Abstract
The keratin-associated proteins (KAPs) are structural components of wool fibers and variation in the genes encoding the KAPs can affect wool traits. In this study, sequence variation in the ovine KAP7-1 gene (KRTAP7-1) was investigated in 222 sheep across 5 different Pakistani breeds and breed crosses. Two previously identified variants (A and B) of the KRTAP7-1 coding sequence were identified. The frequency of the genotypes AA and AB was 76% and 23%, respectively, and that of BB was 1%. The association of sequence variation with various wool traits and measurements included yield (the proportion of greasy fleece weight that is clean fleece), mean staple length (MSL), wool bulk, mean fiber diameter, fiber diameter SD, the coefficient of variation of fiber diameter, medullation, the SD of medullation, the coefficient of variation of medullation, fiber opacity, the SD of opacity, and the coefficient of variation of opacity. Variation in KRTAP7-1 was found to be associated with yield (P = 0.017). The adjusted mean yield of sheep of genotype AA (n = 169) was 79.9 ± 2.72%, while that of genotype AB (n = 51) was 81.9 ± 3.37%. There was also an association between variation in KRTAP7-1 and MSL (P = 0.024), with sheep of genotype AA (n = 169) having an adjusted mean MSL of 47.3 ± 0.57 mm compared with sheep of genotype AB (n = 51, 50.9 ± 0.65 mm). Yield and MSL are both important wool production traits, hence variation in KRTAP7-1 needs to be further investigated in more sheep of differing breed.
Keywords: KAP7-1, KRTAP7-1, polymorphism, wool, yield
Introduction
Pakistan is an agricultural country, where 28 breeds comprising both fat- and thin-tailed sheep exist. These sheep are mainly kept for meat purposes while wool is a secondary product (Younas and Yaqoob, 2019). There are 30.9 million head of sheep, and these produce 46.2 million tons of wool per annum (Economic Survey of Pakistan, 2018–2019). This gives an average wool production of 0.8 to 2.0 kg/animal/year (Akram et al., 2016), which is typically less than the wool produced in other parts of the world (0.9 to 13.6 kg) (Schoenian, 2019).
Wool fiber is comprised primarily of keratins and keratin-associated proteins (KAPs) (Powell and Rogers, 1994). The keratin proteins form intermediate filaments, which are a major structural component of the wool fiber, and have an axial arrangement in the fiber cortex. The KAPs are also found in the cortex and form a matrix, which cross-links the intermediate filaments through disulfide bonds. As a consequence of their effect on keratin assembly, the KAPs can affect wool strength and rigidity (Parry and Steinert, 1995). The KAPs are loosely grouped into 3 categories: the high-sulfur (HS) KAPs, the ultra-high sulfur (UHS) KAPs, and the high glycine/tyrosine (HGT) KAPs (Rogers et al., 2005). Different KAPs are encoded by different KAP genes, and a large number of KAP genes have been described, with these being clustered on different chromosomes (Gong et al., 2012a).
Wool can be improved by routine evaluation of fiber traits and the use of that information for making decisions in breeding programs. This can result in the production of wool of a higher market value (Angel et al., 1990; Kelly et al., 2007; Holman and Malau-Aduli, 2012). Many different wool traits can be assessed, but 1 trait of importance is wool yield (yield) or the proportion of greasy fleece weight (GFW) that is clean fleece after washing or scouring of the wool. It is accordingly a function of the non-fiber constituent levels within a fleece (Thornberry and Atkins 1984, Preston et al., 2016). Knowing wool yields helps farmers, because it affects the value of their wool (Banks and Brown, 2009; Mortimer et al., 2010) and wool of similar fiber diameter, but with greater clean fleece weights (CFWs) are typically more valuable (Holman and Malau-Aduli, 2012).
The HGT-KAPs are predominantly present in the orthocortex of wool fibers (Powell and Rogers, 1990). In the wool follicle, the expression of the HGT-KAPs follows shortly after expression of the keratins, but before the expression of the HS and UHS KAPs (Rogers, 2006). The content of HGT-KAPs in wool fiber varies in sheep, ranging from <3% in Lincoln sheep wool, through to 4% to 12% in Merino wool (Gillespie, 1990). The HGT-KAP content also appears to be influenced by dietary, physiological, and genetic factors (Gillespie, 1990).
In sheep, there are 3 known HGT-KAP families, KAP6, KAP7, and KAP8; with these being located on chromosome 1. The KAP6 family is a multigene family with 5 members (Zhou et al., 2016). On the other hand, 2 genes (KRTAP8-1 and KRTAP8-2) have been described for the KAP8 family (Gong et al., 2014), and only 1 gene (KRTAP7-1) has been identified for KAP7 (Kuczek and Rogers, 1987). The chromosomal location for KRTAP7-1 is aOar_rambouillet_v1.0, GCF_002742125.1. Other KAP genes (KRTAP8-2, KRTAP6-1, and KRTAP6-3) adjacent to KRTAP7-1 on chromosome 1 have been found to be associated with variation in wool characteristics like wool crimping and growth, mean fiber diameter (MFD), fiber diameter SD (FDSD), and prickle factor (Zhou et al., 2015; Tao et al., 2017; Li et al., 2017b). This along with the observed variation in HGT-KAP content of wool in different sheep breeds (Gillespie et al., 1990), and the polymorphism reported for the HGT-KAP genes (Gong et al., 2011, Zhou et al., 2016), suggests that KRTAP7-1 is worth investigating to ascertain if it is associated with wool traits in Pakistani sheep.
