Abstract
Background
Schizophrenia is usually considered an illness of young adulthood. However, onset after the age of 40 years is reported in 23% of patients hospitalised with schizophrenia. At least 0.1% of the world's elderly population have a diagnosis of late‐onset schizophrenia which seems to differ from earlier onset schizophrenia on a variety of counts including response to antipsychotic drugs.
Objectives
To assess the effects of antipsychotic drugs for elderly people with late‐onset schizophrenia.
Search methods
We searched the Cochrane Schizophrenia Group Trials Register (January 2010) which is based on regular searches of CINAHL, EMBASE, MEDLINE and PsycINFO. We inspected references of all identified studies for further trials. We contacted relevant authors of trials for additional information.
We updated this search Janurary 2013 and added 48 new trials to the awaiting classification section.
Selection criteria
All relevant randomised controlled trials that compared antipsychotic drugs with other treatments for elderly people (at least 80% older than 65 years) with a recent (within five years) diagnosis of schizophrenia or schizophrenia like illnesses.
Data collection and analysis
For the 2010 search, two new review authors (AE, AG) inspected all citations to ensure reliable selection. We assessed methodological quality of trials using the criteria recommended in the Cochrane Handbook for Systematic Reviews of Interventions. AE and AG also independently extracted data. For homogenous dichotomous data, we planned to calculate the relative risk (RR) and 95% confidence interval (CI).
Main results
There were no included studies in the original version of this review (2002 search). The 2010 search for the current update produced 211 references, among which we identified 88 studies. Only one study met the inclusion criteria and was of acceptable quality. This was an eight‐week randomised trial of risperidone and olanzapine in 44 inpatients with late‐onset schizophrenia. All participants completed the eight‐week trial, indicating that both drugs were well tolerated. Unfortunately, this study provided little usable data. We excluded a total of 81 studies, 77 studies because they either studied interventions other than antipsychotic medication or because they involved elderly people with chronic ‐ not late‐onset ‐ schizophrenia. We excluded a further four trials of antipsychotics in late‐onset schizophrenia because of flawed design. Five studies are still awaiting classification, and one is on‐going.
Authors' conclusions
There is no trial‐based evidence upon which to base guidelines for the treatment of late‐onset schizophrenia. There is a need for good quality‐controlled clinical trials into the effects of antipsychotics for this group. Such trials are possible. Until they are undertaken, people with late‐onset schizophrenia will be treated by doctors using clinical judgement and habit to guide prescribing.
Note: the 48 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.
Keywords: Aged, Female, Humans, Male, Middle Aged, Age of Onset, Antipsychotic Agents, Antipsychotic Agents/therapeutic use, Benzodiazepines, Benzodiazepines/therapeutic use, Olanzapine, Randomized Controlled Trials as Topic, Risperidone, Risperidone/therapeutic use, Schizophrenia, Schizophrenia/drug therapy
Plain language summary
Antipsychotic drugs for elderly people with late‐onset schizophrenia
A significant proportion of the world's growing elderly population suffers from schizophrenia that started very late in life. Antipsychotic drugs are often used to treat this distressing and severe illness. In this review we attempted to find good quality trial‐based evidence to support this practice but found none. Currently this vulnerable group is not well served by the research community.
Background
Description of the condition
Although schizophrenia is commonly thought of as an illness of young adulthood, it can both extend into and first appear in later life. It has been recognised that schizophrenia may have a late‐onset ever since schizophrenia was first described (Kraepelin 1909), and contemporary diagnostic criteria for schizophrenia, such as DSM‐IV (Diagnostic and Statistical Manual of Mental Disorders) and ICD‐10 (International Classification of Diseases), place no restrictions on age of onset for a diagnosis to be made.
Variously referred to as paraphrenia, late paraphrenia, paranoia, involutional paranoid disorder and late‐onset schizophrenia, there has been no general agreement on the definition of late onset. Different studies defined late‐onset as onset after 40, 45, 60, or 65 years of age (Harris 1988). The International Late‐Onset Schizophrenia Group Consensus Statement recommended the diagnoses of late‐onset schizophrenia (illness onset after 40 years of age) and very‐late‐onset schizophrenia‐like psychosis (onset after 60 years) (Howard 1992b; Howard 1993; Howard 2000). The diagnosis of late‐onset schizophrenia is further complicated by the fact that it is often difficult to determine the age of onset of schizophrenia, especially in older people (Harris 1988a).
At least 12% of elderly people in the community have mental disorders, and about 0.1% of them have a diagnosis of late‐onset schizophrenia (Regier 2000). Only 5.7% of people with late‐onset schizophrenia‐like psychosis live in their own home and 24% live in sheltered accommodation (Henderson 1998). Twenty‐three per cent of patients hospitalised for schizophrenia were reported to have had onset of their illness after the age of 40; 13% in their forties, 7% in their fifties, and 3% after age 60 (Harris 1988). The prevalence of late‐onset schizophrenia‐like psychosis within a community has been reported to be as high as 7.5% (Henderson 1998). The estimated one‐year prevalence rate of schizophrenia in individuals between ages 45 and 64 is 0.6% (Keith 1991), and between 0.1% to 0.5% in individuals over 65 years of age (Castle 1993; Copeland 1992; Copeland 1998; Kua 1992). The incidence rate of DSM‐III‐R‐defined schizophrenia with first onset after 44 years is reported to be 12.6 per 100,000 population per year (Copeland 1998).
Description of the intervention
Neuroleptics remain the most effective treatment for schizophrenia, but their use in elderly people with psychosis is based on clinical data extrapolated from studies of these agents in younger populations (Devanand 1998; Frenchman 1997; Katz 1999). An extremely limited body of published studies suggests that the benefits of neuroleptics may extend to elderly people with late‐life schizophrenia (Jeste 1993). Elderly people with chronic schizophrenia have been shown to benefit from typical neuroleptics such as acetophenazine, trifluoperazine, haloperidol, fluphenazine and thioridazine (Branchey 1978; Honigfeld 1965; Tsuang 1971). The newer atypical antipsychotics are thought to be well tolerated, since they have less potential for producing extrapyramidal side effects and tardive dyskinesias (Jeste 1996; Jeste 1998; Jeste 1999; Jeste 1999a). The atypical antipsychotics do have some side effects of their own; clozapine has a high risk of spontaneous white blood cell decline (agranulocytosis), olanzapine causes significant weight gain (Duggan 2001) and possibly induces diabetes (Koro 2002; Wirshing 1998). Risperidone can also cause significant weight gain (Allison 1999), and movement disorders (Gilbody 2002; Kennedy 2001). In addition, neuroleptics do not produce complete remission of all the symptoms of schizophrenia in most patients. In one series of people with late‐onset schizophrenia, 24% did not show any signs of improvement after typical antipsychotic treatment (Pearlson 1989). Another series found that, after a minimum of three months of typical antipsychotic treatment, about 42% of people with late‐onset schizophrenia‐like psychosis showed no response, 31% of people partially improved, and 27% had remission of their symptoms (Howard 1992a). The foremost barriers to the widespread use of atypical antipsychotic medications in older adults are (1) the lack of well‐designed and well‐conducted studies to demonstrate the effectiveness and safety of these medications in older patients with multiple medical conditions, and (2) the higher cost of these medications relative to typical neuroleptics (Thomas 1998).
How the intervention might work
Neuroleptic drugs are generally thought to work through blocking certain neurotransmitter receptors in the brain. The ability of typical neuroleptics to diminish psychotic symptoms was shown to be initiated by blockade of dopamine D2 receptors in defined areas of the brain (Creese 1976; Davis 1991; Meltzer 1976). Atypical neuroleptics cause potent serotonin (5‐hydroxytryptamine, 5‐HT) receptor subtype 5‐HT2A relative to DA D2 receptor blockade (Arndt 1998; Meltzer 1989), with the exception of aripiprazole, a partial dopamine agonist (Lawler 1999), and amisulpride, a selective D2/D3 antagonist (Di Giovanni 1998).
Why it is important to do this review
The elderly population is expected to grow substantially worldwide. The number of people over 65 increased from about 17 million in 1900 to 342 million in 1992, and is expected to increase to 2.5 billion (20% of the total population) by 2050 (Olshansky 1993). The aging of the general population will be associated with ever elevated numbers of elderly people with late‐onset schizophrenia; the annual incidence of schizophrenia‐like psychosis in people over the age of 60 increases by 11% with each five‐year increase in age (Van Os 1995). Accordingly, the treatment of late‐onset schizophrenia is gaining increased importance, and a review of the effects of neuroleptics on people with late‐onset schizophrenia is particularly important for at least two reasons. First, pharmacotherapy in the older patient is complicated by alterations in pharmacokinetic and pharmacodynamic responses; small doses of neuroleptics are usually sufficient for improvement in older patients, and particular side effects might prove dangerous (Lohr 1992). Second, most published studies do not distinguish between people with late‐onset schizophrenia and elderly people with early‐onset schizophrenia despite studies showing that the inclusion of late‐onset schizophrenia into the diagnosis of early‐onset schizophrenia or delusional disorder has poor empirical and theoretical bases (Almeida 1995). Compared with early‐onset schizophrenia, late‐onset schizophrenia shows a two to 10 times higher prevalence in women than in men (Harris 1988), has a different genetic risk (Howard 1997), has a different symptom profile, (DeLisi 1992; Howard 1993a) and has a better prognosis (Castle 1991; Harris 1988). People with late‐onset schizophrenia seem to respond differently to treatment in comparison with people with an earlier onset of illness (Jeste 1995; Jeste 1997; Jeste 1998; Lohr 1997)
Objectives
To review the beneficial and harmful effects of antipsychotic drugs for elderly people with late‐onset schizophrenia‐like psychosis.
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials. Where trials were described as 'double‐blind' and it was implied that the studies were randomised, we planned to include these trials in a sensitivity analysis. If there was no substantive difference within primary outcomes when these 'implied randomisation' studies were added, then we would have included them in the final analysis. If there was a substantive difference, we planned to use only clearly randomised trials and the results of the sensitivity analysis described in the text. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week.
Types of participants
Elderly people (at least 80% of whom should be over 65 years of age) with a recent (within five years) diagnosis of schizophrenia or schizophrenia like illness such as delusional disorder, schizoaffective disorder, schizophreniform psychosis or paraphrenia.
Types of interventions
Any typical or atypical antipsychotic drug, at any dose and mode or pattern of administration, compared to placebo, no intervention or to any other typical or atypical antipsychotic drug, at any dose and mode or pattern of administration. For the purposes of the planned sensitivity analyses outlined below, we defined low doses as follows; amisulpride, aripiprazole, clozapine (< 50 mg/day), iloperidone (< 4 mg/day), olanzapine (< 5 mg/day), quetiapine (< 50 mg/day), risperidone (< 2 mg/day) , sulpiride, ziprasidone (< 40mg/day), zotepine (< 100 mg/day).
Types of outcome measures
The patient‐oriented outcome measures that are important for this review are mortality (suicide or natural causes), global clinical response as defined by each included study, quality of life, adverse events, cost and service utilisation ( hospital admission, number of days in hospital). As schizophrenia is often a life‐long illness, and antipsychotic drugs are used as an ongoing treatment, we grouped outcomes according to time periods into acute (less than six weeks), short‐term (less than three months), medium‐term (three to six months) and long‐term outcomes (more than 6 months).
Primary outcomes
1. Suicide
2. Hospital admission
3. Clinically significant response in global state ‐ as defined by each of the studies
4. Clinically significant response in mental state ‐ as defined by each of the studies
5. Leaving the study early
Secondary outcomes
1. Death due to natural causes
2. Service utilisation as measured by the number of days in hospital
3. Average score/change in global state
4. Mental state
4.1 Average score/change in mental state 4.2 Clinically significant response in positive symptoms‐ as defined by each of the studies 4.3 Average score/change in positive symptoms 4.4 Clinically significant response on negative symptoms‐ as defined by each of the studies 4.5 Average score/change in negative symptoms
5. Behaviour
5.1 Clinically significant response in behaviour ‐ as defined by each of the studies 5.2 Average score/change in behaviour
6. Extrapyramidal side effects
6.1 Incidence use of antiparkinson drugs 6.2 Clinically significant extrapyramidal side effects ‐ as defined by each of the studies 6.3 Average score/change in extrapyramidal side effects
7. Other adverse effects, general and specific
7.1 Number of patients leaving the study early due to adverse effects 7.2 Cardiac effects 7.3 Anticholinergic effects 7.4 Antihistamine effects 7.5 Prolactin related symptoms
8. Social functioning
8.1 Clinically significant response in social functioning ‐ as defined by each of the studies 8.2 Average score/change in social functioning
9. Economic outcomes
10. Quality of life
10.1 Quality of life/ satisfaction with care for either recipients of care or carers 10.2 Significant change in quality of life/satisfaction ‐ as defined by each of the studies 10.3 Average score/change in quality of life/satisfaction
11. Cognitive functioning
Search methods for identification of studies
Electronic searches
1. Cochrane Schizophrenia Group Trials Register (January 2010)
We searched the register using the phrase:
[((old* or * old * in title) or (*elderly* or *paraphrenia* or *late onset* in title, abstract, and index terms or title) or ((aged* in index terms) terms of REFERENCE) or (elderly in participants of STUDY))].
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module).
For previous electronic searches for earlier versions of this review, please see Appendix 1.
2. Cochrane Schizophrenia Group Trials Register (January 2013)
The Trials Search Co‐ordinator searched the Cochrane Schizophrenia Group’s Trials Register (9th Janurary 2013) using the phrase
[(* old * in title) or (*older adult* or *elderly* or *paraphrenia* or *late onset* in title, abstract, and index terms of REFERENCE) or (*older adults* in participants of STUDY))].
The Cochrane Schizophrenia Group’s Trials Register is compiled by systematic searches of major databases, handsearches and conference proceedings (see group module).
Searching other resources
Please see Appendix 1.
Data collection and analysis
For previous data and collection methods, please see Appendix 2.
Selection of studies
In order to ensure reliable selection, two review authors, AE and GA independently inspected all study citations identified by the searches and obtained full reports of the studies of agreed relevance. Where disputes arose, we resolved disagreements by discussion or, if doubt remained, by acquiring the full article for more detailed scrutiny. If doubt still remained, we added these trials to the list of those awaiting assessment pending acquisition of further information and we contacted the authors of studies. For details of previous author contributions in study selection see Acknowledgements.
Data extraction and management
Data extraction
We extracted data from included trials onto standard, simple forms. The review author GA extracted data from the included study, helped by Mohannad Othman, and this was inspected by AE. We discussed disagreements, documented our decisions and, if necessary, contacted the authors of studies for clarification. When uncertainty persisted, we allocated the trial to Studies awaiting classification while we contacted the authors.
Summary of findings table
If usable data were available, we planned to use the GRADE approach to interpret findings (Schünemann 2008 and used GRADE profiler (GRADEPRO) to import data from RevMan 5 (Review Manager) to create a 'Summary of findings' tables. These tables provide outcome‐specific information concerning the overall quality of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient‐care and decision making.
Assessment of risk of bias in included studies
Working independently, AE and GA assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome, the completeness of outcome data, selective reporting and other biases. Accordingly, we categorised the risk of bias in every study into low, high or unclear risk of bias. We did not include studies where sequence generation was at high risk of bias or where allocation was clearly not concealed. If disputes arose as to which category a trial has to be allocated, again, we resolved this by discussion.
Earlier versions of this review used a different, less well‐developed, means of categorising risk of bias (see Appendix 3).
Measures of treatment effect
Dichotomous (binary) data:
For binary (yes/no) outcomes, we planned to calculate a standard estimation of the fixed‐effect risk ratio (RR) and its 95% confidence interval (95% CI). If heterogeneity had been found, we would have used a random‐effects model.
Continuous data:
We included continuous data from rating scales in this review only if the measuring instrument had been described in a peer‐reviewed journal and the instrument was either a self‐report or completed by an independent rater or relative (not the therapist). Where possible, we made efforts to convert continuous outcome measures to binary data by identifying cut‐off points on rating scales and dividing participants accordingly. Otherwise, endpoint data would have been presented and if both endpoint and change data had been available for the same outcomes, only the former would have been reported in this review. We planned to estimate mean difference (MD) between groups as a summary statistic for continuous data, and, if heterogeneity had been found, we would have applied a random‐effects model.
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, the following standards would have been applied to all data before inclusion: (a) standard deviations (SDs) and means are reported in the paper or are obtainable from the authors; (b) when a scale starts from the finite number zero, the SD, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996); (c) if a scale starts from a positive value the calculation described above in (b) should be modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐Smin), where S is the mean score and Smin is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied to them. When continuous data are presented on a scale which includes a possibility of negative values (such as change on a scale), there is no way of telling whether data are non‐normally distributed (skewed) or not. It is thus preferable to use scale end point data, which typically cannot have negative values. If end point data was not available, then we would have used change data, but these would not have been subjected to a meta‐analysis, and would have been reported in the 'Additional data' tables.
Unit of analysis issues
Cluster trials
Where clustering had been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect. Otherwise, clustering would have been dealt with in this review as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008 Section 16.3).
Cross‐over design
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. This makes cross‐over trials inappropriate for unstable conditions such as schizophrenia. Accordingly, we would have only used data of the first phase of cross‐over studies.
Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we would have presented the additional treatment arms in comparisons. Where the additional treatment arms were not relevant, we would not have reproduced these data.
Dealing with missing data
We planned to carry out an intention to treat analysis. Studies would have been excluded from this review if they reported an attrition rate higher than 50% of participants in any group. In studies with less than 50% attrition rates, people leaving early would have been considered to have had the negative outcome, except for the event of death. We would also have analysed the impact of including studies with high attrition rates in a sensitivity analysis. If inclusion of data from this latter group had resulted in a substantive change in estimate of effects of the primary outcomes, we would have presented their data separately.
Assessment of heterogeneity
We would have considered all included studies without any comparison to judge clinical heterogeneity. We planned to investigate statistical heterogeneity by visual inspection of graphs and by calculating the I2 statistic. The I2 test provides an estimate of the percentage of heterogeneity thought to be due to chance, and it indicates high levels of heterogeneity when it gives a result of 75% or more (Higgins 2008). We would then have re‐analysed the data using a random‐effects model to see if this made a substantial difference. If it did, and the I2 statistic fell below 75% indicating more consistent results, we would have added the studies to the main body of trials. If using the random‐effects model did not make a difference and heterogeneity remained high, data would not have been summated, but presented separately and reasons for heterogeneity investigated.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We would have tried to locate protocols of included trials. If the protocol was available, we would have compared outcomes in the protocol and in the published report. If the protocol was not available, we would have compared outcomes listed in the methods section of the trial report with actually reported results. In addition, we would have attempted to use a funnel plot (included trial effect size versus included trial size) in an attempt to investigate the likelihood of publication bias.
Data synthesis
Where possible, we would have employed a fixed‐effect model for analyses. However, as discussed above, we would have applied a random‐effects model if heterogeneity had been found.
Subgroup analysis and investigation of heterogeneity
It has been proposed that elderly people may be more sensitive to neuroleptic drugs, and that the outcome may be affected by the specific diagnosis of late‐onset psychosis. Accordingly, we would have performed subgroups analyses to test these proposals.
Sensitivity analysis
We intended to undertake an analysis of the primary outcomes comparing the results when completer‐only data were used with analyses on an intention‐to‐treat basis. We would have also performed a sensitivity analysis to compare between low‐quality and high‐quality studies. A third sensitivity analysis would have compared between acute, short‐term, medium‐term and long‐term (as defined above) randomised controlled trials.
Results
Description of studies
Results of the search
The search of the Cochrane Schizophrenia Groups trials register for the initial version of this review resulted in 119 references. Of the 38 studies identified, 37 were excluded and one study added to Ongoing studies (Lacro 2001). The current updated search resulted in 211 references, from which we identified 88 studies, 81 excluded studies, one included study, one additional ongoing study and five awaiting classification.
Included studies
The original version of this review included no studies. We have included one study in the current update (Huang 2007). The characteristics of this study were as follows (Please also see Characteristics of included studies).
1. Length of study
Eight weeks.
2. Setting
Hospital inpatient.
3. Participants
3.1 Diagnosis
People with schizophrenia diagnosed according to CCMD‐3 (Chinese Classification of Mental Disorders (third revision)).
3.2 Age
People with a mean age of 64.3 ± 3.2 years for the risperidone group and a mean age of 63.3 ± 3.9 years for the olanzapine group.
3.3 Sex
Twelve male and 10 female patients in the risperidone group and 11 male and 11 female patients in the olanzapine group.
3.4 History
Participants in the risperidone group had been ill for 1.6 ± 1.1 (mean ± SD) years. Participants in the olanzapine group had been ill for 1.8 ± 1.4 (mean ± SD) years.
4. Study size:
The number of people randomised was 44.
5. Interventions
5.1 Risperidone at a fixed dose of 4.1 ± 2.1 (mean ± SD) mg/day.
5.2 Olanzapine at a fixed dose of 10.2 ± 5.9 mg/day.
6. Leaving the study early
All participants completed the eight‐week study.
7. Outcomes
7.1 General remarks
All measures were obtained at baseline, four and eight weeks following the intervention.
