Abstract
BACKGROUND:
A 5-tier prognostic grade group (GG) system was enacted to simplify the risk stratification of patients with prostate cancer in which Gleason scores of ≤6, 3 + 4, 4 + 3, 8, and 9 or 10 are considered GG 1 through 5, respectively. The authors investigated the utility of biopsy GG for predicting long-term oncologic outcomes after radical prostatectomy in an equal-access health system.
METHODS:
Men who underwent prostatectomy at 1 of 6 Veterans Affairs hospitals in the Shared Equal Access Regional Cancer Hospital database between 2005 and 2015 were reviewed. The prognostic ability of biopsy GG was examined using Cox models. Interactions between GG and race also were tested.
RESULTS:
In total, 2509 men were identified who had data available on biopsy Gleason scores, covariates, and follow-up. The cohort included men with GG 1 (909 patients; 36.2%), GG 2 (813 patients; 32.4%), GG 3 (398 patients; 15.9%), GG 4 (279 patients; 11.1%), and GG 5 (110 patients; 4.4%) prostate cancer. The cohort included 1002 African American men (41%). The median follow-up was 60 months (interquartile range, 33-90 months). Higher GG was associated with higher clinical stage, older age, more recent surgery, and surgical center (P < .001) as well as increased biochemical recurrence, secondary therapy, castration-resistant prostate cancer, metastases, and prostate cancer-specific mortality (all P < .001). There were no significant interactions with race in predicting measured outcomes.
CONCLUSIONS:
The 5-tier GG system predicted multiple long-term endpoints after radical prostatectomy in an equal-access health system. The predictive value was consistent across races.
Keywords: Gleason grade, prostate cancer, race, radical prostatectomy, Shared Equal Access Research (SEARCH), survival
INTRODUCTION
In the 1970s, anatomic pathologists Donald Gleason and George Mellinger published a standardized 5-tier grading system of the histologic architecture of prostate specimens for risk stratification of prostate cancer.1 Although major pathologic revisions were incorporated in 2005 and 2014, Gleason grading remains central to contemporary clinical staging.2,3
However, several aspects of the system present clinical challenges. Gleason sum scores range from 2 to 10, but only summed scores of 6 or greater are reported.2 This creates potential confusion, whereby a diagnosis of Gleason sum 6 may be incorrectly considered intermediate-risk disease.4 In addition, Gleason scores are typically incorporated into broader 3-tiered risk groups, which can obscure important prognostic differences.5 For example, Gleason sum 7 (Gleason 3 + 4 and 4 + 3) is considered intermediate-risk disease, with similar management recommendations, despite evidence suggesting divergent biologic behavior.6 Similarly, Gleason sum 8 cancers seem to have a better clinical course than Gleason 9 and 10 although all 3 are considered high-risk disease.7
Consequently, an updated prognostic grade group (GG) system from 1 to 5 has been proposed corresponding to Gleason scores of ≤6, 3 + 4, 4 + 3, 8, and 9 or 10, respectively, to address deficiencies with classic Gleason scoring. 4,8 The new system includes several pathologic grading updates that were incorporated in 2005 and introduces a simplified single score from 1 to 5 for improved clinical utility. The GG system has been collectively endorsed by the editors of the leading urology journals and is now incorporated into the latest World Health Organization classification of prostate cancer.9,10
The seminal article by Epstein et al in 2016 validated the GG system in 20,845 men after radical prostatectomy (RP) and 5501 men after radiotherapy using post-treatment prostate-specific antigen (PSA) recurrence as an endpoint.4 Recently, validation of the GG system in other cohorts of men has demonstrated encouraging results, and several recent publications have reported the efficacy of prostate GGs for predicting long-term endpoints, including prostate cancer-specific mortality (PCSM).11–16
In this study, we tested the validity of the GG system for predicting multiple long-term oncologic endpoints in the Shared Equal Access Research (SEARCH) database, which consists of patients from 6 Veterans Affairs hospitals in the United States. The population is particularly interesting because of the high rate of sustained continuity of care, the presence of fewer socioeconomic confounders than other health care networks, and the large number of African American (AA) men (>32% in each GG group).
