Abstract
Background & Aims:
Inherited abnormalities in apolipoprotein E (ApoE) or low-density lipoprotein receptor (LDLR) function result in early onset cardiovascular disease and death. Currently, the only curative therapy available is liver transplantation. Hepatocyte transplantation is a potential alternative; however, physiological levels of hepatocyte engraftment and repopulation require transplanted cells to have a competitive proliferative advantage of over host hepatocytes. Herein, we aimed to test the efficacy and safety of a novel preparative regimen for hepatocyte transplantation.
Methods:
Herein, we used an ApoE-deficient mouse model to test the efficacy of a new regimen for hepatocyte transplantation. We used image-guided external-beam hepatic irradiation targeting the median and right lobes of the liver to enhance cell transplant engraftment. This was combined with administration of the hepatic mitogen GC-1, a thyroid hormone receptor-β agonist mimetic, which was used to promote repopulation.
Results:
The non-invasive preparative regimen of hepatic irradiation and GC-1 was well-tolerated in ApoE−/− mice. This regimen led to robust liver repopulation by transplanted hepatocytes, which was associated with significant reductions in serum cholesterol levels after transplantation. Additionally, in mice receiving this regimen, ApoE was detected in the circulation 4 weeks after treatment and did not induce an immunological response. Importantly, the normalization of serum cholesterol prevented the formation of atherosclerotic plaques in this model.
Conclusions:
Significant hepatic repopulation and the cure of dyslipidemia in this model, using a novel and well-tolerated preparative regimen, demonstrate the clinical potential of applying this method to the treatment of inherited metabolic diseases of the liver.
Lay summary:
Hepatocyte transplantation is a promising alternative to liver transplantation for the treatment of liver diseases. However, it is inefficient, as restricted growth of transplanted cells in the liver limits its therapeutic benefits. Preparative treatments improve the efficiency of this procedure, but no clinically-feasible options are currently available. In this study we develop a novel well-tolerated preparative treatment to improve growth of cells in the liver and then demonstrate that this treatment completely cures an inherited lipid disorder in a mouse model.
Keywords: Hepatocyte transplantation, Cell transplantation, Liver-based metabolic disease, Preparative hepatic irradiation, Dyslipidemia, ApoE, Familial hypercholesterolemia
Graphical abstract
Introduction
Lipid homeostasis requires the uptake of chylomicron remnants and other lipoprotein particles by hepatocytes via binding of apolipoprotein E (ApoE) and apolipoprotein B-100 (ApoB-100) lipoprotein ligands to the low-density lipoprotein (LDL) receptor (LDLR) family.1 Inherited loss-of-function mutations in the LDLR, gain-of-function mutations in PCSK9 or ApoB-100 and/ or ApoE deficiency all lead to familial hypercholesterolemia (FH) with elevated levels of plasma LDL cholesterol, the main cause of atherosclerotic disease.1–6
Until statin treatment became available, mortality rates of patients with heterozygous FH aged 20–29 were up to 125 times greater than in a comparable healthy demographic.7 After statins, the mean age of death for homozygous FH increased by almost 14 years to 31.7, and LDL cholesterol (LDL-C) decreased by 10.0–26.4%.1,8 PCSK9 inhibitors that increase cell surface LDLR levels are highly potent in LDL-C reduction but are ineffective in the absence of ApoE or LDLR. Other novel treatments such as lipoprotein apheresis and mipomersen (an anti-sense oligonucleotide for ApoB mRNA) are important advances but not ideal for long-term clinical use due to high cost (apheresis: ~$100,000 per year, requires weekly procedures).9 Liver transplantation restores normal lipid metabolism as it restores the entire metabolic pathway for cholesterol but this treatment is limited by the shortage in donor organs, cost, and the need for lifelong immunosuppression.10,11
Hepatocyte transplantation (HT) has been investigated as a substitute for liver transplantation to restore hepatic function. It is minimally-invasive, cost-effective and with the advent of induced-pluripotent cell (iPSC) technologies, could potentially enable autografts without the need for immunosuppression.12,13 Treatment of LDLR or ApoE deficiency would require a significant mass of transplanted hepatocytes to achieve curative levels of metabolic restoration. However, only a fraction of transplanted hepatocytes undergo initial engraftment and subsequent liver repopulation (proliferation within the liver parenchyma). We therefore developed a preparative regimen of hepatic irradiation (HIR) to transiently disrupt the endothelial barrier, inactivate Kupffer cells and reduce the mitotic potential of resident hepatocytes in order to enhance engraftment and give transplanted cells a proliferative advantage.14,15 In addition, proof-of-concept studies demonstrated that mitogenic stimuli such as partial hepatectomy or adenoviral expression of HGF were essential for efficient liver repopulation by successfully engrafted cells.12,16,17 Recently, clinical trials have been initiated to test the efficacy of HIR alone for the treatment of metabolic disease (, ), but mitotic stimulation was not given due to a lack of clinically suitable reagents.18 Here we demonstrate that HIR and HT followed by a single 3-week course of GC-1 (sobetirome), a selective thyroid hormone receptor β (TRβ)-agonist,19 support curative levels of repopulation in an ApoE-deficient mouse model of human homozygous monogeneic hypercholesterolemia, resulting in a reduction of serum cholesterol levels to normal or near normal levels. Strikingly, a reduction of atherogenic lipoprotein particles completely prevented atherosclerosis in these mice.
Materials and methods
Animals
Nine to 10-week-old male and female ApoE−/− mice were purchased from JAX (#002052). Animals were weighed every 2 weeks and serum was collected 4 weeks after treatment and every 2 weeks thereafter. Mice were housed in the Institute for Animal Studies at Albert Einstein College of medicine (AECOM) in a temperature-controlled room under a 12/12 h dark / light cycle and fed standard, irradiated chow (PicoLab Mouse Diet 20 5058) on an ad libitum basis. All experiments were performed according to protocols approved by Institutional Animal Care and Use Committee at AECOM. Experiments were performed on the following groups, and were independently repeated: Untreated control (n = 10, male), HIR only (n = 5, male), GC-1 only (n = 9, male), HTHir (n = 13, male), HT-GC-1 (n = 8, male), HTHIR+GC-1 (n = 15, male), HTHIR+AdHGF (n = 5, male). For the HIR dose escalation study, HIR 20 Gy (n = 3, male and female), HIR 30 Gy (n = 5, male and female), HIR 40 Gy (n = 5, male and female), HIR 50 Gy (n = 4, male and female).
GC-1 formulation
GC-1 was purchased from Tocris (cat #4554), reconstituted in DMSO and stored at −2 0 °C. IP injections of 1 mg/kg of GC-1 were given for 21 consecutive days at disperse locations.
AdHGF formulation and administration
5 × 106 particles of AdHGF (Genetic Engineering and Gene Therapy Core of AECOM) were injected into the tail vein 24–48 h post transplantation. AdHGF was produced using the Clontech Adeno-X™ system and validated in previous studies.16,17,20
Hepatocyte isolation and transplant
Primary hepatocytes were isolated at the AECOM Cell Therapy Core from C57/B16 male mice 10–12 weeks old using collagenase perfusion as previously described.21 Only cell isolations with >90% viability were used for HT. Individual treatment groups of max n = 5 were transplanted per day. Hepatocytes were diluted to 107 cells/ml in phenol-free DMEM and kept on ice for max 1 h until transplanted. Cells were gently mixed, drawn into an uncapped plastic syringe (to minimize shearing) which was then capped with a 27 G needle. A total of 100 μl (106 hepatocytes) was transplanted into the spleen of healthy adult mice anesthetized with isoflurane. Buprenorphine was given post-operatively as an analgesic.
Hepatic irradiation
A total of 50 Gy irradiation was administered to the median and superior right lobe of the liver using cone-beam CT-guided imaging in a small animal radiation research platform (xStrahl SARRP). See supplementary information for a detailed description.
Statistical analysis
Statistical analysis was performed using PRISM 7 (GraphPad Software, Inc. La Jolla, CA) statistical analysis software. Data is presented as mean ± standard deviation. Standard 1-way or 2-way analysis of variance was used for comparisons. Multiplicity adjusted p values are represented as ****p <0.0001, ***p <0.002, **p <0.0021, or *p <0.0332.
For further details regarding the materials used, please refer to the CTAT table and supplementary information.