7.2 Outcome Scales
No scales provided usable data.
7.3 Missing Outcomes
Missing outcomes were social functioning, economic outcomes, quality of life and cognitive functioning.
Excluded studies
The original version of this review excluded 37 trials, largely because they did not include or report specifically on people with late‐onset schizophrenia. Four randomised controlled trials involved people with schizophrenia, and included a minority who suffered from paraphrenia (Seager 1955; Svestka 1972; Svestka 1973; Svestka 1987). These studies, however, did not report outcomes specifically for people with paraphrenia. Phanjoo 1990 did focus on the evaluation of antipsychotics for people with late‐onset schizophrenia but the two treatments under evaluation, remoxipride and thioridazine, have both been withdrawn from global use. Thioridazine is still available, but remoxipride was last produced by AstraZeneca in 1999.
In the current update, we excluded 81studies. Four studies were not trials (Li 2002; Linden 2001; Shen 2001; Wang 2002), and four trials were not randomised (Chen 2003a; Fleischhacker 2003; Lin 2002; Wang 2005). 35 trials were excluded because the intervention was not neuroleptic medication (Bartels 2005; Bjorndal 1980; Blum 1984; Burns 2007; Chen 2003b; Delapena 2005; Depla 2003; Drake 1993; Dunn 2002; Dunn 2006; Gerlach 1977; Granholm 2004; Granholm 2005; Granholm 2007; Granholm 2008; Inouye 2003; Isley 2003; Leutwyler 2009; Mazeh 2006; McKibbin 2006; McQuaid 2001; Naoki 2003; Ogunmefun 2009; Patterson 2003; Patterson 2005; Patterson 2006; Prat 2009; Pratt 2005; Pratt 2008; Shin 2004; Twamley 2005; Twamley 2008; Wang R 2002; Zisook 2002 ). Another 34 trials were excluded because they did not involve elderly people or involved elderly people with chronic psychoses but not of late‐onset (Anon 2005; Anon 2008a; Anon 2008b; Barak 2002; Brecher 1998; CATIE 2003; Crosthwaite 2000; Docherty 2002; Glorioso 2005; Harrigan 2004; Harvey 2003; Hay 2004; Jeste 2005; Kinon 2000; Kinon 2003; Kopala 2003; Lapolla 1969; Marcelli 2002; Mintzer 2004; Myers 2002; Nordentoft 1999; Pfizer 2006; Ritchie 1999; Sikich 2002; Sun Hui 2008; Tollefson 1997; Tran 1997; Tzimos 2007; Vaglum 2002; Wang LiLi 2008; Wang XiaoQuan 2008; Yamashita 2005; Yang 2002; Yu AiZhen 2008). Four studies of late‐onset schizophrenia were excluded because of flawed sequence generation (Cheng 2007; Lin 2005; Lin 2006; Liu 2007).
Ongoing studies
The original version of this review contained a study that may be ongoing (Lacro 2001). However, the limited description of participants acquired by the reviewers did not suggest that late‐onset schizophrenia was a focus of this work. The current update identified a study that did not seem to have started yet (Barnes 2002).
Studies awaiting classification
This review contains five studies from the 2010 search that need further assessment. In order to clarify the age of the participants and the age of onset of schizophrenia, we have been unable to obtain the full text of one study (Möller 2005) and the authors of two other studies have not responded to our e‐mail enquiries (Riedel 2009; Ritchie 2003). The sequence generation of two Chinese studies of late‐onset schizophrenia was not clear (Chu 2006; Zhe 2007); the authors could not be contacted by phone and they are yet to respond to our email enquiries. From the 2013 search 48 new studies were identified and are now also in awaiting assessment.
Risk of bias in included studies
Only one study fulfilled the criteria for inclusion in this review (Huang 2007). For summary of risk of bias please see Figure 1 and Figure 2.
1.
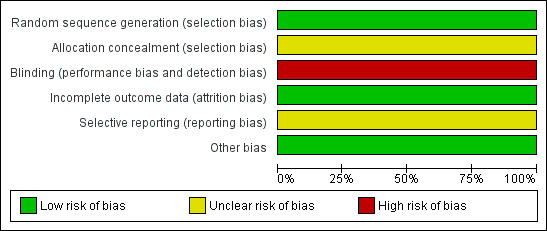
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
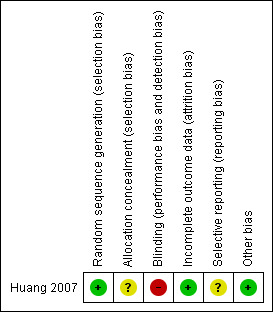
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The only included study allocated participants according to a random number table.
Blinding
The only included study employed no blinding.
Incomplete outcome data
The only included study reported outcomes for all participants, but presented continuous data without SDs.
Selective reporting
We have not been able to obtain the protocol of the only included study, but there is no indication of selective reporting.
Other potential sources of bias
None.
Effects of interventions
1. Leaving the study early
All participants completed the 8 week trial, indicating that both drugs were well tolerated.
2. Mental state: brief psychiatric rating scale (BPRS) date
The only measure of efficacy in this study was the BPRS (Overall 1962). Apparently, similar changes in BPRS scores were reported in the risperidone and the olanzapine groups (Table 1), but we were unable to use this data as they were presented without SDs.
1. Unusable outcome data due to lack of standard deviations.
| Risperidone n (male/female) 22 (12/10) |
Olanzapine n (male/female) 22 (11/11) |
|||
| Before | After | Before | After | |
| BPRS | 65.41 | 36.14 | 64.32 | 33.91 |
| BMI (kg/m2) | 22.97 | 23.09 | 22.65 | 23.62 |
| FBS(Fasting Blood Sugar) (mmol/L) | 4.37 | 4.44 | 4.30 | 4.52 |
| Serum Cholesterol (mmol/L) | 4.31 | 4.37 | 4.29 | 5.10 |
| Serum Triglycerides (mmol/L) | 1.02 | 1.01 | 0.99 | 1.27 |
BPRS: Brief Psychiatric Rating Scale BMI: Body Mass Index FBS: Fasting Blood Sugar
3. Other outcomes
The lack of SDs also prevented us from using data related to harm, which included changes in body mass index, fasting blood glucose, serum cholesterol and serum triglycerides (Table 1).
Discussion
The search
The search for the original version of this review identified only one study that focused on the evaluation of antipsychotics for people with late‐onset schizophrenia (Phanjoo 1990). The current updated search identified seven studies clearly focused on late‐onset schizophrenia; one included study (Huang 2007), four excluded studies (Cheng 2007; Lin 2005; Lin 2006; Liu 2007) and two studies awaiting classification (Chu 2006; Zhe 2007). This observation may be the result of a newly established access to Chinese publication, but may also reflect a growing research interest in late‐onset schizophrenia and can only be welcome.
Lack of trials and unusable data
Antipsychotic drugs are perceived as beneficial for people with schizophrenia, but there are risks associated with the adverse effects (Jeste 1998; Jeste 1999). For older people the effects of these drugs may be different from the effects in younger adults (Salzman 1990). Another Cochrane review evaluated the evidence for antipsychotic drugs in elderly people with schizophrenia regardless of age of onset (Marriott 2006).This review was undertaken to examine the best available evidence on the use of antipsychotic drugs for people whose schizophrenic illness started late in life (onset after 45 years old). Late‐onset schizophrenia may be different from schizophrenia with an earlier onset in term of gender distribution, genetic risk, symptom profile, prognosis and response to treatment (see Why it is important to do this review).
It is unfortunate that the majority of the studies of antipsychotics for late‐onset schizophrenia were excluded from this review because of poor study design (quasi randomisation), and that the only included study comparing olanzapine and risperidone offered little usable data. However, these studies confirm the importance of investigating the effects of antipsychotics for late‐onset schizophrenia and demonstrate that randomised studies are possible.
Authors' conclusions
Implications for practice.
General
Pharmacological treatment of schizophrenia in late life presents some unique challenges. Conventional neuroleptic agents, have proven effective in managing the “positive symptoms” (such as delusions and hallucinations) of many older patients, but these medications have a high risk of potentially disabling and persistent side effects, such as tardive dyskinesia. Recent years have witnessed promising advances in the management of schizophrenia. Studies with mostly younger people with schizophrenia suggest that the newer “atypical” antipsychotics, such as clozapine, risperidone, olanzapine, and quetiapine, may be effective in treating those patients previously unresponsive to traditional neuroleptics. Moreover, the newer medications may be more effective in treating negative symptoms and may even yield partial improvement in certain neurocognitive deficits associated with schizophrenia (Green 1997). Most of the research interest in schizophrenia has been focused on younger adults. With the ageing of the general population, there is now an increase in the attention being given to older people with schizophrenia, including those with a late onset of the disorder (Webster 1998). The literature in this area, however, is still very limited.
For clinicians
It is likely that clinicians will continue with their current practice, using clinical judgement and prescribing patterns to dictate prescribing because there is no trial‐based evidence to help guide their choice of drug. Important ethical questions are raised by current prescribing practices for people with late‐onset schizophrenia. It is difficult to know whether current practice is justified without well‐designed, well‐conducted, reported randomised studies.
For people with late‐onset schizophrenia or their relatives
It would be understandable that people with late‐onset schizophrenia or their relatives closely question their clinicians as regards the effects of antipsychotic drugs. In the light of the limited evidence, people with late‐onset schizophrenia would be justified to be disappointed in the medical/research fraternity.
For policy makers and funders
Currently policy makers have no trial‐based evidence upon which to base guidelines for late‐onset schizophrenia. They are likely to continue to rely on opinion and habit when making their recommendations. Funders of studies may wish to make this important subgroup of people a priority for future research.
Note: the 48 new citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.
Implications for research.
At present, there is no convincing evidence to support the use of antipsychotic medication for people with late‐onset schizophrenia. Clinically meaningful randomised studies are needed to help guide clinicians in their management of elderly people with schizophrenia. Available publications prove that studies of antipsychotic drugs for late‐onset schizophrenia are possible. There is a need for well‐designed randomised clinical trials of late‐onset psychoses, i.e., schizophrenia, delusional disorder, psychotic depression, etc. Comparisons of early‐onset and late‐onset psychoses are warranted, with meaningful outcome measures such as quality of life and activities of daily living functioning.
What's new
| Date | Event | Description |
|---|---|---|
| 9 January 2013 | Amended | Update search of Cochrane Schizophrenia Group's Trial Register (see Search methods for identification of studies), 48 studies added to awaiting classification. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 4 October 2011 | New citation required but conclusions have not changed | As result of search in 2010, one new study added to the included studies table, most data from this study were unusable and conclusions are not altered. |
| 4 October 2011 | New search has been performed | Updated with 211 new references. |
| 4 December 2002 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
This review was initiated by Suwanna Arunpongpaisal who wrote the protocol, undertook the search and data extraction and drafted the previous version of the review. Suchat Paholpak, Irshad Ahmed and Noorulain Aqeel helped write the protocol and the previous version of this review.
We would like to acknowledge the help of the AstraZeneca Medical Information Department who tracked down several trials of remoxipride. Many thanks to Chunbo Li for contacting Chinese authors by phone and email, and to Norah Essali for her help with RevMan. We are most grateful for the ongoing support of Claire Irving, Samantha Roberts and Lindsey Air at the Cochrane Schizophrenia Group editorial base.
We would also like to thank Mohannad Othman who helped in the 2010 search and data extraction.
The Cochrane Schizophrenia group provide a standard template for their methods section and other parts of the protocol. We have used this template and adapted it accordingly.
The additional text for the amendment following 2013 search has been added by the editorial base of the Cochrane Schizophrenia Group.
Appendices
Appendix 1. Previous searches
Electronic searches
We searched the Cochrane Schizophrenia Groups Trials Register (September 2002) using the phrase:
[(((elderly* or *elderly* in abstract terms or title) or (old* or * old * in title) or (*paraphrenia* or *late onset* in abstract, index terms or title) or (aged* in index terms) of REFERENCE) or (elderly in participants of STUDY))].
This register is compiled by up‐to‐date methodical searches of BIOSIS, The Cochrane Library, CINAHL, Dissertation abstracts, EMBASE, LILACS, MEDLINE, PSYNDEX, PsycINFO, RUSSMED, Sociofile, and is supplemented with handsearching of relevant journals and numerous conference proceedings (see Group Module).
Searching other resources
1. Reference searching We also inspected the references of all identified studies for more trials.
2. Personal contact The first author of each included study would have been contacted for information regarding unpublished trials.
3. Drug companies We contacted the manufacturers of amisulpride, or aripiprazole, or clozapine, or iloperidone, or olanzapine, or quetiapine, or risperidone, or sulpiride, or ziprasidone, or zotepine, or typical antipsychotics for additional data.
Appendix 2. Previous data collection and analysis
Dichotomous (binary) data:
For binary (yes/no) outcomes, we planned to to calculate a standard estimation of the fixed‐effect risk ratio (RR) and its 95% confidence interval (95% CI). For statistically significant results, we would have calculated the number needed to treat/harm statistic (NNT/H), and its 95% CI. If heterogeneity had been found, we would have used a random‐effects model.
Continuous data:
We intended to include continuous data from rating scales in this review only if the measuring instrument had been described in a peer‐reviewed journal and the instrument was either a self report or completed by an independent rater or relative (not the therapist). Where possible, we made efforts to convert continuous outcome measures to binary data by identifying cut‐off points on rating scales and dividing participants accordingly. Otherwise, endpoint data would have been presented and if both endpoint and change data had been available for the same outcomes, only the former would have been reported in this review. A weighted mean difference (WMD) between groups was to be estimated as a summary statistics for continuous data, and, if heterogeneity had been found, a random‐effects model would have been applied.
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, the following standards would have been applied to all data before inclusion: (a) standard deviations and means are reported in the paper or are obtainable from the authors; (b) when a scale starts from the finite number zero, the standard deviation, when multiplied by two, is less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution (Altman 1996); (c) if a scale starts from a positive value the calculation described above in (b) should be modified to take the scale starting point into account. In these cases skew is present if 2 SD > (S‐Smin), where S is the mean score and Smin is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied to them. When continuous data are presented on a scale which includes a possibility of negative values (such as change on a scale), there is no way of telling whether data are non‐normally distributed (skewed) or not. It is thus preferable to use scale endpoint data, which typically cannot have negative values. If endpoint data were not available, then we would have used change data, but these would not have been subjected to a meta‐analysis, and would have been reported in the 'Additional data' tables.
Unit of analysis issues
Cluster trials
Where clustering had been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect. Otherwise, clustering would have been dealt with in this review as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008 Section 16.3).
Cross‐over design
A major concern of cross‐over trials is the carry‐over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a wash‐out phase. This makes cross‐over trials inappropriate for unstable conditions such as schizophrenia. Accordingly, we would have only used data of the first phase of cross‐over studies.
Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we would have presented the additional treatment arms in comparisons. Where the additional treatment arms were not relevant, we would not have reproduced these data.
Dealing with missing data
An intention to treat analysis would have been carried out. Studies would have been excluded from this review if they reported a drop out rate higher than 50% of participants in any group. In studies with less than 50% dropout rates, people leaving early would have been considered to have had the negative outcome, except for the event of death. The impact of including studies with high attrition rates would also have been analysed in a sensitivity analysis. If inclusion of data from this latter group had resulted in a substantive change in estimate of effects of the primary outcomes, their data would have been presented separately.
Assessment of heterogeneity
We would have considered all included studies without any comparison to judge clinical heterogeneity. Statistical heterogeneity would have been investigated by visual inspection of graphs and by calculating the I2 statistics. The I2 test provides an estimate of the percentage of heterogeneity thought to be due to chance, and it indicates high levels of heterogeneity when it gives a result of 75% or more (Higgins 2008). We would then have re‐analysed data using a random‐effects model to see if this made a substantial difference. If it did, and the I2 statistics fell below 75% indicating more consistent results, we would have added the studies to the main body trials. If using the random‐effects model did not make a difference and heterogeneity remained high, data would not have been summated, but presented separately and reasons for heterogeneity investigated.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results. These are described in section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions(Higgins 2008). We would have tried to locate protocols of included trials. If the protocol was available, we would have compared outcomes in the protocol and in the published report. If the protocol was not available, we would have compared outcomes listed in the methods section of the trial report with actually reported results. In addition, we would have used a funnel plot (included trial effect size versus included trial size) in an attempt to investigate the likelihood of publication bias.
Data synthesis
Where possible we would have employed a fixed‐effect model for analyses. However, as discussed above, a random‐effects model would have been applied if heterogeneity had been found.
Subgroup analysis and investigation of heterogeneity
It has been proposed that elderly people may be more sensitive to neuroleptic drugs, and that the outcome may be affected by the specific diagnosis of the late‐onset psychosis. Accordingly, we would have performed subgroup analyses to test these proposals.
Sensitivity analysis
We intended to undertake an analysis of the primary outcomes comparing the results when completer‐only data were used with analyses on an intention‐to‐treat basis. We would have also performed a sensitivity analysis to compare between low‐quality and high‐quality studies. A third sensitivity analysis would have compared between acute, short‐term, medium‐term and long‐term (as defined above) randomised controlled trials.
Appendix 3. Assessment of methodological quality
In the first version of this review, we planned to assess the methodological quality of included trials using the criteria described in the Cochrane Handbook (Clarke 2000) and the Jadad Scale (Jadad 1996). The former is based on the evidence of a strong relationship between allocation concealment and direction of effect. The categories are defined below.
A. Low risk of bias (adequate allocation concealment) B. Moderate risk of bias (some doubt about the results) C. High risk of bias (inadequate allocation concealment)
The Jadad Scale measures a wider range of factors that impact on the quality of a trial. It includes three items. 1. Was the study described as randomised? 2. Was the study described as double‐blind? 3. Was there a description of withdrawals and dropouts?
Each item receives one point if the answer is positive. In addition, a point can be deducted if either the randomisation or the blinding/masking procedures described were inadequate. For the purpose of the analysis in this first version of the review, trials would have been included if they met the Cochrane Handbook criteria A or B. If the study did not gain at least two points on the Jadad Scale it would not have been included.
Data and analyses
Comparison 1. RISPERIDONE versus OLANZAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 RISPERIDONE versus OLANZAPINE, Outcome 1 Leaving the study early.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Huang 2007.
| Methods | Allocation: randomised by admission sequence. Blinding: no blinding. Duration: 8 weeks. Design: parallel. Country: China. |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3).