MATERIALS AND METHODS
Study Population
After obtaining institutional review board approval, data from men who underwent primary RP at 1 of 6 participating Veterans Affairs hospitals in California, Georgia, and North Carolina were aggregated from the SEARCH database.17 Available cases diagnosed between 2005 and 2015 were included.
For long-term validation of the GGs, a range of preoperative parameters and the time to occurrence of multiple, clinically relevant, long-term endpoints were included. Clinical covariates included age, race, preoperative PSA, prostate volume, clinical stage, year of surgery, and surgical center. Long-term outcomes included biochemical recurrence (BCR), time to the initiation of secondary therapy, development of castrate-resistant prostate cancer (CRPC), development of metastatic disease, PCSM, and all-cause mortality (ACM). BCR was defined as 2 PSA values of 0.2 ng/mL or 1 PSA value >0.2 ng/mL after RP or the receipt of secondary therapy for an elevated PSA value. CRPC was defined as a PSA increase of 2 ng/mL or as an increase 25% greater than the nadir after hormone treatment despite continuous therapy with a luteinizing hormone releasing agonist or antagonist or after orchiectomy. Metastatic disease was determined from bone scans or other imaging studies. PCSM was defined as the presence of metastatic, progressive CRPC at the time of death with no obvious indication of another cause of death, and ACM was determined from medical records.
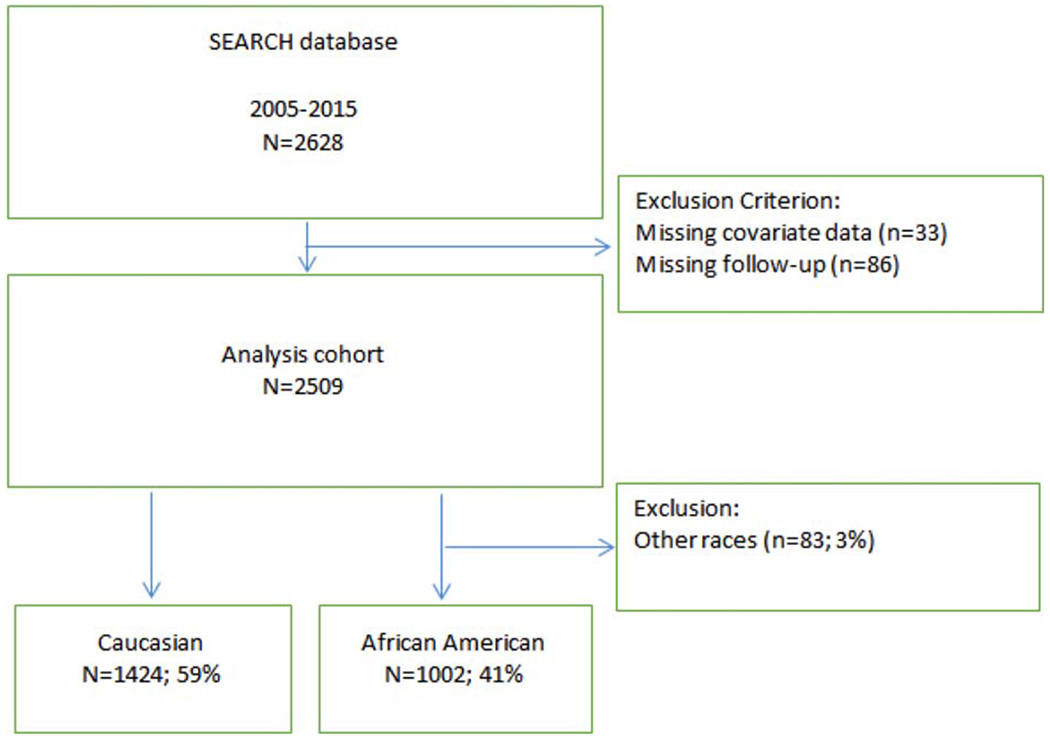
From a total of 2628 cases during the study period, 2509 were identified who had available biopsy Gleason scores, covariate clinical data, and long-term follow-up data (see Fig. 1). Biopsy Gleason scores were then used to stratify the cohort into the 5 corresponding GGs, including GG 1 (909 men; 36.2%), GG 2 (813 men; 32.4%), GG 3 (398 men; 15.9%), GG 4 (279 men; 11.1%), and GG 5 (110 men; 4.4%).
