Abstract
We investigated the effects of inulin on intestinal barrier function and mucosal immunity in Salmonella enterica serovar Enteritidis (SE)–infected specific pathogen-free (SPF) chickens. SPF chickens (n = 240, 1-d-old) were divided into 4 groups (6 replicates per group, 10 chickens per replicate): a control group (CON) fed a basal diet without inulin supplementation and 3 SE-infected groups fed a basal diet supplemented with inulin 0% (SE group), 0.5% (0.5% InSE group), and 1% (1% InSE group), respectively. At 28 d of age, the chickens in SE-infected groups were orally infected with SE and in CON group were administrated with phosphated-buffered saline (PBS). Intestinal morphology, mucosal immunity, and intestinal barrier function-related gene expression were analyzed at 1- and 3-d post-infection (dpi). SE challenge significantly increased the mucosal gene expression, such as interleukin-1β (IL-1β), lipopolysaccharide-induced tumor necrosis factor factor (LITAF), interferon-γ (IFN-γ), and interleukin-6 (IL-6), and increased serum IFN-γ, secretory IgA (sIgA), and IgG concentration, and significantly decreased the gene expression levels of mucin 2 (MUC2) and claudin-1 at 3 dpi compared with the CON group (P < 0.05). Inulin supplementation improved the expression levels of these immunity- and intestinal barrier function-related genes, increased villus height (VH), and decreased crypt depth (CD) in the duodenum, jejunum, and ileum at 1 and 3 dpi within the SE-challenged groups (P < 0.05). SE challenge significantly increased ileal Toll-like receptor 4 (TLR4) mRNA at 1 and 3 dpi, suppressor of cytokine signaling 3 (SOCS3) mRNA at 1 dpi, and phospho-signal transducer and activator of transcription 3 (p-STAT3) and Janus kinase1 (JAK1) protein expression at 3 dpi compared with the CON group (P < 0.05). Inulin supplementation suppressed p-STAT3 and JAK1 protein expression and promoted ileal TLR4 and SOCS3 mRNA expression at 3 dpi compared with SE group (P < 0.05). In conclusion, inulin alleviated SE-induced gut injury by decreasing the proinflammatory response and enhancing mucosal immunity in chickens.
Keywords: chicken, barrier function, gut morphology, immunity, inulin, Salmonella
Introduction
Salmonella enterica serovar Enteritidis (SE) is one of the most prominent food-borne microbial agents worldwide, with dangerous implications for food security and human health (Kirk et al., 2015). Traditional antibiotic therapy for Salmonella infection can lead to resistance and acute diarrhea and may cause chronic toxicity, which is a major concern in poultry production (Castillo et al., 2012). Salmonella are Gram-negative facultative anaerobes, and antibiotics normally increase epithelial oxygenation via decreased butyrate levels, resulting in aerobic post-antibiotic pathogen expansion. Antibiotic treatment may thus promote relapse of Salmonella gastroenteritis (Rivera-Chávez et al., 2016). In addition, although antibiotic therapy can clear Salmonella pathogen stool loads, it cannot effectively eliminate mucosal inflammation, partly because of the Salmonella-induced interferon γ (IFN-γ) response (Dolowschiak et al., 2016). Intestinal inflammation provides Salmonella with a competitive metabolic advantage over the resident microorganisms, thus delaying the resolution of mucosal pathology and gut barrier function. There is thus an urgent need for effective alternative treatments for combating Salmonella infections in poultry production.
Inulin-type fructans are effective potential prebiotics that could be used to control Salmonella colonization and improve meat safety in poultry production (Dankowiakowska et al., 2013). Inulin-type fructans exert modulatory effects on the gut microbiota, intestinal mucosal barrier, and systemic immune response via indirect metabolite fermentation by the gut microbiota or by direct stimulation of the host mucosal immune response (Yang et al., 2016; Chen et al., 2017). Inulin prebiotics can promote the proliferation of beneficial microbiota in the gastrointestinal tract (such as Bifidobacterium and Lactobacillus) (Johnson et al., 2015; Wilson et al., 2017) and inhibit the growth of pathogenic bacteria (such as Salmonella and Escherichia coli) in poultry (Samanta et al., 2013; Bucław et al., 2016; Micciche et al., 2018). Evidence also indicates that inulin-type fructans could modulate the immune response by activating the expression of genes and cytokines implicated in immune processes (Sevane et al., 2014). In general, inulin depresses the expression of pro-inflammatory cytokines, such as lipopolysaccharide-induced tumor necrosis factor (LITAF), interferon-γ (IFN-γ), interleukin-6 (IL-6), and inducible nitric oxide synthase, which in turn modifies the intestinal barrier and immune function (Whelan, 2011; Vogt et al., 2015; Ferenczi et al., 2016; Kareem et al., 2017). However, the ability of inulin to combat Salmonella infection via modulation of the intestinal inflammatory and immune responses is unclear. The current study thus aimed to clarify the mucosal immunoregulatory effects of inulin in SE-infected chickens, identify its restorative effects on the SE-damaged gut barrier, and identify the immune regulation signaling pathways associated with enhanced resistance to SE infection.
Materials and methods
All experimental procedures involving animals were approved by the Animal Management Committee of the Institute of Animal Sciences, Chinese Academy of Agricultural Sciences (IAS-CAAS, Beijing, China). Ethical approval regarding animal survival was given by the animal ethics committee of IAS-CAAS (approval number: IASCAAS-AE20140615).
Animal feeding and management
A total of 240 1-d-old Arbor Acres specific pathogen-free (SPF) chickens (Beijing Merial Vital Laboratory Animal Technology Co., Ltd, Beijing, China) were randomly assigned to 4 groups (6 replicates of 10 birds each) and raised in a sterile IPQ-Type 3 negative pressure isolator (China Agricultural University, Beijing, China). The groups were treated as follows: a control group (CON group) was fed a corn/soybean-based sterile diet, and the other 3 groups were treated with inulin (Sigma-Aldrich, St. Louis, MO) at 0%, 0.5%, and 1% until 28 d and then were orally infected with 1-mL phosphated-buffered saline (PBS) containing 108 cfu SE (CMCC50041, China Institute of Veterinary Drugs Control, Beijing) respectively (SE, 0.5% InSE, and 1% InSE groups) at 28 d of age. Meanwhile, the CON group chickens were orally administrated with 1-mL PBS without SE. Detailed calculated nutrient contents of the basal diet ingredients are shown in Table 1. The temperature in the isolator was 35 °C for the first week and gradually reduced by 2 °C each week until the end of the experiment. All chickens had free access to feed and water (sterilized at 121 °C for 15 min).
Table 1.
