Abstract
Type 1 diabetes is an autoimmune disease caused by the immune-mediated destruction of pancreatic β-cells that results in lifelong absolute insulin deficiency. For nearly a century, insulin replacement has been the only therapy for most people living with this disease. Recent advances in technology and our understanding of β-cell development, glucose metabolism, and the underlying immune pathogenesis of the disease have led to innovative therapeutic and preventative approaches. A paradigm shift in immunotherapy development towards the targeting of islet-specific immune pathways involved in tolerance has driven the development of therapies that may allow for the prevention or reversal of this disease while avoiding toxicities associated with historical approaches that were broadly immunosuppressive. In this review, we discuss successes, failures, and emerging pharmacological therapies for type 1 diabetes that are changing how we approach this disease from improving glycemic control to the ‘holy grail’ of disease prevention.
Keywords: Type 1 diabetes, Immunology, Immunotherapy, Autoimmunity, Glucose, Metabolism, Endocrinology
Type 1 diabetes (T1D) immunotherapies are entering a new era of targeted approaches that take advantage of key mechanistic insights to generate therapies that maintain safety where broadly immunosuppressive approaches have failed. Warshauer et al. discuss successes, failures, and emerging pharmacological therapies for T1D that address the immune and metabolic side of the disease.
Introduction
Type 1 diabetes (T1D) is an autoimmune disease caused by the immune-mediated destruction of pancreatic β-cells within the islets of Langerhans. George Eisenbarth’s initial model of T1D pathogenesis presented in the 1980’s combined with decades of research has led to our current understanding of this disease as a chronic process characterized clinically by the initial appearance of islet autoantibodies, followed by the development of dysglycemia, and finally overt hyperglycemia and ketoacidosis due to absolute insulin deficiency (Eisenbarth, 1986). Nearly a century ago, the discovery of insulin by Frederick Banting and Charles Best (Banting et al., 1922) turned T1D from an acutely fatal disease into a chronic disease that could be managed with exogenous insulin administration. Unfortunately, insulin has its limitations for achieving normal glucose levels, including the risk of hypoglycemia, which has remained a significant barrier to achieving tight glycemic with insulin monotherapy. Recent advances in technology and our understanding of β-cell development, glucose metabolism, and the underlying immune pathogenesis of the disease have led to transformations in therapeutic approaches over time (Figure 1). Immunotherapies target underlying defects in the immune system that lead to β-cell destruction, while currently approved therapies target downstream hyperglycemia that ensues by improving glucose handling. In this review, we discuss successes, failures, and emerging pharmacological therapies for T1D that are changing how we approach this disease from improving glycemic control to the ‘holy grail’ of disease prevention.
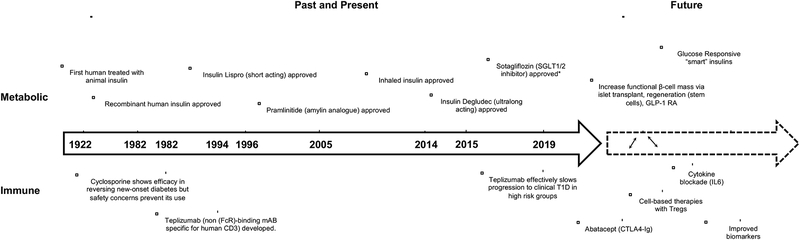
Figure 1.
Timeline of important therapeutic discoveries in T1D and future therapeutic development. *Sotagliflozin only approved in Europe for T1D. FDA denied approval given risk of diabetic ketoacidosis.
Epidemiology
T1D may present at any age with recent data from the UK biobank finding throughout the first six decades of life, 42% of T1D occurs after the age of 30 years and 58% occurs before or at the age of 30 years (Thomas et al., 2018). Overall, there does not appear to be a gender bias with males and females showing similar incidences of disease (Dabelea et al., 2014). Disease incidence varies worldwide with Finland having the highest incidence of 62.5 cases per 100,000 person-years (Harjutsalo et al., 2013) compared with rates as low as 1.0 cases per 100,000 person-years in China (Weng et al., 2018). Genetics plays a strong role in T1D risk as demonstrated in monozygotic twin studies showing 65% of twins of people with T1D will develop T1D themselves by age 60 (Redondo et al., 2008), and U.S. children with an affected family member have a 5% risk of developing T1D by age 20 compared with a 0.3% risk in the general population (Bonifacio and Ziegler, 2010). Additionally, European and North American countries reported increasing incidences of T1D by approximately 2–3% per year during the 20th century, a rate that is far greater than could be attributed purely to genetics, and indicates environmental and/or behavioral cues as likely contributors to T1D development (Libman and LaPorte, 2005; Maahs et al., 2010; Mayer-Davis et al., 2017; Onkamo et al., 1999). Analysis of 84,000 children in 22 European countries from 1989 to 2013 showed the country-pooled incidence rates in all childhood age groups continues to rise, although the incidence rate in high-risk countries (e.g. Finland and Norway) appear to be levelling off (Patterson et al., 2019). Current projections in the U.S. suggest that the incidence of T1D in those less than 20 years of age will increase by 23% by 2050 (Imperatore et al., 2012).
Immune Pathogenesis
Understanding the immune mechanisms of T1D has allowed for the identification of people at increased risk for developing clinical disease and the development of novel therapies that target disease prevention and reversal. The immune pathogenesis of T1D begins with a breakdown in self-tolerance that leads to a T-cell mediated destruction of β-cells (Figure 2). Classical characteristics of this autoimmune disease include the presence of autoantibodies (AAbs) specific for β-cell antigens (Bottazzo et al., 1974) and insulitis (Gepts, 1965; Gepts and De Mey, 1978).
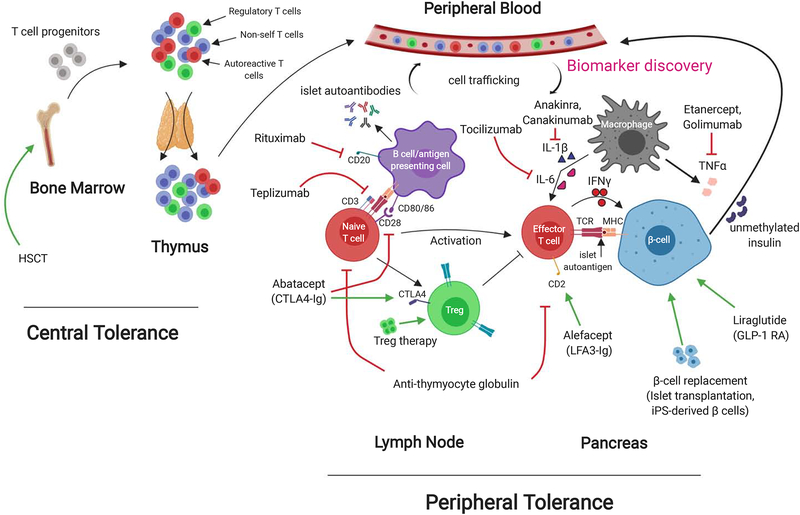
Figure 2:
Mechanisms of immune tolerance and therapeutics in T1D. T cell progenitors are made in the bone marrow from hematopoietic stem cells. They migrate to the thymus where central tolerance mechanisms educate them to self and non-self (negative selection). Regulatory T cells (Tregs) and pathogenic autoreactive T cells may each recognize self antigens but at differing affinities, which could explain their opposing actions. T cells that survive thymic selection then circulate in the blood and lymph nodes, waiting to encounter their corresponding peptide/HLA complex. In T1D, these T cells are specific for β-cell proteins such as insulin. If these islet-specific T cells come into contact with their corresponding epitope displayed by the HLA of an antigen-presenting cell (APC), they will become activated in the lymph node, migrate to the islets and begin the process of β-cell destruction. Tregs represent the suppressive cell primarily responsible for peripheral tolerance and attempt to prevent this process. If the body is unable to curb this autoimmune attack on the β-cells, then insulin deficiency, hyperglycemia, and T1D results. The majority of this process takes place locally in the lymph nodes and pancreas, and has limited the ability for biomarkers in the peripheral blood to accurately reflect disease activity in patients.
