Abstract
According to the World Health Organization, currently, over 880 million people are at risk of acquiring the lymphatic filariasis (LF) infection in over 52 countries worldwide. Current approaches to control LF by 2020 are reaching short of its anticipated goal. Several studies suggest the existence of protective immunity against LF in the human. Thus, it is possible to develop a prophylactic vaccine against LF infections in the human. Several potential vaccine candidates were identified and tested for their vaccine potential against LF. Preclinical studies to date suggest that it is possible to develop a prophylactic vaccine against LF. Much work needs to be done, but it is clear that a prophylactic vaccine combined with targeted chemotherapy is critically required for eliminating LF worldwide.
Keywords: Lymphatic filariasis, vaccine, control, MDA
Current status of LF control and the need for a prophylactic vaccine.
Lymphatic filariasis (LF) is a mosquito-transmitted neglected tropical parasitic infection that affects 120 million people living in 52 countries (Box 1)i. At present, annual mass drug administration (MDA, see Glossary) with morbidity management (Box 2) and vector control is the only main strategy used for controlling LF infection worldwide [1–3]. Targeting the endosymbiont bacteria of lymphatic filariasis, Wolbachia with newer drugs is also showing great promise in clearing adult worms and has enormous potential in the strategy towards the elimination of LF [4]. According to the WHO, 52 countries still have not eliminated LF [2]i. Thus, the goal of LF elimination by 2020 is potentially not achievable. Therefore, there is a critical need to develop a more effective and long-lasting prophylactic strategy such as a vaccine to control and stop the spread of this parasitic infection in the endemic regions [5–7]. It is well established that vaccination can induce herd immunity and have been successful in controlling and eradicating several infectious agents over the past century [8]. Studies during the last five decades have attempted to develop a vaccine for LF, reviewed in [5–7]. However, progress has been considerably slow. Several factors may have contributed to this, such as, (i) the complex life cycle of these parasite, (ii) complex host immune responses to the parasite, (iii) the lack of suitable animals models to study LF, (iv) paucity of evidence supporting the presence of natural protective immunity to LF in the human and (v) the limited information on the characteristics of a protective immune responses to LF parasite in the animals and human [6]. Report by Paciorkowski et al [9] demonstrated that early resistance to LF parasite is mediated by a humoral immune response and they postulated that it is possible to develop a prophylactic vaccine against LF. Subsequent antigen discovery programs identified and characterized several potential candidate antigens of LF [7]. Some of these proved to be excellent vaccine candidates and are moving closer to clinical trials in the human. It is high time that the LF community and concerned stakeholders seriously consider LF vaccine as an important strategy along with chemotherapy for the control and total elimination of LF. Five decades of research have produced enormous data on immunology of lymphatic filariasis and vaccine development. This review will mainly, focus only on the current status of vaccine development against LF and the future prospects for developing a prophylactic vaccine against LF.
Box 1. Overview of the Lymphatic Filariasis infection.
Ancient artifacts recovered from the Nile region and Nok civilization in West Africa suggest that the lymphatic filariasis (LF) disease was present in the human as early as 2000BC [70]. The disease is caused by two major filarial parasites, Wuchereria bancrofti and Brugia malayi. It was not until 1900 that it was discovered that LF is transmitted by a mosquito vector. The adult parasites mainly reside inside the lymphatic vessels of the lower extremities, scrotum, inguinal canal, breast in the case of females, thorax, and upper limb of infected subjects causing severe inflammation and edema leading to hydrocele, cellulitis, lymphangitis, and lymphadenitis (adenolymphangitis and acute dermatolymphangioadenitis) [68, 71]. Immunocompromised status results in increased opportunistic infections in the foot leading to the formation of fibrotic tissue, typical of the elephantiasis. The larval forms of the parasite (microfilaria) circulate in the peripheral blood and are picked up by the mosquito vector during a blood meal. The larvae undergo further development within the mosquito and become infective larvae in about 10 days. According to the World Health Organization (WHO), LF is the second leading cause of permanent disability in the world with DALYs (disability-adjusted life years) estimated to be around 5.8 million [70]. Patients with acute LF infections do not show any clinical symptoms. However, chronically infected subjects are physically disabled [68]. The chronically infected individuals may also suffer from mental conditions due to social stigma and financial losses. Currently, about 120 million people are infected with LF and about 886 million people are at risk of acquiring LF in 52 different countries worldwide [1, 2, 70]i.
Box 2. Current status of Mass Drug Administration (MDA).
The WHO launched its Global Programme to Eliminate Lymphatic Filariasis (GPELF) in 2000, with an aim to eliminate LF by 2020 using MDA preventive chemotherapy as its prime approach to interrupt the transmission of LF [2,70–72]. MDA approach uses a single drug (Diethylcarbamazine, DEC) or a combination of drugs (DEC, Albendazole, Ivermectin) that are given annually or semiannually to all the subjects including children in a community who are at risk of acquiring the infection [2]iv. Treatment for LF is based on the clinical presentation of the disease. Asymptomatic cases are treated with a combination of DEC (6 mg/kg) and albendazole (400 mg/kg) in an outpatient setting [71]. In Africa, where onchocerciasis is also prevalent, a combination of albendazole (400 mg/kg) and ivermectin (150–200 mg/kg) are used instead. Acute adenolymphangitis is treated with a course of DEC combined with albendazole or with ivermectin and analgesics [71]. In addition, antihistaminic and steroids are also given to reduce swelling and hypersensitivity along with antibiotics to treat secondary infections if any. There is no radical cure for patients with lymphedema and the chronic pathology in these patients is largely initiated by the adult parasites. Before initiating the MDA program, about 83 countries were endemic for LF infection [70]. The massive worldwide network of drug distribution and excellent control effort by a number of stakeholders over the past 20 years has significantly paid off in decreasing the spread of the infection in several countries [2, 71]. Since the start of the MDA program, 37 countries have reported a reduction in the transmission of LF, all these attributable to MDA approach thanks to the excellent effort by the GPELF team and several stakeholders [2]. As of 2019, 14 countries including China and the Republic of Korea have declared as having eliminated LF as a public health problem. China adopted DEC-fortified salt as a strategy for eliminating LF, whereas, the Republic of Korea used MDA plus targeted therapy of microfilaria positive individuals with DEC as a strategy for eliminating LF [72]. Thus, the MDA has been successful in decreasing the incidence of LF in several parts of the world, however, has not achieved the goal of total elimination by 2020 as originally proposed [2].