Materials and Methods
Ethical statement
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. No ethical approval was required as only standard samples (wool and blood samples) were obtained from healthy sheep. No experimentation on the animals was performed.
Sampling and wool testing
A total of 222 wool samples were collected from the mid-side of 5 different breeds from Malakand Division of the Khyber Pakhtunkhwa Province of Pakistan. The wool characteristics measured were yield, mean staple length (MSL), wool bulk, MFD, medullation, and opacity.
MSL was measured following the IWTO-30-2007 procedure (IWTO, 2007). Briefly, 3 randomly chosen staples of each sample of the wool were selected for measurement of MSL. The staples were straightened, and the length was measured using a ruler. The mean of the 3 length measurements was taken as the MSL.
The yield of the wool samples is the difference between the CFW and the GFW. Wool samples were scoured as described by Ullah et al. (2019). First GFW was measured using a balance, then the wool samples were scoured in a bowl scouring unit. In the first bowl, the sample were placed in water and allowed to warm. Chemirite Ni 400 (detergent) was added and the temperature was allowed to rise up to 70 °C with the samples being gently agitated for 30 min. The sample was then transferred to new bowl and the wash repeated for another 30 min in water heated to 70 °C. Following this treatment, the samples were rinsed in water at room temperature. After rinsing the samples were dried at 37 °C overnight. Next day, the clean samples were weighed again and yield was calculated using the following formula: YIELD = CFW/GFW × 116% (Khan et al., 2007).
The MFD, medullation, and opacity were measured using an Optical Fibre Digital Analyser (OFDA 100) following IWTO-47-98 and IWTO-57-2000 (IWTO, 1998, 2000). Briefly, the cleaned wool sample was equilibrated at standard atmospheric conditions (temperature = 20 ± 2 °C and humidity = 65 ± 2%) for 24 h. It was then placed in a minicore, and 1.8 to 2.0 mm snippets were cut and spread on a glass slide. The slide was placed under the OFDA camera to ascertain MFD, medullation, and opacity along with their SDs.
The bulk of the carded wool samples was measured following the method of NZS-8716 (NZS 8716, 1994). The bulk of each sample was measured using a bulkometer. First, the clean wool samples were hand carded using a small drum carder, then 10 g of carded wool was placed in the cylinder of the bulkometer for the measurement of wool bulk. The wool sample was subjected to full pressure (30 g force per square cm) for 30 s by lowering the piston (weight 500 g) and added load (weight 1,000 g). Next, the piston plus load was removed, and the wool sample was allowed to relax for 30 s. The sample was again subjected to full pressure, and the height of the wool sample was measured. The load was removed and piston alone (10 g force per square cm) was allowed to exert pressure on the wool sample for 30 s, followed by the sample being allowed to relax for 30 s. The height of the wool was measured for a second time in millimeters. The second measured value was divided by 2, to give a wool bulk measurement.
Blood sampling
Jugular venous blood samples were collected into EDTA tubes at the time of wool sample collection. The samples were stored at the Department of Biotechnology, University of Malakand, Pakistan prior to being sent to the Gene Marker Laboratory, Lincoln University, New Zealand for genotyping. About 200 µL of the blood sample was applied to Whatman FTA classic cards (GE Healthcare Life Sciences, Buckinghamshire, UK) and the FTA cards were kept overnight at room temperature and allowed to dry. Genomic DNA was extracted using a 2-step procedure described by Zhou et al. (2006).
PCR primers and amplification
A previously described (Gong et al., 2012b) primer set (forward: 5′ ACTTGCTCTTCACATTCTATC 3′ and reverse: 5′ GTAGTCATCTGGAGCCATG 3′) was used for amplification of the entire coding sequence of KRTAP7-1 in Pakistani sheep breeds and their crosses. This primer set amplifies 327 bp of DNA sequence from ovine chromosome number 1 (position 122831458–122831784). The PCR amplification was performed in a 15-µL reaction containing the genomic DNA on a 1.2-mm punch of the FTA paper, 0.25 µM primers, 150 µM deoxynucleoside triphosphates (dNTPs) (Bioline, London, UK), 2.5 mM Magnesium chloride, 0.5 U of Taq DNA polymerase (Qiagen, Hilden, Germany), and the reaction buffer supplied with the enzyme. The thermal profile consisted of 2 min at 94 °C; followed by 34 cycles of 30 s at 94 °C, 30 s at 60 °C, and 30 s at 72 °C; with a final extension of 5 min at 72 °C. Amplification was carried out using S1000 thermal cyclers (Bio-Rad, Hercules, CA), and the amplicons were visualized after electrophoresis in 1% agarose gels (Quantum Scientific, Brisbane, Queensland, Australia), using 1× Tris/Borate/EDTA (TBE) buffer [89 mM Tris, 89 mM boric acid, and 2 mM ethylenediaminetetraacetic acid disodium salt (Na2EDTA)] containing 200 ng/mL of ethidium bromide.
