SUMMARY
Hepatocellular carcinoma (HCC) burden is highest in East Asia and Africa, although its incidence and mortality are rapidly rising in the United States and Europe. With implementation of hepatitis B vaccination and hepatitis C treatment programs worldwide, there is a shift in HCC epidemiology from a viral-hepatitis predominant disease to an increasing proportion of cases from non-alcoholic steatohepatitis (NASH). Surveillance using ultrasound with or without alpha fetoprotein every 6 months has been associated with improved early detection and improved overall survival; however, limitations in implementation lead to a high proportion of HCC being detected at late stages in clinical practice. Herein, we review the current state of HCC surveillance and highlight areas for future research including improved risk stratification of at-risk patients, surveillance tools with higher sensitivity and specificity for early HCC, and interventions to increase surveillance utilization.
Liver cancer is the sixth most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide, after lung, colorectal, and stomach cancer.1 Liver cancer is a highly fatal tumor, with most cases detected at late stages and an incidence-to-mortality ratio that approaches 1. For example, there were approximately 854,000 new liver cancer cases in 2015, compared to an estimated 810,000 liver cancer-related deaths per year.2 Hepatocellular carcinoma (HCC) represents about 75–85% of primary liver cancers3 and constitutes a major health problem worldwide.
Incidence
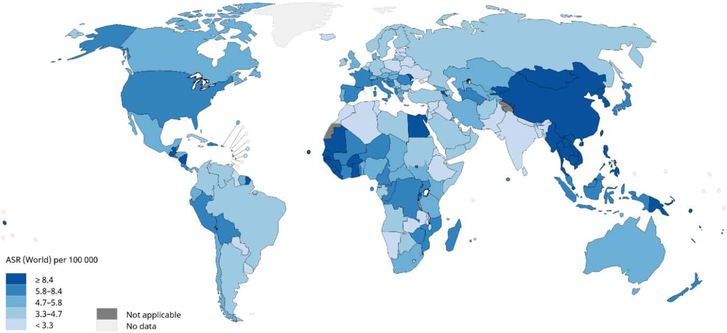
The worldwide incidence of HCC is heterogeneous because of the variable prevalence of underlying risk factors. It is estimated that 72% of cases occurs in Asia (more than 50% in China), 10% in Europe, 7.8% in Africa, 5.1% in North America, 4.6% Latin America and 0.5% in Oceania.4 Figure 1 shows the estimated ASIR for liver cancer in the world in 2018. The highest age-standardized incidence rates (ASIR) per 100,000 occur in Eastern Asia (17.7), with Mongolia (93.4) having the highest ASIR in that area and overall in the word, followed by other regions, East Asia (17.7), South-East Asia (13.3), and Africa (8.4), with Egypt (32.2) and Gambia (23.9) having the highest ASIR in that region. The lowest ASIR are observed in South Central Asia (2.5), followed by Central and Eastern Europe and Western Asia. (equally about 4.0).5
Figure 1.
Worldwide Age-standardized HCC Incidence Rates, 2018
Mortality
Age-standardized mortality rates (ASMR) from HCC in 2018 are also highest in Eastern Asia (16.0) and Northern Africa (13.9) followed by South Eastern Asia (13.2). The lowest ASMR is observed in South Central Asia (2.3), followed by Central, Northern, and Eastern Europe and Western Asia (around 3.8—4.0). Mongolia and Egypt have the highest ASMR, while the lowest are in Morocco and Nepal, countries with low ASIR. Worldwide the ASMR is close to ASIR, reflecting the fact that HCC is a deadly disease.6
Etiology
The large majority of HCC cases occur in the setting of chronic liver disease, with cirrhosis being the primary risk factor for HCC independent of liver disease etiology. It is estimated that one-third of cirrhotic patients will develop liver cancer during their lifetime7, with a 1-8% annual incidence reported in long-term follow-up studies (e.g. 2% in HBV-infected cirrhotic patients and 3—8% in HCV-infected cirrhotic patients).8 The incidence of HCC appears lower in alcohol-related and non-alcohol steatohepatitis (NASH)-related cirrhosis than active viral hepatitis but the incidence appears to be greater than 1.5% across cirrhosis etiologies.
Hepatitis B virus (HBV) is the leading cause of incident cases of liver cancer and deaths in the world (33%), followed by alcohol (30%), hepatitis C virus (HCV) (21%) and other causes (16%). Contribution of different etiologies to HCC incidence varies markedly between countries and regions and are summarized in Table 1. In Africa and East Asia, the largest population attributable fraction is caused by HBV (60%); however, in the Western world only 20% of cases can be attributed to HBV infection, and chronic HCV is the most common underlying liver disease etiology.2 Among HBV-infected individuals, HBV eAg seropositivity9, high viral load10 and genotype C11 are independent predictors of HCC development. Although prior studies among HCV-infected patients similarly reported risk factors, e.g. HCV genotype, the strongest determinants of HCC risk in these patients are currently the presence (vs. absence) of cirrhosis and attaining sustained viral response (SVR). Guidelines uniformly recommend surveillance in patients with HCV-related cirrhosis but differ on HCC risk and recommendations for HCC surveillance in HCV-infected patients with F3 fibrosis;12-14 however, a recent cost-effectiveness analysis suggests that this practice is likely not cost-effective in those without cirrhosis.15
Table 1.
Geographical distribution of risk factors for primary liver cancer
| Variables | Alcohol (%) | Hepatitis B (%) | Hepatitis C (%) | Others (%) |
|---|---|---|---|---|
| Global | 30 | 33 | 21 | 16 |
| Europe: | ||||
| Western | 32 | 13 | 44 | 10 |
| Central | 46 | 15 | 29 | 10 |
| Eastern | 53 | 15 | 24 | 8 |
| America: | ||||
| North America | 37 | 9 | 31 | 23 |
| Andean Latin America | 23 | 45 | 12 | 20 |
| South Latin America | 42 | 6 | 41 | 11 |
| Asia: | ||||
| East Asia | 32 | 41 | 9 | 18 |
| Asia-Pacific | 18 | 22 | 55 | 6 |
| South-East Asia | 31 | 26 | 22 | 21 |
| Africa: | ||||
| North Africa, Middle East | 13 | 27 | 44 | 16 |
| Southern (sub-Saharan) | 40 | 29 | 20 | 11 |
| Western (sub-Saharan) | 29 | 45 | 11 | 15 |
| Oceania | 16 | 38 | 19 | 27 |
Contribution of hepatitis B, C, alcohol and others causes on absolute liver cancer deaths, both sexes, globally and by region 2015 (3). Data refer to all primary liver cancers (hepatocellular carcinoma, intrahepatic cholangiocarcinoma and liver cancer of mixed differentiation).