Results
Non-invasive preparative HIR is well tolerated in ApoE−/− mice
To develop a well-tolerated method of conformal HIR, we designed a non-invasive image-guided radiation treatment using cone-beam CT for treatment planning to deliver 50 Gy HIR via 2 rotating arcs to the upper left lateral region of the median lobe and upper right lobe, totaling approximately 30% of the total liver volume liver (Fig. 1A, C). This method creates a steep dose drop-off which reduces the probability of radiation-induced liver damage and toxicity to surrounding organs, such as the stomach, small intestine, kidney, heart, lungs, and spine (Fig. 1A,B).
Fig. 1. Specific HIR targeting to the median and right lobes.
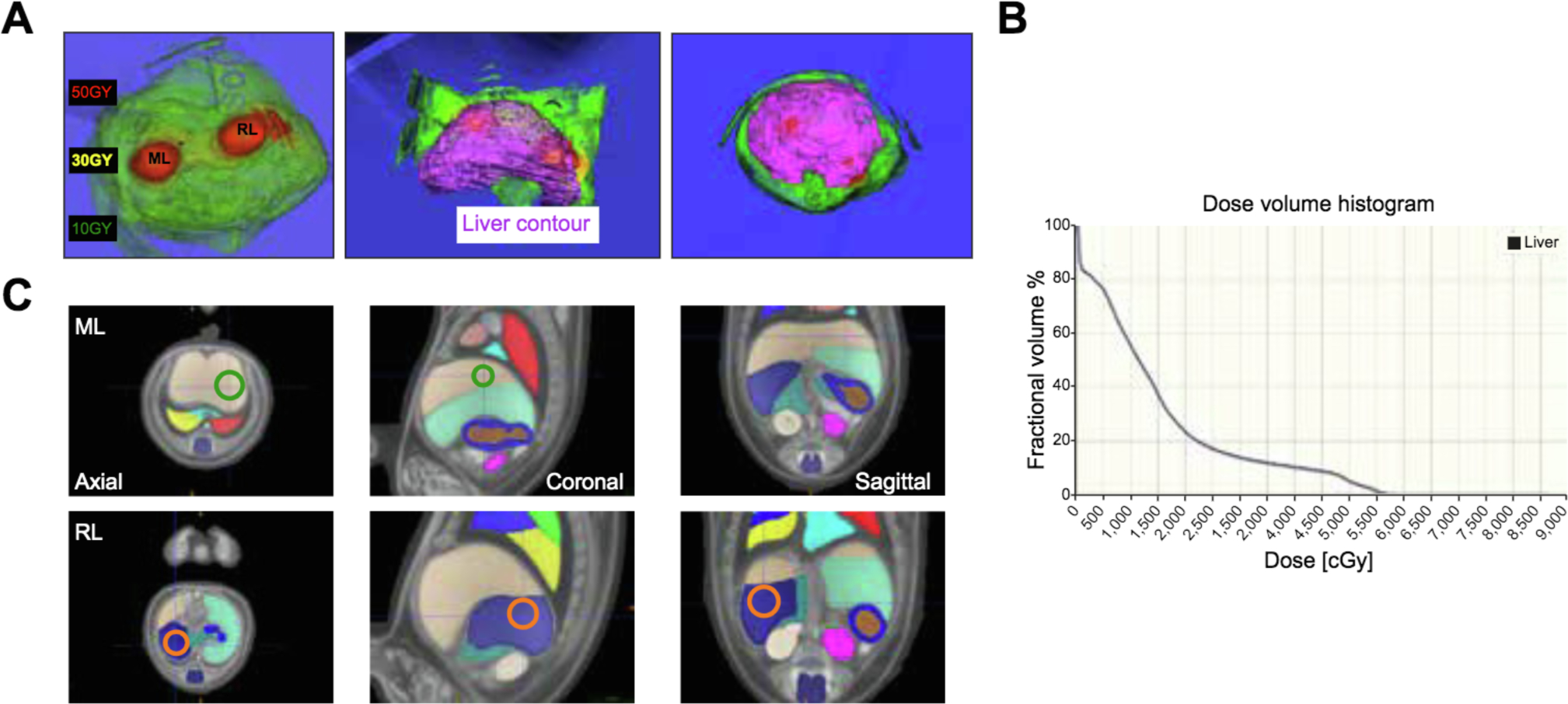
(A) Dose distribution and liver contour. (B) radiation dose/volume histogram. (C) Organs and HIR targets identified using mouse anatomical atlas. Green circle: ML target. Orange circle: RL target. HIR, hepatic irradiation: ML, median lobe; RL, right lobe.
Radiation-induced gastrointestinal syndrome (RIGS) is a potentially lethal acute adverse effect of abdominal irradiation with death between 7–14 days.22 To assess the possibility of RIGS, we treated ApoE−/− mice of both genders with escalating doses (20–50 Gy) of HIR but all irradiated animals survived to day 21, well past the occurrence of RIGS. Furthermore, necropsy at day 21 showed no bleeding, constriction or ulceration of the GI tract (Fig. S1). Together these results demonstrated that our external-beam conformal HIR avoid off-target irradiation of the GI tract.
Atherogenic mice have been shown to have increased sensitivity to irradiation but histological markers of atherosclerosis were absent in the irradiated livers of ApoE−/− mice (Fig. 2B).23–26 A small number of animals were lost over the course of the experiment without any apparent cause of death and we performed necropsy on all animals to examine possible off-target toxicity (Fig. 2B, C). The lung and pancreas were within normal histologic limits while the kidney showed mild to moderate multifocal subcapsular and cortical interstitial fibrosis, consistent with minor radiation-induced fibrosis. Kidney function was assessed using blood urea nitrogen and remained within normal limits over the course of the experiment (Fig. 2D).
Fig. 2. Preparative HIR tolerance in ApoE−/− mice.
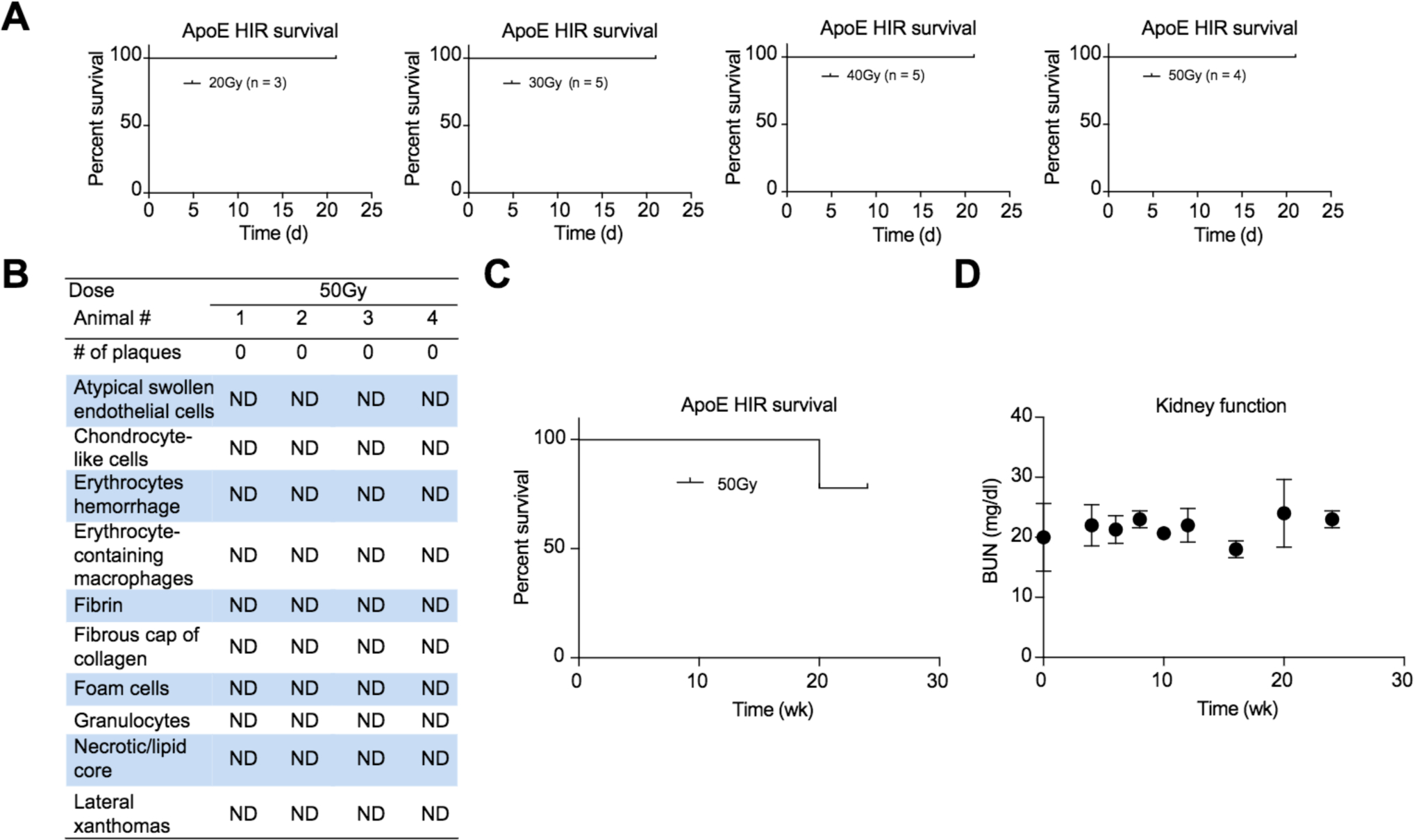
(A) 3-week survival curves after 20–50 Gy HIR. (B) Histological analysis of atherosclerosis formation after 50 Gy HIR (ND = not detected). (C) 24-week survival curve after 50 Gy HIR. (D) Kidney function test over 3 weeks after 50 Gy HIR. HIR, hepatic irradiation.