Detecting variation in KRTAP7-1
The PCR amplicons were screened for sequence variation using a single-strand conformation polymorphism (SSCP) analysis. Each amplicon (0.7 µL) was mixed with 7 µL of loading dye (98% formamide, 10 mM EDTA, 0.025% bromophenol blue, and 0.025% xylene cyanol). After denaturation at 95 °C for 5 min, the samples were cooled on wet ice and then loaded on 16 cm × 18 cm, 14% acrylamide/bisacrylamide (37.5:1) (Bio-Rad) gels. Electrophoresis was performed using Protean II xi cells (Bio-Rad) in 0.5× TBE buffer, at 16 °C for 19 h at 280 V. The gels were silver-stained according to the method of Byun et al. (2009). Samples previously genotyped by Gong et al. (2012b) were run as internal controls to identify PCR-SSCP banding patterns.
Statistical analysis
All the statistical analyses were performed using IBM SPSS Statistics (Version 21, IBM, NY) and the significance threshold was taken as P < 0.05. Pearson correlation coefficients were calculated to test the strength of the phenotypic relationships among the 12 wool traits (i.e., yield, MSL, Bulk, MFD, FDSD, the coefficient of variation of fiber diameter (CVFD), medullation, the SD of medullation (MeSD), coefficient of variation of medullation (CVMed), opacity, the SD of opacity (OpSD), and the coefficient of variation of opacity (CVOp)). For each wool trait, a univariate analysis was carried out separately, using each of the independent variables. Independent variables included gender, location or district, shearing-season, and sheep age. Variables that were potentially affecting the traits (P < 0.2) were then included in the subsequent general linear mixed-effect models (GLMMs). Genotypes occurring at a high enough frequency (>5%) were included in the GLMMs for association studies. Gender had a significant effect on 3 wool traits (MSL, MFD, FDSD, and MeSD) and was thus included in these models. Shearing season was included in the GLMM for yield, MSL, Bulk, MFD, medullation, MeSD, CVMed, opacity, and OpSD. Age had an effect on yield, MSL, Bulk, MFD, and CVFD was included in the GLMMs for these wool traits. The location (District) had an effect on MSL, bulk and opacity was included in GLMMs for these wool traits.
The sheep studied were reared in Malakand Division in private flocks where the farmers keep varying numbers of rams for breeding purposes. Sire/parentage data were not available for these farms. In this context, we chose “flock” as a variable that would accommodate the genetic effect of sire, it being a useful proxy, as no ram would be used on more than 1 farm. Flock was tested against the wool traits in univariate models and was found to affect them all. Breed was also found to affect all the wool traits in the univariate model. In order to check the effect of both flock and breed, these factors were adjusted together (breed + flock) in such a way that flocks that had more than 1 breed, can represent both the effects of flock and breed at the same time. If a flock consisted of a single breed, then the factor represents both the flock and breed. This factor was run in the univariate models and was found to affect all the wool traits; thus, it was included in the GLMMs as a random factor. Fitting all the potentially significant variables in GLMMs, “the best fitted model” was obtained by backward selection method (Zuur et al., 2009).
Results
Correlation results
The Pearson correlation coefficients for the relationships between the wool traits are presented in Table 1. A significant correlation (P < 0.05) was found between yield and medullation while no correlation was found between yield and MSL, bulk, MFD, and opacity. A significant correlation (P < 0.05) was noted between MSL and bulk (r = −0.183), MFD (r = −0.167), medullation (r = 0.159), and opacity (r = 0.134). Furthermore, a significant correlation (P < 0.001) was also observed between MFD and medullation (r = 0.288). However, a significant negative correlation (P = 0.012) was noted between medullation and opacity (r = −0.153) (see Table 1).
Table 1.
Correlations between different wool traits1 in Pakistani sheep breed and breed crosses
| YIELD1 | MSL | Bulk | MFD | FDSD | CVFD | Med | MeSD | CVMed | Opacity | OpSD | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSL | 0.012 | ||||||||||
| Bulk | −0.081 | −0.183* | |||||||||
| MFD | 0.064 | −0.167* | −0.199** | ||||||||
| FDSD | 0.185* | 0.107 | −0.123 | 0.302*** | |||||||
| CVFD | 0.180* | 0.143* | −0.088 | 0.099 | 0.975*** | ||||||
| Med | 0.169* | 0.159* | −0.125 | 0.288*** | 0.478*** | 0.452*** | |||||
| MeSD | 0.128 | 0.240*** | 0.023 | 0.010 | 0.282*** | 0.308*** | 0.448*** | ||||
| CVMed | 0.093 | 0.218** | 0.042 | −0.062 | 0.177* | 0.210** | 0.189* | 0.959*** | |||
| Opacity | 0.123 | 0.134* | 0.122 | 0.077 | 0.097 | 0.095 | −0.153* | 0.022 | 0.067 | ||
| OpSD | 0.197** | −0.061 | −0.208** | 0.241*** | 0.182* | 0.139* | 0.124 | 0.045 | 0.026 | −0.069 | |
| CVOp | 0.057 | −0.078 | −0.097 | 0.068 | 0.041 | 0.026 | 0.067 | 0.004 | −0.010 | −0.545*** | 0.353*** |
1Yield, yield percentage; MSL, mean staple length; MFD, mean fiber diameter; FDSD, fiber diameter standard deviation; CVFD, coefficient of variation of fiber diameter; MeSD, medullation SD; CVMed, coefficient of medullation; OpSD, opacity standard deviation; CVOp, coefficient of opacity. * P < 0.05, ** P < 0.005, *** P < 0.001. │r│> 0.7 bold, 0.3 <│r│≤ 0.7 italicized.