Implementation of infant HBV immunization programs in many countries in East Asia are expected to lower HBV-related HCC in the future, as demonstrated in Taiwan where annual HCC incidence significantly decreased from 0.92 per 105 persons in an unvaccinated cohort of patients to 0.23 per 105 persons in a vaccinated birth cohort.16 However, there are several countries which have yet to implement universal HBV vaccination, so many persons are still infected with HBV (approximately 257 million in 2015), mostly in Asia and Sub-Saharan Africa.17 Unfortunately, a vaccine for HCV does not exist so primary prevention of HCV-related HCC is not possible. Among those with active infection, antiviral therapies are effective in reducing HCC incidence although they do not eradicate the risk, in both HBV- and HCV-infected patients.18-20 Among HCV-infected patients the risk of developing HCC significantly declined from 6.2% to 1.5% with interferon-based SVR19 and a similar reduction is observed for SVR from direct-acting antiviral agents.20 Despite improvement, patients with cirrhosis prior to SVR remain at high HCC risk so surveillance should be continued.21
Alcohol consumption and the resulting cirrhosis seem to have a causal relationship in the development of HCC.22 In France, the estimated HCC incidence in patients with alcoholic cirrhosis was 2.9 per 100 patient-year in a cohort of 652 French patients during a median follow-up of 29 months.23 An increased risk of developing HCC has been reported for most parts of the world (with the exception of Northern Europe), including France24 and Spain.25
There is a growing evidence that non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) contribute to HCC development, and this is becoming an increasing common cause of HCC worldwide. It is estimated that about 10-30% of NAFLD progress to cirrhosis, and in the United States alone approximately 6 million people have NASH.26 Although patients with NASH appear to have a lower risk of HCC than patients with HCV-related cirrhosis, the annual incidence is likely between 1-2%. In a large cohort study of 4235 patient with NASH cirrhosis from the Veterans Affairs health system in the United States, the incidence of HCC was determined to be 1.06 per 100 person-years.27 Whereas NAFLD has a lower risk of developing liver cancer than those with NASH cirrhosis, the high number of people having NAFLD makes it one of the major causes of HCC. There have now been several cohort studies that have shown over one-fourth of NASH-related HCC can occur in the absence of cirrhosis,28,29 which is significantly higher than proportions seen in other liver diseases.30 However, the annual incidence rate of HCC in non-cirrhotic NASH appears to be low. Data from the Veterans Affairs health system among a cohort of 292,366 patients with non-cirrhotic NAFLD demonstrated an incidence of only 0.008 per 100 person-years.27 Similarly, data from Taiwan demonstrated 1-, 3-, and 5-year cumulative incidences of only 0.2%, 0.8%, and 1.0%.31 However, a recent systematic review of this literature highlighted several notable limitations of the current literature including heterogeneous definitions for NAFLD, differential proportions of patients with metabolic syndrome, heterogeneous definitions for cirrhosis, ascertainment bias given intermittent surveillance and selection/referral bias.32 Therefore, the impact of metabolic liver disease on epidemiology of HCC is likely to be underestimated. Components of the metabolic syndrome, such as diabetes and/or obesity, are emerging risk factors for HCC as well and may increase HCC risk if present with other chronic liver diseases, even in the absence of a NAFLD diagnosis. Obesity might account for about 16% of HCC cases in Europe, according to the EPIC study,33 while both, obesity and/or diabetes accounts for about 37% of HCC in the US.34 Based on current data, HCC risk is sufficient to justify HCC surveillance in patients with NASH cirrhosis; however, HCC surveillance is not recommended in those with non-cirrhotic NAFLD given the low annual incidence rate.
Patients with other, less common causes of cirrhosis including primary biliary cirrhosis, autoimmune hepatitis, and hemochromatosis also have increased risk of HCC. Patients with hemochromatosis who progressed to advanced fibrosis/cirrhosis are at extremely high risk and develop HCC in up to 45% of cases35,36, with a higher incidence in those with acute hepatic porphyria and porphyria cutanea tarda.37,38
Among patients with cirrhosis, there is a differential distribution of cases by several sociodemographic factors. HCC has a strong male predominance for incidence and mortality, with a male-to-female ratio exceeding 2.5 for both.2 This differential distribution by sex is believed to be related to a clustering of risk factors among men as well as a potential effect of androgens on HCC risk. Similarly, several studies have reported higher incidence and mortality rates among racial/ethnic minorities in the United States, with higher incidence rates among racial/ethnic minorities than non-Hispanic whites.39
Finally, there are important environmental risk factors for HCC. For example, dietary intake of aflatoxin B1, which originate from fungal contaminations of staple foodstuffs, is a relevant co-factor for HCC development in area of Africa and Asia. Aflatoxin B1 exposure has a strong correlation with and TP53 mutation (codon 249) and HCC development in HBV-infected individuals.40 Several epidemiological studies have also revealed an increased risk of developing HCC among cigarettes smoking, with a meta-analysis reporting a 1.5 adjusted RR (95% CI: 1.37–1.67) among smokers.41
Several epidemiological studies have addressed the topic of HCC prevention in the general population and in patients with chronic liver disease. Coffee consumption, aspirin use and metformin assumption in patients with diabetes have been shown to consistently reduce the HCC incidence.13, 42,43 The highest evidence has been produced for coffee consumption by means of case-control studies in Japanese HCV-patients and a hospital-based-control-study among Italian patients with a variety of liver disease etiologies. These findings have been also confirmed in cohort studies performed in Japan and Southern Europe and a meta-analysis.13, 44-47
Trends
Between 1990 and 2015, liver cancer incidence increased by 75% worldwide. These data reflect changes in etiology, population age distribution, population growth, and age specific incidence rates.2 During this period a significant increase in HCC age-standardized incidence (per 100,000 persons) due to HCV (+15.7%) was observed, while HBV-related HCC significantly decreased (−18.9%) and no significant changes were observed for HCC due to alcohol (+13.5%) and other causes (−12.3%).2 Despite a decrease in age-specific incidence rates for HCC related to HBV and other causes, overall incident HCC cases are increased owing to demographic changes of population growth and aging.2 Based on current trends, the number of new cases and deaths for liver cancer are projected to increase from 841,080 and 781,631 in 2018, to 1,361,836 and 1,284,252 in 2040, with changes of +62% and + 64%, respectively.48
There is geographic variation in these temporal trends. ASIR has increased in many high sociodemographic index countries like North America (USA, Canada), Australia, New Zealand and most European countries (i.e. Austria, Denmark, Germany, Greece, Ireland, Portugal, Norway, Spain, Switzerland, and United Kingdom); conversely, some countries with high incidence rates like China and Eastern and Western Sub-Saharan Africa have experienced a decrease by more than 20%.2 Declines in ASIR have also been observed in Japan, where a decline in HCC incidence has been noted for the first time since 1990,49,50 and in some countries in Europe (i.e. Finland, France, Italy, Netherlands, and Sweden).51
Along the same line is a recent report evaluating projections of primary liver cancer occurrence in 30 Countries worldwide,52 which predicts the percentage change in ASIR over a 25-year period, from 2005 to 2030, increasing >30% among men in 15 countries and among women in 8 countries. The largest rate increases among men are predicted in Norway (2.9% per annum),US whites (2.6%), Canada (2.4%), Russian Federation (2.2%). Equivalent increases in primary liver cancer among women are predicted in fewer, with the greatest increases expected among US blacks (4.0%), Switzerland (3.4%), and Germany (3.0%). In contrast, a decrease in liver cancer among men is predicted in Japan (23.1%), China (22.1%), Singapore (21.6%), Slovakia (21.4%), Czech Republic (21.0%), and Estonia (20.6%), while the largest decreases among women are predicted in Japan (22.3%) and Denmark (21.8%).