A major challenge to uncovering direct mechanisms that might serve as therapeutic targets is due to the polygenic nature of the disease and the contributions of both genetics and environment toward one’s overall risk. Rare cases of monogenic T1D such as those seen with mutations in the Autoimmune Regulator (AIRE) gene (autoimmune polyglandular syndrome type 1) and FoxP3 gene (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome) combined with the use of the nonobese diabetic (NOD) mouse model have led to important insights into central and peripheral mechanisms of immune tolerance that are defective in the disease (Anderson and Bluestone, 2005; Johnson et al., 2016). These insights have formed the rationale for why therapies targeting immune tolerance hold promise in preventing or delaying T1D (Bluestone et al., 2010). Islet autoimmunity in T1D begins months to decades prior to clinical disease manifests with persistent hyperglycemia and low C-peptide due to the permanent loss of more than 70% of β-cell mass (Sherry et al., 2006). Prior to understanding the underlying immune pathogenesis and biomarkers to predict those with disease, blood glucose and C-peptide represented the only way to diagnose these patients with T1D. This limited initial immune therapy studies to those with recent-onset T1D and who lacked sufficient β-cell mass to reach clinically meaningful endpoints.
Natural History and Biomarker development
Understanding the time course of T1D has created an opportunity to intervene at different stages of disease. To date, there is no firm evidence to suggest β-cells have regenerative or proliferative potential during or after their autoimmune destruction in T1D. Therefore, developing therapeutics aimed at curing T1D requires identifying and intervening early on in patients at high-risk for progressing to clinical disease. This is evidenced by the fact that most clinical trials have been unsuccessful at restoring long-lasting euglycemia in people after clinical diagnosis, although there have been small successes using autologous hematopoietic stem cell transplantation showing it is possible (Couri et al., 2009b). Alternative endpoints such as stimulated C-peptide secretion to indicate a slowing in the decline of β-cells mass, immune assays measuring serum levels of inflammatory mediators, and measuring PBMC shifts in T cell subsets and function might provide evidence that the intervention is having the proposed mechanistic effect. A combination of environmental exposures and genetic susceptibility is thought to determine one’s baseline risk of developing preclinical-clinical T1D (Figure 3).
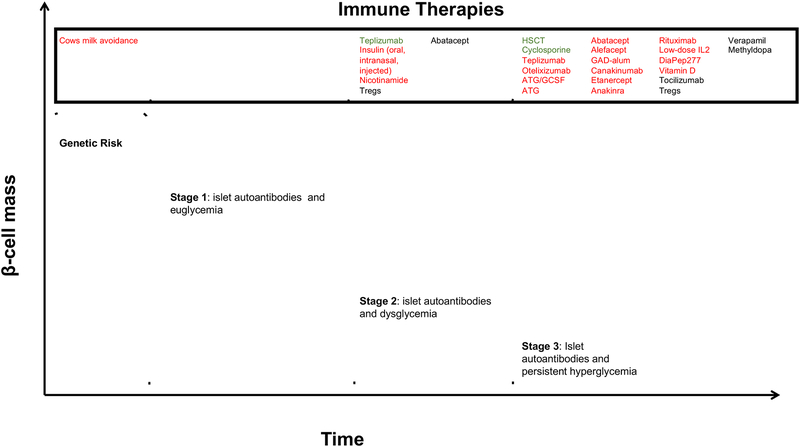
Figure 3.
Natural History of T1D and therapeutics stratified by population. Take home message: population selection is critical in showing therapeutic efficacy in T1D. Green represents efficacy, Red Represents Failure, Black represents under investigation.
Based on genome-wide association studies (GWAS), T1D is a polygenic disease with approximately 50% of genetic risk attributable to the human leukocyte antigen (HLA) class II haplotypes of HLA-DR and HLA-DQ that are located within the major histocompatibility complex (MHC) region and are responsible for antigen presentation by B cells, dendritic cells and macrophages to CD4 T cells (Erlich et al., 2008). The majority of non-HLA-associated genes from GWAS are involved in immune function and regulation such as protein tyrosine phosphatase, non-receptor type (PTPN22), IL2RA, CTLA4, and Ins (Concannon et al., 2009). This data has allowed for the development of genetic risk scores (GRS) that may serve to identify high risk individuals. Recently, a GRS utilizing 67 SNPs (14 DR-DQ, 21 other HLA, and 32 non-HLA SNPs) was validated using the UK Biobank and could detect 77% of future cases by following 9.5% of infants screened, a reduction from the 14.3% of infants that would need to be equivalently followed if only HLA DR-DQ criteria was used (Sharp et al., 2019).
Although determining genetic risk helps identify lifetime risk for disease development, it fails to predict individuals with preclinical disease who are currently experiencing destruction of their β-cells and explains some of the heterogeneity in T1D kinetics. In 2015, a new staging system of T1D was developed to account for preclinical stages of disease beginning with β-cell autoimmunity (defined by >1 islet AAb) with euglycemia (stage 1), through glucose intolerance (stage 2), and finally clinical manifestations of disease (stage 3) (Insel et al., 2015). Interestingly, AAb have not been found to be directly pathogenic and serve primarily as biomarkers for disease with over 90% of newly diagnosed people with T1D testing positive for at least one AAb against either insulin (IAA), glutamate decarboxylase (GAD), islet antigen 2 (IA2), zinc transporter 8 (ZnT8), or the recently reported tetraspanin-7 (Tspan7) (McLaughlin et al., 2016). IAA, GAD, IA2, and ZnT8 are currently the only clinically used AAb in prevention studies and for clinical diagnosis. A clear relationship between the number of detectable AAb and rate of progression exists with data from the TEDDY study showing 5-year incidence of symptomatic diabetes was 11%, 36%, and 47% in children with one, two or three AAb, respectively (Ziegler et al., 2013).
Measurement of β-cell death has been investigated to provide information about disease progression since it is the primary pathologic process in T1D. Utilizing the fact that β cells are the only significant source of unmethylated insulin DNA, the rate of β cell death can be determined by measuring the level of unmethylated insulin DNA in the serum and has been shown to correlate with insulin secretory kinetics and risk of progressing to T1D in high risk individuals (Fisher et al., 2015; Herold et al., 2015). Studies are currently underway to determine if this biomarker could be further utilized in patients progressing to diabetes and in clinical trials for type 1 diabetes.
