ABSTRACT
Oogenesis in the urochordate, Oikopleura dioica, occurs in a large coenocyst in which vitellogenesis precedes oocyte selection in order to adapt oocyte production to nutrient conditions. The animal has expanded Cyclin-Dependant Kinase 1 (CDK1) and Cyclin B paralog complements, with several expressed during oogenesis. Here, we addressed functional redundancy and specialization of CDK1 and cyclin B paralogs during oogenesis and early embryogenesis through spatiotemporal analyses and knockdown assays. CDK1a translocated from organizing centres (OCs) to selected meiotic nuclei at the beginning of the P4 phase of oogenesis, and its knockdown impaired vitellogenesis, nurse nuclear dumping, and entry of nurse nuclei into apoptosis. CDK1d-Cyclin Ba translocated from OCs to selected meiotic nuclei in P4, drove meiosis resumption and promoted nuclear envelope breakdown (NEBD). CDK1d-Cyclin Ba was also involved in histone H3S28 phosphorylation on centromeres and meiotic spindle assembly through regulating Aurora B localization to centromeres during prometaphase I. In other studied species, Cyclin B3 commonly promotes anaphase entry, but we found O. dioica Cyclin B3a to be non-essential for anaphase entry during oogenic meiosis. Instead, Cyclin B3a contributed to meiotic spindle assembly though its loss could be compensated by Cyclin Ba.
KEYWORDS: Urochordate, chromosomal passenger complex, vitellogenesis, cyclin B3, oogenesis, meiosis resumption
Introduction
Oogenesis in many animal species arrests at a prolonged diplotene stage of prophase I (called dictyate in mammals), permitting oocyte differentiation, growth and stockpiling of maternal mRNA and proteins for early embryonic development [1]. The duration of prophase I arrest varies among species: ~ 23 minutes in the hermaphroditic nematode, C. elegans, when sperm is present, ~ 2 days in Drosophila, several months in mice, or up to decades in humans. Release from prophase I arrest in response to appropriate stimuli relies on activation of maturation promoting factor (MPF), consisting of Cyclin-Dependant Kinase 1 (CDK1) kinase and its regulatory subunit Cyclin B. After primary arrest, most species, except nematodes, will experience a secondary arrest at metaphase I (most invertebrates), metaphase II (most vertebrates) or the pronuclear stage (sea urchins and sea stars). Secondary arrest is maintained by high activity of MPF and cytostatic factor (CSF) including early mitotic inhibitor 2 (Emi2), responsible for APC/C inhibition [2]. The roles of CDK1-Cyclin B complexes during oocyte maturation have been studied in a variety of species including mice, Xenopus, clams, starfish, and several fish species [3]. CDK1 collaborates with multiple kinases to complete meiosis, and the interrelations are focused on where these mitotic kinases lie in signaling pathways. For instance, the mitogen-activated protein kinase (MAPK) is activated ahead of CDK1 activation by progesterone stimulation in Xenopus or sperm-derived major sperm protein (MSP) in C. elegans, whereas in starfish, Mos synthesis and MAPK activation depend on CDK1 activation [4]. Polo-like kinases (Plk) are also involved in CDK1 activation, either as a trigger kinase initiating CDK1 activation during mitotic entry [5], or in a CDK1 positive feedback loop during meiosis [6].
In the urochordate, appendicularian, Oikopleura dioica, among the closest extant relatives to vertebrates [7], oogenesis occurs over a period of 2–3 days in a single-cell coenocyst, where thousands of meiotic and nurse nuclei share a common cytoplasm, surrounded by a monolayer of follicle cells [8]. Cellularization of oocytes occurs at the end of oogenesis shortly before spawning [9]. This is different from other chordate species and most deuterostomes, where germline, cystoblast, cyst breakdown occurs during early stages of oogenesis, around the period of oocyte determination and before vitellogenesis [10]. There are similarities to Drosophila, where nurse cells synthesize RNAs and proteins, followed by transport through ring canals to the growing pro-oocyte by cytoplasmic streaming to support vitellogenesis [11]. The cytoplasmic transport process is slow until stage 10, but from stage 11, the entire cytoplasmic content of nurse cells is transferred within 30 minutes to the oocyte by contraction of the actin network at the end of vitellogenesis [12]. An important distinction is that in Drosophila, oocyte determination occurs prior to vitellogenesis, whereas in O. dioica, oocyte selection occurs after vitellogenesis has begun, and final oocyte number is determined very late in the life cycle as a function of accumulated energetic resources [13]. In all studied deuterostomes, except O. dioica, maturing oocytes contain a transcriptionally active meiotic nucleus termed the germinal vesicle (GV). O. dioica meiotic nuclei harbor three bivalents, and with the exception of two small foci [13], they are transcriptionally quiescent, a probable adaptation to coenocystic oogenesis. Regardless of the transcriptional status of the meiotic nucleus, meiosis resumption is shortly followed by kinase-induced nuclear envelope breakdown (NEBD) and termination of transcription.
During female meiosis, substrate specificities of CDK1 are largely determined by their Cyclin partner [14], principally the B-type Cyclins. Three B-type Cyclins, B1, B2, and B3, (amphibians also possess Cyclins B4 and B5 [15]), have been studied in metazoans, with different degrees of functional redundancy being reported. From an evolutionary standpoint, it remains unclear to what extent diversification of Cyclin B functions and/or redundancies, might in part, reflect different structural organizations of oogenesis. In Drosophila, where the cyst phase (15 nurse nuclei to 1 meiotic nucleus) persists late in oogenesis, Cyclin B restrains APC/C activity and is required for metaphase maintenance and meiotic spindle organization, whereas Cyclin B3, which is destroyed after Cyclin B, promotes the metaphase-anaphase transition by activating APC/C [16,17]. In C. elegans, where the cyst phase is resolved near the spatial midpoint of oogenesis (ratio of the cytoplasm of 1 nucleus in the rachis sacrificed for 1 nucleus continuing to complete meiosis) at the bend in the gonad arm, CYB-1, CYB-2.1, CYB-2.2 and CYB-3, show somewhat greater functional overlap in meiosis than in mitosis. In both meiosis and mitosis CYB-1 is required for chromosome congression, and CYB-3 is required for chromosome segregation [18]. CYB-3 also supports S-phase progression and is a major contributor to promote NEBD in mitotic entry [19]. In higher vertebrates, where the cyst phase is resolved very early in oogenesis, Cyclin B1 is essential, while Cyclin B2 is dispensable [20,21]. The division of functions between Cyclins B1 and B2 lies in nuclear mitotic functions for Cyclin B1, such as driving NEBD, spindle assembly, spindle checkpoint induction and M-phase maintenance, whereas Cyclin B2 is involved in earlier interphase events such as Golgi fragmentation [22] and centrosome separation [23,24]. Vertebrate Cyclin B3 shares properties of both Cyclin As and Bs, localizes in nucleus throughout the cell cycle [25] and drives the metaphase to anaphase transition by activating APC/Ccdc [20] independently of the spindle assembly checkpoint (SAC) during meiosis I in mouse oocytes [26].
CDK-type kinases have experienced functional differentiation during eukaryote evolution. In yeast, a single CDK1 homolog (Cdc28 in budding yeast and Cdc2 in fission yeast) and its associated cyclins drive the entire cell cycle [27]. In metazoans, CDK1 interacts with mitotic Cyclins A and B to drive M-phase, though knockouts of interphase CDKs has revealed that, as in yeast, CDK1 alone retains the ability to regulate the entire embryonic cell cycle [28]. Although the specific requirements for CDK1 in meiosis are not completely resolved due to early embryonic lethality of CDK1 knockout mice, the consensus is that CDK1 relieves prophase I arrest after vitellogenesis in Xenopus, starfish [29], Drosophila [30] and C. elegans [31]. Surprisingly, O. dioica is unique among metazoans in possessing five CDK1 paralogs (CDK1a-CDK1e) [32], none of which retains the invariant, canonical Cyclin B-interacting PSTAIRE motif of the single CDK1 homolog found in other metazoans. Its complement of B-type Cyclins has also expanded to five paralogs (Cyclins Ba, Bb, Bc, B3a and B3b) that show distinct expression patterns during somatic and germline development [32]. Briefly, all five CDK1 paralogs exhibit expression during mitotic divisions in early embryonic development, though CDK1d and e expression is most prevalent in the ovary and declines rapidly in early development, indicative of maternal transcripts. Surprisingly, CDK1a,b and c paralogs are expressed throughout the life cycle, including stages dominated by rapid growth through endocycling, a cell cycle variant where CDK1 activity is suppressed in other model organisms that have been studied. In comparison, all cyclin B paralogs are expressed in mitotic stages but, as would be expected, are suppressed during portions of the life cycle dominated by endocycling, when they are restricted to tissues, such as the mitotically proliferating germline nuclei in the gonad. In O. dioica, active MAPK and Plk1 emerge on Organizing Centres (OCs) where CDK1s reside before meiosis resumption. At this point, Plk1 translocates into selected meiotic nuclei after oocyte selection, whereas MAPK is retained on OCs in maturing oocytes [33]. The consequences of differential translocation of these meiotic kinases and their interplays with different CDK1 paralogs are unknown.
In O. dioica, oogenesis is subdivided into 5 phases P1 to P5 (summarized in Table 1 of Ganot et al. [9]). Briefly, during P1, ovarian germline nuclei undergo an asymmetric division, generating a 1:1 ratio of endocycling nurse nuclei and meiotic nuclei. During P2, meiotic nuclei progress to zygotene and in P3, the coenocyst expands in size. Upon transition to P4, a subset of pro-oocytes increases in size via transfer of cytoplasm through their ring canal connections to the general coenocyst cytoplasm and meiotic chromatin condenses. In the final P5 stage, oocyte maturation is completed, nurse and excess meiotic nuclei undergo apoptosis, and spawning occurs via rupture of the ovarian wall. In this study, we found that two CDK1 paralogs, CDK1a and CDK1d, and a mitotic cyclin, cyclin Ba exhibited distinct spatial and temporal dynamics during oocyte selection and meiosis resumption. By RNAi knockdowns, we demonstrated the sub-functionalization of the two CDK1 paralogs in oogenic meiosis. CDK1a regulated coenocystic vitellogenesis by participating in nurse nuclei dumping during pro-oocyte growth, whereas CDK1d together with cyclin Ba, and to a lesser, non-essential extent, cyclin B3a, promoted meiosis resumption, NEBD and meiotic spindle assembly during prometaphase I.
Results
CDK1 and cyclin B paralog expressions and localizations during coenocystic oogenesis in oikopleura dioica
Previous work revealed that CDK1a and CDK1d resided on Organizing Centers (OCs) in P3, but their localizations were unclear after oocyte selection [33]. To clarify their localizations during meiosis, we injected a CDK1a capped mRNA with a full-length endogenous 3ʹUTR into P3 gonads, and observed CDK1a to locate to selected meiotic nuclei and their adjacent OCs during P4 (Figure 1(a)). CDK1d was also observed in selected meiotic nuclei during P4, but not on OCs (Figure 1(b)), further supporting its role in driving meiosis resumption.
Figure 1.