The predicted changes in increased incidence mainly reflect demographic increase due to population growth and aging in most countries and changes in risk factors. Changing distributions of risk factors, especially HBV, HCV, alcohol consumption, and obesity, could alter future trends and projections. Upfront to predicted declines in the prevalence of HBV and HCV infections, mainly due to HBV immunization and increased efforts to screen and treat patients with active HCC, the importance of non-viral risk factors for HCC is expected to increase in the future, mainly due to NAFLD. In the United States, it was estimated an increased incidence of hepatocellular carcinoma due to NAFLD by 122% between 2016 and 2030, from 5,510 to 12,240 cases.53
HCC Surveillance Data and Intervals
Cancer surveillance programs aim to detect tumors at an early stage when they are amenable to curative therapy known to improve survival,54 The evidence highlighting a survival benefit associated with HCC screening in patients with cirrhosis remains controversial.55 Apart from numerous methodological biases discussed below, analysis of the literature shows in fact that negative studies often underscore inappropriate or suboptimal implementation of screening procedures rather than failure of surveillance programs to be translated into survival benefit.56 The only randomized controlled trial supporting HCC surveillance using abdominal ultrasound every 6 months was obtained from a trial performed in more than 18,000 Chinese patients and displayed a 37% reduction risk in mortality in screened patients.57 However, this trial was conducted in a HBV-infected patient population and it is unclear if these results would apply to patients with cirrhosis given increased nodularity which could impact surveillance effectiveness as well as a higher competing risk of liver-related mortality. Because the implementation of trials comparing screening versus no surveillance would not be ethical,58 the level of evidence mostly relies on retrospective observational studies which have concluded that surveillance for HCC was an independent predictor of survival.59-63 More recently, the long prospective follow-up of patients with compensated viral cirrhosis showed that patients who respected the recommended 6-months screening interval had higher proportion of HCC detected at an early stage, which translated into a survival benefit due to more frequent implementation of first-line curative procedures.64 However, numerous limitations and biases affecting observational studies dedicated to cancer screening must be acknowledged. Among them, lead-time bias suggests that a given proportion of the survival benefit could be ascribed to earlier diagnosis due to surveillance. In addition, length time bias supports that tumors diagnosed early in the setting of surveillance programs might differ in their prognosis from tumors diagnosed later. The most recent studies assessing the impact of HCC screening on outcomes usually took into account these biases in an attempt to reinforce strength in the drawn conclusions.65
Western recommendations support a 6-month time frame for screening interval based on HCC volume doubling-time, which is estimated to be around 6 months.66 In order to minimize the risk of detecting HCC at an advanced stage, a 3-month interval has been proposed by Japanese guidelines for specific groups considered at higher risk.67 However, a French randomized trial had previously compared intervals of 3 and 6 months in more than 1200 cirrhotic patients, and concluded that surveillance performed every 3 months, detected increased small-size focal lesions compared with US every 6 months but did not improve detection of early HCC and did not translate into survival benefits.68 Similarly, a large retrospective study assessed the impact of different surveillance intervals in patients at risk of HCC.69 Shorter US screening intervals were associated with reduced overall mortality in these patients, and as a whole provided additional arguments to support the 6-month time frame as the optimal cut-off for HCC screening interval.
Surveillance Tests
Abdominal ultrasound has been the historic cornerstone for HCC surveillance and continues to be recommended as the primary surveillance test by the AASLD, EASL, and APASL. It has several advantages including being cheap, readily available, and safe with minimal direct physical harms. Although ultrasound has an acceptable sensitivity of 84% (95%CI 76 – 92%) for detecting HCC at any stage, its sensitivity for detection of early stage HCC is significantly lower at only 47% (95%CI 33 – 61%).70 Further, its effectiveness can be affected by operator expertise as well as several patient-level factors such as obesity and liver disease severity, leading to wide variation in its sensitivity between centers and patients.71-73 The reported lower sensitivity of ultrasound in patients with obesity and non-viral liver disease is particularly concerning in light of epidemiologic shifts with an increasing proportion of HCC related to underlying NASH. However, there are few data specifically evaluating surveillance effectiveness among cohorts with emerging risk factors such as those with NASH and post-SVR HCV infection.
Given these concerns, there has been increasing use of alternative imaging modalities such as computed tomography (CT) or magnetic resonance imaging (MRI) in clinical practice;74 however, there are currently limited data supporting routine use of cross-sectional imaging for HCC surveillance. A small single-center randomized trial comparing CT and ultrasound-based surveillance among 163 patients with cirrhosis failed to find a significant difference in early detection (62.5.5% vs. 55.5%, p=0.93) or HCC-related mortality (8.8% vs. 6.0%, p=0.46) despite significantly higher costs in the surveillance CT group ($57,383 vs $17,041 per HCC detected).75 Subsequently, a recent retrospective cohort study including 636 HBV-infected patients found that patients who underwent surveillance using alternating CT and ultrasound every 6 months had improved early HCC detection than those who underwent ultrasound-based surveillance (HR 2.52, 95%CI 1.41 – 4.51); however, these data are limited by potential selection bias and residual confounding so still require prospective validation.76 Finally, Kim and colleagues conducted a prospective cohort study comparing MRI-based and ultrasound-based surveillance among 407 patients with cirrhosis (predominantly HBV-related) and found MRI-based surveillance had a significantly higher sensitivity for early HCC detection than ultrasound (83.7% vs. 25.6%).77 However, further data about MRI performance in non-HBV patients and its cost-effectiveness are still needed prior to routine use of surveillance MRI in clinical practice. Other concerns about potential physical harms (radiation and contrast exposure), financial harms (costs), and limited radiologic capacity may also limit routine use of CT or MRI for HCC surveillance in all patients with cirrhosis. Early studies evaluating alternative imaging strategies, such as abbreviated MRI protocols, have suggested high sensitivity for early HCC detection, approaching that of diagnostic MRI;78-80 however, these data are limited by selection bias and verification bias, which may overestimated the accuracy of abbreviated MRI. Until data evaluating these novel imaging techniques in larger cohorts mature, ultrasound remains the standard radiographic surveillance modality.
Therefore, there has been increasing interest in serum biomarkers that may improve sensitivity for early HCC detection. The best studied biomarker to date remains alpha fetoprotein (AFP), which has garnered limited enthusiasm given poor sensitivity for HCC when used alone. However, a meta-analysis of studies that directly compared the performance of ultrasound alone versus ultrasound plus AFP for early HCC detection found concomitant use of ultrasound and AFP improved early HCC detection compared to ultrasound alone, with sensitivities of 63%, (95% CI 48%–75%) and 45%, (95% CI 30%–62%), respectively.70 The improved sensitivity was offset by a decrease in specificity (84% vs. 92%, RR 1.08, 95% 1.05 – 1.09), although the clinical significance of this decrease is thought to be minimal. The diagnostic odds ratio, which accounts for sensitivity and specificity, of the two tests in combination was higher than that of ultrasound alone. Further, several methods have been proposed to minimize false-positive results of AFP. First, using the trend of AFP values, rather than a single test result at a fixed threshold, better reflects how AFP is interpreted in clinical practice and can more accurately identify patients with early stage HCC.81,82 Patients with consistent increases in AFP level, even if below 20 ng/mL, can be concerning and prompt cross-sectional imaging, whereas stable to decreasing AFP levels, even if greater than 20 ng/mL, would be reassuring and may be monitored instead of requiring diagnostic evaluation. Second, AFP is traditionally interpreted at a cut-off of 20 ng/mL for all patients with cirrhosis, despite a recognition that is often elevated in the absence of HCC among patients with viral hepatitis.83 Therefore, use of different AFP cutoffs by liver disease etiology can improve specificity with one study suggesting a higher cut-off of 59 ng/mL in patients with cirrhosis from viral hepatitis and a lower cut-off of 11 ng/mL in those with non-viral cirrhosis.84 Finally, there has been increasing interest in developing AFP-adjusted algorithms to improve its accuracy for early HCC detection. For example, an HCC early detection screening (HES) model that incorporates the rate of AFP change along with AFP most recent value, age of the patient, alanine aminotransferase blood level, and platelet count is associated with improved sensitivity for early HCC detection compared to the current standard of care.85
Due to HCC intra-tumoral heterogenicity, there has been increasing recognition that a single biomarker may not be sufficient and a combination of biomarkers may be needed to optimize sensitivity for early HCC detection. GALAD, which includes gender, age, AFP-L3%, AFP, and DCP, is one of the best studied biomarker panels to date. In a multi-national phase II study with 6834 patients (2430 HCC and 4404 chronic liver disease), GALAD achieved sensitivities ranging from 60% to 80% for early HCC detection.86 Another panel including AFP, fucosylated kininogen, age, gender, alkaline phosphatase, and ALT demonstrated a c-statistic of 0.97 (95%CI 0.95 – 0.99) for early HCC detection in a small phase II biomarker study of 162 patients (69 early HCC, 93 cirrhosis).87 Finally, a methylated DNA marker panel had a c-statistic of 0.96 (95%CI 0.93 – 0.99), with a sensitivity exceeding 90%, for early HCC detection in a phase II study with 146 patients (95 HCC and 51 cirrhosis).88 Similar to individual biomarker studies, these data are promising but still require validation in large phase III biomarker studies.
