Abstract
Objective:
Symptoms of psychopathology covary across diagnostic boundaries, and a family history of elevated symptoms for a single psychiatric disorder places an individual at heightened risk for a broad range of other psychiatric disorders. Both twin-based and genome-wide molecular methods indicate a strong genetic basis for the familial aggregation of psychiatric disease. This has led researchers to prioritize the search for highly heritable childhood risk factors for transdiagnostic psychopathology. Cognitive abilities that involve the selective control and regulation of attention, known as executive functions (EFs), are a promising set of risk factors.
Method:
In a population-based sample of child and adolescent twins (n = 1,913, M age = 13.1 years), we examined genetic overlap between both EFs and general intelligence (g) and a transdiagnostic dimension of vulnerability to psychopathology comprising symptoms of anxiety, depression, neuroticism, aggression, conduct disorder, oppositional defiant disorder, hyperactivity and inattention. Psychopathology symptoms were rated by both children and their parents.
Results:
Latent factors representing general EF and g were highly heritable (h2 = 86–92%), and genetic influences on both sets of cognitive abilities were robustly correlated with transdiagnostic genetic influences on psychopathology symptoms (genetic rs ranged from −.20 to −.38).
Conclusion:
Both EF and g robustly index genetic risk for transdiagnostic symptoms of psychopathology in childhood. Delineating the developmental and neurobiological mechanisms underlying observed associations between cognitive abilities and psychopathology remains a priority for ongoing research.
Lay Summary
General intelligence and executive functions were measured in a large (N = 1,913) sample of children and adolescents from the Texas Twin Project. The cognitive abilities were found to index transdiagnostic genetic risk for emotional and behavioral problems, including symptoms of depression, anxiety, attention deficit hyperactivity disorder, and conduct disorder. These results indicate that children with low cognitive abilities are at elevated risk for developing psychopathology. Genetic research on cognitive abilities has the potential to illuminate transdiagnostic mechanisms of risk for psychopathology.
Introduction
Supporting a transdiagnostic and dimensional perspective on psychopathology,1 mental disorders are highly comorbid, and psychiatric symptoms have the strong tendency to co-occur across diagnostic boundaries and across the full (sub-clinical to clinical) range of variation. Such pervasive comorbidity can be represented by a general dimension of liability, p, which indexes a broad tendency to experience an array of psychopathology symptoms across diagnostic categories.2–10 Research relying on both family-based and genomic approaches indicates that p arises in large part from a genetic architecture shared across diagnostic boundaries.4,7,11–13 Identifying heritable traits that can be measured during childhood, when individuals are typically in earlier phases of symptom progression, that index broad genetic vulnerability to psychopathology has therefore become a priority for current psychiatric research.
Poor executive functioning (EF) is one genetically-influenced childhood risk factor that might index vulnerability to multiple forms of psychopathology. Executive functions are supervisory cognitive functions that selectively control and direct attention and that regulate basic cognitive processes.14,15 Major domains of EF include: (a) switching, defined as the ability to shift rapidly between cognitive operations; (b) updating, the ability to monitor incoming stimuli and replace old information with new information; (c) inhibition, the ability to withhold a prepotent response; and (d) working memory, the ability to simultaneously store and manipulate information.16 EFs play a central role in formal models of higher-order reasoning, abstract thinking, and other complex cognitive operations. Indeed, EFs have been proposed as fundamental to maintenance of mental health,17 particularly against a backdrop of stressful or traumatic contexts and life events.
EF deficits are pervasively observed across psychiatric disorders.17 Clinical research has often relied on individual EF measures; however, performance on a single EF task is influenced by a mixture of both executive and nonexecutive factors—an issue known as the “task impurity” problem.18 The task impurity problem can be overcome by using latent variable approaches that extract common executive variance from multiple indicators of each EF domain.19,20 Studies using such an approach have found that multiple EF tests converge on a single, highly heritable factor (h2 > 90%).19,20 Few studies have examined the multidimensional structure of EFs in relation to a broad array of psychiatric symptoms. Nevertheless, research to-date is mostly consistent with conceptualizing deficits in general EF as a transdiagnostic risk factor, rather than as a specific vulnerability to any one disorder.17,21,22 A notable exception is Attention Deficit Hyperactivity Disorder (ADHD), which might be associated with EF deficits even after accounting for comorbid conditions.23–27
Like EF, general intelligence (g) is broadly related to multiple forms of psychopathology, thus potentially contributing to their comorbidity.2,11 Moreover, genetic influences on EF substantially overlap with genetic influences on g, even after accounting for individual differences in more basic cognitive processes, such as speed of information processing.28 Previous studies have established that both EF and intelligence are correlated with specific forms of psychopathology and with a general p factor, but it has not yet been established the extent to which EFs and intelligence account for the genetic vulnerability shared across mental disorders or the extent to which cognitive abilities are associated with psychopathology above and beyond shared genetic influences. The current paper examines this hypothesis using data from a population-based sample (N = 1,913) of child and adolescent twins, ages 8 to 20, who participated in in-laboratory studies of cognitive and psychiatric functioning.
Methods
Participants
The current sample consists of N = 1,913 twins and multiples from N = 937 families from the Texas Twin Project,29 a registry of school-aged twins from the greater Austin and Houston metropolitan areas. Twins and multiples in grades 3–12 were identified from public school rosters and invited to participate in one or more on-going mail-based or lab-based studies. All participants were either currently enrolled in grade school or had graduated high school within the past three months but had not yet left home for college or full-time work. Ages ranged from 7.8 to 20.1 years (M = 13.1 years); less than 4% of the sample was over age 18. The sample was nearly evenly split by sex (51% male participants, 49% female participants). The sample was racially diverse: 58% of the sample identified as non-Hispanic White, 18% as Hispanic/Latino, 11% as African American, 3% as Asian / Asian American, and 10% as another or multiple race/ethnicity. Approximately one-third of families reported having received food stamps or another form of means-tested government assistance at some point since the twins were born.
Of the 937 families, 902 families each had a single pair of twins; 3 families each had 2 sets of twins; 31 families had triplets that each contributed 3 pairwise combinations of individuals; and 1 family had quadruplets that contributed 6 pairwise combinations of individuals, for a total of 1,007 pairs. Zygosity was classified using latent class analysis of twins’, parents’, and research assistants’ ratings of physical similarity and ease of being mistaken for one another.30 Of the 1007 pairs, 188 were monozygotic female-female (MZF) pairs, 166 were monozygotic male-male pairs (MZM), 166 were dizygotic female-female pairs (DZF), 182 were dizygotic male-male pairs (DZM), and 305 were dizygotic male-female pairs (DZO). That is, 35% of pairs were MZ and 65% were DZ.
