INTRODUCTION
The human heart has one of highest metabolic requirements in the body: even in a resting physiologic state it must work to circulate approximately 5 to 8 L/min of blood against systemic vascular afterload. In addition, overall cardiac mechanical efficiency is only 20% to 25%,1 with cellular maintenance and heat production accounting for the balance of energetic requirements. A dense network of mitochondria, which contain the machinery of oxidative phosphorylation, is needed to maintain the balance of cellular ATP utilization and production in the setting of such a high energetic demand. Indeed, cardiac myocytes have one of highest mitochondrial volume densities of any cell in the body, with mitochondria occupying almost one-third of the cell volume.2 This network is highly vulnerable to injury, and therefore impaired function, due to a combination of patient factors and stressors encountered in the context of cardiac surgery. A discussion of normal and pathologic cardiac mitochondrial function, specific perioperative stressors, and protective strategies for cardiac surgical patients follows.
THE ROLES OF CARDIAC MITOCHONDRIA IN HEALTH AND DISEASE
Under normoxic conditions, cardiac ATP production is accomplished primarily through oxidation of fatty acids and, to a lesser degree, carbohydrates.3 Ketone bodies, lactate, and amino acids can also be used as substrate, but they contribute little to maintaining the ATP/ADP balance at baseline.4 However, cardiac metabolism is dynamic, and adapts in response to both chronic and acute pathologic perturbations.
Chronic pathologies most relevant to cardiac surgical patients include heart failure and diabetes. In heart failure, there is an increase in glucose utilization and a decrease in fatty acid oxidation, which has been compared to a shift back toward fetal cardiac metabolism.5 As a result, in the long-term failing hearts demonstrate an overall reduction in energy reserves as reflected by the phosphocreatinine (PCr)/ATP ratio. Clinically, a decline in the PCr/ATP ratio predicts mortality in heart failure, suggesting that such a metabolic shift is ultimately maladaptive.6 In diabetes on the other hand, fatty acid uptake and oxidation is increased, and glucose metabolism is decreased, with an overall decrease in metabolic efficiency and increased oxidative stress. In addition, despite the increased fatty acid oxidation, there is a relative abundance of lipid substrate. These factors lead to accumulation of toxic metabolic intermediates and contractile failure, which has been termed “lipotoxic cardiomyopathy.”7 Clinically, impaired mitochondrial function and dynamics are associated with contractile dysfunction8,9 and an increased risk of arrhythmia and sudden cardiac death10 in type 2 diabetic patients.
Acutely, ischemia and reperfusion are the most well-studied pathologies that impact cardiac surgical patients. Within seconds of the onset of ischemia, high-energy phosphate reserves are exhausted and anaerobic glycolysis becomes the only source of ATP. As a consequence, intracellular H+ accumulates, pH decreases, and contractile function is impaired.11 With reperfusion, oxidative phosphorylation is restored rapidly, but mechanical efficiency is diminished due to disproportionately increased fatty acid oxidation with continued upregulation of anaerobic glycolysis. The preexisting and ongoing accumulation of intracellular H+ is normalized, but at the expense of increased intracellular Ca2+ (via the H+/Na+ and 2Na+/Ca2+ exchangers). Intracellular Ca2+ overload increases the risk of mitochondrial permeability transition pore (MPTP) opening and activation of cell death.12,13
In all, maintaining the cellular ATP supply is one of the fundamental roles of mitochondria. The chronic and acute perturbations discussed demonstrate how dynamic cardiac metabolism can be, and illuminate therapeutic opportunities for improving mitochondrial efficiency and limiting damage in the setting of cardiac surgery (please see the “Opportunities for intervention”section).
In addition to their central role in energy supply, mitochondria are important to several other cellular processes, including reactive oxygen species (ROS) signaling, calcium hemostasis, and regulation of apoptosis and necrosis pathways. First, mitochondria are a source of intracellular ROS, which are normal byproducts of the electron transport chain (ETC) complex in oxidative phosphorylation. Although ROS can contribute to oxidative stress, it is important not to overlook that at low levels, ROS activate crucial intracellular signaling pathways (termed redox signaling,14,15 which may underlie the mechanisms of ischemic pre,16 post,17 and remote ischemic preconditioning18). ROS are balanced by antioxidant systems, preventing damage to cellular components. However, ROS production is tied to the rate of respiration, and increases disproportionately when there are perturbations in respiratory chain complex activity or cofactor availability. Ischemia and reperfusion are examples of such perturbations, in which ROS production is increased first due to inadequate substrate availability,19 followed by increased electron leakage by ETC complexes and decreased ROS scavenging (antioxidant) capacity during the hyperoxic period of reperfusion.20
Second, mitochondria play a role in Ca2+ hemostasis21; mitochondrial Ca2+ uptake serves as a buffer for cytoplasmic levels, and increased Ca2+ within the mitochondrial matrix activates ATP synthesis. As mentioned, this capacity can be overwhelmed in pathologic states, and Ca2+ overload can contribute to mitochondrial activation of cell death. In addition, excessive levels of ROS, such as those produced in the reperfusion phase of ischemia/reperfusion (I/R) injury,22 heart failure,23 and other cardiomyopathies24 result in damage to lipids, proteins, mitochondrial DNA (mtDNA), and ETC complexes themselves, perpetuating oxidative stress. When there is overwhelming oxidative stress or mitochondrial Ca2+ overload, as can occur during reperfusion, mitochondrial “metabolic checkpoints”25 activate cell death programming. Well-known mitochondrial pathways of cell death activation include the following:
Permeabilization of the mitochondrial outer membrane, with release of cytochrome c and other mitochondrial proteins into the cytosol, leading to caspase activation.
-
Induction of the mitochondrial permeability transition, with uncoupling of oxidative phosphorylation, mitochondrial swelling, rupture, and release of cytochrome c.25
Ultimately these can lead to apoptosis or regulated necrosis26 depending on the degree and mechanism of stress.
MITOCHONDRIAL DYSFUNCTION IN CARDIAC SURGERY PATIENTS
It is clear that mitochondria have important impacts on myocardial function through their roles in energy and calcium balance, ROS signaling, and regulation of cell death. Cardiac surgical patients may present with preexisting mitochondrial dysfunction due to prior insult or chronic pathology,27 as discussed. In addition, cardiac surgery involves a period of cardiac I/R, as well as the induction of systemic inflammatory responses due to surgical tissue trauma and cardiopulmonary bypass (CPB) exposure, all of which can impact mitochondrial function.