Composition and nutrient levels of basal diets (as-fed basis) (g/kg)
| Treatment | |||
|---|---|---|---|
| Item | CON | 0.5% Inulin | 1% Inulin |
| Ingredients | |||
| Corn | 680.1 | 669.8 | 660.2 |
| Choline chloride | 0.3 | 0.3 | 0.3 |
| Soybean meal | 275.6 | 277.8 | 279.6 |
| Corn oil | 0 | 3 | 5.8 |
| Salt | 2 | 2 | 2 |
| Limestone powder | 4.8 | 4.8 | 4.8 |
| Calcium dihydrogen phosphate | 16 | 16 | 16 |
| Cystine | 3 | 3.1 | 3.1 |
| Methionine | 0.2 | 0.2 | 0.2 |
| Vitamin premix1 | 10 | 10 | 10 |
| Microelement premix2 | 5 | 5 | 5 |
| Feed grade silicondioxide/titanium | 3 | 3 | 3 |
| Inulin | 0 | 5 | 10 |
| Calculated nutrient level | |||
| ME, MJ/kg | 11.98 | 11.98 | 11.98 |
| CP | 181.0 | 181.0 | 181.0 |
| Available phosphorus | 4.0 | 4.0 | .4.0 |
| Calcium | 9.0 | 9.0 | 9.0 |
| Lysine | 9.1 | 9.0 | 9.1 |
| Methionine | 3.1 | 3.1 | 3.1 |
| Cystine | 6.2 | 6.2 | 6.2 |
1Vitamin premix provided the following per kilogram of diet: vitamin A, 12,000 IU; vitamin D3, 3500 IU; vitamin E, 25 IU; nicotinic acid, thiamin 60 mg; vitamin B12, 0.014 mg; calcium pantothenate, 20 mg; vitaminK3,2.0 mg; thiamin, 2.0 mg; riboflavin, 8.0 mg; vitamin B6, 7.0 mg; folic acid, 0.8 mg; biotin, 0.2 mg.
2Microelement premix provided the following per kilogram of diet: Fe, 100 mg; Cu, 8 mg; Mn, 120 mg; Zn, 100mg; I, 0.7 mg; Se, 0.3 mg.
Sample collection
One chicken from each replicate was randomly selected at 1- and 3-d post-infection (dpi) and slaughtered for sample collection. Blood was also sampled from 1 chicken chosen at random from each replicate, incubated at 37 °C for 10 min (GRP-9050; Shanghai Senxin Test Instrument Co., Ltd, Shanghai, China), and then serum were separated at 1000 g/min for 15 min and stored at −20 °C to analyze IFN-γ and antibody contents. Ileum segments (3–4 cm, near the cecum) were dissected aseptically and rinsed with sterile phosphate-buffered saline. Mucosa samples were then collected by opening the segments lengthwise and scraping with a glass slide. The samples were snap-frozen in liquid nitrogen and then stored at −80 °C for gene and protein expression analyses.
Intestinal morphological analysis
The chickens were infected with SE at 3 dpi and the intestinal morphology was analyzed according to Song et al (2018). Briefly, duodenum, jejunum, and ileum segments (2 cm long) were cut and flushed three times with phosphate-buffered saline to wipe off the intestinal contents. The contents from each sampling day were fixed in 2-mL sterile tubes with 4% paraformaldehyde for histological examination and stain with hematoxylin and eosin (performed by Beijing Xuebang Technology Co., Ltd., Beijing, China). For each staining section, 10 representative intact villi and their related crypt were selected to measure villus height (VH) and crypt depth (CD) using a Leica DMI6000B (Wetzlar, Germany) light microscope equipped with image-processing software (Leica application suite V4.2). Then, the ratio of the VH to CD (V/C) was calculated.
Enzyme-linked immunosorbent assay
Serum levels of immunoglobulin (Ig) A, IgM, IgG, secretory IgA (sIgA), and IFN-γ were measured using enzyme-linked immunosorbent assay (ELISA) kits (Cusabio Biotech Co., Ltd., Wuhan, China) following the manufacturer’s procedures. Absorbance was measured at 450 nm within 5 min using an automated microplate reader (Multiskan GO; Thermo Fisher Scientific Oy, Van-taa, Finland).
Quantitative real‑time polymerase chain reaction
Total RNA was extracted from the ileum using a MiniBEST Universal RNA Extraction Kit (TaKaRa, Dalian, China). Extracted RNA was dissolved in RNase-free water and quantified using a Nanodrop spectrophotometer (ND-2000 UV-Vis; Thermo Fisher Scientific, Waltham, MA). RNA purity was verified by determining the 260:280 nm absorbance ratio. RNA integrity was evaluated based on the spectral curve. cDNA samples were obtained by reverse transcription of total RNA using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham) and stored at −20 °C until analysis. Intestinal gene expression levels were measured by real-time polymerase chain reaction (PCR) using SYBR1 Premix Ex Taq (Tli RNaseH Plus; Takara Biotechnology Inc., Osaka, Japan) and an ABI 7500 Real Time PCR Systems (Applied Biosystems, Foster City, CA) according to manufacturer’s instructions. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression levels were used as an internal control to normalize the amount of initial RNA for each sample. Primer sequences for the target and reference genes are shown in Table 2. The mRNA expression levels of intestinal genes were calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
Primers used in PCR analysis
| Target genes1 | Primer sequence, 5′–3′ | Gene Bank ID |
|---|---|---|
| MUC2 | F: ACTCCTCCTTTGTATGCGTGA | NM_001318434.1 |
| R: GTTAACGCTGCATTCAACCTT | ||
| Claudin-1 | F: GGTGTACGACTCGCTGCTTA | NM_001013611.2 |
| R: CGAGCCACTCTGTTGCCATA | ||
| IL-6 | F: AATCCCTCCTCGCCAATCT | NM_204628.1 |
| R: TCACGGTCTTCTCCATAAACG | ||
| LITAF | F: TGTGTATGTGCAGCAACCCGTAGT | NM_205134.1 |
| R: GGCATTGCAATTTGGACAGAAGT | ||
| IL-1β | F: TGGGCATCAAGGGCTACAAG | HQ739080.1 |
| R: CCAGGCGGTAGAAGAAGATGAAG | ||
| IFN-γ | F: ATGACTTGCCAGACTTACAAC | NM_205149.1 |
| R: GCTACATCTGAATGACTTGAG | ||
| TLR4 | F: ACGGCATTTCAGAACGGACT | NM_001030693.1 |
| R: ACAGCTTCTCAGCAGGCAAT | ||
| SOCS3 | F: TGCGCCTCAAGACGTTCA | NM_204600.1 |
| R: GTACTCGCTCTTAGAGCT | ||
| GAPDH | F: GGAGAAACCAGCCAAGTAT | NM_204305.1 |
| R: CCATTGAAGTCACAGGAGA |
1MUC2, mucin 2; IL-6, interleukin-6; LITAF, lipopolysaccharide-induced tumor necrosis factor factor; IL-1β, interleukin-1β; IFN-γ, interferon-γ; TLR4, Toll-like receptor 4; SOCS3, suppressor of cytokine signaling 3.