Potential Surveillance Harms
As for all screening programs, HCC surveillance might cause objective or subjective discomfort encompassing 1) depression or anxiety during the screening process, 2) financial or physical harms resulting from investigation of false-positive or indeterminate results and 3) overdiagnosis and overtreatment of a tumor that never would have progressed to clinical attention in the absence of screening, although the latter is likely a rare situation in the case of HCC.89-91 Overall, the risk of these potential harms when deciding to perform recall procedures for a focal lesion and/or elevated serum biomarker has to be weighed against the dismal prognosis in the case of unscreened liver cancer and the possibility of remission from curative procedures of a tumor detected during surveillance.
All guidelines recommend the performance of contrast-enhanced imaging techniques when a focal lesion, larger than 1 cm, is detected by US.12-14 CT scans lead to radiation exposure and might be responsible for potential renal toxicity due to contrast injection.92 While MRI scan has no radiation exposure, the test is costly, a contrast injection is still required, and it can be particularly considered by patients as a cause of distress.93 Recent data suggest a risk of gadolinium accumulation, although the long-term clinical consequences of this phenomenon are currently unknown. In cases of atypical radiologic finding, a liver biopsy of the lesion is recommended. The risk of false negative biopsies are common in clinical practice, particularly in case of small-size lesion, and may lead to delays in both diagnosis and treatment.94 Tumor seeding along the biopsy tract is a rare event (1%-5%), with important consequences as it may preclude the implementation of subsequent curative procedures such as ablation, resection or transplantation.95 Finally, the risk of bleeding is considered low but can be life-threatening.96
A recent report suggested that false-positive or indeterminate results are likely frequently observed among patients included in HCC surveillance programs.97 This study included 680 cirrhotic patients, among whom 78 (11.5%) developed HCC over a 3-year period. As a measure of screening benefit, it was noted that 48 (61.5%) of the HCCs were identified by surveillance US and/or AFP. However, surveillance harm events over the same period, defined largely as “unnecessary testing”, were identified in 187 (27.5%) patients and nearly 10% had moderate-tosevere harm, defined as repeated imaging and/or invasive testing such as a biopsy. Of note, some patients in this study had diagnostic evaluation for indeterminate surveillance tests, such as sub-centimeter lesions on ultrasound, suggesting surveillance harm could have been mitigated by closer observation of guideline recommendations. These data were recently confirmed in another single-center study, which similarly found nearly 20% of patients underwent diagnostic testing for indeterminate lesions detected as part of an HCC surveillance program.98 Although subjective discomfort was not assessed in these studies, the results compensate the lack of data regarding surveillance-related harms highlighted by most reports, which have to date mostly focused on the potential benefits of HCC surveillance.
Implementation of and compliance to surveillance programs
Numerous studies from the West suggest that less than 30% of patients with cirrhosis are included in HCC surveillance programs and actually receive semi-annual screening.99-101 Factors explaining low adherence to HCC screening are multiple. Access to care seems to impact HCC screening, in particular in the US for uninsured patients and in African Americans.102 In addition, it is suspected that only 20-50% of cirrhotic patients are seen by hepatologists or gastroenterologists, who are usually prone to include their patients in screening programs. In this setting, the majority of cirrhotic patients are followed by primary care providers, in whom knowledge and beliefs regarding HCC surveillance are usually less developed.103-105 In a recent US study, a survey performed in 1000 primary care providers showed that most practitioners see patients with cirrhosis, but only a minority enroll them in surveillance protocols, which could be related to suboptimal knowledge of effective HCC therapy options.106
Patients adherence and compliance to surveillance also seem to be determinant. In this setting, based on the prospective follow-up of a large cohort of 1671 patients with compensated viralinduced cirrhosis included in protocolized screening procedures, an impaired compliance to the 6-months rule was observed in nearly 40% of the 216 patients who were diagnosed with HCC during a nearly 60 months follow-up.107 Such observation is particularly worrisome when considering that only patients with viral-related cirrhosis (usually more prone to be compliant) who accepted long-term, periodical follow-up, were recruited. It is possible, if not likely, that adherence to surveillance regimens may be lower in patients with alcohol- or NASH-related cirrhosis.108 Similarly, patients with a history of HCV-related cirrhosis remain at risk for HCC after SVR but may not be followed as closely and therefore more prone to lapses in surveillance. Further data characterizing surveillance utilization and barriers to surveillance in cohorts with these emerging risk factors are needed.
Overall these prospective analyses revealed a survival advantage associated with compliance with HCC screening guidelines after correction for lead-time bias. Indeed, respect of the 6-month screening rule was associated with early HCC diagnosis, allocation of curative treatment and longer lead-time adjusted overall survival. In this context, deciphering the mechanisms explaining lower compliance in some patients is pivotal. Patient knowledge, attitudes, and perceived barriers were recently assessed through a survey performed in a tertiary American center and were correlated with receipt of surveillance during a one-year period.109 Overall, this study demonstrated that surveillance rates were higher in patients displaying high levels of cirrhosis/HCC-related knowledge; conversely, the quality of screening was impaired by several pragmatic aspects reported as “barriers” including difficulty with the scheduling process, costs of surveillance testing, and transportation difficulties. Finally, the strongest argument highlighting the impact of patient knowledge is derived from an aborted surveillance trial, in which it was demonstrated that a randomized study of comparing HCC screening versus no surveillance was not feasible once informed consent had been provided.110
Perspectives and areas of research
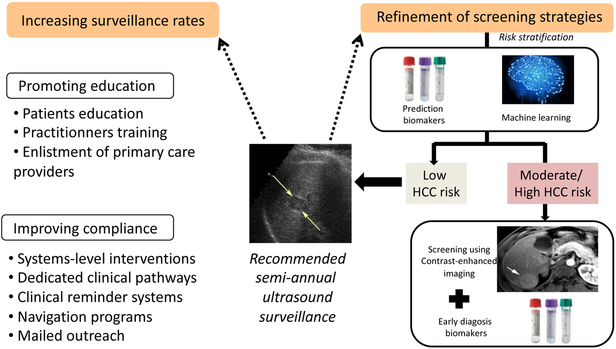
Optimizing HCC surveillance is one of the major challenges our community will have to deal with in order to improve the dismal prognosis of this cancer. Increasing uptake and refining strategies to tailor personalized management are the two cornerstones that must guide our action (Figure 2). The latter must be scientifically implemented and evaluated in the forthcoming years; they will furthermore have to account for the evolution of healthcare and medicoeconomic contexts characterized by limited resources.