The T1D Biomarker Working Group and the Core for Assay validation (www.t1dbiomarkers.org) is currently working to establish and validate T-cell biomarkers and assays that more directly reflect the underlying autoimmune pathogenesis and could be used for monitoring T1D progression, onset and response to therapy (Ahmed et al., 2019). Extremely low frequencies of diabetogenic T cells in the peripheral blood, low-avidity interactions between autoreactive TCRs and the HLA/peptide complex, and significant disease heterogeneity have made this task challenging. However, recent advances in single-cell technologies are allowing for the high dimensional characterization of diabetogenic T cells at phenotypic, transcriptional, and epigenetic levels that could enable the discovery of sensitive and specific immune biomarkers. Resources like The Network for Pancreatic Organ Donors (nPOD, http://www.jdrfnpod.org/) has allowed for an organized umbrella to obtain tissues from subjects with T1D or at-risk for T1D and this has allowed for direct study of islet infiltrating T cells. Cloning of islet infiltrating T cells or single-cell sequencing of alpha and β chains of the TCR from islet infiltrating T cells and generating cell lines with these individual TCRs has allowed for improving our understanding of the islet-specific TCR repertoire in T1D. Testing the response of these T cells to various autoantigens has shown reactivity to the C-peptide region of proinsulin (Pathiraja et al., 2015), post-translationally modified islet antigens (Babon et al., 2016), hybrid insulin peptides (Delong et al., 2016), and insulin B: 9–23 (Michels et al., 2017). Understanding the TCR repertoire of pathogenic T cells in T1D may allow for isolation and use of these cells in individuals on immune therapies as surrogate biomarkers that reflect drug efficacy.
Imaging studies of the pancreas in T1D to understand the temporal dynamics of the pancreas in relation to disease stage has shown pancreatic volume declines within 100 days of T1D diagnosis, a finding that could lead to a noninvasive imaging biomarker of underlying disease pathophysiology (Virostko et al., 2019). Despite the fact that islets only comprise about 1–2% of the pancreas, magnetic resonance imaging (MRI) studies in adults with T1D have found pancreatic volume to be 26% less than in controls within months of diagnosis (Williams et al., 2012) and 48% less than in controls after more than 10 years of disease (Williams et al., 2007), suggesting exocrine pancreas changes as well. Noninvasive visualization of pancreatic inflammation using MRI and the magnetic nanoparticle ferumoxytyl showed increased pancreatic inflammation and significant regional heterogeneity in those with recent-onset T1D, and may represent a method for monitoring treatment response in individuals receiving immunomodulatory therapies (Gaglia et al., 2015).
Finally, metabolic biomarkers that indicate glucose intolerance and insufficient functional β-cell mass include a decline in stimulated C-peptide level (surrogate for insulin secretion) and rising HbA1C, fasting plasma glucose (FPG), and/or glucose levels during oral glucose tolerance testing (OGTT). The Diabetes Control and Complications Trial (DCCT) found stimulated C-peptide concentrations were inversely associated with the risk of short and long-term complications, evidence supporting its use as a clinically relevant surrogate endpoint in T1D β-cell preservation trials (Lachin et al., 2014). Currently, a combination of islet autoantibodies and OGTT is the best predictor of disease progression and T cell assays are, paradoxical to their key pathogenic role, not helpful in predicting disease progression at this point. The Diabetes Prevention Trial, type 1 diabetes (DPT-1) found 2h-OGTT glucose levels best predicted disease progression approximately 0.8 years prior to diagnosis at which point there was a rapid rise in glucose levels (Ferrannini et al., 2010). Studies from TrialNet Natural History Study found a 20% increase in HbA1C from baseline was associated with an 84% risk of T1D at 3 years, and a 20% decrease in C-peptide from baseline was associated with a 47% risk of T1D at 4 years and had a positive predictive value of symptomatic T1D of 78% at 5 years (Krischer, 2013). A number of factors including absolute β-cell mass, insulin sensitivity, ambient glucose, and underlying inflammatory state may influence insulin secretion and limit the ability of metabolic biomarkers to predict disease progression. Combining these various biomarkers allows for the most accurate prediction of individual disease kinetics as demonstrated by the DPT-1 which showed relatives of individuals with T1D, impaired glucose tolerance, and positive islet cell autoantibodies had a 78% risk of developing T1D over 5 years. Recently, proinsulin has shown promise as another biomarker of β-cell dysfunction with measurable levels even when C-peptide levels fall, although as C-peptide eventually becomes undetectable so too dose proinsulin (Sims et al., 2019). Thus, understanding the natural history of T1D affords the opportunity to intervene before actual diabetes ensues.
Environmental Interventions
The search for environmental influences leading to T1D development has focused on viruses (Coxsackie B virus, Rubella viruses, Enterovirus, Rotavirus, CMV, Retroviruses), and factors that might influence the gut immune system including diet and hygiene. Studies in the nonobese diabetic (NOD) mouse model have shown that diabetes incidence increases in cleaner mouse colonies and alterations in the gut microbiome can significantly shift T1D incidence (Kriegel et al., 2011; Wen et al., 2008), consistent with observations that countries with stricter hygiene practices show a higher incidence of T1D (Bach, 2002). Human studies of the gut microbiome in T1D using 16S ribosomal RNA gene and shotgun metagenomic sequencing have shown reduced diversity of bacterial composition in children who progress to T1D, although specific bacteria genera have not been consistently observed across studies (de Goffau et al., 2014; Kostic et al., 2015; Vatanen et al., 2018). Recent approaches measuring anti-commensal antibodies have shown an association between immune responses to intestinal bacteria, antibody responses to islet autoantigens, high-risk HLA haplotypes and T1D, and may represent a robust platform that allows for monitoring dynamic responses to therapeutic interventions (Paun et al., 2019). The Diabetes Autoimmunity Study in the Young (DAISY) and The Environmental Determinants of Diabetes in the Young (TEDDY) study are ongoing prospective cohort studies designed to identify environmental factors influencing islet autoimmunity and T1D. Initial results from DAISY showed an association between enterovirus infection and acceleration in disease onset in high risk individuals (Stene et al., 2010) suggesting an enterovirus vaccine may serve as a potential primary or secondary prevention therapy for T1D (Dunne et al., 2019). Thus far, only modest associations of unclear significance have been found in these studies between environment (changes in microbiome, diet (e.g. formula, breast-milk, and cow’s milk exposure), probiotics, household exposures (e.g. pets), infections, and geographical location) and one’s predetermined genetic risk (defined by factors such as family history, HLA genotype (DR3/4 vs. others), and high-risk single nucleotide polymorphisms (SNPs)), but might provide clues to explain the variable kinetics of T1D progression and ways in which lifestyle interventions may reduce one’s overall risk (Krischer et al., 2019; Vatanen et al., 2018).