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of the current study.
Statistical Analysis
Characteristics among the 5 GGs were compared using rank-sum tests for continuous variables and chi-square tests for categorical variables. Continuous variables were summarized using median and 25th and 75th percentiles, and categorical variables were summarized using counts and percentages. Continuous covariates included age, prostate volume, preoperative PSA level (log-transformed), and year of surgery. Categorical covariates included race, clinical tumor classification (T1, T2-T4), and surgical center. Kaplan-Meier estimates were then graphed by GG for each endpoint, including BCR, secondary therapy, CRPC, metastatic disease, PCSM, and ACM. Differences in progression to the various endpoints were tested using the log-rank test. Cox proportional-hazards models were used to test the associations between GG and each outcome. Models were adjusted for clinical covariates. To account for changing of Gleason grading over time and the lack of centralized pathology review and the year of surgery and surgical center were also included as covariates. It is noteworthy that there were insufficient events to model PCSM and CRPC, and only univariable analyses were performed for metastatic disease and ACM because of low numbers of events.
We also performed a sensitivity analysis for BCR, secondary therapy, and ACM adjusting for transrectal ultrasound-measured prostate volume, percentage of positive biopsy cores, number of positive cores, and percentage of positive cores among patients who had these covariates available to determine whether the addition of these covariates impacted the results.
In a secondary analysis, we tested whether the prognostic ability of GG varied by race (AA compared with Caucasian) in predicting each of the endpoints by including both main factors along with an interaction term in the same multivariable model.
RESULTS
Patient Characteristics
Clinical characteristics of the study cohort stratified by GG are listed in Table 1. Higher GG was associated with older age, more recent year of surgery, higher clinical stage, higher PSA, and surgical center (all P < .001). Higher GG was also associated with a higher number of positive cores and a higher percentage of positive cores (P < .001). AA men comprised 41% (n = 1102) of the overall cohort, ranging from 32% to 43% of each GG. Distribution according to race among the GGs was not statistically different (P = .069).