All participants completed measures of psychopathology and intelligence. A subsample of n = 1,019 younger participants (538 pairwise combinations of individuals from 497 families, including 19 families with triplets and 3 families each with 2 sets of twins), ages 7.8 to 15.3 years old (M = 10.8 years), completed a battery of EF tasks. The EF sub-sample was 55% non-Hispanic White, 16% Hispanic/Latino, 1% Asian/ Asian American, 7% African American, and 21% another or multiple race/ethnicity. One-third (33%) of the EF sub-sample pairs were MZ, and 66% were DZ.
Measures
Psychopathology.
Psychopathology was measured using child self-reports of their own psychopathology symptoms, as well as parent-reports of the child’s symptoms, on (1) abbreviated versions of the Child Behavior Checklist (CBCL),31,32 which measures depression, anxiety, somatic complaints, thought problems, aggression, rule-breaking, hyperactivity, and inattention; (2) the Conner’s 3 rating scales,33 which measures DSM-IV symptoms of Conduct Disorder, Oppositional Defiant Disorder, and Attention Deficit Hyperactivity Disorder; and the neuroticism scale of the Big Five Inventory (BFI),34 which measures anxiety, sadness, and emotional lability. For parent-reports, one parent or caregiver reported on child psychopathology (71% of parent-reports were by biological mothers, 21% by biological fathers, 8% by caregivers with a different relationship to the twins, including adoptive parents, grandparents, aunts/uncles, and older siblings). The number of items for each scale (ranging from 4 to 13 items per scale), sample items, descriptive statistics, reliabilities, and twin correlations for the 10 self-reported and 12 parent-reported symptom scales are provided in Supplementary Table S1, available online. Symptom scale scores for primary analyses were obtained by averaging across non-missing items; log-transforming to reduce positive skew, residualizing for sex, age, age-squared, and dummy-coded race/ethnic group membership; and then standardizing the residuals. Scale scores were adjusted for covariates to prevent bias in behavioral genetic models.
WASI-II.
The Wechsler Abbreviated Scale of Intelligence-II (WASI-II)35 was administered to assess general intelligence. The WASI-II is normed for ages 6 through 89 and has a high short-term test-retest correlation (r = .94).35 The assessment consists of Block Design and Matrix Reasoning subtests to assess visuospatial reasoning, and Vocabulary and Similarities subtests to assess verbal ability (descriptive statistics are provided in Table S2, available online). Visuospatial reasoning and verbal ability scales can be combined to form Full-Scale IQ (FSIQ). FSIQ in the current sample reflects population norms (M = 102.8, SD = 13.7), indicating that the sample is representative of cognitive functioning in the U.S. population.
Executive Functioning.
A 12-task battery was administered to measure four EF domains: (1) Inhibition, the ability to stop or prevent a prepotent behavior; (2) Switching, the ability to shift attention across task rules or stimulus features; (3) Working Memory, the ability to process and store information simultaneously; and (4) Updating, the ability to monitor incoming stimuli and replace old information with new information. Descriptive statistics for EF variables are provided in Table S2, available online. Detailed descriptions of each EF task can be found elsewhere.20,28 A brief summary follows:
Inhibition was assessed using Animal Stroop,36 Stop Signal,37,38 and Mickey (an anti-saccade paradigm39). For Stroop and Mickey tasks, inhibition cost was calculated as the difference in response times between inhibit and non-inhibit trial types. For the Stop Signal task, “go” and “stop” trials were dynamically presented to estimate the speed with which a person could prevent a pre-potent response. Stop signal reaction time (SSRT) was calculated by Block scores were averaged, after excluding scores on the basis of consistent stop failures, misidentification of arrow direction, failure to respond to “go” trials, and low SSRTs.40
Switching was assessed using Trail Making, Local-Global, and Plus-Minus tasks.16,41 Each task contained non-switch trials (e.g., connecting letters alphabetically in Trail Making) and switch trials (e.g., connecting letters and numbers in an alternating fashion), and switching costs were measured using response time differences between trial types.
Working memory was assessed using Digit Span Backward, Symmetry Span, and Listening Recall.42–44 Tasks required storing and manipulating numerical, spatial, and verbal information, respectively. Number of items correctly recalled was the measure of performance.
Updating was assessed using 2-Back, Keeping Track, and Running Memory for Letters. 16,45,46 While stimulus presentation continued, participants were asked to maintain the most recent stimuli from one or more specified sets in working memory. For the latter two tasks, performance was assessed as the number of items correctly recalled. For 2-Back, performance was assessed as the number of true matches minus false alarms (i.e., incorrectly identifying non-matches).
Statistical Analyses
Zero-order correlations among all measures are provided in Table S3, available online. Data were analyzed using Mplus version 7.1 (Muthén & Muthén, 1998–2015). For phenotypic analyses that treated each individual as a case, standard errors and model fit statistics were corrected for nesting within families using cluster robust standard errors. For behavioral genetic analyses, triplets were weighted 0.5 and quadruplets were weighted 0.33, to correct for each triplet’s/quadruplet’s representation in more than one pair, and standard errors and model fit statistics were corrected for nesting of pairs within triplet and quadruplet sets using cluster robust standard errors. The fits for all reported models were good (RMSEA < .08, SRMR < .05, CFI >.90, TLI > .90; fit statistics provided in Table S4, available online). To guard against false positives resulting from multiple testing, we used a false discovery rate correction (FDR) for 79 statistical tests and present q-values (FDR-adjusted p-values).47 Tests with a q-value < .05 (corresponding to a p-value < .017) are described as “significant.”
We conducted four sets of analyses. First, we aimed to replicate previous work finding that a general factor, p, can represent covariation among symptoms of different forms of psychopathology. For each reporter separately, we fit a bifactor model that allowed each symptom scale to load on both a general p-factor and on one or more domain-specific factors (Attention Problems, Externalizing, or Internalizing). Refined models added residual covariances as suggested by modification indices. (Structural models of psychopathology were adapted prior to incorporating cognitive measures, in order to minimize bias in the estimates of psychopathology-cognitive ability associations and their standard errors.) The best-fitting models for each reporter were then combined in a single model, in order to estimate correlations across reporters for the Internalizing, Externalizing, Attention Problems, and p factors, and for the residual variances in the observed symptom scales.