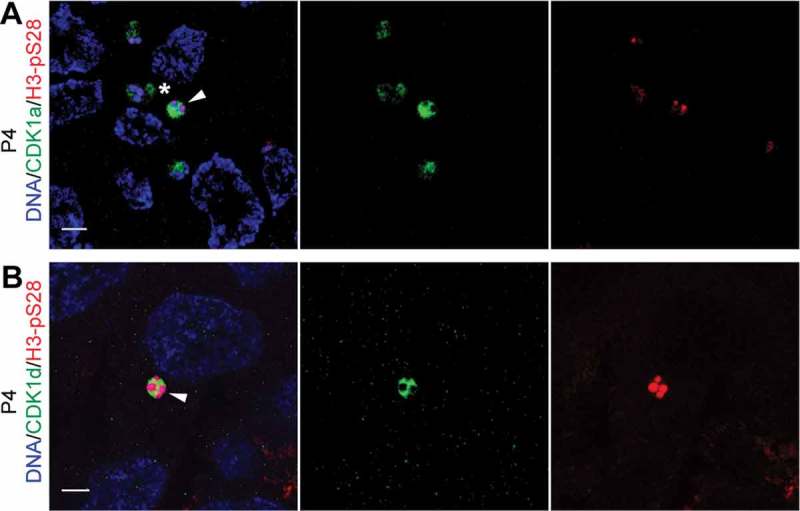
CDK1a and d paralogs locate to selected meiotic nuclei after oocyte selection. (A) CDK1a-GFP localized to H3-pS28 positive selected meiotic nuclei (arrowhead) and their adjacent OCs (*) in P4 ovaries. (B) CDK1d-GFP localized to H3-pS28 positive selected meiotic nuclei (arrowhead) in P4 ovaries. Scale bars: 5 µm.
We had previously identified 3 homologs (Cyclins Ba, Bb and Bc) of Cyclin B1, and 2 homologs (Cyclins B3a and B3b) of Cyclin B3 in the O. dioica genome [32]. Cyclin Ba and Bb share 97% amino acid identity, and their exon/intron arrangements are very similar, supporting a recent duplication. First, we quantified mRNA levels of B-type cyclins during oogenesis by RT-qPCR. Cyclin Ba mRNA levels were high during early stages of oogenesis, reaching a peak when pro-oocytes were growing, and then gradually declined thereafter, whereas Cyclin Bb mRNA levels were barely detected throughout oogenesis (Figure 2(a)). Cyclin B3a mRNA levels were high during late stages of oogenesis, reaching and maintaining a peak from P5 to metaphase I, whereas Cyclin B3b mRNA levels were barely detected throughout oogenesis. Cyclin Bc mRNA levels were low, with a slight increase late in oogenesis (P5) and in oocytes. We therefore focused on the Cyclin Ba, and B3a paralogs in further analyzes.
Figure 2.
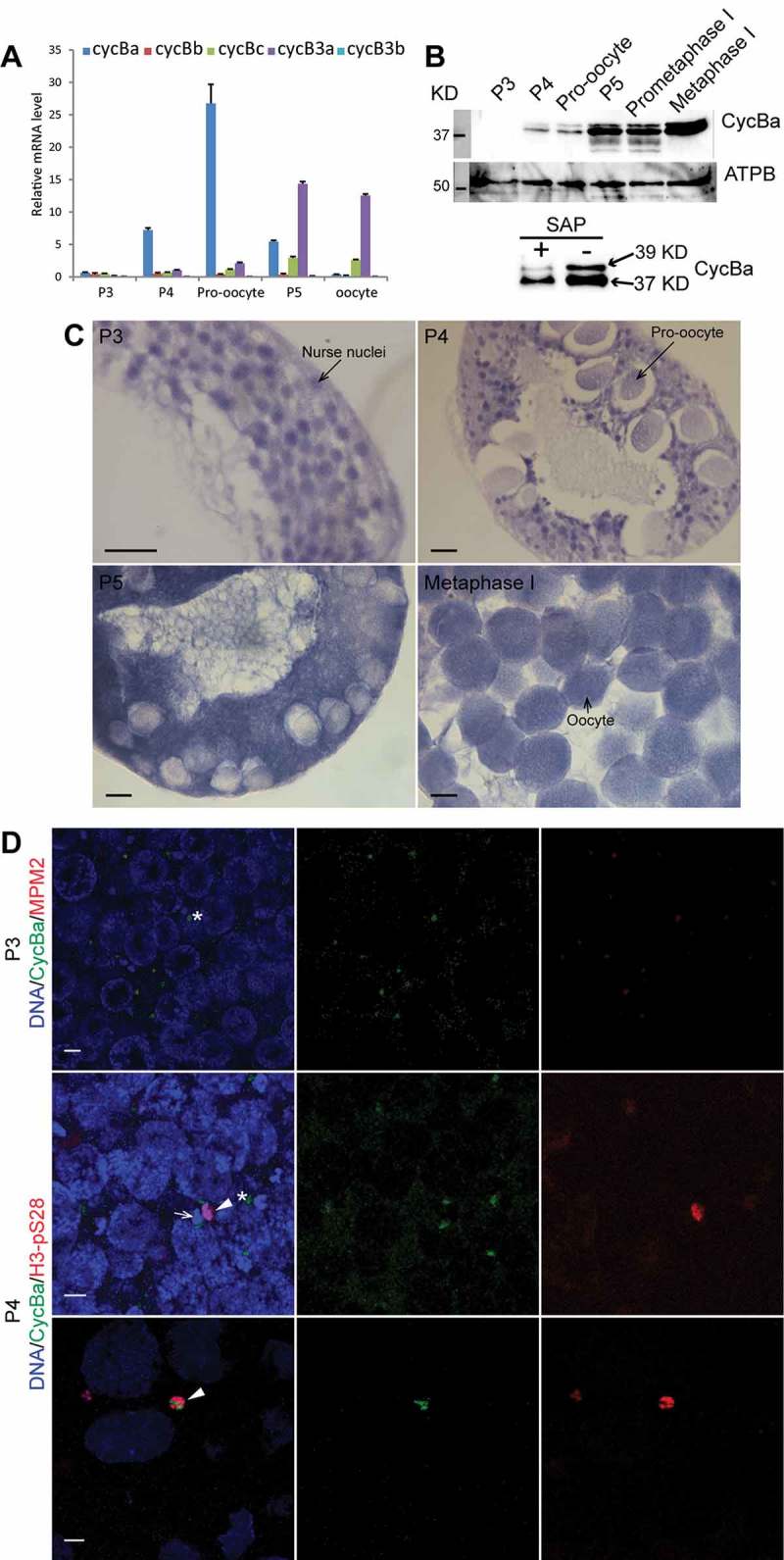
Expression profiles and distribution of Oikopleura dioica B-type cyclin transcripts and Cyclin Ba protein during oogenesis. (A) Expression levels of O. dioica homologs of Cyclins B1 and B3 during oogenesis. Mean values normalized to EF1β transcripts (n = 3) are shown with standard error bars. (B) Top panels: Cyclin Ba increased during maturation from P3 to metaphase I. Whole animal lysates were immunoblotted and detected with Cyclin Ba antibody (top) or ATP synthase β antibody as a loading control (bottom). Bottom panel: Shrimp alkaline phosphatase (SAP) treatment reduced the Cyclin Ba upper band, indicating this is phosphorylated form of Cyclin Ba. (C) In situ hybridization showed that cyclin Ba was transcribed in nurse nuclei in P3 and P4 ovaries, transported into the coenocyst cytoplasm in P5, and subsequently concentrated in mature oocytes. Scale bars: 50 µm. (D) Cyclin Ba-GFP localized at MPM2-stained OCs (*) in P3 (top panels) and early P4 ovary (mid panels), where it was adjacent to both H3-pS28 stained selected meiotic nuclei (arrowhead) and non-selected meiotic nuclei (arrow). Cyclin Ba-GFP translocated into selected meiotic nuclei in late P4 ovaries (bottom panel). Scale bars: 5 µm.
Cyclin Ba was mainly transcribed in nurse nuclei during P3 and P4 (Figure 2(c)). In P5, cyclin Ba mRNA was exported to the coenocyst cytoplasm, and then transferred and evenly distributed in mature oocytes just prior to spawning. These distribution patterns are similar to those of cyclin B mRNA in syncytial Drosophila ovarioles [34]. Western blots showed that Cyclin Ba was detectable in P4, before increasing dramatically in P5, to achieve its highest levels in metaphase I arrested spawned oocytes (Figure 2(b)). The significant increase of Cyclin Ba during late prophase I and prometaphase I might indicate potential roles during the final stages of oocyte maturation. It is noteworthy that Cyclin Ba exhibited two bands (~ 37 KD and 39 KD), and the proportion of upper band significantly increased during prometaphase I. The upper band decreased upon phosphatase treatment, indicating a phosphorylated form of Cyclin Ba. To explore the spatial localization of Cyclin Ba during oogenesis, capped mRNA encoding Cyclin Ba fused to eGFP at its C-terminal, was injected into P3 ovaries at Day 4. Injected animals were cultured to Day 5 and harvested in late P3 and P4 for immunostaining with anti-GFP antibodies (Figure 2(d)). Cyclin Ba was found on OCs in late P3, co-staining with M-phase specific, MPM2 phospho-epitopes. At the beginning of P4, we used histone H3-pS28 staining to determine the meiotic nuclei selected to populate growing pro-oocytes. At this stage, Cyclin Ba was found on OCs adjacent to both selected (histone H3-pS28 positive) and non-selected (H3-pS28 negative) meiotic nuclei. At late P4, Cyclin Ba translocated into selected meiotic nuclei. Thus, translocation of Cyclin Ba from OCs to selected meiotic nuclei occurs after oocyte selection in late P4, consistent with a possible role of Cyclin Ba as the regulatory subunit of MPF.
CDK1a, CDK1d, cylinBa and cyclinB3a knockdown oocytes differ significantly in kinase activity
The dynamics of CDK1a and d localizations and Cyclin Ba and B3a paralog expressions and localizations during oogenesis led us to examine the effects of their respective knockdowns on meiosis and oocyte maturation. CDK1 kinase assays of oocytes (Figure 3) revealed that all individual knockdowns reduced kinase activity. The impact of cycB3a knockdown was relatively minor, reducing kinase activity to about 80% of that in wild type oocytes. All other knockdowns reduced kinase activity to less than 20% of wild type activity. There was no significant difference in the kinase activity of cycBa RNAi versus cycBa;B3a double RNAi knockdowns. Loss of kinase activity was more severe in Cdk1d knockdowns (9% of wild type) and almost eliminated in CDK1a knockdowns (1% of wild type).
Figure 3.