The rationale for developing a vaccine for LF
Several infectious diseases were successfully eliminated and eradicated from the world using the prophylactic vaccine [8]ii. Thus, in addition to the MDA and anti-Wolbachia approach, a prophylactic vaccine has great promise in the control and elimination of LF. However, there is no commercially licensed prophylactic vaccine available for LF. With over 880 million people at risk, there is great potential for developing a vaccine for LF. An effective prophylactic vaccine should confer long-lasting and robust protective immune responses in the vaccinated individual.
Immune response to infections with LF parasites in the human
The canonical host immune responses to LF infection in the human is the development of a strong Th2 type response mediated by Type 2 innate lymphoid cells (ILCs), which in turn results in the expansion of IL-10 producing CD4+ T cells and T regulatory cells (Tregs) that promote a generalized diminished Th1 response to the antigens [10–14]. This downregulation of key immune cells, molecules and mediators potentially help the parasite to survive in the hostile environment of the lymphatic system, where potential effector cells circulate around the parasite [10]. The larval form of the parasite, microfilaria is notorious for impairing the immune responses in the infected individuals [13]. Analysis of the parasite genome and proteome reveal the presence of several molecules including homolog of macrophage migration inhibition factor (MIF), homolog of cytokine and chemokine mimics and antagonists, regulatory and suppressive factors (serpins and cystatins), developmental regulators and molecules such as ES-62, can suppress the proliferation of all major immune cells and downregulate expression of effector molecules in the host cells [12]. This subdued response to the parasite is also supported by the development of specific antibody isotypes (IgG1, IgG4, and IgE) secreted by B cells and the development of macrophages that are alternatively activated [10–14]. The immunosuppressive effect induced by the LF parasite also results in poor immune responses to vaccination, allergens and other infectious agents in the infected individuals [15]. The remarkable ability of the parasite to manipulate host immune responses, and its persistence for several years within the lymphatics, lend to the controversy as to whether it is possible to develop protective immunity against LF?
Can humans develop protective immunity against LF?
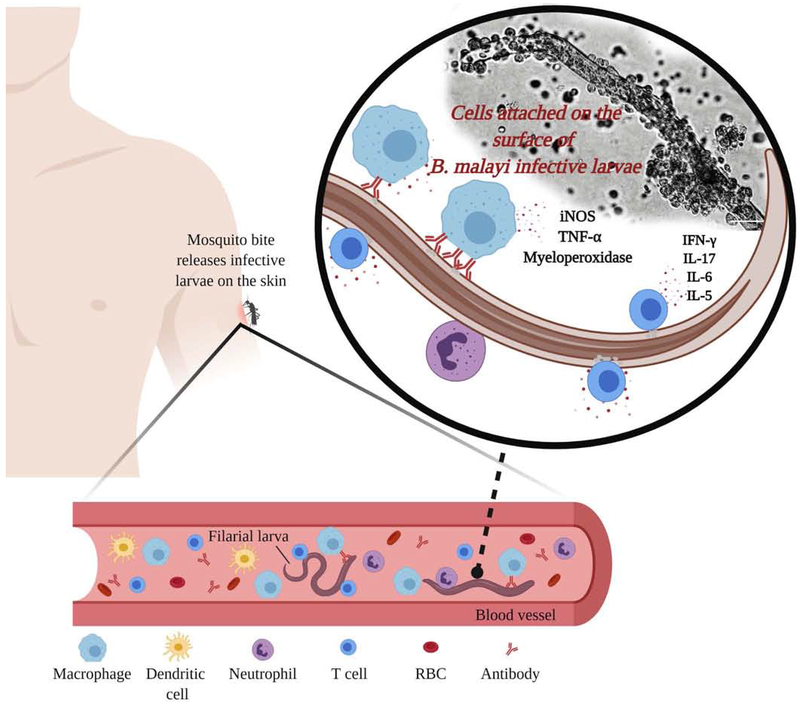
Several published evidence confirm that protective immunity against LF does exist in the human [11, 16]. The most convincing evidence is the finding that there is an age-dependent decrease in the prevalence of infection. Younger individuals are more susceptible to LF infection compared to older subjects [16]. Thus, the development of protective immunity to LF appears to increase with age. Further confirmation comes from the mathematical modeling studies by Michael et al, which suggests the existence of herd immunity for LF in the human communities [11]. Similarly, studies in the animal models using Acanthocheilonema viteae show that the presence of parasites (even if they are trapped in the tissue) from a previous infection can partially protect the host against homologous superinfection as a result of concomitant immunity [6]. It is not known if concomitant immunity plays a role in the diminished clinical presentation of LF infection in the human. Finally, aside from the concomitant immunity, certain individuals living in the endemic regions are resistant to the LF infection. These individuals called the “Endemic Normals” (EN) and they carry high titer of circulating IgG antibodies against the infective third-stage larvae (L3) of the parasite in their blood [17–19]. When the peripheral blood mononuclear cells and sera from these EN subjects were exposed ex vivo to the infective stages of the parasite in antibody-dependent cell-mediated cytotoxicity (ADCC) assay, significant numbers of the larvae were killed, suggesting the presence of protective antibodies [19–21]. Several macrophages were found attached to the dead larvae [22]. These macrophages secreted myeloperoxidases, nitric oxide, and damaging nitrogen intermediates, which are believed to play a role in the killing of the L3 stages of the parasite. Thus, the protective immune responses in the EN subjects appear to be targeted towards the L3 stages (Figure 1). The human can also develop partial protection against microfilaria and adult stages of the parasite [23]. However, the most efficient form of protective immunity would be directed against the infective or early developing larval stages of the parasite [16,19] because L3 is the most vulnerable stage of the parasite in the mammalian host in terms of its size as well as biological requirements. Similarly, L3 must rapidly adapt to its changing environment, from mosquitoes to the mammalian host. After entry into the body, L3 must wade its way through the subcutaneous tissue into presumably the nearest lymphatic vessel, where it needs to rapidly metabolize, grow in size and develop and molt within about 10–12 days into L4 stage [5, 16]. The existence of protective antibodies in EN subjects demonstrated for the first time that it is possible to develop a vaccine against LF in the human. There appear to be no clear genetic differences in the development of protective immunity in the EN subjects since these putative immune individuals are reported from different communities around the world [18].