Second, phenotypic associations with cognitive abilities were examined separately by reporter. WASI subtests were modeled as indicators of a g factor, with residual covariances estimated between the two verbal ability tests (Vocabulary and Similarities) and between the two visuospatial reasoning tests (Matrix Reasoning and Block Design; Table S4, available online). For latent factor models of g, subtest scores were residualized for age, sex, and race/ethnicity prior to model fitting. The hierarchical factor model of EF was specified as in our previous publications with these data (Figure S1, available online).20,28 This factor structure has been found to be invariant across younger (< 11 years old) versus older (> 11 years old) participants.20 EF task scores were residualized for sex and race/ethnicity prior to model fitting. Sensitivity analyses probed whether there were non-linear or domain-specific associations between cognitive abilities and p.
Third, we fit biometric models that use information on the relative similarity of MZ versus DZ twins to decompose the variances in and covariances among EF, g, and p.48 Biometric models capitalize on the difference in the genetic relationship between MZ twins and DZ twins, in order to decompose variation in a phenotype into three latent components. A, or additive genetic variation, reflects the extent to which more genetically similar people (MZ twins vs. DZ twins) are more phenotypically similar. The ratio of A variance to the total variance in a phenotype is its heritability (h2). C, or shared environmental variation, reflects the extent to which children raised in the same home are phenotypically similar, regardless of their genetic relationship. Finally, E variance reflects the extent to which even MZ twins differ in their phenotypes. The biometric models fit in this paper were applied to latent variables, so E does not reflect MZ differences due to measurement error.
Previous analyses of EF in this sample found that shared environmental (C) influences on EF (at all levels of the hierarchical model) were negligible and could be omitted.20,28 As preliminary analyses, we fit separate biometric models to data on (1) general intelligence, (2) parent-reported psychopathology, and (3) self-reported psychopathology. Best-fitting biometric models were then combined in pairwise models that estimated the genetic and environmental correlations between p (self- and parent-reported) and cognitive abilities (g and EF).
Results
A Transdiagnostic Dimension of Psychopathology Captures Substantial Symptom Variation and Converges Across Reporters
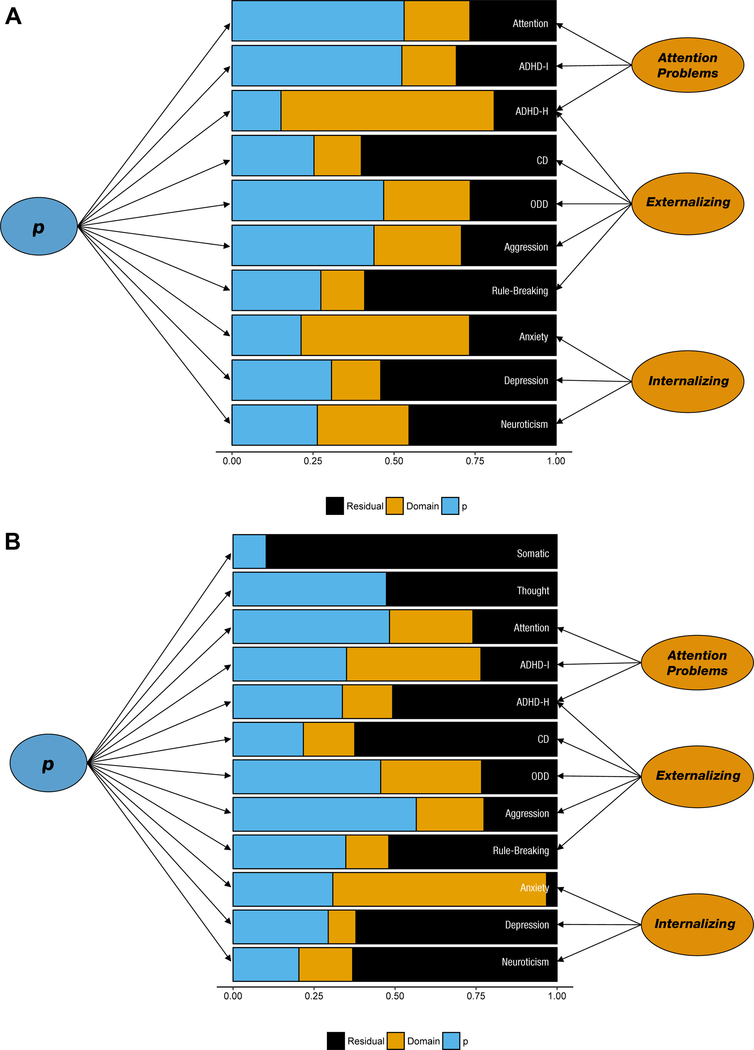
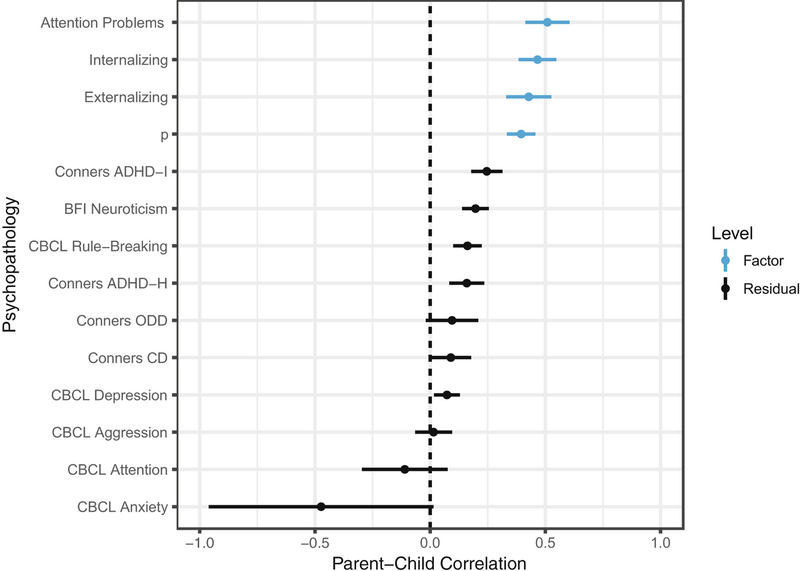
Results from the p-factor models indicate that up to half of the variance in each scale was general across psychopathology domains rather than unique (Figure 1). All factor loadings were significantly different from zero (Table S5–S6, available online). Model fit was improved by allowing for residual covariances between self-reported CBCL Rule-Breaking and Conner’s Conduct Disorder and between parent-reported CBCL Withdrawn symptoms and Conner’s ADHD Hyperactivity. Agreement between children and their parents was moderate at the factor level (all ps < .0005, q-values < .002, Figure 2): Internalizing r = .47 (SE = .04); Externalizing r = .43 (SE = .05); Attention Problems r = .51 (SE = .06), p-factor r = .40 (SE = .03). Relative to parent-child agreement at the factor level, parent-child correlations for residual variances were minimal (median = .10; Figure 2; Table S7, available online).