N = 44. Age: adults over 60 years (risperidone group mean age ˜ 64.3 years (SD = 3.2), olanzapine group mean age ˜ 63 years (SD = 3.9)) ‐ check 80% value etc Sex: 23M, 21F. History: recent onset: risperidone group mean duration of illness ˜1.6 years (SD = 1.1), olanzapine group mean duration of illness 1.8 years (SD = 1.4). |
|
| Interventions | 1. Risperidone: fixed dose mean 4.1 mg/day (SD = 2.1). N = 22. 2. Olanzapine: fixed dose mean 10.2 mg/day (SD = 5.9). N = 22. | |
| Outcomes | Leaving the study early. Unable to use: Mental state: BPRS: (no SD). BMI (kg/m2): (no SD). FBS (mmol/L): (no SD). Serum Cholesterol (mmol/L): (no SD). Serum Triglycerides (mmol/L): (no SD). |
|
| Notes | This study was included although we were unable to confirm that 80% of the participants were 65 or older. The age of onset, however, is clearly after the age of 60. This study provided unusable data (Table 1). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via random number table. Chumbo Li phoned the author on 26th July, 2010. The author stated that allocation was according to random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not stated. |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants left the study early. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not located, unsure if selective reporting occurred. |
| Other bias | Low risk | There was no industry funding. |
BMI: Body Mass Index BPRS: Brief Psychiatric Rating Scale CCMD‐3: Chinese Classification of Mental Disorders (third revision) FBS: Fasting Blood Sugar SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anon 2005 | Participants: not late‐onset schizophrenia. |
| Anon 2008a | Allocation: randomised. Double blind. Participants: elderly people (mean age 70 years) with schizophrenia ‐ not late‐onset schizophrenia. Interventions: paliperidone. |
| Anon 2008b | Allocation: randomised. Double blind. Participants: people with early‐onset schizophrenia. Interventions: risperidone and olanzapine. |
| Bamrah 2000 | Allocation: randomised. Participants: 114 elderly people with dementia and psychosis, not late‐onset schizophrenia. |
| Barak 2000 | Allocation: randomised. Participants: 51 "geriatric inpatients with chronic schizophrenia", not late‐onset schizophrenia. |
| Barak 2002 | Allocation: randomised. Participants:elderly people with chronic schizophrenia, mean age 72.7 ± 5.9 years, mean disease duration 33.1 ± 12.0 years ‐ not late‐onset schizophrenia. Interventions: olanzapine and haloperidol. |
| Bartels 2005 | Allocation: randomised. Participants: elderly people with serious mental illness. Interventions: health management and supported rehabilitation. |
| Berman 1995 | Allocation: randomised. Participants: 20 "geriatric patients with schizophrenia", not late‐onset schizophrenia. |
| Bjorndal 1980 | Allocation: randomised. Participants: psychiatric patients with tardive dyskinesia. Interventions: naloxone, enkephalins, morphine. |
| Blum 1984 | Interventions: naloxone, placebo. |
| Branchey 1978 | Allocation: unclear, "double‐blind, crossover". Participants: 30 elderly inpatients with chronic schizophrenia, not late‐onset schizophrenia. |
| Brecher 1998 | Allocation: randomised. Double blind. Participants: adult and elderly people with schizophrenia or schizoaffective disorder, mean age < 41 years. Interventions: risperdone and olanzepine. |
| Burns 1998 | Allocation: randomised. Participants: 59 "geriatric patients with schizophrenia, schizophreniform disorder, schizoaffective disorder", not late‐onset schizophrenia. |
| Burns 2007 | Allocation: randomised. Participants: people with severe mental illness ‐ not elderly people. Interventions: supported employment (Individual Placement and Support programme) vs. vocational training. |
| Byerly 2001 | Allocation: unclear, "comparing the efficacy....". Participants: 60 people with schizophrenia, not late‐onset schizophrenia. |
| Cantillon 1998 | Allocation: randomised. Participants: 351 (in trial 1 ‐fixed dose), 296 (in trial 2 ‐titrated dose) people with acute exacerbation of schizophrenia, age group not specified, not late‐onset schizophrenia. |
| CATIE 2003 | Allocation: randomised. Double blind. Participants: people with schizophrenia ‐ not late‐onset schizophrenia. Interventions: typical and atypical antipsychotic medications. |
| Chen 2003a | Allocation: not randomised. Participants: elderly people with schizophrenia. Interventions: olanzapine and clozapine. Age at onset: unclear. |
| Chen 2003b | Allocation: unclear. Participants: elderly people with psychotic disease. Interventions: nursing education. |
| Cheng 2007 | Allocation: not randomised ‐ according to illness severity. Participants: elderly people with first episode schizophrenia. Interventions: olanzapine vs risperdone. |
| Crosthwaite 2000 | Allocation: unclear. Participants: older patients with psychosis (age.>.50 years) ‐ not late‐onset psychosis. |
| Davidson 2000 | Allocation: unclear, "double‐blind". Participants: 180 chronic psychotic patients aged 54 to 89 years, not late‐onset schizophrenia. |
| Delapena 2005 | Allocation: randomised. Participants: older patients with schizophrenia. Interventions: group therapy and cognitive behavioral social skills training. |
| Depla 2003 | Allocation: not randomised ‐ post test only design to assess community integration of elderly mentally ill persons. |
| Docherty 2002 | Allocation: randomised. Double blind. Participants: elderly people with schizophrenia or schizoaffective disorder ‐ not late‐onset. Interventions: risperidone and olanzapine. |
| Drake 1993 | Allocation: unclear. Participants: homeless people with dual diagnosis, age range 18 to 50 years. Interventions: social network case management and intensive case management. |
| Dunn 2002 | Allocation: randomised. Participants: middle‐aged and elderly people with schizophrenia. Interventions: enhanced consent procedure and routine consent procedure. |
| Dunn 2006 | Allocation: randomised. Participants: middle‐aged and older patients with schizophrenia and schizoaffective disorder. Interventions: Brief Patient Education, Computer‐Assisted Instruction. Outcome: understanding of placebo control. |
| Evans 2000 | Allocation: randomised. Participants: people with Alzheimer's disease and psychosis, not late‐onset psychosis. |
| Fleischhacker 2003 | Allocation: not randomised, open‐label. Participants: symptomatically stable people with schizophrenia or schizoaffective disorder. Interventions: long‐acting risperidone at three different doses. |
| Gerlach 1977 | Allocation: randomised. Participants: hospitalised psychiatric patients with neuroleptic‐induced tardive dyskinesia ‐ not elderly patients. Interventions: alpha‐Methyl‐para‐tyrosine, Baclofen, Biperiden. |
| Glorioso 2005 | Allocation: randomised. Participants: middle‐aged and elderly patients with schizophrenia or dementia ‐ not late‐onset schizophrenia. Interventions: four atypical antipsychotic drugs. |
| Granholm 2004 | Allocation: randomised. Participants: older people with schizophrenia. Interventions: group cognitive‐behavioural social skills training. |
| Granholm 2005 | Allocation: randomised. Participants: middle‐aged and elderly people with chronic schizophrenia. Interventions: cognitive behavioural social skills training. |
| Granholm 2007 | Allocation: randomised. Participants: not aged patients. Interventions: Cognitive Behavioral Therapy. |
| Granholm 2008 | Allocation: randomised. Single blind (outcome assessor). Participants: older patients with schizophrenia. Interventions:computer‐assisted cognitive behavioral social skill training, goal‐focused supportive contact. |
| Harrigan 2004 | Allocation: randomised No blinding. Participants: people with psychotic disorders, age range 18 to 65 ‐ not late‐onset. |
| Harvey 2000a | Allocation: randomised. Participants: 367 people with schizophrenia living in a community, not late‐onset schizophrenia. |
| Harvey 2000b | Allocation: randomised. Participants: 377 people with schizophrenia, schizoaffective outpatients. Subgroup analysis for 153 elderly patients with schizophrenia, but not late‐onset schizophrenia. |
| Harvey 2000c | Allocation: randomised. Participants: 222 people with schizophrenia, not late‐onset schizophrenia. |
| Harvey 2002 | Allocation: randomised. Participants: 269 patients with schizophrenia or schizoaffective disorder, not late‐onset schizophrenia. |
| Harvey 2003 | Allocation: randomised. Double blind. Participants: elderly people with schizophrenia or schizoaffective disorder ‐ not late‐onset schizophrenia (mean duration of illness 36.5 years, age of onset 33 to 36 years). Interventions: risperidone and olanzapine. |
| Hay 2004 | Allocation: randomised. Participants: acutely psychotic or agitated elderly people. Interventions: olanzapine and conventional antipsychotic drugs. |
| Hellewell 2000 | Allocation: randomised. Participants: 455 patients with schizophrenia, schizoaffective disorder, age 18 to 89 years old, including only 18 elderly patients, not late‐onset schizophrenia. |
| Howanitz 1996 | Allocation: randomised. Participants: 42 chronic schizophrenic inpatients aged 55 years or older, not late‐onset schizophrenia. |
| Inouye 2003 | Delirium Prevention Trial. Interventions: adherence to nonpharmacologic interventions. |
| Isley 2003 | Allocation: randomised. Double blind.
Participants: older patients with psychosis. Interventions: Medication Adherence Therapy. |
| Jeste 2001 | Allocation: randomised, Participants: 176 "geriatric" patients with schizophrenia or schizoaffective disorder, not late‐onset schizophrenia. |
| Jeste 2005 | Allocation: randomised. Participants: middle‐aged and elderly psychiatric patients ‐ not late‐onset psychosis. |
| Kinon 2000 | Allocation: randomised.
Blinding: Double blind. Method and participants: data were obtained from patients (n = 760 participants) enrollment into a large (n = 1996 not aged participants). not late‐onset schizophrenia. |
| Kinon 2001 | Allocation: randomised. Participants: 43 "elderly dementia patients with behavioural disturbance and psychosis living in nursing homes", not late‐onset schizophrenia. |
| Kinon 2003 | Allocation: randomised. Participants: acutely psychotic or agitated elderly people without tardive dyskinesia ‐ not late‐onset psychosis. |
| Kopala 2003 | Allocation: randomised. Blinding: unclear. Participants: people with recent onset schizophrenia. Not late‐onset schizophrenia. |
| Kuntz 1998 | Allocation: randomised. Participants: 59 "geriatric" patients with schizophrenia, schizophreniform disorder, schizoaffective disorder, not late‐onset schizophrenia. |
| Lane 2001 | Allocation: randomised. Participants: 24 people with first episode schizophrenia, age 18 to 45 years old, not late‐onset schizophrenia. |
| Lapolla 1969 | Allocation: not randomised. Participants: elderly (age range 55 to 80 years) ‐ not late‐onset psychosis. Interventions: acetophenazine and thioridazine. |
| Leutwyler 2009 | Allocation: randomised. (Secondary data analysis of a lifestyle intervention programme). Participants: persons over age 40 with schizophrenia or schizoaffective disorder. Interventions: Rehabilitation Training or Usual Care plus Information. |
| Li 2002 | Design: cross‐sectional study. Participants: elderly people with schizophrenia and normal adults. Outcome: assessment of cognitive functions. |
| Lin 2002 | Allocation: not randomised. Participants: elderly people with schizophrenia. Interventions: quetiapine and perphenazine. |
| Lin 2005 | Allocation: 'randomised' by admission sequence ‐ odd for olanzapine, even for risperdone. |
| Lin 2006 | Allocation: 'randomised' by admission sequence ‐ odd for risperidone, even for perphenazine Participants: elderly people with schizophrenia. |
| Linden 2001 | Allocation: not randomised. Participants: people who had left early from a clinical trial. |
| Liu 2007 | Allocation: 'randomised' by sequence ‐ The author stated that allocation was according to odd or even numbers (phone call by Chunbo Li on 26th July, 2010). Participants: elderly people with late‐onset schizophrenia. Interventions: aripiprazole vs risperidone. |
| Lovett 1987 | Allocation: randomised. Participants: 54 "geriatric patients with chronic brain syndrome and senile psychosis", not late‐onset schizophrenia. |
| Marcelli 2002 | Allocation: randomised. Participants: adolescent and young adults (age 14 to 24 years) with first acute psychotic episode. |
| Matkovits‐Gupta 1999 | Allocation: randomised. Participants: people with schizophrenia including the elderly, not late‐onset schizophrenia. |
| Mazeh 2006 | Allocation: randomised. Double blind, cross‐over. Participants: elderly patients with schizophrenia. Interventions: donepezil, placebos. |
| McKibbin 2006 | Allocation: randomised. Participants: people with schizophrenia or schizophrenic disorder and diabetes mellitus. Age: older than 40 years. Interventions: diabetes awareness and rehabilitation training vs. usual care plus information. |
| McQuaid 2001 | Allocation: randomised. Participants: older people with schizophrenia, age 45 years and above. Interventions: Cognitive Behavioural Social Skills Training. |
| Mintzer 2000 | Allocation: randomised. Participants: 92 elderly people, 31 with schizophrenia, 22 who with schizoaffective disorder, 5 with delusional disorder, 12 with mood disorders and 12 with dementia, not late‐onset schizophrenia. |
| Mintzer 2004 | Design: post hoc analysis of a subset of older patients from and open‐label RCT. Participants: elderly people with psychosis, age 60 to 80 years ‐ not late‐onset psychosis. |
| Mohler 1970 | Allocation: non randomised, case‐control comparative study. |
| Myers 2002 | Design: RCT. Allocation: blinding ‐ double. Participants: not aged patients. |
| Naoki 2003 | Allocation: randomised. Interventions: community re‐entry programme. |
| Nordentoft 1999 | Allocation: randomised. Participants: 18 to 45 years old with first time diagnosis of schizophrenia spectrum disorder. |
| Ogunmefun 2009 | Allocation: randomised. Double‐blind, cross‐over. Participants: people with tardive dyskinesia. Interventions: placebos and donepezil. |
| Patterson 2003 | Allocation: randomised. Participants: middle‐aged and older patients with chronic schizophrenia. Interventions: functional adaptation skills training and group therapy. |
| Patterson 2005 | Allocation: randomised. Participants: middle aged and older Latino patients with psychosis. Interventions: psychosocial intervention. |
| Patterson 2006 | Allocation: randomised Participants: middle‐aged and elderly people with chronic psychotic disorders. Interventions: functional adaptation skills training. |
| Pfizer 2006 | Allocation: randomised. Participants: elderly people with psychosis, age > 65 years ‐ not late‐onset schizophrenia. Interventions: asenapine. |
| Phanjoo 1990 | Allocation: randomised. Participants: 18 "geriatric inpatients with schizophrenia and paranoid disorder ", 16 with late‐onset schizophrenia. Interventions: remoxipride versus thioridazine, remoxipride withdrawn from the market (1994), last batch produced (1999). |
| Prat 2009 | Allocation: randomised. Participants: older people with serious mental illness. Interventions: psychosocial rehabilitation. |
| Pratt 2005 | Allocation: randomised. Participants: older people with serious mental illness, age 50 years and above. Interventions: the Using Medications Effectively programme. |
| Pratt 2008 | Allocation: randomised. Participants: older people with serious mental illness. Interventions: social rehabilitation and health care management (Helping Older People Experience Success; HOPES) vs. usual care. |
| Reams 1998 | Allocation: randomised. Participants: 59 "geriatric" patients with schizophrenia, schizophreniform disorder, schizoaffective disorder, not late‐onset schizophrenia. |
| Ritchie 1999 | Allocation: not randomised (a cohort study). Participants: elderly people on antipsychotic medications ‐ not late‐onset psychosis. Interventions: olanzapine and risperidone. |
| Salganik 1998 | Allocation: randomised. Participants: 17 "geriatric patients with chronic refractory schizophrenia", not late‐onset schizophrenia. |
| Salzman 2000 | Allocation: not randomised, descriptive study. |
| Seager 1955 | Allocation: unclear, "divided into two groups". Participants: 48 people including 13 with schizophrenia or paraphrenia. Interventions: chlorpromazine different doses. Outcome: no data reported for subgroup of people with paraphrenia. |
| Shen 2001 | Design: cross‐sectional study. Participants: young, middle‐aged and elderly male people with schizophrenia. Outcome: serum testosterone levels. |
| Shin 2004 | Allocation: randomised. Interventions: psycho education and supportive therapy. |
| Sikich 2002 | Allocation: randomised. Double blind. Participants: children and adolescents with psychosis. Interventions: risperidone, olanzapine and haloperidol. |
| Sun Hui 2008 | Allocation: randomised. Participants: elderly men with schizophrenia ‐ not late‐onset schizophrenia. Interventions: quetiapine and risperidone. |
| Sutton 2001 | Allocation: randomised. Participants: 339 people with schizophrenia, schizoaffective outpatients age 18 to 65. Subgroup analysis for 39 elderly patients(age 50 to 65) with schizophrenia, not late‐onset schizophrenia. |
| Svestka 1972 | Allocation: randomised. Participants: 44 elderly women with schizophrenia including 21 with paraphrenia. Interventions: pimozide versus perphenazine. Outcome: no data reported for subgroup of women with parapharenia. |
| Svestka 1973 | Allocation: unclear "double‐blind". Participants: 46 people with schizophrenia or paraphrenia. Interventions: oxyprothepine versus perphenazine. Outcome: no data reported for subgroup of women with parapharenia. |
| Svestka 1987 | Allocation: unclear "double‐blind". Participants: 40 people with schizophrenia, schizoaffective disorder, including 9 paraphrenia. Interventions: isofloxythepine versus haloperidol. Outcomes: no data reported for subgroup of people with parapharenia. |
| Svestka 1989a | Allocation: randomised. Participants: 40 people with chronic schizophrenia or schizoaffective disorder, aged 17 to 67. No mention of late‐onset schizophrenia. |
| Svestka 1989b | Allocation: unclear "cross‐over". Participants: 34 inpatients with chronic schizophrenia, no mention of late‐onset schizophrenia. |
| Svestka 1990 | Allocation: unclear, "double‐blind". Participants: 36 patients with schizophrenia or schizoaffective disorder, not late‐onset schizophrenia. |
| Tariot 2000 | Allocation: not randomised, open‐label trial. |
| Tollefson 1997 | Allocation: randomised. Double blind. Participants: people with schizophrenia, onset of illness by the age of 45 years in 87% of the participants ‐ not late‐onset schizophrenia. |
| Tran 1997 | Allocation: randomised. Double blind. Participants: people with chronic schizophrenia and related psychotic disorders, aged 18 to 65 years ‐ not late‐onset schizophrenia. Interventions: risperidone, olanzapine. |
| Twamley 2005 | Allocation: randomised. Participants: middle‐aged and older people with schizophrenia. Interventions: three work rehabilitation approaches. |
| Twamley 2008 | Allocation: randomised. Participants: middle‐aged and older people with severe mental illness. Interventions: a supported employment model (Individual Placement and Support) and conventional vocational rehabilitation. |
| Tzimos 2007 | Allocation: randomised. Double blind. Interventions: placebos and paliperidone. Participants: elderly people with schizophrenia. mean age at onset of schizophrenia 34 years ‐ not late‐onset schizophrenia. |
| Vaglum 2002 | Allocation: not randomised. Participants: people with first episode schizophrenia, without age limit. Interventions: early detection programme. |
| Wang 2002 | Design: cross‐sectional study. Participants: people with schizophrenia taking novel or traditional antipsychotic drugs. Outcome: direct cost of treatment. |
| Wang 2005 | Allocation: not randomised. Participants: elderly people with primary‐onset schizophrenia. Interventions: risperidone and perphenazine. |
| Wang LiLi 2008 | Allocation: randomised. Participants: elderly people with schizophrenia ‐ not late‐onset schizophrenia. Interventions: risperidone oral solution and risperidone tablets. |
| Wang R 2002 | Interventions: family support and psychological treatment. |
| Wang XiaoQuan 2008 | Allocation: randomised. Participants: elderly people with schizophrenia ‐ not late‐onset schizophrenia. Interventions: quetiapine and risperidone. |
| Warner 2006 | Allocation: randomised. Participants: older people with mental illness. Interventions: home treatment and standard care. This is only a study proposal. |
| Yamashita 2005 | Allocation: unclear. Participants: people with schizophrenia receiving conventional antipsychotic drugs. Mean age 61.4 years ‐ not late‐onset schizophrenia. Interventions: olanzapine, perospirone, quetiapine and risperidone. Outcome: sleep quality. |
| Yamawaki 1996 | Allocation: unclear, "double‐blind". Participants: 35 elderly people with schizophrenia, not late‐onset schizophrenia. |
| Yang 2002 | Allocation: randomised. Participants: 20 to 53 years old people with schizophrenia. Interventions: nicotine patch and placebo patch. |
| Yu AiZhen 2008 | Allocation: randomised. Participants: elderly people with schizophrenia ‐ not late‐onset schizophrenia. Interventions: olanzapine and perphenazine. |
| Zisook 2002 | Allocation: randomised. Participants: older people with schizophrenia, age 40 years and above. Interventions: placebo vs citaloprams. |
Characteristics of studies awaiting assessment [ordered by study ID]
Anon 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Barak 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Chu 2006.
| Methods | Allocation: quasi‐randomised according to admission sequence.
Blindness: none.
Duration: 8 weeks.
Design: parallel. Country of origin: China. |
| Participants | N = 60. Diagnosis: schizophrenia (CCMD‐3). Age: 63.7 (SD = 2.1) for the quetiapine group and 64.2 (SD = 2.5) for the risperidone group. Sex (male/female): quetiapine group (16/14), risperidone group (17/13). Duration of illness: quetiapine group 4.3 months (SD = 2.2), risperidone group 4.0 months (SD = 2.3). Age at onset: older than 60 years. |
| Interventions | 1. Quetiapine: fixed dose, 200 ˜ 350mg/day. N = 30. 2. Risperidone: fixed dose, 2 ˜ 3 mg/day. N = 30. |
| Outcomes | Mental state: PANSS (Positive and Negative Syndrome Scale for Schizophrenia). Adverse effects: extra pyramidal side effects, sleepiness, orthostatic hypotension, dry mouth, constipation, ECG changes, elevated alanine aminotransferase, weight gain. Leaving the study early. |
| Notes | Further details about the allocation procedure are required. Chunbo Li couldn't contact the author by telephone, and an inquiry email was sent to the author on 26th July, 2010 with no response. The outcome data of this study is presented in Table 3. |
2. Outcomes of Chu 2006.
| Quetiapine n (male/female): 30 (16/14) |
Risperidone n (male/female): 30 (17/13) |
|
| Endpoint score of PANSS (+) | 8.1 (SD = 1.7) | 8.1 (SD = 2.9) |
| Endpoint score of PANSS (‐) | 11.1 (SD = 5.1) | 10.2 (SD = 5.1) |
| General Mental state | 21.0 (SD = 6.7) | 20.1 (SD = 5.9) |
| Endpoint score of PANSS | 38.2 (SD = 11.7) | 38.1 (11.7) |
| TESS | ||
| Extra Pyramidal Symptoms | 4 | 8 |
| Sleepiness | 3 | 1 |
| Orthostatic hypotension | 3 | 1 |
| Dry mouth | 1 | 1 |
| Constipation | 1 | 0 |
| ECG changes | 4 | 3 |
| Elevated alanine aminotransferase | 1 | 0 |
| Gain weight | 0 | 2 |
| Leaving the study early | 0 | 0 |
PANSS: Positive and Negative Syndrome Scale
Dubovsky 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Dubovsky 2010a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Dubovsky 2010b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Dubovsky 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Kircher 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Luo 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
McGuire 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Mintzer 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Möller 2005.
