardiography. J Am Soc Echocardiogr. 2017;30(11):1119–29. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003; 108(8):977–82. [DOI] [PubMed] [Google Scholar]
- 15.Levy D RJ G, Savage D, Kannel WB, Castelli WP. Prognostic Implications of Echocardiographically Determined Left Ventricular Mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. [DOI] [PubMed] [Google Scholar]
- 16.De Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: The Cardiovascular Health Study. Eur Heart J. 2008;29(6):741–7. [DOI] [PubMed] [Google Scholar]
- 17.Cheng S, Mccabe EL, Larson MG, Merz AA, Osypiuk E, Lehman BT, et al. Distinct Aspects of Left Ventricular Mechanical Function Are All-Cause Mortality in the Community. JAHA. 2015;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biering-sørensen T, Biering-sørensen SR, Olsen FJ, Sengeløv M, Jørgensen PG, Mogelvang R, et al. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population The Copenhagen City Heart Study. Circ Cardiovasc Imaging. 2017;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedberg P, Selmeryd J, Leppert J, Henriksen E. Left atrial minimum volume is more strongly associated with N-terminal pro-B-type natriuretic peptide than the left atrial maximum volume in a community-based sample. Int J Cardiovasc Imaging. 2016;32(3):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, et al. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2013;34(4):278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma RK, Volpe G, Rosen BD, Ambale-Venkatesh B, Donekal S, Fernandes V, et al. Prognostic implications of left ventricular dyssynchrony for major adverse cardiovascular events in asymptomatic women and men: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2014;3(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–90. [DOI] [PubMed] [Google Scholar]
- 23.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: Design, recruitment, and initial examination. Am J Epidemiol. 2007;165(11):1328–35. [DOI] [PubMed] [Google Scholar]
- 24.Quan SF, Howard B V, Iber C, Kiley JP, Nieto FJ, O’Connor GT, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–85. [PubMed] [Google Scholar]
- 25.Kannel WB, Wolf PA, Garrison RJ. The Framingham Study: an epidemiological investigation of cardiovascular disease. Section 34. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements: Framingham Heart Study, 30-yea. Bethesda, Md Natl Hear Lung, Blood Institute, 1987 1987;(87):2703. [Google Scholar]
- 26.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58(6):1072–83. [DOI] [PubMed] [Google Scholar]
- 27.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–8. [DOI] [PubMed] [Google Scholar]
- 28.Teichholz LE, Herman V, Kreulen T, Gorlin R. Problems in Echocardiographic Presence or Absence Volume Determinations: Correlations in the of Asynergy. Am J Cardiol. 1976;37:7–11. [DOI] [PubMed] [Google Scholar]
- 29.Tsao CW, Lyass A, Larson MG, Cheng S, Lam CSP, Aragam JR, et al. Prognosis of Adults With Borderline Left Ventricular Ejection Fraction. JACC Hear Fail. 2016;4(6):502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu K, Liu D, Herrmann S, Niemann M, Gaudron PD, Voelker W, et al. Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging. 2013;14(3):205–12. [DOI] [PubMed] [Google Scholar]
- 31.Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, et al. Reproducibility of speckle-tracking-based strain measures of left ventricular function in a community-based study. J Am Soc Echocardiogr. 2013;26(11):1258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: The atherosclerosis risk in communities stud. Circ Cardiovasc Imaging. 2014;7(1):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng S, McCabe EL, Larson MG, Merz AA, Osypiuk E, Lehman BT, et al. Distinct Aspects of Left Ventricular Mechanical Function Are Differentially Associated With Cardiovascular Outcomes and All-Cause Mortality in the Community. J Am Heart Assoc. 2015;4(10):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, et al. Reproducibility of speckle-tracking-based strain measures of left ventricular function in a community-based study. J Am Soc Echocardiogr. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, et al. Age- and sex-based reference limits and clinical correlates of myocardial strain and synchrony: The framingham heart study. Circ Cardiovasc Imaging. 2013;6(5):692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, et al. Relations of Central Hemodynamics and Aortic Stiffness with Left Ventricular Structure and Function: The Framingham Heart Study. J Am Heart Assoc. 2016;5(3): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–71. [DOI] [PubMed] [Google Scholar]
- 38.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: A meta-analysis. J Am Soc Echocardiogr. 2013;26(2): 185–91. [DOI] [PubMed] [Google Scholar]