GC-1 is an effective and well tolerated hepatic mitogen
Previous studies have established that thyroid hormone, T3, is a hepatic mitogen but prolonged administration induces severe cardiac arrhythmia.27–30 In contrast, the synthetic T3 mimetic GC-1, a thyroid hormone receptor β (TR-β) selective agonist, is a similarly strong hepatic mitogen but has no impact on cardiac function.31,32 To verify the mitogenic effect of GC-1, we first tested its effects on primary hepatocytes in vitro and found increased proliferation compared to controls (Fig. S2A). This proliferative effect was abrogated by H89, a protein kinase A (PKA) inhibitor, or NH3, a TR-antagonist (Fig. S2A). GC-1 also led to increased Cyclin D1 levels at 3 h post treatment to the same extent as T3 hormone (Fig. S2B). Together, these results suggest that the mitogenic effects of T3 and GC-1 are identical; induction of TR-β signaling leading to β-catenin phosphorylation by PKA resulting in Cyclin D1 upregulation and proliferation.31,32
We confirmed the mitogenic effect of GC-1 on hepatocyte proliferation in vivo on C57/B16 mice, treated with various combinations of HIR, HT and GC-1, using BrdU incorporation as a measure of proliferation (Fig. 3). In our study, about 5% of cells are actively cycling at any given time in normal liver, that fraction increases to about 15% after GC-1 administration. As expected, HIR suppressed hepatic proliferation to 1–3%, even after GC-1 or HT alone. In contrast, when hepatocytes were transplanted after HIR+GC-1, the proliferative index rose to about 15% in the liver (Fig. 3B,C).
Fig. 3. Hepatic mitogenic effects of GC-1.
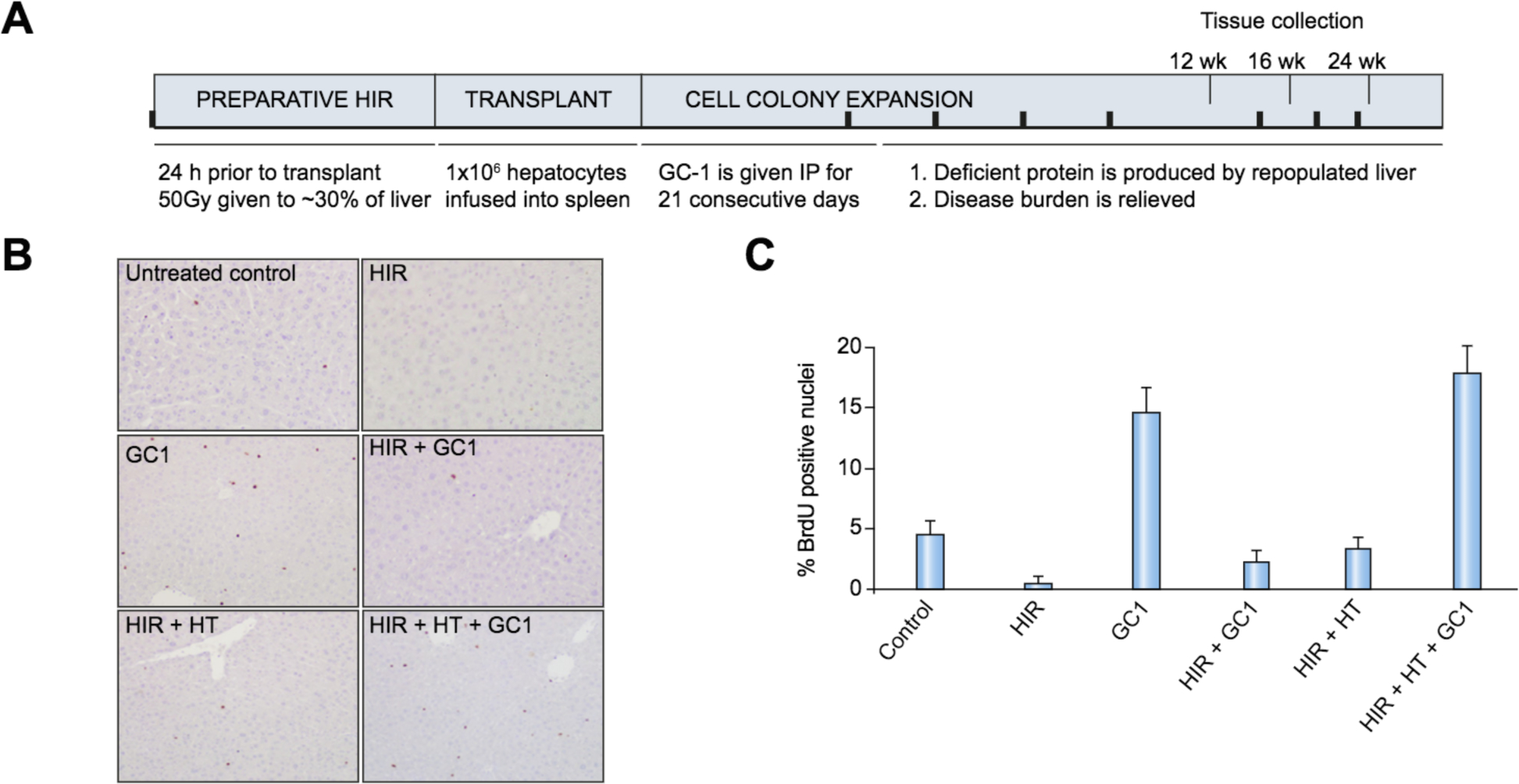
(A). Experimental schema. Black bars: sera collected at baseline, 4 weeks after treatment, and every 2 weeks thereafter. (B). BrdU immunohistochemistry of liver sections. Mice (n = 5) were labeled with BrdU for 24 h, 2 weeks post HIR, ±1 week of GC-1 administration. Livers were sectioned and stained for BrdU. (C). Quantitation of BrdU incorporation. HIR, hepatic irradiation; HT, hepatocyte transplantation; i.p., intraperitoneal.
Preparative HIR+GC-1 significantly reduces serum cholesterol after HT
Hepatocytes were transplanted intrasplenically into ApoE−/− mice, 24 h post HIR followed by 21 consecutive daily doses of GC-1. Blood plasma was collected pre-treatment and at intervals post treatment to determine total cholesterol, LDL and high-density lipoprotein (HDL) cholesterol, and triglycerides (Fig. 3A). As expected from the known cholesterol-lowering effect of GC-1 33,34 all groups receiving GC-1 had an ~10% reduction in total cholesterol over the course of the treatment but only mice receiving HT+HIR and either AdHGF or GC-1 showed a continuous and sustained reduction in cholesterol and triglyceride levels to normal or near normal levels over the 12 week study period (Fig. 4A). The plasma of ApoE−/− mice was turbid before treatment because of high very low-density lipoprotein (VLDL) and chylomicron content but became clear 12 weeks after treatment in the HTHIR+GC-1 group (Fig. 4B). Interestingly, serum cholesterol levels decreased faster with GC-1 as a hepatic mitogen compared to AdHGF (Fig. 4, and Fig. S3) while control groups receiving GC-1 or HTGC-1 alone exhibited a transient reduction of cholesterol levels. A control group receiving HThir but no mitogen showed no improvement (Fig. 4A and Fig. S3).
Fig. 4. Blood cholesterol measurements after HTHIR+mitogen in ApoE−/− mice.