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 6 weeks. Design: parallel. |
| Participants | N = 36. Diagnosis: schizophrenia (DSM‐IV), schizophreniform disorder, schizoaffective disorder, delusional disorder or shared psychotic disorder. Age: 65 years or more. Sex: not described. Duration of illness: not described. Age at onset: not described. |
| Interventions | 1. Amisulpride: flexible dose. Allowed dose range: 100 to 400 mg/day. N = 24. 2. Risperidone: flexible dose. Allowed dose range: 1 to 4 mg/day. N = 12. |
| Outcomes | Leaving the study early: adverse events. Cognitive functioning: MMSE (Mini Mental State Examination). Adverse effects: open interviews. Unable to use‐ Mental State: PANSS total score, BPRS total score (no usable data). Cardiac effects: (QTc), laboratory tests (no data). |
| Notes | Age at onset: Unclear ‐ requires full texts. |
NCT01625897.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Riedel 2009.
| Methods | Allocation: random, no further details. Blindning: double blind. Duration: 6 weeks. Design: parallel. |
| Participants | N = 38. Diagnosis: DSM IV‐diagnostic criteria for psychotic disorders with psychotic symptoms severe enough to require antipsychotic medication. Age: 72.7 ± 7.4 years. Sex: predominantly female (80.6%). Duration of illness: not described. Age of onset: not clear. |
| Interventions | 1. Risperidone. 2. Amisulpride. |
| Outcomes | Global state: Improvement, Clinical Global Impression (CGI), Clinical Global Impression ‐ Change Scale (CGI‐C).
Mental state: Brief Psychiatric Rating Scale (BPRS), Positive and Negative Syndrome Scale for Schizophrenia (PANSS), Simpson & Angus Neurological Rating Scale for Extrapyramidal Effects /Simpson‐Angus Scale (SAS), Depressive symptoms, Positive symptoms, Anxiety, Mini Mental State Examination (MMSE), Calgary Depression Scale (CDS). Leaving the study early (or loss of contact between carer and patient/client). Adverse effects: Barnes Akathisia Scale (BAS), Abnormal Involuntary Movement Scale (AIMS), Tardive dyskinesia, Agitation, Parkinsonism, Insomnia, Nausea Restlessness, Headache, Hypotension, Dizziness, Tolerability, Treatment Emergent Adverse Event (TEAT), Tachycardia, Diarrohea, Fatigue, Tiredness, Influenza, Dyspnea, Prostate symptom, Nutrition, Conjunctivitis. Cognitive: Memory, Cognitive function. Death. Medication used. Laboratory tests: Blood pressure, Electrocardiogram (ECG), QTc Interval, Cardiac functioning, Metabolism, Heamatology, Hypertension, Biochemistry, Uric acid levels, Respiration. |
| Notes | Age at onset: unclear ‐ author was contacted at riedel@med.uni‐muenchen.de on June 16, 2010 with no response. |
Ritchie 2003.
| Methods | Allocation: random, no further details.
Blinding: no. Duration: unclear. Design: open‐label cross‐over. |
| Participants | N = 66. Diagnosis: schizophrenia treated with conventional neuroleptics. Age: elderly people (mean age 69.6 SD 6.2). Sex: unclear. Age of onset of schizophrenia: unclear. |
| Interventions | Switching elderly patients from typical antipsychotics (either as depot or oral preparations) to: 1. olanzapine. 2. risperidone. |
| Outcomes | Mental state: Brief Psychiatric Rating Scale (BPRS), Mini Mental State (MMS), Scale for the Assessment of Negative Symptoms (SANS), Montgomery and Asberg Depression Rating Scale (MADRS) Leaving the study early. Adverese effects: Barnes Akathisia Scale (BAS), Abnormal Involuntary Movement Scale (AIMS), Parkinsonism, Simpson & Angus Neurological Rating Scale for Extrapyramidal Effects / Simpson‐Angus Scale (SAS), Extrapyramidal side effects (EPS). Quality of Life: WHO Quality of Life [Brief] Scale (WHO‐QOL‐BREF). |
| Notes | Age at onset: unclear ‐ the author was contacted three times at C.Ritchie@medsch.ucl.ac.uk in June 2010 with no response. |
Ritchie 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Sutton 2001a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Zhang 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
Zhe 2007.
| Methods | Allocation: randomised by admission sequence ‐ no further detail. Blinding: No blinding. Duration: 6 weeks. Design: parallel. Country of origin: China. |
| Participants | N = 62. Diagnosis: schizophrenia (CCMD‐3), antipsychotic naive. Age: 64.2 (SD = 1.9) for the aripiprazole group and 63.9 (SD = 2.2) years for the risperidone group. Sex (male/female): 17/13 in the aripiprazole group and 18/14 in the risperidone group. Duration of illness: 5.5 months (SD = 2.1) for the aripiprazole group and 4.9 months (SD = 2.3) for the risperidone group. |
| Interventions | 1. Aripiprazole fixed dose (within 14 days) 15˜20 mg/d. 2. Risperidone fixed dose (within 14 days) 2˜3 mg/d. |
| Outcomes | Mental state: PANSS (Positive and Negative Syndrome Scale for Schizophrenia). Adverse effects/events: anxiety, headache, sleepiness, nausea. |
| Notes | Further details about the allocation procedure are required. Chunbo Li couldn't contact the author by telephone, and an inquiry email was sent to the author on 26th July, 2010 with no response. The outcome data of this study is presented in Table 4. |
3. Outcomes of Zhe 2007.
| Aripiprazole n (male/female): 30 (17/13) |
Risperidone n (male/female): 32 (18/14) |
|
| Endpoint score of PANSS (+) | 10.5 (SD = 2.9) | 12.6 (SD = 4.7) |
| Endpoint score of PANSS (‐) | 9.8 (SD = 8.3) | 9.9 (SD = 7.3) |
| Endpoint score of Psychopathology | 26.1 (SD = 7.3) | 22.7 (SD = 7.3) |
| Endpoint score of PANSS | 46.4 (SD = 20.7) | 45.2 (SD = 19.4) |
| TESS | ||
| Anxiety | 5 | 4 |
| Headache | 4 | 1 |
| Sleepiness | 4 | 5 |
| Nausea | 1 | 1 |
PANSS: Positive and Negative Syndrome Scale
伏彩霞 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
余成国 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
刁端忠 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
刘金光 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
吕斌军 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
周益辉 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
宋丽 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
崔明伟 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
张保利 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
张峻岭 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
张疆莉 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
张艳琦 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
张艳琦 2010a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
徐清 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
曾庆翰 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
李国兰 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
李永红 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
李海涛 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
杨仁登 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
杨雀屏 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
梁勇 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
石保青 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
管銮友 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
罗琦 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
董晓茹 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
董继承 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
蒋幸衍 2009.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
诸春明 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
谢国建 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
陈永红 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
黄峰 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
黄峰 2010a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
黄峰 2010b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
黄振英 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | To be assessed. |
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders
Characteristics of ongoing studies [ordered by study ID]
Barnes 2002.
| Trial name or title | A prospective study of tardive dyskinesia in the elderly; single‐blind comparison of amisulpride and risperidone. |
| Methods | Prospective single‐blind study. |
| Participants | Elderly people with tardive dyskinesia, not clear if including late‐onset schizophrenia. |
| Interventions | 1. Amisulpride. 2. Risperidone. |
| Outcomes | Tardive dyskinesia. |
| Starting date | Unclear. |
| Contact information | National Research Register 2002;3. National Research Register 2003;1. |
| Notes |
Lacro 2001.
| Trial name or title | Antipsychotic treatment in late life schizophrenia. |
| Methods | Randomised, double‐blind. |
| Participants | Older people with schizophrenia, not clear if including late‐onset schizophrenia. |
| Interventions | 1. Risperidone. 2. Haloperidol. |
| Outcomes | 1. Clinical response (reduction in psychopathology). 2. Cognitive performance (a battery of neuropsychological tests). 3. Health‐related quality of life. 4. Plasma level of antipsychotic drug. 5. Adverse effects. |
| Starting date | Unclear ˜ 2001. |
| Contact information | CRISP database (https://www‐commons.cit.nih.gov/crisp/index.html. |
| Notes |
Differences between protocol and review
The protocol used for this update has been changed and updated: taken from the most recent methodology of the Cochrane Schizophrenia Group, it acknowledges changes in Cochrane methodology since this review was first published ‐ for example use of 'Summary of findings' tables instead of calculating NNT/NNH and changes in the 'Risk of bias' tables. We do not think the use of these new methods affects the results or integrity of the review.
For previous methodology please see Appendix 1.
Contributions of authors
Adib Essali ‐ undertook the 2010 update search and data extraction, updated the full review.
Ghassan Ali ‐ trial selection and data extraction.
Sources of support
Internal sources
Faculty of Medicine, Khon Kaen University, Thailand.
Yale University School of Medicine, USA.
Brown University School of Medicine, USA.
External sources
University of Leeds, UK.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Huang 2007 {published and unpublished data}
- Huang X, Zhong Z, Zhang J. The effects of risperidone and olanzapine on the glucose metabolism and lipid metabolism in elderly patients with schizophrenia. Journal of Clinical Psychosomatic Diseases 2007;13(1):1‐3. [Google Scholar]
References to studies excluded from this review
Anon 2005 {published data only}
- Anon. Drug update This older schizophrenia drug is as effective as newer ones. RN 2005;68(11):81, 84. [Google Scholar]
Anon 2008a {published data only}
- Anon. Paliperidone extended release in elderly schizophrenia patients. Brown University Geriatric Psychopharmacology Update 2008;12(4):3. [Google Scholar]
Anon 2008b {published data only}
- Anon. Atypical vs older antipsychotics for early‐onset schizophrenia. Brown University Child and Adolescent Psychopharmacology Update 2008;10(11):3‐4. [Google Scholar]
Bamrah 2000 {published data only}
- Bamrah S. A multicentre, double‐blind, randomised, parallel group comparison of seroquel and haloperidol in the treatment of elderly patients presenting with dementia and psychosis. National Research Register 2000.
Barak 2000 {published data only}
- Barak Y, Shamir E, Weizman R. Risperidone compared with typical neuroleptic treatment in elderly schizophrenic patients. International Journal of Neuropsychopharmacology 2000;3(Suppl. 1):122. [Google Scholar]
Barak 2002 {published data only}
- Barak Y//Shamir E//Zemishlani H//Mirecki I//Toren P//Weizman R. Olanzapine vs. haloperidol in the treatment of elderly chronic schizophrenia patients. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2002;26:1199‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bartels 2005 {published data only}
- Bartels SJ. Rehabilitation and health care for elderly with SMI. http://www.clinicaltrials.gov 2005.
Berman 1995 {published data only}
- Berman I, Allan ER, Pappas D, Sison CE, Merson A. The cognitive effect of risperidone in elderly schizophrenic patients a pilot double‐blind comparison study with haloperidol. 150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22; San Diego, USA. 1997. [MEDLINE: ]
- Berman I, Merson A, Allan E, Alexis C, Losonczy M. Effect of risperidone on cognitive performance in elderly schizophrenic patients: a double‐blind comparison study with haloperidol. Psychopharmacology Bulletin 1995;31(3):552. [MEDLINE: ] [Google Scholar]
- Berman I, Pappas D, Patel C, Chang H, Goff D. Effect of risperidone on cognitive function in schizophrenia. 151st Annual Meeting of the American Psychiatric Association; 1998 May 30‐Jun 4; Toronto, Canada. 1998. [MEDLINE: ]
Bjorndal 1980 {published data only}
- Bjørndal N, Casey DE, Gerlach J. Enkephalin, morphine, and naloxone in tardive dyskinesia. Psychopharmacology 1980;69(2):133‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blum 1984 {published data only}
- Blum I, Munitz H, Shalev A, Roberts E. Naloxone may be beneficial in the treatment of tardive dyskinesia. Clinical Neuropharmacology 1984;7(3):265‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Blum I, Nisipeanu PF, Roberts E, Casey DE, Korsgaard S, Gerlach J. Des‐tyr‐gamma‐endorphin in tardive dyskinesia. Proceedings of the 3rd World Congress of Biological Psychiatry; 1981 Jun 28 ‐ Jul 3; Stockholm, Sweden 1981;93:538. [Google Scholar]
Branchey 1978 {published data only}
- Branchey MH, Lee JH, Amin R, Simpson GM. High and low potency neuroleptics in elderly psychiatric patients. Journal of the American Medical Association 1978;239(18):1860‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Brecher 1998 {published data only}
- Berry SA, Gudelsky GA, Mahmoud RA. Serum prolactin levels in schizophrenia. Biological Psychiatry 2001;49(8):22S. [Google Scholar]
- Conley RR, Mahmoud R. Risperidone and olanzapine in people with schizophrenia or schizoaffective disorder: a randomised double‐blind study. Data on file. Baltimore, USA: Maryland Psychiatric Research Center, 2001. [DOI] [PubMed]
- Conley RR, Mahmoud R. Risperidone versus olanzapine in patients with schizophrenia and schizoaffective disorder. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Conley RR, Mahmoud R. Risperidone vs olanzapine in patients with schizophrenia & schizoaffective disorder. Proceedings of the 40th Annual Meeting of the New Clinical Drug Evaluation Unit; 2000 May 30 ‐ Jun 2; Boca Raton, Florida, USA. 2000.
- Conley RR, Mahmoud R. Risperidone vs olanzapine in patients with schizophrenia and schizo‐affective disorder. International Drug Therapy Newsletter 2000;35(10):77‐8. [Google Scholar]
- Conley RR, Mahmoud R. Risperidone Study Group. Risperidone vs. olanzapine in patients with schizophrenia and schizoaffective disorder. Biological Psychiatry 2000;47:32S. [Google Scholar]
- Harvey P, Meltzer H, Green M. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. Biological Psychiatry 2001;49(8):123S. [Google Scholar]
- Harvey P, Meltzer H, Green M. Risperidone cognitive effects in schizophrenia and schizoaffective patients. International Drug Therapy Newsletter 2001;36(8):59. [Google Scholar]
- Harvey PD. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Harvey PD, Gharabawi G. Risperidone and cognition in schizophrenic elderly. Proceedings of the 12th World Congress of Psychiatry; 2002 Aug 24‐29; Yokohama, Japan. 2002. [MEDLINE: ]
- Harvey PD, Green MF, McGurk SR, Meltzer HY. Changes in cognitive functioning with risperidone and olanzapine treatment: a large‐scale, double‐blind, RANDOMized study. Psychopharmacology 2003;169(3‐4):404‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Harvey PD, Meltzer HY, Green MF. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. Proceedings of the 40th Annual Meeting of the New Clinical Drug Evaluation Unit; 2000 May 30 ‐ Jun 2; Boca Raton, Florida, USA. 2000.
- Kelly DL, Conley RR, Love RC, Morrison JA, McMahon RP. Metabolic risk with second‐generation antipsychotic treatment: a double‐blind RANDOMized 8‐week trial of risperidone and olanzapine. Annals of Clinical Psychiatry 2008;20(2):71‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lasser RA, Mao L, Gharabawi G. Smokers and nonsmokers equally affected by olanzapine‐induced weight gain: metabolic implications. Schizophrenia Research 2004;66(2‐3):163‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Martinez RA, Berry SA, Gudelsky GA, Myers JE, Mahmoud RA. Serum prolactin levels in schizophrenia. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Myers J, Mahmoud R, Berry S, Conley R. Risperidone versus olanzapine for the treatment of mood symptoms in patients with schizophrenia and schizoaffective disorder. Bipolar Disorders 2001;3(Suppl 1):49. [Google Scholar]
- Myers JE, Mahmoud RA, Berry SA, Conley RR. Risperidone versus olanzapine for the treatment of mood symptoms in patients with schizophrenia and schizoaffective disorder. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Myers JE, Mahmoud RA, Keith SJ, Csernansky JG. Long‐term benefit of risperidone versus haloperidol for affective symptoms in patients with schizophrenia and schizoaffective disorder. Proceedings of the 155th Annual Meeting of the American Psychiatric Association. Philadelphia, Pennsylvania, USA, 2002.
Burns 1998 {published data only}
- Burns PR, Reams SG, Sanger TM, Beasley CM Jr. Olanzapine in the treatment of elderly patients with schizophrenia and related psychotic disorders. 21st Congress of the Collegium Internationale Neuro‐psychopharmacologicum; 1998 Jul 12‐16; Glasgow, Scotland. 1998. [MEDLINE: ]
- Burns PR, Reams SG, Sanger TM, Beasley CM Jr. Olanzapine in the treatment of elderly patients with schizophrenia and related psychotic disorders. 9th Biennial Winter Workshop on Schizophrenia; 1998 Feb 7‐13; Davos, Switzerland. 1998. [MEDLINE: ]
- Lane LM, Burns PR, Reams SC, Sanger TM, Beasley CM Jr. Olanzapine in the treatment of elderly patients with schizophrenia and related psychotic disorders. 11th Congress of The European College of Neuropsychopharmacology; 1998 Oct 31‐ Nov 4; Paris, France. 1998.
Burns 2007 {published data only}
- Burns T, Catty J, Becker T, Drake RE, Fioritti A, Knapp M, Lauber C, Rossler W, Tomov T, Van Busschbach, White S, Wiersma D. The effectiveness of supported employment for people with severe mental illness: a randomised controlled trial. Lancet 2007;370(9593):1146‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Burns T, White SJ, Catty J. Individual placement and support in Europe: the EQOLISE trial. International Review of Psychiatry 2008;20(6):498‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Byerly 2001 {published data only}
- Byerly MJ, Weber MT, Brooks DL, Snow LR, Worley MA, Lescouflair E. Antipsychotic medications and the elderly: effects on cognition and implications for use. Drugs and Aging 2001;18(1):45‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cantillon 1998 {published data only}
- Cantillon M. Quetiapine fumarate reduces aggression. 11th Annual Meeting of the American Association for Geriatric Psychiatry; 1998 Mar 8‐11; San Diego, USA. 1998. [MEDLINE: ]
- Cantillon M, Arvanitis LA, Miller BG, Kowalcyk BB. 'Seroquel' (Quetiapine Fumarate) reduces hositility and aggression in patients with acute schizophrenia. 36th Annual Meeting of the American College of Neuropsychopharmacology; 1997. 1997:171. [MEDLINE: ]
- Cantillon M, Goldstein JM. Efficacy of quetiapine fumarate in affective symptoms of schizophrenia. 151st Annual Meeting of the American Psychiatric Association; 1998 May 30‐Jun 4; Toronto, Canada. 1998.
- Cantillon M, Goldstein JM. Quetiapine fumarate reduces aggression and hostility in patients with schizophrenia. 151st Annual Meeting of the American Psychiatric Association; 1998 May 30‐Jun 4; Toronto, Canada. 1998.
CATIE 2003 {published data only}
- Anon. Newer antipsychotics similar to older agents. Journal of Family Practice 2005;54(12):1026. [MEDLINE: ] [PubMed] [Google Scholar]
- Bick P, Knoesen N, Castle D. Clinical implications of the CATIE schizophrenia trials: day‐to‐day management lessons for Australasian psychiatrists. Australasian Psychiatry : Bulletin of Royal Australian and New Zealand College of Psychiatrists 2007;15(6):465‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chwastiak LA, Rosenheck RA, McEvoy JP, Stroup TS, Swartz MS, Davis SM, Lieberman JA. The impact of obesity on health care costs among persons with schizophrenia. General Hospital Psychiatry 2009;31(1):1‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covell NH. Antipsychotic medications do not differ substantially in ability to reduce violent behaviour in people with schizophrenia. Evidence‐Based Mental Health 2009;12(1):13. [DOI] [PubMed] [Google Scholar]
- Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, Rosenheck R, Davis SM, Hsiao JK, Stroup TS, Lieberman JA. Antipsychotic effects on estimated 10‐year coronary heart disease risk in the CATIE schizophrenia study. Schizophrenia Research 2008;105(1‐3):175‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SM, Koch GG, Davis CE, LaVange LM. Statistical approaches to effectiveness measurement and outcome‐driven re‐RANDOMizations in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) studies. Schizophrenia Bulletin 2003;29(1):73‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Nasrallah HA. The CATIE schizophrenia effectiveness trial. Schizophrenia Research 2005;80(1):5‐6. [DOI] [PubMed] [Google Scholar]
- Glazer WM, Conley RR, Citrome L. Are we treating schizophrenia effectively? Understanding the primary outcomes of the catie study. CNS Spectrums 2006;11(Suppl 10):1‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Haddad PM, Dursun S. Selecting antipsychotics in schizophrenia: lessons from CATIE. Journal of Psychopharmacology 2006;20(3):332‐4. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW. Cognitive improvement in response to antipsychotic drugs: neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Archives of General Psychiatry 2007;64(6):631‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Heres S, Kissling W, Leucht S. Different antipsychotics produce similar, small improvements in psychosocial functioning at one year in people with schizophrenia. Evidence‐Based Mental Health 2007;10(4):112. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keefe R, Bilder RM, Harvey PD, Davis S, Palmer B, McEvoy JP, Goldberg TE, Gold JM, Green MF, Swartz M, Stroup S, Rosenheck R, Perkins DO, Meltzer HY, Miller D, Canive J, Walker TM, Lieberman JA. Baseline neurocognitive assessment of 1364 patients with schizophrenia in the clinical antipsychotic trials for intervention effectiveness ( CATIE ) project. Schizophrenia Bulletin 2005;31:361‐2. [Google Scholar]
- Keefe RSE, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Archives of General Psychiatry 2007;64(6):633‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Mohs RC, Bilder RM, Harvey PD, Green MF, Meltzer HY, Gold JM, Sano M. Neurocognitive assessment in the clinical antipsychotic trials of intervention effectiveness (CATIE) project schizophrenia trial: Development, methodology, and rationale. Schizophrenia Bulletin 2003;29(1):45‐55. [DOI] [PubMed] [Google Scholar]
- Lieberman J, McEnvoy J, Stroup S. Protocol: comparative effectiveness of antipsychotic medications in patients with schizophrenia: revised in response to DSMB comments. National Institute of Mental Health. Chapel Hill, USA: University of North Carolina, 2000.