Multiple dietary interventions such as vitamin D, omega-3 fatty acids, and gluten have been proposed based on observational data that they may be associated with T1D risk, but the Trial to Reduce Insulin-Dependent Diabetes Mellitus in the Genetically at Risk (TRIGR) study is the only randomized, controlled, adequately powered study that has tested whether a dietary intervention could directly affect T1D development. TRIGR was a large international trial in high risk infants comparing extensively hydrolyzed casein formula containing small pieces of cow’s milk proteins versus regular cow’s milk formula with intact proteins. Subjects were followed for at least 10 years and findings showed cow’s milk had no impact on T1D development (Knip et al., 2018). Overall, environmental trials to date have failed to show any meaningful impact on the natural course of T1D and this remains a challenging approach to modulate disease onset or severity.
The Setting of immune therapies
Fundamental to understanding drug development in T1D is knowing the setting that a given drug will be effective in. Immune evolution from thymic development to diet, hygiene and environment generate the backdrop for our immune system to mature and become educated. Designing successful immune interventions for autoimmune diseases like T1D relies on understanding the interactions between environment, genes, and immune regulation.
Historical Immunotherapy Approaches
The first immunotherapy used in patients with established T1D was the immunosuppressive agent cyclosporine, a calcineurin inhibitor directed primarily at T-cells, which was first tested in the 1980s in those on insulin therapy less than 2 months since diagnosis (Feutren et al., 1986). It successfully increased rates of diabetes remission (HbA1C ≤ 7.5% and off insulin therapy) throughout the treatment course, but upon cessation of cyclosporine, the disease progressed, resulting in the ultimate destruction of the residual β-cell mass. Because the risks associated with prolonged drug treatment were significant, including nephrotoxicity and an increased risk of cancer, the use of cyclosporine as a true curative therapy did not reach equipoise. However, these landmark studies validated the notion that T1D was indeed an autoimmune disease. It also drove research efforts towards the development of therapies that promoted immune tolerance, rather than broad immunosuppressive strategies, and these efforts focused on short term therapies that would re-education the immune system to provide long term remission without unwanted adverse events.
Initial studies from autologous bone marrow transplantation in T1D reversal studies suggested a broad reprogramming of the immune system to correct defects in immune tolerance may also lead to diabetes remission. These studies showed promise in autologous non-myeloablative hematopoietic stem cell transplantation (HSCT) using cyclosphosphamide, granulocyte colony stimulating factor (G-CSF) , and rabbit anti-thymocyte globulin (ATG) (Couri et al., 2009a). In this single-arm study lacking a control group, 23 recently diagnosed (<6 weeks) subjects with T1D, 20 were insulin-free (12 continuously, 8 transiently) at 4 years, but only 3 remained insulin-free at a mean follow-up of approximately 5.6 years (Malmegrim et al., 2017). Additionally, adverse events including cyclophosphamide-related oligospermia, infections, and rashes were significant. Differing results were reported in another study that found worse HbA1C in recent-onset T1D after autologous HSCT vs. control patients, and no difference in C-peptide or insulin dosage (Gu et al., 2014). A critical issue in the above studies was that they only targeted the immune system in overtly diabetic populations with potentially too few β-cells remaining to see a clinical effect in glycemic control.
β-cell replacement using either allogenic solid organ pancreas or islet transplantation has shown the ability to reverse T1D but requires lifelong immunosuppression to prevention graft rejection. Historical immunosuppressive regimens for islet transplantation used ATG induction immunosuppression followed by long-term cyclosporine A, steroids, and azathioprine, but only 10% of patients remained insulin free at one year (Bretzel et al., 1999). A significant improvement in the rate of insulin independence to 58% at one year occurred with the Edmonton protocol, a corticosteroid-free immunosuppressive regimen that used daclizumab (an anti-CD25 monoclonal antibody that serves to delete activated T cells) for induction, and sirolimus (an mTOR inhibitor that blocks IL2 signaling) and low-dose tacrolimus (a calcineurin inhibitor, similar to cyclosporine, that blocks T cell receptor signaling) for maintenance immunosuppression (Shapiro et al., 2006). Immunosuppressive toxicities including neutropenia, severe infections, and nephrotoxicity have led to a focus on more targeted therapies that may be effective and safe. Indeed, a calcineurin-free maintenance regimen using Belatacept, a CTLA-4Ig that blocks costimulation, with mycophenolate mofetil and/or sirolimus showed 4/5 patients were insulin independent at 1 year and 2/5 patients were insulin independent at 10 years post-transplant (Gardner et al., 2018). The current position of the American Diabetes Association regarding who should receive a pancreas or islet transplantation is largely driven by the risks associated with chronic immunosuppression and recommends only individuals who are simultaneously undergoing renal transplantation, have already received a renal transplantation, or have recurrent ketoacidosis or severe hypoglycemia despite intensive glycemic management should undergo transplantation (2019). In addition to the risks of chronic immunosuppression, major challenges remain for β-cell transplantation including donor organ shortage and lack of perfect HLA matching (allogenic), both of which result from the current necessity for cadaveric donors. β-cell replacement by differentiating induced-pluripotent stem (iPS) cells into β-cells that are isogenic may overcome these challenges. However, to date this has remained a challenge with many significant barriers including showing true β-cell functionality and an ability to circumvent autoimmune destruction in the already autoimmune primed recipient.
Overall, these historical therapies have established T1D as an autoimmune disease that can respond to immune interventions (in addition to β -cell replacement in later stages), but also highlight the fact that altering the immune system needs to be done in a balanced way that preserves safety with efficacy. Recent advances in our understanding of the immune mechanisms that cause this disease is leading to novel, targeted therapies that may alter disease course without undo toxicity.
New age of immunotherapy for T1D: Targeted Therapies
The past two decades have seen the development of more refined therapies used to treat autoimmune and inflammatory diseases (e.g. Rheumatoid arthritis treatment with anti-TNF therapy or rituximab) that have more restricted side effects than broadly immunosuppressive regimens of the past. Bringing this premise to T1D, a paradigm shift in T1D immunotherapy development towards the targeting of islet-specific immune pathways involved in tolerance has led to the development of therapies that may allow for the prevention or reversal of this disease while avoiding toxicities associated with historical immunosuppressive approaches. Over the last decade, the field has tested these drugs in the high risk and new-onset settings so that the biomarker of C-peptide preservation can be measured as an outcome.