Polymorphism of KRTAP7-1 in Pakistani sheep breeds and crosses
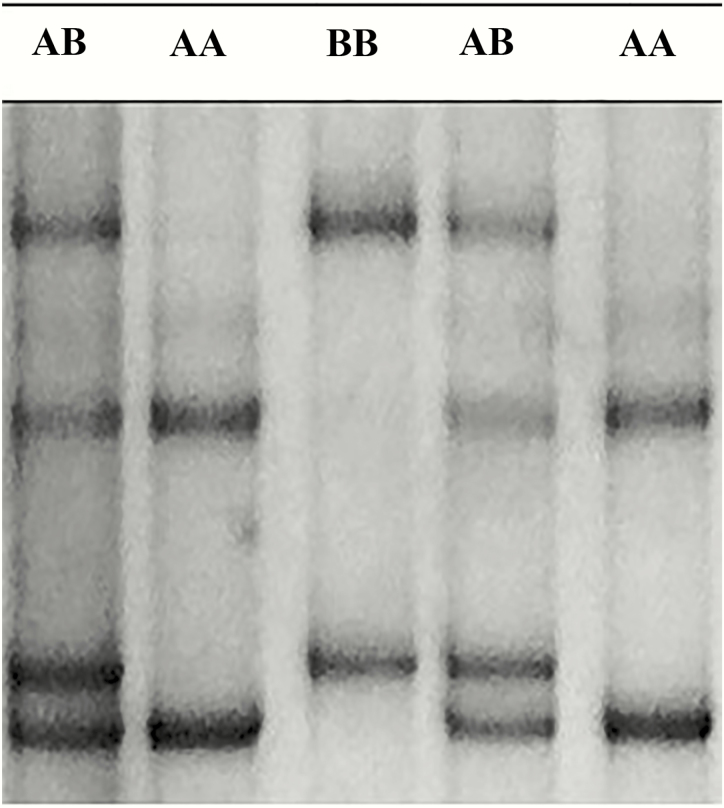
Three different banding patterns were revealed in the SSCP analysis (Figure 1). Two patterns appeared to be from homozygous sheep, while the third pattern appeared to be for heterozygous sheep. The PCR-SSCP patterns obtained in the Pakistani sheep breeds were compared with the patterns previously identified by Gong et al. (2012b) and were found to be identical to the patterns produced by the A and B variants. The frequency of the genotypes AA, AB, and BB in the total 222 sheep studied was 76.1%, 23.0%, and 1.0%, respectively. The frequency of genotype AA was the highest (90.2%) in the Ramghani-cross sheep, and lowest in the Balkhi-cross sheep (75%). The frequency of genotype AB was the highest in Kutta (36.9%) and the lowest in the Ramghani-cross (9.8%). Genotype BB was only present in Balkhi-cross with a very low frequency (4.2%), as shown in Table 2.
Figure 1.
SSCP patterns for KRTAP7-1 genotypes AA, BB, and AB.
Table 2.
Number and frequency (%) of KRTAP7-1 genotypes (AA, AB, and BB) in 5 different sheep breeds/crosses in Malakand Division, Pakistan
| Genotypes | AA | AB | BB | ||||
|---|---|---|---|---|---|---|---|
| Breed/cross | Total | Number present | Frequency, % | Number present | Frequency, % | Number present | Frequency, % |
| Ramghani-cross | 51 | 46 | 90.2 | 5 | 9.8 | ||
| Kutta | 65 | 41 | 63.1 | 24 | 36.9 | ||
| Balkhi-cross | 48 | 36 | 75 | 10 | 20.8 | 2 | 4.2 |
| Kari | 21 | 16 | 76.2 | 5 | 23.8 | ||
| Balkhi | 37 | 30 | 81.1 | 7 | 18.9 |
Association of KRTAP7-1 genotypes with wool traits
In the GLMM analyses, variation in KRTAP7-1 genotype was found to be associated with yield (P = 0.017) and MSL (P = 0.024). Animals with the AA genotype had lower adjusted mean yields and MSLs, when compared with AB animals. The adjusted mean yield of the AA sheep (n = 169) was 79.9 ± 2.72%, while that of the genotype AB sheep (n = 51) was 81.9 ± 3.37%. The adjusted mean MSL of genotype AA sheep was 47.3 ± 0.57 mm and that of genotype AB sheep was 50.9 ± 0.65 mm (Table 3).
Table 3.
