Abstract
Antimicrobial resistance is of increasing global concern. To incentivize the creation of new treatments, the US Congress enacted the Generating Antibiotic Incentives Now Act (GAIN Act) of 2012, which provides benefits to manufacturers of Qualified Infectious Disease Products (QIDPs) including 5 years of additional nonpatent exclusivity. The results of this program have so far been disappointing, largely because QIDP eligibility criteria were not sufficiently targeted to unmet need. The time value of money also means that QIDP exclusivity disproportionately rewards modifications to existing drugs rather than the creation of new drugs. To improve the outlook, GAIN Act criteria should be limited to a more narrowly tailored list of qualifying pathogens to ensure that QIDPs offer clinical value not available from existing treatments. Additional options for improvement include greater reliance on animal data when determining QIDP eligibility and conditioning GAIN Act benefits on the availability of companion diagnostics.
Keywords: antibiotic, exclusivity, incentives, net present value, QIDP
Congress enacted the GAIN Act in 2012 to incentivize new antibiotics. However, results have been disappointing because eligibility criteria are not sufficiently focused on unmet need and the 5-year exclusivity extension disproportionately rewards modifications rather than new drugs.
In 2012, the US Congress enacted the Generating Antibiotic Incentives Now Act (GAIN Act) to promote the development of certain new antimicrobial products known as Qualified Infectious Disease Products (QIDPs). During the deliberations leading to its passage, legislators explained that the law’s purpose was to incentivize new drugs to “conquer…germs that are resistant to antibiotics” [1] and thereby fulfill “unmet needs” [2].
To achieve these goals, the Act provided financial incentives in the form of 5-year exclusivity extensions for QIDPs that are added to the end of nonpatent exclusivities, which Food and Drug Administration (FDA)–approved drugs may be eligible to receive under the Hatch-Waxman and Orphan Drug Acts. These exclusivities prevent the FDA from approving competing generic versions for at least 3 years in the case of drug modifications that require the submission of new clinical data, 4–5 years for new chemical entities, and 7 years for drugs targeted to rare diseases, yielding total exclusivity periods of 8, 9–10, and 12 years, respectively.
The GAIN Act also sought to advance the development of QIDPs in other ways. QIDPs were made automatically eligible for priority review, which provides 6-month FDA review targets for new drug applications, rather than the standard 10-month target, and fast-track designation, which allows approval following well-controlled phase 2 trials. The FDA was also required to revise 3 guidance documents per year to clarify regulatory requirements, including the selection of appropriate animal models, the use of noninferiority vs superiority trials, and the choice of appropriate noninferiority margins.
Despite these features, the GAIN Act has so far fallen short of its goals. In 2018, the FDA reported that it designated 147 experimental therapies as QIDPs, 74 (50%) of which addressed novel drugs, and 73 (50%) of which addressed modifications such as new doses or indications. The 12 approved QIDPs discussed in the FDA’s report included 9 new chemical entities, none of which work via a new mechanism of action. Since the FDA’s report, plazomicin (Zemdri), lefamulin (Xenleta), and cefiderocol (Fetroja) were approved as QIDPs on the basis of noninferiority to older comparators. Imipenem/cilastatin/relebactam (Recarbrio) was approved as a QIDP in 2019 on the basis of previous efficacy findings for imipenem/cilastatin and new clinical evidence of the drug’s effect against imipenem-susceptible pathogens that suggested but did not clearly demonstrate noninferiority, relying on in vitro and animal data to suggest that relebactam could restore the activity of imipenem against certain imipenem-nonsusceptible bacteria. Clinical use of approved QIDPs has been slow, suggesting that these products are not satisfying urgent needs [3].