Western blotting analysis of JAK-STAT pathway proteins
The ileal mucosal tissues of SE-infected chickens at 3 dpi were analyzed to western blotting and carried out according to previous studies (Xue et al., 2017). Briefly, ileal mucosal tissue (5 mg) was treated with 300-µL RIPA lysis buffer and homogenized using an electrical homogenizer (SCIENZT-48, Ningbo, China) and the lysate was then kept on ice for 30 min and cell debris was removed by centrifugation at 16,000 × g for 30 min at 4 °C. Protein concentrations were determined by Bradford assay. Equal amounts (30 μg) of protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a BioRad mini protein electrophoresis separation unit and then transferred onto a nitrocellulose membrane. After blocking in 5% nonfat milk with Tween 20 buffer and 3% bovine serum albumin at room temperature for 1 h, the membranes were incubated with primary antibodies against signal transducer and activator of transcription 3 (STAT3) (Ab68153, 1:5000), phospho (p)-STAT3 (ab76315, 1:500), and Janus kinase 1 (JAK1) (Ab133666, 1:500), respectively, followed by appropriate horseradish peroxidase-conjugated secondary antibodies. The chemiluminescent substrate (Immobilon Western HRP substrate, Millipore, MA) was applied to the blot according to manufacturer’s recommendations. Protein signals were detected using the Image Quant Tanon-5200 system (Tanon Technology Inc., Shanghai, China). Protein expression levels were normalized to β-actin (YM3028, 1:5000) using image analysis software (GIS 1D, ver.4.00).
Statistical analysis
All statistical analyses were performed using SAS V8. Means were compared by one-way analysis of variance followed by Duncan’s multiple tests. Statistical significance was considered at P < 0.05.
Results
Villus morphology in the small intestine
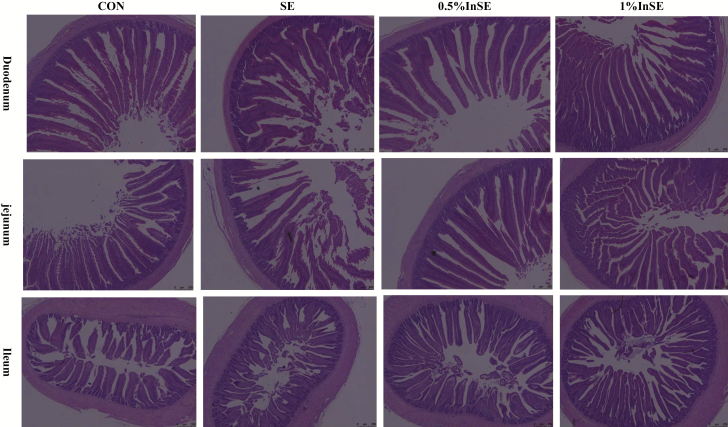
As shown in Figure 1, inulin supplementation alleviated the villus atrophy and shedding in the duodenum, jejunum, and ileum induced by SE-infection. SE infection significantly increased the CD and decreased the V/C ratio in the duodenum, jejunum, and ileum at 1 and 3 dpi compared with the CON group (P < 0.05) (Table 3). Although the VHs in the duodenum, jejunum, and ileum were greater in the CON group compared with the SE group at 1 and 3 dpi, the difference was only significant in the ileum at 1 dpi (P < 0.05).Compared with the SE group, VH and V/C ratio (except ileum at 1 dpi) were significantly increased and the CD were significantly decreased (P < 0.05) in the duodenum, jejunum, and ileum at 1 and 3 dpi by inulin supplementation at all concentrations (Table 3).
Figure 1.
Effects of inulin on gut morphology at 3 d post-infection with Salmonella.
Table 3.
Effects of inulin on intestinal morphology of SE-infected chickens1
| Treatment2 | |||||||
|---|---|---|---|---|---|---|---|
| Item | CON | SE | 0.5% InSE | 1% InSE | SEM | P-value | |
| VH3, μm | |||||||
| Duodenum | 1 dpi4 | 1006b,c | 975c | 1080a | 1054a,b | 40 | 0.003 |
| 3 dpi | 1010a,b | 939b | 1050a | 1072a | 56 | 0.019 | |
| Jejunum | 1 dpi | 999a,b | 905b | 1057a | 1079a | 81 | 0.023 |
| 3 dpi | 938b,c | 903c | 1092a | 1002b | 54 | 0.001 | |
| Ileum | 1 dpi | 620a | 510b,c | 537b | 478c | 32 | 0.001 |
| 3 dpi | 452b,c | 425c | 540a | 499a,b | 37 | 0.005 | |
| CD3, μm | |||||||
| Duodenum | 1 dpi | 101b | 134a | 110b | 98b | 11 | 0.001 |
| 3 dpi | 90b | 119 a | 93b | 98b | 8 | 0.001 | |
| Jejunum | 1 dpi | 98b | 119a | 95b | 97b | 7 | 0.001 |
| 3 dpi | 94b | 119a | 85b | 92b | 9 | 0.001 | |
| Ileum | 1 dpi | 86b | 99a | 83b | 85b | 7 | 0.011 |
| 3 dpi | 85b | 106a | 90b | 93b | 6 | 0.001 | |
| V/C3 | |||||||
| Duodenum | 1 dpi | 9.99a | 7.38b | 9.84a | 10.84a | 0.85 | 0.001 |
| 3 dpi | 11.32a | 7.89b | 11.28a | 10.94a | 0.98 | 0.001 | |
| Jejunum | 1 dpi | 10.22a | 7.60b | 11.10a | 11.23a | 0.89 | 0.001 |
| 3 dpi | 10.10b | 7.64c | 12.98a | 10.95b | 1.25 | 0.001 | |
| Ileum | 1 dpi | 7.24a | 5.19b | 6.51a | 5.63b | 0.55 | 0.001 |
| 3 dpi | 5.19a | 4.01b | 6.06a | 5.41a | 0.49 | 0.001 | |
1Means were calculated on n = 6 replicates per treatment.
2CON, basal diet without inulin supplementation; SE, Salmonella-infected chicken fed with basal diet; 0.5% InSE, Salmonella-infected chicken fed with 0.5% inulin; 1% InSE, Salmonella-infected chickens fed with 1% inulin.
3VH, villus height (μm); CD, crypt depth (μm); V/C, VH/CD.
4dpi: days post-infection.
a–cMeans within rows with different superscript letters were significantly different (P < 0.05).