TABLE 1.
Characteristics of the 2509 Patients Stratified by Grade Group
| No. of Patients (%) |
||||||
|---|---|---|---|---|---|---|
| Characteristic | GG 1 | GG 2 | GG 3 | GG 4 | GG 5 | P |
| All patients | 909 (36.2) | 813 (32.4) | 398 (15.9) | 279 (11.1) | 110 (4.4) | |
| Age, y | < .001a | |||||
| Median | 61 | 62 | 63 | 63 | 65 | |
| Q1, Q3 | 58, 65 | 58, 66 | 59, 66 | 59, 67 | 60, 68 | |
| Race | .069b | |||||
| White | 541 (60) | 433 (53) | 228 (57) | 150 (54) | 72 (65) | |
| Black | 345 (38) | 352 (43) | 152 (38) | 118 (42) | 35 (32) | |
| Other | 23 (3) | 28 (3) | 18 (5) | 11 (4) | 3 (3) | |
| Year of surgery | < .001a | |||||
| Median | 2009 | 2011 | 2010 | 2011 | 2012 | |
| Q1, Q3 | 2006, 2011 | 2008, 2013 | 2008, 2012 | 2008, 2013 | 2009, 2013 | |
| Clinical tumor classification | < .001b | |||||
| T1 | 657 (72) | 522 (64) | 240 (60) | 182 (65) | 58 (53) | |
| T2-T4 | 252 (28) | 291 (36) | 158 (40) | 97 (35) | 52 (47) | |
| PSA, ng/mL | < .001a | |||||
| Median | 5.6 | 6.0 | 6.7 | 6.7 | 6.9 | |
| Q1, Q3 | 4.4, 7.8 | 4.8, 8.6 | 4.8, 9.6 | 4.8, 9.6 | 4.9, 11.3 | |
| Surgery center | < .001b | |||||
| West LA | 116 (13) | 138 (17) | 71 (18) | 74 (27) | 35 (32) | |
| Palo Alto | 153 (17) | 69 (8) | 34 (9) | 17 (6) | 10 (9) | |
| Augusta | 202 (22) | 212 (26) | 93 (23) | 73 (26) | 16 (15) | |
| Durham | 190 (21) | 194 (24) | 58 (15) | 29 (10) | 8 (7) | |
| San Diego | 106 (12) | 124 (15) | 73 (18) | 61 (22) | 32 (29) | |
| Asheville | 142 (16) | 76 (9) | 69 (17) | 25 (9) | 9 (8) | |
| Percentage positive biopsy cores | < .001a | |||||
| Median | 0.3 | 0.4 | 0.4 | 0.4 | 0.5 | |
| Q1, Q3 | 0.1, 0.4 | 0.3, 0.6 | 0.3, 0.6 | 0.2, 0.5 | 0.3, 0.8 | |
| Total no. of biopsy cores | < .001a | |||||
| Median | 12 | 12 | 12 | 12 | 12 | |
| Q1, Q3 | 10, 12 | 11, 12 | 11, 12 | 12, 12 | 12, 12 | |
| No. of positive biopsy cores | < .001a | |||||
| Median | 3 | 4 | 5 | 4 | 6 | |
| Q1, Q3 | 1, 4 | 3, 6 | 3, 7 | 2, 6 | 4, 9 | |
| TRUS prostate volume, cc | ||||||
| Median | 34.0 | 32.0 | 33.8 | 32.0 | 35.0 | .197a |
| Q1, Q3 | 25.1, 47.0 | 25.0, 43.5 | 25.0, 43.0 | 25.3, 42.2 | 28.3, 44.5 | |
Abbreviations: GG, grade group; PSA, prostate-specific antigen; Q1, 25th percentile; Q3, 75th percentile; TRUS, transrectal ultrasound.
P values were determined with the Kruskal-Wallis test.
P values were determined with the chi-square test.