Figure 1. Proportions of Variance Due to General Factor (p), Domain-Specific Factors, and Unique Residual Variance.
Note: (A) Parent-reported psychopathology. (B) Self-reported psychopathology. Proportion of variance calculated from results of bifactor models. The variance in each symptom scale is divided into three components: (1) shared with all other forms of psychopathology (p), (2) shared with other symptom scales within the internalizing domain, and (3) unique to that particular symptom scale. Between 20% and 50% of the variance in each symptom scale was attributable to the p factor. Factor loading estimates can be found in Supplementary Tables 5 and 6.
Figure 2. Correlations between Parent-Reported and Self-Reported Psychopathology.
Note: Parents and their children showed moderate agreement on whether the child was experiencing psychopathology in general, but modest agreement regarding specific symptom scales. Bands represent 95% confidence intervals around the point estimates of parent-child correlations. Estimates also reported in Supplementary Table 7.
Youth with Higher Cognitive Abilities Have Lower Transdiagnostic Vulnerability to Psychopathology
General intelligence.
There was a negative association between p and g (parent-report: r = −.21, SE = .04, p < .0005, q = .002; self-report: r = −.21, SE = .04, p < .0005, q = .002). When restricting the analysis to the younger sub-sample for whom EF data was also available, this correlation was unchanged (parent-report: r = −.23, SE = .05, p < .0005, q = .002; self-report: r =−.21, SE = .05, p < .0005, q = .002).
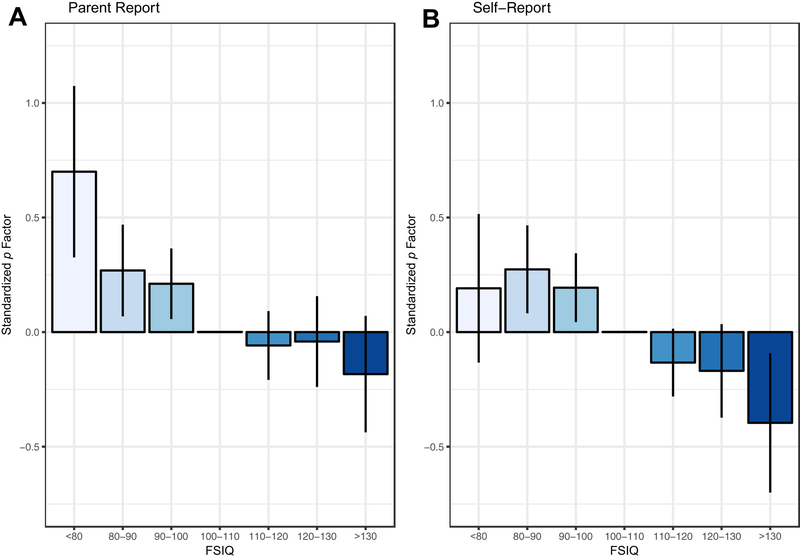
Illustrating this association using FSIQ bins (Figure 3) suggested reporter-specific non-linearity in the relationship between g and p: Parent-reported psychopathology was particularly elevated for children with very low intelligence (FSIQ < 80), but this elevation was not evident in the children’s own reports of psychopathology. Modeling this non-linear association with a quadratic regression in the full sample revealed evidence for a quadratic effect of g on parent-reported p (linear β = −0.24, SE = .048, p < .0005; quadratic β = 0.13, SE = .05, p = .007, q =.024). In contrast, there was no evidence for a quadratic relationship between g and child-reported p (linear β = −0.22, SE = .04, p < .0005; quadratic β = 0.02, SE = .041, p = 0.64, q =.77).
Figure 3. Parent- and Self-Reported Psychopathology by Full-Scale IQ Bins.
Note: Children experience fewer symptoms of psychopathology with increasing intelligence. Lines represent 95% confidence intervals around the means. Factor mean fixed to zero in average IQ category (100–110) for model identification. FSIQ = Full-scale Intelligence Quotient.
Despite the broad age range of the sample, there was no evidence for an interaction between g and age in predicting either parent-reported p (β = −0.010, SE = .012, p = .432, q =.614) or self-reported p (β = −0.013, SE = .013, p = .316, q = .52). Subsequent models tested whether, above and beyond the general tendency for more intelligent youth to have a lower vulnerability to psychopathology, intelligence was uniquely associated with certain symptom domains. For parent-reported symptoms, higher g was uniquely associated only with lower Attention Problems (β = −0.185, SE = .050, p < .0005, q = .002). For self-reported symptoms, higher g was uniquely associated with higher Internalizing (β = 0.112, SE = .046, p = .016, q = .049). Finally, sensitivity analyses indicated that visuospatial reasoning and verbal ability had equivalent associations with p and with specific symptom domains (Table S8, available online).
Executive functions.
Youth with better overall EF had a lower general vulnerability to psychopathology (parent-report: r = −.26, SE = .05, p < .0005, q = .002; self-report: r = −.29, SE =.04, p < .0005, q = .002). As with g, we tested for non-linear associations between EF and p using a quadratic model. There was a significant linear (β = −.41, SE = .06, p < .0005) and quadratic (β = .24, SE = .05, p < .0005, q = .002) association between EF and parent-reported p, such that the strongest relationships with psychopathology were observed for the low range of EF abilities. In contrast, the relationship between EF and self-reported psychopathology was only linear (linear β = −.28, SE = .05, p < .0005; quadratic β = −.001, SE = .03, p = .99, q = .995). There was no significant evidence for an interaction between EF and age in predicting either parent-reported p (β = −0.010, SE = .032, p = .753, q = .859) or self-reported p (β = 0.053, SE =.023, p = .023, q = .065).