Gene expression related to ileal mucosal immune and barrier functions
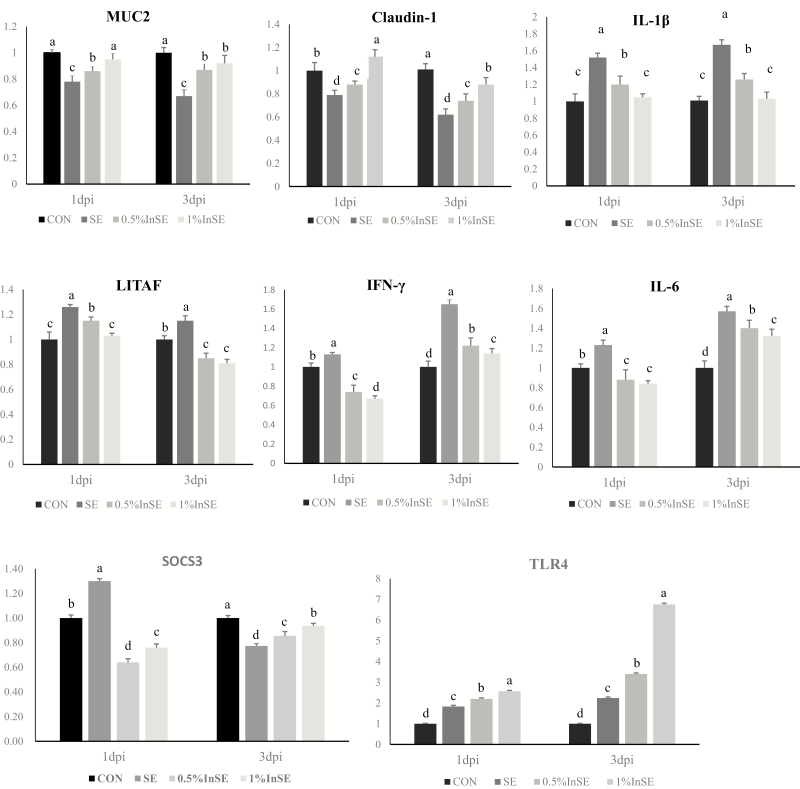
The results of inulin effecting on mucosal immune and barrier functions in ileum are shown in Figure 2. SE challenge significantly decreased (P < 0.05) expression levels of MUC2 and claudin-1 at 1 and 3 dpi and SOCS3 at 3 dpi and significantly increased (P < 0.05) levels of IL-1β, LITAF, IL-6, TLR4, and IFN-γ level at 1 and 3 dpi and SOCS3 at 1 dpi in the ileum compared with the CON group.
Figure 2.
Ileal mRNA expression levels of genes related to mucosal immune and barrier functions in SE-infected chickens at 1- and 3-dpi. Different superscripts above bars indicate significant differences (P < 0.05). All data expressed as mean ± standard error (n = 6). MUC2, mucin 2; IL-6, interleukin-6; LITAF, lipopolysaccharide-induced tumor necrosis factor; IL-1β, interleukin-1β; IFN-γ, interferon-γ; SOCS3, suppressor of cytokine signaling 3; TLR4, Toll-like receptor 4; dpi, days post-infection.
Supplementation with 0.5% and 1% inulin after SE challenge significantly increased the expression levels of MUC2, claudin-1, and TLR4 at 1 and 3 dpi and SOCS3 at 3 dpi (P < 0.05), and significantly decreased expression levels of IL-1β, LITAF, IL-6, and IFN-γ at 1 and 3 dpi and SOCS3 at 1 dpi (P < 0.05) compared with the SE group.
Serum parameters
Serum levels of IgA, sIgA, IgG, IgM, and IFN-γ at 1 and 3 dpi are shown in Table 4. SE challenge significantly increased the levels of IgA at 3 dpi, sIgA at 1 and 3 dpi, IgM at 1 dpi, IgG at 3 dpi, and IFN-γ at 1 and 3 dpi compared with the CON group (all P’s < 0.05). Compared with the SE group, 0.5% and 1% inulin supplements significantly increased the levels of IgA, SIgA, and IgG (except at 1 dpi) and decreased IFN-γ levels (all P’s < 0.05). However, SE challenge and inulin supplementation had no significant effects on serum IgM at 3 dpi and IgG at 1 dpi (P > 0.05).
Table 4.
Effects of inulin on serum Ig and IFN-γ levels in SE-infected chickens1
| Treatment2 | ||||||
|---|---|---|---|---|---|---|
| Item | CON | SE | 0.5% InSE | 1% InSE | SEM | P-value |
| IgA3, mg/mL | ||||||
| 1 dpi4 | 7.07c | 7.33c | 7.83b | 9.84a | 0.14 | 0.001 |
| 3 dpi | 6.40c | 8.68b | 9.51a | 9.80a | 0.15 | 0.001 |
| SIgA3, mg/mL | ||||||
| 1 dpi | 6.41d | 7.44c | 8.40b | 10.53a | 0.15 | 0.001 |
| 3 dpi | 3.78d | 5.63c | 6.51b | 8.81a | 0.10 | 0.001 |
| IgM3, mg/mL | ||||||
| 1 dpi | 0.93b | 1.04a | 1.00a | 1.00a | 0.02 | 0.001 |
| 3 dpi | 0.98 | 1.03 | 1.01 | 1.00 | 0.03 | 0.597 |
| IgG3, mg/mL | ||||||
| 1 dpi | 0.28 | 0.29 | 0.28 | 0.31 | 0.02 | 0.377 |
| 3 dpi | 0.24d | 0.34c | 0.40b | 0.46a | 0.01 | 0.001 |
| IFN-γ 3, pg/mL | ||||||
| 1 dpi | 56.63c | 74.42a | 61.29b | 46.96d | 0.99 | 0.001 |
| 3 dpi | 58.81b | 68.32a | 55.04c | 37.07d | 0.87 | 0.001 |
1Means were calculated on n = 6 replicates per treatment.
2CON, basal diet without inulin supplementation; SE, Salmonella-infected chicken fed with basal diet; 0.5% InSE, Salmonella-infected chicken fed with 0.5% inulin; 1% InSE, Salmonella-infected chicken fed with 1% inulin.
3IgA, immunoglobulin A; SIgA, secretory immunoglobulin A; IgM, immunoglobulin M; IgG, immunoglobulin G; IFN-γ, interferon-γ.
4dpi, days post-infection.
a–dMeans within rows with different superscript letters were significantly different (P < 0.05).