Figure 2.
Potential Interventions to Increase HCC Surveillance Effectiveness
Risk stratification, cost-effectiveness and personalized screening
All patients with cirrhosis do not have the same risk of developing HCC and it remains difficult to assess the specific risk at an individual level.111 Furthermore, and as mentioned earlier, despite enrollment in surveillance programs, some patients are diagnosed with advanced HCC irrespective of their compliance, particularly because of the poor sensitivity of US. Such pitfall could be overcome by the use of more sophisticated contrast-enhanced imaging techniques such as MRI or the use of new circulating biomarkers useful for HCC prediction as well as early detection. However, implementing such costly surveillance programs may not be cost-effective in certain subsets of cirrhotic patients because of their particularly low annual incidence of HCC, for example in the case of cirrhotic patients controlled or eradicated for HBV-/HCV-infection.106-112 In this setting, personalized assessment of the individual risk of HCC and refinement of screening policies might be discussed. Until now, various HCC scoring systems have been based on the combination of routine clinical features to stratify cirrhotic patients into various HCC risk classes.113 However, it is unclear if clinical features can accurately risk stratify patients in isolation or if other features such as genomics or molecular signatures are needed.114-116 Further, it is unclear if these risk stratification tools could be applied to patients with non-cirrhotic NAFLD to identify a subgroup in whom HCC surveillance may be cost-effective. Based on the stratification into low-, intermediate- or high-HCC risks, it is tempting to speculate that adaptation of screening strategies might optimize both cost-effectiveness and the allocation of limited medical resources. In this context, the intensification of screening programs in intermediate- or high-risk groups is a timely challenge in view of the changing epidemiology of chronic liver disease: using expensive but highly sensitive imaging technique such as MRI or performing sequential assessment of circulating biomarkers yet to be discovered might then be justified in populations with the highest risk of HCC.117
Development of new biomarkers for early diagnosis
Despite improved accuracy compared to ultrasound alone, it is clear that a surveillance strategy of ultrasound and AFP remains far from where we need to be. In fact, the surveillance strategy of ultrasound with AFP still misses approximately one-third of HCC at an early stage. A number of novel biomarkers, such as des-gamma carboxy prothrombin (DCP) and lectin-bound AFP (AFP-L3), have been promising in phase II studies but still require validation in larger phase III cohort studies.86 Cell free DNA released from tumor cells may be detected in peripheral blood samples and is another promising biomarker; however, it is also in early phases of evaluation for surveillance as its unclear if it will be found in sufficient quantities in patients with early stage tumors or simply in those with larger, advanced stage tumors.118 The development of large prospective cohorts with stored biobanks of longitudinal serum and plasma samples, such as the Early Detection Research Network (EDRN) Hepatocellular cancer Early Detection Study (HEDS) and Cancer Prevention Research Institute of Texas (CPRIT) Texas HCC Consortium (THCCC) cohorts, should facilitate phase III validation of these biomarkers in the near future.119 Such efforts are also being initiated in Europe, with the constitution of the STHEPBIO consortium, which will encompass several prospective cohorts of cirrhotic patients with adjoining sequential biobanks from France, Italy, Belgium and Spain.
Intervention to increase surveillance rates
Optimizing HCC surveillance will likely necessitate the creation of specific networks involving physicians, patients and healthcare systems. Improving patient education using dedicated tools encompassing the intervention of trained personnel, websites, patient groups sessions, education screencasts, and smart phone applications must be encouraged. Involvement of patients into decision-making process in the setting of the aforementioned personalization of screening programs is also strongly recommended. Integrating HCC surveillance into complete work-up sessions mixing diverse interventions (nutritionists, alcohol liaison service, portal hypertension screening) into one-stop clinics might facilitate clinical pathways and ultimately favor compliance on the long term. The intensification of specific interventions aimed at improving compliance are also needed. For instance, mailed outreach strategies and patient navigation have been proven to successfully increase HCC surveillance uptake.120,121 In the same line, implementation of clinical reminder systems for physicians seems to positively impact the respect of surveillance timeframes in routine practice.122 Finally, enlisting primary care providers in HCC surveillance through reinforced partnership and training programs might also facilitate screening uptake.106 However, these interventions, particularly when implemented in broad populations followed by primary care providers alone, have still have surveillance rates below 50%, highlighting the need for more intensive intervention strategies in the future.
Conclusions
The global incidence and mortality of HCC is rising, particularly in the United States and Europe. Given the strong association between early detection and survival, improving uptake and performance of HCC surveillance must be defined as a priority for our community. Our actions will benefit from improved risk stratification of at-risk patients, discovery of more sensitive and specific surveillance tools (e.g. circulating blood-based biomarkers and new imaging techniques) and implementation of interventions to increase surveillance utilization that involve a broader range of physicians and patient participation. The adaptation of healthcare systems to the changing epidemiology of chronic liver disease and challenges in economic burden will be mandatory to step into personalized medicine aimed at increasing rates of HCC patients eligible for curative procedures, which can be considered as the most effective action to improve the prognosis of this difficult-to-treat cancer.
Acknowledgments
Financial Source: Dr. Singal’s research is supported by National Cancer Institute (NCI) R01 CA212008 and CA222900. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript. Dr Nahon’s research is sponsored by the ANRS (France REcherche Nord & sud SIDA-HIV Hépatites: FRENSH) and Banque Publique d’Investissement. The funding sponsors had no role in the design and conduct of the study, the collection, management, analysis, interpretation of data, or the preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest:
Amit G. Singal has has served on advisory boards for Gilead, Abbvie, Bayer, Eisai, Bristol Meyers Squibb, Wako Diagnostics, and Exact Sciences. He serves as a consultant to Bayer, Eisai, Exelixis, Roche, Exact Sciences, Glycotest, and TARGET. He has received research funding from Gilead and Abbvie.
Pietro Lampertico has no relevant conflicts of interest
Pierre Nahon has received honoraria from and/or consults for Abbvie, Astra-Zeneca, Bayer, Bristol-Myers Squibb, Gilead, Ipsen and MSD. He received research grants from Abbvie and Bristol-Myers Squibb.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global Burden of Disease Liver Cancer Collaboration 2015, Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Mortality and Causes of Death Collaborators.Global, regional, and national life expectancy, all-causemortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388 (10053):1459–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015.JAMA Oncol. 2017. December 1;3(12):1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2018. November;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 4.https://gco.iarc.fr/today/online-analysis-treemap?v=2018&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=5&group_cancer=1&include_nmsc=1&include_nmsc_other=1&reloaded. Access on 06/04/2019
- 5.https://gco.iarc.fr/today/online-analysis-map?v=2018&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=11&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=5&group_cancer=1&include_nmsc=1&include_nmsc_other=1&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&rotate=%255B10%252C0%255D. Access on 06/04/2019
- 6.https://gco.iarc.fr/today/online-analysis-map?v=2018&mode=population&mode_population=continents&population=900&populations=900&key=asr&sex=0&cancer=11&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=5&group_cancer=1&include_nmsc=1&include_nmsc_other=1&projection=natural-earth&color_palette=default&map_scale=quantile&map_nb_colors=5&continent=0&rotate=%255B10%252C0%255D. Access on 06/04/2019.