The inflammatory response to CPB includes activation of both humoral and cellular components in early and late phases, attributed to blood interaction with nonendothelial bypass circuit surfaces and I/R injury, respectively. The result is a cascading release of cytokines, enzymes, and other vasoactive substances, which manifests as a systemic inflammatory response syndrome picture.28 The impact of systemic inflammatory states on mitochondrial function can be substantial.29 Generally, mitochondrial respiration is impaired by inflammation, a response that is conserved across a variety of inflammatory states and organisms, suggesting that energetic conservation may confer adaptive advantages as long as the response is temporary.30 Inflammation can also activate mitochondrial quality control and antioxidant programs to facilitate recovery. However, if inflammation is severe, cellular (including mitochondrial) components can be damaged, which further interferes with energy production and may lead to cell death. As such, the level of inflammatory response due to surgical tissue trauma and exposure to CPB would be expected to correlate with the degree of mitochondrial dysfunction and cell death in cardiac surgical patients.
Furthermore, mitochondrial dysfunction and damage can itself exacerbate the inflammatory response. Certain pathways of cell death can result in the extracellular release of intracellular products, which are then known as damage-associated molecular patterns (DAMPs). DAMPs interact with pattern recognition receptors to upregulate proinflammatory or anti-inflammatory responses31–33 and inflammasome activation.34 DAMPs of mitochondrial origin include mtDNA, cytochrome c, mitochondrial transcription factor A (mtTFA), ATP, and high-mobility group box 1 (HMGB1). Intracellularly, mitochondrial dysfunction causes translocation of mtDNA into the cytoplasm, where it upregulates proinflammatory signaling through nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and inflammasome activation.
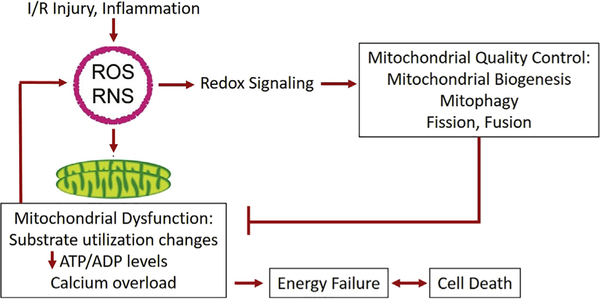
Fortunately, there is some capacity for recovery after mitochondrial injury (Fig. 1). Recovery is facilitated through the induction of a group of mitochondrial quality control (MQC) mechanisms that separate and degrade unhealthy mitochondria and mitochondrial components while retaining healthy elements (through the processes of fission, fusion, and mitophagy), and generate new mitochondrial components (biogenesis). The actions of these processes are at once oppositional and complementary; as such, they must be tightly regulated by a complex machinery both at baseline and in response to stress to maintain an optimally functioning mitochondrial network. Indeed, proteins that regulate mitochondrial fission and fusion are the targets of signaling pathways activated in normal development, exercise/metabolic demand, or by heart failure, diabetes, cardiomyopathy, or I/R, among other pathologies.35 Similarly, mitophagy is upregulated through several different pathways depending on the stimulus (eg, the Parkin-PINK1 pathway for decreased membrane potential and I/R and Fundc1 in hypoxic stress), and proteins that are well-known as regulators of mitochondrial biogenesis are also impacted by elements of the Parkin mitophagy pathway.36 MQC processes are of obvious interest due to their potential to improve cardiac recovery in a variety of clinical settings and have recently been extensively reviewed.37
Fig. 1.
Perioperative I/R and inflammation induce oxidative stress (reactive oxygen and nitrogen species), which modulate MQC programs through redox signaling. Excessive oxidative stress may also contribute to mitochondrial dysfunction, resulting in energetic failure and cell death. RNS, reactive nitrogen species.
Given the variety of perturbations that may impact mitochondrial function in the perioperative period, what is the evidence that mitochondrial dysfunction is clinically relevant for cardiac surgical patients? First, in adults undergoing coronary artery bypass or valve surgery on CPB, mitophagy, mitochondrial biogenesis, and mtDNA damage (strand breaks) immediately post-CPB are increased compared with pre-CPB in atrial tissue.38 The upregulation in mitochondrial biogenesis, in particular, seems to be driven by post-transcriptional mechanisms, highlighting those mechanisms as potential therapeutic targets.
Cardiac surgical patients are also at high risk for atrial fibrillation (AF), which is associated with increased morbidity and mortality. The risk of developing AF may be increased by inflammation or changes in calcium signaling,39 both of which are central features of mitochondrial dysfunction and I/R injury. Recent studies have demonstrated that high levels of mtDNA in peripheral blood before cardiac surgery,40 or significant increases from precardiac to postcardiac surgery (indicating tissue injury)41 are predictive of developing new-onset postoperative AF. In addition, cardiac surgery patients with preexisting mitochondrial dysfunction (decreased respiration and increased sensitivity to MPTP opening with calcium) in right atrial tissue also had a higher incidence of new-onset postoperative AF,42 and in patients with preexisting AF, mitochondrial ETC activity is lower and oxidative stress higher than in those without AF before cardiac surgery.43
SPECIFIC CARDIAC SURGICAL PATIENT POPULATIONS AND MITOCHONDRIAL DYSFUNCTION
As previously discussed, mitochondrial dysfunction also plays a role in the pathogenesis of heart failure3,6,7; long-term mechanical unloading with a left ventricular assist device may improve mitochondrial function44 and ultrastructural remodeling, particularly in ischemic cardiomyopathy patients.45 For heart failure patients who progress to heart transplantation, mitochondrial dysfunction can occur in the donor heart, increasing the risk of early graft failure (formally defined as primary graft dysfunction when significant inotropic or mechanical circulatory assistance are required for failure without other discernible cause within 24 hours posttransplant46). Donor factors that may contribute to donor heart mitochondrial dysfunction include increased catecholamine exposure and cytosolic calcium, as well as decreased hormone levels following brain death. Contributing procedural factors are primarily the cold and warm ischemic times for organ transport and implantation, and the duration of CPB (unlike in other cardiac surgical procedures, graft ischemic time and recipient CPB time may be mutually exclusive). Recipient factors that may contribute to mitochondrial dysfunction include high pulmonary vascular resistance, which increases myocardial afterload, and the systemic inflammatory response to intraprocedure CPB and any preoperative exposure to mechanical circulatory support.