Primary Outcome Measures: Secondary Therapy, Clinical Progression, and Survival
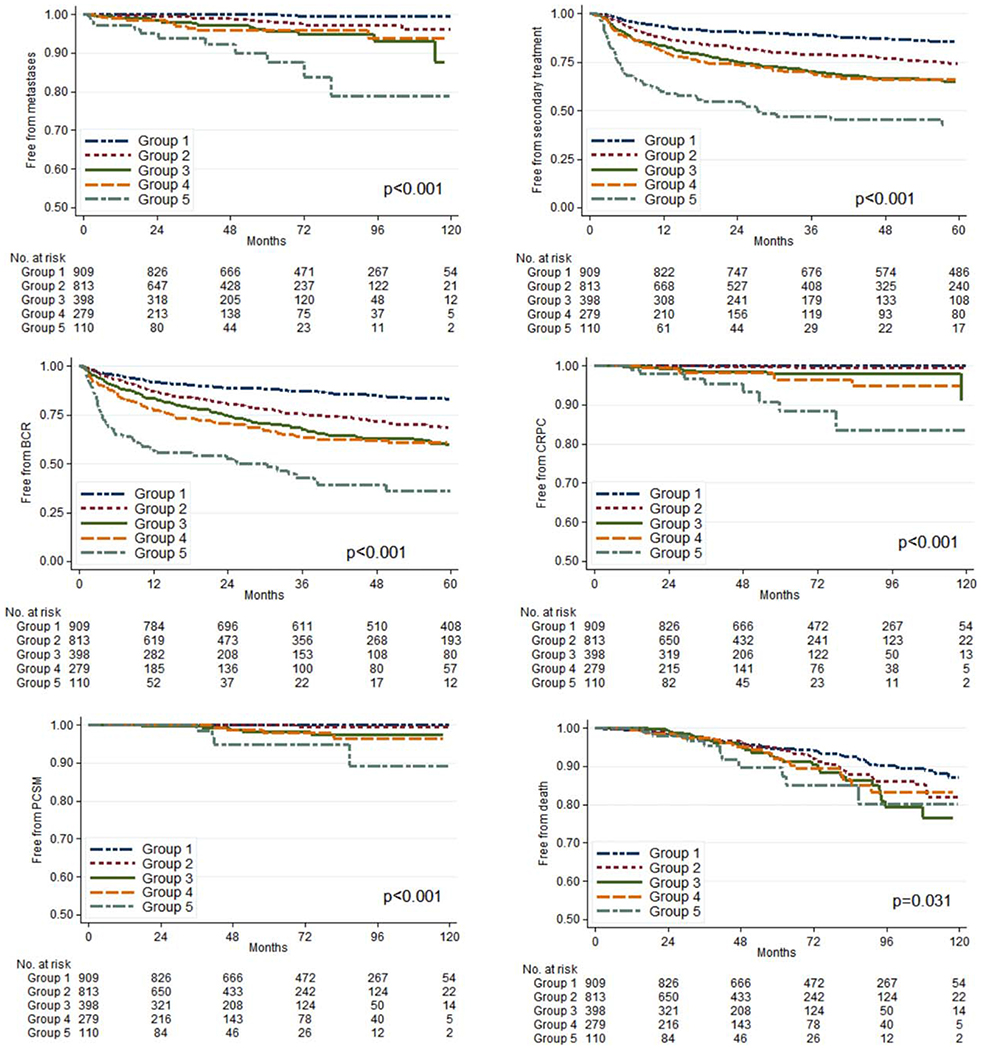
Primary outcome measures stratified by prostate GG are listed in Table 2. The median overall follow-up was 60 months (interquartile range, 33-90 months), and the median PSA follow-up was 46 months (interquartile range, 22-78 months). In total, 645 men (25.7%) had a BCR. Higher GG was associated with higher risk of BCR on univariable and multivariable analyses (P < .001). Two hundred seventy-nine (11.1%) patients received androgen-deprivation therapy, and 532 (21.2%) received radiotherapy. Higher GG was associated with a greater risk of receiving secondary therapy on univariable and multivariable analyses (P < .001). During follow-up, 24 men (1%) developed CRPC, 51 (2%) developed metastases, 14 (0.56%) died from prostate cancer, and 184 (7.3%) died from any cause. On univariable analysis, higher GG was strongly associated with an increased risk of metastases (P < .001). Hazard ratios for select endpoints are listed in Tables 3 and 4. Kaplan-Meier estimates graphed by GG for each endpoint are depicted in Figure 2.
TABLE 2.
Event Counts and Follow-Up for the 2509 Patients Stratified by Grade Group
| No. of Patients (%) |
||||||
|---|---|---|---|---|---|---|
| Outcome Measure | GG 1 | GG 2 | GG 3 | GG 4 | GG 5 | P |
| All patients | 909 (36.2) | 813 (32.4) | 398 (15.9) | 279 (11.1) | 110 (4.4) | |
| Follow-up, mo | < .0011a | |||||
| Median | 73.8 | 51.2 | 50.0 | 51.0 | 40.7 | |
| Q1, Q3 | 46.0, 100.9 | 27.6, 78.8 | 29.3, 78.4 | 26.5, 77.3 | 27.9, 70.2 | |
| BCR | 150 (17) | 212 (26) | 131 (33) | 94 (34) | 58 (53) | < .001b |
| ADT | 53 (6) | 75 (9) | 65 (16) | 46 (16) | 40 (36) | < .001b |
| XRT | 120 (13) | 173 (21) | 110 (28) | 79 (28) | 50 (45) | < .001b |
| CRPC | 0 (0) | 2 (0) | 7 (2) | 7 (3) | 8 (7) | < .001c |
| Metastases | 2 (0) | 13 (2) | 15 (4) | 10 (4) | 11 (10) | < 0.001c |
| Died of PC | 0 (0) | 1 (0) | 5 (1) | 4 (1) | 4 (4) | < .001c |
| ACM | 65 (7) | 55 (7) | 33 (8) | 21 (8) | 10 (9) | < .8292b |
Abbreviations: ACM, all-cause mortality; ADT, androgen-deprivation therapy; BCR, biochemical recurrence; CRPC, castration-resistant prostate cancer; GG, grade group; PC, prostate cancer; Q1, 25th percentile; Q3, 75th percentile; XRT, radiation therapy.
P values were determined with the Kruskal-Wallis test.
P values were determined with the chi-square test.