Figure 1: Human can develop protective immune responses against lymphatic filariasis (LF).
In the endemic region, certain individuals who are naturally immune to the LF infection carry high titer of parasite-specific IgG1, IgG2 and IgG3 antibodies in their peripheral circulation. These antibodies were protective in an antibody-dependent cell-mediated cytotoxicity mechanism. When an infected mosquito transfer the infective larvae of LF into the body of immune individuals, the protective antibodies and CD11b+, FcγR1 bearing macrophages that produce copious amounts of myeloperoxidases and nitric oxide can bind to the surface of B. malayi L3 larva resulting in the death of the larva. IFN-γ, IL-17, IL-6 and IL-5 secreting cells were also found attached to the dead larva. Depletion of IgG antibodies prevented cell adherence and larval death suggesting that IgG antibodies and macrophages are critical for the protective immune responses in the human. The figure was created with BioRender.com.
Lack of suitable animal models handicapped the development of a vaccine for LF
Mice, jirds, and mastomys are extensively used as experimental models to characterize potential vaccine candidates against LF [6]. Nearly 90% of infections in the human are due to Wuchereria bancrofti. However, nearly all of the vaccine development studies used the B. malayi or B. pahangi model to evaluate vaccine efficacy. This is mainly because of the difficulty in maintaining the W. bancrofti life cycle under laboratory conditions in rodents. Rodents are good model, but the Brugia parasites do not develop into adult worms in immunocompetent mice. However, the brugia parasite can develop into adult worms in nude mice, but this model cannot be used for vaccine development studies [6, 22]. Jird and mastomys are permissive hosts for brugia parasites. The parasites can develop into adults in these animals and produce microfilariae that appear in the peripheral blood similar to that in the human. Therefore, these models are widely used in challenge studies, where parasite establishment can be determined in vaccinated animals [6, 22]. This model, however, has some drawbacks in that there are no specific reagents available to measure immunological parameters in jirds and mastomys. Although not well characterized, cross-reactive mouse immunological reagents (antibodies) were used in several publications to analyze the immune responses in jirds and mastomys [6, 24–35]. Similarly, jirds and mastomys do not develop the typical lymphatic pathology that is present in LF infected humans, so it is not possible to study the LF pathology in this model. Non-human primates can be infected with both W. bancrofti and B. malayi and the infections resemble a human infection with typical lymphatic pathology. Therefore, non-human primates are considered as one of the best models available to study the host-parasite relationships and immunity to LF [36–38], despite the cost and problems associated with handling the animals.
Dogs, cats, and ferrets are natural hosts of B. malayi parasites [6,39,40] and zoonotic infections are common in the endemic areas, where dogs and cats carry the infection in nature and transmit the infection to humans [41]. Infected dogs and ferrets develop clinical lymphedema, scrotal enlargement, conjunctivitis, and lymphangitis similar to the human [42]. However, the pathology is transient, and are not as severe as in the human. Dogs and cats can also be infected with B. malayi under laboratory conditions [6,42]. Since dogs and cats are natural hosts for B. malayi infection, they are probably an ideal model for studying the immune responses to vaccine candidates and to develop a prophylactic vaccine against LF infection.
Historical attempts to develop a vaccine for LF
Initial attempts to develop a protective immune response against LF using homogenates of adult worms or infective larvae were largely unsuccessful, reviewed in [5–7]. Subsequently, several attempts were made to induce protective immune responses by (i) implanting the parasite into an abnormal site, (ii) infecting with a strain of the parasite which would not become patent in the host, (iii) injecting dead parasites, (iv) inoculating live worms attenuated by artificial means (X-irradiation or γ-irradiation), (v) chemically abbreviated infections, and/or (vi) by injecting the metabolites collected from the culture supernatants of live worms gave varying results [5,7,16]. In 1969, Dr. Ramachandran and his team attempted to immunize rhesus macaque with various antigenic preparations including X-ray concomi live parasites and demonstrated that five out of seven rhesus macaques immunized with the radiation attenuated larvae of B. malayi did not show any signs of the infection for up to six months after challenge with 100 infective larvae [36]. This showed for the first time that it is possible to induce protective immunity against LF, at least in experimental animal models. Subsequently, several studies demonstrated that γ-irradiation (10-15 kR) attenuated parasites can confer over 91% and 98% protection in jirds and cats respectively against challenge infections [5–7]. These initial studies strongly suggested that it is possible to develop an effective prophylactic vaccine against LF. However, live attenuated or dead parasites are not suitable for developing as a vaccine for the human. Nevertheless, these earlier studies provided a strong base for subsequent studies that attempted to develop a vaccine for LF.
Identification of vaccine antigens
Completion of the B. malayi genome project, and the characterization of the transcriptome and secretome of the parasite exploded the field of vaccine antigen discovery by allowing researchers to screen the genome of the parasite to identify potential vaccine candidates [43]. Although inter- and intra-species variations in the genome of LF parasites have not been done, available data on the comparison of select genes between W. bancrofti and B. malayi show significant homology (>95% similarity) [44]. Therefore, in selecting a vaccine candidate, it is important to select antigens that are highly homologous in both the species, because W. bancrofti is the major parasite that infects the human. Similarly, extensive studies using the rodent filarial parasite, Litomosoides sigmodontis [45] and the bovine parasite Setaria cervi [5] also provided several valuable data in identifying potential candidate antigens for developing the vaccine against LF and in understanding the host immune responses to filarial parasites. Previous studies showed that IgG1 and IgG3 antibodies in the peripheral circulation of EN subjects are critical for killing the B. malayi infective larvae in an ADCC reaction [46–48]. Development of these cytophilic antibodies in the human correlated with absence of infection [48, 49]. Thus, antigens or peptides that can elicit these protective antibodies in the EN subjects are an attractive target for developing as prophylactic vaccine candidates [6]. Screening the genome of the W. bancrofti parasite with the protective antibodies from EN subjects, or immune animals can be a good starting point to identify the candidate antigens [47]. Using a similar approach we reported several promising vaccine antigens of W. bancrofti and B. malayi that showed high similarity (Figure 2). Some of these candidate antigens include abundant larval transcript 2 (ALT2) [50], thioredoxin peroxidase (TPX) [51], collagen 4 (Col4) [47], tetraspanin (TSP) [52], vespid venom allergen (VAH) [53], heat shock protein (HSP) [54], and glutathione S-transferases (GST) [55]. One of the major considerations in selecting the vaccine candidate is that the antigens should be expressed on the surface of the infective stages of the parasite and are thus easily accessible to the human immune system. Similarly, one of the most consistent findings in filarial infections is the elevated level of IgE that is observed following L3 exposure [10]. Therefore, the selected antigens should have minimal or no homology to human proteins and should not elicit any IgE or IgG4 responses to prevent any adverse effects [56].