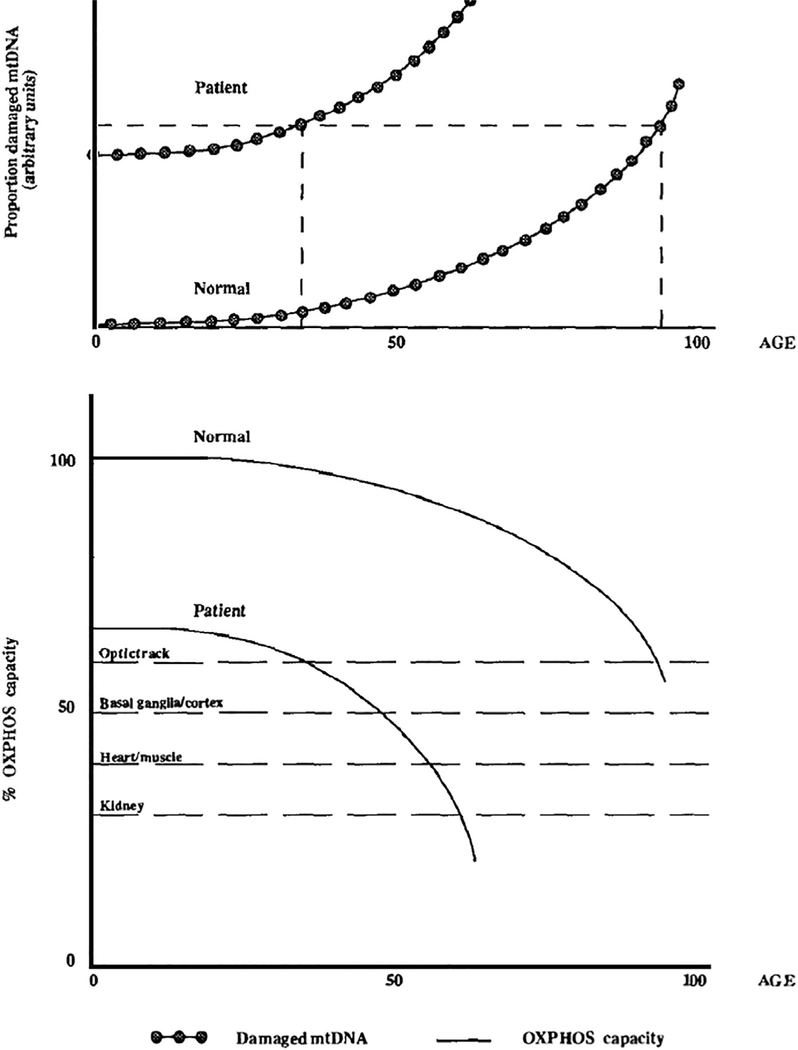
Finally, apart from the comorbidities already discussed (heart failure, diabetes), 2 other conditions that may predispose to mitochondrial dysfunction in cardiac surgical patients are worth discussion: aging and cardiomyopathies. With aging, oxidative phosphorylation is increasingly disrupted,47,48 which plays a part in declining organ functional reserve; this observed decreased energetic capacity may be explained by increases in mtDNA deletions in cardiac tissue from aging patients (Fig. 2).49 Conversely, ROS production is progressively elevated with age.50 These changes may increase the propensity for injury after cardiac I/R with aging, which has been shown in both rats and human patients.51,52 Cardiomyopathies can reflect a wide variety of underlying disease processes, some with a notable component of mitochondrial dysfunction. Viral53 or bacterial infections (particularly sepsis54) can cause myocarditis and/or a systemic inflammatory response, and thereby contribute to perioperative cardiac mitochondrial dysfunction. A number of mitochondrial diseases are associated with cardiomyopathies (Table 1).55 These are important to consider for 2 reasons: (1) because the heart has a relatively high energy requirement, cardiac dysfunction may be the only clinical manifestation of mitochondrial dysfunction that is as yet sub-clinical in other organ systems, and (2) cardiomyopathies (or other organ dysfunctions) in these patients can be precipitated by stressors including febrile illness or surgery, with metabolic decompensation and/or acute heart failure. One important cardiomyopathy subset that is regularly encountered in the perioperative space is hypertrophic cardiomyopathy (HCM). Although a variety of genetic mutations cause the HCM phenotype, it is thought that common features are excessive sarcomeric energy use, mitochondrial dysfunction and morphologic disorganization, and overall myocardial remodeling.56 The clinical impact of myocardial bioenergetics deficits and reduced metabolic reserve has not been described specifically for patients with HCM in the context of cardiac surgery, but should be considered when there is otherwise unexplained poor perioperative cardiac function.
Fig. 2.
Hypothesis for the mechanism of age-related progression of oxidative phosphorylation (OXPHOS) diseases. The upper panel shows the proposed accumulation of somatic mtDNA mutations with age.47,48 Patients are born with a certain percentage of mutant mtDNAs, some patients with more than others. The dashed lines indicate the relative ages when sufficient mutations accumulate to cause disease. The lower panel shows the decline of OXPHOS capacity of healthy individuals and patients with underlying mtDNA mutations. Different tissues have different minimum energy thresholds, below which dysfunction is clinically apparent, shown by dashed lines. OXPHOS declines for both healthy individuals and patients, consistent with the accumulation of somatic mtDNA damage. However, because of the inherited mtDNA mutation, patients start with a lower initial OXPHOS capacity and thus drop below the expression thresholds much earlier than normal individuals. (From Wallace DC. Diseases of the mitochondrial DNA. Annu Rev Biochem 1992;61:1175–212; with permission.)
Table 1.