- 7.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology 2006;43:1303–1310. [DOI] [PubMed] [Google Scholar]
- 8.Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2007;5:938–945. [DOI] [PubMed] [Google Scholar]
- 9.Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med 2002;347:168–174. [DOI] [PubMed] [Google Scholar]
- 10.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65–73. [DOI] [PubMed] [Google Scholar]
- 11.Yu M-W, Yeh S-H, Chen P-J, Liaw Y-F, Lin C-L, Liu C-J, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst 2005;97:265–272. [DOI] [PubMed] [Google Scholar]
- 12.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, Md). 2018;67(1):358–380. [DOI] [PubMed] [Google Scholar]
- 13.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of hepatology. 2018;69(1):182–236. [DOI] [PubMed] [Google Scholar]
- 14.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatology international. 2017;11(4):317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zangneh F, Wong WWL, Sander B, et al. Cost Effectiveness of Hepatocellular Carcinoma Surveillance After a Sustained Virologic Response to Therapy in Patients With Hepatitis C Virus Infection and Advanced Fibrosis. Clin Gastroenterol Hepatol. 2018. December 20. pii: S1542-3565(18)31394-6. doi: 10.1016/j.cgh.2018.12.018 [DOI] [PubMed] [Google Scholar]
- 16.Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, et al. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology. 2016;151:472–480. [DOI] [PubMed] [Google Scholar]
- 17.Yuen M-F, Chen D-S, Dusheiko GM, et al. Hepatitis B virus infection. Nat Rev Dis Primers 2018; 4: 18035. [DOI] [PubMed] [Google Scholar]
- 18.Liaw Y-F, Sung JJY, Cheng Chow W, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351: 1521–31. [DOI] [PubMed] [Google Scholar]
- 19.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013; 158: 329–37. [DOI] [PubMed] [Google Scholar]
- 20.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology 2017; 153(4): 996–1005. [DOI] [PubMed] [Google Scholar]
- 21.Ioannou G, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, Sterling RK, Feld JJ, Kaplan DE, Taddei TH, Berry K. Elevated HCC risk persists up to 10 years after HCV eradication in patients with baseline cirrhosis or elevated FIB-4. Gastroenterology (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKillop I, Schrum L. Role of Alcohol in Liver Carcinogenesis. Semin Liver Dis 2009; 29: 222–232. [DOI] [PubMed] [Google Scholar]
- 23.Ganne-Carrié N, Chaffaut C, Bourcier V, Archambeaud I, Perarnau JM, Oberti F, et al. ; for CIRRAL Group. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J Hepatol. 2018;69:1274–1283. [DOI] [PubMed] [Google Scholar]
- 24.Nahon P, Sutton A, Rufat P, Ziol M, Akouche H, Laguillier C, et al. Myeloperoxidase and superoxide dismutase 2 polymorphisms comodulate the risk of hepatocellular carcinoma and death in alcoholic cirrhosis. Hepatology 2009;50:1484–1493. [DOI] [PubMed] [Google Scholar]
- 25.Mancebo A, Gonzalez-Dieguez ML, Cadahia V, Varela M, Perez R, Navascues CA, et al. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin Gastroenterol Hepatol 2012;11:95–101. [DOI] [PubMed] [Google Scholar]
- 26.World Gastroenterology Organisation Global Guidelines. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis.2012. Available from: http://www.worldgastroenterology.org/assets/export/userfiles/2012_NASH%20and%20NAFLD_Final_long.pdf. (accessed: 25.05.2018). [DOI] [PubMed]
- 27.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of Hepatocellular Cancer in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology. 2018; 155(6): 1828–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal S, El_Serag HB, Sada YH, Hepatocellular Carcinoma in the Absence of Cirrhosis in US Veterans is Associated with Non-Alcoholic Fatty Liver Disease Clin Gastroenterol Hepatol. 2016. January ; 14(1): 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyson J, Jaques B, Chattopadyhay D, et al. Hepatocellular cancer: the im- pact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110–117. [DOI] [PubMed] [Google Scholar]
- 30.Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Alimentary pharmacology & therapeutics. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee TY, Wu JC, Yu SH, et al. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer. 2017;141:1307–1314. [DOI] [PubMed] [Google Scholar]
- 32.Reig M, Gambato M, Man N. Should Patients With NAFLD/NASH Be Surveyed for HCC? Translantation 2019; 103(1): 39–44. [DOI] [PubMed] [Google Scholar]
- 33.Schlesinger S, Aleksandrova K, Pischon T, et al. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol 2013; 24: 2449–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welzel TM, Graubard BI, Quraishi S, Zeuzem S, Davila JA, El-Serag HB, et al. Population-Attributable Fractions of Risk Factors for Hepatocellular Carcinoma in the United States. Am J Gastroenterol 2013; 108: 1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deugnier YM, Guyader D, Crantock L, Lopez JM, Turlin B, Yaouanq J, et al. Primary liver cancer in genetic hemochromatosis: a clinical, pathological, and pathogenetic study of 54 cases. Gastroenterology 1993;104:228–234. [DOI] [PubMed] [Google Scholar]
- 36.Fracanzani AL, Conte D, Fraquelli M, Taioli E, Mattioli M, Losco A, et al. Increased cancer risk in a cohort of 230 patients with hereditary hemochromatosis in comparison to matched control patients with noniron-related chronic liver disease. Hepatology 2001;33:647–651. [DOI] [PubMed] [Google Scholar]
- 37.Andant C, Puy H, Bogard C, Faivre J, Soulé JC, Nordmann Y, et al. Hepatocellular carcinoma in patients with acute hepatic porphyria: frequency of occurrence and related factors. J Hepatol 2000;32:933–939. [DOI] [PubMed] [Google Scholar]
- 38.Fracanzani AL, Taioli E, Sampietro M, Fatta E, Bertelli C, Fiorelli G, et al. Liver cancer risk is increased in patients with porphyria cutanea tarda in comparison to matched control patients with chronic liver disease. J Hepatol 2001:498–503. [DOI] [PubMed] [Google Scholar]
- 39.Rich NE, Hester C, Odewole M,et al. Racial and Ethnic Differences in Hepatocellular Carcinoma Presentation and Outcomes. Clinical Gastroenterology Hepatology 2019; 17(3): 551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature 1991;350:427–428. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y-CA, Cohet C, Yang Y-C, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol 2009; 38: 1497–1511. [DOI] [PubMed] [Google Scholar]
- 42.Tseng C-H. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver International 2018;38:2018–2027. [DOI] [PubMed] [Google Scholar]
- 43.Simon TG, Ma Y, Ludvigsson JF, Chong DQ, Giovannucci EL, Fuchs CS, Meyerhardt JA, Corey KE, Chung RT, Zhang X, Chan AT. Association Between Aspirin Use and Risk of Hepatocellular Carcinoma. JAMA Oncol. 2018;4:1683–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue M, Yoshimi I, Sobue T, Tsugane S. Influence of coffee drinking on subsequent risk of hepatocellular carcinoma: a prospective study in Japan. J Natl Cancer Inst 2005;97:293–300. [DOI] [PubMed] [Google Scholar]
- 45.Gelatti U, Covolo L, Franceschini M, Pirali F, Tagger A, Ribero ML, et al. Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: a case-control study. J Hepatol 2005;42:528–534. [DOI] [PubMed] [Google Scholar]
- 46.Bravi F, Bosetti C, Tavani A, Bagnardi V, Gallus S, Negri E, et al. Coffee drinking and hepatocellular carcinoma risk: A meta-analysis. Hepatology 2007;46:430–435. [DOI] [PubMed] [Google Scholar]
- 47.Bravi F, Tavani A, Bosetti C, Boffetta P, La Vecchia C. Coffee and the risk of hepatocellular carcinoma and chronic liver disease: a systematic review and meta-analysis of prospective studies. Eur J Cancer Prev 2017;26:368–377. [DOI] [PubMed] [Google Scholar]