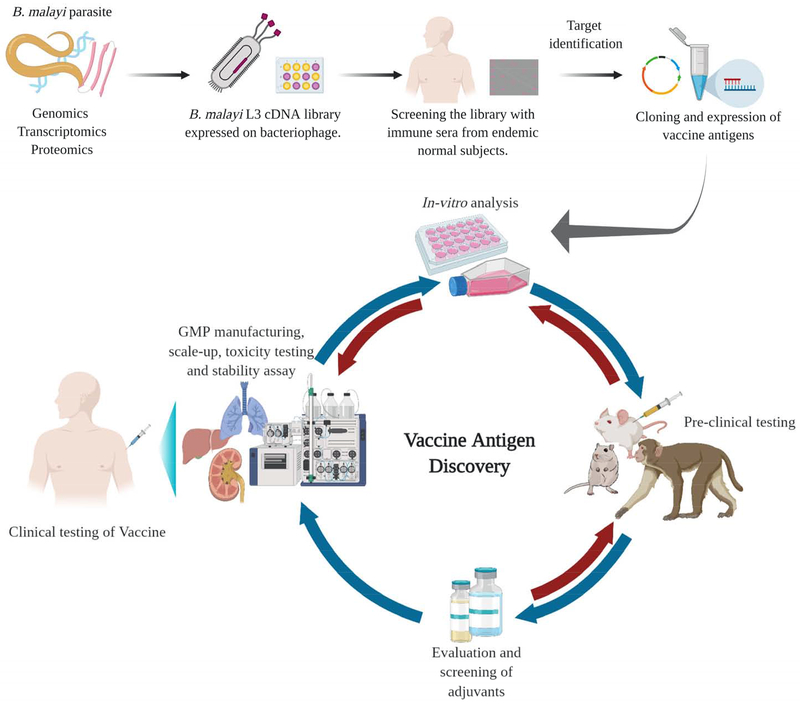
Figure 2: Vaccine antigen discovery pipeline for developing a prophylactic vaccine for LF.
The completion of the genome project, identification of the transcriptome and secretome of the B. malayi and W. bancrofti parasites gave a tremendous boost to the identification of target vaccine candidate antigens of LF. It is now well established that in the endemic area certain subjects (EN) carry protective antibodies against LF in their peripheral blood and remain uninfected. Recent advances in the genomic screening have enabled us to screen the genome of LF parasites with the immune sera from EN subjects and identify antigens that are specifically recognized by the protective antibodies. Using a bioinformatics approach, the target antigens are then identified, cloned, expressed and evaluated for their vaccine potential using in vitro assays. Promising candidates among these along with suitable adjuvants are then tested in various experimental animal models for their pre-clinical vaccine efficacy. The most promising vaccine candidates are then taken up for GMP manufacturing, scale-up production, toxicity, and stability testing before being developed for clinical testing in the human. The figure was created with BioRender.com.
Current approaches to develop a vaccine for LF
Multivalent antigens gave better protection
Nearly all attempts to develop a vaccine against LF with a single antigen (monovalent) gave unsatisfactory results [50–55]. LF is a multicellular organism that uses multiple approaches to evade host immune responses and survive in the host. Therefore, targeting a single critical antigen will not have the desired effect, since the LF parasites are notorious for using redundant mechanisms to escape host insults. Subsequent approaches to combine potential vaccine antigens as cocktail vaccines [24,30,51], chimeric antigen [60], multisubunit and multi epitope vaccines [31,32,35,60,62] or multivalent vaccines [37,38,46,61], gave excellent results when tested in experimental rodent models. Thus, vaccine development against LF started focusing more on combining one or more antigens and testing their vaccine potential in experimental animals. Various combinations of antigens were evaluated as multivalent vaccines. Among these, the combination of ALT-2 [50], small heat shock protein 12.6 (HSP) [54], thioredoxin peroxidase 2 [51] and tetraspanin large extracellular loop (TSP) [52] as a recombinant multivalent fusion protein vaccine (rBmHAXT) gave close to 88-94% protection against challenge infections in rodents [46, 61] and about 57% protection against challenge infections in rhesus macaques [38]. To date, this is probably one of the leading prophylactic vaccine formulations for LF. Table 1 is a partial list that summarizes the most promising prophylactic vaccines that were developed for LF during the last 10 years. There is a need to identify and test more vaccine candidates against LF.
Table 1.
Summary of the most successful recombinant protein vaccines for LF reported in the last 10 years
| Recombinant vaccine candidates | Percent Protection | Animal model used | References |
|---|---|---|---|
| Monovalent vaccine | |||
| TGA | 30% | Jirds | [24] |
| TPX | 43% | Jirds | [24] |
| GST | 61% | Jirds | [25] |
| HSP12.6 | 58% | Mice | [54] |
| TSP | 60% | Mice | [52] |
| TPP | 71% | Mastomys coucha | [26] |
| DIM-1 | 50% | Mastomys coucha | [27] |
| Troponin 1 | 65% | Mice | [59] |
| Heavy chain myosin | 84% | Mice | [57] |
| ALT-2 with Tuftsin | 65% | Mice | [58] |
| FAR binding proteins | 68% | Jirds | [28] |
| Calponin | 42% | Mastomys coucha | [29] |
| Cocktail vaccine | |||
| TGA+TPX | 74% | Jirds | [24] |
| ALT-2+TPX | 80% | Mice | [51] |
| TRX+TPX | 71% | Mastomys coucha | [30] |
| Myosin+ TPP | 70% | Jirds | [32] |
| iPGM + TPP | 70% | Jirds | [32] |
| VAL-1+ALT-2 | 77-80% | Jirds | [33] |
| Bivalent vaccine | |||
| HSP12.6+ALT-2 | 90% | Mice | [46] |
| HSP12.6+TSP | 80% | Mice | [46] |
| TSP+ALT-2 | 82% | Mice | [46] |
| Bm-103, Bm-RAL-2 | 61% | Jirds | [28] |
| Multivalent vaccine | |||
| Multi antigen peptide (MAP) | 63% | Jirds | [31] |
| Multiepitope | 75% | Mastomys coucha | [35] |
| HAT | 94% | Mice | [46] |
| Chimeric multistage filarial epitope protein (FEP) | 70% | Jirds | [60] |
| HAT | 45% | Rhesus macaque | [37] |
| HAXT | 88% | Mice | [61] |
| HAXT | 57% | Rhesus macaque | [38] |
Adjuvant formulations for LF vaccine
Peptide antigens require an adjuvant to induce early activation of the innate immunity and to induce broader adaptive protective immune responses. Adjuvants are also critical for boosting the select type of immune responses that are critical for generating protective immune responses. Analysis of the immune responses against LF in the human suggested that broader and balanced immune responses (IgG1, IgG2, and IgG3) against the parasite antigens is essential for the protective immunity [46–48]. These studies also demonstrated that depletion of any one of these antibody isotypes diminished the protective immune responses [46]. Thus, choosing the right adjuvant is critical for eliciting the best vaccine-induced responses. The majority of vaccination trials against LF in the experimental rodents used various formulations of alum. Unfortunately, alum did not promote the desired level of protection in non-human primates [37]. In an attempt to improve the vaccine-induced protection, some of the recent studies used analogs of TLR-4 or tuftsin to improve the protective immune responses and these studies demonstrated promising results [38,58,63]. In selecting the adjuvant, it is important to consider the fact that the potential end-users of the adjuvant will be the human.