Mitochondrial diseases associated with cardiomyopathies or other cardiac disease
| Mitochondrial Disease | Cardiac Manifestations | Other System Manifestations |
|---|---|---|
| ETC complex deficiencies | ||
| Complex I deficiency | Hypertrophic cardiomyopathy | Growth failure, developmental delay, epilepsy, ataxia, weakness, spasticity, leukoencephalopathy, macrocephaly, sensorineural deafness, hepatic dysfunction, lactic acidosis, hypoglycemia |
| Complex II deficiency | Hypertrophic, dilated, and noncompaction cardiomyopathies | Growth failure, developmental delay, weakness, spasticity, ataxia, epilepsy, leukodystrophy, contractures, ophthalmoplegia, pigmentary retinopathy, optic atrophy, lactic acidosis |
| Complex III deficiency | Hypertrophic, dilated, and histiocytoid cardiomyopathies | Growth failure, exercise intolerance, optic atrophy, strokelike episodes, epilepsy, lactic acidosis, hypoglycemia |
| Complex IV deficiency | Dilated, hypertrophic, and histocytoid cardiomyopathies | Growth failure, developmental delay, ataxia, epilepsy, hypotonia, sensorineural hearing loss, optic atrophy, pigmentary retinopathy, liver dysfunction, renal tubulopathy, lactic acidosis |
| Leigh syndrome | Cardiomyopathy and arrhythmias | Respiratory failure, dysphagia, hypotonia, dystonia, ataxia, peripheral neuropathy, ophthalmoparesis, nystagmus, optic atrophy |
| Mitochondrial tRNA genes | ||
| MERRF (myoclonic epilepsy with ragged red fibers) syndrome | Dilated and histiocytoid cardiomyopathy | Epilepsy, ataxia, weakness, sensorineural hearing loss, short stature, lactic acidosis |
| MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes) syndrome | Hypertrophic cardiomyopathy | Muscle weakness, strokelike episodes, dementia, epilepsy, sensorineural hearing loss, lactic acidosis, diabetes, short stature |
| Mitochondrial DNA depletion | ||
| Mitochondrial neurogastrointestinal encephalopathy syndrome (MNGIE) | Hypertrophic cardiomyopathy | Gastrointestinal dysmotility, cachexia, ptosis, ophthalmoplegia, hearing loss, peripheral neuropathy, leukoencephalopathy |
| CoQ10 deficiency | ||
| Coenzyme Q10 deficiency | Hypertrophic cardiomyopathy | Growth failure, developmental delay, weakness, epilepsy, ataxia, pigmentary retinopathy, sensorineural hearing loss, liver dysfunction, renal impairment, pancytopenia, lactic acidosis |
| 3-Methylglutaconic acidurias | ||
| 3-Methylglutaconic aciduria type II (Barth syndrome) | Noncompaction, dilated, and hypertrophic cardiomyopathies | Growth failure, weakness, arrhythmias, neutropenia |
| 3-Methylglutaconic aciduria, type V (dilated cardiomyopathy and ataxia syndrome) | Dilated and noncompaction cardiomyopathies | Growth failure, ataxia, testicular dysgenesis, anemia |
| Mitochondrial complex V deficiency | Hypertrophic cardiomyopathy | Growth failure, developmental delay, hypotonia, ataxia, epilepsy, leukodystrophy distinctive facial features, lactic acidosis, hyperammonemia |
| Sengers syndrome | Hypertrophic cardiomyopathy | Growth failure, cataracts, hypotonia, weakness, lactic acidosis |
| Defects in iron-sulfur cluster | ||
| Friedreich ataxia | Hypertrophic cardiomyopathy | Ataxia, dysarthria, peripheral sensory neuropathy, diabetes mellitus |
| Multiple deletions | ||
| Kearns-Sayre syndrome | Arrhythmias | Progressive external ophthalmoplegia, pigmentary retinopathy, ataxia, weakness, deafness, renal insufficiency, dementia, short stature, diabetes |
Abbreviations: ETC, electron transport chain; tRNA, transfer RNA.
Adapted from El-Hattab AW, Scaglia F. Mitochondrial Cardiomyopathies. Front Cardiovasc Med 2016;3:25; and Meyers DE, Basha HI, Koenig MK. Mitochondrial cardiomyopathy: pathophysiology, diagnosis, and management. Tex Heart Inst J 2013;40(4):389.
OPPORTUNITIES FOR INTERVENTION
Mitochondrial dysfunction is clearly pervasive in the cardiac surgical patient population. The mechanisms discussed previously naturally prompt consideration of opportunities for intervention to prevent or modulate damage, or encourage repair after mitochondrial injury. A brief categorical overview follows here, but a detailed discussion of evidence for (or against) these strategies is beyond the scope of this work; recent reviews are referenced in each section.
First, a number of physiologic, anesthetic, and surgical factors can be modulated in the perioperative period to protect mitochondrial function. Hyperglycemia in the perioperative setting has several detrimental effects, including increased oxidative stress and I/R injury in cardiac tissue.57 In adult cardiac surgery patients, acute hyperglycemia is particularly common during CPB, even in nondiabetic patients. As might be expected, there is a well-established association with hyperglycemia during CPB and increased morbidity and mortality.58,59 As such, intraoperative glycemic control, perhaps particularly during the I/R phase, is recommended for diabetic and nondiabetic patients alike, although there is some debate over treatment thresholds due to the increased risk of hypoglycemia with aggressive treatment.60
Hypercalcemia at the time of I/R and inflammatory stress would also be expected to exacerbate mitochondrial injury due to intracellular calcium overload and increased activation of cell death. It is common practice, however, for calcium supplementation to given at the time of separation from CPB (after cross-clamp removal and some period of reperfusion has elapsed), with the goals of augmenting myocardial contractility and systemic vascular resistance. In considering the competing risk of injury versus augmented contractility, calcium supplementation should be judicious with regard to magnitude, administration (avoiding large, rapid boluses), and timing. A phase 4 clinical trial investigating the clinical efficacy of calcium administration at the time of separation from CPB (ICARUS Trial) is registered in ClinicalTrials.gov at the time of this writing.61
Perioperative anesthetic administration may also have an impact on mitochondrial function. Propofol is known to have antioxidant effects due to a structural similarity to vitamin E, and demonstrates myocardial mitochondrial preservation in preclinical studies. Outcomes in studies with cardiac surgical patients have been mixed.62–64 Somewhat confusingly, there is also clinical evidence that inhalational agents may confer a preconditioning effect, and provide enhanced cardioprotection when compared with propofol65,66; the results of these studies have not yet overwhelmingly impacted clinical practice in favor of either inhalational agent or propofol infusion as the preferred anesthetic technique in cardiac surgery.
The impact of I/R injury on mitochondria can be limited by minimizing ischemic time, cooling of myocardial tissue, and induction of diastolic electromechanical arrest to lower metabolic requirements, through the administration of cardioplegia. The protective effects of cardioplegia depend first on distribution in the myocardium; patients with coronary pathology or other microvascular abnormalities may benefit from retrograde administration. Although there is wide variation in practice, cardioplegia solutions are typically either crystalloid or blood-based, and most contain a high concentration of potassium to induce cardiac arrest. The previously mentioned flexibility in myocardial substrate metabolism results in increased carbohydrate metabolism in the immediate reperfusion phase after aortic cross-clamp and removal.67 Such transient perioperative changes in substrate use, as well as preexisting alterations in myocardial metabolism due to prior ischemia or other comorbid conditions point to the potential for therapeutic adjustments of substrate supply during cardiac surgery.3 A number of cardioplegia and organ preservation solutions incorporate components to facilitate substrate utilization, such as glycolytic substrates, amino acids, and specifically ketoglutarate in histidine-tryptophan-ketoglutarate (HTK) cardioplegia.68,69 More generally, the addition or adjustment of several other cardioplegia components have been studied, including potassium, calcium, sodium, histidine, tryptophan, adenosine, magnesium, and lidocaine (Table 2). These have shown promise in animal studies, but variable impact on outcomes in clinical studies.70,71 Some of these elements are components of so-called “long-acting” cardioplegia solutions, which are given less frequently during surgery to minimize surgical interruption, shortening ischemic times for long or complex procedures; despite this, clinical studies have not reliably shown an improvement in myocardial injury.72 Overall, there is not yet clear clinical evidence for one specific cardioplegia solution or strategy that is most advantageous for myocardial protection.73
Table 2.