- 39.Choi EY, Rosen BD, Fernandes VRS, Yan RT, Yoneyama K, Donekal S, et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: The Multi-Ethnic Study of Atherosclerosis. Eur Heart J. 2013;34(30):2354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartlage GR, Kim JH, Strickland PT, Cheng AC, Ghasemzadeh N, Pernetz MA, et al. The prognostic value of standardized reference values for speckle-tracking global longitudinal strain in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2015;31(3):557–65. [DOI] [PubMed] [Google Scholar]
- 41.Sardana M, Nah G, Tsao CW, Ogunsua AA, Vittinghoff E, Thomas RC, et al. Clinical and Echocardiographic Correlates of Left Atrial Function Index: The Framingham Offspring Study. J Am Soc Echocardiogr. 2017;30(9):904–912.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lubitz SA, Magnani JW, Fontes J, Magnani JW, Rienstra M, Villalon ML, et al. Association Between Familial Atrial Fibrillation and Risk of New-Onset Atrial Fibrillation. Jama. 2010;304(20):2263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKee PA, Castelli WP, McNamara PM, Kannel WB, McKee PA, Castelli WP, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–6. [DOI] [PubMed] [Google Scholar]
- 44.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 45.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet. 2015;386(9989):154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei L. On the C-statistics for Evaluating Overall Adequacy of Risk Prediction Procedures with Censored Survival Data. Stat Med. 2011. ;30(10):1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hedberg P, Selmeryd J, Leppert J, Henriksen E. Long-term prognostic impact of left atrial volumes and emptying fraction in a community-based cohort. Heart. 2016;687–93. [DOI] [PubMed] [Google Scholar]
- 48.Yamano M, Yamano T, Iwamura Y, Nakamura T, Shiraishi H, Shirayama T, et al. Impact of Left Ventricular Diastolic Property on Left Atrial Function from Simultaneous Left Atrial and Ventricular Three-Dimensional Echocardiographic Volume Measurement. Am J Cardiol. 2017;119(10):1687–93. [DOI] [PubMed] [Google Scholar]
- 49.Wong JM, Welles CC, Azarbal F, Whooley MA, Schiller NB, Turakhia MP. Relation of Left Atrial Dysfunction to Ischemic Stroke in Patients With Coronary Heart Disease (from the Heart and Soul Study). Am J Cardiol. 2014; 113(10):1679–84. [DOI] [PubMed] [Google Scholar]
- 50.Russo C, Jin Z, Homma S, Rundek T, Elkind MS V, Sacco RL, et al. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98(10):813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction. J Am Coll Cardiol. 2019;73(15):1961–77. [DOI] [PubMed] [Google Scholar]
- 52.Sardana M, Lessard D, Tsao CW, Parikh NI, Barton BA, Nah G, et al. Association of left atrial function index with atrial fibrillation and cardiovascular disease: The framingham offspring study. J Am Heart Assoc. 2018;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bekwelem W, Misialek JR, Konety S, Solomon SD, Soliman EZ, Loehr LR, et al. Echocardiographic measures of cardiac structure and function are associated with risk of atrial fibrillation in blacks: The Atherosclerosis Risk in Communities (ARIC) study. PLoS One. 2014;9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah AM, Claggett B, Kitzman D, Biering-Sorensen T, Jensen JS, Cheng S, et al. Contemporary Assessment of Left Ventricular Diastolic Function in Older Adults: The Atherosclerosis Risk in Communities Study. Circulation. 2017;135(5):426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandhi J, Gottdiener JS, Bartz TM, Kop WJ, Mehra MR. Comparison of characteristics and outcomes of asymptomatic versus symptomatic left ventricular dysfunction in subjects 65 years old or older (from the Cardiovascular Health Study). Am J Cardiol. 2011;107(11):1667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kardys I, Deckers JW, Stricker BHC, Vletter WB, Hofman A, Witteman JCM. Echocardiographic parameters and all-cause mortality: The Rotterdam Study. Int J Cardiol. 2009;133(2):198–204. [DOI] [PubMed] [Google Scholar]
- 57.Vasan RS, Xanthakis V, Lyass A, Andersson C, Tsao C, Cheng S, et al. Epidemiology of Left Ventricular Systolic Dysfunction and Heart Failure in the Framingham Study. JACC Cardiovasc Imaging. 2017;118529:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brand B, Rydberg E, Ericsson G, Gudmundsson P, Willenheimer R. Prognostication and risk stratification by assessment of left atrioventricular plane displacement in patients with myocardial infarction. Int J Cardiol. 2002;83(1):35–41. [DOI] [PubMed] [Google Scholar]
- 59.Svealv BG, Olofsson EL, Andersson B. Ventricular long-axis function is of major importance for long-term survival in patients with heart failure. Heart. 2008;94(3):284–9. [DOI] [PubMed] [Google Scholar]
- 60.Gorcsan J, Oyenuga O, Habib PJ, Tanaka H, Adelstein EC, Hara H, et al. Relationship of echocardiographic dyssynchrony to long-term survival after cardiac resynchronization therapy. Circulation. 2010;122(19):1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.