- Lieberman J, McEnvoy J, Stroup S. Trial design summary: comparative effectiveness of antipsychotic medications in patients with schizophrenia: draft. National Institute of Mental Health. Chapel Hill, USA: University of North Carolina, 2002.
- Lieberman JA. Comparative effectiveness of antipsychotic medications in patients with schizophrenia (CATIE schizophrenia trial). http://www.clinicaltrials.gov 2001.
- Lieberman JA. Research gaps and current research initiatives to improve the treatment of schizophrenia. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California, USA. 2003.
- Lieberman JA, Schneider LS, McEroy J, Pariot P, Stroup S, Adiao J, Lebowitz BD. Effectiveness trials of antipsychotic drugs. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Lieberman JA, Stroup TS. Schizophrenia, VI: Treatments. American Journal of Psychiatry 2003;160(10):1748. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Keefe R, Perkins DO, Davis S, Davis CE, Lebowitz B, Hsiao J. CATIE trial results. European Neuropsychopharmacology 2006;16(Suppl 4):S184. [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz B, Hsiao J, Severe J. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia: primary efficacy and safety outcomes of the clinical antipsychotic trials of intervention effectiveness (CATIE) schizophrenia trial. Neuropsychopharmacology 2005;30(Suppl 1):S32. [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Hsiao J, Severe J, Lebowitz BD. Dr. Lieberman and colleagues reply. American Journal of Psychiatry 2006;163(3):555‐6. [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353(12):1209‐23. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Davis VG, McEvoy JP, Goff DC, Nasrallah HA, Davis SM, Daumit GL, Hsiao J, Swartz MS, Stroup TS, Lieberman JA. Impact of antipsychotic treatment on nonfasting triglycerides in the CATIE schizophrenia trial phase 1. Schizophrenia Research 2008;103(1‐3):104‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Nasrallah HA, McEvoy JP, Goff DC, Davis SM, Chakos M, Patel JK, Keefe RS, Stroup TS, Lieberman JA. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophrenia Research 2005;80(1):9‐18. [DOI] [PubMed] [Google Scholar]
- Miller DD, McEvoy JP, Davis SM, Caroff SN, Saltz BL, Chakos MH, Swartz MS, Keefe RSE, Rosenheck RA, Stroup TS, Lieberman JA. Clinical correlates of tardive dyskinesia in schizophrenia: baseline data from the CATIE schizophrenia trial. Schizophrenia Research 2005;80(1):33‐43. [DOI] [PubMed] [Google Scholar]
- Miller DD, Caroff SN, Davis SM, Rosenheck RA, McEvoy JP, Saltz BL, Riggio S, Chakos MH, Swartz MS, Keefe RSE, Stroup TS, Lieberman JA. Extrapyramidal side‐effects of antipsychotics in a randomised trial. British Journal of Psychiatry 2008;193(4):279‐88. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed S, Rosenheck R, McEvoy J, Swartz M, Stroup S, Lieberman JA. Cross‐sectional and longitudinal relationships between insight and attitudes toward medication and clinical outcomes in chronic schizophrenia. Schizophrenia Bulletin 2009;35(2):336‐46. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed S, Rosenheck R, Swartz M, Stroup S, Lieberman JA, Keefe RS. Relationship of cognition and psychopathology to functional impairment in schizophrenia. American Journal of Psychiatry 2008;165(8):978‐87. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Meyer JM, McEvoy JP, Davis VG, Goff DC, Davis SM. Inflammatory markers in schizophrenia: comparing antipsychotic effects in the CATIE schizophrenia trial. Schizophrenia Bulletin 2009;35(Suppl 1):37. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SG, Rosenheck RA, Canive JM, Souza C, Stroup TS, McEvoy J, Davis S, Keefe RSE, Swartz M, Lieberman J. Employment outcomes in a randomized trial of second‐generation antipsychotics and perphenazine in the treatment of individuals with schizophrenia. Journal of Behavioral Health Services and Research 2008;35(2):215‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rosenheck RA. Cost‐effectiveness of atypical antipsychotics in the CATIE schizophrenia trial. Neuropsychopharmacology 2005;30(Suppl 1):S32. [Google Scholar]
- Rosenheck RA, Davis S, Covell N, Essock S, Swartz M, Stroup S, McEvoy J, Lieberman J. Does switching to a new antipsychotic improve outcomes? Data from the CATIE Trial. Schizophrenia Research 2009;107(1):22‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stroup TS. Baseline decision making capacity in the CATIE schizophrenia trial. Schizophrenia Research 2004;67(1):209. [DOI] [PubMed] [Google Scholar]
- Stroup TS, Lieberman JA, McEvoy JP, Davis SM, Swartz MS, Keefe RSE, Miller AL, Rosenheck RA, Hsiao JK. Results of phase 3 of the CATIE schizophrenia trial. Schizophrenia Research 2009;107(1):1‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, McGee MF, Simpson GM, Stevens MC, Lieberman JA. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophrenia Bulletin 2003;29(1):15‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stroup TS. CATIE Study Group. Statistical methods and analytic plan. CATIE Protocol. NIMH, 2005. [MEDLINE: ] [Google Scholar]
- Swanson JW, Swartz MS, Dorn RA, Volavka J, Monahan J, Stroup TS, McEvoy JP, Wagner HR, Elbogen EB, Lieberman JA. Comparison of antipsychotic medication effects on reducing violence in people with schizophrenia. British Journal of Psychiatry 2008;193(1):37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MS, Perkins DO, Stroup TS, McEvoy JP, Nieri JM, Haak DC. Assessing clinical and functional outcomes in the clinical antipsychotic trials of intervention effectiveness (CATIE) schizophrenia trial. Schizophrenia Bulletin 2003;29(1):33‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Swartz MS, Stroup TS, McEvoy JP, Davis SM, Rosenheck RA, Keefe RSE, Hsiao JK, Lieberman JA. What CATIE found: results from the schizophrenia trial. Psychiatric Services 2008;59(5):500‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MS, Wagner HR, Swanson JW, Stroup TS, McEvoy JP, Reimherr F, Miller DD, McGee M, Khan A, Canive JM, Davis SM, Hsiao JK, Lieberman JA. The effectiveness of antipsychotic medications in patients who use or avoid illicit substances: results from the CATIE study. Schizophrenia Research 2008;100(1‐3):39‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2003a {published data only}
- Chen F, Liang L, Zhu XH. A control study of elderly patients with schizophrenia treated with olanzapine or clozapine. Journal of Clinical Psychological Medicine 2003;13(5):298‐9. [Google Scholar]
Chen 2003b {published data only}
- Chen CY, Chen WX, Mao L. Nursing about side effects of old psychotic disease patients when taking medicine. Journal of Qilu Nursing 2003;9(1):11‐2. [Google Scholar]
Cheng 2007 {published data only}
- Cheng W. Comparative study on olanzapine and risperidone of treating elderly first ‐ episode schizophrenia. Journal of Qiqihar Medical College [Qiqihar Yixueyuan xuebao] 2007;28(18):2185‐7. [Google Scholar]
Crosthwaite 2000 {published data only}
- Crosthwaite CG, Reveley MA. Improved Sleep with Risperidone Compared with Conventional Antipsychotic Drugs in Older Patients with Psychosis. Schizophrenia Research 2000;41(1):202. [MEDLINE: ] [Google Scholar]
Davidson 2000 {published data only}
- Davidson M, Harvey PD, Vervarcke J, Gagiano CA, Hooge JD, Bray G, Dose M, Barak Y, Haushofer M. A long‐term, multicenter, open‐label study of risperidone in elderly patients with psychosis. On behalf of the Risperidone Working Group. International Journal of Geriatric Psychiatry 2000;15(6):506‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Delapena 2005 {published data only}
- Delapena J, Edwards S, Stirling C, Loh C, Granholm E. Functional rehabilitation of older patients with schizophrenia. http://www.clinicaltrials.gov 2005.
Depla 2003 {published data only}
- Depla MFIA, Graaf R, Busschbach JT, Heeren TJ. Community integration of elderly mentally ill persons in psychiatric hospitals and two types of residences. Psychiatric Services 2003;54(5):730‐5. [DOI] [PubMed] [Google Scholar]
Docherty 2002 {published data only}
- Docherty JP, Napolitano J, Mahmoud RA, Martinez RA, Lasser RA, Pandina GJ, Gharabawi G. Anticholinergic effect of atypical antipsychotics in elderly patients. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Tune L//Mulsant B//Gharabawi G. Anticholinergic effect of atypical antipsychotics in elderly patients. European Neuropsychopharmacology 2002;12(Suppl 3):S314. [Google Scholar]
Drake 1993 {published data only}
- Drake RE, Bebout RR, Roach JP. A research evaluation of social network case management for homeless persons with dual disorders. In: Harris M//Bergman HC editor(s). Case Management For Mentally Ill Patients: Theory and Practice. Vol. 1, Harwood Academic Publishers, 1993:83‐98. [Google Scholar]
Dunn 2002 {published data only}
- Dunn LB, Liindamer LA, Schneiderman LJ, Jeste DV. Enhanced informed consent for research in older patients with psychotic disorders. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Dunn LB, Lindamer LA, Palmer BW, Golshan S, Schneiderman LJ, Jeste DV. Improving understanding of research consent in middle‐aged and elderly patients with psychotic disorders. American Journal of Geriatric Psychiatry 2002;10(2):142‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Dunn 2006 {published data only}
- Dunn LB, Palmer BW, Keehan M. Understanding of PLACEBO controls among older people with schizophrenia. Schizophrenia Bulletin 2006;32(1):137‐46. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2000 {published data only}
- Evans M. Multi‐centre double blind randomised parallel group comparison of seroquel and haloperidol in the treatment of elderly patients presenting with dementia, psychoses. National Research Register 2000.
Fleischhacker 2003 {published data only}
- Fleischhacker WW, Eerdekens M, Karcher K, Remington G, Llorca P‐M, Chrzanowski W, Martin S, Gefvert O. Treatment of schizophrenia with long‐acting injectable risperidone: a 12‐month open‐label trial of the first long‐acting second‐generation antipsychotic. Journal of Clinical Psychiatry 2003;64(10):1250‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lasser RA, Bossie CA, Zhu Y, Gharabawi G, Eerdekens M, Davidson M. Efficacy and safety of long‐acting risperidone in elderly patients with schizophrenia and schizoaffective disorder. International Journal of Geriatric Psychiatry 2004;19(9):898‐905. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J‐P, Jarboe K, Bossie CA, Zhu Y, Mehnert A, Lasser R. Minimal injection site pain and high patient satisfaction during treatment with long‐acting risperidone. International Clinical Psychopharmacology 2005;20(4):213‐21. [DOI] [PubMed] [Google Scholar]
Gerlach 1977 {published data only}
- Gerlach J, Thorsen K. The movement pattern of oral tardive dyskinesia in relation to anticholinergic and antidopaminergic treatment. International Pharmacopsychiatry 1976;11(1):1‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Glorioso 2005 {published data only}
- Glorioso DK, Jeste DV. Metabolic effects of newer antipsychotics in older patients. http://www.clinicaltrials.gov 2005.
Granholm 2004 {published data only}
- Granholm E, McQuaid JR, Auslander LA, McClure FS. Group cognitive‐behavioral social skills training for older outpatients with chronic schizophrenia. Journal of Cognitive Psychotherapy 2004;18(3):265‐79. [Google Scholar]
Granholm 2005 {published data only}
- Granholm E, McQuaid JR, Link PC, Fish S, Patterson T, Jeste DV. Neuropsychological predictors of functional outcome in cognitive behavioral social skills training for older people with schizophrenia. Schizophrenia Research 2008;100(1‐3):133‐43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, McQuaid JR, McClure FS, Auslander LA, Perivoliotis D, Pedrelli P, Patterson T, Jeste DV. A randomized, controlled trial of cognitive behavioral social skills training for middle‐aged and older outpatients with chronic schizophrenia. American Journal of Psychiatry 2005;162(3):520‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Granholm 2007 {published data only}
- Granholm E. Neurocognitive predictors of outcome in cognitive behavioral social skills training for people with chronic schizophrenia. Schizophrenia Bulletin 2007;33(2):431. [Google Scholar]
- Granholm E, McQuaid JR, McClure FS, Link PC, Perivoliotis D, Gottlieb JD, Patterson TL, Jeste DV. Randomized Granholm E//McQuaid JR//McClure FS//Link PC//Perivoliotis D//Gottlieb JD//Patterson TL//Jeste DVcontrolled trial of cognitive behavioral social skills training for older people with schizophrenia: 12‐month follow‐up. Journal of Clinical Psychiatry 2007;68(5):730‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Granholm 2008 {published data only}
- Granholm EL, Edwards S, Loh C. Functional rehabilitation for older patients with schizophrenia. http://www.clinicaltrials.gov 2008.
Harrigan 2004 {published data only}
- Chiu CC, Chang WH, Huang MC, Chiu YW, Lane HY. Regular‐dose risperidone on QTc intervals. Journal of Clinical Psychopharmacology 2005;25(4):391‐3. [DOI] [PubMed] [Google Scholar]
- Harrrigan EP, Miceli JJ, Anziano R, Watsky E, Reeves KR, Cutler NR, Sramek J, Shiovitz T, Middle M. A randomized evaluation of the effects of six antipsychotic agents on QTC, in the absence and presence of metabolic inhibition. Journal of Clinical Psychopharmacology 2004;24(1):62‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harvey 2000a {published data only}
- Harvey P, Meltzer H, Green M. Long term cognitive effects of risperidone treatment in schizophrenia. International Journal of Neuropsychopharmacology 2000;3(Suppl. 1):154. [Google Scholar]
- Harvey PD. Long‐term cognitive effects of risperidone treatment in schizophrenia. 52nd Institute on Psychiatric Services; 2000 Oct 25‐29; Philadelphia, USA. 2000. [MEDLINE: ]
- Harvey PD, Lyons BE, Mahmoud R. Long term cognitive effects of risperidone. Schizophrenia Research 2000;41(1):200‐1. [Google Scholar]
- Harvey PD, Meltzer HY, Green MF. Long‐term cognitive effects of risperidone treatment in schizophrenia. 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000; Dec 10‐14; San Juan; Puerto Rico. 2000.
Harvey 2000b {published data only}
- Harvey PD. Cognitive effects of risperidone and olanzapine in patients with schizophrenia. 52nd Institute on Psychiatric Services; 2000 Oct 25‐29; Philadelphia, USA. 2000. [MEDLINE: ]
- Harvey PD. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18; Chicago, USA. 2000.
- Harvey PD, Gharabawi G. Cognition in elderly patients with schizophrenia: risperidone versus olanzapine. International Journal of Neuropsychopharmacology. Abstracts of the 23rd Congress of the Collegium Internationale Neuro‐Psychopharmacologicum; 2002 Jun 23‐27; Montreal, Canada 2002;5(Suppl. 1):77. [Google Scholar]
- Harvey PD, Mahmoud R, Meltzer HY, Green MP. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. European Neuropsychopharmacology. Abstracts of the 14th Congress of the European College of Neuropsychopharmacology; 2001 Oct 13‐17; Istanbul, Turkey 2001;11(3):257. [Google Scholar]
- Harvey PD, Mao L, Napolitano J, Gharabawi G. Improved cognition in elderly schizophrenic patients: risperidone versus olanzapine. Schizophrenia Research. Abstracts of the 11th Biennial Winter Workshop on Schizophrenia 2002;3(Suppl. 1):28. [Google Scholar]
- Harvey PD, Meltzer HY, Green M. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000; Dec 10‐14; San Juan; Puerto Rico. 2000.
- Harvey PD, Meltzer HY, Green M. Cognitive effects of risperidone and olanzapine in patients with schizophrenia or schizoaffective disorder. 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000; Dec 10‐14; San Juan; Puerto Rico. 2000.
Harvey 2000c {published data only}
- Harvey PD, Meltzer HY, Romano S. Improvement in cognition following a switch to open label ziprasidone from olanzapine, risperidone and conventional antipsychotics. International Journal of Neuropsychopharmacology 2000;3(Suppl. 1):161. [Google Scholar]
- Harvey PD, Meltzer HY, Romano S. Improvement in cognition following a switch to open label ziprasidone from olanzapine, risperidone and conventional antipsychotics. Journal of the European College of Neuropsychopharmacology 2000;10(Suppl. 3):288. [Google Scholar]
- Harvey PD, Meltzer HY, Romano SJ. Improvement in cognition following a switch to open label ziprasidone in outpatients with schizophrenia treated with conventional antipsychotics, olanzapine or risperidone. 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18; Chicago, USA. 2000.
Harvey 2002 {published data only}
- Harvey P, Simpson GM, Loebel A. Effect of ziprasidone vs olanzapine on cognition in schizophrenia: a double‐blind study. International Journal of Neuropsychopharmacology. Abstracts of the 23rd Congress of the Collegium Internationale Neuro‐Psychopharmacologicum; 2002 Jun 23‐27; Montreal, Canada 2002;5(Suppl. 1):125. [Google Scholar]
- Harvey PD, Simpson G, Horne R, Weiden PJ, Bari M, Romano SJ. Effects of ziprasidone on cognitive function in patients with schizophrenia: results of a double‐blind trial vs. olanzapine. Schizophrenia Research. Abstracts of the 11th Biennial Winter Workshop on Schizophrenia 2002;53(3 Suppl.1):195. [Google Scholar]
- Harvey PD, Simpson GM, Weiden PJ, Loebel A. Ziprasidone vs olanzapine for cognitive function in schizophrenia. European Neuropsychopharmocology 2002;12(Suppl. 3):293. [Google Scholar]
Harvey 2003 {published data only}
- Harvey PD, Napolitano JA, Mao L, Gharabawi G. Comparative effects of risperidone and olanzapine on cognition in elderly patients with schizophrenia or schizoaffective disorder. International Journal of Geriatric Psychiatry 2003;18(9):820‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jeste D, Madhusoodanan S, Barak Y, Martinez RA, Mahmoud R, Kershaw P. Risperidone and olanzapine in elderly patients with schizophrenia and schizoaffective disorder. International Psychogeriatrics 2001;13(2):295S. [Google Scholar]
- Jeste DV, Barak Y, Madhusoodanan S, Grossman F, Gharabawi G. International multisite double‐blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. American Journal of Geriatric Psychiatry 2003;11(6):638‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jeste DV, Madhusoodanan S, Barak F, Martinez RA. Risperidone versus olanzapine in elderly patients with schizophrenia. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
Hay 2004 {published data only}
- Hay D, Kinon B, Stauffer V, Kaiser C, Kollack‐Walker S. Incidence of presumptive tardive dyskinesia in elderly patients treated with olanzapine or conventional antipsychotics. Schizophrenia Research 2004;67(1):185‐6. [Google Scholar]
Hellewell 2000 {published data only}
- Hellewell JS, Westhead E. Safety during long‐term exposure to quetiapine. 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000; Dec 10‐14; San Juan; Puerto Rico. 2000. [MEDLINE: ]
- Hellewell JSE, Westhead E. Safety during long‐term exposure to quetiapine. Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; LA, USA. Marathon Multimedia, 2001.
Howanitz 1996 {published data only}
- Howanitz E, Pardo M, Smelson DA, Engelhart C, Eisenstein N, Losonczy MF. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. Journal of Clinical Psychiatry 1999;60(1):41‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Howanitz E, Pardo M, Smelson DA, Engelhart C, Eisenstein N, Losonczy MF. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia: erratum. Journal of Clinical Psychiatry 1999;60(5):341. [DOI] [PubMed] [Google Scholar]
- Howanitz EM, Pardo M, Litwin P, Stern RG, Wainwright KM, Flosonczy M. Efficacy of clozapine versus chlorpromazine in geriatric schizophrenia. 149th Annual Meeting of the American Psychiatric Association; 1996 May 4‐9; New York, USA. 1996. [MEDLINE: ]
Inouye 2003 {published data only}
- Inouye SK, Bogardus ST Jr, Williams CS, Leo‐Summers L, Agostini JV. The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the Delirium Prevention Trial. Archives of Internal Medicine 2003;163:958‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Isley 2003 {published data only}
- Isley D, Lacro J. Medication adherence in older psychotic people. http://www.clinicaltrials.gov 2003.
Jeste 2001 {published data only}
- Jeste DV, Madhusoodanan S, Barak F, Martinez RA. Risperidone versus olanzapine in elderly patients with schizophrenia. Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; LA, USA. Marathon Multimedia, 2001.
Jeste 2005 {published data only}
- Jeste DV. Movement disorders caused by antipsychotic drugs in older patients. http://www.clinicaltrials.gov 2005.