Categories of cardioplegia strategies used for cardioprotection, with example solutions, representative component concentrations, and additives for each category
| Cardioplegia Type | Example Solutions and Concentrations |
Na+, mM/L |
K+, mM/L |
Mg2+, mM/L |
Ca2+, mM/L |
HTK, mM/L |
Mannitol | Glucose, mM/L |
Lidocaine | Adenosine | NaHCO3 | Modifications: |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystalloid-based | ||||||||||||
| Intracellular | Custodiol, HTK, Bretschneider’s, University of Wisconsin Solution | 15 | 9 | 4 | 0.015 | 198/2/1 | x | Glutamate, aspartate | ||||
| Extracellular | Plegisol, Celsior, St Thomas, Stanford solution | 110–120 | 16–26 | 32 | 2.4 | x | Glutamate, aspartate | |||||
| Blood-based (includes endogenous oxygen carrying, substrates, buffers, antioxidants, oncotic pressure, inflammatory factors) | ||||||||||||
| 4:1 Blood:crystalloid | Buckberg | 140 | 20–10 (i-m) | 13–9 (i–m) | 6 | 260 mg/L (induction) | Glutamate, aspartate, CPD, tromethamine | |||||
| 1:4 Blood:crystalloid, long-acting | del Nido (in 1 L Plasma-Lyte A) | Plasma-Lyte | 26 meq | 2 mg | 3.2 g | 130 mg | 13 meq | Glutamate, aspartate | ||||
| Microplegia (blood-based cardioplegia principles apply; with relatively less edema, increased neutrophil accumulation and endothelial dysfunction) | ||||||||||||
| Various additive and concentration options | ALM with insulin (8 mL additive:l L blood) | 30–8 meq/L (i-m) | 2 g/20 mL | 25 mg/l mL | 6 mg/2 mL | 2.5 IU Insulin added to ALM volume | ||||||
Abbreviations: ALM, adenosine and lidocaine with magnesium; CPD, citrate-phosphate-dextrose; HTK, histidine, tryptophan, ketoglutarate; i-m, concentrations for induction or maintenance of cardioplegia; Plasma-lyte A concentrations/L (Baxter), 140 mEq sodium, 5 mEq potassium, 3 mEq magnesium, 98 mEq chloride, 27 mEq acetate, and 23 mEq gluconate; x, may be added as a modifier.
Similarly, strategies to optimize the composition and conditions of reperfusion at the end of ischemia have also been investigated in efforts to minimize reperfusion injury, and may be of particular use in settings of prolonged ischemia or hypoxia (heart or lung transplants, or cyanotic pediatric cardiac surgery).74 Administration of warm cardioplegia before cross-clamp removal (terminal warm induction or “hot shot”) is meant to allow washout of ischemic metabolic byproducts and augment aerobic metabolism while continued electromechanical arrest minimizes energy demand. Clinical evidence is limited: some small studies show improvements in biochemical markers of I/R injury and/or hemodynamic parameters, but none have demonstrated outcome differences.75 Finally, perioperative infusions of glucose, insulin, and potassium (GIK) also have been used to support metabolism during cardiac surgery, with mixed results for different outcomes in clinical studies.76
The application of antioxidant therapies is an intuitive approach to counteracting the damaging effect of increased oxidative stress in tissues secondary to I/R injury, inflammation, and/or mitochondrial dysfunction.62 It is common practice for free-radical scavenging drugs like mannitol to be included in CPB circuit priming fluid77 or in cardioplegia solutions (eg, HTK, del Nido).78 Beyond this, clinical outcomes with experimental antioxidant therapies have not been promising. This is perhaps not surprising when we consider oxidative stress by the following definition: “an imbalance between oxidants and antioxidants in favor of the oxidants, leading to a disruption of redox signaling and control and/or molecular damage.”79 With this view, it becomes clear that the most fundamental concern in broadly applied or poorly timed antioxidant treatment is the potential to mask or prevent critical intracellular ROS signaling mechanisms, and interfere with cellular stress response pathways. Targeted approaches that take these issues into consideration through improved precision of delivery and dosing, such as synthetic mitochondrial antioxidants (eg, MitoQ or MitoVit-E), have been tested but have not yet translated into widespread clinical application.80
On the other side of the oxidant:antioxidant balance lies the potential for therapeutic manipulation of ROS signaling, on which the concept of ischemic conditioning relies. Pre, post, and remote ischemic conditioning have all been investigated widely in several settings,81 and generally involve the application of short cycles of ischemia and reperfusion (directly to the tissue at risk, or to a remote tissue in the case of remote ischemic preconditioning), with the goal of increasing ischemic tolerance and minimizing I/R injury. The complex and fascinating mechanisms of these conditioning phenomena converge on a number of subcellular targets, notably including mitochondria; these pathways have been intensely studied and reviewed.82 As is also the case for many of the therapies and approaches already discussed, cardiac surgery represents an appealing platform for application of these strategies, because the timing of perioperative stress and potential tissue injury can be anticipated, and along with it the timing of therapy. Clinical studies of both preconditioning and postconditioning in adult and pediatric cardiac surgery demonstrate promising results, but adoption has not been robust due to the requirement for repeated clamping and unclamping of the aorta. Remote (limb) conditioning has obvious practical advantages, and has been promising in preclinical and small clinical trials, but recently failed to demonstrate protective effects in 3 large multicenter trials in cardiac surgical patients.83 This was likely due to several factors, including limited additional advantage over the multitude of cardioprotective strategies already in place in the perioperative period, and the impact of patient comorbidities and medications (including the use of propofol, which has a free-radical scavenging role, as discussed previously).