As was observed for g, models testing unique associations with symptom domains found evidence that parent-reported Attention Problems had a unique negative association with general EF (r = −.185, SE = .068, p = .007, q = .024). There were no significant unique associations between general EF and any domain-specific factor of self-reported psychopathology. Finally, in each of four separate models, general EF and one domain-specific EF factor were entered as simultaneous predictors of the psychopathology factors. No domain-specific EF factor predicted any form of psychopathology, either self- or parent-reported, above and beyond the effect of general EF (all ps > .05, all qs > .14).
Genetic Influences on Cognitive Abilities Confer Transdiagnostic Vulnerability to Psychopathology
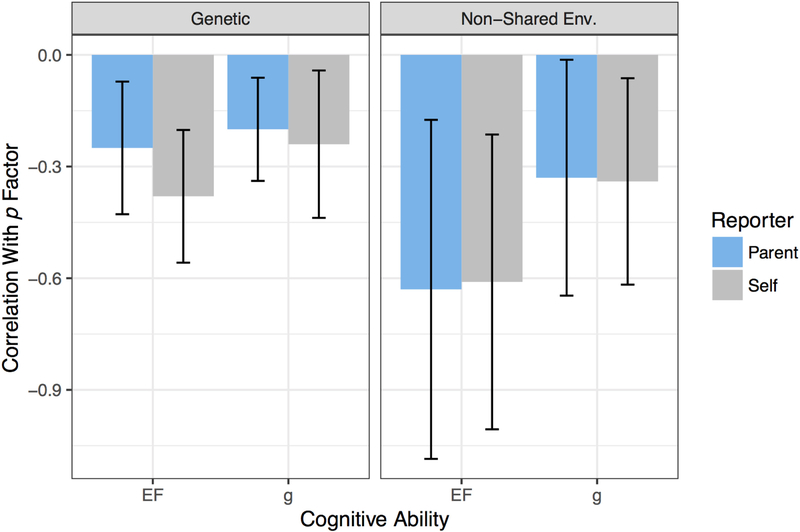
Results from biometric models of each phenotype are shown in Supplemental Figures 1–4, available online. At the level of the latent construct, all phenotypes showed substantial heritability (h2): EF = 92%; g = 86%, parent-reported p = 72%, self-reported p = 49%). These heritability estimates are higher than commonly reported in the twin literature because they decompose variation in latent factors, and so are now downwardly biased by measurement error.49 As shown in Figure 4, there were negative and significant genetic correlations between EF and p (self-report: rA = −.38, SE = .09, p < .0005 , q = .002; parent-report: rA = −.25, SE =.09, p = .004, q = .016) and between g and p (self-report: rA = −.24, SE = .10, p = .008 q = .026; parent-report: rA = −.20, SE = .07, p = .013, q = .041).
Figure 4. Genetic and Environmental Associations between p, g, and EF.
Note: Cognitive abilities are associated with lower psychopathology due to correlated genetic influences but remain associated even comparing within MZ twin pairs (non-shared environmental correlations). Error bars show 95% confidence interval. g = general intelligence; EF = executive functions; p = general factor of psychopathology.
Additionally, even though MZ twins differed only modestly in their EF abilities, as represented by the non-shared environmental component of variance (e2 = 8%), these within-MZ-twin pair differences were reliably associated with differences in parent-reported and self-reported psychopathology, as indicated by the significant non-shared environmental correlations between EF and p-factors (self-report: rE = −.61, SE = .20, p = .003 , q = .012; parent-report: re = −.63, SE = .23, p = .005, q = .019). This result indicates that the inverse relation between EF and psychopathology is not entirely due to genetic influences on both sets of phenotypes. In contrast to what was observed for EF, the non-shared environmental correlations with g were not reliably different from zero (parent-report: rE = −.33, SE = .16, p = .041, q = .108; self-report: rE = −.34, SE = .14, p = .018, q = .053). That said, given the high heritability of general EF, the phenotypic associations between cognitive abilities and p were primarily genetically mediated.
Discussion
In a population-based sample of child and adolescent twins, we investigated associations between both executive functioning (EF) and general intelligence (g) and a transdiagnostic vulnerability to symptoms of internalizing, externalizing, and attention-deficit psychopathology (p). Relations with p were highly consistent across cognitive abilities, and the pattern of genetic correlations was pervasive across all investigated forms of psychopathology. Thus, just as previous epidemiological work has established lower childhood cognitive ability as a robust risk factor for medical disease across the lifespan,50 our findings extend this pattern of disease sequelae of low childhood cognitive abilities to symptoms of psychopathology distributed across a broad range of domains.
The inverse phenotypic associations between cognitive abilities and p were evident across the full range of the ability distribution and were primarily the result of overlapping genetic architecture. That is, genetic variants related to low EF and low general intelligence also confer general vulnerability to a child and adolescent psychiatric symptomology. Insights from molecular genetic research will further advance understanding of the mechanisms that generate genetic correlations between mental health and cognitive abilities. First, as genetic discoveries from GWAS of psychiatric disorders and cognitive abilities continue to accelerate, methods that leverage GWAS summary statistics to test directional causal hypotheses will become more powerful.51–53 Second, polygenic scores, in combination with well-phenotyped longitudinal data from child and adolescent samples, would allow researchers to trace how genetic risk for adult psychiatric disorders is prospectively associated with the development of cognitive abilities, and similarly how genetic risks for low cognitive ability is associated with the emergence of mental health problems.54
High cognitive ability has been proposed to index an individual’s “system integrity,” i.e., the overall quality of the body’s physiological functioning at the intracellular, cellular, or visceral levels that contributes to the general ability of an organism to resist disease and respond to environmental challenges.55 Such a perspective is consistent with a “watershed” model of genetic architecture,56 which positions complex, integrative traits like psychiatric diseases and intelligence as “downstream” phenotypes that are influenced by multiple, progressively narrow “upstream” processes (as in tributaries to a river). Not only are complex traits thus expected to be highly polygenic, as variants affecting the function of any upstream process will ultimately affect a complex emergent system, but each upstream process is also expected to contribute to multiple complex downstream traits, resulting in widespread pleiotropy.