Effects of inulin on signaling pathway proteins
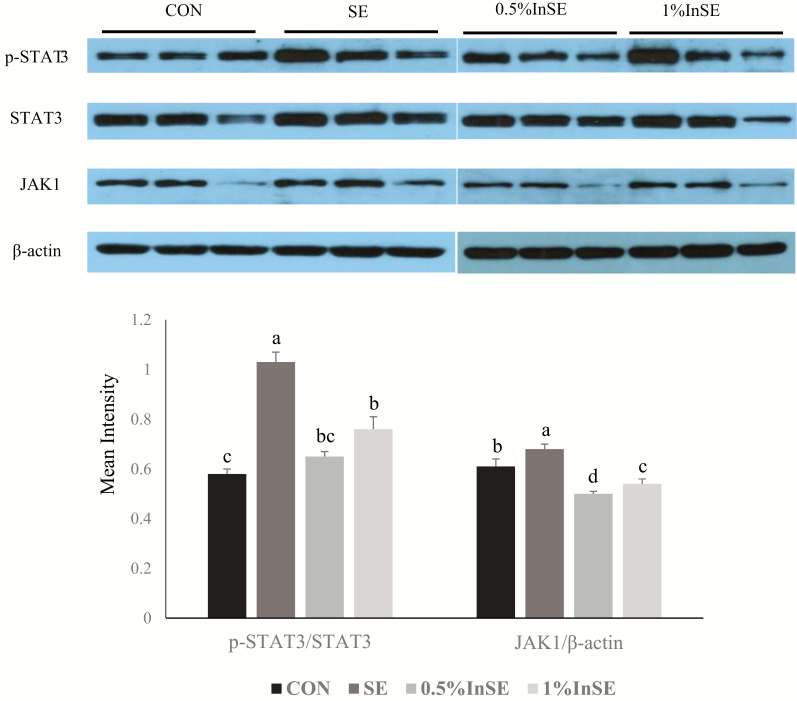
Levels of JAK1/STAT3 signaling pathway proteins in the ileum were determined by western blotting analysis (Figure 3). SE infection with 3 dpi significantly increased p-STAT3 and JAK1 protein expression levels compared with the CON group (P < 0.05), and these increases were significantly suppressed by inulin (0.5% and 1%) (P < 0.05).
Figure 3.
Protein expression levels of p-STAT3 and JAK in ileum of SE-infected chickens at 3 d post-infection. Different superscripts above bars indicate significant differences (P < 0.05). All data expressed as mean ± standard error (n = 3). JAK, Janus kinase; STAT3, signal transducer and activator of transcription 3; p-STAT3, phospho-STAT3.
Discussion
The modern poultry industry aims to provide high-quality and efficient animal protein for human consumption. However, Salmonella infections pose a major challenge to poultry production worldwide, with important implications for food security and human health, including via the production of subclinical infections in the avian intestine (Micciche et al., 2018). Even in the absence of clinical signs of infection, chronic, persistent Salmonella infection may damage the integrity of the gut mucosal barrier and disrupt intestinal homeostasis (Schultz et al., 2018). Salmonella can be recognized by pattern recognition receptors in the epithelial cells, resulting in the production of inflammatory cytokines such as ILs, tumor necrosis factor (TNF)-α, and IFN-γ, which disrupt the gut mucosal homeostasis among the microbial community, epithelial cells, and the gut immune system (Ibrahim et al., 2018). Salmonella thus triggers an inflammatory immune response, which in turn affects gut mucosal barrier integrity. Many reports showed that SE-challenged chickens exhibited higher IFN-γ expression in spleen after 18-h infection (Cheeseman et al., 2007) and higher TLR4, IL-8 expression in cecum after 48-h infection (Tohidi et al., 2013). In the present study, we showed that Salmonella challenge at 1- and 3-dpi significantly upregulated inflammatory cytokine genes such as IL-1β, IL-6, LITAF, and IFN-γ and downregulated Claudin-1 and MUC2. IFN-γ has been shown to play a key role in the resolution of intestinal Salmonella infections (Dolowschiak et al., 2016). Moreover, chronic intestinal inflammation supports Salmonella colonization and helps it to compete against the resident microbiota, leading to the persistence of Salmonella in the intestine. Although traditional antibiotic therapies can effectively eliminate the pathogen, they cannot eliminate the mucosal inflammation and may lead to immune tolerance and chronic toxicity. Therefore, the present study demonstrated that the inulin could effectively eliminate the susceptibility to intestinal inflammation and enhance the immune response against Salmonella in intestinal tissues, which provides a new strategy to prevent SE-infection in poultry production (Micciche et al., 2018; Tran et al., 2018).
Inulins have been shown to be effective in preventing SE infection by modulating the intestinal inflammation that allows SE to colonize and propagate (Teng et al., 2018; Tran et al., 2018). In general, intestinal inflammation provides Salmonella with a metabolic advantage over the resident microorganisms. Inulin could thus be used to prevent SE infection by reducing the expression of pro-inflammatory cytokines. The present results showed that inulin had the potential to reduce the gene expression levels of pro-inflammatory cytokines such as LITAF, IFN-γ, IL-6, and IL-1β, which play essential roles in controlling intestinal inflammation (Whelan, 2011; Vogt et al., 2015; Ferenczi et al., 2016; Kareem et al., 2017). Moreover, inulin-type fructans have been shown to possess anti-inflammatory properties and to increase the activity of goblet cells and the thicker mucus layer (Bhatia et al., 2015). Inulin might thus improve the intestinal inflammatory environment to make it unfavorable for Salmonella colonization.
Furthermore, many studies have shown that inulin strengthens the intestinal barrier function to limit invasion and colonization by SE pathogens by activating the expression of MUC2, Claudin-1, and sIgA (Awad et al., 2017; Genda et al., 2017). sIgA acts as a first-line barrier to protect the intestinal mucosal surface against invading pathogens by inhibiting their attachment to the gut epithelium (Adhikari et al. 2018). The present study showed that serum sIgA and IgG, which play important roles in protecting against SE infection, were increased in chickens fed with inulin (Shao et al., 2016). This also confirmed that inulin enhanced sIgA levels to ameliorate SE-induced damage to the gut barrier by downregulation of pro-inflammatory cytokines such as TNF-ɑ, IFN-γ, nuclear factor-κB, and IL-6 (Janardhana, et al., 2009; Mantis et al., 2011; Adhikari et al. 2018). MUC2 and Claudin-1 are also important intestinal epithelial barrier components that prevent harmful substances from reaching the surface of the epithelium. The function of inulin modification of epithelial tight junction integrity is attributed to the expression of tight junction proteins and secretion of mucus protein (Wu et al., 2017a). The immunomodulatory effect of inulin on the gene expression levels of MUC2 and claudin-1 may be associated with the secretion of the pro-inflammatory cytokines LITAF, IL-6, which in turn effects the expression of tight junction proteins (Poritz et al., 2011; Garcia-Hernandez et al., 2017). Therefore, inulin exerted beneficial effects on the integrity of the gut barrier, increased the height of microvilli, and alleviated the damage to the mucosal morphology caused by SE (Teng et al., 2018), which is consistent with the present results. Therefore, inulin thus appears to repair SE-induced damage to the mucosal morphology via its effects on pro-inflammatory cytokine expression.