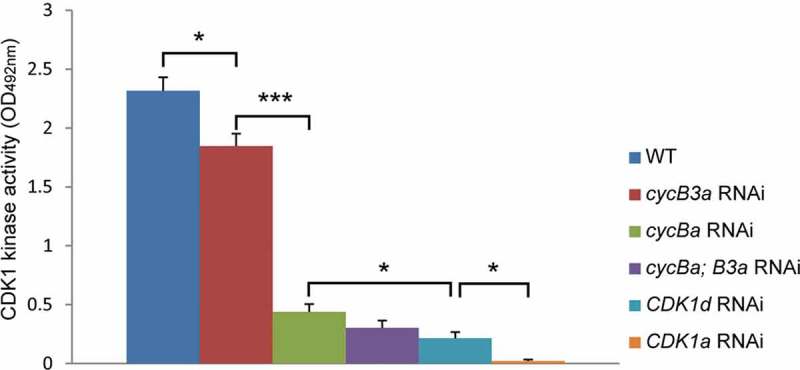
CDK1 kinase activity assays of CDK1 and cycB paralog RNAi oocytes (n = 30 per category). The values were calculated as means from three independent assays with standard errors shown. Significant pairwise step differences in kinase activities (student’s t-test) are indicated (*, p < 0.05; ***, P < 0.001).
CDK1a knockdown results in small infertile oocytes and disrupts cyclin B paralog dynamics
After observing that CDK1a knockdown severely impaired the kinase activity levels in the ovarian coenocyst, we then characterized the phenotypic effects resulting from this loss of activity. CDK1a knockdown females produced small infertile oocytes, which were often surrounded by excess coenocyst cytoplasm (Figure 4(a) and [33]). Lamin1 staining revealed that the nuclear envelope was intact, with OC closely associated (Figure 4(b)), whereas OC disassembled during prometaphase I in wild type oogenesis (Figure S1). In wild type selected meiotic nuclei at the P4 stage, MPM2 foci were present on chromosomes (Figure S1) whereas in CDK1a knockdown ovaries, MPM2 foci were absent in meiotic nuclei, but remained present on OC (Figure 4(b)). In knockdown ovaries, active Polo-like kinase 1 (Plk1) was not observed on meiotic chromatin, being retained in the nucleoplasm and on OC (Figure 4(c)). In contrast, active Plk1 translocated from OC to selected meiotic nuclei and distributed evenly on both chromosomes and in the nucleoplasm at P4 in wild type ovaries (see Figure 10(c) below). In spawned CDK1a RNAi oocytes, cyclin Ba mRNA levels were high and cyclin B3a mRNA levels very low (Figure S2A), similar to their expression profiles during pro-oocyte growth (Figure 2(a)). However, Cyclin Ba protein was very low, similar to Cyclin Ba protein levels at the beginning of P4 (Figure S2B). This was not the case for CDK1d knockdowns, where Cyclin Ba protein levels were similar to those found in wild type (Figure S2B). These data indicate that CDK1a RNAi oocytes were arrested at diplotene during pro-oocyte growth.
Figure 4.
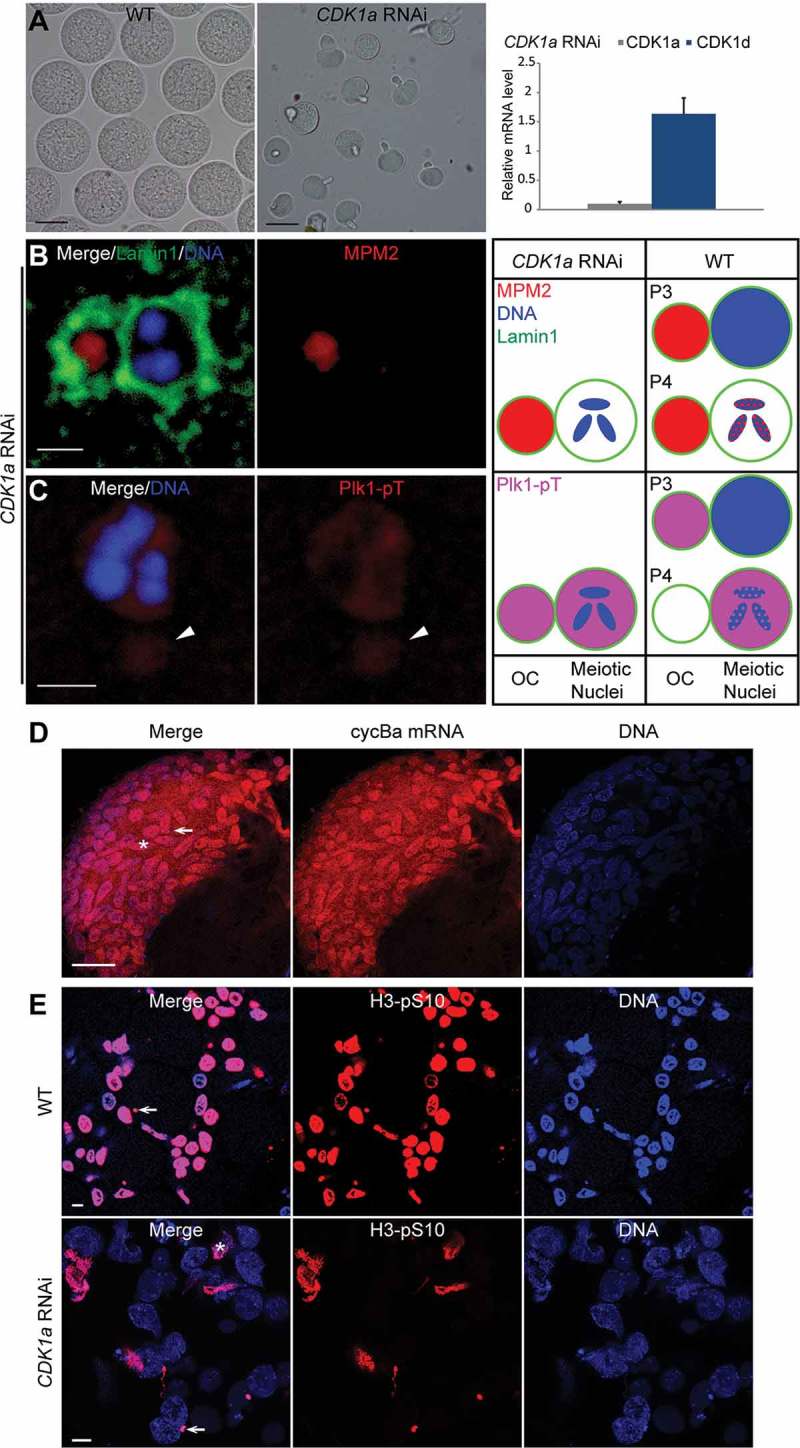
Meiotic defects resulting from CDK1a RNAi. (A) CDK1a RNAi oocytes (mid panel) were smaller than wild-type oocytes (left panel). Scale bars: 50 µm. In the right panel, CDK1a knockdown efficiency was confirmed by RT-qPCR. No significant off-target effects on other CDK1 paralogs were detected. (B) Lamin1 staining showed that CDK1a RNAi oocytes retained OC around meiotic nuclei. MPM2 epitopes were present on OC but absent in meiotic nuclei. Scale bar: 2 µm. (C) Plk1 was absent on chromosomes, but present in nucleoplasm and on OC (arrowhead). Scale bar: 2 µm. Schemas on the right compare the localizations of MPM2 epitopes (red) and Plk1 (pink) in meiotic nuclei and on OC between CDK1a RNAi oocytes and wild type in P3 and P4. (D) in situ hybridization showed that cyclin Ba mRNA (red) was retained in nurse nuclei of CDK1a RNAi ovaries in late P5 prior to spawning. Arrow indicates a meiotic nucleus, aster indicates a nurse nucleus. Scale bar: 50 µm. (E) In WT, all nurse nuclei underwent apoptosis and became H3-pS10 positive just prior to spawning. In CDK1a RNAi ovaries, only a small proportion of nurse nuclei underwent apoptosis and became H3-pS10 positive just before spawning. Arrow indicates a meiotic nucleus, aster indicates a nurse nucleus. Scale bars: 10 µm.
Figure 10.
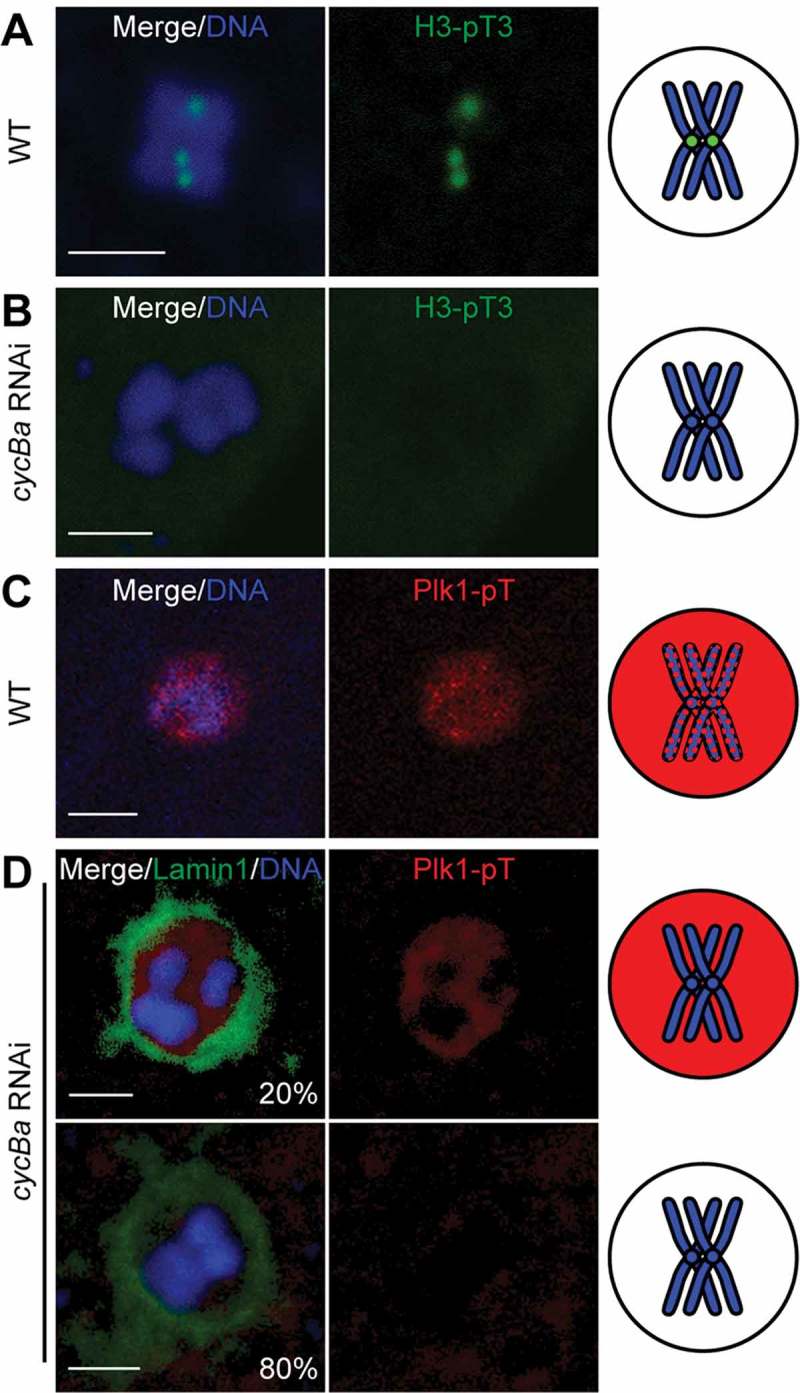
Knockdown of Cyclin Ba results in loss of H3T3 phosphorylation and phospho-Plk1 from centromeric regions. (A) In wild-type oocytes, H3-pT3 (green) was present on centromeric regions at metaphase I. (B) In cycBa RNAi oocytes, H3-pT3 was absent from chromosomes. (C) In wild-type oocytes, Plk1 located in meiotic nucleus at prometaphase I. (D) In cycBa RNAi oocytes (n = 30), two phenotypes were observed: Plk1 was absent from chromosomes (20%, top panel) or from the entire meiotic nucleus (80%, bottom panel). Lamin1 staining (green) indicated retention of the nuclear envelope in all cases. Schemas on the right summarize H3-pT3 (green) and Plk1-pT (red) signals in meiotic nuclei with only one bivalent (blue) shown for simplification. Scale bars: 2 µm.