Finally, strategies that would enhance recovery in the setting of chronic pathology or after acute injury are also intuitive and appealing. As outlined briefly previously, MQC mechanisms work in concert to clear defective mitochondrial components and generate new ones, maintaining a functional mitochondrial network. There is documented evidence of MQC dysregulation in several pathologies and stressors that are highly relevant to cardiac surgical patients, including diabetes, heart failure, aging, and I/R injury.35,37,84 As such, interest exists in targeting elements of the MQC pathways. However, MQC mechanisms can have both pathologic or beneficial effects, depending on the conditions under which they are applied; for example, over-expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1a), a transcriptional regulator of mitochondrial biogenesis and antioxidant defense mechanisms, results in cardiac hypertrophy and heart failure, but with PGC-1a knockdown and impaired biogenesis upregulation, mice also develop heart failure in response to increased afterload, and increased tissue damage in sepsis.85,86 Similarly dichotomous results have been observed for mitophagy and mitochondrial dynamics pathways,84 suggesting that perhaps even more so than in the case of antioxidant therapies, highly specific application of MQC therapies (patient selection, dosing, timing, precision of treatment at the subtissue level) will be the key to their effective use.
SUMMARY
In summary, mitochondria are key to the cellular response to energetic demand, but are also vital to ROS signaling, calcium hemostasis, and regulation of apoptosis and necrosis pathways in cell death. Mitochondrial dysfunction and disruption of any of these vital processes can lead to chronic or acute pathology, particularly in tissues with high metabolic demand and mitochondrial content, such as the heart. In cardiac surgery, several perioperative factors can impact mitochondrial function, including I/R injury and an increased systemic inflammatory response due to exposure to CPB and surgical tissue trauma. Patients with diabetes, heart failure, advanced age, or cardiomyopathies may have underlying mitochondrial dysfunction or be more sensitive to perioperative injury. Mitochondrial dysfunction most immediately impacts postoperative myocardial contractility, but also predisposes to arrhythmias. A multitude of strategies to minimize the impact of perioperative factors on mitochondrial function have been incorporated into routine clinical care (hypothermia, avoidance of hyperglycemia or hypercalcemia, substrate and other additions to cardioplegia solutions, anesthetic choice), whereas other more targeted therapies (antioxidants, ischemic conditioning, modulators of MQC mechanisms), remain under investigation or are in development for use in selected settings.
KEY POINTS.
Mitochondria are key to cellular energy production, but are also vital to reactive oxygen species signaling, calcium hemostasis, and regulation of cell death.
Cardiac surgical patients with chronic comorbidities (diabetes, heart failure, advanced age, cardiomyopathies) may have preexisting mitochondrial dysfunction or be more sensitive to perioperative injury.
Mitochondrial dysfunction from ischemia/reperfusion injury and inflammatory responses to cardiopulmonary bypass and surgical tissue trauma impact myocardial contractility and predispose to arrhythmias.
Strategies for perioperative mitochondrial protection or recovery after injury include well-established cardioprotective protocols as well as a number of targeted therapies that remain under investigation.
Acknowledgments
Funding: This document was written with the support of: (1) NIH 5T32GM008600 (PI Warner).
(2) American Society of Transplantation: Transplantation and Immunology Research Network (AST TIRN) Basic Science Faculty Development Research Grant (PI Cherry). (3) American Society of Anesthesiologists: Foundation for Anesthesia Education and Research (FAER) Mentored Research Training Grant (PI Cherry).
Footnotes
Disclosure Statement: None.
REFERENCES
- 1.Westerhof N Cardiac work and efficiency. Cardiovasc Res 2000;48(1):4–7. [DOI] [PubMed] [Google Scholar]
- 2.Schaper J, Meiser E, Stammler G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res 1985;56(3):377–91. [DOI] [PubMed] [Google Scholar]
- 3.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 2005;85(3):1093–129. [DOI] [PubMed] [Google Scholar]
- 4.Wentz AE, d’Avignon DA, Weber ML, et al. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem 2010;285(32): 24447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Razeghi P, Young ME, Alcorn JL, et al. Metabolic gene expression in fetal and failing human heart. Circulation 2001;104(24):2923–31. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997; 96(7):2190–6. [DOI] [PubMed] [Google Scholar]
- 7.Kolwicz SC Jr, Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ Res 2013;113(5): 603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montaigne D, Marechal X, Coisne A, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 2014;130(7):554–64. [DOI] [PubMed] [Google Scholar]
- 9.Croston TL, Thapa D, Holden AA, et al. Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart. Am J Physiol Heart Circ Physiol 2014;307(1):H54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J, Yang R, Yang J, et al. Mitochondrial dysfunction-associated arrhythmogenic substrates in diabetes mellitus. Front Physiol 2018;9:1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank A, Bonney M, Bonney S, et al. Myocardial ischemia reperfusion injury: from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth 2012;16(3): 123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crompton M, Andreeva L. On the involvement of a mitochondrial pore in reperfusion injury. Basic Res Cardiol 1993;88(5):513–23. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths EJ, Halestrap AP. Protection by cyclosporin A of ischemia/reperfusioninduced damage in isolated rat hearts. J Mol Cell Cardiol 1993;25(12):1461–9. [DOI] [PubMed] [Google Scholar]
- 14.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014;24(10):R453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 1991;10(8):2247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Gabel S, Steenbergen C, et al. A redox-based mechanism for cardioprotection induced by ischemic preconditioning in perfused rat heart. Circ Res 1995; 77(2):424–9. [DOI] [PubMed] [Google Scholar]
- 17.Penna C, Rastaldo R, Mancardi D, et al. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol 2006; 101(2):180–9. [DOI] [PubMed] [Google Scholar]
- 18.Weinbrenner C, Schulze F, Sarvary L, et al. Remote preconditioning by infrarenal aortic occlusion is operative via delta1-opioid receptors and free radicals in vivo in the rat heart. Cardiovasc Res 2004;61(3):591–9. [DOI] [PubMed] [Google Scholar]
- 19.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res 2014;114(3):524–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol 2015;6:524–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mammucari C, Raffaello A, Vecellio Reane D, et al. Mitochondrial calcium uptake in organ physiology: from molecular mechanism to animal models. Pflugers Arch 2018;470(8):1165–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bliksoen M, Baysa A, Eide L, et al. Mitochondrial DNA damage and repair during ischemia-reperfusion injury of the heart. J Mol Cell Cardiol 2015;78:9–22. [DOI] [PubMed] [Google Scholar]