- 48.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Tomorrow. International Agency for Research on Cancer, Lyon 2018. Available from: https://gco.iarc.fr/tomorrow (accessed:29.09.2018). [Google Scholar]
- 49.Tanaka H, Imai Y, Hiramatsu N, Ito Y, Imanaka K, Oshita M, et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med 2008;148:820–826. [DOI] [PubMed] [Google Scholar]
- 50.Qiu D, Katanoda K, Marugame T, Sobue T. A Joinpoint regression analysis of long-term trends in cancer mortality in Japan (1958–2004). Int J Cancer 2009;124:443–448. [DOI] [PubMed] [Google Scholar]
- 51.Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980–2004. Hepatology 2008;48:137–145. [DOI] [PubMed] [Google Scholar]
- 52.Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67:600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018; 69: 896–904. [DOI] [PubMed] [Google Scholar]
- 54.Prasad V, Lenzer J, Newman DH. Why cancer screening has never been shown to “save lives” – and what we do about it. BMJ 2016352: h6080. [DOI] [PubMed] [Google Scholar]
- 55.Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Annals of internal medicine. 2014;161(4):261–269. [DOI] [PubMed] [Google Scholar]
- 56.Moon AM, Weiss NS, Beste LA, et al. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-related Mortality in Patients with Cirrhosis. Gastroenterology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. Journal of cancer research and clinical oncology. 2004;130(7):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology (Baltimore, Md). 2011;54(6):1998–2004. [DOI] [PubMed] [Google Scholar]
- 59.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS medicine. 2014;11(4):e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Meer S, de Man RA, Coenraad MJ, et al. Surveillance for hepatocellular carcinoma is associated with increased survival: results from a large cohort in the Netherlands. J Hepatol 2015;63:1156–1163. [DOI] [PubMed] [Google Scholar]
- 61.Wu CY, Hsu YC, Ho HJ, et al. Association between ultrasonography screening and mortality in patients with hepatocellular carcinoma: a nationwide cohort study. Gut 2016;65:693–701 [DOI] [PubMed] [Google Scholar]
- 62.Mittal S, Kanwal F, Ying J, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: a United States cohort. J Hepatol 2016. 65:1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi DT, Kum H, Park S, Ohsfeldt RL, Parikh ND, Singal AG. Hepatocellular Carcinoma Screening is Associated with Increased Survival of Patients with Cirrhosis. Clinical Gastroenterology Hepatology 2019; 17(5): 976–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costentin CE, Layese R, Bourcier V, et al. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-Time Adjusted Survival of Patients With Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology 2018; 155(2): 431–442. [DOI] [PubMed] [Google Scholar]
- 65.Duffy SW, Nagtegaal ID, Wallis M, et al. Correcting for lead time and length bias in estimating the effect of screen detection on cancer survival. Am J Epidemiol. 2008;168(1):98–104. [DOI] [PubMed] [Google Scholar]
- 66.Barbara L, Benzi G, Gaiani S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: A multivariate analysis of prognostic factors of tumor growth rate and patient survival Hepatology 1992; 16(!): 132–7. [DOI] [PubMed] [Google Scholar]
- 67.Kudo M, Izumi N, Kokudo N, et al. Management of Hepatocellular Carcinoma in Japan: Consensus-Based Clinical Practice Guidelines Proposed by the Japan Society of Hepatology (JSH) 2010 Updated Version. Dig Dis 2011;29:339e64. [DOI] [PubMed] [Google Scholar]
- 68.Trinchet JC, Chaffaut C, Bourcier V, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology 2011;54(6):1987–1997. [DOI] [PubMed] [Google Scholar]
- 69.Wu CY, Hsu YC, Ho HJ, et al. Association between ultrasonography screening and mortality in patients with hepatocellular carcinoma: a nationwide cohort study Gut 2016;65:693–701. [DOI] [PubMed] [Google Scholar]
- 70.Tzartzeva K, Obi J, Rich NE, et al. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154(6):1706–1718.e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Alimentary pharmacology & therapeutics. 2017;45(1):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Del Poggio P, Olmi S, Ciccarese F, et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clinical gastroenterology and hepatology 2014;12(11):1927–1933.e1922. [DOI] [PubMed] [Google Scholar]
- 73.Singal AG, Nehra M, Adams-Huet B, Yopp AC, Tiro JA, Marrero JA, Lok AS, Lee WM. Detection of Hepatocellular Carcinoma at Advanced Stages among Patients in the HALT-C Trial: Where Did Surveillance Fail? American J. Gastroenterology 2013; 108(3): 425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joshi K, Mendler M, Gish R, et al. Hepatocellular carcinoma surveillance: a national survey of current practices in the USA. Dig Dis Sci 2014; 59(12): 3073–7. [DOI] [PubMed] [Google Scholar]
- 75.Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Alimentary pharmacology & therapeutics. 2013;38(3):303–312. [DOI] [PubMed] [Google Scholar]
- 76.Lee M, Kim JH, Kang S, Jun BG, Kim TS, et al. Alternate dynamic computed tomography and ultrasonography for surveillance of chronic hepatitis B patients with cirrhosis at high risk for hepatocellular carcinoma. AASLD 2018, abstract 894 [Google Scholar]
- 77.Kim SY, An J, Lim YS, et al. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA oncology. 2017;3(4):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Besa C, Lewis S, Pandharipande PV, Chhatwal J, Kamath A, et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol (NY) 2017;42:179–190 [DOI] [PubMed] [Google Scholar]
- 79.Marks RM, Ryan A, Heba ER, Tang A, Wolfson TJ, et al. Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid-enhanced MRI for hepatocellular carcinoma surveillance. AJR Am J Roentgenol 2015;204:527–35. [DOI] [PubMed] [Google Scholar]
- 80.Khatri G, Pedrosa I, Xi Y, Singal AG, Yopp A, Yokoo T. Abbreviated-protocol screening MRI versus complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis - an equivalence study using LI-RADS v2018. Journal of Magnetic Resonance Imaging (in press) [DOI] [PubMed] [Google Scholar]
- 81.Tayob N, Lok AS, Do KA, Feng Z. Improved Detection of Hepatocellular Carcinoma by Using a Longitudinal Alpha-Fetoprotein Screening Algorithm. Clinical gastroenterology and hepatology 2016;14(3):469–475.e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee E, Edward S, Singal AG, Lavieri MS, Volk M. Improving screening for hepatocellular carcinoma by incorporating data on levels of alpha-fetoprotein, over time. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(4):437–440. [DOI] [PubMed] [Google Scholar]
- 83.Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha- fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol 2005;43:434–441. [DOI] [PubMed] [Google Scholar]
- 84.Gopal P, Yopp AC, Waljee AK, et al. Factors that affect accuracy of alpha-fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clinical gastroenterology and hepatology 2014;12(5):870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.White DL, Richardson P, Tayoub N, Davila JA, Kanwal F, El-Serag HB. The Updated Model: An Adjusted Serum Alpha-Fetoprotein-Based Algorithm for Hepatocellular Carcinoma Detection With Hepatitis C Virus-Related Cirrhosis. Gastroenterology. 2015;149(7):1986–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clinical Gastroenterology and Hepatology 2016;14:875–886.e6. [DOI] [PubMed] [Google Scholar]