Future prospects of a prophylactic vaccine against LF
We now know much about the human immune responses to LF and the potential mechanism by which the parasites modulate the host immune responses for its survival in the host [10–14, 16–19]. It is also well established that protective immune responses against LF exist in the human [11, 19–21]. However, the characteristics of the protective immune response to LF in the human is only partially defined. Understanding the basic characteristics of the protective immune responses to LF will help in the identification of a vaccine candidate and an adjuvant that can induce the desired protective responses in the human. There is also a need to study the longevity of these protective immune responses since LF is a slow-developing disease. Genome-wide screening offers an excellent tool to identify potential vaccine targets [64,65]. Completion of the genome of the parasites and the availability of transcriptome and secretome data on the larval stages of the parasite does provide an excellent tool for screening and identifying key molecules of the parasite that can be targeted to develop a prophylactic vaccine against the LF parasite [47,66]. The focus of such a vaccine should be to prevent the establishment of infective larvae (L3) following the bite of an infective mosquito [19,20]. If the vaccine is also effective against the microfilariae, transmission of the infection can be blocked as well [23]. The MDA approach has substantially reduced the incidence of LF infection in several parts of the world. In order to maintain the status quo and prevent future infections, it is critical that a more sustatinable approach such as a prophylactic vaccine be available. All available data to date suggest that it is feasible to develop a prophylactic vaccine against LF. History has proven time again that several infectious agents can be eliminated or eradicated from the world with a prophylactic vaccine [8], LF is not different [67]. LF infection is a slow-developing disease, unlike bacterial or viral infections and causes severe morbidity with little or no mortality [68]. Therefore, relapse or reemergence of the disease will take several years before it becomes a national concern. Inducing herd immunity with a prophylactic vaccine is a critical step in stopping the establishment and spread of the infection and for achieving elimination in a community [7,22,69].
Hurdles and roadblocks to the development of the LF vaccine
A major hurdle in the development of a prophylactic vaccine for LF is the willingness to manufacture a vaccine for the poor [56]. LF infection mainly affects subjects in economically poor countries, these individuals really need the vaccine but cannot afford the premium cost of the vaccine. Therefore, the manufacturing cost of the vaccine needs to be as minimum as possible so it can reach all population in the endemic region. A second major hurdle is the lack of a clear definition for the protective immune responses in the human [10,11]. This is very critical in designing and tailoring the vaccine to obtain maximum protective immune responses in the human. A third major hurdle is the lack of an ideal experimental animal model to study the vaccine-induced protection against LF [6]. Our work and others have demonstrated that findings in the mouse model do not translate well to vaccine-induced protection in the non-human primates [37]. Thus, the mouse model can be used to screen the vaccine antigens initially but is not a good model to study the protective immune response for a human vaccine. Finally, the setting of a global deadline for elimination of LF is not critically needed, this can be done regionally based on the needs. A vector transmitted infection with potential for zoonotic transmission in a community is very complex and requires a multipronged approach. This need to be taken into consideration in planning and executing the next steps in the control of LF in endemic regions, where the infection still remains and is spreading.
Concluding Remarks
LF infection continues to threaten 880 million people living in 52 different countries. About 40 million people suffer from the chronic consequences of the disease and several million people harbor the infection without showing any clinical symptoms. These asymptomatic individuals are what the parasite is counting on to spread the disease and to maintain their life cycle in the world. The WHO spearheaded, mass chemotherapy (MDA) in a community was to target these asymptomatic carriers with drugs to break the lifecycle and to achieve transmission interruption. For this, to occur, all asymptomatic carriers must be identified and treated on a regular basis. MDA approach successfully interrupted transmission in several parts of the world, but yet, total elimination has not achieved in the majority of the world. This could be partially due to the difficulty in diagnosing many patent and latent infections. Thus, there is a critical need for a more reliable, and affordable rapid diagnostic kits for detecting acute and latent cases (see Outstanding Questions). Drugs used in the current MDA approach clears only circulating microfilariae and are not efficient in killing adult parasites. Therefore, additional effective chemotherapeutic agents are needed to clear adult and larval parasites from infected individuals.
Outstanding Questions Box.
Can we develop a more integrated control approach for LF worldwide that will include diagnosis, targeted therapy, vector control, and a prophylactic vaccine?
Can we determine if zoonotic infections play a role in the persistence of LF transmission in the human?
Can we develop a suitable animal model to study lymphatic filarial infection under laboratory conditions?
Can we clearly define the benchmark for protective immune responses against LF in the human?
Can we identify more candidate vaccine antigens for LF that can confer better protection?
Is it possible to manufacture a better rapid diagnostic tool for mass detection of latent infections following mass vaccination.
Can we develop a prophylactic vaccine for LF that is fairly inexpensive and can be afforded by the population-at-risk in the endemic regions?