Several factors explain these disappointing results. GAIN Act eligibility criteria target pathogens that cause “serious or life-threatening infections” but do not exclude pathogens adequately treated with existing medicines, such as those resistant to some but not most current treatments. Tasked with establishing and regularly updating a list of “qualifying pathogens,” the FDA currently lists 21 pathogens including Staphylococcus aureus, Streptococcus pneumonia, and Vibrio cholera, which can often be treated or prevented with existing medicines [4]. QIDPs are automatically eligible for priority review and fast-track status even when they do not demonstrate significant improvements over existing therapies or address unmet needs—required criteria when these programs are applied to non-QIDP drugs. FDA clarification of guidance documents may reduce uncertainty and encourage development of new products [5] but provides these benefits whether manufacturers pursue new or existing classes of antibiotics.
When QIDPs do not demonstrably outperform existing products, many of which are inexpensive generics, they are unlikely to succeed commercially. For example, after plazomicin, a semisynthetic addition to the aminoglycoside class, was approved on the basis of noninferiority to meropenem in June 2018, its manufacturer reported <$1 million in sales during the rest of the calendar year; it filed for bankruptcy in 2019.
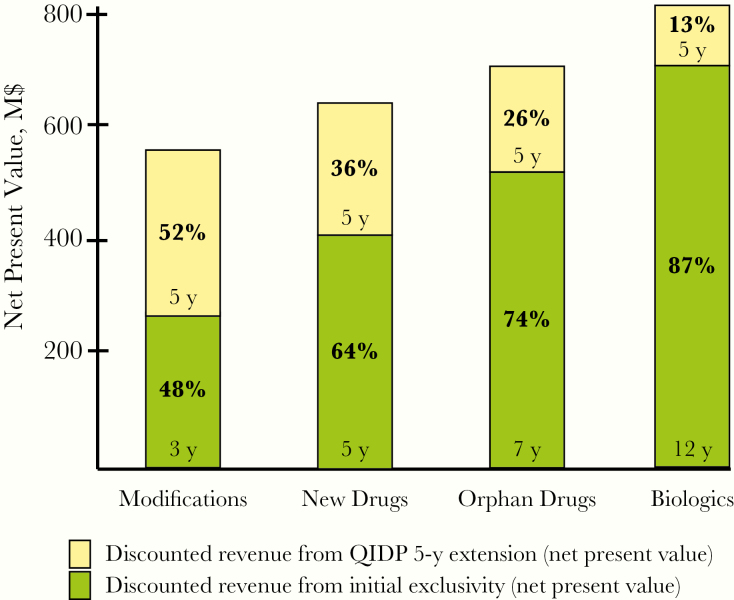
Another problematic aspect of the GAIN Act is the disproportionate value that the 5-year exclusivity extensions contribute to drugs that Congress has already determined deserve shorter exclusivity. Due to the time value of money, a uniformly applied extension contributes a decreasing share of the net present value (NPV) of expected revenue streams when added to longer exclusivity periods. The incentive is therefore more valuable for modifications earning the 3-year Hatch-Waxman exclusivity than for new drugs receiving 5-year exclusivity or rare disease products receiving 7-year exclusivity. For example, assuming a discount rate of 12%, the additional 5-year GAIN Act exclusivity would contribute 52% of NPV when added to 3-year exclusivity, but just 26% of NPV when added to 7-year exclusivity (Figure 1). Although these nonpatent exclusivities run concurrently with patent exclusivities, patents can sometimes be invalidated, and nonpatent exclusivity therefore provides greater certainty that generics will not enter the market.
Figure 1.
Contribution of 5-year Generating Antibiotic Incentives Now (GAIN) Act exclusivity extension to net present value: hypothetical model of value at year 0 assuming a 12% discount rate, $100 million in annual revenue during the exclusivity period paid on the last day of the year, and no revenue thereafter. Biologics are not eligible for GAIN Act exclusivity and are included for illustrative purposes only. Abbreviations: M$, millions of dollars; QIDP, Qualified Infectious Disease Product.
To maximize the chance that the GAIN Act will appropriately incentivize therapeutically valuable products, infectious disease professional societies should urge Congress to consider several modifications to the law. Most importantly, legislators should narrow the definition of QIDP to exclude pathogens for which adequate treatments are available, helping to ensure that exclusivity extensions, priority review, and fast-track status incentivize products that address unmet need. Because approved products occasionally encounter manufacturing difficulties or must be withdrawn for safety reasons, the FDA should remove pathogens from the list after 2 new drugs adequately addressing a particular qualifying pathogen have been approved.