Approaches to Reverse T1D
Targeting T cells
More specific and less toxic T-cell directed therapies are currently being tested in T1D and show promise. Targeted CD3 blockade began with an engineered, humanized antibody that was first developed in the mid 1980’s as a modified form of OKT3, the first approved monoclonal antibody used to treat acute kidney allograft rejection. However, this initial form of the drug had significant side effects due to the cytokine storm induced as a consequence of the mAbs activating properties and subsequent immunogenicity. Thus, teplizumab [hOKT3γ (Ala-Ala)] was developed and represented a modified humanized form of the antibody with a mutated human Fc receptor that resulted in reduced T cell activation and immunogenicity. In a small study conducted in 1995, teplizumab was shown in a pilot study to reverse kidney transplant rejection similarly to the parent OKT3 mAb without the adverse events described above. Additionally, a preclinical study in the NOD mouse during the 1990s showed a short course of an anti-CD3 mAb could reverse and induce long term remission of autoimmune diabetes (Chatenoud et al., 1994), a finding that created the foundation for anti-CD3 mAb translation into T1D. Approximately seven years later after Teplizumab’s original development, clinical safety and efficacy were shown in patients with new-onset T1D following a single two-week course of therapy. C-peptide was preserved at 2 years (and up to 5 years in some subjects) in newly diagnosed (≤12 weeks) T1D subjects (Herold et al., 2013; Sherry et al., 2011), although neither actual disease reversal (HbA1C<6.5%) nor reduced insulin use (<0.5u/kg/day) was observed. Nevertheless, this sustained effect after a short course of therapy supported teplizumab’s ability to induce a state of long-term immune tolerance. Mechanistic studies supported the notion that the long-term effects were due to a combination of Teff depletion and induced unresponsiveness, combined with preservation and enhanced Treg activity. The limitation in the drug treatment not reversing disease in the majority of patients was possibly due to an insufficient starting β-cell mass. Currently, a Phase III trial is underway to determine if teplizumab can significantly alter disease progression in the new onset setting (). Results in patients treated with otelixizumab, a similar mAb, demonstrated preserved β–cell function in initial Phase 2 Trials (Keymeulen et al., 2005; Keymeulen et al., 2010), however, there was increased EBV reactivation due to the immunosuppressive and cytokine activating properties of the mAb. Follow up studies using lower doses failed to reach primary endpoints of preserved C-peptide in the DEFEND-1 and DEFEND-2 Phase 3 trials (Ambery et al., 2014; Aronson et al., 2014) and there are currently no ongoing registered clinical trials.
Low-dose ATG is another T-cell approach being trialed in T1D prevention and reversal but is less specific than anti-CD3 therapies like teplizumab. ATG is a purified rabbit sera containing cytotoxic IgG antibodies directed against human T-cells and is made by immunizing rabbits with human thymocytes; therefore, there is not a specific epitope that ATG targets and repeated exposures may have an increased risk for serum sickness (Haller et al., 2018). In a recent three-arm study in new-onset T1D (<100 days) with stimulated C-peptide ≥ 0.2 nmol/L, subjects were given one treatment of low-dose ATG with or without six doses of GCSF, or randomized to the placebo arm (Haller et al., 2019). Although, restoration of normoglycemia was not seen, the ATG alone group was able to slow the rate of β-cell function and reduce HbA1C to statistical significance compared to the placebo group. Interestingly, the ATG+GCSF group failed to show the ability to preserve C-peptide secretion suggesting it may actually diminish the modest effect of ATG. Given the success, specificity, and safety observed with teplizumab, low-dose ATG appears to be an inferior therapy attempting to target the same mechanism.
Preferentially targeting CD4+ and CD8+ Teff cells (rather than Tregs) remains a theme in therapeutic development aimed at manipulating immune tolerance mechanisms and selectively eliminating pathogenic T cells. Alefacept is an LFA3-Ig fusion protein that binds CD2, which predominates on these effector T cells (Rigby et al., 2015). Following two 12-week course treatments over 36 weeks in recently diagnosed (<100 days) T1D, the predicted rise in Treg/Teff ratios was seen in peripheral blood along with a slowed decline in C-peptide versus placebo. Unfortunately, overall insulin requirement did not decrease within the alefacept group, no improvement in HbA1C was seen to suggest disease reversal, and no current trials are underway. Abatacept, a CTLA4-Ig fusion protein, prevents T cell activation by blocking CD80 and CD86 on antigen presenting cells (APCs) thereby preventing their interaction with the T cell costimulatory molecule CD28. Naïve T cell (vs Teff) activation is thought to be preferentially inhibited, and therefore abatacept is thought to affect pathogenic T cells at early stages of activation. Results from 24 months of abatacept in recently diagnosed T1D found the decline in β-cell function paralleled the placebo group, and possible explanations for the lack of efficacy may be due to the dosing not being saturating causing reduced effects on Teffs and the possibility that other co-stimulatory pathways (such as IL-2, CD137 and CD154) bypass the need for co-stimulation in some pathogenic cells, especially CD8 T cells (Orban et al., 2011).
Targeting B cells
Although T1D is considered a T cell mediated autoimmune disease, B cells also have a pathogenic role in T1D related to their function as antigen-presenting cells and modulators of the pancreatic microenvironment (Mariño et al., 2011). A short course of 4 infusions over a month with the anti-CD20 monoclonal antibody rituximab was found to delay the fall in C-peptide by 8.2 months in recent onset T1D , but after two years the decline in C-peptide and β-cell function was essentially the same as placebo (Pescovitz et al., 2014). It remains unclear if repeat dosing would lead to a sustained effect on C-peptide. A clinical trial combining rituximab with abatacept to test efficacy in either preventing or reversing T1D is being pursued ().
Antigen Specific Therapy
Antigen-specific interventions that can suppress that autoimmune response is a promising approach that may avoid the off-target effects seen with systemic immunomodulating therapies. Despite T1D being a polyclonal autoimmune disease with multiple T-cell epitopes and autoantibodies that are islet specific, single antigen-specific therapies are capable of suppressing the polyclonal immune response and reverse diabetes in preclinical models (Tang et al., 2004). This strategy has centered around the generation of islet-specific Tregs that can restore immune tolerance by mechanisms including: “bystander suppression” that is mediated by soluble products or cell-cell contact including release of cytokines such as IL-10 and TGF-β which can disrupt diabetogenic T cells function or development (“infectious tolerance”), and contact-dependent mechanisms that modulate Teff function (Homann et al., 1999; Masteller et al., 2005; Waldmann and Cobbold, 1998)
One approach to developing antigen-specific therapies has been with peptide immunotherapy, which is premised on the idea that exposure to peptides of disease specific auto-antigens (e.g. β cell auto-antigens in T1D) can induce an expansion of the Tregs and the deletion and/or anergy of pathogenic T cells thereby restoring immune tolerance. Insulin has served as the primary antigen target based on studies in the NOD mouse ((Nakayama et al., 2005). Attempts targeting insulin or its associated peptides in those with clinical T1D include trials with oral insulin (Chaillous et al., 2000; Ergun-Longmire et al., 2004), intranasal insulin (Fourlanos et al., 2011), altered insulin B9–23 epitope (NBI-6024) (Walter et al., 2009) and proinsulin peptide (Alhadj Ali et al., 2017), but have not shown overall success. Immunization with GAD65, another major autoantigen in T1D, was also attempted with GAD formulated with aluminum hydroxide (GAD-alum) that was delivered subcutaneously, but this approach also did not result in preserved insulin secretion (Ludvigsson et al., 2012; Wherrett et al., 2011). Despite limited to no efficacy, these therapies were considered safe and without toxicities frequently seen with systemic immunomodulatory therapy. While peptide immunotherapy that targets insulin and GAD65 has been unsuccessful to date, other antigen-specific approaches are being pursued. Current phase 1 studies include the use of a mixture of peptides from islet autoantigens (MultiPepT1De) (), the use of modified peptides from major islet autoantigens that can activate antigen-specific CD4 cells against APCs and their respective pathogenic T cells attached (IMCY-0098) (). A nanoparticular, emulsion-based adjuvant combined with the insulin B chain (MER3101) has also been tested and was found to induce Th2 and Treg responses as well as prevent diabetes in NOD mice, and a Phase 1 trial is currently underway (). Additionally, nanoparticles coated with T1D-relevant peptide MHC class I and class II complexes have been shown to generate antigen-specific Tregs and regulatory B cells in the NOD mouse with reversal of diabetes(Clemente-Casares et al., 2016). Other nanoparticles loaded with a neoepitope for the 2.5HIP, a hybrid insulin peptide fused to chromogranin A and a major contributor to autoimmunity in the NOD mouse, was found to induce tolerance by promoting T cell anergy and increasing Treg/Teff ratio and represents an exciting finding that awaits clinical translation (Jamison et al., 2019).