Kinon 2000 {published data only}
- Kinon BJ, Milton DR, Gilmore JA. Continued improvement in quality of life despite weight change during olanzapine treatment. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):S154. [Google Scholar]
Kinon 2001 {published data only}
- Kinon BJ, Wang L, Stauffer VL. A comparison of prolactin concentrations in the elderly during treatment with olanzapine, haloperidol, and risperidone. 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26; San Francisco, USA. 2001. [MEDLINE: ]
Kinon 2003 {published data only}
- Kinon BJ, Stauffer VL, Kaiser C, Hay DP, Kolack‐Walker S. Incidence of presumptive tardive dyskinesia in elderly patients treated with olanzapine or conventional antipsychotics. International Psychogeriatrics 2003;15(Suppl. 2):271. [MEDLINE: ] [Google Scholar]
- Kinon BJ, Stauffer VL, Kaiser C, Walker SK. Incidence of presumptive tardive dyskinesia in elderly patients treated with olanzapine or conventional antipsychotics. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California, USA. 2003.
Kopala 2003 {published data only}
- Kopala L, Rabinowitz J, Davidson M. Extra‐pyramidal signs and symptoms (EPS) in recent onset schizophrenia: a comparison of risperidone and haloperidol. European Neuropsychopharmacology 2003;13(4):S338. [Google Scholar]
Kuntz 1998 {published data only}
- Kuntz AJ, Sanger TM, Beasley CM. Olanzapine in the treatment of elderly patients with schizophrenia and related psychotic disorders. 11th Annual Meeting of the American Association for Geriatric Psychiatry; 1998 Mar 8‐11; San Diego, USA. 1998. [MEDLINE: ]
Lane 2001 {published data only}
- Lane H‐Y, Chang W‐H, Chui C‐C, Huang M‐C, Lee S‐H, Chen J‐Y. A pilot double‐blind, dose comparison study of risperidone in drug niave, first‐episode schizophrenia. Journal of Clinical Psychiatry 2001;62(12):994‐5. [DOI] [PubMed] [Google Scholar]
Lapolla 1969 {published data only}
- Lapolla. Acetophenazine versus thioridazine in the elderly. Psychopharmacology Bulletin 1969;6(3):101‐3. [Google Scholar]
Leutwyler 2009 {published data only}
- Leutwyler H, Wallhagen M, McKibbin C. The impact of symptomatology on response to a health promoting intervention among older adults with schizophrenia. Proceedings of the 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16‐21; San Francisco, CA 2009. [MEDLINE: ] [DOI] [PubMed]
Li 2002 {published data only}
- Li X, Xiao S, Lu Z. Cognitive function of elderly schizophrenics. Shanghai Archives of Psychiatry 2002;14(2):85‐7. [Google Scholar]
Lin 2002 {published data only}
- Lin J, Huang Y, Chen G. A control study of treating elderly patients with schizophrenia with quetiapine or perphenazine. Chinese Journal of Psychiatry 2002;35(2):99‐102. [Google Scholar]
Lin 2005 {published data only}
- Lin Z‐Y, Li F‐Q, Yu Y‐Y, Zhong T‐P. A controlled study of olanzapine and risperidol in the treatment of elderly patients with schizophrenia. Hainan Medical Journal 2005;16(8):37‐8. [Google Scholar]
Lin 2006 {published data only}
- Lin L. Comparative study on risperidone and perphenazine in treatment of elderly schizophrenia. Journal of Qiqihar Medical College [Qiqihar Yixueyuan xuebao] 2006;27(18):2184‐5. [Google Scholar]
Linden 2001 {published data only}
- Linden M, Godemann F, Gaebel W, Kopke W, Muller P, Muller‐Spahn F, Pietzcker A, egeler J. A prospective study of factors influencing adherence to a continuous neuroleptic treatment program in schizophrenia patients during 2 years. Schizophrenia Bulletin 2001;27(4):585‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liu 2007 {published and unpublished data}
- Liu L, Xinag H. Compared effect and safety analysis of aripiprazole in late‐onset schizophrenia. Sichuan Mental Health 2007;20(3):142‐4. [Google Scholar]
Lovett 1987 {published data only}
- Lovett WC, Stokes DK, Taylor LB, Young ML, Free SM, Phelan DG. Management of behavioral symptoms in disturbed elderly patients: comparison of trifluoperazine and haloperidol. Journal of Clinical Psychiatry 1987;48(6):234‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Marcelli 2002 {published data only}
- Marcelli D, Rose‐Reinhardt H, Fahs H, Kasolter‐Pere MA. Adolescents and young adults (14‐24‐years old), acute psychotic episodes: Diagnostic therapeutic and ethical questions [Episode psychotique aigu de l'adolescent et du jeune adulte. Questions diagnostiques, therapeutiques et ethiques]. Annales Medico‐Psychologiques 2002;160(5‐6):386‐95. [Google Scholar]
Matkovits‐Gupta 1999 {published data only}
- Matkovits‐Gupta T, Lasser R, Young F, Happy J, Gharabawi G, Cucchiaro J, Fairweather D. Managing psychotic disorders through balanced receptor blockade: the zomaril tm clinical program. 11th World Congress of Psychiatry; 1999 Aug 6‐11; Hamburg, Germany 1999;2:163. [Google Scholar]
Mazeh 2006 {published data only}
- Mazeh D, Zemishlani H, Barak Y, Mirecki I, Paleacu D. Donepezil for negative signs in elderly patients with schizophrenia: an add‐on, double‐blind, crossover, placebo‐controlled study. International Psychogeriatrics 2006;18(3):429‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McKibbin 2006 {published data only}
- McKibbin CL, Patterson TL, Norman G, Patrick K, Jin H, Roesch S, Mudaliar S, Barrio C, Hanlon K, Griver K, Sirkin A, Jeste DV. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: a randomized controlled trial. Schizophrenia Research 2006;86(1‐3):36‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McQuaid 2001 {published data only}
- McQuaid J, Jeste DV, Patterson TL, McClure FS. Rehabilitation of older patients with schizophrenia. http://www.clinicaltrials.gov 2001.
Mintzer 2000 {published data only}
- Matkovits‐Gupta T, Cucchiaro J, El‐Bizri H, Fairweather D, Klonowski E, Gharabawi G, Lasser R, E‐VMCRFG. Safety and efficacy of iloperidone in patients with psychotic disorders: a randomized, double‐blind multicenter study. Schizophrenia Research. Abstracts of 8th International Congress on Schizophrenia Research; 2001 Apr 28 ‐ May 2; British Columbia, Canada 2001;49(Suppl. 1‐2):238. [MEDLINE: ] [Google Scholar]
- Mintzer JE, Yeung PP, Mullen JA, Sweitzer D. EPS variations in elderly. 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐18; Chicago, USA. 2000.
Mintzer 2004 {published data only}
- Mintzer JE, Mullen JA, Sweitzer DE. A comparison of extrapyramidal symptoms in older outpatients treated with quetiapine or risperidone. Current Medical Research and Opinion 2004;20(9):1483‐91. [DOI] [PubMed] [Google Scholar]
Mohler 1970 {published data only}
- Mohler G. Clinical trial of thiothixene (Navane) in elderly chronic schizophrenics. Current Therapeutic Research Clinical and Experimental 1970;12(6):377‐86. [MEDLINE: ] [PubMed] [Google Scholar]
Myers 2002 {published data only}
- Myers JE, Mahmoud RA, Keith SJ, Csernansky JG. Long‐term benefit of risperidone versus haloperidol for affective symptoms in patients with schizophrenia and schizoaffective disorder. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
Naoki 2003 {published data only}
- Naoki K, Nobuo A, Emi I. Randomized controlled trial on effectiveness of the community re‐entry program to inpatients with schizophrenia spectrum disorder, centering around acquisition of illness self‐management knowledge. Psychiatria et Neurologia Japonica [Seishin Shinkeigaku Zasshi] 2003;105(12):1514‐31. [MEDLINE: ] [PubMed] [Google Scholar]
Nordentoft 1999 {published data only}
- Nordentoft M, Jeppesen P, Petersen L, Thorup A, Abel M, Ohlenschlaeger JK, Jorgensen P, Christensen T. OPUS project: A randomised controlled trial of integrated psychiatric treatment in first episode psychosis. Schizophrenia Research 2003;60:297. [Google Scholar]
Ogunmefun 2009 {published data only}
- Ogunmefun A, Hasnain M, Alam A, Osuala T, Regenold WT. Effect of donepezil on tardive dyskinesia. Journal of Clinical Psychopharmacology 2009;29(1):102‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patterson 2003 {published data only}
- Patterson TL, McKibbin C, Taylor M, Goldman S, Davila‐Fraga W, Bucardo J, Jeste DV. Functional adaptation skills training (FAST): a pilot psychosocial intervention study in middle‐aged and older patients with chronic psychotic disorders. American Journal of Geriatric Psychiatry 2003;11(1):17‐23. [PubMed] [Google Scholar]
Patterson 2005 {published data only}
- Patterson TL, /Bucardo J, McKibbin CL, Mausbach BT, Moore D, Barrio C, Goldman SR, Jeste DV. Development and pilot testing of a new psychosocial intervention for older Latinos with chronic psychosis. Schizophrenia Bulletin 2005;31(4):922‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patterson 2006 {published data only}
- Mausbach BT, Cardenas V, McKibbin CL, Jeste DV, Patterson TL. Reducing emergency medical service use in patients with chronic psychotic disorders: results from the FAST intervention study. Behaviour Research and Therapy 2008;46(1):145‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Mausbach BT, McKibbin C, Goldman S, Bucardo J, Jeste DV. Functional adaptation skills training (FAST): a RANDOMized trial of a psychosocial intervention for middle‐aged and older patients with chronic psychotic disorders. Schizophrenia Research 2006;86(1‐3):291‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pfizer 2006 {published data only}
- Pfizer. A randomized, parallel group, multiple dose, 6‐week study to evaluate safety, tolerability, and pharmacokinetics of asenapine in elderly subjects with psychosis. http://www.clinicaltrials.gov 2006.
Phanjoo 1990 {published data only}
- Phanjoo AL, Link C. Remoxipride versus thioridazine in elderly psychotic patients. Acta Psychiatrica Scandinavica Supplementum 1990;358:181‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prat 2009 {published data only}
- Prat SI, Hapenny AM. Individually based psychosocial rehabilitation for older people with serious mental illness (SMI). http://www.clinicaltrials.gov 2009.
Pratt 2005 {published data only}
- Pratt SI. Medication adherence in older adults. http://www.clinicaltrials.gov 2005.
Pratt 2008 {published data only}
- Pratt SI, Bartels SJ, Mueser KJ, Forester B. Helping older people experience success: an integrated model of psychosocial rehabilitation and health care management for older adults with serious mental illness. American Journal of Psychiatric Rehabilitation 2008;11(1):41‐60. [Google Scholar]
Reams 1998 {published data only}
- Reams SG, Sanger TM, Beasley CM. Olanzapine in the treatment of elderly patients with schizophrenia and related psychotic disorders. Schizophrenia Research 1998;29(1‐2):151. [MEDLINE: ] [Google Scholar]
Ritchie 1999 {published data only}
- Ritchie C, Ames D, Chiu E, O'Connor D, Hall K, Hassett A. Schizophrenia cohort study of olanzapine v risperidone in the elderly (score): analysis of conversion period. International Psychogeriatrics 1999;11(Suppl 1):157‐8. [Google Scholar]
Salganik 1998 {published data only}
- Salganik I, Modai I, Bercovici BR, Kutzuk D, Weizman A. Clozapine vs haloperidol therapy in elderly chronic schizophrenic inpatients: preliminary results. A double blind, cross over randomized study. International Journal of Geriatric Psychopharmacology 1998;1(4):185‐7. [Google Scholar]
Salzman 2000 {published data only}
- Salzman C, Naidoo U, Schwab EP, Czworka KJ. Elderly patients with schizophrenia. 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000; Dec 10‐14; San Juan; Puerto Rico. 2000. [MEDLINE: ]
Seager 1955 {published data only}
- Seager CP. Chlorpromazine in treatment of elderly psychotic women. British Medical Journal 1955;1:882‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shen 2001 {published data only}
- Shen G, Han J, Song L. A comparative the results of serum testosterone levels in different age‐group of male schizophrenics. Sichuan Mental Health 2001;14(4):205‐6. [Google Scholar]
Shin 2004 {published data only}
- Shin S‐K. Effects of culturally relevant psychoeducation for Korean American families of persons with chronic mental illness. Research on Social Work Practice 2004;14(4):231‐9. [Google Scholar]
Sikich 2002 {published data only}
- Sikich L. Critical decisions in the treatment of adolescent and pediatric psychosis. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
- Sikich L, Horrigan JP, Lieberman JA, Barnhill LJ Jr, Sheitman BB, Courvoisie HE. Comparative use of olanzapine and risperidone in psychotic youth. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
Sun Hui 2008 {published data only}
- Sun H, Jiao Y, Li J. A comparative study of quetiapine and risperidone in the treatment of old ‐ men schizophrenia. Journal of Clinical Psychiatry 2008;20(1):14‐5, 46. [Google Scholar]
Sutton 2001 {published data only}
- Sutton VK, Street JS, Kennedy JS, Feldman PD, Breier A. Superiority of olanzapine over risperidone in the control of negative symptoms of schizophrenia and related psychotic disorders in older patients. European Neuropsychopharmacology. Abstracts of the 14th Congress of the European College of Neuropsychopharmacology; 2001 Oct 13‐17; Istanbul, Turkey 2001;11(3):276. [Google Scholar]
Svestka 1972 {published data only}
- Svestka J, Nahunek K. A comparison of pimozide with perphenazine in the treatment of acute schizophrenic psychoses. Activitas Nervosa Superior 1972;14(2):93‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Svestka 1973 {published data only}
- Svestka J, Rodova A, Nahunek K. Comparison of the short‐term therapeutic effect of oxyprothepine and perphenazine in schizophrenic patients A controlled study. Activitas Nervosa Superior 1973;15(2):103‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Svestka 1987 {published data only}
- Svestka J, Nahunek K, Rysanek R, Ceskova E. Double blind comparison of isofloxythepin and haloperidol in the treatment of acute schizophrenic and schizoaffective psychoses. 29th Annual Psychopharmacology Meeting (1987, Jesenik, Czechoslovakia). Activitas Nervosa Superior 1987;29(3):205‐6. [Google Scholar]
Svestka 1989a {published data only}
- Svestka J, Nahunek K, Ceskova E, Rysanek R, Balsikova J. Controlled cross over comparison of isofloxythepin and perphenazine in the treatment of schizophrenic psychoses. Activitas Nervosa Superior 1989;31(1):32‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Svestka 1989b {published data only}
- Svestka J, Rysanek R, Ceskova E, Nahunek K. Cross‐over comparison of the therapeutic effects of sulpiride and perphenazine in schizophrenic and schizoaffective psychoses. Acta Nervosa Superior 1989;31(1):35‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Svestka J, Rysanek R, Nahunek K, Ceskova E. Therapeutic results in schizophrenic and schizoaffective psychoses with sulpiride (Eglonyl Alkaloid), as compared with perphenazine (Perfenazin Spofa) [Vysledky lecby schizofrennich a schizoafektivnich nemocnych sulpiridem (Eglonyl alkaloid) ve srovnani s perfenazinem (Perfenazin Spofa)]. Ceskoslovenska Psychiatrie 1990;86(3):145‐56. [MEDLINE: ] [PubMed] [Google Scholar]
Svestka 1990 {published data only}
- Svestka J, Ceskova E, Rysanek R, Obrovska V. Double blind clinical comparison of risperidon and haloperidol in acute schizophrenic and schizoaffective psychoses. 32nd Annual Psychopharmacological Meeting; 1990, Jesenik, Czechoslovakia. Activitas Nervosa Superior 1990;32(3):237‐8. [Google Scholar]
Tariot 2000 {published data only}
- Tariot PN, Salzman C, Yeung PP, Pultz JP, Rak IW. Long‐term use of quetiapine in elderly patients with psychotic disorders. Clinical Therapeutics 2000;22(9):1068‐83. [DOI] [PubMed] [Google Scholar]
Tollefson 1997 {published data only}
- Almond S, O'Donnell O. Cost analysis of the treatment of schizophrenia in the UK: a comparison of olanzapine and haloperidol. Pharmacoeconomics 1998;13(5 Pt 2):575‐88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Basson B, Kinon BJ, Gilmore JA, Taylor CC, Tollefson GD, Czekalla J. Factors influencing weight change in patients with schizophrenia treated with olanzapine verus haloperidol or risperidone. Journal of Psychopharmacology 2000;14(3 Suppl):A60. [Google Scholar]
- Beasley CM. Safety of olanzapine. Journal of Clinical Psychiatry 1997;15(2):19‐21. [MEDLINE: ] [PubMed] [Google Scholar]
- Beasley CM, Sayler ME, Keisler GM, Potvin JH, Sanger TM, Tollefson GD. The influence of pharmacotherapy on self‐directed and externally‐directed aggression in schizophrenia. Schizophrenia Research 1998;29(1‐2):28. [Google Scholar]
- Cohen LS, Goldstein JM, Lee H, Tohen M, Andersen S, Tollefson GD. Sex and neuroendocrine differences following treatment with typical and atypical antipsychotics. 151st Annual Meeting of American Psychiatric Association; 1998;Toronto; Ontorio, Canada:May 30‐June 4, 1998. [MEDLINE: ] [Google Scholar]
- Crawford AMK, Gomez JC, Beasley CM Jr, Tollefson GD. Olanzapine versus haloperidol: analysis of schizophrenic patients from the multi‐center international trial. European Neuropsychopharmacology 1997;7:P.2.015. [DOI] [PubMed] [Google Scholar]
- Dellva MA, Beasley CM, Tamura RN, Tollefson GD. What is the differential risk of tardive dyskinesia with the novel antipsychotic olanzapine?. Proceedings of XXIst Collegium Internationale Neuro‐psychopharmacologicum; 1998;Glasgow; Scotland, UK:July 12‐16, 1998. [MEDLINE: ] [Google Scholar]
- Eli Lilly, Company. Tollefson 1997 ‐ olz vs hpl. Data on file 2001:22‐9.
- Goldstein JM, Cohen LS, Horton NJ, Lee H, Andersen S, Tohen M, Crawford A, Tollefson G. Sex differences in clinical response to olanzapine compared with haloperidol. Psychiatry Research 2002;110(1):27‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gregor KJ, Hamilton SH, Edgell ET. Functional outcomes in schizophrenia: a European comparison of olanzapine and haloperidol. Schizophrenia Research 2000;41(1):189. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Inada T, Beasley CM, JR//Tanaka Y, Walker DJ. Extrapyramidal symptom profiles assessed with the Drug‐Induced Extrapyramidal Symptom Scale: comparison with Western scales in the clinical double‐blind studies of schizophrenic patients treated with either olanzapine or haloperidol. International Clinical Psychopharmacology 2003;18(1):39‐48. [DOI] [PubMed] [Google Scholar]
- Jaton LA, Kinon BJ, Rotelli MD, Kaiser C, Kollack‐Walker S. Differential rate of weight gain present among patients treated with olanazpine. Schizophrenia Research 2003;60(1):357. [MEDLINE: ] [Google Scholar]
- Jaton LA, Kinon BJ, Rotelli MD, Kaiser C, Kollack‐Walker S. Differential rate of weight gain present among patients treated with olanzapine. Schizophrenia Research. 2003. [MEDLINE: ]
- Johnstone BM, Obenchain RL, Croghan TW, Tunis SL, Kniesner TJ. To evaluate the cost‐effectiveness of olanzolpine compared to haloperidol for schizophrenia. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; 1998 May 30 ‐ Jun 4; Toronto, Ontario, Canada. 1998, issue Nr541.