The recent discovery of new macrofilaricidal agents targeting the endosymbiont, Wolbachia has great potential for controlling LF. Nevertheless, drugs can only cure current infections and will not prevent future infections unless the drugs or their active metabolites are cleared slowly from the system and they remain in the circulation for a longer duration. Therefore, there is a need to develop a more sustainable prophylactic approach. Research over 250 years has clearly proven that infectious diseases can be eliminated and even eradicated with prophylactic vaccines [8]iii. Studies during the last 50 years have identified several potential vaccine candidates for LF and clearly provided evidence that it is possible to develop a prophylactic vaccine against LF. However, there is a need to more clearly define the protective immune responses in the human and identify more candidate vaccine antigens that can induce protective immune responses in the human. It is also important to develop a better animal model to study the protective immune responses to LF. In order to more efficiently control and eliminate LF from all endemic regions of the world, there is a need for improved diagnostic tools that can help in the rapid detection of all infected individuals including latent infections in a community. Finally, the availability of a targeted chemotherapy that is effective against adult worms, supported by a strong vector control program and an effective prophylactic vaccine is essential for eliminatng LF from the endemic regions of the world. Given all the uncertainties around the globe, the timeline for achieving the LF elimination goal should be region-specific.
Highlights.
Current control strategy against lymphatic filariasis (LF) is effective but is progressing slowly causing concerns as to its longterm benefits worldwide on the LF control.
Vaccines have been successfully used over two centuries to control, eliminate and eradicate infectious diseases. Therefore, developing a prophylactic vaccine against LF can benefit millions of people at risk.
Completion of the genome, transcriptome, proteome, and secretome of LF parasite have helped identify several potential vaccine candidates that proved promising results in preclinical studies.
In order to achieve total elimination of LF from the world, there is an immediate need for a prophylactic vaccine that needs to be combined with a reliable diagnostic tool that helps in targeted chemotherapy along with a strong vector control program.
Acknowledgment
This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases grants R01AI072613 (RK).
Glossary
- Antibody-dependent cell-mediated cytotoxicity (ADCC)
is an immunological phenomenon where both cells and antibodies participate in the killing the target cells or organisms. In the protective immune response to LF, following the transfer of infective larvae into the human body by the mosquitoes during a blood meal, the protective antibodies in the sera of immune subjects will bind to the surface of the infective larvae. Fc receptor-bearing effector cells can now bind to the parasite bound antibodies, get activated, and eventually kill the antibody-coated infective larvae thus achieving protection
- Herd immunity
is a type of immunity that occurs when a significant portion of a population in a community (or herd) are vaccinated and they exhibit protection against the disease. Herd immunity will stop the spread of the disease in the community, especially for people who cannot receive the vaccine, such as children who are too young to be vaccinated, people with immune system problems, and those who are too ill to receive the vaccine
- Concomitant immunity
is the ability of a host to mount an effective protective defence against the newly entering infective stages of a parasite, while the parasite from the prior infection still persists in the body. This is a paradoxic immune status in the host, belived to be generated in response to the antigens released from the already established parasite. Concomitant immunity is demonstrated in infections with several helminth and protozoan parasites and tumors
- Mass drug administration (MDA)
is an approach is a strategy currently recommended by the World Health Organization (WHO) to control and eliminate neglected tropical diseases (NTDs) in a community. In this approach, selective chemotherapeutic drugs are given to all the subjects (children and adults) living in an area annually or at specific time intervals
- T regulatory cells (Tregs)
are a specialized subpopulation of T lymphocytes that can suppress the immune responses, thereby maintaining homeostasis and self-tolerance. Tregs promote this effect by inhibiting T cell proliferation and cytokine production by secreting inhibitory molecules. Tregs also play a central role in downregulating inflammation in autoimmunity
- Wolbachia
is a gram-negative bacteria that primarily infects mosquitoes and can spread to the lymphatic filariasis (LF) parasites. Wolbachia bacteria remains as an endosymbiont (an organism that lives within the body or cells of another organism in a mutualistic relationship) and is one of the most common endosymbiotic bacteria in nematode parasites that play a major role in the reproductive health of the parasite. Clearing of this bacterium from the parasite can affect the fertility of the female parasites and possibly can be lethal to the parasite
- Zoonotic infections
is a disease that can be transmitted from animals to human. Typically, the disease normally exists in animals but human gets infected with the zoonotic infection from animals. LF is considered a zoonotic infection. In nature, LF is maintained in cats and dogs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis EL et al. (2019) Evaluating the evidence for lymphatic filariasis elimination. Trends Parasitol. 35, 860–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichimori K et al. (2014) Global programme to eliminate lymphatic filariasis: The processes underly1ng programme success. PLoS Negl. Trop. D1s 8, e3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pi-Bansa S et al. (2019) Potential factors influencing lymphatic filariasis transmission in “hotspot” and “control” areas in Ghana: the importance of vectors. Infect. Dis. Poverty 8, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor MJ et al. (2019) Preclinical development of an oral anti-Wolbachia macrolide drug for the treatment of lymphatic filariasis and onchocerciasis. Sci. Translational Med 11, eaau2086. [DOI] [PubMed] [Google Scholar]
- 5.Babayan SA et al. (2012) Future prospects and challenges of vaccines against filariasis. Parasite Immunol. 34, 243–253 [DOI] [PubMed] [Google Scholar]