Adjusted means ± 95% CIs for various wool traits from sheep of 2 KRATP7-1 genotypes derived from the general linear mixed-effects models
| Mean ± 95% CI | |||
|---|---|---|---|
| Traits | AA, n = 169 | AB, n = 51 | P-value1 |
| Yield2, % | 79.1 ± 2.72 | 81.9 ± 3.37 | 0.017 |
| MSL3, mm | 47.3 ± 0.57 | 50.9 ± 0.65 | 0.024 |
| Bulk | 23.6 ± 1.72 | 23.1 ± 1.74 | 0.176 |
| MFD, μm | 25.8 ± 0.88 | 25.3 ± 0.98 | 0.286 |
| FDSD, μm | 12.5 ± 1.78 | 12.4 ± 2.43 | 0.886 |
| CVFD | 44.8 ± 5.94 | 45.8 ± 8.13 | 0.778 |
| Medullation | 44.6 ± 2.47 | 44.9 ± 3.63 | 0.879 |
| MeSD | 19.6 ± 3.12 | 22.8 ± 5.43 | 0.294 |
| CVMed | 42.3 ± 5.82 | 49.4 ± 10.39 | 0.217 |
| Opacity | 61.6 ± 5.61 | 60.9 ± 5.98 | 0.658 |
| OpSD | 14.4 ± 0.69 | 14.2 ± 0.86 | 0.599 |
| CVOp | 29.2 ± 3.25 | 32.9 ± 5.96 | 0.278 |
1Significant differences (P < 0.05) are shown in bold.
2Adjusted for age.
3Adjusted for age and gender.
Discussion
In this study, 5 Pakistani sheep breeds/crosses were investigated for selected wool traits and genotyped for KRTAP7-1. Pearson correlation coefficients were calculated to ascertain the relationship between the different wool traits, and then GLMMs were used to test associations between KRTAP7-1 genotypes and the wool traits.
Correlation
The correlations found between the wool traits were similar to previous studies (Huisman and Brown 2009; Gong et al., 2015; Li et al., 2017c), with some exceptions. A slight negative correlation (r = −0.167) between MSL and MFD was observed in this study, while Li et al. (2017c) reported the correlation of −0.270 between these wool traits. The correlation coefficient between MFD and FDSD in this study was 0.320, which is less than that reported by Gong et al. (2015) (r = 0.733) but is concordance with Li et al. (2017c) (r = 0.350). In this study, a strong correlation was found between FDSD and CVFD, with a correlation coefficient (r = 0.975) greater than that reported by Li et al. (2017c) (r = 0.815) and Gong et al. (2015), (r = 0.813). These correlations are strong as expected because CVFD values are derived from FDSD.
The correlation of yield and FDSD in the present study is lower than that reported by Li et al. (2017c), although Gong et al. (2015) reported an even lower correlation between these 2 traits. The absence of a correlation between yield and MFD in the present study is in line with the findings of Wuliji et al. (2001), but contrasts the findings of Gong et al (2015), who reported a moderate negative correlation. The correlation between MFD and MSL was low (r = −0.167), but contrasts the findings of Wuliji et al. (2001), who found no correlation between these traits. The reasons for the above differences are not clear, but they suggest that the traits explored in the different studies, might also be affected by other factors including breed, the age of sheep when the wool was collected, the frequency of shearing per year, the season when shearing was undertaken, the gender of the sheep and the number of samples analyzed, all of which can affect correlation between wool traits. For example, while Roldan et al. (2010) reported a moderate correlation (r = 0.390) between CFW and yield in wool samples collected at first shearing, no correlation was found between CFW and yield in wool samples collected at a second shearing.
Wool characteristics also vary considerably among breeds and localities and are influenced by many factors including genetics, environment, disease (bacterial, viral, and fungal), exogenous chemicals, hormones, and photo-period (Khan et al., 2012). These factors also likely account for the differences between studies.
Polymorphism in KRTAP7-1
KAP genes, in-part responsible for the mechanical properties of hair and wool, are capable of influencing wool quality and quantity. In this study, KRTAP7-1 was found to be variable in Pakistani sheep breeds. The PCR-SSCP patterns obtained were found to be similar to patterns reported previously (Gong et al., 2012b), the frequencies of genotypes AA (76%) and AB (23%) are similar to variants frequencies of New Zealand Romney sheep (77% and 23%, for variants A and B, respectively). The genotype BB was only detected in 2 Balkhi-cross sheep from different flocks. This is a limitation in the study, and given the potential of these 2 sheep to adversely impact the statistical models they were not included in the various analyses. In this study, the frequency of each genotype in each breed/cross is different from each other as well as from the previous finding of Gong et al. (2012b). The differences in the genotype frequencies in the present and previous study (Gong et al., 2012b) may be breed specific due to varying selective pressures.
The variation in KRTAP7-1 appears to be sheep specific as opposed to breed specific, as no new variation was found in the Pakistani sheep breeds based on the PCR-SSCP patterns obtained. It should however be noted that the nucleotide sequences of the KRTAP7-1 variants (accession numbers JN091630 and JN091631) reported by previously by Gong et al. (2012b), and that gave similar PCR-SSCP patterns to the variants detected in this study, do not have restriction sites for restriction enzymes, MspI and BglII reported by McLaren et al. (1997). One might therefore conclude that greater variation in KRTAP7-1 is likely to exist in sheep.