DNA vaccination intramuscularly with a proinsulin-encoding plasmid (BHT-3021) was done in a phase 1 study in adults ≤5 years since T1D diagnosis (Roep et al., 2013). This trial showed overall safety suggesting this approach may have potential. While a decrease in peripheral proinsulin-reactive CD8+ T cells and preserved C-peptide levels were reported, these were exploratory measures and more work is still needed to validate these results.
Cell-based therapies centered around infusion of autologous ex vivo-expanded polyclonal Tregs have shown good safety and tolerability in children and adults (Bluestone et al., 2015; Marek-Trzonkowska et al., 2016). A few patients had persistent C-peptide levels after 1–2 years. Most recently, a Phase 2a trial in adolescents with recent onset T1D found low doses (2.5 million cells/kg) and high doses (20 million cells/kg) of autologous ex vivo-expanded polyclonal Tregs were well tolerated and safe but unable to preserve C-peptide vs. placebo at one year using a mixed meal tolerance test (Caladrius Biosciences, 2019). Moving forward, Treg therapy may have to include Tregs that are enriched for islet-specificity (rather than polyclonal Tregs). Additionally, autologous dendritic cells directed ex vivo toward a tolerogenic state were injected intradermally into the abdominal region in those with longstanding T1D and was found to be safe and well tolerated in a phase 1 trials, but further study is needed to see if they can actually alter the course of disease (Giannoukakis et al., 2011). Current efforts to identify islet-specific TCRs and critical epitopes in T1D may also allow for the in vitro generation of antigen-specific Tregs using gene editing tools such as TCR gene transfer and CRISPR-Cas9 (Hull et al., 2017; Safari et al., 2018).
Although ideal, antigen-specific therapies have been a major challenge to develop. Firstly, the key antigens to target may still be unknown, which could leave certain efforts futile. Secondly, it is still not known who should be introduced antigen in a true tolerogenic form. Cellular approaches (i.e. Tregs) may be the most attractive approach because they bypass these two unknowns yet are still able to restore immune tolerance in a targeted approach.
Targeting inflammation
In contrast to the successes of therapies targeting the adaptive immune compartment’s B and T cells, therapies targeting the generalized inflammatory response have not experienced the same success. The innate immune system has been targeted with IL-1 blockade using anakinra and canakinumab. Two multicenter, randomized, double-blind, placebo-controlled trials showed failure to impact C-peptide response (Moran et al., 2013). Blockade of the pro-inflammatory cytokine Tumor Necrosis Factor (TNF)-a was first attempted with etanercept in 18 children with new-onset type 1 diabetes and results showed a reduction in A1C and increase in C-peptide AUC after 24 weeks of treatment (Mastrandrea et al., 2009). Trials with Golimumab, another TNF-a blocker, are ongoing in children and young adults with newly diagnosed diabetes (Phase 2 Trial, ) and Stage 2 T1D (Phase 1 Trial, ). Other anti-cytokine trials including treatment with anti-IL6 (tocilizumab, ) or anti-IL21 (NNC0114–0006, NovoNordisk) with liraglutide () are also ongoing or awaiting report. Based on evidence that alpha-1 antitrypsin may protect islets from apoptosis via caspase-3 inhibition, it was translated into humans with a phase 1 trial showing it was safe and well tolerated, but further study is needed to determine whether it is efficacious (Weir et al., 2018). Overall, targeting inflammatory pathways are more refined immunotherapies than broadly immunosuppressive regimens of the past, but it remains to be seen whether they can restore immune tolerance in T1D.
Targeting cell intrinsic metabolism
Therapies targeting cell intrinsic metabolism have also been investigated. Rapamycin blocks mTOR signaling and inhibits proliferation of Th1 and Th17 Teff cells while having a weaker effect on Tregs that are not reliant on mTOR signaling. When used in combination with IL-2 therapy in T1D, increases in Tregs were found but unfortunately promotion of an inflammatory environment resulting in accelerated T1D progression and β-cell dysfunction occurred (Long et al., 2012). Another drug being tested in a phase 2 trial in recent-onset T1D is the tyrosine kinase inhibitor imatinib. Studies in the NOD mouse found imatinib could restore immune tolerance as complete protection against T1D was seen prediabetic mice seen after 7 weeks of treatment and diabetes reversal was seen in 80% of diabetic mice by 2 weeks after treatment (Louvet et al., 2008). These effects are thought to be due to a combination of anti-inflammatory effects short term, due at least in part to its PDGFR antagonism, combined with longer term effects, perhaps due to its c-abl- and c-kit-specific activities (Hagerkvist et al., 2007). At the β-cell level, imatinib has been thought to enhance β-cell survival via a mechanism similar to ischemic preconditioning, as shown by NF-κB activation, increased nitric oxide and reactive oxygen species production, and depolarization of the inner mitochondrial membrane (Hagerkvist et al., 2006; Hagerkvist et al., 2007)
Three conclusions may be drawn from new-onset studies: 1) T-cell directed therapies show the most promise 2) Immune therapies alone cannot overcome the severe deficiency of β-cells to reverse the disease, but may reduce the rate of β-cell loss 3) β-cell replacement strategies will be required in addition to immune therapy to create a meaningful, long-term impact on glucose control at this stage of disease.
Window of Opportunity – Prevention
Numerous therapies have shown the ability to prevent T1D in preclinical studies using the NOD mouse model but failed when translated into humans. Most prevention trials focus on those individuals at highest risk of developing disease to decrease the time to achieve the given study’s outcome and observe an answer. As discussed above, this risk is usually a combination of family history, HLA, islet autoantibodies, glucose tolerance (Figure 4). Together, these factors can usually determine subjects with >50% risk of developing T1D in the next 5 years.
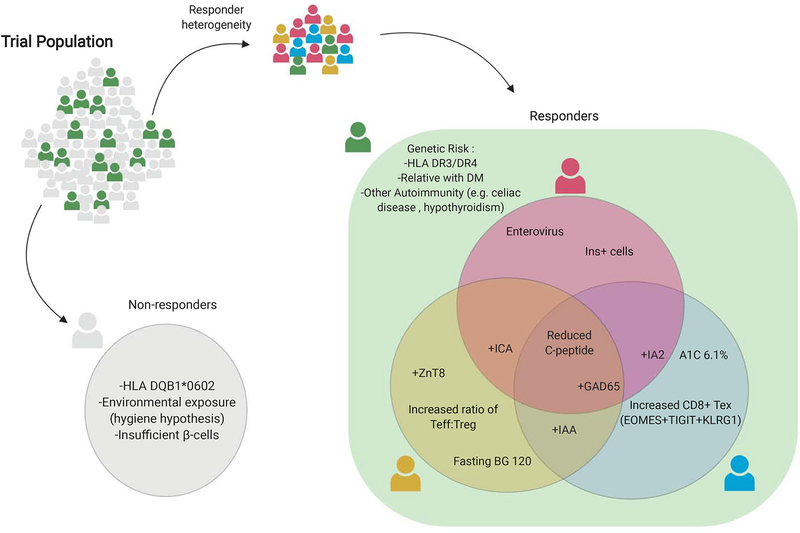
Figure 4.