In situ hybridizations on CDK1a RNAi ovaries prior to spawning showed that most cyclin Ba mRNA was retained in nurse nuclei (Figure 4(d)), instead of being transported to the coenocystic cytoplasm during pro-oocyte growth. This likely contributes to the lower levels of Cyclin Ba protein found in CDK1a knockdown oocytes. During late stages of wild type oogenesis, all nurse nuclei become histone H3-pS10 positive and undergo apoptosis [13]. In CDK1a RNAi ovaries just before spawning, a small proportion of nurse nuclei became deformed, and were positively stained with H3-pS10, indicating they were undergoing apoptosis, whereas most nurse nuclei remained oval and did not stain for the H3-pS10 marker indicative of apoptosis (Figure 4(e)). Apoptosis and rapid cytoplasmic transport are interconnected processes, and the failure of nurse nuclei dumping blocks apoptosis [35]. Thus, CDK1a knockdown females produce small oocytes, similar to the “dumpless” class of oogenesis mutants described in Drosophila [36]. We have shown previously [33] that ring canal diameters of growing oocytes in CDK1a RNAi ovaries were not affected, excluding the possibility of pre-mature constriction of ring canals and nurse nuclei did not clog the ring canals. We tried to identify the regulatory subunit of CDK1a by knocking down other mitotic cyclins including Cyclin Bc and Cyclin A, but neither knockdown showed similar defects to knockdown of CDK1a. Taken together, our data suggest that CDK1a is involved in the regulation of nurse nuclear dumping and cytoplasmic flow during later stages of coenocystic oogenesis in O. dioica.
Cyclin Ba is required for meiosis completion whereas cyclin B3a is dispensable
To address meiotic roles of Cyclin Ba, we injected cyclin Ba dsRNA into P3 ovaries, corresponding to the diplotene stage of prophase I. Efficient knockdown of cyclin Ba transcripts was observed with no off-target effects on paralogous genes, and western blots showed that Cyclin Ba protein could not be detected in cyclin Ba RNAi oocytes (Figure 5(a)). Upon exposure to wild type sperm, cyclin Ba RNAi oocytes were infertile, indicating that Cyclin Ba is an important regulator of oocyte maturation (Figure 5(b)). To further analyze the infertile phenotype, progress of meiosis I in cyclin Ba RNAi oocytes was examined by immunostaining the nuclear lamina. Anti-odLamin1 immunostaining showed that the nuclear envelope was retained when cyclin Ba was depleted, in contrast to its disassembly in wild type oocytes arrested at metaphase I, indicating NEBD did not occur (Figure 5(c)).
Figure 5.
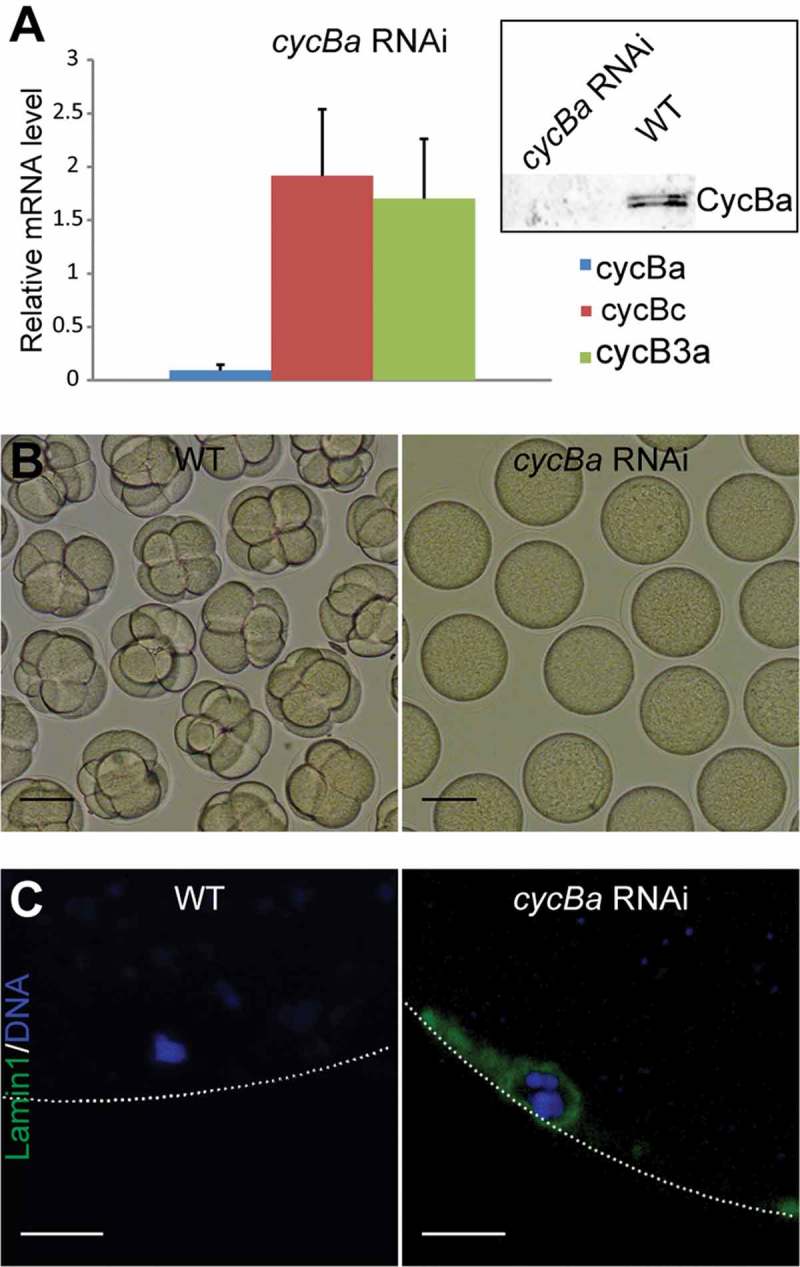
Depletion of Cyclin Ba blocks NEBD and generates non-fertile oocytes. (A) Cyclin Ba transcripts were efficiently knocked down by RNAi with no significant off-target effects on other cyclin B paralogs. Upper right corner: Western-blotting of cycBa RNAi oocytes (left lane) showed that Cyclin Ba was absent, compared to equal loading of wild-type control oocytes (right lane). (B) At 1 h post exposure to wild-type sperm, cycBa RNAi oocytes were infertile, whereas wild-type oocytes had developed normally to the 8-cell stage. Scale bars: 50 µm. (C) Lamin1 immunostaining revealed retention of the nuclear envelope in cycBa RNAi oocytes, compared to successful NEBD in wild-type oocytes at metaphase I. Dotted lines indicate the position of the oocyte plasma membrane. Scale bars: 5 µm.
Cyclin B3a is also actively transcribed during late stages of oogenesis and early stages of embryogenesis. Knockdown of Cyclin B3a lowered the kinase activity in oocytes to 80% of wild type levels (Figure 3) but did not affect the fertility of spawned oocytes nor impact early embryonic development (Figure S3). This suggests that Cyclin Ba is capable of compensating the loss of Cyclin B3a during oogenic meiosis.
Effects of CDK1a, CDK1d, cyclinBa and cyclinB3a knockdowns on meiotic bivalents and acentrosomal spindle formation
It has been demonstrated that the chromosomal passenger complex (CPC) is directly involved in meiotic spindle assembly during coenocystic oogenic meiosis I of Drosophila [37], and we wished to characterize its role in this process during the coenocystic meiosis of O. dioica. We observed that Aurora B, the kinase module in CPC, moved to centromeres, concurrent with the progress of meiotic acentrosomal spindle assembly during prometaphase I (Figure 6). This coincided with a change in uniform H3-pS28 staining along chromosomes to a concentration of this mark at the centromeres at prometaphase I (Figure S4). In wild type metaphase I arrested oocytes, bivalents exhibited high levels of H3-pS10 along entire chromosomes, and concentrated H3-pS28 at centromeres (Figure 7(a)). Cyclin Ba depletion resulted in a lack of concentrated H3-pS28 staining at the centromeres of bivalents (Figure 7(b)), indicating arrest of cyclin Ba RNAi oocytes at prometaphase I. This same effect was observed in cycBa;B3a double knockdowns and in knockdowns of CDK1d or CDK1a (Figure 7(c-e))
Figure 6.
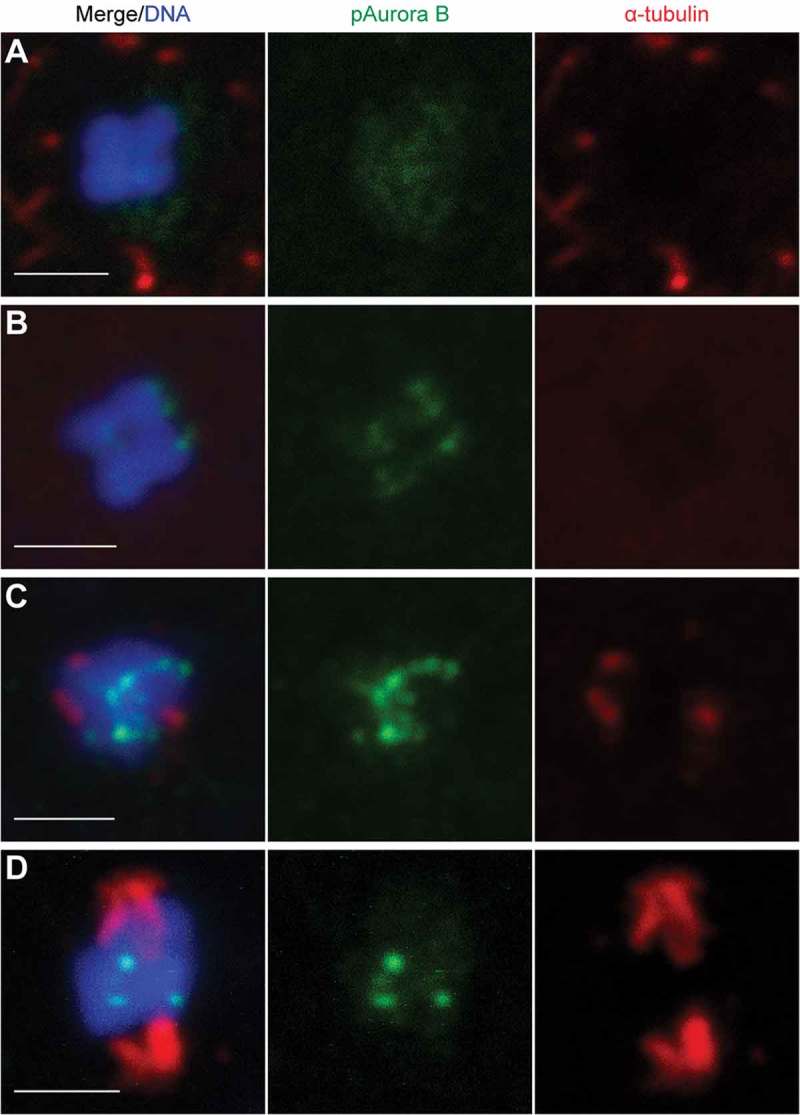
Acentrosomal spindle assembly during prometaphase I of Oikopleura oocytes is concurrent with Aurora B moving to centromeres. (A) Before NEBD, Aurora B distributed evenly through the nucleus, and a microtubule network surrounded the nuclear membrane. (B) After NEBD, Aurora B moved towards centromeres, and the microtubule network disappeared. (C) When the Aurora B signal became strong on centromeres, short microtubule fibers started to form from opposite poles. (D) When Aurora B was established on the centromeres of bivalents, the spindle elongated. Scale bars: 2 µm.