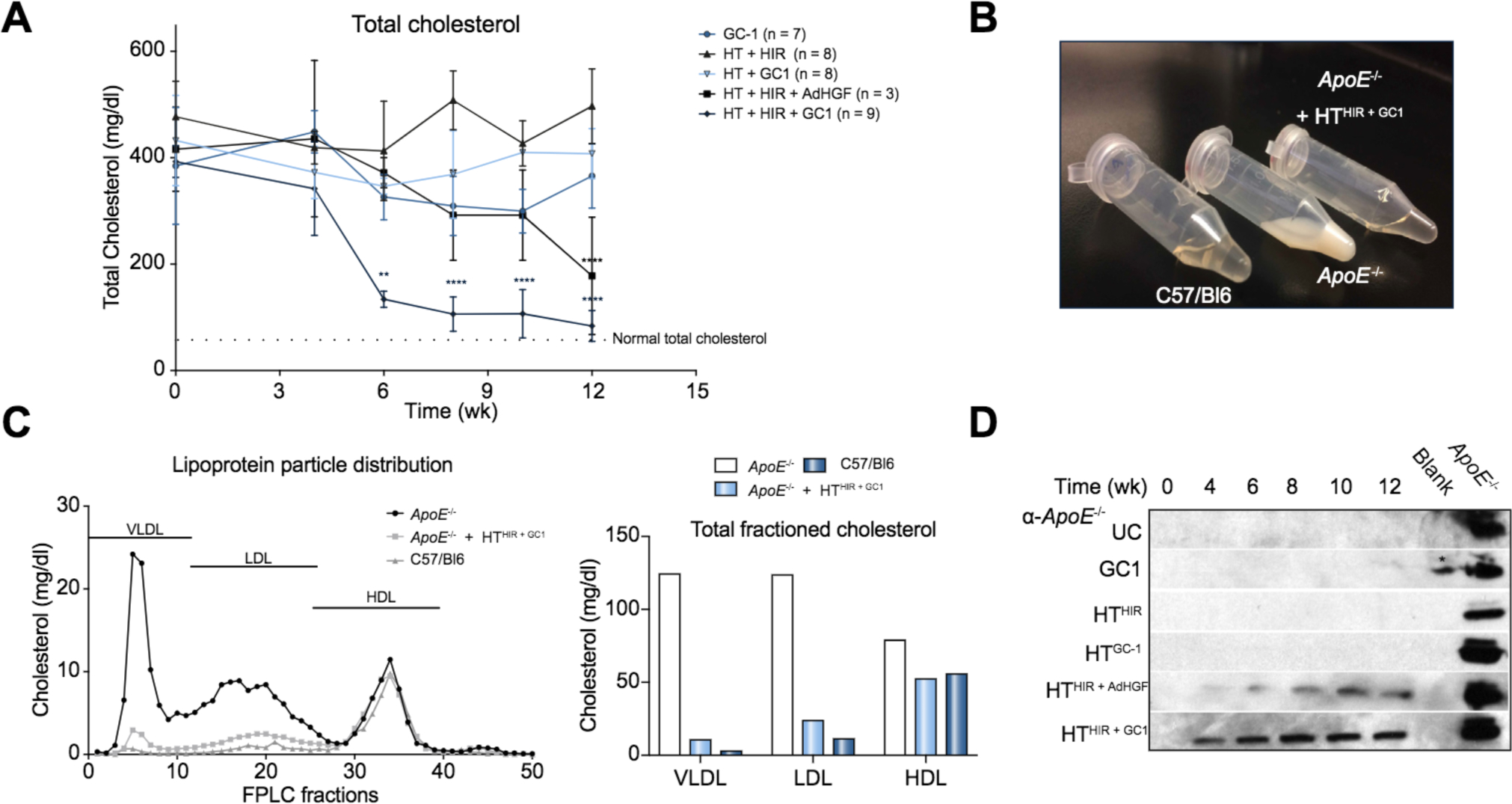
(A) Total cholesterol over 12 weeks post treatment. (B) Pictograph showing raw plasma of C57/B16, ApoE−/− and HTHIR+GC1 -treated mice, 12 weeks post treatment. (C) FPLC analysis of lipoprotein particle size distribution in plasma 16 weeks post treatment. (D). ApoE Western blot. * = spill from positive control. FPLC, fast-protein liquid chromatography; HDL, high-density lipoprotein; HIR, hepatic irradiation; HT, hepatocyte transplantation; LDL, low-density lipoprotein; UC, untreated control; VLDL, very low-density lipoprotein.
Fast-protein liquid chromatography (FPLC) fractionation was performed to analyze plasma lipoprotein particle composition before and after treatment and cholesterol reduction. In wild-type (WT) mice, cholesterol circulates predominantly as HDL particles, whereas the significantly increased cholesterol levels in ApoE−/− circulate mostly as VLDL particles.35 As expected, the vast majority of cholesterol in age and sex-matched control C57/B16 mice (~80%) was present as HDL particles (55.73 mg/dl) and only minor amounts as LDL and VLDL particles (11.24 mg/dl and 2.93 mg/dl respectively) (Fig. 4C). In contrast, the cholesterol in ApoE−/− mice was distributed in all 3 particle categories with a 40-fold increase in VLDL and 10-fold increase in LDL fractions compared with to WT mice: VLDL-124 mg/dl, LDL-123.6 mg/dl (Fig. 4C). HDL levels in ApoE−/− mice were only increased by about 30% (HDL-78.8 mg/dl). Sixteen weeks after HT in the HTHIR+GC1 group, hypercholesteremia in the ApoE−/− mice was resolved to near normal levels (Fig. 4A) and, remarkably, plasma cholesterol particle distribution shifted to a pattern highly similar to WT mice: VLDL (10.6 mg/dl, 92% reduction), LDL (23.8 mg/dl, 81% reduction) and HDL (52.31 mg/dl) (Fig. 4C). This clearly demonstrates that HTHIR+GC1 cures hypercholesterolemia through reduction in VLDL, LDL and to a lesser extent, HDL particles, consistent with the role of ApoE as an LDLR ligand present in VLDL and LDL, but not HDL particles. Six months after treatment, total serum cholesterol in the HTHIR+GC1 treated mice was 87 ± 23 mg/dl while that of controls continued to be elevated ~400 mg/dl (Fig. S3E).
ApoE detection in circulation starting at 4 weeks in animals given HTHIR+GC1
ApoE was detectable in serum by western blot 4 weeks post treatment in mice receiving HTHIR+ADHGF or GC-1, with levels increasing at week 4 and reaching steady-state levels at week 6 (Fig. 4D) with significant cholesterol reduction occurring between week 4–6. Serum ApoE concentrations were higher in the HTHIR+GC1 group compared to the HTHIR+ADHGF group, paralleling the greater cholesterol reduction observed in the HTHIR+GC1 group (fig. 4A). Importantly, although ApoE concentrations in the HTHIR+GC1 group at 12 weeks were still significantly lower compared to WT mice, cholesterol levels were still normalized (Fig. 4A, D) suggesting that the threshold concentration of ApoE required for cholesterol homeostasis is significantly lower than normal concentrations.
Robust engraftment and repopulation of transplanted hepatocytes in animals given HTHIR+mitogen
Animals were sacrificed 12 weeks post treatment and liver tissue was analyzed for repopulation by ApoE immunostaining. In animals receiving HTHIR or HTGC-1 only a few ApoE+ cells were detected as single cells or 2-cell clusters. (Fig. 5A, middle panels). In contrast, mice1 receiving the full treatment protocol (HTHIR+ADHGF or HTHIR+GC-1) had large clusters of ApoE+ cells containing up to 1,000 cells per colony/section restricted to irradiated liver parenchyma, demonstrating HIR dependent robust proliferation of transplanted hepatocytes (Fig. 5A, bottom panels, Fig. S4). Quantitative image analysis shows 10% to 40% ApoE+ within the irradiated liver volume (Fig. 5B). Cell number and cluster size in the AdHGF group were overall smaller compared to GC-1 treated animals, likely due to faster turnover and/or lower levels of HGF vs. the 21-day administration of GC-1. These results correlate with the greater cholesterol reduction and ApoE plasma concentration noted in the HTHIR+GC-1 group (Fig. 4). At 24 weeks post treatment, control groups still showed scant engraftment of 1–2 cells (data not shown), while irradiated liver lobes of HTHIR+GC-1 treated animals showed near total repopulation (Fig. S4D–E).
Fig. 5. Liver repopulation analysis after HTHIR+mitogen.