The relationship between cognitive abilities and psychopathology might therefore not involve a direct causal or mechanistic relationship between the two. Rather, both might be complex representations of brain function that have overlapping genetic etiologies because they rely on similar physiological functions. As a specific example, the CADM2 gene encodes the cell adhesion molecule 2, which is involved in cells attaching to other cells and is critical for the organization of neuronal synapses. This “upstream” process (cell adhesion) is relevant for an array of complex phenotypes, and genome-wide association studies (GWAS) have found associations between CADM2 variants and age at first sexual intercourse, body mass index, cannabis use, educational attainment, hyperactivity, longevity, risk-taking propensity, and processing speed.57–63
Alternatively, there might be causal effects of lower cognitive function on risk for mental health problems. Cognitive models of depression emphasize difficulties with redirecting attention away from negative stimuli, failures to incorporate new information into negative cognitive schemas, and biased memory for negative information.64 Many of these cognitive processes are now recognized to be trans-diagnostic,65 leading to so-called “unified” treatment protocols for emotional disorders, which aim to build cognitive flexibility (e.g., learning new ways to appraise emotion-relevant information) and inhibitory control (e.g., stopping emotion-driven behaviors, including avoidance), regardless of specific diagnosis.66,67 One possibility, then, is that individuals who have more adept executive functioning and abstract reasoning in “cold” contexts (i.e., contexts lacking affective information) also have stronger cognitive skills in the face of emotion regulation demands. Reciprocal effects -- in which psychopathology impairs cognitive development or performance on cognitive tests -- are also plausible. The significant non-shared environmental correlation is consistent with a causal effect of psychopathology on EF and/or of EF on psychopathology,68 but could also result from both phenotypes being caused by the same set of unique environmental impacts.
We modeled both cognitive abilities and psychopathology using latent factors for the purposes of obtaining a parsimonious representation of the multivariate covariance structure of the respective constellations of phenotypes. By implementing latent factor modelling, we do not automatically presume that the factors are real or etiologically homogeneous. With respect to psychiatric comorbidity, the p factor could represent a coherent underlying entity that confers vulnerability to a wide variety of psychiatric symptoms, or it could just as plausibly be a statistical placeholder for an emergent pattern of the correlations that arise from, for example, mutual causation between symptoms.69 We take an agnostic approach here and treat p as a parsimonious statistical summary of a complex pattern of widespread covariation between psychiatric symptoms: “Factors may or may not be weighted with surplus meaning. Certainly, when they are regarded as ‘real dimensions’ a great deal of surplus meaning is implied, and the interpreter must shoulder a substantial burden of proof. The alternative view is to regard factors as defining a working reference frame, located in a convenient manner in the ‘space’ defined by all behaviors of a given type” (p. 277–278).70
The p-factor defined in this study captures continuous variation in psychopathology symptoms in the general population, but some forms of childhood psychopathology (e.g., Autistic Spectrum Disorders, Tourette Syndrome and other tic disorders) were not assessed. Whether the results observed here generalize across all disorders and across the full range of clinical severity has not yet been established. Additionally, although we controlled for mean differences between race/ethnic groups in study variables, it is not yet clear whether the pattern of associations seen in the combined, ethnically-diverse sample generalize to all race/ethnic groups.
One strength of this study was its use of multiple reporters for psychopathology symptoms. Overall, children and parents agreed moderately regarding whether children were generally experiencing emotional and behavioral problems, but they agreed minimally on specific symptoms scales. The correlation between the parent-reported and child-reported p factors was 0.40, which mirrors the global meta-analytic estimate for parent-child agreement on CBCL total scores (r = .41).71 Although parents and children have unique perspectives on child psychopathology, the pattern of results was consistent across reporters, with two notable exceptions. First, parent-reported psychopathology was particularly elevated for children with low IQ, but this exacerbation of the intelligence-psychopathology association was not observed for child-reported psychopathology. Second, both EF and intelligence were associated with parent-reported attention problems, but not child-reported attention problems, above and beyond their relationship with p. These discrepancies between parent- and self-reported psychopathology at the low end of child intelligence might result from children with low cognitive abilities having poor insight into their own functioning.
The current study has several other notable strengths, including its large, population-based genetically informative sample, and its comprehensive, in-laboratory battery of cognitive tests. We find that performance on tests of EF and general intelligence indexes an underlying genetic signal that is related to risk for psychopathology, even at an age (8- to 13-years-old) when children have not yet passed through the peak period of risk for the onset of mental health problems. Accordingly, measures of EF and g hold promise as prospective predictors of the future onset of psychopathology as youth mature through adolescence and young adulthood. Evaluating this possibility will require genetically-informative longitudinal studies that track the emergence of psychopathology in youth that in relation to variation in childhood EF.
Supplementary Material
Acknowledgements
We gratefully acknowledge all participants members of the Texas Twin Project.
Funding
KPH and EMT are Faculty Research Associates of the Population Research Center at the University of Texas at Austin, which is supported by a grant, 5-R24-HD042849, from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). KPH and EMT are also supported by Jacobs Foundation Research Fellowships. This research was supported by NIH grant R01HD083613.
Footnotes
Statement regarding Conflicts of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
K. Paige Harden, University of Texas at Austin.
Laura E. Engelhardt, University of Texas at Austin.
Frank D. Mann, University of Texas at Austin.
Megan W. Patterson, University of Texas at Austin.
Andrew D. Grotzinger, University of Texas at Austin.
Stephanie L. Savicki, University of Texas at Austin.
Megan L. Thibodeaux, University of Texas at Austin.
Samantha M. Freis, University of Texas at Austin.
Jennifer L. Tackett, Northwestern University.
Jessica A. Church, University of Texas at Austin.
Elliot M. Tucker-Drob, University of Texas at Austin.