Policy-makers should also avoid increasing payments for drugs that have not demonstrated added clinical value. For example, the proposed DISARM Act would increase Medicare reimbursement for infectious disease products based on the same expansive criteria as the GAIN Act, potentially squandering limited resources on products that do not provide any additional patient benefit. Although the GAIN Act does not require QIDPs to demonstrate an advantage over less expensive alternatives, the QIDP designation itself may be incorrectly perceived as a signal of value and contribute to excessive spending.
Other reforms would require greater agency discretion. When insufficient clinical evidence is available to demonstrate that a drug addresses unmet need, Congress could condition QIDP eligibility (but not necessarily FDA approval) on the FDA’s determination that animal or other nonclinical data suggest that such benefit is likely. If drugs are useful in broad patient populations but offer benefit over existing drugs only in certain populations, QIDP incentives should be conditional on the availability of companion diagnostics that allow for rapid identification of those populations and thereby help to promote stewardship, slow resistance, and minimize use in populations for which existing treatments are effective. Exclusivity extensions for modifications to existing products should be reduced to 1 year or eliminated.
If these changes rehabilitate the GAIN Act into an effective incentive mechanism for valuable new products, its scope should be enlarged to include antiparasitic, antihelminthic, and antiviral products, as well as vaccines and other antimicrobial biologics. If not, the GAIN Act should be repealed, as the provision of incentives irrespective of therapeutic value diverts scarce development funding and imposes administrative costs such as those needed to maintain the qualifying pathogen list and evaluate designation requests.
The emergence of treatment-resistant pathogens is inevitable, and innovative approaches to developing new products are needed. The GAIN Act offers 1 such approach, but is not sufficiently targeted to pathogens for which current treatment options are inadequate, and disproportionately rewards modifications rather than new drugs. Legislators should consider more appropriately tailoring the GAIN Act to elicit antimicrobial products with improved clinical benefits.
Acknowledgments
The authors gratefully acknowledge John H. Powers, III, MD, for helpful feedback on the completed version of the manuscript.
Financial support. This work was funded by a Novo Nordisk Foundation grant for a scientifically independent Collaborative Research Programme (grant NNF17SA0027784). Dr. Darrow and Dr. Kesselheim’s work is also supported by Arnold Ventures, the Harvard-MIT Center for Regulatory Science, and the Engelberg Foundation. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Potential conflicts of interest. Both authors: no reported conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.US Senate. Congressional Record. Food and Drug Administration Safety and Innovation Act–Motion to Proceed–Resumed. May 22, 2012; 158:S3389–S3400. Available at: https://www.congress.gov/112/crec/2012/05/22/CREC-2012-05-22-pt1-PgS3389-6.pdf. Accessed 13 January 2020.
- 2.US Senate. Congressional Record. Food and Drug Administration Safety and Innovation Act of 2012–Continued. June 26, 2012; 158:S4610–S4627. Available at: https://www.congress.gov/112/crec/2012/06/26/CREC-2012-06-26-pt1-PgS4610-3.pdf. Accessed 13 January 2020.
- 3. Schulz LT, Kim SY, Hartsell A, Rose WE. Antimicrobial stewardship during a time of rapid antimicrobial development: potential impact on industry for future investment. Diagn Microbiol Infect Dis 2019; 95:114857. [DOI] [PubMed] [Google Scholar]
- 4.List of qualifying pathogens that have the potential to pose a serious threat to public health. 21 C.F.R. § 317.2 (2019). Available at: https://www.ecfr.gov/cgi-bin/text-idx?SID=94f0fcb497ad38c675e12e2a006a7c48&mc=true&node=se21.5.317_12&rgn=div8. Accessed 13 January 2020.
- 5. Tillotson GS. GAIN Act legislation: is it enough? Lancet Infect Dis 2012; 12:823–4. [DOI] [PubMed] [Google Scholar]