Variation in KRTAP7-1 and its effect on wool traits
In this study, while variation in ovine KRTAP7-1 was found to be associated with both yield and MSL, no correlation was observed between yield and MSL. Yield and MSL have previously been associated with variation in KRTAP1-1 (Itenge et al., 2010), KRTAP1-2 (Gong et al., 2015), KRTAP1-3 (Farag et al., 2018), KRTAP15-1 (Li et al., 2018), and KRTAP22-1 (Li et al., 2017a, associated with yield only). The absence of correlation contrasts the findings of Li et al. (2017c), who reported a moderate correlation (r = 0.341) between yield and MSL. These findings are in line with those of Rolden et al. (2010), who reported a QTL near KRTAP6 and KRTAP8 (which are clustered with KRTAP7). Farag et al. (2016) reported association between growth hormone genes and wool traits, and variation in these genes was reported to affect CFW, which could therefore affect yield. Taken together, it would suggest that yield and MSL may be controlled by more than 1 gene, regardless of whether KRTAP7-1 is associated with these traits. Other factors that may also influence yield and MSL include breed, and the amount and quality of feed intake (Macpherson 2012), therefore to ascertain the specific effect of individual or grouped KRTAPs genes, studies need to be designed to control the effects of all these factors. The findings of this study suggest that additional genotyping of KRTAP7-1 should be undertaken in a larger population of sheep of differing breed to ascertain how the gene is affecting wool traits.
Conclusion
This investigation suggests that variation in KRTAP7-1 is associated with yield and MSL in some Pakistani sheep breeds and crosses. Analysis of a larger population of sheep of different breeds is required to reach to confirm the findings.
Acknowledgments
This work was in part supported by the Higher Education Commission (HEC), Islamabad, Pakistan under grant no. 20-3370/NRPU/R&D/HEC2014/455 to S.M.J. and the HEC’s International Research Support Program (IRSIP) grant no. 1-8/HEC/HRD/2017/8208 to FU for 6 month work at Gene-Marker Laboratory, Lincoln University, New Zealand.
Conflict of interest statement
None declared.
Literature Cited
- Akram S., Butt I. A. and Shabbir M.. . 2016. Wool sector analysis for Pakistan. Adv. Soc. Sci. Res. 3(8):22–32. doi: 10.14738/assrj.38.2050. [DOI] [Google Scholar]
- Angel C., Beare S. and Zwart A.. . 1990. Product characteristics and arbitrage in the Australian and New Zealand wool markets. Aust. J. Agric. Resour. Econ. 34:67–79. doi: 10.1111/j.1467-8489.1990.tb00493.x [DOI] [Google Scholar]
- Banks R., and Brown D.. . 2009. Genetic improvement in the Australasian Merino management of a diverse gene pool for changing markets. Anim. Genet. Resour. Info. 45:29–36. doi: 10.1017/S1014233909990290 [DOI] [Google Scholar]
- Byun S. O., Fang Q., Zhou H., and Hickford J. G. H.. . 2009. An effective method for silver staining DNA in large numbers of polyacrylamide gels. Analyt. Biochem. 385:174–175. doi: 10.1016/j.ab.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Economic Survey of Pakistan 2018–2019. Chapter 2, Agriculture. Ministry of Finance, Government of Pakistan; p. 11–33. http://finance.gov.pk/survey/chapters_19/Economic_Survey_2018_19.pdf. [Google Scholar]
- Farag I. M., Darwish A. M., Darwish H. R., AbdelAziz K. B., Ramadan W. A., Mohamed M. I., and Othman O. E.. . 2016. Polymorphism of growth hormone gene and its association with wool traits in Egyptian sheep breeds. Afr. J. Biotechnol. 15:549–556. doi: 10.5897/AJB2015.14928. [DOI] [Google Scholar]
- Farag I. M., Darwish H. R., Darwish A. M., Eshak M. G., Ahmed R. W.. . 2018Genetic Polymorphism of KRT1. 2 Gene and its Association with Improving of Some Wool Characteristics in Egyptian Sheep. Asian J. Sci. Res. 11:295–300. doi: 10.3923/ajsr.2018.295.300. [DOI] [Google Scholar]
- Gillespie J. M. 1990. The proteins of hair and other hard a-keratins. In: Goldman R. D. and Steinert P. M., editors. Cellular and molecular biology of intermediate filaments. New York: Plenum Press; p. 95–128. doi: 10.1007/978-1-4757-9604-9_4 [DOI] [Google Scholar]
- Gong H., Zhou H., Dyer J. M., Hickford J. G.. . 2014. The sheep KAP8-2 gene, a new KAP8 family member that is absent in humans. Springer Plus, 3:528. doi: 10.1186/2193-1801-3-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H., Zhou H., and J.Hickford G.. . 2011. Diversity of the glycine/tyrosine-rich keratin associated protein 6 gene (KAP6) family in sheep. Mol. Biol. Rep. 38:31–35. doi: 10.1007/s11033-010-0074-6. [DOI] [PubMed] [Google Scholar]