Figure 7.
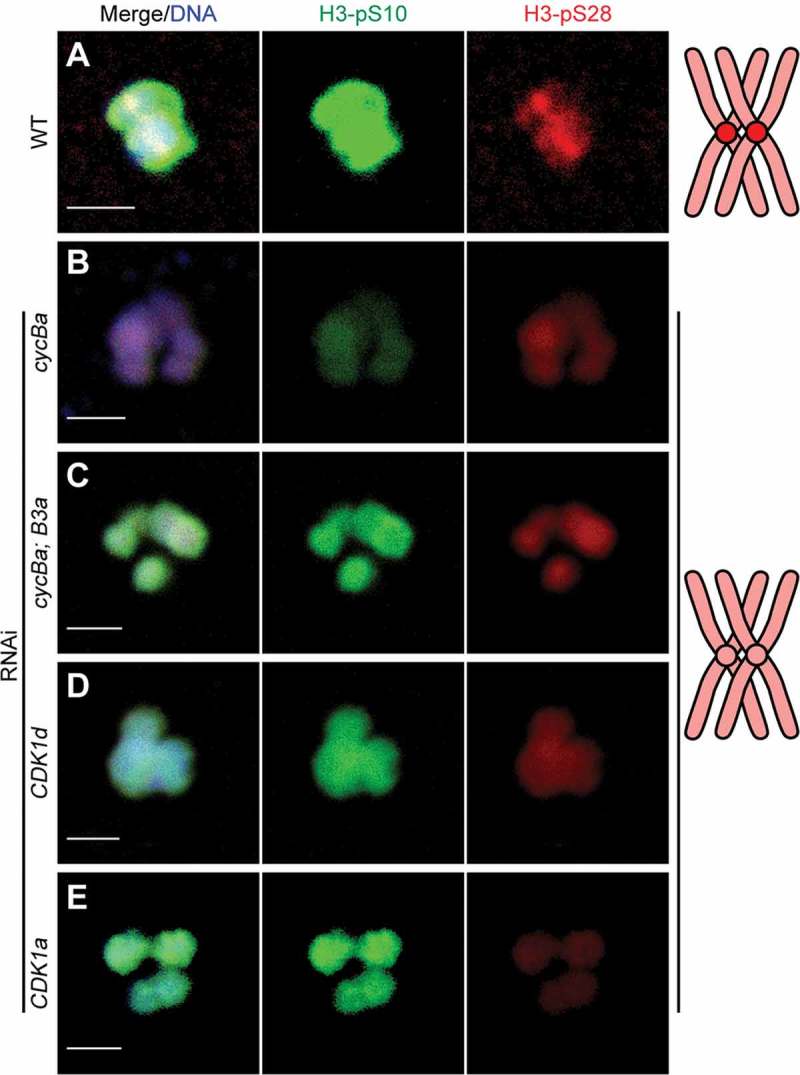
Histone H3-S10 and H3-S28 phosphorylations on bivalents in wild type and knockdown oocytes. (A) Wild type oocytes showed strong H3-pS10 staining on chromosome arms and H3-pS28 staining on centromeres of bivalents at metaphase I. H3-pS28 staining on centromeres was abolished after single knockdowns of cyclin Ba (B), CDK1d (D) or CDK1a (E), or double knockdown of cyclin Ba and cyclin B3a (C). Schemas on the right summarize H3-pS28 localization (red) on bivalents of wild type and knockdown oocytes. Scale bars: 2 µm.
We observed that bivalents in cyclin Ba RNAi oocytes were not well aligned on the metaphase I plate as compared to wild type (Figure 8(a,b)). Aurora B was delocalized from centromeres and the spindle was severely compromized. Complete loss of the spindle, indicated by an absence of any tubulin staining in the region surrounding the chromosomes was observed in 39% of oocytes. In 57% of oocytes there was weak tubulin staining around chromosomes, but it was not organized into any recognizable fibre-like structures. Finally, in a very minor proportion of oocytes (4%) we observed enhanced tubulin staining around chromosomes but an absence of any assembly into fibres. In cycBa;B3a double knockdowns, more severe defects on meiotic spindle assembly were observed, with 84% of double RNAi oocytes showing complete loss of tubulin staining, and 16% retaining weak, diffuse tubulin staining around chromosomes (Figure 8(c)) with an absence of any detectable microtubule fibres. This suggests that Cyclin B3a may assist Cyclin Ba in promoting meiotic spindle assembly during prometaphase I, though it is not strictly required for this purpose. CDK1d is required for meiosis resumption in O. dioica, and its knockdown blocked NEBD [33]. The defects of CDK1d RNAi oocytes were similar to cyclin Ba RNAi oocytes regarding the distribution of H3-pS10 and H3-pS28 on chromosomes, but meiotic spindle assembly defects were more severe (Figure 8(d)) with no tubulin staining observed in any of the CDK1d RNAi oocytes.
Figure 8.
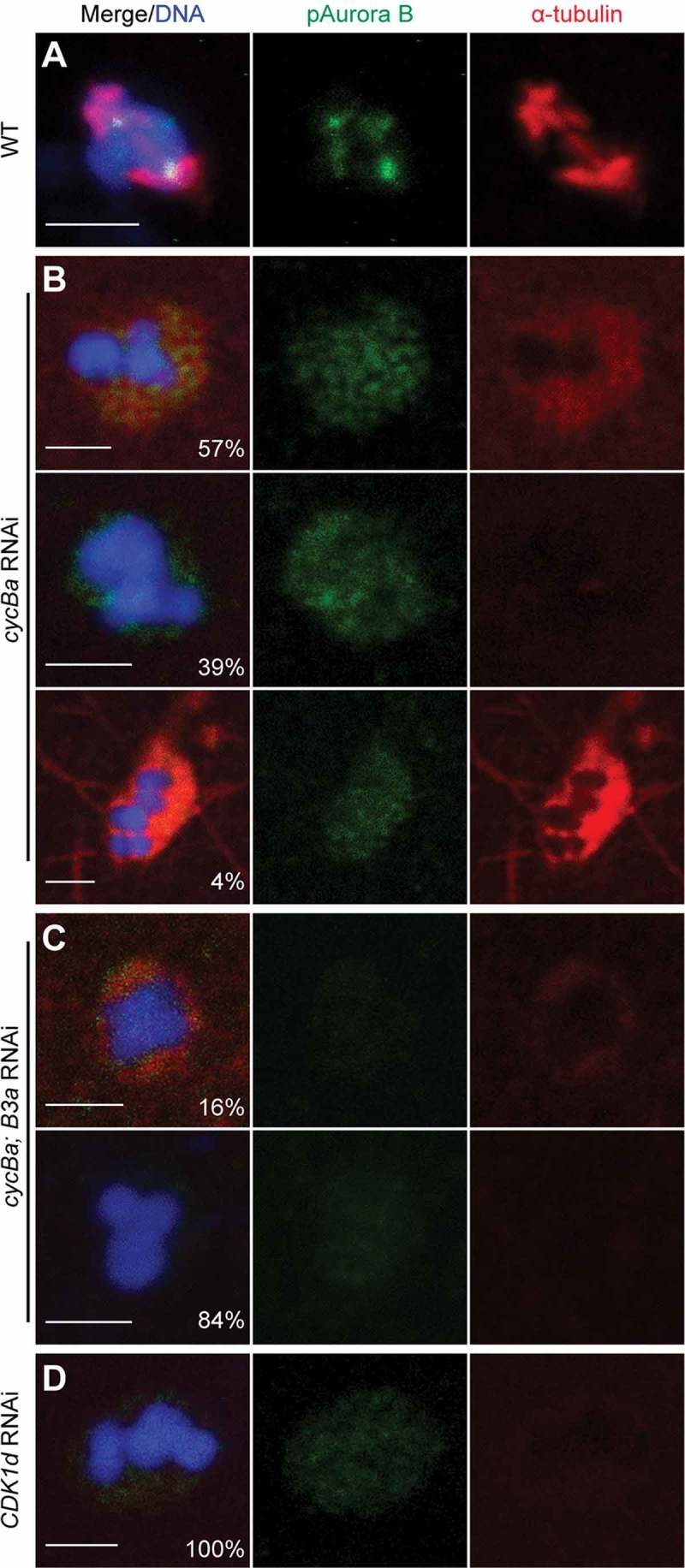
Aurora B is delocalized from centromeres and acentrosomal spindle assembly is blocked after RNAi of cycBa, cycBa and cycB3a, or CDK1d. (A) In wild-type oocytes, Aurora B localized on centromeres and a bipolar spindle formed, with microtubule fibers attaching to bivalents at metaphase I. (B) Representative defects in cycBa RNAi oocytes (n = 123) are shown. Proportions of each category are indicated in the merge panels. Aurora B remained dispersed in the nucleus and diffuse α-tubulin staining surrounding chromosomes was generally weak or absent. In rare cases, α-tubulin staining around chromosomes was strong and proximal microtubule fibers were observed. (C) In cycBa; cycB3a double RNAi oocytes (n = 104), Aurora B staining was even weaker, and the proportion of oocytes without any α-tubulin staining around chromosomes increased. (D) In CDK1d RNAi oocytes (n = 50), α-tubulin staining around chromosomes was absent. Scale bars: 2 µm.