- 23.Keith M, Geranmayegan A, Sole MJ, et al. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol 1998;31(6):1352–6. [DOI] [PubMed] [Google Scholar]
- 24.Maack C, Kartes T, Kilter H, et al. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 2003;108(13):1567–74. [DOI] [PubMed] [Google Scholar]
- 25.Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science 2014;345(6203):1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Liu D, Zhang M, et al. Programmed necrosis in cardiomyocytes: mitochondria, death receptors and beyond. Br J Pharmacol 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chistiakov DA, Shkurat TP, Melnichenko AA, et al. The role of mitochondrial dysfunction in cardiovascular disease: a brief review. Ann Med 2018;50(2):121–7. [DOI] [PubMed] [Google Scholar]
- 28.Warren OJ, Smith AJ, Alexiou C, et al. The inflammatory response to cardiopulmonary bypass: part 1–mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009;23(2):223–31. [DOI] [PubMed] [Google Scholar]
- 29.Cherry AD, Piantadosi CA. Regulation of mitochondrial biogenesis and its intersection with inflammatory responses. Antioxid Redox Signal 2015;22(12):965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singer M Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med 2007;35(9 Suppl):S441–8. [DOI] [PubMed] [Google Scholar]
- 31.Tolerance Matzinger P., danger, and the extended family. Annu Rev Immunol 1994;12:991–1045. [DOI] [PubMed] [Google Scholar]
- 32.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 2004;4(6): 469–78. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010;464(7285):104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012;36(3):401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nan J, Zhu W, Rahman MS, et al. Molecular regulation of mitochondrial dynamics in cardiac disease. Biochim Biophys Acta Mol Cell Res 2017;1864(7):1260–73. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb RA, Thomas A. Mitophagy and mitochondrial quality control mechanisms in the heart. Curr Pathobiol Rep 2017;5(2):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tahrir FG, Langford D, Amini S, et al. Mitochondrial quality control in cardiac cells: mechanisms and role in cardiac cell injury and disease. J Cell Physiol 2019; 234(6):8122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andres AM, Tucker KC, Thomas A, et al. Mitophagy and mitochondrial biogenesis in atrial tissue of patients undergoing heart surgery with cardiopulmonary bypass. JCI Insight 2017;2(4):e89303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denham NC, Pearman CM, Caldwell JL, et al. Calcium in the Pathophysiology of Atrial Fibrillation and Heart Failure. Front Physiol 2018;9:1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Xu S, Xu Y, et al. Relation of mitochondrial DNA copy number in peripheral blood to postoperative atrial fibrillation after isolated off-pump coronary artery bypass grafting. Am J Cardiol 2017;119(3):473–7. [DOI] [PubMed] [Google Scholar]
- 41.Sandler N, Kaczmarek E, Itagaki K, et al. Mitochondrial DAMPs are released during cardiopulmonary bypass surgery and are associated with postoperative atrial fibrillation. Heart Lung Circ 2018;27(1):122–9. [DOI] [PubMed] [Google Scholar]
- 42.Montaigne D, Marechal X, Lefebvre P, et al. Mitochondrial dysfunction as an arrhythmogenic substrate: a translational proof-of-concept study in patients with metabolic syndrome in whom post-operative atrial fibrillation develops. J Am Coll Cardiol 2013;62(16):1466–73. [DOI] [PubMed] [Google Scholar]
- 43.Emelyanova L, Ashary Z, Cosic M, et al. Selective downregulation of mitochondrial electron transport chain activity and increased oxidative stress in human atrial fibrillation. Am J Physiol Heart Circ Physiol 2016;311(1):H54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SH, Doliba N, Osbakken M, et al. Improvement of myocardial mitochondrial function after hemodynamic support with left ventricular assist devices in patients with heart failure. J Thorac Cardiovasc Surg 1998;116(2):344–9. [DOI] [PubMed] [Google Scholar]
- 45.Heerdt PM, Schlame M, Jehle R, et al. Disease-specific remodeling of cardiac mitochondria after a left ventricular assist device. Ann Thorac Surg 2002;73(4): 1216–21. [DOI] [PubMed] [Google Scholar]
- 46.Kobashigawa J, Zuckermann A, Macdonald P, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant 2014;33(4):327–40. [DOI] [PubMed] [Google Scholar]
- 47.Lesnefsky EJ, Chen Q, Hoppel CL. Mitochondrial metabolism in aging heart. Circ Res 2016;118(10):1593–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linnane AW, Marzuki S, Ozawa T, et al. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet 1989;1(8639): 642–5. [DOI] [PubMed] [Google Scholar]
- 49.Sugiyama S, Hattori K, Hayakawa M, et al. Quantitative analysis of age-associated accumulation of mitochondrial DNA with deletion in human hearts. Biochem Biophys Res Commun 1991;180(2):894–9. [DOI] [PubMed] [Google Scholar]
- 50.Judge S, Jang YM, Smith A, et al. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J 2005;19(3):419–21. [DOI] [PubMed] [Google Scholar]
- 51.Lesnefsky EJ, Lundergan CF, Hodgson JM, et al. Increased left ventricular dysfunction in elderly patients despite successful thrombolysis: the GUSTO-I angiographic experience. J Am Coll Cardiol 1996;28(2):331–7. [DOI] [PubMed] [Google Scholar]
- 52.Lesnefsky EJ, He D, Moghaddas S, et al. Reversal of mitochondrial defects before ischemia protects the aged heart. FASEB J 2006;20(9):1543–5. [DOI] [PubMed] [Google Scholar]
- 53.Wei J, Gao DF, Wang H, et al. Impairment of myocardial mitochondria in viral myocardial disease and its reflective window in peripheral cells. PLoS One 2014;9(12):e116239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watts JA, Kline JA, Thornton LR, et al. Metabolic dysfunction and depletion of mitochondria in hearts of septic rats. J Mol Cell Cardiol 2004;36(1):141–50. [DOI] [PubMed] [Google Scholar]
- 55.El-Hattab AW, Scaglia F. Mitochondrial cardiomyopathies. Front Cardiovasc Med 2016;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vakrou S, Abraham MR. Hypertrophic cardiomyopathy: a heart in need of an energy bar? Front Physiol 2014;5:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Z, Laubach VE, French BA, et al. Acute hyperglycemia enhances oxidative stress and exacerbates myocardial infarction by activating nicotinamide adenine dinucleotide phosphate oxidase during reperfusion. J Thorac Cardiovasc Surg 2009;137(3):723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doenst T, Wijeysundera D, Karkouti K, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2005;130(4):1144. [DOI] [PubMed] [Google Scholar]
- 59.Navaratnarajah M, Rea R, Evans R, et al. Effect of glycaemic control on complications following cardiac surgery: literature review. J Cardiothorac Surg 2018; 13(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Girish G, Agarwal S, Satsangi DK, et al. Glycemic control in cardiac surgery: rationale and current evidence. Ann Card Anaesth 2014;17(3):222–8. [DOI] [PubMed] [Google Scholar]
- 61.Lomivorotov V Calcium administration in patients undergoing cardiac surgery under cardiopulmonary bypass (ICARUS Trial): prospective randomized, double-blind placebo-controlled superiority trial. 2019. NCT Number: NCT03772990 Available at: https://clinicaltrials.gov/ct2/show/NCT03772990. Accessed January 15, 2019.