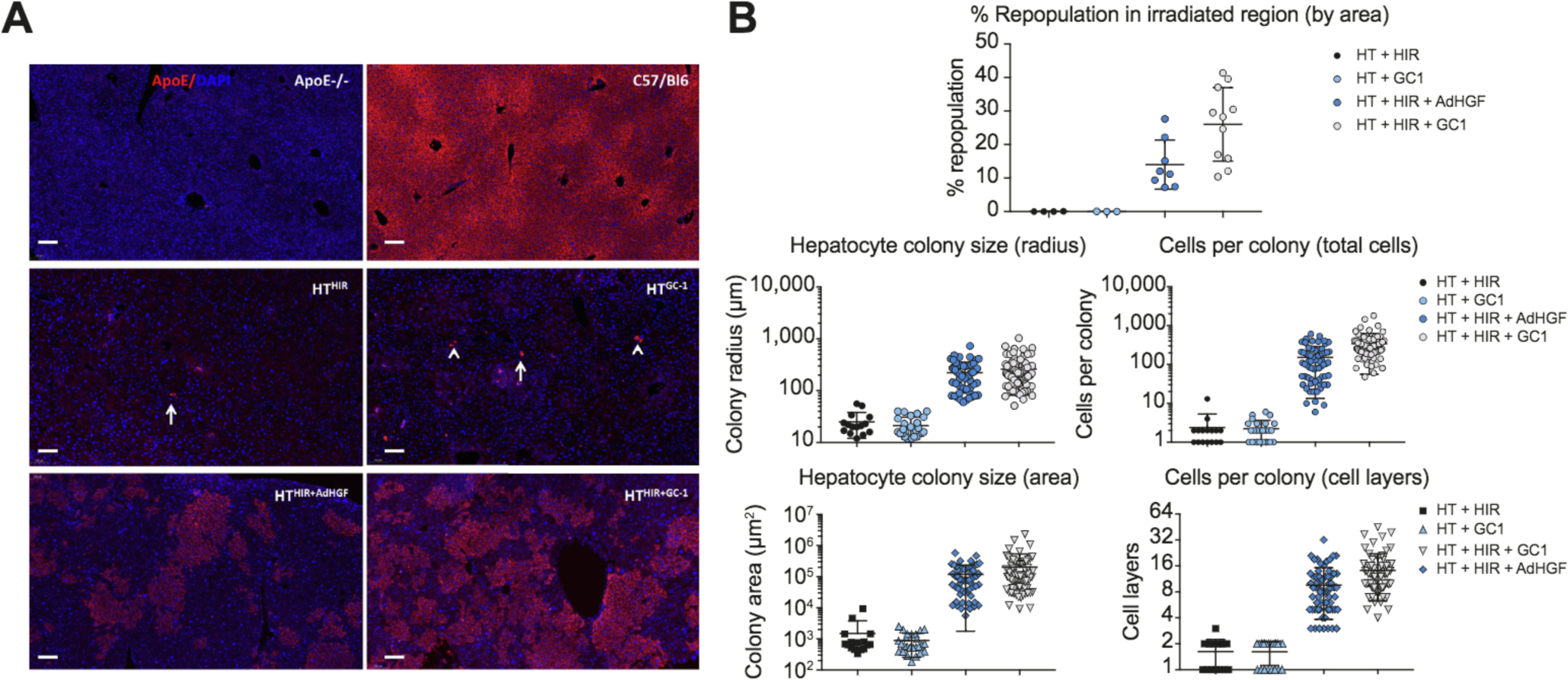
(A) ApoE immunofluorescence of liver sections. Arrow: single cell, arrowhead: cluster of 2 cells. Scale bar = 100 μm. (B) Quantitative analysis of ApoE positive cell clusters. HIR, hepatic irradiation: HT, hepatocyte transplantation.
No α-ApoE antibodies or liver inflammation were detected in transplanted animals
ApoE secreted by transplanted hepatocytes could be potentially recognized as a foreign protein in ApoE null mice. We therefore tested for humoral immune response against ApoE, as well as cell-mediated rejection of the transplanted cells. Western blot showed no detectable antibodies against α-ApoE in sera of mice irrespective of degree of repopulation (Fig. S5A, B). In addition, there were no histological signs of transplant rejection in control or transplanted HTHIR+GC-1 liver tissue (Fig. 6).
Fig. 6. Analysis of immune rejection of repopulating ApoE+ cells in ApoE−/− liver parenchyma.
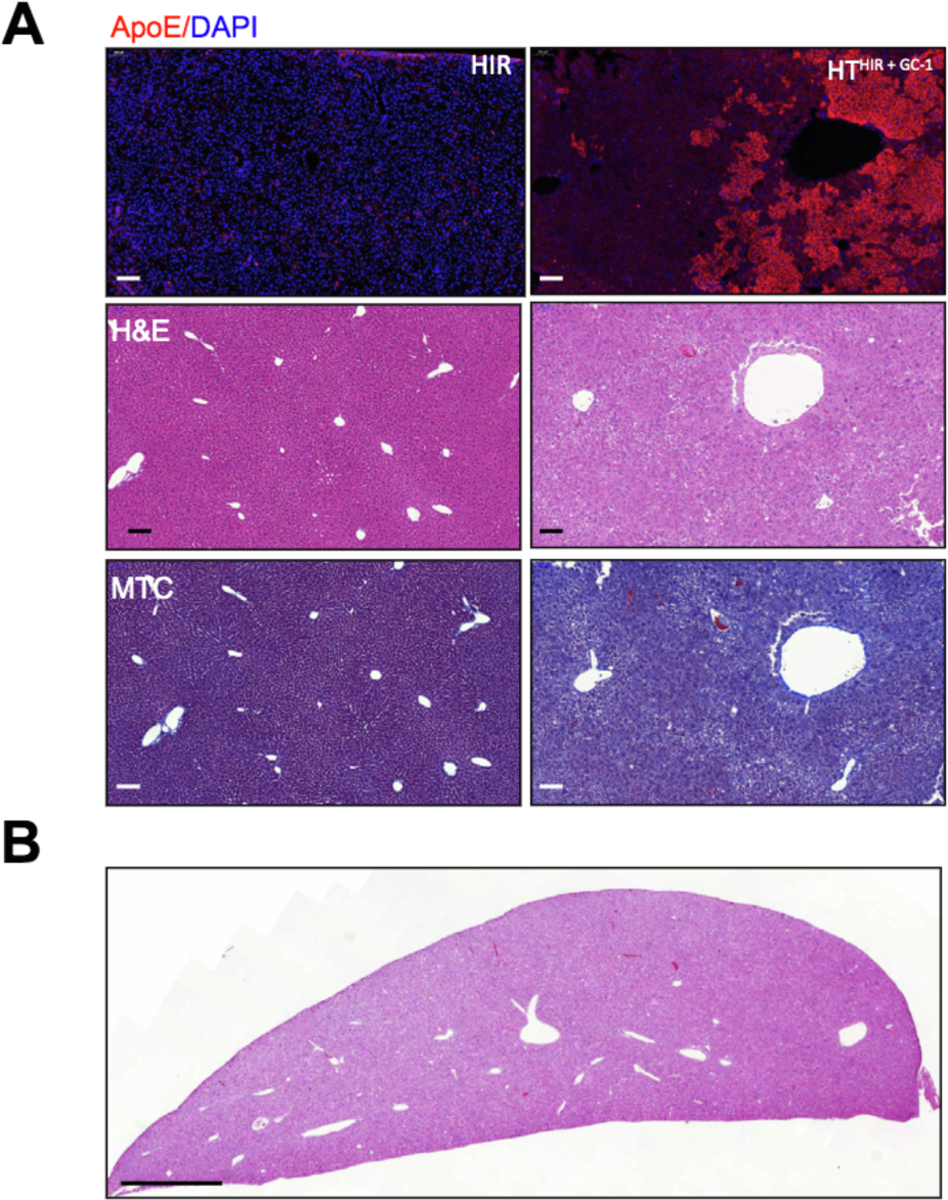
(A) Side-by-side comparison of untreated liver (left side) and repopulated liver (right side) by ApoE immunofluorescence, H&E, and MTC staining of the same region (10×). Scale bar = 100 μm. (B). Low power view of repopulated lobe showing normal histology. Scale bar = 1 mm. H&E, hematoxylin and eosin; HIR, hepatic irradiation; HT, hepatocyte transplantation; MTC, Masson’s trichrome.