- Kennedy JS, Jeste D, Kaiser CJ, Golsham S, Maguire GA, Tollefson G, Sanger T, Bymaster FP, Kinon BJ, Dossenbach M, Gilmore JA, Breier A. Olanzapine vs haloperidol in geriatric schizophrenia: analysis of data from a double‐blind controlled trial. International Journal of Geriatric Psychiatry 2003;18:1013‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Basson BR, Tollefson GD. Effect of long‐term olanzapine treatment on weight change in schizophrenia. Schizophrenia Research 2000;41(1‐ Special Issue):195‐6. [MEDLINE: ] [Google Scholar]
- Kinon BJ, Milton DR, Hill AL, Williamson DJ. Effective resolution of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia with olanzapine. Journal of Psychopharmacology 2000;14(3 Suppl):A60. [Google Scholar]
- Leucht S, Shamsi SAR, Busch R, Kissling W, Kane JM. Predicting antipsychotic drug response‐‐replication and extension to six weeks in an international olanzapine study. Schizophrenia Research 2008;101(1‐3):312‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mallinckrodt C, David S, Burns P, Breier A. Long‐term efficacy and safety of olanzapine in patients with inadequate initial response to haloperidol or olanzapine. European Neuropsychopharmacology 1998;8(Suppl 2):S228. [MEDLINE: ] [Google Scholar]
- Mallinckrodt CH, David SR, Burns PR, Breier A. Long‐term efficiacy and safety of olanzapine in patients with inadequate initial response to haloperidol or olanzapine. Schizophrenia Research 1999;36:288. [MEDLINE: ] [Google Scholar]
- Pilowsky LS, Busatto GF, Taylor M//Costa DC, Sharma T, Sigmundsson T, Ell P, Nohria V, Kerwin RW. Dopamine D‐2 receptor occupancy in vivo by the novel atypical antipsychotic olanzapine ‐ A (123)I IBZM single photon emission tomography (SPET) study. Psychopharmacology 1996;124(1‐2):148‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Street JS, Tamura R, Sanger T, Tollefson G. Long‐term treatment‐emergent dyskinetic symptoms in patients treated with olanzapine and haloperidol. Psychopharmacology Bulletin 1996;32(3):522. [MEDLINE: ] [Google Scholar]
- Street JS, Tollefson GD, Tohen M, Sanger TM, Clark WS, Gannon KS, Wei HX. Olanzapine for psychotic conditions in the elderly. Psychiatric Annals 2000;30:191‐6. [MEDLINE: ] [Google Scholar]
- Tollefson G, Lu Y. Comorbid mood disturbance in schizophrenia. Schizophrenia Research 1997;24:192. [Google Scholar]
- Tollefson GD, Lu Y. Comorbid mood disturbance in schizophrenia. Biological Psychiatry 1997;41:101S. [Google Scholar]
- Tollefson GD, Sanger TD, Lieberman JA. Olanzapine versus haloperidol in the treatment of first episode psychosis. Biological Psychiatry 1997;41:73S. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Sanger TM. A blinded trial on the course and relationship of depressive symptoms in schizophrenia. Schizophrenia Research 1998;29(1,2):205. [Google Scholar]
- Tollefson GD, Sanger TM, Andersen SW. Depressive signs and symptoms in schizophrenia: a prospective blinded trial of olanzapine and haloperidol. European Neuropsychopharmacology 1998;8(Suppl 2):225. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Tran PV, Hamilton S, Kuntz A. Olanzapine versus risperidone in the treatment of psychosis. Preliminary report. Biological Psychiatry 1997;41:20S. [Google Scholar]
- Tran P, Lu Y, Sanger T, Beasley C, Tollefson G. Olanzapine in the treatment of schizoaffective disorder. Psychopharmacology Bulletin 1996;32(3):527. [Google Scholar]
- Tran PV, Dellva MA, Tollefson GD, Wentley AL, Beasley CM Jr. Oral olanzapine versus oral haloperidol in the maintenance treatment of schizophrenia and related psychoses. British Journal of Psychiatry 1998;172:499‐505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tunis SL. The impact of schizophrenic patient functionality on service utilization and cost. Based on a presentation by Sandra L. Tunis, PhD. American Journal of Managed Care 1999;5(10 Suppl):S583‐90. [MEDLINE: ] [PubMed] [Google Scholar]
- Wright P, Tollefson GD, Beasley CM, Tamura RN, Tran PV, Potvin JII. A blinded, controlled, long‐term study of the comparative incidence of treatement‐emergent tardive dyskinesia with olanzapine or haloperidol. Schizophrenia Research 1998;29(1‐2):206. [DOI] [PubMed] [Google Scholar]
Tran 1997 {published data only}
- Ahmed S, Zhang F, Lindborg S, Tohen M, Breier A. A comparison of olanzapine versus risperidone on improvement in negative symptoms and emotional discomfort in patients with schizophrenia. Schizophrenia Research 2003;60:270. [Google Scholar]
- Ahmed S, Zhang F, Walker D, Beglinger L, Earley WR, Tran PV. Olanzapine versus risperidone for treatment of negative symptoms in schizophrenia. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California, USA. 2003.
- David S, Crawford AM, Breier A. Prolactin levels in olanzapine versus typical and atypical antipsychotics. European Neuropsychopharmacology 1998;8(Suppl 2):s229. [Google Scholar]
- David SR, Meehan KM, Sutton VK/, Taylor CC. Treatment of negative symptoms with olanzapine in comparison with other novel antipsychotic agents. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):s140. [Google Scholar]
- Edgell ET, Andersen SW, Johnstone BM, Dulisse B, Revicki D, Breier A, Gavart S. Olanzapine versus risperidone: a prospective comparison of clinical and economic outcomes in schizophrenia. European Psychiatry 2000;15(Suppl 2):408s. [DOI] [PubMed] [Google Scholar]
- Eli Lilly, Company. Tran 1997 ‐ olz vs risp. Data on file 2001:1‐9.
- Feldman PD, Kaiser C, Kennedy JS, Sutton VK, Tran PV, Tollefson GD, Zhang F, Breier A. Comparison of risperidone and olanzapine in the control of negative symptoms of chronic schizophrenia and related psychotic disorders in patients aged 50 to 65 years. Journal of Clinical Psychiatry 2003;64(9):998‐1004. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kennedy J, Basson B, Tran P, Beasley C, Bymaster F, Breier A. The comparative anti‐muscarinic‐like adverse event profiles of olanzapine and risperidone treatment in patients with schizophrenia spectrum psychosis. Proceedings of the 39th Annual Meeting of the New Clinical Drug Evaluation Unit; 1999 Jun 1‐4; Boca Raton, Florida, USA. 1999:89.
- Kinon BJ, Zhao Z, Barber BL, Wirtz HS. Acute response to olanzapine predicts the continued improvement in patients with schizophrenia. Schizophrenia Research. 2003. [MEDLINE: ]
- Kollack‐Walker S, Lipkovich I, Ahmed S. Treatment‐emergent EPS symptoms during treatment with olanzapine or risperidone. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California, USA. 2003.
- Owens DG. Olanzapine produced a higher clinical response rate than risperidone in schizophrenia. Evidence‐Based Mental Health 1998;1(2):55. [Google Scholar]
- Pickar D. Clinical profiles of the new antipsychotic agents. Proceedings of the 150th Annual Meeting of the American Psychiatric Association; 1997 May 17‐22; San Diego, California, USA. USA, 1997.
- Tran P, Dellva M, Rampey V, Tollefson G, Beasley C. Long‐term continuation therapy with the novel antipsychotic olanzapine: a review of the clinical experience. Proceedings of the 9th European College of Neuropsychopharmacology Congress; 1996 Sep 21‐25; Amsterdam, The Netherlands. 1996.
- Zhao Z, Kinon BJ, Barber BL, Wirtz HS. Acute response to olanzapine predicts continued improvement in schizophrenia. Schizophrenia Research 2003;60:308. [Google Scholar]
Twamley 2005 {published data only}
- Twamley EW, Padin DS, Bayne KS, Narvaez JM, Williams RE, Jeste DV. Work rehabilitation for middle‐aged and older people with schizophrenia: a comparison of three approaches. Journal of Nervous and Mental Disease 2005;193(9):596‐601. [DOI] [PubMed] [Google Scholar]
Twamley 2008 {published data only}
- Twamley EW, Narvaez JM, Becker DR, Bartels SJ, Jeste DV. Supported employment for middle‐aged and older people with schizophrenia. American Journal of Psychiatric Rehabilitation 2008;11(1):76‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tzimos 2007 {published data only}
- Tzimos A, Kramer M, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. A 6‐week placebo‐controlled study on the safety and tolerability of flexible doses of oral paliperidone extended‐release tablets in the treatment of schizophrenia in elderly patients. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
- Tzimos A, Kramer M, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. A 6‐week placebo‐controlled study on the safety and tolerability of flexible doses of oral paliperidone extended‐release tablets in the treatment of schizophrenia in elderly patients. Proceedings of the Collegium Internationale Neuro‐Psychopharmacologium 25th Biennial Congress; 2006 July 9‐13; Chicago, Illinois. 2006. [MEDLINE: ]
- Tzimos A, Kramer M, McLemore J, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. A 6‐week placebo‐controlled study of the safety and tolerability of flexible doses of oral paliperidone extended‐release tablets in the treatment of schizophrenia in elderly patients. Schizophrenia Bulletin 2007;33(2):464. [Google Scholar]
- Tzimos A, Samokhvalov V, Kramer M, Ford L, Gassmann‐Mayer C, Lim P, Eerdekens M. Safety and tolerability of oral paliperidone extended‐release tablets in elderly patients with schizophrenia: a double‐blind, placebo‐controlled study with six‐month open‐label extension. American Journal of Geriatric Psychiatry 2008;16(1):31‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vaglum 2002 {published data only}
- Vaglum P, Friis S, Melle I, Opjordsmoen S, Larsen TK, Simonsen E, McGlashan TH. Does duration of untreated psychosis bias schizophrenia study samples?. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
Wang 2002 {published data only}
- Wang J, Xie S. Health economic impact of novel versus older anti‐psychotic medications in schizophrenia. Sichuan Mental Health 2002;15(1):8‐10. [Google Scholar]
Wang 2005 {published data only}
- Wang Z‐J, Du X‐S, Wang J‐L. Comparative study on treatment of aged schizophrenic patients with risperidone. Journal of Clinical Psychological Medicine 2005;15(5):289‐90. [Google Scholar]
Wang LiLi 2008 {published data only}
- Wang L, Xun Z, Wang Y. A comparative study of risperidone oral solution and risperidone tablets in the treatment of elderly schizophrenia. Journal of Clinical Psychiatry 2008;21(2):92‐4. [Google Scholar]
Wang R 2002 {published data only}
- Wang R, Geng Y, Zhang S, Zhang R. Family support and psychological treatment of elderly schizophrenia recovery efficacy analysis. Modern Rehabilitation 2002;6(9):1317. [Google Scholar]
Wang XiaoQuan 2008 {published data only}
- Wang X‐Q, Song C. Comparative study of efficacy and safety of quetiapine and risperidone in the treatment of elderly schizophrenia. Medical Journal of Chinese People's Health 2008;16(12):1394‐6. [Google Scholar]
Warner 2006 {published data only}
- Warner J. Randomised controlled evaulation of home treatment for older people with mental illness. National Research Register 2006; Vol. 2.
Yamashita 2005 {published data only}
- Yamashita H, Mori K, Nagao M, Okamoto Y, Morinobu S, Yamawaki S. Influence of aging on the improvement of subjective sleep quality by atypical antipsychotic drugs in patients with schizophrenia: comparison of middle‐aged and older adults. American Journal of Geriatric Psychiatry 2005;13(5):377‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamawaki 1996 {published data only}
- Yamawaki S, Hayashi T, Yokota N, Takahashi T, Sato G, Tahara Y. Serotonin‐dopamine antagonists in elderly schizophrenics. 20th Congress of the Collegium Internationale Neuro‐psychopharmacologicum; 1996 Jun 23‐27; Melbourne, Australia. 1996. [MEDLINE: ]
Yang 2002 {published data only}
- Yang YK, Nelson L, Kamaraju L, Wilson W, McEvoy JP. Nicotine decreases bradykinesia‐rigidity in haloperidol‐treated patients with schizophrenia. Neuropyschopharmacology 2002;27(4):684‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yu AiZhen 2008 {published data only}
- Yu A‐Z, Peng Y‐Y, Zeng C‐Y, Luo Y‐Z. Olanzapine and perphenazine in the treatment of senile schizophrenia. Chinese Healthcare Innoviation 2008;3(8):107, 118. [Google Scholar]
Zisook 2002 {published data only}
- Zisook S. Citalopram augmentation in older patients with schizophrenia. http://www.clinicaltrials.gov 2002.
- Zisook S, Kasckow JW, Golshan S, Fellows I, Solorzano E, Lehman D, Mohamed S, Jeste DV. Citalopram augmentation for subsyndromal symptoms of depression in middle‐aged and older outpatients with schizophrenia and schizoaffective disorder: a randomized controlled trial. Journal of Clinical Psychiatry 2009;70(4):562‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Anon 2009 {published data only}
- Anon. A multicentre, double‐blind, randomised, parallel‐group comparison of quetiapine and haloperidol in the treatment of elderly patients presenting with dementia and psychoses (5077il/0049). AstraZeneca Clinical Trials [www.astrazenecaclinicaltrials.com] 2009.
Barak 2001 {published data only}
- Barak Y, Zemishlani C, Mirecki I, Shamir E, Toren P, Weizman R. Olanzapine vs. haloperidol for elderly schizophrenia patients. Proceedings of the 10th Congress of the International Psychogeriatric Association; 2001 Sept 9‐14; Nice, France. 2001:202S.
Chu 2006 {published data only}
- Chu J. Comparative study of quetiapine and risperidone in the treatment of late onset schizophrenias. Journal of Clinical Psychiatry 2006;16(1):34‐5. [Google Scholar]
Dubovsky 2010 {published data only}
- Dubovsky SL, Frobose C, Phiri P, Panagides J. Short‐term tolerability, safety, and pharmacokinetic profile of asenapine in older patients with psychosis. Schizophrenia Research 2010;117(2‐3):263. [DOI] [PubMed] [Google Scholar]
Dubovsky 2010a {published data only}
- Dubovsky S, Colleen Frobose C, Phiri P, Panagides J. Short‐term tolerability, safety, and pharmacokinetic profile of asenapine in older patients with psychosis. Proceedings of the 163rd Annual Meeting of the American Psychiatric Association; 2010 May 22‐26; New Orleans, LA. 2010.
Dubovsky 2010b {published data only}
- Dubovsky S, Frobose C, Phiri P, Panagides J. Asenapine: Short‐term tolerability, safety, and pharmacokinetic profile in older patients with psychosis. European Neuropsychopharmacology 2010;1:489‐90. [Google Scholar]
Dubovsky 2011 {published data only}
- Dubovsky SL, Frobose C, Phiri P, Greef R, Panagides J. Short‐term safety and pharmacokinetic profile of asenapine in older patients with psychosis. International Journal of Geriatric Psychiatry 2011;27(5):472‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kircher 2002 {published data only}
- Kircher TTJ, Meisner C, Schwrzler F, Buchkremer G, Wormstall H. The efficacy of specialised old age psychiatric wards: a multicenter randomised clinical trial. European Psychiatry 2002;17(Suppl 1):146s. [Google Scholar]
Luo 2009 {published data only}
- Luo Y. quetiapine and risperidone in the treatment of elderly schizophrenia [Google Translate] [喹硫平与利培酮治疗老年精神分裂症的对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2009;12(24):3110‐214. [Google Scholar]
McGuire 2002 {published data only}
- McGuire G, Kennedy JS, Shaw SC, Kaiser CJ, Sutton V, Street J, et al. Acute double‐blind comparison of olanzapine and haloperidol in schizophrenia patients age >= 60. American Journal of Geriatric Psychiatry 2002;10(2 Suppl 1):168. [Google Scholar]
Mintzer 2001 {published data only}
- Mintzer JE, Mullen JA, Sweitzer DE. Extrapyramidal symptoms in elderly patients treated with quetiapine or risperidone. Proceedings of the 10th Congress of the International Psychogeriatric Association; 2001 Sept 9‐14; Nice, France. 2001:201S.
Möller 2005 {published data only}
- Möller HJ, Riedel M, Eich FX. A randomised, double‐blind clinical trial comparing treatment with amisulpride or risperidone for six weeks in elderly patients with schizophrenia. European Neuropsychopharmacology 2005;15(Suppl 3):S511. [Google Scholar]
NCT01625897 {published data only}
- NCT01625897. A long‐term study of mp‐214 in patients with chronic phase or elderly schizophrenia. http://ClinicalTrials.gov/show/NCT01625897 2012.
Riedel 2009 {published data only (unpublished sought but not used)}
- Riedel M, Eich FX, Moller HJ. A pilot study of the safety and efficacy of amisulpride and risperidone in elderly psychotic patients. European Psychiatry 2009;24(3):149‐53. [DOI] [PubMed] [Google Scholar]
Ritchie 2003 {published data only}
- Ritchie CW, Chiu E, Harrigan S, Hall K, Hassett A, Macfarlane SMM, O'Connor DW, Opie J, Ames D. The impact upon extra‐pyramidal side effects, clinical symptoms and quality of life of a switch from conventional to atypical antipsychotics (risperidone or olanzapine) in elderly patients with schizophrenia. International Journal of Geriatric Psychiatry 2003;18(5):432‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ritchie CW, Chiu E, Harrigan S, Macfarlane S, Mastwyk M, Halliday G, Hustig H, Hall K, Hassett A, O'Connor DW, Opie J, Nagalingam V, Snowdon J, Ames D. A comparison of the efficacy and safety of olanzapine and risperidone in the treatment of elderly patients with schizophrenia: an open study of six months duration. International Journal of Geriatric Psychiatry 2006;21(2):171‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ritchie 2010 {published data only}
- Ritchie CW, Harrigan S, Mastwyk M, Macfarlane S, Cheesman M, Ames D. Predictors of adherence to atypical antipsychotics (risperidone or olanzapine) in older patients with schizophrenia: an open study of 31/2 years duration. International Journal of Geriatric Psychiatry 2010;25(4):411‐8. [DOI] [PubMed] [Google Scholar]
Sutton 2001a {published data only}
- Sutton VK, Street JS, Kennedy JS, Feldman PD, Breier A, Eli L&C. Superiority of olanzapine over risperidone in the control of negative symptoms in older patients. Proceedings of the 10th Congress of the International Psychogeriatric Association; 2001 Sept 9‐14; Nice, France. 2001:201S.
Zhang 2009 {published data only}
- Zhang B. aripiprazole in the treatment of schizophrenia in old age [Google Translate] [阿立哌唑治疗老年期精神分裂症的研究]. 中国当代医药 2009; Vol. 16, issue 18:47‐8.
Zhe 2007 {published data only}
- Zhe J, Shi X‐Y, Tan T‐C, Liu W‐X, Tao P‐Y, Wang J, Liu G‐F, Cao J‐X, Xue Q‐X, Rong L‐Z. Comparative study of aripiprazole and risperidone in treatment of late onset schizophrenia. Linchuang Jingshen Yixue Zazhi 2007;17(5):327‐8. [Google Scholar]
伏彩霞 2010 {published data only}
- 伏彩霞, 翟歆明, 李拉珂, 王宝安. Aripiprazole in the treatment of senile schizophrenia [Google Translate] [阿立哌唑治疗老年期精神分裂症的疗效观察]. Modern Medicine and Health [现代医药卫生] 2010;26(7):1001‐2. [Google Scholar]
余成国 2009 {published data only}
- 余成国, 蒋国庆, 冉江峰. Aripiprazole and risperidone in the treatment of senile schizophrenia [Google Translate] [阿立哌唑与利培酮治疗老年性精神分裂症对照研究]. Chongqing Medical Journal 2009;38(7):785. [Google Scholar]
刁端忠 2010 {published data only}
- 刁端忠. Controlled observation of quetiapine and perphenazine in the treatment of senile schizophrenia [奎硫平与奋乃静治疗老年期精神分裂症的对照观察]. 中国民康医学 2010;22(23):3047‐8. [Google Scholar]
刘金光 2010 {published data only}
- 刘金光, 克纳新. Olanzapine and risperidone in the treatment of elderly schizophrenia [Google Translate] [奥氮平与利培酮治疗老年精神分裂症的对照研究]. Shanxi Clinical Medicine 2010; Vol. 19, issue 8A:575‐8.
吕斌军 2009 {published data only}
- 吕斌军, 王建林, 林永清. Quetiapine and risperidone in the treatment of elderly schizophrenia [Google Translate] [喹硫平与利培酮治疗老年精神分裂症的对照研究]. Shanghai Archives of Psychiatry [上海精神医学] [上海精神醫學] 2009; Vol. 21, issue 1:34‐6.
周益辉 2010 {published data only}
- 周益辉, 王兵华, 曾德志, 钟跃峰. Aripiprazole impact on the quality of life of elderly patients with schizophrenia [阿立哌唑对老年精神分裂症患者生活质量的影响]. 海南医学院学报 2010;16(11):1424‐32. [Google Scholar]
宋丽 2009 {published data only}
- 宋丽, 樊凌姿. Olanzapine and risperidone in the treatment of elderly schizophrenia [Google Translate] [奥氮平与利培酮治疗老年精神分裂症的对照研究]. Medicine World 2009;11(10):583‐5. [Google Scholar]
崔明伟 2011 {published data only}
- 崔明伟. Ziprasidone, and perphenazine in the treatment of elderly schizophrenia [齐拉西酮与奋乃静治疗老年精神分裂症对照研究]. 中国民康医学 2011;23(16):1970‐2055. [Google Scholar]
张保利 2010 {published data only}
- 张保利, 兰志敏, 李相桦, 冯锋. Aripiprazole and quetiapine in the treatment of senile schizophrenia [阿立哌唑与奎硫平治疗老年期精神分裂症对照研究]. 临床精神医学杂志 2010; Vol. 20, issue 05:340‐2.
张峻岭 2009 {published data only}
- 张峻岭. Clinical analysis of 104 cases older schizophrenia [Google Translate] [老年精神分裂症104例疗效分析]. 中国现代医生 2009; Vol. 47, issue 18:66‐7.
张疆莉 2010 {published data only}
- 张疆莉, 张建宏, 申彪. Aripiprazole orally disintegrating tablets in the treatment of schizophrenia in old age were observed [Google Translate] [阿立哌唑口腔崩解片治疗老年期精神分裂症疗效的对照观察]. 中国现代药物应用 2010; Vol. 4, issue 14:119‐20.