- Gong H., Zhou H., Hodge S., Dyer J. M., and Hickford J. G.. . 2015. Association of wool traits with variation in the ovine KAP1-2 gene in Merino cross lambs. Small Rumin. Res., 124:24–29. doi: 10.1016/j.smallrumres.2015.01.009 [DOI] [Google Scholar]
- Gong H., Zhou H., McKenzie G. W., Yu Z., Clerens S., Dyer J. M., and Chen Y.. . 2012a. An updated nomenclature for keratin-associated proteins (KAPs). Int. J. Biol. Sci. 8:258. doi: 10.7150/ijbs.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H., Zhou H., Plowman J. E., Dyer J. M., and Hickford J. G.. . 2012b. Search for variation in the ovine KAP7-1 and KAP8-1 genes using polymerase chain reaction–single-stranded conformational polymorphism screening. DNA Cell Biol. 31:367–370. doi: 10.1089/dna.2011.1346 [DOI] [PubMed] [Google Scholar]
- Holman B., and Malau-Aduli A.. . 2012. A review of sheep wool quality traits. Annu. Rev. Res. Biol. 2:1–14. [Google Scholar]
- Huisman A., and Brown D.. . 2009. Genetic parameters for bodyweight, wool, and disease resistance and reproduction traits in Merino sheep. 4. Genetic relationships between and within wool traits. Anim. Prod. Sci. 2009, 49:289–296. doi: 10.1071/EA08173 [DOI] [Google Scholar]
- Itenge T., Hickford J., Forrest R., McKenzie G., and Frampton C.. . 2010. Association of variation in the ovine KAP1. 1, KAP1. 3 and K33 genes with wool traits. Int. J. Sheep Wool Sci. 58:1–20. doi: 10.1016/j.mcp.2007.04.002. [DOI] [Google Scholar]
- IWTO 1998. Specification IWTO-47–98, Measurement of the mean and distribution of fiber diameter of wool using an Optical Fibre Diameter Analyzer (OFDA). Brussels (Belgium): International Wool Textile Organization. [Google Scholar]
- IWTO 2000. IWTO-57-2000.Brussels (Belgium): International Wool Textile Organization. [Google Scholar]
- IWTO 2007. IWTO 30–2007. Brussels (Belgium): International Wool Textile Organization. [Google Scholar]
- Kelly M., Swan A., and Atkins K.. . 2007. Optimal use of on-farm fibre diameter measurement and its impact on reproduction in commercial Merino flocks. Aust. J. Exp. Agric. 47:525–534. doi: 10.1071/EA06222. [DOI] [Google Scholar]
- Khan M. F., Rafique M., and Ashfaq F.. . 2007. Study of some important wool characteristics of selected sheep breeds in Pakistan. Proceedings of National Seminar on Sheep and Wool in Pakistan; p. 44. [Google Scholar]
- Khan M. J., Abbas A., Ayaz M., Naeem M., Akhter M. S., and Soomro M. H.. . 2012. Factors affecting wool quality and quantity in sheep. Afr. J. Biotechnol. 11:13761–13766. doi: 10.5897/AJBX11.064 [DOI] [Google Scholar]
- Kuczek E. S., and Rogers G. E.. . 1987. Sheep wool (glycine+ tyrosine) ‐rich keratin genes: a family of low sequence homology. Eur. J. Biochem. 1:79–85. doi: 10.1111/j.1432-1033.1987.tb13486.x [DOI] [PubMed] [Google Scholar]
- Li W., Gong H., Zhou H., Wang J., Liu X., Li S., and Hickford J. G. H.. . 2018. Variation in the ovine keratin-associated protein 15-1 gene affects wool yield. J. Agric. Sci. 156:922–928. doi: 10.1017/S0021859618000953. [DOI] [Google Scholar]
- Li S., Zhou H., Gong H., Zhao F., Wang J., Liu X., Luo Y., and Hickford J. G. H.. . 2017a. Identification of the ovine keratin-associated protein 22-1 (KAP22-1) gene and its effect on wool traits. Genes, 8:27. doi: 10.3390/genes8010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhou H., Gong H., Zhao F., Wang J., Luo Y., and Hickford J. G.. . 2017b. Variation in the ovine KAP6-3 gene (KRTAP6-3) is associated with variation in mean fibre diameter-associated wool traits. Genes, 8:204. doi: 10.3390/genes8080204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhou H., Gong H., Zhao F., Hu J., Luo Y., Hickford J. G.. . 2017c. Identification of the ovine keratin-associated protein 26-1 gene and its association with variation in wool traits. Genes, 8:225. doi: 10.3390/genes8090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson S. 2012. Wool classing, wool marketing & clip preparation. The Australian Wool Education Trust licensee for educational activities, University of New England. [Google Scholar]
- McLaren R. J., Rogers G. R., and Davies K. P., Maddox J. F., Montgomery G. W.. . 1997. Linkage mapping of wool keratin and keratin-associated protein genes in sheep. Mamm. Genom. 8:938–940. doi: 10.1007/s003359900616 [DOI] [PubMed] [Google Scholar]