- 6.Morris CP et al. (2013) A Comprehensive, Model-Based Review of Vaccine and Repeat Infection Trials for Filariasis. Clin. Microbiol. Rev 26, 381–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalyanasundaram R (2018) Next step Lymphatic Filariasis Eradication: Current status in the development of a vaccine against Lymphatic Filariasis, Lymphatic Filariasis;. (Tyagi BK. ed) pp. 59–80, Springer Nature. [Google Scholar]
- 8.Andre FE et al. (2008) Vaccination greatly reduces disease, disability, death and inequity worldwide. WHO Bull. 86, 81–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paciorkowski N et al. (2000) B1 B lymphocytes play a critical role in host protection against lymphatic filarial parasites. J. Exp. Med 191, 731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babu S and Nutman T (2014) Immunology of lymphatic filariasis. Parasite Immunol. 36, 338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravindran B et al. (2003) Protective immunity in human lymphatic filariasis: problems and prospects. Med Microbiol Immunol. 192, 41–46 [DOI] [PubMed] [Google Scholar]
- 12.Nutman TB (2016) Immunology of lymphatic filariasis. Encyclopedia Immunobiol. 4, 142–149 [Google Scholar]
- 13.Metenou S and Nutman TB (2013) Regulatory T cell subsets in filarial infection and their function. Front. Immunol 4, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen JE and Maizels RM (2011) Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol 11, 375–388 [DOI] [PubMed] [Google Scholar]
- 15.Cooper PJ et al. (1999) Human onchocerciasis and tetanus vaccination: impact on the post vaccination anti-tetanus antibody response. Infect. Immun 67, 5951–5957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwarteng A and Ahuno ST (2017) Immunity in Filarial Infections: Lessons from Animal Models and Human Studies. Scand. J. Immunol 85, 251–257 [DOI] [PubMed] [Google Scholar]
- 17.Day KP (1991) The endemic normal in lymphatic filariasis: A static concept. Parasitol. Today 7, 341–343 [DOI] [PubMed] [Google Scholar]
- 18.Steel C and Ottesen EA (2001) Evolution of immunologic responsiveness of persons living in an area of endemic bancroftian filariasis: a 17-year follow-up. J. Infect. Dis 184, 73–79 [DOI] [PubMed] [Google Scholar]
- 19.Helmy H et al. (2000) Human antibody responses to Wuchereria bancrofti infective larvae. Parasite Immunol. 22,89–96 [DOI] [PubMed] [Google Scholar]
- 20.Sim BK et al. (1982) Immune responses in human Brugia malayi infections: serum dependent cell-mediated destruction of infective larvae in vitro. Trans. R. Soc. Trop. Med. Hyg 76, 362–370 [DOI] [PubMed] [Google Scholar]
- 21.Hoerauf A (2002) Immune effectors important in protective resistance The filaria. Eds Klei TR and Rajan TV pp 109–126, Kluwer Academic Publishers, New York. [Google Scholar]
- 22.Kalyanasundaram R (2016) Lymphatic Filariasis: Current Status of Elimination Using Chemotherapy and the Need for a Vaccine. Chapter 3 Communicable Diseases of the Developing World. Topics in Medicinal Chemistry. (Saxena AK ed) vol 29, pp 1–36. DOI: 10.1007/7355_2015_5002. Springer, Cham. [DOI] [Google Scholar]
- 23.Dissanayake S et al. (1995). Differential recognition of microfilarial chitinase, a transmission-blocking vaccine candidate antigen, by sera from patients with Brugian and Bancroftian filariasis. Am. J. Trop. Med. Hyg 53,289–29425. [PubMed] [Google Scholar]
- 24.Vanam U et al. (2009) Evaluation of immunoprophylactic efficacy of Brugia malayi transglutaminase (BmTGA) in single and multiple antigen vaccination with BmALT-2 and BmTPX for human lymphatic filariasis. Am. J. Trop. Med. Hyg 80, 319–324. [PubMed] [Google Scholar]
- 25.Veerapathran A et al. (2009) Evaluation of Wuchereria bancrofti GST as a vaccine candidate for lymphatic filariasis. PLoS Negl. Trop. Dis 3, e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushwa V et al. (2013) Immunization of Mastomys coucha with Brugia malayi Recombinant trehalose-6-phosphate phosphatase results in significant protection against homologous challenge infection. PLoS One 8, e72585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kushwaha V et al. (2014) Disorganized muscle protein-1 (DIM-1) of filarial parasite Brugia malayi: cDNA cloning, expression, purification, structural modeling and its potential as vaccine candidate for human filarial infection. Vaccine 32, 1693–1699. [DOI] [PubMed] [Google Scholar]
- 28.Zhan B et al. (2018) Ligand binding properties of two Brugia malayi fatty acid and retinol (FAR) binding proteins and their vaccine efficacies against challenge infection in gerbils. PLoS Negl. Trop. Dis 12, e0006772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma SK et al. (2017) Recombinant Calponin of human filariid Brugia malayi: Secondary structure and immunoprophylactic potential. Vaccine 35, 5201–5208 [DOI] [PubMed] [Google Scholar]
- 30.Prince PR et al. (2014) Tandem antioxidant enzymes confer synergistic protective responses in experimental filariasis. J. Helminthol 88, 402–410 [DOI] [PubMed] [Google Scholar]
- 31.Immanuel C et al. (2017) Immunoprophylaxis of multi-antigen peptide (MAP) vaccine for human lymphatic filariasis. Immunol. Res 65, 729–738 [DOI] [PubMed] [Google Scholar]
- 32.Shrivastava N et al. (2013) Immunization with a multisubunit vaccine considerably reduces establishment of 639 infective larvae in a rodent model of Brugia malayi. Comp. Immunol. Microbiol. Infect. Dis 36, 507–519 [DOI] [PubMed] [Google Scholar]
- 33.Kalyanasundaram R and Balumuri P (2011) Multivalent vaccine formulation with BmVAL-1 and BmALT-2 confer significant protection against challenge infections with Brugia malayi in mice and jirds. Res. Rep. Trop. Med 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arumugam S et al. (2016) Vaccination of Gerbils with Bm-103 and Bm-RAL-2 Concurrently or as a Fusion Protein Confers Consistent and Improved Protection against Brugia malayi Infection. PLoS Negl. Trop. Dis 10, e0004586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madhumathi J et al. (2017) Epitope mapping of Brugia malayi ALT-2 and the development of a multi-epitope vaccine for lymphatic filariasis. J. Helminthol 91, 43–54. [DOI] [PubMed] [Google Scholar]
- 36.Wong MM et al. (1969) Studies on immunization against Brugia malayi infection in the rhesus monkey. Bull. World Health Organ 40, 493–501 [PMC free article] [PubMed] [Google Scholar]
- 37.Dakshinamoorthy G et al. (2014) Evaluation of a multivalent vaccine against lymphatic filariasis in rhesus macaque model. PLoS One 2014; 9, e112982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khatri V et al. (2018) Prospects of developing a prophylactic vaccine against human lymphatic filariasis - evaluation of protection in non-human primates. Int. J. Parasitol 48, 773–783. [Corrigendum in Int. J. Parasitol. 2018; 48: 1071]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ambily VR et al. (2011) Detection of human filarial parasite Brugia malayi in dogs by histochemical staining and molecular techniques. Vet. Parasitol 181, 210–214 [DOI] [PubMed] [Google Scholar]