Cholesterol reduction prevents formation of atherosclerotic plaques
Cholesterol levels have a strong correlation with atherosclerosis and early mortality in patients with monogeneic dyslipidemias.36 ApoE−/− mice begin to develop fatty streaks in the abdominal aorta at 12 weeks of age35 and invasive atherosclerotic lesions around 5–8 months and we wanted to determine if reduction of cholesterol levels by our liver repopulation protocols had an effect on this process in ApoE−/− mice. Histological analysis of the thoracic aorta in untreated control mice 12 weeks post treatment showed early signs of atherosclerosis, including lesions containing foam cells, granulocytes, necrotic core lesions, and fibrous caps but such lesions were completely absent in HTHIR+GC-1 treated mice (Fig. 7A). Sixteen weeks after treatment, control mice had large, pronounced atherosclerotic plaques with subendothelial accumulation of foamy macrophages, smooth muscle cells hypertrophy, and occasional min-eralization of the vessel wall were detected in the aortic arch of untreated mice while only a few, small plaques were seen in the HTHIR+GC-1 treated group (Fig. 7B). At 24 weeks post treatment, untreated mice had large aortic plaques, thickening of the coronary vessel walls with foam cells, pronounced atherosclerotic accumulations and thickening around the aortic valve (Fig. 7C), consistent with previous reports on ApoE−/− mice fed standard chow or high-fat d iet37 Remarkably, these features were fully absent in the HTHIR+GC-1 group (Fig. 7D) demonstrating that our transplant protocol and associated cholesterol reduction was able to completely resolve atherosclerosis in these mice.
Fig. 7. Atherogenesis in ApoE−/− mice after treatment.
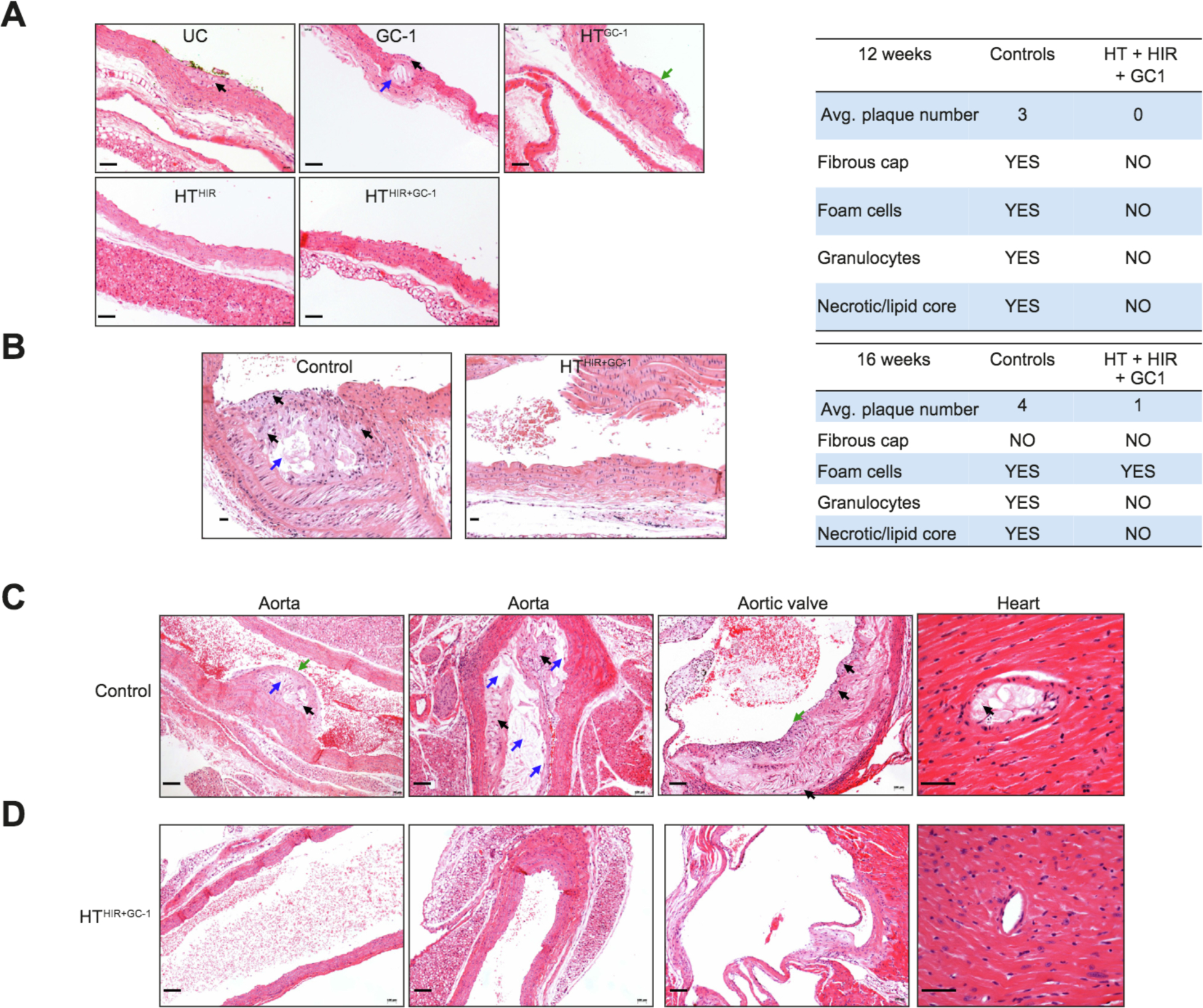
H&E staining was performed to evaluate atheromatous plaque formation in tissue samples from untreated and treated mice. H&E of aorta of untreated ApoE−/− animals and -HTHIR+GC1 treated animals, at 12 weeks (A) and at 16 weeks (B) post treatment. Samples were scored blindly (Table). (C, D). H&E of aorta, aortic valve, and heart tissue at 24 weeks post treatment in HTHIR+GC1 and untreated control animals, respectively. Black arrow: foam cells. Blue arrow: Necrotic core. Green arrow: Fibrous cap. Scale bar=100μm. HIR, hepatic irradiation: HT, hepatocyte transplantation: UC, untreated control.
Discussion
The standard treatment for inherited hypercholesterolemias is daily administration of pharmaceutical agents for the lifetime of the patient. Even with maximum dose therapy, therapeutic guidelines are often not met, and even when they are, the risk of developing early cardiovascular disease remains substantial.38 Liver transplantation is a curative treatment option, but its use is limited by cost, shortage of donor organs and the need for lifelong immunosuppression. In addition, maintaining immune suppressive medication compliance over a lifetime is difficult for adults and in children it is only about 50%.39 Thus, a single treatment with lasting results would be ideal for long-term cholesterol reduction and atherosclerosis prevention.
In the present study, we have demonstrated complete restoration of serum cholesterol metabolism and atherosclerosis prevention in a murine model of a homozygous monogeneic hypercholesterolemia, the ApoE-deficient mouse. ApoE expression was restored to therapeutic levels by a single HT procedure coupled with a well-tolerated preparative regimen consisting of targeted, non-invasive conformal HIR to a part of the liver mass and a post-transplantation 3-week course of GC-1 administration, resulting in substantial liver repopulation by the transplanted cells.
Premature atherosclerotic cardiovascular disease is the main cause of death in patients with inherited hypercholesterolemia. Atherosclerosis is a progressive process dependent on an inflammatory cascade at a primary lesion caused by subintimal cholesterol deposition.40 Early diagnosis, and rapid, consistent treatment is essential for long-term control of atherosclerosis and in young patients with inherited dyslipidemias it would be ideal to prevent the development of atherosclerosis alto-gether.1,6,41 In this study, cholesterol reduction starting at 4–6 weeks after treatment prevented the formation of early atherosclerotic plaques as well as progression of atherosclerosis in the aorta, the aortic valve, and occlusive changes in the coronary vasculature.
We sought to develop and test a therapeutic regimen of HIR, HT and hepatic mitogen towards a clinical application by mini-mizing off-target side-effects and toxicity. In previous studies of preparative HIR, the liver was irradiated after it was exposed with all non-target areas shielded.17 That technique provided a proof-of-concept of the safety and efficacy of preparative partial HIR for HT in an invasive procedure. We therefore evaluated the efficacy and potential toxicides associated with non-invasive external-beam HIR techniques that are currently in clinical use. Liver irradiation requires careful treatment planning due to the radiosensitivity of the liver and adjacent GI tract. We demonstrate that treatment planning targeting approximately 30% of the liver volume using external-beam irradiation is both well tolerated and sufficiently voluminous to allow therapeutic levels of liver repopulation with subsequent reversal of the disease phenotype without off-target effects. In particular, radiation-induced atherosclerosis is more likely to form in irradiated tissue of patients with high atherosclerotic propensity and can cause premature vascular disease but no atherosclerotic features were detected in irradiated liver tissue in this study.23,24,26 However, care should be taken to minimize the radiation dose to muscular arteries, such as the hepatic artery and the renal arteries that are prone to atherosclerosis in any future clinical applications.