- Mortimer S., Atkins K., Semple S., and Fogarty N.. . 2010. Predicted responses in Merino sheep from selection combining visually assessed and measured traits. Anim. Prod. Sci. 50:976–982. doi: 10.1071/AN10085. [DOI] [Google Scholar]
- NZS 8716 1994. Measurement of the bulk of raw wool. Wellington: Standards Association of New Zealand. [Google Scholar]
- Parry D. A., and Steinert P. M.. . 1995. Intermediate filament structure. New York: Springer-Verlag; Intermediate Filament Associated Proteins; p. 123–44. [Google Scholar]
- Powell B. C., and Rogers G. E.. . 1990. Hard keratin IF and associated proteins. In Goldman R. D. and Stelnert P. M., editors, Cellular and molecular biology of intermediate filaments. New York:Plenum Press; p. 267–300. doi: 10.1007/978-1-4757-9604-9_10. [DOI] [Google Scholar]
- Powell B. C., and Rogers G. E.. . 1994. Differentiation in hard keratin tissues: hair and related structures. In: Leigh M., Lane E. B. and Watt F. M., editors, The Keratinocyte Handbook. Cambridge University Press; p. 401–436. [Google Scholar]
- Preston J., Hatcher S., and McGregor B.. . 2016. Fabric and greasy wool handle, their importance to the Australian wool industry: a review. Anim. Prod. Sci. 56:1–17. doi: 10.1071/AN14777. [DOI] [Google Scholar]
- Rogers G. E. 2006. Biology of the wool follicle: an excursion into a unique tissue interaction system waiting to be re‐discovered. Exp. Dermatol. 15:931–949. doi: 10.1111/j.1600-0625.2006.00512.x. [DOI] [PubMed] [Google Scholar]
- Rogers M. A., Edler L., Winter H., Langbein L., Beckmann L., and Schweizer J.. . 2005. Characterization of new members of the human type II keratin gene family and a general evaluation of the keratin gene domain on chromosome 12q13. 13. J. Invest Dermatol. 124:536–544. doi: 10.1111/j.0022-202X.2004.23530.x [DOI] [PubMed] [Google Scholar]
- Rogers G. R., Hickford J. G. H., and Bickerstaffe R.. . 1994. Polymorphism in two genes for B2 high sulphur proteins of wool. Anim. Genet. 25:407–415. doi: 10.1111/j.1365-2052.1994.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Roldan D., Dodero A., Bidinost F., Taddeo H., Allain D., Poli M., and Elsen J.. . 2010. Merino sheep: a further look at quantitative trait loci for wool production. Animal 4:1330–1340. doi: 10.1017/S1751731110000315. [DOI] [PubMed] [Google Scholar]
- Schoenian S. 2019. “Real men wear wool. Sheep 101. Info” http://www.sheep101.info/wool.html (accessed November 05, 2019).
- Tao J., Zhou H., Yang Z., Gong H., Ma Q., Ding W., and Hickford J. G.. . 2017. Variation in the KAP8-2 gene affects wool crimp and growth in Chinese Tan sheep. Small Ruminant Res. 149:77–80. doi: 10.1016/j.smallrumres.2017.01.001. [DOI] [Google Scholar]
- Thornberry K., and Atkins K.. . 1984. Variation in non-wool components of the greasy fleece over the body of Merino sheep. Aust. J. Exp. Agri. 24:62–76. doi: 10.1071/EA9840062 [DOI] [Google Scholar]
- Ullah F., Khan M. F. U. Khan M. H., and Jamal S. M.. . 2019. Factors affecting mean fiber diameter in selected Pakistani sheep breeds/crosses. J. Nat. Fibers. doi: 10.1080/15440478.2019.1658259. (in press) [DOI] [Google Scholar]
- Wuliji T., Dodds K., Land J., Andrews R., and Turner P.. . 2001. Selection for ultrafine Merino sheep in New Zealand: heritability, phenotypic and genetic correlations of live weight, fleece weight and wool characteristics in yearlings. Anim. Sci. 72:241–250. doi: 10.1017/S1357729800055739 [DOI] [Google Scholar]
- Younas M., and Yaqoob M.. . 2019. “Rural livestock production in Pakistan”http://www.pakissan.com/english/allabout/livestock/rural.livestock.pakistan.shtml – [accessed June 30, 2019].
- Zhou H., Gong H., Li S., Luo Y., and Hickford J. G. H.. . 2015. A 57‐bp deletion in the ovine KAP 6‐1 gene affects wool fibre diameter. J. Anim. Breed. Genet. 132:301–307. doi: 10.1111/jbg.12138. [DOI] [PubMed] [Google Scholar]
- Zhou H., Gong H., Wang J., Dyer J. M., Luo Y., and Hickford J. G.. . 2016. Identification of four new gene members of the KAP6 gene family in sheep. Sci. Rep. 6:24074. doi: 10.1038/srep24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Hickford J. G., and Fang Q.. . 2006. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Analyt. Biochem. 354:159–161. doi: 10.1016/j.ab.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Zuur A., Ieno E. N., Walker N., Saveliev A. A., and Smith G. M.. . 2009. Mixed effects models and extensions in ecology with R. New York: Springer Science & Business Media. [Google Scholar]