The above results led us to investigate the centromeric region of bivalents in more detail. These were decorated by strong MPM2 epitopes during prometaphase I (Figure 9(a)). Since the MPM2 antibody mostly recognizes CDK1 substrates, it is possible that CDK1 phosphorylates substrates at the centromeric region during prometaphase I. In cyclin Ba RNAi oocytes, MPM2 foci were absent on chromosomes, but remained in the nucleoplasm (Figure 9(b)). The same result was observed in cycBa;B3a double knockdowns (Figure 9(c)). When CDK1d was knocked down, MPM2 foci disappeared from centromeric regions and nucleoplasmic MPM2 staining became weak (Figure 9(d)). These results indicate that Cyclin Ba is important for CDK1 activity at centromeres and may contribute to the delocalization of Aurora B from centromeres in cyclin Ba or CDK1d knockdowns (Figure 8). We then examined upstream regulators that recruit Aurora B to centromeres. One of them is phosphorylation of histone H3 on Thr3 (H3-pT3) in the inner centromere region by Haspin [38], and this modification recruits CPC to centromeres (Figure 10(a)). We found that H3-pT3 was absent at centromeres in cyclin Ba RNAi oocytes (Figure 10(b)). Another key regulator, Plk1, localized in the nucleoplasm and along chromosomes during wild type prometaphase I (Figure 10(c)), but was either completely absent from meiotic nuclei (80%) or present in the nucleoplasm but absent from chromosomes (20%) in cyclin Ba RNAi oocytes (Figure 10(d)). These results indicate that in O. dioica ovaries, Cyclin Ba is a critical partner of CDK1 in regulating the localization and activity of Aurora B, for H3-S28 phosphorylation on centromeres, chromosome congression and meiotic spindle assembly during prometaphase I, and that CDK1d-Cyclin Ba activity is upstream of Plk1 localization to chromosomes.
Figure 9.
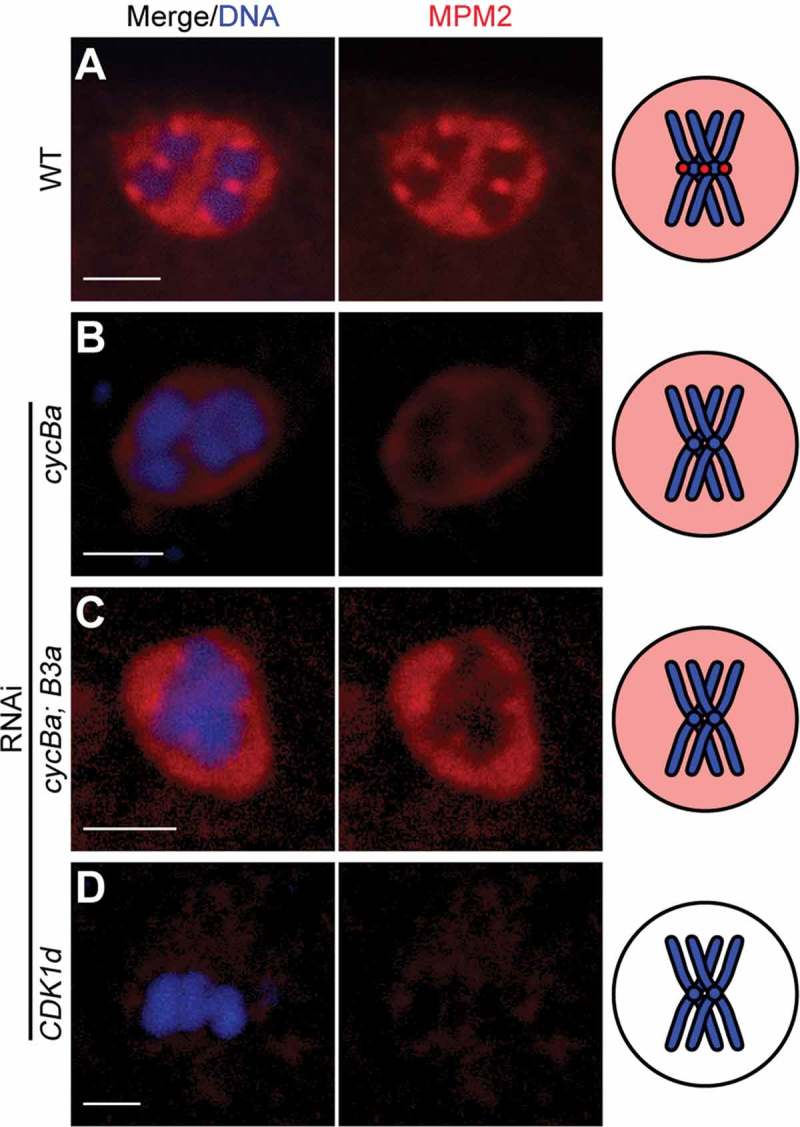
Knockdown of Cyclin Ba results in loss of MPM2 foci from centromeres. (A) MPM2 was present in the nucleoplasm and on the centromeres of bivalents during prometaphase I of wild-type meiotic nuclei. MPM2 foci were present in the nucleoplasm, but absent from chromosomes in cycBa RNAi oocytes (B) and cycBa; cycB3a double RNAi oocytes (C). (D) MPM2 was absent in meiotic nuclei in CDK1d RNAi oocytes. Schemas on the right summarize MPM2 signals (red) with only one bivalent (blue) shown for simplification. Scale bars: 2 µm.
Discussion
In this study we have shown a specialization of CDK1 paralog function during coenocystic oogenesis in O. dioica, in which CDK1a acts upstream of CDK1d to regulate vitellogenesis and Cyclin Ba dynamics. CDK1d, together with Cyclin Ba drives resumption of meiosis from prophase I arrest and final oocyte maturation. Cyclin B3a has a non-essential role in meiosis completion, though it may participate in regulating acentrosomal spindle assembly.
Knockdown of CDK1a disrupted vitellogenesis by interfering with nurse nuclear dumping to support pro-oocyte growth. Since the failure of rapid cytoplasmic transport inhibits the initiation of apoptosis in most nurse nuclei of CDK1a RNAi ovaries, we suggest that the last step of nurse nuclei contraction driven by subcortical actin-myosin network is defective. Indeed, disruption of the F-actin scaffold in the O. dioica ovary led to inhibition of oocyte growth [9]. Given that the fundamental molecular machinery of force-production by the actin-myosin network is conserved in a variety of cellular contexts and similarities in the coenocystic organization between Drosophila and Oikopleura, we speculate that CDK1a works upstream of actin-myosin contraction to promote nurse nuclei dumping and/or rapid cytoplasmic flow during pro-oocyte growth. The losses of MPM2 foci and active Plk1 on chromosomes following knockdown of CDK1a could be indirectly caused by the subsequent, reduced expression of Cyclin Ba in selected meiotic nuclei, since both phenotypes were more severe in cyclin Ba RNAi oocytes. Plk1 relies on its polo-box domain (PBD) to dock on phosphorylated targets, and CDK1 is a major kinase to generate the docking site for the recruitment of Plk1 to specific substrates or cellular structures [39]. Plk1 activation did not depend on CDK1 during meiosis I of O. dioica, since active Plk1-pT210 was present on OCs and in the nucleoplasm of meiotic nuclei in the absence of CDK1 activity. This contrasts with starfish [40] and Xenopus [6], where Plk1 is a component of an MPF amplification loop during meiosis resumption and its activation depends on the prior activation of CDK1.
Several cell cycle regulators appeared first on OCs during diplotene arrest of prophase I, including CDK1a, CDK1d, Cyclin Ba, Aurora and Plk1. This is consistent with evidence that active CDK1-Cyclin B first appears on centrosomes of mammalian cells in prophase [41] and meiotic asters close to nuclear membranes before GVBD in immature oocytes of mouse [42] and starfish [43]. OCs might integrate early meiotic pathways and coordinate the temporal order of kinase translocations into meiotic nuclei. Although CDK1a, CDK1d and Cyclin Ba locate on OCs during P3 phase, their knockdowns did not have any obvious effects on OCs, since MPM2 foci and active Plk1 remained present, OCs associated with meiotic nuclei after oocyte selection, and they disassembled during prometaphase I with normal kinetics. This suggests that neither CDK1 paralog is essential for the structure or function of OCs.
All the characteristics of CDK1d-Cyclin Ba are consistent with it being MPF: 1) translocation was observed into meiotic nuclei during diplotene arrest; 2) NEBD was promoted; and 3) it drove meiotic spindle assembly and chromosome congression during prometaphase I. Levels of phosphorylated Cyclin Ba increased dramatically during late stages of oogenesis. In mitosis of mammalian cells, phosphorylated Cyclin B1 prefers to bind active CDK1, Cyclin B1 phosphorylation promotes nuclear translocation and nuclear translocation promotes Cyclin B1 phosphorylation [44]. It is possible that CDK1d-Cyclin Ba also form a spatial positive feedback loop to ensure its nuclear translocation and activation is robust and irreversible. The observed Cyclin Ba kinetics are consistent with models where rapid synthesis of Cyclin B forms excess active CDK1-Cyclin B required for meiosis resumption [45].
As in many metazoans, O. dioica oocytes lack centrosomes during the asymmetric partitioning of cytoplasm over the two meiotic divisions [46]. In the absence of centrosomes, chromatin-based signals play a major role in recruiting microtubules to organize a bipolar spindle. While the RanGTP pathway is not essential for meiosis I [47,48], the CPC, including Aurora B kinase, and three regulatory and targeting components INCENP, Survivin and Borealin, has been shown to be critical for acentrosomal meiotic spindle assembly during meiosis I. INCENP regulates the assembly of spindle microtubules and establishment of spindle bipolarity and chromosome biorientation in Drosophila [49]. Aurora B phosphorylates and suppresses the microtubule destabilizing factors mitotic centromere-associated kinesin (MCAK) at centromere to stabilize microtubule-kinetochore attachment [50]. In O.dioica meiotic spindles, we observed that microtubules focused at the two poles formed a broad shape and did not anchor to an acentriolar MTOC since the OC was disassembled during prometaphase I (Figure S1). Following NEBD in O. dioica, we found that prometaphase I spindle assembly was accompanied by the movement of Aurora B from an even distribution in meiotic nuclei to concentration on centromeres. CDK1d-Cyclin Ba was upstream of the recruitment of the CPC to centromeres during prometaphase I. In cyclin Ba RNAi oocytes, H3-pT3 was absent on centromeres and active Plk1 failed to localize to chromosomes. Histone H3-pT3 is the centromeric target of Haspin kinase and H3-pT3 binds Survivin to recruit Aurora B to centromeres [38]. Thus Haspin was not activated in the absence of CDK1d-Cyclin Ba. It has been shown in Xenopus egg extracts and human cells that during mitotic entry, Haspin is activated by sequential phosphorylation by CDK1 and Plk1 on its N terminal [51]. Analogously, CDK1d-Cyclin Ba probably initiates activation of the H3T3 kinase Haspin, recruiting the CPC to centromeres to promote meiotic spindle assembly during prometaphase I.
Information on the roles of Cyclin B paralogs across taxonomic groups is rather patchy and is derived from mitotically proliferating cells (often embryonic) and meiotic cells (usually oogenic), though, within a given model organism, not always both. The generally emerging picture is that Cyclin B paralogs display functional redundancy but also exhibit distinct roles. In single cell budding yeast, none of Clbs 1–4 are essential and Clb2 alone is sufficient to drive meiosis [52], whereas in fission yeast a single cyclin, Cdc13, is sufficient for the entire cell cycle [53]. Cyclin B3 arose at the origin of metazoans [54], and to date, is found as a single gene in all species, except, O. dioica, where there are Cyclin B3a and B3b paralogs. Cyclin B3a is expressed during oogenesis and embryogenesis whereas Cyclin B3b is specifically expressed in the testis, reminiscent of the expression pattern of mammalian Cyclin B3 at leptotene and zygotene stages in spermatocytes [55]. As in other species, the Cyclin B3 genes of O. dioica show higher phylogenetic affinity to Cyclin B3 of other species than to other Cyclin B paralogs within O. dioica [32]. Selective pressures for a distinct Cyclin B3 throughout metazoan evolution might be expected to imply specific essential functions, but our results support studies in other species to suggest that this is not always the case.