We and others have shown that proliferative stimulation is essential for effective liver repopulation but so far sufficiently safe options for clinical use have not been demonstrated.12,16,17,42,43 Partial hepatectomy or adenoviral expression of HGF, although efficient, is not suitable for clinical use. In one study, partial hepatectomy was tolerated in 2 patients with Crigler-Najjar.44 However, it is a highly invasive surgery subject to significant risks and complications. Recombinant HGF is unstable and expensive and viral expression of HGF is biologically hazardous.45,46 Additional safety concerns exist regarding the clinical use of HGF such as serious negative effects on cardiac function and broad and unpredictable tissue effects.47,48 The thyroid hormone, triiodothyronine (T3), is a known hepatic mitogen and has been reported to stimulate proliferation of hepatocytes transplanted into the liver of rats pretreated with the plant alkaloid retrorsine.28,49 However, the required effective dose produced severe tachycardia by direct action of T3 on TRa the T3 receptor dominant in cardiac tissue, which can cause lethal arrythmias. In contrast, GC-1, an FDA approved T3 mimetic, is selective for TRβ, which is preferentially expressed in the liver, with an affinity that is equal to that of endogenous triiodothyronine, whereas its affinity to TRαl is 10-fold lower.19 Therefore, because GC-1 functions as a hepatic mitogen without impacting cardiac function, we evaluated whether it could provide the required proliferative stimuli for transplanted hepatocytes without causing tachycardia associated with thyrotoxicosis.19,32 Notably, we did not find any evidence of hepatotoxicity in mice treated with GC-1 in our study, consistent with reports of extensive pre-clinical and clinical testing.50 GC-1 is currently an investigational drug and there is also an ongoing trial on the use of GC-1 for the treatment of X-linked adrenoleukodystrophy () which will further improve our understanding of its potential clinical use and safety.
Besides its role as a hepatic mitogen, GC-1 also has inherent lipid-lowering activity of its own, making it especially attractive in the context of dyslipidemia treatment.33,34 Although we consistently observed some cholesterol reduction during the GC-1 administration period in all groups, a pronounced and sustained hypocholesterolemic effect was only seen at the 12- and 24- week end points when combined with HIR and HT. The combined HTHIR+GC-1 treatment led to detectable plasma ApoE levels within 4 weeks of treatment and significant reduction of serum cholesterol at 6 weeks. At the 12 week time point, the extent of liver repopulation in the irradiated area of the liver was similar to other studies utilizing preparative HIR in other metabolic disease models such as the UGT1A1-deficient jaundiced Gunn rat and Agxt-deficient mouse model of primary hyperoxaluria type 1.42,51 jn a recent study, iPSC-derived hepatocytes were transplanted into immunodeficient Ldlr−/− mice with 3 Gy preparative HIR but without mitogenic stimulation.52 The comparatively low repopulation reported in that study could have resulted from an HIR dose that was insufficient to reduce the mitotic potential of the host hepatocytes adequately, a lack of mitotic stimulation or both. This highlights the importance of an optimized regimen combining a sufficiently high radiation dose with mitotic stimulation of transplanted cells.
A recent report of a set of HT clinical trials emphasizes the importance of allograft rejection monitoring, as graft loss will negate any therapeutic benefits.18 Although we used congeneic WT donors, the results could still be potentially affected by an immune response against ApoE being recognized as a non-self-antigen by the recipient immune system. However, our assessment of histopathological markers of graft rejection within areas showing repopulation and the tests for antibodies against ApoE showed no evidence of this which could be explained by immune tolerance, which is often induced toward non-self proteins expressed in the liver.53
In summary, our results show that HT coupled with preparative conformal external-beam HIR and a short course of GC-1 administration results in long-term cure of hypercholesterolemia in ApoE−/− mice, preventing the development of atherosclerosis. Importantly, to our knowledge, this is the first report of curative HT in a liver-based metabolic disease model, in which each component of the regimen is well tolerated. Although the recipient mice in our study did not exhibit any significant toxicity, for future clinical application, it will be necessary to assess possible thyroid toxicity, and radiation injury of the liver and large arteries, and to monitor graft immunoreactivity.
Supplementary Material
Highlights.
Conformal hepatic irradiation (HIR) with a hepatic mitogen, GC-1 is safe.
HIR+GC-1 enables massive liver repopulation by transplanted hepatocytes.
HIR+HT+GC-1 leads to complete correction of inherited dyslipidemia.
Preparative regimen of HIR+GC-1 has clinical potential.
Acknowledgements
The authors would like to thank Dr. David S. Neufeld for performing the liver perfusions, and Dr. N. Patrik Brodin for assistance with designing and testing the conformal radiation plan, Laibin Liu for technical assistance, the Biomarker Analytic Research Core (supported by UL1TR001073 and DK020541).
Financial support
M.B was supported by the Medical Scientist Training Program of the Albert Einstein College of Medicine (NIH T32-GM007288). The P250 high capacity scanner was acquired through a shared instrumentation grant (1S100D019961-01). Additional research support to N.R-C by R01 DK092469, P30 DK41296 to J.R-C and U01AI133608 and U01AI138324 to C.G.
Footnotes
Conflict of interest
The authors have no conflicts to declare.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2019.01.010.
References
- [1].Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014;35:2146–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Austin MA, Hutter CM, Zimmern RL, Humphries SE. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol 2004;160:407–420. [DOI] [PubMed] [Google Scholar]
- [3].Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol 2010;55:2833–2842. [DOI] [PubMed] [Google Scholar]
- [4].Garcia CK, Wilund K, Area M, Zuliani G, Fellin R, Maioli M, et al. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 2001;292:1394–1398. [DOI] [PubMed] [Google Scholar]
- [5].Hobbs HH, Russell DW, Brown MS, Goldstein JL. The LDL receptor locus in familial hypercholesterolemia: mutational analysis of a membrane protein. Ann Rev Genetics 1990;24:133–170. [DOI] [PubMed] [Google Scholar]
- [6].Santos RD, Gidding SS, Hegele RA, Cuchel MA, Barter PJ, Watts GF, et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol 2016;4:850–861. [DOI] [PubMed] [Google Scholar]
- [7].Risk of fatal coronary heart disease in familial hypercholesterolaemia. Scientific Steering Committee on behalf of the Simon Broome Register Group. Bmj 1991;303:893–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Raal FJ, Pilcher GJ, Panz VR, van Deventer HE, Brice BC, Blom DJ, et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation 2011;124:2202–2207. [DOI] [PubMed] [Google Scholar]
- [9].Brown WV, Brook R, Hemphill LC, Moriarty PM. The use of lipopheresis in the practice of clinical lipidology. J Clin Lipidolo 2012;6:98–104. [DOI] [PubMed] [Google Scholar]
- [10].Moini M, Mistry P, Schilsky ML Liver transplantation for inherited metabolic disorders of the liver. Curr. Opin. Organ Transplant 2010;15:269–276. [DOI] [PubMed] [Google Scholar]
- [11].Turner RA, Wauthier E, Lozoya O, McClelland R, Bowsher JE, Barbier C, et al. Successful transplantation of human hepatic stem cells with restricted localization to liver using hyaluronan grafts. Hepatology 2013;57:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chen Y, Li Y, Wang X, Zhang W, Sauer V, Chang CJ, et al. Amelioration of hyperbilirubinemia in gunn rats after transplantation of human induced pluripotent stem cell-derived hepatocytes. Stem Cell Rep 2015;5:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roy-Chowdhury N, Wang X, Guha C, Roy-Chowdhury J. Hepatocyte-like cells derived from induced pluripotent stem cells. Hep Inti 2017;11:54–69. [DOI] [PubMed] [Google Scholar]
- [14].Guha C, Parashar B, Deb NJ, Sharma A, Gorla GR, Alfieri A et al. Liver irradiation: a potential preparative regimen for hepatocyte transplantation. Int J Radiat Oncol Biol Phys 2001;49:451–457. [DOI] [PubMed] [Google Scholar]