Trial Population is critical for successful efficacy.
Improved biomarkers will reflect disease activity and allow for prediction of who will respond to a particular immunotherapy, with some biomarkers also serving as endpoints.
Based on preclinical data in NOD mice that suggested exogenous insulin might tolerize the body to insulin the Diabetes Prevention Trial-1 (DPT-1) was completed. This randomized, controlled study found oral insulin was not effective in preventing T1D (Krischer et al., 2017).
The only successful human trial to date of clinical T1D prevention has been with teplizumab, which was recently granted Breakthrough Therapy Designation (BTD) by the FDA for this indication and may allow it to reach patients more quickly (Herold et al., 2019). In its Phase 2 trial, a single course of teplizumab was able to delay progression to clinical T1D by 2 years in high risk relatives of people with T1D, defined as at least two positive AAb and an abnormal OGTT at trial entry. In comparison, 72% of high-risk individuals with Stage 2 disease were found to progress to clinical T1D at 2 years in the placebo arm of the teplizumab prevention trial. Despite prior trials showing teplizumab was unable to restore euglycemia in recently diagnosed patients (Hagopian et al., 2013), this teplizumab trial in high risk patients with preclinical disease represented the first successful prevention trial and highlights the utility of combining genetic, metabolic, and immune biomarkers to identify patients who will benefit from therapies aimed at disease prevention. The primary mechanism of action of teplizumab is incompletely understood, but thought to involve changing the phenotype of autoreactive CD8+ T cells and favoring depletion of Teff while preserving Tregs (Gitelman and Bluestone, 2016). The antibody binds to a conserved component of the T cell receptor signaling complex and this binding likely imposes these phenotypic changes in the T cell compartment. In addition, the modified Fc-receptor of the antibody results in lower depletion activity of the antibody such that the potential immunosuppressive effect of the antibody is restrained. The most favorable responses to teplizumab in the prevention trial by Herold et al. were seen in those with either: HLA-DR4 present with absent HLA-DR3, absent anti-ZnT8 AAb, and low C-peptide responses. Low C-peptide may reflect the importance of active disease during the critical treatment window when immune tolerance mechanisms respond favorably to prevention therapies. In a similar fashion, a current clinical trial with CTLA4-Ig (Abatacept) is being used to test efficacy in prevention (). Given abatacept’s preferential targeting of naïve T cells, there is a solid mechanistic basis that this will yield promising results in the T1D prevention space.
Future of T1D Immunotherapies
Significant progress in our understanding of the immunology of T1D is allowing for innovative therapeutic development that may be effective and acceptably safe for curing type 1 diabetes, however many unanswered questions remain. A continued focus on understanding diabetogenic T cell specificity may allow for both biomarker development and the creation of antigen-specific therapies that can restore immune tolerance via DNA vaccination, peptide immunotherapy, or Treg-based adoptive cell transfer. Additionally, the β-cell itself may have an important role at driving the autoimmune response with recent NOD mouse studies showing a critical role of senescent β cells in T1D pathogenesis, and applying a senolytic drug could prevent T1D and preserve β cell mass (Thompson et al., 2019).
Combining immunotherapies represents a way to enhance current monotherapies that currently have only a small effect, and/or target different mechanisms of T1D with the ultimate goal of optimally suppressing pathogenic pathways and stimulating regulatory pathways that may result in immune tolerance restoration. Two examples are the combination of polyclonal Tregs with IL2 () or Rapamycin ( and ) to improve their survival and function in T1D. Another approach may include the combination of systemic therapies (e.g. anti-CD3, anti-CD20, anti-inflammatory) with antigen-specific therapies with the hope of enhanced antigen-specific Treg induction (e.g. tolerogenic peptides) under an immune modulator umbrella. Alternatively, combining immunotherapies with metabolic therapies that may help improve β cell mass represents another important approach to improve efficacy in reversal studies. Early stage studies include the use of umbilical cord blood Treg plus a GLP-1 RA (liraglutide) (). Additionally, exenatide with allogenic islet transplantation and chronic immunosuppression may also improve long-term graft survival in T1D and is under investigation (Zoso et al., 2016).
Moving forward, trial design efforts to enrich for potential responders versus non-responders may also be critical. As outlined in Figure 4, approaches that segregate T1D patient groups for biomarkers could be important. For example, the HLA DQB1*0602 haploptype is protective of T1D so including these subjects may minimize the treatment effect size and prevent the ability of the trial to achieve its primary endpoint. Alternatively, if a trial includes subjects with insufficient starting β-cell mass, then no degree of immune intervention alone will allow for improved β-cell function and could lead to the misleading conclusion that the immune therapy does not help alter the disease course. Subgroup analyses from immune therapy trials have suggested active disease is important for predicting treatment response. Factors such as the C-peptide secretion and glycemic severity, immune biomarkers (e.g. Teff and Treg subsets) ,specific AAb positivity (e.g. anti-ZnT8), and environmental exposures (e.g. viruses) may help with identifying these subjects, monitoring their disease course, and optimizing the timing of an immune intervention.
Glucose Metabolism
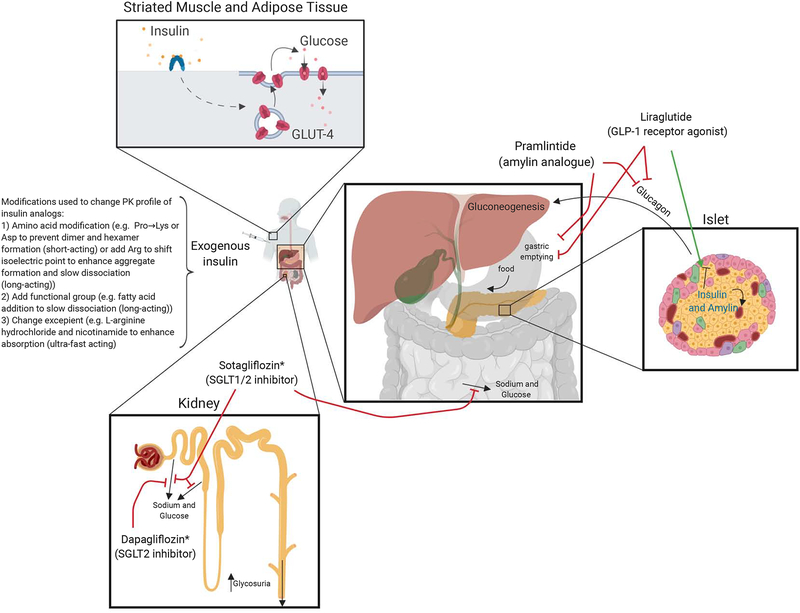
Data from the Diabetes Control and Complications Trial (DCCT) has established that maintaining a HbA1C <7% (especially early after diagnosis - “metabolic memory”) substantially reduces the occurrence of microvascular and macrovascular complications of T1D, but only about 25% of adults with T1D are able to reach this goal (Garg et al., 2017). Normal endogenous release of insulin from the β-cells allows it to remain at high local concentrations and serve as a potent paracrine suppressor of glucagon release from neighboring α-cells within the islet (Figure 5). Although insulin becomes progressively diluted as it travels out of the pancreas, overall its concentration remains relatively high as it circulates through the portal vein and liver relative to downstream concentrations sensed by peripheral tissues. These high concentrations are required to adequately suppress hepatic glucose production and minimize glycemic variability. Unfortunately, in T1D, exogenous insulin injected subcutaneously arrives at the pancreas and liver at concentrations significantly lower than are required to adequately suppress these glucagon-mediated mechanisms driving hyperglycemia and remains one important cause of glycemic variability in people with T1D. This limitation of insulin monotherapy results in some amount of hyperglycemia in even the most well-controlled and diligent people with T1D. The body’s primary mechanism to handle excess glucose in T1D is through its renal elimination after glucose reabsorption via sodium-glucose co-transporter-2 (SGLT-2) reaches capacity, typically at blood glucose levels above 180 mg/dl (Ferrannini, 2017). Development of insulins with improved pharmacokinetic and pharmacodynamics profiles, as well as the targeting of crucial mechanisms involved in either reducing gluconeogenesis or increasing glucose excretion are leading to improved therapies for those with T1D.