A growing consensus from loss-of-function analyzes indicates an anaphase promoting role of Cyclin B3 [16–18,26,56]. Some recent evidence that Cyclin B3 regulates timely progression of S-phase and NEBD, might imply that anaphase defects observed in Cyclin B3 mutants may be indirectly caused by under-replicated chromosomes in the preceding S-phase [19]. In mitotic C. elegans embryonic cells, this anaphase-promoting activity appears to be SAC-dependent [56], whereas in meiotic mouse oocytes, Cyclin B3 regulation of the metaphase-anaphase transition is proposed to operate through a SAC-independent pathway [26]. The expression profile of O. dioica Cyclin B3a is consistent with a role in later meiotic events (Figure 2(a)), but we observed that it is not required to promote the metaphase to anaphase transition in this chordate species.
Cyclin B3 also collaborates with other mitotic cyclins (Cyclins B1 and B2 in C. elegans [18], Cyclin A and B in Drosophila [17]) to drive NEBD during oocyte maturation. In O. dioica, Cyclin B3a shows overlapping functions with Cyclin Ba in promoting meiotic spindle assembly. There are no functional studies of B-type Cyclins in other urochordates and cephalochordates, but the Cyclin B1 homolog maintains maximum CDK1 activity at metaphase I in the ascidian Ciona intestinalis [57]. It is tempting to suggest that in urochordates, Cyclin B1 homologs are critical for both NEBD and spindle assembly in oogenic meiosis I. Our observations have potential implications for the evolutionary trajectory of metazoan B-type Cyclins in oogenic meiosis. In invertebrates, Cyclin B1 collaborates with other mitotic Cyclins (Cyclin B2 and B3 in C. elegans, Cyclin A and B3 in Drosophila) to promote NEBD. Cyclin B1 seems more critical for spindle organization in Drosophila [17], but not in C. elegans [18]. In clam and starfish, Cyclin A is not expressed during meiosis I, such that Cyclin B is the sole regulator of CDK1 to drive meiosis resumption [58,59]. In sea urchin, new Cyclin B synthesis is not required for GVBD, but is required for spindle formation, and Cyclin A, though present, can’t compensate for the loss of Cyclin B in meiosis I [60]. In non-mammalian vertebrates such as Xenopus, Cyclin B2 is the dominant subtype in meiosis I, and its neosynthesis is critical to promote GVBD and spindle assembly [45,61]. In mice, Cyclin B2 is critical for GVBD and initial stage of spindle assembly during early metaphase I [62], whereas the rate of Cyclin B1 synthesis after GVBD determines the progression of spindle assembly and the length of meiosis I [63]. Thus, Cyclin B1 homologs appear to become more dominant in driving meiotic progression over the course of animal evolution. Our results suggest that the chordate common ancestor had Cyclin B1 and Cyclin B3, with Cyclin B1 playing a major role in both GVBD and spindle assembly. Two rounds of whole genome duplication in the vertebrate stem gave rise to Cyclin B2, which shares responsibilities with Cyclin B1 in meiotic events to different extents in different vertebrates.
Materials and methods
Animal culture and sample collection
Oikopleura dioica were maintained in culture at 15°C [64]. Days 4–6 animals were placed in artificial seawater, removed from their houses, anesthetized in ethyl 3-aminobenzoate methanesulfonate salt (MS-222, 0.125 mg/ml; Sigma), and then fixed in 4% paraformaldehyde (PFA) for in situ hybridization or immunofluorescence, or snap frozen in liquid nitrogen for western blots or RNA extraction. For dsRNA injected animals, mature females were transferred to artificial seawater in 6-well plates coated with 0.1% gelatin. Equal numbers of spawned oocytes were collected for western blot and kinase activity assays, and remaining oocytes were used for RT-qPCR, immunofluorescence or in vitro fertilization as described previously [33].
In situ hybridization on paraffin sections or of whole-mount fluorescence
After fixation at 4°C overnight, samples were washed 3 times in PBS, dehydrated in 70%-95%-100% gradient ethanol (1 h each), cleared in xylene for 30 minutes, and incubated in paraffin at 55°C for 2 h before solidification on ice. Sections (5 µm) were cut using a Leica microtome RM2155 and collected on PolysineTM slides (ThermoFisher). After drying at 55°C, paraffin sections were de-paraffinized in xylene, rehydrated in 100%-95%-70% gradient ethanol, and processed for in situ hybridization as previously [65]. Digoxygenin-labeled anti-sense RNA probes of full-length cyclin Ba were used for hybridization. For whole-mount fluorescence in situ, hybridization signal was detected by anti-Digoxigenin-POD and TSA Plus Cyanine 5 Evaluation Kit, and collected by excitation at 561 nm and emission from 563 nm to 620 nm.
Microinjections
Injection solutions were prepared by mixing 400 ng/µl capped mRNA or dsRNA with 100 ng/µl Alexa Fluor 568 dye (Molecular Probes) in RNase-free PBS. Microinjection was performed as described previously [33].
eGFP fusion constructs, cmRNA and dsRNA synthesis
3ʹ UTRs of CDK1a, CDK1d and cycBa were obtained by 3ʹ RACE (SMARTer RACE 5ʹ/3ʹ Kit, Clontech). For eGFP fusion constructs, the coding region (with its endogenous 3ʹ UTR) was driven by the T7 promoter, followed by eGFP at the C-terminal. The construct was linearized and used as template to synthesize cmRNA (Ambion mMessage mMachine T7 transcription kit) according to the manufacturer’s protocol. The cmRNA was purified using Ambion MEGAclear kit. About 300 bp fragments in the N terminal of the coding region of cycBa and cycB3a were amplified as template to synthesize dsRNA as described previously [33].
Antibodies
Custom rabbit polyclonal affinity–purified anti-cyclin Ba/b (acetyl-NRDLNIQESGPVKAVVNAC-amide and acetyl-CLEFLRRFSRVAEETIDPKEY-amide), and rabbit polyclonal affinity–purified anti-lamin1 (acetyl-QSPISLPPLSGSTC-amide) were produced by 21st Century Biochemicals (Marlboro, MA). Other antibodies included anti-Histone H3-pT3 (Abcam), anti-Histone H3-pS10 (Millipore), anti-Histone H3-pS28 (Abcam), anti-phospho-Ser/Thr-Pro MPM-2 (Millipore), anti-Plk1-pT210 (BioLegend), anti-Aurora A (pT288)/Aurora B (pT232)/Aurora C (pT198) (Cell Signaling Technology), anti-MAPK-pTEpY (pERK1/2) (Promega), anti-PSTAIRE (Abcam), anti-eGFP (AMS Biotechnology), anti-Tubulin (Abcam). Secondary antibodies against rabbit, rat and mouse IgG (conjugated Alexa Fluor 488, 568 or HRP) were from Molecular Probes. For immunofluorescence, primary antibodies were used at 1:100 dilution and secondary antibodies at 1:300 dilution. For western blot, primary and secondary antibodies were used at 1:1000 and 1:5000 dilutions, respectively.
CDK1 kinase assay
CDK1 kinase activity was determined using the MESACUP CDK1 Kinase Assay Kit (MBL). Briefly, equal numbers of oocytes were frozen and thawed twice. Then the samples were mixed with biotinylated MV peptide and 1 mM ATP and incubated at 30°C for 30 min. After stopping the phosphorylation reaction, ELISA was performed using anti-phosphorylated MV peptide antibody. Finally, POD conjugated streptavidin was used to detect phosphorylated MV peptide and color development measured at OD492nm. Statistical significances of CDK1 kinase activities from different knockdown oocytes were analyzed by the Student’s T-test.
Biography
H.F., and E.M.T. conceived and designed the experiments. H.F. performed experiments and H.F. and E.M.T. analyzed the data. H.F. and E.M.T. wrote the manuscript. All authors approved the manuscript.
Funding Statement
This work was supported by a PhD fellowship from Department of Biological Sciences, University of Bergen (H.F.) and grants 183690/S10 NFR-FUGE and 133335/V40 from the Norwegian Research Council (E.M.T.).