- 40.Ravindran R et al. (2014) Canine filarial infections in a human Brugia malayi endemic area of India. Biomed. Res. Int 630160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pisarski K (2019) The Global Burden of Disease of Zoonotic Parasitic Diseases: Top 5 Contenders for Priority Consideration. Trop. Med. Infect. Dis 4, E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grenfell BT et al. (1991) A model for the dynamics of human lymphatic filariasis. Parasitol. Today 7, 318–323 [DOI] [PubMed] [Google Scholar]
- 43.Blaxter M et al. (2002) The Brugia malayi genome project: expressed sequence tags and gene discovery. Trans. R. Soc. Trop. Med. Hyg 96, 7–17 [DOI] [PubMed] [Google Scholar]
- 44.McNulty SN et al. (2013) Inter and Intra-specific diversity of parasites that cause lymphatic filariasis. Infect. Genet. Evol 14, 137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armstrong SD et al. (2014) Comparative analysis of the secretome from a model filarial nematode (Litomosoides sigmodontis) reveals maximal diversity in gravid female parasites. Mol. Cell. Proteomics 13, 2527–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dakshinamoorthy G et al. (2013) Multivalent fusion protein vaccine for lymphatic filariasis. Vaccine 31, 1616–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gnanasekar M et al. (2004) Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infect. Immun 72, 4707–4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hitch WL et al. (1991) Analysis of isotype-specific antifilarial antibody levels in a Haitian pediatric population. Am. J. Trop. Med. Hyg 44, 161–167 [DOI] [PubMed] [Google Scholar]
- 49.Jaoko WG et al. (2006) Filarial-specific antibody response in East African bancroftian filariasis: effects of host infection, clinical disease, and filarial endemicity. Am. J. Trop. Med. Hyg 75, 97–107 [PubMed] [Google Scholar]
- 50.Gregory WF et al. (2000) The abundant larval transcript-1 and −2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infect. Immun 68,4174–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anand SB et al. (2008) Comparison of immunogenicity, protective efficacy of single and cocktail DNA vaccine of Brugia malayi abundant larval transcript (ALT-2) and thioredoxin peroxidase (TPX) in mice. Acta. Trop 107, 106–112 [DOI] [PubMed] [Google Scholar]
- 52.Dakshinamoorthy G et al. (2013) Large extracellular loop of tetraspanin as a potential vaccine candidate for filariasis. PLoS One 8, e77394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anand SB et al. (2011) A combination of two Brugia malayi filarial vaccine candidate antigens (BmALT-2 and BmVAH) enhances immune responses and protection in jirds. J. Helminth 85, 442–452. [DOI] [PubMed] [Google Scholar]
- 54.Dakshinamoorthy G et al. (2012) Biochemical characterization and evaluation of a Brugia malayi small heat shock protein as a vaccine against lymphatic filariasis. PLoS One 7, e34077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veerapathran A et al. (2009) Evaluation of Wuchereria bancrofti GST as a vaccine candidate for lymphatic filariasis. PLoS Negl. Trop. Dis 3, e457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hotez PJ et al. (2016) Human anthelminthic vaccines: Rationale and challenges. Vaccine 34, 3549–3555 [DOI] [PubMed] [Google Scholar]
- 57.Gupta J et al. (2019) CpG enhances the immunogenicity of heterologous DNA-prime/protein-boost vaccination with the heavy chain myosin of Brugia malayi in BALB/c mice. Parasitol. Res 118, 1943–1952 [DOI] [PubMed] [Google Scholar]
- 58.Paul R et al. (2018) Cloning, large-scale production and characterization of fusion protein (P-TUFT-ALT-2) of Brugian abundant larval transcript-2 with tuftsin in Pichia pastoris. Prep. Biochem. Biotechnol 48, 823–833 [DOI] [PubMed] [Google Scholar]
- 59.Kushwaha V et al. (2019) Troponin 1 of human filarial parasite Brugia malayi: cDNA cloning, expression, purification, and its immunoprophylactic potential. Parasitol. Res 118, 1849–1863 [DOI] [PubMed] [Google Scholar]
- 60.Anugraha G et al. (2015) Chimeric epitope vaccine from multistage antigens for lymphatic filariasis. Scand. J. Immunol 82, 380–389 [DOI] [PubMed] [Google Scholar]
- 61.Chauhan N et al. (2018) Evaluating the vaccine potential of a tetravalent fusion protein (rBmHAXT) vaccine antigen against lymphatic filariasis in a mouse model. Front. Immunol 9, 1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shey RA (2019) In-silico design of a multi-epitope vaccine candidate against onchocerciasis and related filarial diseases. Scientific Reports 9, 4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chauhan N, et al. (2017) Improving the efficacy of a prophylactic vaccine formulation against lymphatic filariasis. Parasitol. Res 116, 2821–2830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schussek S et al. (2014) Genome- and proteome-wide screening strategies for antigen discovery and immunogen design. Biotechnol. Adv 32, 403–414 [DOI] [PubMed] [Google Scholar]
- 65.Versteeg L et al. (2019) Enlisting the mRNA Vaccine Platform to Combat Parasitic Infections. Vaccines 7, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li BW et al. (2012) Transcription profiling reveals stage- and function-dependent expression patterns in the filarial nematode Brugia malayi. BMC Genomics 13, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keiser PB and Nutman TB (2002) Vaccines for Filarial Infections The filaria. Eds Klei TR and Rajan TV. pp 167–178, Kluwer Academic Publishers, New York. [Google Scholar]
- 68.Klion A (2019) Lymphatic filariasis: Epidemiology, clinical manifestations, and diagnosis. UpToDate 2019 [Google Scholar]
- 69.Michael E et al. (2006) Mathematical models and lymphatic filariasis control: endpoints and optimal interventions. Trends Parasitol. 22, 226–233 [DOI] [PubMed] [Google Scholar]
- 70.Mathew CG et al. (2019) The health and economic burden of lymphatic filariasis prior to mass drug administration programmes. Clin Infect Dis pii: ciz671. doi: 10.1093/cid/ciz671. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edi C et al. (2019) Pharmacokinetics, safety, and efficacy of a single co-administered dose of diethylcarbamazine, albendazole and ivermectin in adults with and without Wuchereria bancrofti infection in Cote d’Ivoire. PLoS Negl. Trop. Dis 13, e0007325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang Y and Zhang Yi. (2019) Lessons from lymphatic filariasis elimination and the challenges of post-elimination surveillance in China. Infectious Dis. Poverty 8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]