- [15].Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, et al. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res 1999;59:5871–5874. [PubMed] [Google Scholar]
- [16].Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L Yamamoto T, et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology 2009;49:258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou H, Dong X, Kabarriti R, Chen Y, Avsar Y, Wang X, et al. Single liver lobe repopulation with wildtype hepatocytes using regional hepatic irradiation cures jaundice in Gunn rats. PLoS ONE 2012;7 e46775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Soltys KA, Setoyama K, Tafaleng EN, Soto Gutierrez A Fong J, Fukumitsu K, et al. Host conditioning and rejection monitoring in hepatocyte transplantation in humans. J Hepatol 2017;66:987–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Scanlan TS. Sobetirome: a case history of bench-to-dinic drug discovery and development. Heart Fail Rev 2010;15:177–182. [DOI] [PubMed] [Google Scholar]
- [20].Ding J Yannam GR, Roy-Chowdhury N, Hidvegi T, Basma H, Rennard SI, et al. Spontaneous hepatic repopulation in transgenic mice expressing mutant human alpha 1-antitrypsin by wild-type donor hepatocytes. J Clin Investig 2011;121:1930–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Berry MN, Friend DS. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J. Cell Biol. 1969;43:506–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res 2004;161:123–136. [DOI] [PubMed] [Google Scholar]
- [23].Hoving S, Heeneman S, Gijbels MJ, te Poele JA Russell NS, Daemen MJ, et al. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE(−/−) mice. Int J Radiat Oncol Biol Phys 2008;71:848–857. [DOI] [PubMed] [Google Scholar]
- [24].Hoving S, Heeneman S, Gijbels MJ, Te Poele JA, Visser N, Cleutjens J, et al. Irradiation induces different inflammatory and thrombotic responses in carotid arteries of wildtype C57BL/6J and atherosclerosis-prone ApoE(−/−) mice. Radiother. Oncol 2012;105:365–370. [DOI] [PubMed] [Google Scholar]
- [25].Scharpfenecker M, Kruse JJ, Sprong D, Russell NS, Ten Dijke P, Stewart FA. Ionizing radiation shifts the PAI-l/ID-1 balance and activates notch signaling in endothelial cells. Int J Radiat Oncol Biol Phys 2009;73:506–513. [DOI] [PubMed] [Google Scholar]
- [26].Stewart FA, Heeneman S, Te Poele J, Kruse J, Russell NS, Gijbels M, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE−/− mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am. J. Pathol 2006;168:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fanti M, Singh S, Ledda-Columbano GM, Columbano A Monga SP. Triiodothyronine induces hepatocyte proliferation by protein kinase A-dependent beta-catenin activation in rodents. Hepatology 2014;59:2309–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Francavilla A Carr BI, Azzarone A, Polimeno L, Wang Z, Van Thiel DH, et al. Hepatocyte proliferation and gene expression induced by triiodothyronine in vivo and in vitro. Hepatology 1994;20:1237–1241. [PubMed] [Google Scholar]
- [29].Kowalik MA, Perra A, Pibiri M, Cocco MT, Samarut J, Plateroti M, et al. TRbeta is the critical thyroid hormone receptor isoform in T3-induced proliferation of hepatocytes and pancreatic acinar cells. J Hepatol 2010;53:686–692. [DOI] [PubMed] [Google Scholar]
- [30].Pibiri M, Ledda-Columbano GM, Cossu C, Simbula G, Menegazzi M, Shinozuka H, et al. Cyclin Dl is an early target in hepatocyte proliferation induced by thyroid hormone (T3). FASEBJ 2001;15:1006–1013. [DOI] [PubMed] [Google Scholar]
- [31].Alvarado TF, Puliga E, Preziosi M, Poddar M, Singh S, Columbano A, et al. Thyroid hormone receptor beta agonist induces beta-catenin-dependent hepatocyte proliferation in mice: implications in hepatic regeneration. Gene Expr 2016;17:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Columbano A Pibiri M, Deidda M, Cossu C, Scanlan TS, Chiellini G, et al. The thyroid hormone receptor-beta agonist GC-1 induces cell proliferation in rat liver and pancreas. Endocrinology 2006;147:3211–3218. [DOI] [PubMed] [Google Scholar]
- [33].Baxter JD, Webb P, Grover G, Scanlan TS. Selective activation of thyroid hormone signaling pathways by GC-1: a new approach to controlling cholesterol and body weight. Trends Endocrinol Metabol: TEM 2004;15:154–157. [DOI] [PubMed] [Google Scholar]
- [34].Kannisto K, Rehnmark S, Slatis K, Webb P, Larsson L Gafvels M, et al. The thyroid receptor beta modulator GC-1 reduces atherosclerosis in ApoE deficient mice. Atherosclerosis 2014;237:544–554. [DOI] [PubMed] [Google Scholar]
- [35].Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992;258:468–471. [DOI] [PubMed] [Google Scholar]
- [36].Expert Panel on Integrated Guidelines for Cardiovascular H, Risk Reduction in C, Adolescents, National Heart L, Blood I. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128(Suppl 5):S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang SH, Reddick RL Burkey B, Maeda N. Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein E gene disruption. J Clin Investig 1994;94:937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Defesche JC, Gidding SS, Harada-Shiba M, Hegele RA, Santos RD, Wierzbicki AS. Familial hypercholesterolaemia. Nat Rev Dis Primers 2017; 3:17093. [DOI] [PubMed] [Google Scholar]
- [39].Matsui DM. Drug compliance in pediatrics. Clinical and research issues. Pediatr Clin North Am 1997;44:1–14. [DOI] [PubMed] [Google Scholar]
- [40].Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- [41].Watts GF, Gidding S, Wierzbicki AS, Toth PP, Alonso R Brown WV, et al. Integrated guidance on the care of familial hypercholesterolemia from the International FH Foundation. Clin Lipidol 2014;8:148–172. [DOI] [PubMed] [Google Scholar]
- [42].Guha C, Parashar B, Deb NJ, Garg M, Gorla GR, Singh A, et al. Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology 2002;36:354–362. [DOI] [PubMed] [Google Scholar]
- [43].Krause P, Wolff HA, Rave-Frank M, Schmidberger H, Becker H, Hess CF, et al. Fractionated external beam radiotherapy as a suitable preparative regimen for hepatocyte transplantation after partial hepatectomy. Int J Radiat Oncol Biol Phys 2011;80:1214–1219. [DOI] [PubMed] [Google Scholar]
- [44].Jorns C, Nowak G, Nemeth A, Zemack H, Mork LM, Johansson H, et al. De novo donor-specific HLA antibody formation in two patients With Crigler-Najjar syndrome type I following human hepatocyte transplantation with partial hepatectomy preconditioning. Am J Transplantation 2016;16:1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu CJ, Jones DS 2nd, Tsai PC, Venkataramana A, Cochran JR An engineered dimeric fragment of hepatocyte growth factor is a potent c-MET agonist. FEBS Lett 2014;588:4831–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A et al. Molecular cloning and expression of human hepatocyte growth factor. Nature 1989;342:440–443. [DOI] [PubMed] [Google Scholar]
- [47].Ido A, Moriuchi A Numata M, Murayama T, Teramukai S, Marusawa H, et al. Safety and pharmacokinetics of recombinant human hepatocyte growth factor (rh-HGF) in patients with fulminant hepatitis: a phase I/II clinical trial, following predinical studies to ensure safety. J Transi Med 2011; 9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Japan Acad Series B, Phys Biol Sei 2010;86:588–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Oren R Dabeva MD, Karnezis AN, Petkov PM, Rosencrantz R Sandhu JP, et al. Role of thyroid hormone in stimulating liver repopulation in the rat by transplanted hepatocytes. Hepatology 1999;30:903–913. [DOI] [PubMed] [Google Scholar]
- [50].Lammel Lindemann J, Webb P. Sobetirome: the past, present and questions about the future. Exp Opin Ther Targets 2016;20:145–149. [DOI] [PubMed] [Google Scholar]
- [51].Jiang J, Salido EC, Guha C, Wang X, Moitra R, Liu L, et al. Correction of hyperoxaluria by liver repopulation with hepatocytes in a mouse model of primary hyperoxaluria type-1. Transplantation 2008;85:1253–1260. [DOI] [PubMed] [Google Scholar]
- [52].Yang J, Wang Y, Zhou T, Wong LY, Tian XY, Hong X, et al. Generation of human liver chimeric mice with hepatocytes from familial hypercholesterolemia induced pluripotent stem cells. Stem Cell Rep 2017;8:605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Grant CR, Liberal R Liver immunology: how to reconcile tolerance with autoimmunity. Clin Res Hepatol Gastroenterol 2017;41:6–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