Acknowledgments
We thank Xiaofei Ma, Sars Centre, for helpful discussions and A. Aasjord and K. N. Nøkling for providing animals from the Oikopleura culture facility.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Von Stetina JR, Orr-Weaver TL.. Developmental control of oocyte maturation and egg activation in metazoan models. Cold Spring Harb Perspect Biol. 2011;3:a005553 PMID:21709181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kubiak JZ, Ciemerych MA, Hupalowska A, et al. On the transition from the meiotic to mitotic cell cycle during early mouse development. Int J Dev Biol. 2008;52:201–217. PMID:18311711. [DOI] [PubMed] [Google Scholar]
- [3].Yamashita M. Molecular mechanisms of meiotic maturation and arrest in fish and amphibian oocytes. Semin Cell Dev Biol. 1998;9:569–579. PMID:9835645. [DOI] [PubMed] [Google Scholar]
- [4].Kishimoto T. Cell-cycle control during meiotic maturation. Curr Opin Cell Biol. 2003;15:654–663. PMID:14644189. [DOI] [PubMed] [Google Scholar]
- [5].Seki A, Coppinger JA, Jang C-Y, et al. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. PMID:18566290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Abrieu A, Brassac T, Galas S, et al. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J Cell Sci. 1998;111:1751–1757. PMID:9601104. [DOI] [PubMed] [Google Scholar]
- [7].Delsuc F, Tsagkogeorga G, Lartillot N, et al. Additional molecular support for the new chordate phylogeny. Genesis. 2008;46:592–604. PMID:19003928. [DOI] [PubMed] [Google Scholar]
- [8].Ganot P, Bouquet J-M, Thompson EM. Comparative organization of follicle, accessory cells and spawning anlagen in dynamic semelparous clutch manipulators, the urochordate Oikopleuridae. Biol Cell. 2006;98:389–401. PMID:16478443. [DOI] [PubMed] [Google Scholar]
- [9].Ganot P, Kallesøe T, Thompson EM. The cytoskeleton organizes germ nuclei with divergent fates and asynchronous cycles in a common cytoplasm during oogenesis in the chordate Oikopleura. Dev Biol. 2007;302:577–590. PMID:17123503. [DOI] [PubMed] [Google Scholar]
- [10].Pepling ME, de Cuevas M, Sprading AC. Germline cysts: a conserved phase of germ cell development? Trends Cell Biol. 1999;9:257–262. PMID:10370240. [DOI] [PubMed] [Google Scholar]
- [11].Bastock R, St Johnston D. Drosophila oogenesis. Curr Biol. 2008;18:R1082–R1087. PMID:19081037. [DOI] [PubMed] [Google Scholar]
- [12].Matova N, Cooley L. Comparative aspects of animal oogenesis. Dev Biol. 2001;231:291–320. PMID:11237461. [DOI] [PubMed] [Google Scholar]
- [13].Ganot P, Moosmann-Schulmeister A, Thompson EM. Oocyte selection is concurrent with meiosis resumption in the coenocystic oogenesis of Oikopleura. Dev Biol. 2008;324:266–276. PMID:18845138. [DOI] [PubMed] [Google Scholar]
- [14].Loog M, Morgan DO. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature. 2005;434:104–108. PMID:15744308. [DOI] [PubMed] [Google Scholar]
- [15].Hochegger H, Klotzbücher A, Kirk J, et al. New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development. 2001;128:3795–3807. PMID:11585805. [DOI] [PubMed] [Google Scholar]
- [16].Yuan K, O’Farrell PH. Cyclin B3 is a mitotic cyclin that promotes the metaphase-anaphase transition. Curr Biol. 2015;25:811–816. PMID:25754637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bourouh M, Dhaliwal R, Rana K, et al. Distinct and overlapping requirements for Cyclins A, B, and B3 in Drosophila female meiosis. G3 Genes|Genomes|Genetics. 2016;6:3711–3724. PMID:27652889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].van der Voet M, Lorson MA, Srinivasan DG, et al. C. elegans mitotic cyclins have distinct as well as overlapping functions in chromosome segregation. Cell Cycle. 2009;8:4091–4102. PMID:19829076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Michael WM. Cyclin CYB-3 controls both S-phase and mitosis and is asymmetrically distributed in the early C. elegans embryo. Development. 2016;143:3119–3127. PMID:27578178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brandeis M, Rosewell I, Carrington M, et al. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc Natl Acad Sci U S A. 1998;95:4344–4349. PMID:9539739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang Y, Sramkoski RM, Jacobberger JW. The kinetics of G2 and M transitions regulated by B cyclins. PLoS One. 2013;8:e80861 PMID:24324638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jackman M, Firth M, Pines J. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO J. 1995;14: 1646–1654. PMID:7737117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Spalluto C, Wilson DI, Hearn T. Evidence for centriolar satellite localization of CDK1 and cyclin B2. Cell Cycle. 2013;12:1802–1803. PMID:23656781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nam H-J, van Deursen JM. Cyclin B2 and p53 control proper timing of centrosome separation. Nat Cell Biol. 2014;16:538–549. PMID:24776885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gallant P, Nigg EA. Identification of a novel vertebrate cyclin: cyclin B3 shares properties with both A- and B-type cyclins. EMBO J. 1994;13: 595–605. PMID:8313904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang T, Qi S-T, Huang L, et al. Cyclin B3 controls anaphase onset independent of spindle assembly checkpoint in meiotic oocytes. Cell Cycle. 2015;14:2648–2654. PMID:26125114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Morgan DO. CYCLIN-DEPENDENT KINASES: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. PMID:9442875. [DOI] [PubMed] [Google Scholar]
- [28].Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. PMID:19238148. [DOI] [PubMed] [Google Scholar]
- [29].Kishimoto T. A primer on meiotic resumption in starfish oocytes: the proposed signaling pathway triggered by maturation-inducing hormone. Mol Reprod Dev. 2011;78:704–707. PMID:21714029. [DOI] [PubMed] [Google Scholar]
- [30].Stern B, Ried G, Clegg NJ, et al. Genetic analysis of the Drosophila cdc2 homolog. Development. 1993;117:219–232. PMID:8223248. [DOI] [PubMed] [Google Scholar]
- [31].Boxem M, Srinivasan DG, van den Heuvel S. The Caenorhabditis elegans gene ncc-1 encodes a cdc2-related kinase required for M phase in meiotic and mitotic cell divisions, but not for S phase. Development. 1999;126: 2227–2239. PMID:10207147. [DOI] [PubMed] [Google Scholar]
- [32].Campsteijn C, Øvrebø JI, Karlsen BO, et al. Expansion of cyclin D and CDK1 paralogs in Oikopleura dioica, a chordate employing diverse cell cycle variants. Mol Biol Evol. 2012;29:487–502. PMID:21734012. [DOI] [PubMed] [Google Scholar]
- [33].Øvrebø JI, Campsteijn C, Kourtesis I, et al. Functional specialization of chordate CDK1 paralogs during oogenic meiosis. Cell Cycle. 2015;14:880–893. PMID:25714331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Becalska AN, Gavis ER. Lighting up mRNA localization in Drosophila oogenesis. Development. 2009;136:2493–2503. PMID:19592573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Foley K, Cooley L. Apoptosis in late stage Drosophila nurse cells does not require genes within the H99 deficiency. Development. 1998;125: 1075–1082. PMID:9463354. [DOI] [PubMed] [Google Scholar]
- [36].Cooley L, Verheyen E, Ayers K. chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell. 1992;69: 173–184. PMID:1339308. [DOI] [PubMed] [Google Scholar]
- [37].Colombié N, Cullen CF, Brittle AL, et al. Dual roles of Incenp crucial to the assembly of the acentrosomal metaphase spindle in female meiosis. Development. 2008;135:3239–3246. PMID:18755775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yamagishi Y, Honda T, Tanno Y, et al. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330:239–243. PMID:20929775. [DOI] [PubMed] [Google Scholar]
- [39].Barr FA, Silljé HHW, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. PMID:15173822. [DOI] [PubMed] [Google Scholar]
- [40].Okano-Uchida T, Okumura E, Iwashita M, et al. Distinct regulators for Plk1 activation in starfish meiotic and early embryonic cycles. EMBO J. 2003;22:5633–5642. PMID:14532135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jackman M, Lindon C, Nigg EA, et al. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. PMID:12524548. [DOI] [PubMed] [Google Scholar]
- [42].Marangos P, Carroll J. The dynamics of cyclin B1 distribution during meiosis I in mouse oocytes. Reproduction. 2004;128:153–162. PMID:15280554. [DOI] [PubMed] [Google Scholar]
- [43].Ookata K, Hisanaga S, Okano T, et al. Relocation and distinct subcellular localization of p34cdc2-cyclin B complex at meiosis reinitiation in starfish oocytes. EMBO J. 1992;11:1763–1772 PMID:1316272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Santos SDM, Wollman R, Meyer T, et al. Spatial positive feedback at the onset of mitosis. Cell. 2012;149:1500–1513. PMID:22726437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gaffré M, Martoriati A, Belhachemi N, et al. A critical balance between Cyclin B synthesis and Myt1 activity controls meiosis entry in Xenopus oocytes. Development. 2011;138:3735–3744. PMID:21795279. [DOI] [PubMed] [Google Scholar]
- [46].Bennabi I, Terret M-E, Verlhac M-H. Meiotic spindle assembly and chromosome segregation in oocytes. J Cell Biol. 2016;215:611–619. PMID:27879467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dumont J, Petri S, Pellegrin F, et al. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J Cell Biol. 2007;176:295–305. PMID:17261848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cesario J, McKim KS. RanGTP is required for meiotic spindle organization and the initiation of embryonic development in Drosophila. J Cell Sci. 2011;124:3797–3810. PMID:22100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Radford SJ, Jang JK, McKim KS. The chromosomal passenger complex is required for meiotic acentrosomal spindle assembly and chromosome biorientation. Genetics. 2012;192:417–429. PMID:22865736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Andrews PD, Ovechkina Y, Morrice N, et al. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–268. PMID:14960279. [DOI] [PubMed] [Google Scholar]
- [51].Ghenoiu C, Wheelock MS, Funabiki H. Autoinhibition and polo-dependent multisite phosphorylation restrict activity of the histone H3 kinase haspin to mitosis. Mol Cell. 2013;52:734–745. PMID:24184212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Richardson H, Lew DJ, Henze M, et al. Cyclin-B homologs in Saccharomyces cerevisiae function in S phase and in G2. Genes Dev. 1992;6:2021–2034. PMID:1427070. [DOI] [PubMed] [Google Scholar]
- [53].Fisher DL, Nurse P. A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J. 1996;15: 850–860. PMID:8631306. [PMC free article] [PubMed] [Google Scholar]
- [54].Lozano J-C, Vergé V, Schatt P, et al. Evolution of Cyclin B3 shows an abrupt three-fold size increase, due to the extension of a single exon in placental mammals, allowing for new protein–protein interactions. Mol Biol Evol. 2012;29:3855–3871. PMID:22826462. [DOI] [PubMed] [Google Scholar]
- [55].Nguyen TB, Manova K, Capodieci P, et al. Characterization and expression of mammalian cyclin B3, a prepachytene meiotic cyclin. J Biol Chem. 2002;277:41960–41969. PMID:12185076. [DOI] [PubMed] [Google Scholar]
- [56].Deyter GM, Furuta T, Kurasawa Y, et al. Caenorhabditis elegans cyclin B3 is required for multiple mitotic processes including alleviation of a spindle checkpoint-dependent block in anaphase chromosome segregation. PLoS Genet. 2010;6:e1001218 PMID:21124864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Russo GL, Kyozuka K, Antonazzo L, et al. Maturation promoting factor in ascidian oocytes is regulated by different intracellular signals at meiosis I and II. Development. 1996;122:1995–2003. PMID:8681780. [DOI] [PubMed] [Google Scholar]
- [58].Hunt T, Luca FC, Ruderman JV. The requirements for protein synthesis and degradation, and the control of destruction of cyclins A and B in the meiotic and mitotic cell cycles of the clam embryo. J Cell Biol. 1992;116: 707–724. PMID:1530948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Okano-Uchida T, Sekiai T, Lee K, et al. In vivo regulation of Cyclin A/Cdc2 and Cyclin B/Cdc2 through meiotic and early cleavage cycles in starfish. Dev Biol. 1998;197:39–53. PMID:9578617. [DOI] [PubMed] [Google Scholar]
- [60].Voronina E, Marzluff WF, Wessel GM. Cyclin B synthesis is required for sea urchin oocyte maturation. Dev Biol. 2003;256:258–275. PMID:12679101. [DOI] [PubMed] [Google Scholar]
- [61].Yoshitome S, Furuno N, Prigent C, et al. The subcellular localization of cyclin B2 is required for bipolar spindle formation during Xenopus oocyte maturation. Biochem Biophys Res Commun. 2012;422:770–775. PMID:22627133. [DOI] [PubMed] [Google Scholar]
- [62].Gui L, Homer H. Hec1-dependent Cyclin B2 stabilization regulates the G2-M transition and early prometaphase in mouse oocytes. Dev Cell. 2013;25:43–54. PMID:23541922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Polanski Z, Ledan E, Brunet S, et al. Cyclin synthesis controls the progression of meiotic maturation in mouse oocytes. Development. 1998;125:4989–4997. PMID:9811583. [DOI] [PubMed] [Google Scholar]
- [64].Bouquet J-M, Spriet E, Troedsson C, et al. Culture optimization for the emergent zooplanktonic model organism Oikopleura dioica. J Plankton Res. 2009;31:359–370. PMID:19461862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ganot P, Bouquet J-M, Kallesøe T, et al. The Oikopleura coenocyst, a unique chordate germ cell permitting rapid, extensive modulation of oocyte production. Dev Biol. 2007;302:591–600. PMID:17126826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