P values were determined with the Fisher exact test.
TABLE 3.
Association of Grade Group With Biochemical Recurrence and Secondary Treatment
| BCR |
Secondary Treatment |
|||
|---|---|---|---|---|
| GG | HR (95% CI) | P | HR (95% CI) | P |
| Unadjusted | < .001 | < .001 | ||
| 1 | Ref | Ref | ||
| 2 | 2.00 (1.62-2.47) | 1.90 (1.52-2.38) | ||
| 3 | 2.75 (2.17-3.48) | 2.76 (2.16-3.53) | ||
| 4 | 2.94 (2.27-3.80) | 2.89 (2.21-3.79) | ||
| 5 | 6.26 (4.61-8.49) | 6.36 (4.67-8.66) | ||
| Adjusteda | < .001 | < .001 | ||
| 1 | Ref | Ref | ||
| 2 | 1.92 (1.47-2.26) | 1.78 (1.41-2.24) | ||
| 3 | 2.43 (1.90-3.10) | 2.54 (1.97-3.28) | ||
| 4 | 2.68 (2.05-3.51) | 2.87 (2.17-3.80) | ||
| 5 | 5.42 (3.93-7.47) | 6.23 (4.42-8.47) | ||
Abbreviations: BCR, biochemical recurrence; CI, confidence interval; GG, grade group; HR, hazard ratio.
Analyses were adjusted for age, race, prostate-specific antigen level, clinical stage, year of surgery, and surgical center.
TABLE 4.
Association of Grade Group With Metastases and All-Cause Mortalitya
| Metastases |
ACM |
|||
|---|---|---|---|---|
| GG | HR (95% CI) | P | HR (95% CI) | P |
| Unadjusted | < .001 | .034 | ||
| 1 | Ref | Ref | ||
| 2 | 9.79 (2.21-43.5) | 1.38 (0.96-1.97) | ||
| 3 | 23.4 (5.33-102) | 1.70 (1.12-2.58) | ||
| 4 | 23.1 (5.05-106) | 1.59 (0.97-2.61) | ||
| 5 | 72.2 (15.9-327) | 2.20 (1.13-4.29) | ||
Abbreviations: ACM, all-cause mortality; CI, confidence interval; GG, grade group; HR, hazard ratio; Ref, reference category.
Fifty-one patients developed metastases, and there were184 deaths.
Figure 2.
Kaplan-Meier estimates of each endpoint stratified by Grade Group. BCR indicates biochemical recurrence; CRPC, castration-resistant prostate cancer; PCSM, prostate cancer-specific mortality.
Secondary Outcome: Impact of AA Race on the Predictive Utility of Prostate GG
In total, there were 1596 AA men (38.1%) in the analysis cohort. Interactions between race and GG were tested for select outcomes (Table 5). After adjusting for age, PSA, clinical stage, year of surgery, and surgical center, no significant interactions were observed between GG and race that predicted any measured outcomes, suggesting that GG has similar prognostic abilities in AA and Caucasian men.
TABLE 5.
Interactions Between Race and Grade Group
| Outcome | PInteractiona |
|---|---|
| BCR | .38 |
| Adjuvant treatment | .36 |
| ACM | .92 |
Abbreviations: ACM, all-cause mortality; BCR, biochemical recurrence.
Values were adjusted for age, PSA, clinical stage, year of surgery, and surgical center.
DISCUSSION
Analyzing outcomes from an equal-access health system, we validated the GG stratification system for predicting multiple long-term oncologic outcomes after RP. Our findings support the use of GG for patient counseling and treatment planning and suggest the independent integration of each group into management algorithms. This represents 1 of the first published studies defining the predictive utility of the 5 updated GG tiers for multiple long-term endpoints.