References
- 1.Krueger RF, Eaton NR. Transdiagnostic factors of mental disorders. World Psychiatry 2015; 14: 27–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, et al. The p Factor. Clin Psychol Sci 2014; 2: 119–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carragher N, Krueger RF, Eaton NR, Slade T. Disorders without borders: current and future directions in the meta-structure of mental disorders. Soc Psychiatry Psychiatr Epidemiol 2015; 50: 339–50. [DOI] [PubMed] [Google Scholar]
- 4.Lahey BB, Hulle CA Van, Singh AL, Waldman ID, Rathouz PJ. Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Arch Gen Psychiatry 2011; 68: 181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray AL, Eisner M, Ribeaud D. The development of the general factor of psychopathology p factor through childhood and adolescence. J Abnorm Child Psychol 2016; 44: 1573–86. [DOI] [PubMed] [Google Scholar]
- 6.Patalay P, Fonagy P, Deighton J, Belsky J, Vostanis P, Wolpert M. A general psychopathology factor in early adolescence. Br J Psychiatry 2015; 207: 15–22. [DOI] [PubMed] [Google Scholar]
- 7.Pettersson E, Larsson H, Lichtenstein P. Common psychiatric disorders share the same genetic origin: a multivariate sibling study of the Swedish population. Mol Psychiatry 2016; 21: 717–21. [DOI] [PubMed] [Google Scholar]
- 8.Stochl J, Khandaker GM, Lewis G, Perez J, Goodyer IM, Zammit S, et al. Mood, anxiety and psychotic phenomena measure a common psychopathological factor. Psychol Med 2015; 45: 1483–93. [DOI] [PubMed] [Google Scholar]
- 9.Tackett JL, Lahey BB, van Hulle C, Waldman I, Krueger RF, Rathouz PJ. Common genetic influences on negative emotionality and a general psychopathology factor in childhood and adolescence. J Abnorm Psychol 2013; 122: 1142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caspi A, Moffitt TE. All for One and One for All: Mental Disorders in One Dimension. Am J Psychiatry 2018; 175: 831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann A, Pappa I, Lahey BB, Verhulst FC, Medina-Gomez C, Jaddoe VW, et al. Single nucleotide polymorphism heritability of a general psychopathology factor in children. J Am Acad Child Adolesc Psychiatry 2016; 55: 1038–1045.e4. [DOI] [PubMed] [Google Scholar]
- 12.Grotzinger AD, Rhemtulla M, Vlaming R de, Ritchie SJ, Mallard TT, Hill WD, et al. Genomic SEM provides insights into the multivariate genetic architecture of complex traits. Cold Spring Harbor Laboratory, 2018. doi: 10.1101/305029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smoller JW, Andreassen OA, Edenberg HJ, Faraone SV, Glatt SJ, Kendler KS. Psychiatric genetics and the structure of psychopathology. Mol Psychiatry 2019; 24: 409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond A. Executive functions. Annu Rev Psychol 2013; 64: 135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engle RW. Working memory capacity as executive attention. Curr Dir Psychol Sci 2002; 11: 19–23. [Google Scholar]
- 16.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognit Psychol 2000; 41: 49–100. [DOI] [PubMed] [Google Scholar]
- 17.Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol 2015; 6: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikaye A, Friedman NP. The nature and organization of individual differences in executive functions four general conclusions. Curr Dir Psychol Sci 2012; 21: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen 2008; 137: 201–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelhardt LE, Briley DA, Mann FD, Harden KP, Tucker-Drob EM. Genes unite executive functions in childhood. Psychol Sci 2015; 26: 1151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipszyc J, Schachar R. Inhibitory control and psychopathology: a meta-analysis of studies using the stop signal task. J Int Neuropsychol Soc 2010; 16: 1064–76. [DOI] [PubMed] [Google Scholar]
- 22.McGrath JJ, Alati R, Clavarino A, Williams GM, Bor W, Majman JM, et al. Age at first tobacco use and risk of subsequent psychosis-related outcomes: A birth cohort study. Aust N Z J Psychiatry 2016; 50: 577–83. [DOI] [PubMed] [Google Scholar]
- 23.Nigg JT, Jester JM, Stavro GM, Ip KI, Puttler LI, Zucker RA. Specificity of executive functioning and processing speed problems in common psychopathology. Neuropsychology 2017; 31: 448–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nigg JT, Stavro G, Ettenhofer M, Hambrick DZ, Miller T, Henderson JM. Executive functions and adhd in adults: Evidence for selective effects on ADHD symptom domains. J Abnorm Psychol 2005; 114: 706–17. [DOI] [PubMed] [Google Scholar]
- 25.Barkley RA. Behavioral Inhibition, Sustained Attention, and Executive Functions: Constructing a Unifying Theory of ADHD. ; : 30. [DOI] [PubMed] [Google Scholar]
- 26.Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for Attention-Deficit/Hyperactivity Disorder? Behav Brain Res 2002; 130: 3–28. [DOI] [PubMed] [Google Scholar]
- 27.Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res 2009; 166: 210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelhardt LE, Mann FD, Briley DA, Church JA, Harden KP, Tucker-Drob EM. Strong genetic overlap between executive functions and intelligence. J Exp Psychol Gen 2016; 145: 1141–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harden KP, Tucker-Drob EM, Tackett JL. The Texas Twin Project. Twin Res Hum Genet 2013; 16: 385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harden KP, Patterson MW, Briley DA, Engelhardt LE, Kretsch N, Mann FD, et al. Developmental changes in genetic and environmental influences on rule-breaking and aggression: age and pubertal development. J Child Psychol Psychiatry 2015; 56: 1370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achenbach TM, Rescorla LA. Manual for ASEBA school age forms & profiles. University of Vermont Research Center for Children, Youth and Families, 2001. [Google Scholar]
- 32.Lizotte AJ, Chard-Wierschem DJ, Loeber R, Stern SB. A shortened child behavior checklist for delinquency studies. J Quant Criminol 1992; 8: 233–45. [Google Scholar]
- 33.Laskar J, Robutel P. High order symplectic integrators for perturbed Hamiltonian systems. J Abnorm Child Psychol 2000; 26: 257–68. [Google Scholar]
- 34.John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative big five trait taxonomy In Handbook of personality: Theory and research. (3rd edn) (eds John OP, Robins RW, Pervin LA): 114–58. Guilford Press, 2008. [Google Scholar]
- 35.Wechsler D. WASI-II: Wechsler Abbreviated Scale of Intelligence. 2011. [Google Scholar]
- 36.Wright I, Waterman M, Prescott H, Murdoch-Eaton D. A new Stroop-like measure of inhibitory function development: typical developmental trends. J Child Psychol Psychiatry 2003; 44: 561–75. [DOI] [PubMed] [Google Scholar]