- 87.Wang M, Sanda M, Comunale MA, Harrera H, Swindell C, et al. Changes in the glycosylation of kininogen and the development of a kininogen-based algorithm for the early detection of HCC. Cancer Epi Biomarkers Prevention 2017; 26(5): 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kisiel JB, Allawi HT, Giakoumopoulos M, et al. 1044-Hepatocellular Carcinoma Detection by Plasma Assay of Methylated Dna Markers: Phase II Clinical Validation. Gastroenterology. 2018;154(6):S-1113–S-1114. [Google Scholar]
- 89.Harris RP, Sheridan SL, Lewis CL, Barclay C, Vu MB et al. The harms of screening: a proposed taxonomy and application to lung cancer screening. JAMA Intern Med 2014; 174(2): 281–5. [DOI] [PubMed] [Google Scholar]
- 90.Heleno B, Thomsen MF, Rodrigues DS, Jorgensen KJ, Brodersen J. Quantification of harms in cancer screening trials: literature review. BMJ 2013; 347: f5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rich NE and Singal AG. Overdiagnosis: An understudied issue in Hepatocellular carcinoma surveillance. Seminars in Liver Disease 2017; 37(4): 296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis Gut. 2010; 59:638–644. [DOI] [PubMed] [Google Scholar]
- 93.Geh D, Rana FA, Reeves HL. Weighing the benefits of hepatocellular carcinoma surveillance against potential harms. Journal of Hepatocellular Carcinoma 2019. ; 6 : 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97–104. [DOI] [PubMed] [Google Scholar]
- 95.Maturen K, Nghiem H, Marrero JA, et al. Lack of Tumor Seeding of Hepatocellular Carcinoma after Percutaneous Needle Biopsy Using Coaxial Cutting Needle Technique. AJR Am J Roentgenol 2006;187:1184–1187. [DOI] [PubMed] [Google Scholar]
- 96.Buscarini L, Fornari F, Bolondi L, et al. Ultrasound-guided fine-needle biopsy of focal liver lesions: techniques, diagnostic accuracy and complications. J Hepatol. 1990; 11:344–348. [DOI] [PubMed] [Google Scholar]
- 97.Atiq O, Tiro J, Yopp AC, Muffler A, Marrero JA, Parikh ND, Murphy C, McCallister K, Singal AG. An Assessment of Benefits and Harms of Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Hepatology 2017; 65(4): 1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Konerman M, Verma A, Zhao B, Singal AG, Lok AS, Parikh ND. Frequency and Outcome of Abnormal Imaging Impact in Patients with Cirrhosis Enrolled in a Hepatocellular Carcinoma Surveillance Program. Liver Transplantation 2019; 25(3): 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singal AG, Yopp A, Skinner CS, Packer M, Lee WM, Tiro JA. Utilization of Hepatocellular Carcinoma Surveillance Among American Patients: A Systematic Review. J General Internal Medicine 2012; 27(7): 861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goldberg D, Taddei TH, Serper M, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis Hepatology 2017. ; 65(3) : 864–874. [DOI] [PubMed] [Google Scholar]
- 101.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Annals of internal medicine. 2011;154(2):85–93. [DOI] [PubMed] [Google Scholar]
- 102.Singal AG, Li X, Tiro JA, Kandunoori P, Huet B, Nehra M, Yopp AC. Racial, Social, and Clinical Determinants of Hepatocellular Carcinoma Surveillance. American Journal of Medicine 2015; 128(1): 90e1–90e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Palmer L, Kappelman M, Sandler RS, Hayashi P. Surveillance for Hepatocellular Carcinoma in a Medicaid Cirrhotic Population, J Clin Gastroenterol. 2013; 47(8):713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singal AG, Tiro J, Li X, Adams-Huet B, Chubak J. Hepatocellular Carcinoma Surveillance among Patients with Cirrhosis in a Population Based Integrated Healthcare Delivery System. J Clin Gastro 2017; 51(7): 650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simmons OL, Feng Y, Parikh ND, Singal AG. Primary Care Provider Practice Patterns and Barriers to Hepatocellular Carcinoma Surveillance. Clinical Gastroenterology Hepatology 2019; 17(4): 766–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McGowan C, Edwards T, Luong M, Hayashi P. Suboptimal Surveillance for and Knowledge of Hepatocellular Carcinoma Among Primary Care Providers. Clinical Gastroenterology and Hepatology 2015; 13(4): 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Costentin CE, Layese R, Bourcier V, et al. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-Time Adjusted Survival of Patients With Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology 2018; 155(2): 431–442. [DOI] [PubMed] [Google Scholar]
- 108.Singal AG, Yopp A, Gupta S, Skinner CS, Halm EA, et al. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prevention Research 2012; 5(9): 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Farvardin S, Patel J, Khambaty M, Yerokun O, Mok H, Tiro JA, Yopp AC, Parikh ND, Marrero JA, Singal AG. Patient-Reported Barriers are Associated with Lower HCC Surveillance Rates in Patients with Cirrhosis. Hepatology 2017; 65(3): 875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology (Baltimore, Md). 2011;54(6):1998–2004. [DOI] [PubMed] [Google Scholar]
- 111.Nahon P and Zucman-Rossi J. Single nucleotide polymorphisms and risk of hepatocellular carcinoma in cirrhosis. J Hepatol. 2012. September;57(3):663–74. [DOI] [PubMed] [Google Scholar]
- 112.Papatheodoridis G, Lampertico P, Manolakopoulos S, Lok AS, Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: A systematic review. Journal of Hepatology 53(2): 348–356 [DOI] [PubMed] [Google Scholar]
- 113.Papatheodoridis GV, Idilman R, Dalekos GN, Buti M, Chi H, The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology. 2017. November;66(5):1444–1453. [DOI] [PubMed] [Google Scholar]
- 114.Nahon P, Bourcier V, Layese R, Audureau E, Cagnot C, et al. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology. 2017. January;152(1):142–156. [DOI] [PubMed] [Google Scholar]
- 115.Singal AG, Mukherjee A, Elmunzer BJ, et al. Machine Learning Algorithms Outperform Conventional Regression Models in Predicting Development of Hepatocellular Carcinoma. American J Gastroenterology 2013; 108: 172–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoshida Y, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. New England Journal of Medicine, 2008. 359(19): p. 1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goosens N, Singal AG, King L, Andersson KL, Fuchs B, Besa-Correa C, Taouli B, Chung R, Hoshida Y. Cost-effectiveness of risk score-stratified hepatocellular carcinoma screening in patients with cirrhosis. Clinical Translational Gastroenterology 2017; 8(6): e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tang JC, Feng Y, Guo T, Xie A, Cai X. Circulating tumor DNA in hepatocellular carcinoma: trends and challenges. Cell Biosci. 2016; 6: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feng Z, Marrero JA, Khaderi S, Singal AG, Kanwal F, Loo N, Beretta L, Ning J, El-Serag H. Design of the Texas Hepatocellular Carcinoma Consortium Cohort Study. Am J Gastro 2019; 114(3): 530–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singal AG, Tiro JA, Marrero JA, McCallister K, Mejias C, Sanders J, Bishop WP, Santini NO, Halm EA. Mailed Outreach Program Increases Ultrasound Screening of Patients with Cirrhosis for Hepatocellular Carcinoma. Gastroenterology 2017; 152(3): 608–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singal AG, Tiro JA, Murphy CC, Marrero JA, McCallister K, Fullington H, Meijas C, Waljee AK, Bishop W, Santini N, Halm EA. Mailed Outreach Invitations Significantly Improve HCC Surveillance Rates in Patients with Cirrhosis: A Randomized Clinical Trial. Hepatology 2019; 69(1): 121–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beste LA, Ioannou GN, Yang Y, Chang MF, Ross D, Dominitz JA. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clinical gastroenterology and hepatology 2015;13(1):172–179. [DOI] [PubMed] [Google Scholar]