The current results are consistent with emerging findings from several other large studies. Spratt et al examined the impact of GG on biochemical recurrence-free survival in 3694 men after RP from 1994 to 2013. Those authors demonstrated statistically significant differences in 5-year actuarial biochemical recurrence-free survival stratified by GG for both preoperative biopsy grade and RP grade, with better utility than the 3-tier risk system.16 Loeb and colleagues examined the National Prostate Cancer Registry of Sweden and identified 4325 men who underwent RP and 1555 men who received radiation therapy from 2005 to 2007.15 For both RP and radiation therapy, biopsy GG was a significant, independent predictor of 4-year biochemical recurrence-free survival. Mathieu et al examined data from more than 27,000 men at 7 European centers and validated GG at both biopsy and RP for predicting 4-year biochemical recurrence-free survival.18
Recent reports have begun to examine long-term oncologic endpoints. Ham and coworkers examined mortality outcomes in men who had GG 4 and GG 5 disease diagnosed on either biopsy or RP specimens. The cohort spanned procedures performed between 1984 and 2014 and included 721 men who had original biopsy Gleason scores of 8 to 10 and 1047 men who had original RP Gleason scores of 8 to 10.12 Those investigators observed worse PCSM and ACM in men with GG 5 (Gleason score ≥9) compared with GG 4 (Gleason score 8) for both biopsy and RP grading.
Leapman et al examined oncologic outcomes in the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) registry and noted that GG was associated with PCSM and metastatic bone progression across multiple primary treatment strategies, including conservative management and primary androgen deprivation.14 In the largest population study to date, He et al reviewed the Surveillance, Epidemiology, and End Results database and observed that GG tiers predicted PCSM independent of primary treatment and clinical stage.13
Our current study is unique and relevant for several important reasons. First, we have defined multiple, long-term, nonsurrogate endpoints for each biopsy GG that can be used as references for the management of all grades of prostate cancer. Complementing the findings by Ham et al for the highest Gleason groups, our findings for lower GG tiers are particularly useful for counseling the increasing number of men diagnosed with lower grade tumors who are considering active surveillance or partial gland ablation. Second, the analysis highlights the divergent biologic behavior within D’Amico “intermediate-risk” disease when comparing GG 2 (G sum 3 + 4) and GG 3 (G sum 4 + 3) relative to GG 1 for important oncologic endpoints, including BCR, secondary therapy, and metastatic disease (Tables 3 and 4). This has important clinical implications, because some centers have expanded inclusion criteria for active surveillance. Third, the series is comprised of 41% AA men, including at least 32% in each GG tier, providing 1 of the largest published multiracial series to date. Poor inclusion of AA men in prostate cancer studies remains a significant research concern, and lack of available race-specific outcomes hinders both pretreatment and post-treatment counseling.19 We identified no significant interactions between race and GG in predicting any outcomes, supporting the independent predictive utility of GG in AA men. Finally, our analysis was carried out at equal-access Veterans Affairs hospitals, removing potentially confounding socioeconomic barriers to treatment and continuity that may exist in other health care networks.
There are several limitations to the current study that should be acknowledged. We performed a multicenter, retrospective study based on “standard-read” rather than “specialized-read” pathology reports, with no centralized pathology review, thus introducing confounding variability into the design. This may have contributed to the lack of prognostic difference observed between GG 3 and GG 4. We restricted the analysis to men who were diagnosed in 2005 and later to capture the impact of changes in pathologic grading introduced at that time. Although this limited the length of follow-up and frequency of adverse oncologic events likely to be observed with longer observation, the timeframe best reflects the predictive utility of the updated GG system. GG stratification and oncologic outcomes for patients in the SEARCH database from 1988 to 2015 are included in the Supporting Materials and in Supporting Figures 1 and 2 and Supporting Tables 1 through 4 (see online supporting information).
We also restricted our analysis to the predictive utility of biopsy rather than RP Gleason scores and similarly used transrectal ultrasound rather than pathologic prostate volume, because these are the variables available to physicians for patient counseling and management decisions at the time of initial diagnosis. Although the RP Gleason score provides more accurate disease grading, the GG is used most commonly in the pretreatment phase, with an increasing number of men undergoing active surveillance or nonextirpative treatments.
A final limitation was that we did not examine the influence of secondary therapies, including androgen deprivation and radiation therapy, on survival outcomes. Nonetheless, we observed that men with higher GG had greater receipt of secondary therapy and a trend toward worse PCSM. If not for the higher rate of secondary therapies, the worse survival with higher GG may have been even more pronounced.