Inulin may directly regulate host mucosal immune signaling to modulate the effects of SE infection. Several reports showed that inulin protected the intestinal epithelial barrier and dampened bacterial infection by modulating protein kinase C and mitogen-activated protein kinase signaling in the intestinal epithelium (Wu et al.,2017a, 2017b). Inulin-type fructans could be directly recognized by TLRs, NODs, and CLRs on the surface of immune cells (B cells, T cells, and NK cells) and thus elicit an immune regulation response by increasing the levels of serum IgA, IgG, and IgM antibodies (Seifert et al., 2007; Janardhana, et al., 2009; Adhikari et al., 2018). The present study also showed that inulin (0.5% and 1%) increased serum IgA and IgG levels compared with control groups. We previously demonstrated that TLR4 signaling plays a key role in resistance to Salmonella infection in poultry (Li et al., 2017). We also showed that SE challenge increased TLR4 mRNA expression levels, which play an important role in regulating mucosal immune responses, mucosal barrier function, and inflammatory cytokine expression in the gut (de Kivit et al., 2014; Adhikari et al., 2019). Inulin may directly modulate the proinflammatory cytokine production and epithelial cell kinase signaling via upregulation of TLR-4 expression (Capitán-Cañadas et al., 2014; de Kivit et al., 2014; Ortega-González et al., 2014; Lehmann et al., 2015; Adhikari et al., 2018). These results suggest that prebiotics may directly activate TLR signaling to modulate cytokine expression and epithelial barrier integrity to regulate the effects of SE infection (Capitan-Canadas et al., 2014; Wu et al., 2017a).
But some researcher also showed that inulin modulates the host gut inflammatory response and intestinal health via beneficial intestinal microbes including Bifidobacteria and Lactobacilli and the end-products of fermentation of inulins, such as SCFAs, mainly acetate, propionate, and butyrate (Bhatia et al., 2015; Wu et al., 2017a), which exert important regulatory functions in intestinal epithelium cell proliferation and anti-inflammatory properties in the intestinal mucosa (Ferreira et al., 2014). Song et al (2018) also showed that inulin significantly increased serum butyrate and acetate levels. Butyrate is the main fuel source for the growth of intestinal epithelium cells, which exert anti-inflammatory effects by decreasing the expression of pro-inflammatory cytokines (Ferreira et al., 2014). Therefore, inulin exerted beneficial effects on the integrity of the gut barrier, increased the height of microvilli, and alleviated the damage to the mucosal morphology caused by SE (Teng et al., 2018). Several reports have shown that SCFAs, especially butyrate, stimulate the growth of Bifidobacteria and Lactobacilli, which in turn promote an increase in SCFAs and enhance the mucosal immune function (Ricke, 2015).
The JAK/STAT signaling pathway not only plays an important role in the expression of cytokine genes, but can also be activated by many cytokines (Murray et al., 2007). The induction of proinflammatory cytokines (such as IL6 and IFN-γ) by SE infection may thus activate the JAK/STAT signaling pathway and increase p-STAT protein expression (Hotson et al., 2016; Kogut et al., 2016; Ibrahim et al., 2018a), consistent with the present results. Aberrant activation of the JAK/STAT signaling pathway disrupts the immune and inflammatory responses (Murray et al., 2007; Villarino et al., 2015). Inulin alleviated the inflammatory response in SE-infected chickens by inhibiting JAK1/STAT3 signaling pathways, which in turn regulated the intestinal mucosal immune and inflammatory responses to mitigate SE infection (Liu et al., 2010). SOCS3 has been demonstrated to be involved in the negative feedback regulation of the JAK/STAT signaling pathway (Liu et al., 2017). Accordingly, increased SOCS3 gene expression levels in the inulin groups were associated with inhibition of JAK1/STAT3 expression. JAK1/STAT3 inhibition also has the potential to increase TLR-mediated production of pro- and anti-inflammatory cytokines (Wang et al., 2013), which may also help us to explain the role of inulins in controlling SE-infection in chickens intestines.
In conclusion, our results indicated that Salmonella challenge destroyed the mucosal barrier function and caused gut inflammation, which in turn supported Salmonella colonization in the gut in chickens. Inulin alleviated SE-induced gut injury by directly decreasing the proinflammatory response and enhancing mucosal immunity in chickens. In the following research, we will study the effects of metabolites (such as SCFA) or microbiota on the immunity and gut barrier of SE-infected chickens. This study also indicated that inulins, as important prebiotics, may represent promising new therapeutic candidates for manipulating the mucosal immune responses and gut SE infection in chickens.
Acknowledgments
We thank Jin Zhang, Fei Wang, Bo Zhu, and Siyuan Xing in my lab for their assistance in the process of animals feeding and samples collection. We thank the College of Animal Science and Technology (China Agricultural University, Beijing, China) that provided sterile isolation chamber for SPF chicken feeding. We also thank W. Bruce Currie (the Emeritus Professor, Cornell University, USA) for his contributions to presentation and statistical suggestions on the manuscript. This research was supported by the National Key Technology R&D Program (2015BAD03B03), China Agriculture Research System (CARS-41), and the Agricultural Science and Technology Innovation Program (ASTIPIAS04 and CAAS-XTCX2016010-03).
Conflict of interest statement. None declared.
Literature cited
- Adhikari P., Cosby D. E., Cox N. A., Franca M. S., Williams S. M., Gogal R. M. Jr, Ritz C. W., and Kim W. K.. . 2018. Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella enteritidis. Poult. Sci. 97:2525–2533. doi: 10.3382/ps/pey101 [DOI] [PubMed] [Google Scholar]
- Adhikari P., Lee C. H., Cosby D. E., Cox N. A., and Kim W. K.. . 2019. Effect of probiotics on fecal excretion, colonization in internal organs and immune gene expression in the ileum of laying hens challenged with Salmonella Enteritidis. Poult. Sci. 98:1235–1242. doi: 10.3382/ps/pey443 [DOI] [PubMed] [Google Scholar]
- Awad W. A., Hess C., and Hess M.. . 2017. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. 9:E60. doi: 10.3390/toxins9020060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia S., Prabhu P. N., Benefiel A. C., Miller M. J., Chow J., Davis S. R., and Gaskins H. R.. . 2015. Galacto-oligosaccharides may directly enhance intestinal barrier function through the modulation of goblet cells. Mol. Nutr. Food Res. 59:566–573. doi: 10.1002/mnfr.201400639 [DOI] [PubMed] [Google Scholar]