张艳琦 2010 {published data only}
- 张艳琦, 于振东. Aripiprazole and perphenazine in schizophrenia compared treatment of elderly [Google Translate] [阿立哌唑与奋乃静治疗老年精神分裂症疗效比较]. 中外医疗 2010;5:114. [Google Scholar]
张艳琦 2010a {published data only}
- 张艳琦, 于振东. Ziprasidone treatment of elderly schizophrenia [Google Translate] [齐拉西酮治疗老年精神分裂症对照研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2010; Vol. 20, issue 3:198‐9.
徐清 2010 {published data only}
- 徐清, 蒋幸衍, 陆雅娜, 邵薇. Aripiprazole and risperidone in the treatment of senile schizophrenia [Google Translate] [阿立哌唑与利培酮治疗老年期精神分裂症的对照研究]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2010;18(9):1036‐7. [Google Scholar]
曾庆翰 2010 {published data only}
- 曾庆翰, 刘峥嵘, 郄晓明. Olanzapine and perphenazine in treatment of elderly schizophrenia [Google Translate] [奥氮平与奋乃静治疗老年精神分裂症对照研究]. 中外医疗 2010;15:125‐6. [Google Scholar]
李国兰 2010 {published data only}
- 李国兰, 孟凤华, 冷树东. Quetiapine and perphenazine in the treatment of senile schizophrenia observed [Google Translate] [喹硫平与奋乃静治疗老年期精神分裂症对照观察]. Medical Journal of Chinese People's Health [中国民康医学] 2010;22(2):127. [Google Scholar]
李永红 2010 {published data only}
- 李永红. Risperidone treatment in elderly schizophrenia clinical observation [利培酮治疗老年精神分裂症临床观察]. 青海医药杂志 2010;40(08):41‐2. [Google Scholar]
李海涛 2010 {published data only}
- 李海涛, 赵明伟. Aripiprazole and quetiapine in the treatment of elderly schizophrenia [Google Translate] [阿立哌唑与喹硫平治疗老年精神分裂症对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2010; Vol. 22, issue 10:1250‐326.
杨仁登 2010 {published data only}
- 杨仁登, 崔林梅, 李恩. Ziprasidone, and perphenazine in the treatment of elderly schizophrenia [齐拉西酮与奋乃静治疗老年精神分裂症对照研究]. 中国民康医学 2010;22(24):312‐3. [Google Scholar]
杨雀屏 2010 {published data only}
- 杨雀屏, 张恒, 包炤华, 周兆新. Aripiprazole and olanzapine in elderly patients clinical analysis of first‐episode schizophrenia [Google Translate] [阿立哌唑与奥氮平治疗老年期首发精神分裂症的临床分析]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2010; Vol. 18, issue 7:773‐5.
梁勇 2011 {published data only}
- 梁勇. Olanzapine in the Psychogeriatric [奥氮平片在老年精神科中的应用]. 当代医学 2011;17(21):11‐2. [Google Scholar]
石保青 2011 {published data only}
- 石保青, 蒋成娣, 李燕. Aripiprazole and risperidone treatment in elderly schizophrenia [阿立哌唑与利培酮治疗老年精神分裂症的对照研究]. 中国民康医学 2011; Vol. 23, issue 16:1971‐6.
管銮友 2009 {published data only}
- 管銮友. Ziprasidone and quetiapine in the treatment of senile schizophrenia [Google Translate] [齐拉西酮与奎硫平治疗老年性精神分裂症的对照研究]. Journal of Military Surgeon in Southwest China 2009; Vol. 11, issue 5:843‐5.
罗琦 2010 {published data only}
- 罗琦, 陈筱章, 熊典樟, 邹果果. Palley risperidone and perphenazine in the treatment of senile [Google translate] [帕利哌酮与奋乃静治疗老年期]. Journal of the Yichun University [宜春学院学报] 2010;32(4):80, 153. [Google Scholar]
董晓茹 2009 {published data only}
- 董晓茹, 孙利, 武晓杰. The clinical investigation of benazepril and amiodipine on elderly hypertension by 24‐hours ambulatory blood pressure monitoring [奥氮平与氯丙嗪治疗精神分裂症对照研究]. 中国医药导报 2009;6(20):56‐7. [Google Scholar]
董继承 2009 {published data only}
- 董继承, 席巧真, 缪竞诚. Quetiapine and risperidone in the treatment of elderly schizophrenia [Google Translate] [喹硫平与利培酮治疗老年精神分裂症对照研究]. Shandong Archives of Psychiatry [山东精神医学] 2009; Vol. 22, issue 4:289‐90.
蒋幸衍 2009 {published data only}
- 蒋幸衍, 黄寅平, 徐清, 陆雅娜. Aripiprazole in elderly patients with schizophrenia quality of life [Google Translate] [阿立哌唑对老年精神分裂症患者生活质量的影响]. Chinese Journal of Health Psychology [中国健康心理学杂志] 2009;17(12):1421‐3. [Google Scholar]
诸春明 2010 {published data only}
- 诸春明. Quetiapine in elderly patients with schizophrenia quality of life [Google Translate] [奎硫平对老年精神分裂症患者生活质量的影响]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2010;20(4):262‐4. [Google Scholar]
谢国建 2010 {published data only}
- 谢国建, 罗捷, 冉江峰. Domestic quetiapine and perphenazine treatment in elderly schizophrenia control study [国产奎硫平与奋乃静治疗老年精神分裂症对照研究]. 四川精神卫生 2010;23(04):238‐9. [Google Scholar]
陈永红 2010 {published data only}
- 陈永红. Aripiprazole and risperidone in the treatment of senile schizophrenia [Google Translate] [阿立哌唑和利培酮治疗老年性精神分裂症的对照研究]. 中国实用医药 2010; Vol. 5, issue 27:31‐2.
黄峰 2010 {published data only}
- 黄峰. Ziprasidone clinical study of elderly patients with schizophrenia [Google Translate] [齐拉西酮治疗老年精神分裂症的临床研究]. 内科 2010;5(1):5‐6. [Google Scholar]
黄峰 2010a {published data only}
- 黄峰. Ziprasidone and risperidone in the treatment of elderly clinical controlled study of schizophrenia [Google Translate] [齐拉西酮与利培酮治疗老年精神分裂症的临床对照研究]. Medical Journal of Chinese People's Health [中国民康医学] 2010;22(10):1214‐5. [Google Scholar]
黄峰 2010b {published data only}
- 黄峰. Controlled study of ziprasidone treatment of senile negative symptoms of schizophrenia [齐拉西酮治疗老年期精神分裂症阴性症状的对照研究]. 现代医药卫生 2010;26(18):2745‐7. [Google Scholar]
黄振英 2010 {published data only}
- 黄振英, 顾广善. Ziprasidone and quetiapine in the treatment of elderly schizophrenia control analysis [齐拉西酮与喹硫平治疗老年精神分裂症的对照分析]. 中国医药指南 2010;8(35):297‐8. [Google Scholar]
References to ongoing studies
Barnes 2002 {published data only}
- Barnes T. A prospective study of tardive dyskinesia in the elderly; single blind comparison of amisulpride and risperidone. National Research Register 2003; Vol. 1.
- Barnes T. A prospective study of tardive dyskinesia in the elderly; single‐blind comparison of amisulpride and risperidone. National Research Register 2002; Vol. 3.
Lacro 2001 {published data only}
- Lacro J. Antipsychotic treatment in late life schizophrenia. CRISP database. https://www‐commons.cit.nih.gov/crisp/index.html accessed 19th February 2001.
Additional references
Allison 1999
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ. Antipsychotic‐induced weight gain: a comprehensive research synthesis. American Journal of Psychiatry 1999;156(11):1686‐96. [DOI] [PubMed] [Google Scholar]
Almeida 1995
- Almeida OP, Howard RJ, Levy R. David AS. Psychotic states arising in late life (late paraphrenia) psychopathology and nosology. The British Journal of Psychiatry 1995;166:205‐14. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [HLDS020600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arndt 1998
- Arndt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 1998;18:63–101. [DOI] [PubMed] [Google Scholar]
Castle 1991
- Castle DJ, Murray RM. The neurodevelopmental basis of sex differences in schizophrenia. Psychological Medicine 1991;21:565‐75. [DOI] [PubMed] [Google Scholar]
Castle 1993
- Castle DJ, Murray RM. The epidemiology of late‐onset schizophrenia. Schizophrenia Bulletin 1993;19:691–700. [DOI] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD. Cochrane Reviewers' Handbook 4.0. Cochrane Database of Systematic Reviews. Oxford: Update Software, 2000, issue 1.
Copeland 1992
- Copeland JRM, Davidson IA, Dewey ME, Gilmore C, Larkin BA, McWilliam C, Saunders PA, Scott A, Sharma V, Sullivan C. Alzheimer’s disease, other dementias, depression and pseudodementia: prevalence, incidence and three‐year outcome in Liverpool. British Journal of Psychiatry 1992;161:230–9. [DOI] [PubMed] [Google Scholar]
Copeland 1998
- Copeland JRM, Dewey ME, Scott A, Gilmore C, Larkin BA, Cleave N, McCracken CFM, McKibbin PE. Schizophrenia and delusional disorder in older age: community prevalence, incidence, comorbidity and outcome. Schizophrenia Bulletin 1998;24:153–61. [DOI] [PubMed] [Google Scholar]
Creese 1976
- Creese I, Burt IR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 1976;192:481–3. [DOI] [PubMed] [Google Scholar]
Davis 1991
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. American Journal of Psychiatry 1991;148:1474–86. [DOI] [PubMed] [Google Scholar]
DeLisi 1992
- DeLisi LE. The significance of age of onset for schizophrenia. Schizophrenia Bulletin 1992;18:209–15. [DOI] [PubMed] [Google Scholar]
Devanand 1998
- Devanand DP, Marder K, Michaels KS, Sackeim HA, Bell K, Sullivan MA, Cooper TB, Pelton GH, Mayeux R. A randomized, placebo‐controlled dose comparison trial of haloperidol for psychosis and disruptive behaviors in Alzheimer's disease. American Journal of Psychiatry 1998;155:1512‐20. [DOI] [PubMed] [Google Scholar]
Di Giovanni 1998
- Giovanni G, Mascio M, Matteo V, Esposito E. Effects of acute and repeated administration of amisulpride, a dopamine D2/D3 receptor antagonist, on the electrical activity of midbrain dopaminergic neurons. Journal of Pharmacology and Experimental Therapeutics 1998;28:51–7. [PubMed] [Google Scholar]
Duggan 2001
- Duggan L, Fenton M, Dardennes RM, El‐Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews. Oxford, UK: Update Software, 2001, issue 3. [DOI: 10.1002/14651858.CD001359.pub2] [DOI]
Frenchman 1997
- Frenchman IB, Prince T. Clinical experience with risperidone, haloperidol, and thioridazine for dementia‐associated behavioral disturbances. International Journal of Psychogeriatrics 1997;9:431‐5. [DOI] [PubMed] [Google Scholar]
Gilbody 2002
- Gilbody SM, Bagnall AM, Duggan L, Tuunainen A. Gilbody SM, Bagnall AM, Duggan L,Tuunainen A Risperidone versus other atypical antipsychotic medication for schizophrenia. Cochrane Database of Systematic Reviews. Oxford, UK: Update software, 2002, issue 1. [DOI: 10.1002/14651858.CD002306] [DOI]
Green 1997
- Green MF, Marshall BD Jr, Wirshing WC, Ames D, Marder SR, McGurk S, Kern RS, Mintz J. Does risperidone improve verbal working memory in treatment‐resistant schizophrenia?. American Journal of Psychiatry 1997;154:799‐804. [DOI] [PubMed] [Google Scholar]
Harris 1988
- Harris MJ, Jeste DV. Late‐onset schizophrenia: an overview. Schizophrenia Bulletin 1988;14:39‐55. [DOI] [PubMed] [Google Scholar]
Harris 1988a
- Harris MJ, Cullum CM, Jeste DV. Clinical presentation of late‐onset schizophrenia. Journal of Clinical Psychiatry 1988;49:356‐60. [PubMed] [Google Scholar]
Henderson 1998
- Henderson AS, Korten AE, Levings C, Jorm AF, Christensen H, Jacomb PA, Rodgers B. Psychotic symptoms in the elderly: a prospective study in a population sample. International Journal of Geriatric Psychiatry 1998;13(7):484‐92. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. The Cochrane Collaboration, 2008. [Available from www.cochrane‐handbook.org] [Google Scholar]
Honigfeld 1965
- Honigfeld G, Rosebaum MP, Blumenthal IJ, Lambert HL, Roberts AJ. Behavioral improvement in the older schizophrenic patient: drug and social therapies. Journal of the American Geriatrics Society 1965;13:57‐71. [DOI] [PubMed] [Google Scholar]
Howard 1992a
- Howard RJ, Forstl H, Naguib M, Burns A, Levy R. First‐rank symptoms of Schneider in late paraphrenia. Cortical structural correlates. British Journal of Psychiatry 1992;160:108‐9. [DOI] [PubMed] [Google Scholar]
Howard 1992b
- Howard RJ. Late paraphrenia: an update. Journal of the Royal Society of Medicine 1992;3:63‐5. [Google Scholar]
Howard 1993
- Howard RJ, Almeida O, Levy R. Phrenomenology of late‐onset schizophrenia. Schizophrenia Research 1993;9:100. [Google Scholar]
Howard 1993a
- Howard R, Castle D, Wessely S, Murray R. A comparative study of 470 cases of early‐onset and late‐onset schizophrenia. The British Journal of Psychiatry 1993;163:352‐7. [DOI] [PubMed] [Google Scholar]
Howard 1997
- Howard RJ, Graham C, Sham P, Dennehey J, Castle DJ, Levy R, Murray R. A controlled family study of late‐onset non‐affective psychosis (late paraphrenia). The British Journal of Psychiatry 1997;170:511‐4. [DOI] [PubMed] [Google Scholar]
Howard 2000
- Howard R, Rabins PV, Seeman MV Jeste DV and the International Late‐Onset, Schizophrenia Group. Late‐onset schizophrenia and very‐late‐onset schizophrenia‐like psychosis: an international consensus. American Journal of Psychiatry 2000;157:172‐8. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Jeste 1993
- Jeste DV, Lacro JP, Gilbert PL, Kline J, Kline N. Treatment of late‐life schizophrenia with neuroleptics. Schizophrenia Bulletin 1993;19:817‐30. [DOI] [PubMed] [Google Scholar]
Jeste 1995
- Jeste DV, Harris MJ, Krull A, Kuck J, McAdams LA, Heaton R. Clinical and neuropsychological characteristics of patients with late‐onset schizophrenia. American Journal of Psychiatry 1995;152:722‐30. [DOI] [PubMed] [Google Scholar]
Jeste 1996
- Jeste DV, Eastham JH, Lacro JP, Gierz M, Field MG, Harris MJ. Management of late life psychosis. Journal of Clinical Psychiatry 1996;57(suppl 3):39‐45. [PubMed] [Google Scholar]
Jeste 1997
- Jeste DV, McClure FS. Psychoses: Diagnosis and treatment in the elderly. In: Schneider L editor(s). Updates in Geriatric Psychiatry, New Directions for Mental Health Services. 1. San Francisco: Jossey‐Bass, 1997. [DOI] [PubMed] [Google Scholar]
Jeste 1998
- Jeste DV, Lohr JB, Eastham JH, Rockwell E, Caligiuri MP. Adverse effect of long‐term use of neuroleptics: human and animal studies. Journal of Psychiatric Research 1998;32:201‐14. [DOI] [PubMed] [Google Scholar]
Jeste 1999
- Jeste DV, Rockwell E, Harris MJ, Lohr JB, Lacro J. Conventional versus newer antipsychotics in elderly. American Journal of Geriatric Psychiatry 1999;7:70‐6. [PubMed] [Google Scholar]
Jeste 1999a
- Jeste DV, Lacro JP, Bailey A, Rockwell E, Harris MJ, Caligiuri MP. Lower incidence of tardive dyskinesia with risperidone compared with haloperidol in older patients. Journal of the American Geriatrics Society 1999;47:716‐9. [DOI] [PubMed] [Google Scholar]
Katz 1999
- Katz IR, Jeste DV, Minter JE, and the Risperidone Study Group. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: A randomized, double‐blind trial. Journal of Clinical Psychiatry 1999;60:107‐15. [DOI] [PubMed] [Google Scholar]
Keith 1991
- Keith SJ, Regier DA, Rae DS. Schizophrenic disorders, in Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. Edited by Robins LN, Regier DA, pp 33–52. New York: Free Press, 1991. [Google Scholar]
Kennedy 2001
- Kennedy E, Song F, Hunter R, Clarke A, Gilbody S. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database of Systematic Reviews. Oxford, UK: Update Software, 2001, issue 3. [DOI: 10.1002/14651858.CD000440] [DOI] [PMC free article] [PubMed]
Koro 2002
- Koro CE, Fedder DO, L'Italien GJ, Weiss SS, Magder LS, Kreyenbuhl J, Revicki DA, Buchanan RW. Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case‐control study. BMJ 2002;325(7358):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kraepelin 1909
- Kraepelin E. Psychiatrie ein Lehrbuch fur Studierende und Aertzte, 8th ed. Leipzig: Barth, 1909. [Google Scholar]
Kua 1992
- Kua EHA. Community study of mental disorders in elderly Singaporean Chinese using the GMS‐AGECAT package. Australian and New Zealand Journal of Psychiatry 1992;26:502–6. [DOI] [PubMed] [Google Scholar]
Lawler 1999
- Lawler CP, Prioleau C, Lewis MM, Mak C, Jiang D, Schetz JA, Gonzalez AM, Sibley DR, Mailman RB. Interactions of the novel antipsychotic aripiprazole (OPC‐14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 1999;20:612–27. [DOI] [PubMed] [Google Scholar]
Lohr 1992
- Lohr JB, Jeste DV, Harris MJ, Salzman C. Treatment of disordered behavior. In: Salzman C, ed. Clinical geriatric psychopharmacology. pp.79‐113. Baltimore: Williams & Wilkins, 1992. [Google Scholar]
Lohr 1997
- Lohr JB, Alder M, Flynn K, Harris MJ, McAdams LA. Minor physical anomalies in older patients with late‐onset schizophrenia, early onset schizophrenia, depression, and Alzheimer's disease. American Journal of Geriatric Psychiatry 1997;5:318‐23. [DOI] [PubMed] [Google Scholar]
Marriott 2006
- Marriott R, Neil W, Waddingham S. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005580] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meltzer 1976
- Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophrenia Bulletin 1976;2:19–76. [DOI] [PubMed] [Google Scholar]
Meltzer 1989
- Meltzer HY, Matsubara S, Lee J‐C. Classification of typical andatypical antipsychotic drugs on the basis of dopamine D‐1, D‐2 and serotonin‐2 pKi values. Journal of Pharmacology and Experimental Therapeutics 1989;251:238–46. [PubMed] [Google Scholar]
Olshansky 1993
- Olshansky SJ. The human life span: are we reaching the outer limits?. Geriatrics 1993;48(3):85‐8. [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812. [Google Scholar]
Pearlson 1989
- Pearlson GD, Kreger L, Rabins PV, Chase GA, Cohen B, Wirth JB, Schlaepfer TB, Tune LE. A chart review study of late‐onset and early‐onset schizophrenia. American Journal of Psychiatry 1989;146(12):1568‐74. [DOI] [PubMed] [Google Scholar]
Regier 2000
- Regier DA. Community diagnosis counts. Archives of General Psychiatry 2000;57(3):223‐4. [DOI] [PubMed] [Google Scholar]
Salzman 1990
- Salzman C. Principles of psychopharmacology. In: Bienenfeld D editor(s). Verwoerdt's Clinical Geropsychiatry. 1. Baltimore: Williams and Wilkins, 1990:235‐49. [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83. [Google Scholar]
Thomas 1998
- Thomas CS, Lewis S. Which atypical antipsychotic?. British Journal of Psychiatry 1998;172:106‐9. [DOI] [PubMed] [Google Scholar]
Tsuang 1971
- Tsuang MM, Lu LM, Stotsky BA, Cole JO. Haloperidol versus thioridazine for hospitalized psychogeriatric patients: double‐blind study. Journal of the American Geriatrics Society 1971;19:593‐600. [DOI] [PubMed] [Google Scholar]
Van Os 1995
- Os J, Howard R, Takei N, Murray RM. Increasing age is a risk factor for psychosis in the elderly. Social Psychiatry and Psychiatric Epidemiology 1995;30:161–4. [DOI] [PubMed] [Google Scholar]
Webster 1998
- Webster J, Grossberg GT. Late‐life onset of psychotic symptoms. American Journal of Geriatric Psychiatry 1998;6(3):196‐202. [PubMed] [Google Scholar]
Wirshing 1998
- Wirshing DA, Spellberg BJ, Erhart SM, Marder SR, Wirshing WC. Novel antipsychotics and new onset diabetes. Biological Psychiatry 1998;44(8):778‐83. [DOI] [PubMed] [Google Scholar]