- 62.Zakkar M, Guida G, Suleiman MS, et al. Cardiopulmonary bypass and oxidative stress. Oxid Med Cell Longev 2015;2015:189863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogers CA, Bryan AJ, Nash R, et al. Propofol cardioplegia: a single-center, placebo-controlled, randomized controlled trial. J Thorac Cardiovasc Surg 2015; 150(6):1610–9.e13. [DOI] [PubMed] [Google Scholar]
- 64.Ansley DM, Raedschelders K, Choi PT, et al. Propofol cardioprotection for on-pump aortocoronary bypass surgery in patients with type 2 diabetes mellitus (PRO-TECT II): a phase 2 randomized-controlled trial. Can J Anaesth 2016; 63(4):442–53. [DOI] [PubMed] [Google Scholar]
- 65.Li F, Yuan Y. Meta-analysis of the cardioprotective effect of sevoflurane versus propofol during cardiac surgery. BMC Anesthesiol 2015;15:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang XL, Wang D, Zhang GY, et al. Comparison of the myocardial protective effect of sevoflurane versus propofol in patients undergoing heart valve replacement surgery with cardiopulmonary bypass. BMC Anesthesiol 2017;17(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pietersen HG, Langenberg CJ, Geskes G, et al. Myocardial substrate uptake and oxidation during and after routine cardiac surgery. J Thorac Cardiovasc Surg 1999;118(1):71–80. [DOI] [PubMed] [Google Scholar]
- 68.Rosenkranz ER. Substrate enhancement of cardioplegic solution: experimental studies and clinical evaluation. Ann Thorac Surg 1995;60(3):797–800. [DOI] [PubMed] [Google Scholar]
- 69.Ali JM, Miles LF, Abu-Omar Y, et al. Global cardioplegia practices: results from the global cardiopulmonary bypass survey. J Extra Corpor Technol 2018;50(2): 83–93. [PMC free article] [PubMed] [Google Scholar]
- 70.Siddiqi S, Blackstone EH, Bakaeen FG. Bretschneider and del Nido solutions: are they safe for coronary artery bypass grafting? If so, how should we use them? J Card Surg 2018;33(5):229–34. [DOI] [PubMed] [Google Scholar]
- 71.Dobson GP, Faggian G, Onorati F, et al. Hyperkalemic cardioplegia for adult and pediatric surgery: end of an era? Front Physiol 2013;4:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoyer A, Lehmann S, Mende M, et al. Custodiol versus cold Calafiore for elective cardiac arrest in isolated aortic valve replacement: a propensity–matched analysis of 7263 patients. Eur J Cardiothorac Surg 2017;52(2):303–9. [DOI] [PubMed] [Google Scholar]
- 73.Ferguson ZG, Yarborough DE, Jarvis BL, et al. Evidence-based medicine and myocardial protection–where is the evidence? Perfusion 2015;30(5):415–22. [DOI] [PubMed] [Google Scholar]
- 74.Beyersdorf F The use of controlled reperfusion strategies in cardiac surgery to minimize ischaemia/reperfusion damage. Cardiovasc Res 2009;83(2):262–8. [DOI] [PubMed] [Google Scholar]
- 75.Volpi S, Ali JM, De Silva R. Does the use of a hot-shot lead to improved outcomes following adult cardiac surgery? Interact Cardiovasc Thorac Surg 2019;28(3): 473–7. [DOI] [PubMed] [Google Scholar]
- 76.Fan Y, Zhang AM, Xiao YB, et al. Glucose-insulin-potassium therapy in adult patients undergoing cardiac surgery: a meta-analysis. Eur J Cardiothorac Surg 2011;40(1):192–9. [DOI] [PubMed] [Google Scholar]
- 77.Miles LF, Coulson TG, Galhardo C, et al. Pump priming practices and anticoagulation in cardiac surgery: results from the global cardiopulmonary bypass survey. Anesth Analg 2017;125(6):1871–7. [DOI] [PubMed] [Google Scholar]
- 78.Larsen M, Webb G, Kennington S, et al. Mannitol in cardioplegia as an oxygen free radical scavenger measured by malondialdehyde. Perfusion 2002; 17(1):51–5. [DOI] [PubMed] [Google Scholar]
- 79.Jones DP. Redefining oxidative stress. Antioxid Redox Signal 2006;8(9–10): 1865–79. [DOI] [PubMed] [Google Scholar]
- 80.Muntean DM, Sturza A, Daunilau MD, et al. The role of mitochondrial reactive oxygen species in cardiovascular injury and protective strategies. Oxid Med Cell Longev 2016;2016:8254942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sprick JD, Mallet RT, Przyklenk K, et al. Ischaemic and hypoxic conditioning: potential for protection of vital organs. Exp Physiol 2019;104(3):278–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heusch G Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 2015;116(4):674–99. [DOI] [PubMed] [Google Scholar]
- 83.Candilio L, Hausenloy D. Is there a role for ischaemic conditioning in cardiac surgery? F1000Res 2017;6:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Picca A, Mankowski RT, Burman JL, et al. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol 2018;15(9): 543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res 2010;107(7):825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cherry AD, Suliman HB, Bartz RB, et al. Peroxisome proliferator-activated receptor gamma co-activator 1 alpha as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in S. aureus sepsis. J Biol Chem 2014;289(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Habertheuer A, Kocher A, Laufer G, et al. Cardioprotection: a review of current practice in global ischemia and future translational perspective. Biomed Res Int 2014;2014:325725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim K, Ball C, Grady P, et al. Use of del Nido cardioplegia for adult cardiac surgery at the Cleveland Clinic: perfusion implications. J Extra Corpor Technol 2014; 46(4):317–23. [PMC free article] [PubMed] [Google Scholar]
- 89.Onorati F, Santini F, Dandale R, et al. “Polarizing” microplegia improves cardiac cycle efficiency after CABG for unstable angina. Int J Cardiol 2013;167(6): 2739–46. [DOI] [PubMed] [Google Scholar]
- 90.Vinten-Johansen J Whole blood cardioplegia: do we still need to dilute? J Extra Corpor Technol 2016;48(2):P9–14. [PMC free article] [PubMed] [Google Scholar]