- 37.Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol Sci 1997; 8: 60–4. [Google Scholar]
- 38.Verbruggen F, Logan GD, Stevens MA. STOP-IT: Windows executable software for the stop-signal paradigm. Behav Res Methods 2008; 40: 479–83. [DOI] [PubMed] [Google Scholar]
- 39.Lee K, Bull R, Ho RMH. Developmental changes in executive functioning. Child Dev 2013; 84: 1933–53. [DOI] [PubMed] [Google Scholar]
- 40.Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, Poldrack RA. Measurement and reliability of response inhibition. Front Psychol 2012; 3. doi: 10.3389/fpsyg.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salthouse T. What cognitive abilities are involved in trail-making performance? Intelligence 2011; 39: 222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wechsler D. Wechsler Intelligence Scale for Children® - Fourth Edition Pearson, 2003. (https://www.pearsonclinical.com/psychology/products/100000310/wechsler-intelligence-scale-for-children-fourth-edition-wisc-iv.html). [Google Scholar]
- 43.Kane MJ, Hambrick DZ, Tuholski SW, Wilhelm O, Payne TW, Engle RW. The generality of working memory capacity: A latent-variable approach to verbal and visuospatial memory span and reasoning. J Exp Psychol Gen 2004; 133: 189–217. [DOI] [PubMed] [Google Scholar]
- 44.Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verbal Learn Verbal Behav 1980; 19: 450–66. [Google Scholar]
- 45.Broadway JM, Engle RW. Validating running memory span: Measurement of working memory capacity and links with fluid intelligence. Behav Res Methods 2010; 42: 563–70. [DOI] [PubMed] [Google Scholar]
- 46.Jaeggi SM, Studer-Luethi B, Buschkuehl M, Su Y-F, Jonides J, Perrig WJ. The relationship between n-back performance and matrix reasoning — implications for training and transfer. Intelligence 2010; 38: 625–35. [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol 1995; 57: 289–300. [Google Scholar]
- 48.Neale MC, Maes HHM. Methodology for Genetic Studies of Twins and Families. Kluwer Academic Publishers B.V, 2004. [Google Scholar]
- 49.Schmidt F,E Hunter J. Measurement Error in Psychological Research. Psychol Methods 1996; 1: 199–223. [Google Scholar]
- 50.Deary IJ, Weiss A, Batty GD. Intelligence and personality as predictors of illness and death: How researchers in differential psycology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychol Sci Public Interest 2010; 11: 53–79. [DOI] [PubMed] [Google Scholar]
- 51.DiPrete TA, Burik CAP, Koellinger PD. Genetic instrumental variable regression: Explaining socioeconomic and health outcomes in nonexperimental data. Proc Natl Acad Sci 2018; 115: E4970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 2018; 50: 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Connor LJ, Price AL. Distinguishing genetic correlation from causation across 52 diseases and complex traits. Nat Genet 2018; 50: 1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belsky DW, Harden KP. Phenotypic annotation: Using polygenic scores to translate discoveries from genome-wide association studies from the top-down. Curr Dir Psychol Sci 2019. doi:https://doi.org/10.1177%2F0963721418807729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deary IJ. Looking for system integrity in cognitive epidemiology. Gerontology 2012; 58: 545–53. [DOI] [PubMed] [Google Scholar]
- 56.Cannon TD, Keller MC. Endophenotypes in the Genetic Analyses of Mental Disorders. Annu Rev Clin Psychol 2006; 2: 267–90. [DOI] [PubMed] [Google Scholar]
- 57.Albayrak Ö, Pütter C, Volckmar A-L, Cichon S, Hoffmann P, Nöthen MM, et al. Common obesity risk alleles in childhood attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 2013; 162: 295–305. [DOI] [PubMed] [Google Scholar]
- 58.Broer L, Buchman AS, Deelen J, Evans DS, Faul JD, Lunetta KL, et al. GWAS of Longevity in CHARGE Consortium Confirms APOE and FOXO3 Candidacy. J Gerontol Ser A 2015; 70: 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies G, Marioni RE, Liewald DC, Hill WD, Hagenaars SP, Harris SE, et al. Genome-wide association study of cognitive functions and educational attainment in UK Biobank (N= 112 151). Mol Psychiatry 2016; 21: 758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Day FR, Helgason H, Chasman DI, Rose LM, Loh P-R, Scott RA, et al. Physical and neurobehavioral determinants of reproductive onset and success. Nat Genet 2016; 48: 617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ibrahim-Verbaas CABJ, Debette S, Schuur M, Smith A V., Bis JC. GWAS for executive function and processing speed suggests involvement of the CADM2 gene HHS Public Access. Mol Psychiatry 2016; 21: 189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal eighteen new loci associated with body mass index. Nat Genet 2010; 42: 937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stringer S, Minică CC, Verweij KJH, Mbarek H, Bernard M, Derringer J, et al. Genome-wide association study of lifetime cannabis use based on a large meta-analytic sample of 32 330 subjects from the International Cannabis Consortium. Transl Psychiatry 2016; 6: e769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Disner SG, Beevres CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci 2011; 12: 467–77. [DOI] [PubMed] [Google Scholar]
- 65.Carver CS, Johnson SL, Timpano KR. Toward a Functional View of the P Factor in Psychopathology. Clin Psychol Sci J Assoc Psychol Sci 2017; 5: 880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barlow DH, Allen LB, Choate ML. Toward a unified treatment for emotional disorders. Behav Ther 2004; 35: 205–30. [DOI] [PubMed] [Google Scholar]
- 67.Moses EB, Barlow DH. A new unified treatment approach for emotional disorders based on emotion science. Curr Dir Psychol Sci 2006; 15: 146–50. [Google Scholar]
- 68.Turkheimer E, Harden KP. Behavior genetic research methods: Testing quasi-causal hypotheses using multivariate twin data In Handbook of research methods in social and personality psychology (2nd edn) (eds Reis HT, Judd CM): 159–87. Cambridge University Press, 2014. [Google Scholar]
- 69.Fried EI, van Borkulo CD, Cramer AOJ, Boschloo L, Schoevers RA, Borsboom D. Mental disorders as networks of problems: a review of recent insights. Soc Psychiatry Psychiatr Epidemiol 2017; 52: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cronbach LJ, Meehli PE. Construct validity in psychological tests. Psychol Bull 1955; 52: 281–302. [DOI] [PubMed] [Google Scholar]
- 71.Rescorla LA, Ginzburg S, Achenbach TM, Ivanova MY, Almqvist F, Begovac I, et al. Cross-informant agreement between parent-reported and adolescent self-reported problems in 25 societies. J Clin Child Adolesc Psychol Off J Soc Clin Child Adolesc Psychol Am Psychol Assoc Div 53 2013; 42: 262–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.