- Bucław M. 2016. The use of inulin in poultry feeding: a review. J. Anim. Physiol. Anim. Nutr. (Berl). 100:1015–1022. doi: 10.1111/jpn.12484 [DOI] [PubMed] [Google Scholar]
- Capitán-Cañadas F., Ortega-González M., Guadix E., Zarzuelo A., Suárez M. D., de Medina F. S., and Martínez-Augustin O.. . 2014. Prebiotic oligosaccharides directly modulate proinflammatory cytokine production in monocytes via activation of TLR4. Mol. Nutr. Food Res. 58:1098–1110. doi: 10.1002/mnfr.201300497 [DOI] [PubMed] [Google Scholar]
- Castillo N. A., de Moreno de LeBlanc A., Galdeano C. M., and Perdigón G.. . 2012. Probiotics: an alternative strategy for combating salmonellosis:Immune mechanisms involved. Food Res. Int. 45:831–841. doi: 10.1016/j.foodres.2011.04.031 [DOI] [Google Scholar]
- Cheeseman J. H., Kaiser M. G., Ciraci C., Kaiser P., and Lamont S. J.. . 2007. Breed effect on early cytokine mRNA expression in spleen and cecum of chickens with and without Salmonella enteritidis infection. Dev. Comp. Immunol. 31:52–60. doi: 10.1016/j.dci.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Chen K., Chen H., Faas M. M., de Haan B. J., Li J., Xiao P., Zhang H., Diana J., de Vos P., and Sun J.. . 2017. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 61:1601006. doi: 10.1002/mnfr.201601006 [DOI] [PubMed] [Google Scholar]
- Dankowiakowska A., Kozłowska I., and Bednarczyk M.. . 2013. Probiotics, prebiotics and snybiotics in Poultry–mode of action, limitation, and achievements. J. Cent. Eur. Agr. 14:46. doi: 10.5513/JCEA01/14.1.1222 [DOI] [Google Scholar]
- Dolowschiak T., Mueller A. A., Pisan L. J., Feigelman R., Felmy B., Sellin M. E., Namineni S., Nguyen B. D., Wotzka S. Y., Heikenwalder M., . et al. 2016. IFN-γ hinders recovery from mucosal inflammation during antibiotic therapy for salmonella gut infection. Cell Host Microbe. 20:238–249. doi: 10.1016/j.chom.2016.06.008 [DOI] [PubMed] [Google Scholar]
- Ferenczi S., Szegi K., Winkler Z., Barna T., and Kovács K. J.. . 2016. Oligomannan prebiotic attenuates immunological, clinical and behavioral symptoms in mouse model of inflammatory bowel disease. Sci. Rep. 6:34132. doi: 10.1038/srep34132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira C. M., Vieira A. T., Vinolo M. A., Oliveira F. A., Curi R., and Martins F. d. o. s. S.. . 2014. The central role of the gut microbiota in chronic inflammatory diseases. J. Immunol. Res. 2014:689492. doi: 10.1155/2014/689492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hernandez V., Quiros M., and Nusrat A.. . 2017. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 1397:66–79. doi: 10.1111/nyas.13360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genda T., Sasaki Y., Kondo T., Hino S., Nishimura N., Tsukahara T., Sonoyama K., and Morita T.. . 2017. Fructo-oligosaccharide-induced transient increases in cecal immunoglobulin a concentrations in rats are associated with mucosal inflammation in response to increased gut permeability. J. Nutr. 147:1900–1908. doi: 10.3945/jn.117.253955 [DOI] [PubMed] [Google Scholar]
- Hotson A. N., Gopinath S., Nicolau M., Khasanova A., Finck R., Monack D., and Nolan G. P.. . 2016. Coordinate actions of innate immune responses oppose those of the adaptive immune system during Salmonella infection of mice. Sci. Signal. 9:ra4. doi: 10.1126/scisignal.aaa9303 [DOI] [PubMed] [Google Scholar]
- Ibrahim H., Askar B., Barrow P., and Foster N.. . 2018. Dysregulation of JAK/STAT genes by vasoactive intestinal peptide (VIP) in Salmonella-infected monocytes may inhibit its therapeutic potential in human sepsis. Cytokine 105:49–56. doi: 10.1016/j.cyto.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Ibrahim H., Askar B., Hulme S., Neilson P., Barrow P., and Foster N.. . 2018a. Differential immune phenotypes in human monocytes induced by non-host-adapted salmonellaenterica serovar choleraesuis and host-adapted S. typhimurium. Infect. Immun. 86:e00509–18. doi: 10.1128/IAI.00509-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janardhana V., Broadway M. M., Bruce M. P., Lowenthal J. W., Geier M. S., Hughes R. J., and Bean A. G.. . 2009. Prebiotics modulate immune responses in the gut-associated lymphoid tissue of chickens. J. Nutr. 139:1404–1409. doi: 10.3945/jn.109.105007 [DOI] [PubMed] [Google Scholar]
- Johnson L. P., Walton G. E., Psichas A., Frost G. S., Gibson G. R., and Barraclough T. G.. . 2015. Prebiotics modulate the effects of antibiotics on gut microbial diversity and functioning in vitro. Nutrients 7:4480–4497. doi: 10.3390/nu7064480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareem K. Y., Loh T. C., Foo H. L., Asmara S. A., and Akit H.. . 2017. Influence of postbiotic RG14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poult. Sci. 96:966–975. doi: 10.3382/ps/pew362 [DOI] [PubMed] [Google Scholar]
- Kirk M. D., Pires S. M., Black R. E., Caipo M., Crump J. A., Devleesschauwer B., Dӧpfer D., Fazil A., Fischer-Walker C. L., Hald T., . et al. 2015. World health organization estimates of the global and and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med. 12:e1001921. doi: 10.1371/journal.pmed.1001921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kivit S, Tobin M. C., Forsyth C. B., Keshavarzian A., and Landay A. L.. 2014. Regulation of intestinal immune responses through TLR activation: implications for pro- and prebiotics. Front. Immunol. 5:60. doi: 10.3389/fimmu.2014.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M. H., Swaggerty C. L., Byrd J. A., Selvaraj R., and Arsenault R. J.. . 2016. Chicken-specific kinome array reveals that salmonella enterica serovar enteritidis modulates host immune signaling pathways in the cecum to establish a persistence infection. Int. J. Mol. Sci. 17:1207. doi: 10.3390/ijms17081207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S., Hiller J., van Bergenhenegouwen J., Knippels L. M., Garssen J., and Traidl-Hoffmann C.. . 2015. In vitro evidence for immune-modulatory properties of non-digestible oligosaccharides: direct effect on human monocyte derived dendritic cells. PLoS One. 10(7):e0132304. doi: 10.1371/journal.pone.0132304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Wang H., Zhao X., Gou Z., Liu R., Song Y., Li Q., Zheng M., Cui H., Everaert N., Zhao G., and Wen J.. . 2017. Allelic variation in TLR4 is linked to resistance to Salmonella Enteritidis infection in chickens. Poult. Sci. 96:2040–2048. doi: 10.3382/ps/pex010 [DOI] [PubMed] [Google Scholar]