Conclusion
This study validates the contemporary prostate biopsy GG system for predicting multiple long-term oncologic outcomes after RP. The data confirm the independent clinical utility of the GG in a large cohort of AA men, demonstrating that race does not impact prognostic utility. Continued integration of the GG system into clinical practice is warranted.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This study was supported by the National Institute of Health (grant K24CA1600653 to Stephen J. Freedland and P50CA92131 to William J. Aronson).
Footnotes
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. [DOI] [PubMed] [Google Scholar]
- 3.Zhou P, Chen MH, McLeod D, Carroll PR, Moul JW, D’Amico AV. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23:6992–6998. [DOI] [PubMed] [Google Scholar]
- 4.Epstein JI, Zelefsky MJ, Sjoberg DD, et al. a contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol. 2016;69:428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. [DOI] [PubMed] [Google Scholar]
- 6.Chan TY, Partin AW, Walsh PC, Epstein JI. Prognostic significance of Gleason score 3+4 versus Gleason score 4+3 tumor at radical prostatectomy. Urology. 2000;56:823–827. [DOI] [PubMed] [Google Scholar]
- 7.Jackson W, Hamstra DA, Johnson S, et al. Gleason pattern 5 is the strongest pathologic predictor of recurrence, metastasis, and prostate cancer-specific death in patients receiving salvage radiation therapy following radical prostatectomy. Cancer. 2013;119:3287–3294. [DOI] [PubMed] [Google Scholar]
- 8.Pierorazio PM, Walsh PC, Partin AW, Epstein JI. Prognostic Gleason grade grouping: data based on the modified Gleason scoring system. BJU Int. 2013;111:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zietman A, Smith J, Klein E, Droller M, Dasgupta P, Catto J. Consensus guidelines for reporting prostate cancer Gleason grade [serial online]. BJU Int. 2016;117:849. [DOI] [PubMed] [Google Scholar]
- 10.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016;70:106–119. [DOI] [PubMed] [Google Scholar]
- 11.Berney DM, Beltran L, Fisher G, et al. Validation of a contemporary prostate cancer grading system using prostate cancer death as outcome. Br J Cancer. 2016;114:1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ham WS, Chalfin HJ, Feng Z, et al. New prostate cancer grading system predicts long-term survival following surgery for Gleason score 8-10 prostate cancer. Eur Urol. 2017;71:907–912. [DOI] [PubMed] [Google Scholar]
- 13.He J, Albertsen PC, Moore D, Rotter D, Demissie K, Lu-Yao G. Validation of a contemporary 5-tiered Gleason grade grouping using population-based data. Eur Urol. 2017;71:760–768. [DOI] [PubMed] [Google Scholar]
- 14.Leapman MS, Cowan JE, Simko J, et al. Application of a prognostic Gleason grade grouping system to assess distant prostate cancer outcomes. Eur Urol. 2017;71:750–759. [DOI] [PubMed] [Google Scholar]
- 15.Loeb S, Folkvaljon Y, Robinson D, Lissbrant IF, Egevad L, Stattin P. Evaluation of the 2015 Gleason grade groups in a nationwide population-based cohort. Eur Urol. 2016;69:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spratt DE, Cole AI, Palapattu GS, et al. Independent surgical validation of the new prostate cancer grade-grouping system. BJU Int. 2016;118:763–769. [DOI] [PubMed] [Google Scholar]
- 17.Wadhwa H, Terris MK, Aronson WJ, et al. Long-term oncological outcomes of apical positive surgical margins at radical prostatectomy in the Shared Equal Access Regional Cancer Hospital cohort. Prostate Cancer Prostatic Dis. 2016;19:423–428. [DOI] [PubMed] [Google Scholar]
- 18.Mathieu R, Moschini M, Beyer B, et al. Prognostic value of the new grade groups in prostate cancer: a multi-institutional European validation study. Prostate Cancer Prostatic Dis. 2017;20:197–202. [DOI] [PubMed] [Google Scholar]
- 19.Ahaghotu C, Tyler R, Sartor O. African American participation in oncology clinical trials—focus on prostate cancer: implications, barriers, and potential solutions. Clin Genitourin Cancer. 2016;14:105–116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.