- Liu B. H., and Cai J. P.. . 2017. Identification of transcriptional modules and key genes in chickens infected with salmonella enterica serovar pullorum using integrated coexpression analyses. Biomed. Res. Int. 2017:8347085. doi: 10.1155/2017/8347085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lu R., Xia Y., Wu S., and Sun J.. . 2010. Eukaryotic signaling pathways targeted by Salmonella effector protein AvrA in intestinal infection in vivo. BMC Microbiol. 10:326. doi: 10.1186/1471-2180-10-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Mantis N. J., Rol N., and Corthesy B.. . 2011. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4:603–11. doi: 10.1038/mi.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micciche A. C., Foley S. L., Pavlidis H. O., McIntyre D. R., and Ricke S. C.. . 2018. A review of prebiotics against salmonella in poultry: current and future potential for microbiome research applications. Front. Vet. Sci. 5:191. doi: 10.3389/fvets.2018.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. J. 2007. The JAK-STAT signaling pathway: input and output integration. J. Immunol. 178:2623–2629. doi: 10.4049/jimmunol.178.5.2623 [DOI] [PubMed] [Google Scholar]
- Ortega-González M., Ocón B, Romero-Calvo I., Anzola A., Guadix E., Zarzuelo A., Suárez M. D., Sánchez de Medina F., and Martínez-Augustin O.. 2014. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFκB. Mol. Nutr. Food Res. 58:384–393. doi: 10.1002/mnfr.201300296 [DOI] [PubMed] [Google Scholar]
- Poritz L. S., Harris L. R. 3rd, Kelly A. A., and Koltun W. A.. . 2011. Increase in the tight junction protein claudin-1 in intestinal inflammation. Dig. Dis. Sci. 56:2802–2809. doi: 10.1007/s10620-011-1688-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke S. C. 2015. Potential of fructooligosaccharide prebiotics in alternative and nonconventional poultry production systems. Poult. Sci. 94:1411–1418. doi: 10.3382/ps/pev049 [DOI] [PubMed] [Google Scholar]
- Rivera-Chávez F., Zhang L. F., Faber F., Lopez C. A., Byndloss M. X., Olsan E. E., Xu G., Velazquez E. M., Lebrilla C. B., Winter S. E., . et al. 2016. Depletion of butyrate-producing clostridia from the gut microbiota drives an aerobic luminal expansion of salmonella. Cell Host Microbe. 19:443–454. doi: 10.1016/j.chom.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta A. K., Jayapal N., Senani S., Kolte A. P., and Sridhar M.. . 2013. Prebiotic inulin: useful dietary adjuncts to manipulate the livestock gut microflora. Braz. J. Microbiol. 44:1–14. doi: 10.1590/S1517-83822013005000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz B. M., Salazar G. A., Paduro C. A., Pardo-Roa C., Pizarro D. P., Salazar-Echegarai F. J., Torres J., Riedel C. A., Kalergis A. M., Álvarez-Lobos M. M., . et al. 2018. Persistent salmonella enterica serovar typhimurium infection increases the susceptibility of mice to develop intestinal inflammation. Front. Immunol. 9:1166. doi: 10.3389/fimmu.2018.01166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert S., and Watzl B.. . 2007. Inulin and oligofructose: review of experimental data on immune modulation. J. Nutr. 137(11 Suppl):2563S–2567S. doi: 10.1093/jn/137.11.2563S [DOI] [PubMed] [Google Scholar]
- Sevane N., Bialade F., Velasco S., Rebolé A., Rodríguez M. L., Ortiz L. T., Cañón J., and Dunner S.. . 2014. Dietary inulin supplementation modifies significantly the liver transcriptomic profile of broiler chickens. PLoS One 9:e98942. doi: 10.1371/journal.pone.0098942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Wang Z., Tian X., Guo Y., and Zhang H.. . 2016. Yeast β-d-glucans induced antimicrobial peptide expressions against Salmonella infection in broiler chickens. Int. J. Biol. Macromol. 85:573–584. doi: 10.1016/j.ijbiomac.2016.01.031 [DOI] [PubMed] [Google Scholar]
- Song J., Li Q. H., Li P.. Liu R. R., Cui H. X., Zheng M. Q., Nadia E., Zhao G. P., and Wen J.. . 2018. The effects of inulin on the mucosal morphology and immune status of specific pathogen-free chickens. Poult. Sci. 97:3938–3946. doi: 10.3382/ps/pey260 [DOI] [PubMed] [Google Scholar]
- Teng P. Y., and Kim W. K.. . 2018. Review: roles of prebiotics in intestinal ecosystem of broilers. Front. Vet. Sci. 5:245. doi: 10.3389/fvets.2018.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidi R., Idris I. B., Malar Panandam J., and Hair Bejo M.. . 2013. The effects of polymorphisms in 7 candidate genes on resistance to Salmonella Enteritidis in native chickens. Poult. Sci. 92:900–909. doi: 10.3382/ps.2012-02797 [DOI] [PubMed] [Google Scholar]
- Tran T. H. T., Everaert N., and Bindelle J.. . 2018. Review on the effects of potential prebiotics on controlling intestinal enteropathogens Salmonella and Escherichia coli in pig production. J. Anim. Physiol. Anim. Nutr. 102:17–32. doi: 10.1111/jpn.12666 [DOI] [PubMed] [Google Scholar]
- Villarino A. V., Kanno Y., Ferdinand J. R., and O’Shea J. J.. . 2015. Mechanisms of Jak/STAT signaling in immunity and disease. J. Immunol. 194:21–27. doi: 10.4049/jimmunol.1401867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt L., Meyer D., Pullens G., Faas M., Smelt M., Venema K., Ramasamy U., Schols H. A., and De Vos P.. . 2015. Immunological properties of inulin-type fructans. Crit. Rev. Food Sci. Nutr. 55:414–436. doi: 10.1080/10408398.2012.656772 [DOI] [PubMed] [Google Scholar]
- Wang H., Brown J., Gao S., Liang S., Jotwani R., Zhou H., Suttles J., Scott D., and Lamont R. J.. . 2013. The role of janus kinase (JAK)-3 in regulating toll-like receptor-mediated inflammatory cytokine production in innate immune cells. J. Immunol. 191:1164–1174. doi: 10.4049/jimmunol.1203084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan K. 2011. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr. Opin. Clin. Nutr. Metab. Care. 14:581–587. doi: 10.1097/MCO.0b013e32834b8082 [DOI] [PubMed] [Google Scholar]
- Wilson B., and Whelan K.. . 2017. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 32Suppl 1:64–68. doi: 10.1111/jgh.13700 [DOI] [PubMed] [Google Scholar]
- Wu R. Y., Abdullah M., Määttänen P., Pilar A. V., Scruten E., Johnson-Henry K. C., Napper S., O’Brien C., Jones N. L., and Sherman P. M.. . 2017a. Protein kinase C δ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function. Sci. Rep. 7:40820. doi: 10.1038/srep40820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. Y., Määttänen P., Napper S., Scruten E., Li B., Koike Y., Johnson-Henry K. C., Pierro A., Rossi L., Botts S. R., . et al. 2017b. Non-digestible oligosaccharides directly regulate host kinome to modulate host inflammatory responses without alterations in the gut microbiota. Microbiome 5:135. doi: 10.1186/s40168-017-0357-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Song J., Liu L., Luo J., Tian G., and Yang Y.. . 2017. Effect of epigallocatechin gallate on growth performance and antioxidant capacity in heat-stressed broilers. Arch. Anim. Nutr. 71:362–372. doi: 10.1080/1745039X.2017.1355129 [DOI] [PubMed] [Google Scholar]
- Yang G. Q., Yin Y., Liu H. Y., and Liu G. H.. . 2016. Effects of dietary oligosaccharide supplementation on growth performance, concentrations of the major odor-causing compounds in excreta, and the cecal microflora of broilers. Poult. Sci. 95:2342–2351. doi: 10.3382/ps/pew124 [DOI] [PubMed] [Google Scholar]