Figure 5.
Glucose metabolism and targeted therapies. *Sotagliflozin and Dapagliflozin approved in Europe for T1D (not approved by FDA in the U.S. due to increased risk for diabetic ketoacidosis)
Metabolic Therapies - Insulins
Exogenous insulin therapy is the mainstay of therapy for people with T1D and is typically delivered via a subcutaneous insulin infusion (CSII) or multiple daily injections. Since the initial use of crude insulin extracts from animal pancreas in the 1920s, many humanized insulin analogues have been developed with varying pharmacokinetic profile ranging from ultrafast-acting insulins that begin lowering blood glucose within minutes of administration to ultralong-acting insulin that last up to 42 hours with minimal peaks in action. These different profiles utilize amino acid modifications to alter absorption or clearance and have improved the quality of life for the heterogeneous group of people that need insulin. For instance, insulin human inhalation powder (Afreeza, MannKind) was approved for use in adults with T1D in 2014, has an extremely rapid onset of action and elimination and may be ideal for patients with a phobia to needles, tendency for initial postprandial hyperglycemia or tendency to stack insulin doses (FDA prescribing information for Afrezza, 2014). Fast-acting injectable insulins are ideal for use with CSII and CGMS that allow for minute-to-minute insulin titrations, especially with artificial pancreas algorithms. Fast-acting insulin aspart (Fiasp) is the most rapidly acting insulin currently FDA approved, the result of altering excipients in the aqueous solution that allows it to appear in circulation within 5 minutes of injection, reduce BG by 15 minutes, and Phase III data showed improved overall glycemic control compared to conventional insulin aspart (Mathieu et al., 2018). Similarly, a faster-acting insulin lispro (LY900014) contains locally acting excipients (citrate and treprostinil) to accelerate insulin lispro absorption, and Phase 3 trial results in T1D using CSII is pending (). Long-acting insulins have progressed from FDA approval of Glargine in 2000, which lasts 18–26 hours (FDA prescribing information Lantus, 2009), to Glargine U300, a 3 times more concentrated version of Glargine that lasts approximately 5 hours longer (FDA prescribing information Toujeo, 2015), and most recently Degludec Insulin approved in 2015 that lasts up to 42 hours (FDA prescribing information Tresiba, 015).
Development of glucose responsive (i.e. “smart”) insulins is an exciting area of research but data is limited to preclinical models (Bakh et al., 2017; Wang et al., 2019). The only human trial is with MK-2640, a novel insulin saccharide conjugate that, in addition to binding the insulin receptor, can bind the mannose receptor C type 1 (MRC1) too detect glucose concentration (Krug et al., 2019). Although preclinical data was promising in pigs and dogs (Moore et al., 2018), Phase 1 trial data in T1D showed at plasma concentrations that might be used in T1D its clearance was nearly saturated. This resulted in poor glucose-responsive pharmacokinetics and prevented its further clinical development.
Because human insulin is a protein hormone, it cannot be delivered orally due to the acidic pH of the stomach and proteases that cause its breakdown. To address these barriers, pH sensitive enteric coatings and protease inhibitors protecting a new short-acting insulin formulation (ORMD-0801) may allow for its oral delivery. Additionally, the first pass effect through the liver of an oral insulin could result in improved suppression of hepatic gluconeogenesis, similar to endogenous insulin secretion, which could lead to improved glucose control (Kidron, 2018).
Metabolic Therapies - Noninsulins
The large degree of glucose variability and risk of hypoglycemia in even the most well controlled people with T1D on insulin monotherapy has led to the search for noninsulin therapies that may help reduce insulin requirements and these associated risks. Targeting insulin independent mechanisms to reduce hyperglycemia in T1D has been accomplished through approaches that either reduce gluconeogenesis or increase glucose excretion.
Reducing Gluconeogenesis
Blocking gluconeogenesis could possibly serve as an adjunctive treatment of T1D, however, the most widely prescribed drug, metformin has not been shown to be effective in the treatment of T1D (Libman et al., 2015; Petrie et al., 2017; Vella et al., 2010). Despite this, data from 2016–2018 reported in the T1D Exchange clinical registry found in those >25 years old, metformin is the most commonly prescribed noninsulin medication for BG control (6%), followed by GLP-1 agonists (4%), SGLT2 inhibitors (3%), and Pramlintide (2%) (C. et al., 2019).
Amylin (also known as islet amyloid polypeptide, or IAPP) is co-secreted (1:100) with insulin by the β-cell in response to meals and has been shown to decrease glucagon secretion, in addition to slowing nutrient absorption and causing satiety (Heptulla et al., 2005; Koda et al., 1992). In 2005, the FDA approved pramlintide, an amylin analog, as an adjunct prandial therapy in people with T1D (Chapman et al., 2005; Edelman et al., 2006; Ratner et al., 2004). Clinical trials showed modest improvements in HbA1C (≤0.5%) with the ability to lower total daily insulin doses compared to insulin monotherapy (Whitehouse et al., 2002). Associated nausea and the need for increased injections have likely contributed to its limited use. It remains the only noninsulin therapy approved in the U.S. for T1D.
Glucagon-like peptide-1 (GLP-1) is a gut hormone that is secreted by enteroendocrine L-cells in the small intestine and colon in a biphasic manner following meal ingestion – early phase after 10–15 minutes and longer second phase after 30–60 minutes (Drucker, 2018). Data in T2D has shown GLP-1 receptor agonists (GLP1-RA) stimulate insulin secretion and suppress glucagon secretion in a glucose dependent manner, in addition to delaying gastric emptying (Kielgast et al., 2011). Effects of liraglutide on postprandial glucagon excursions in T1D have been mixed with some trials showing a reduction in postprandial glucagon release (Kuhadiya et al., 2016) while others have not (Galderisi et al., 2018). ADJUNCT ONE trial showed liraglutide can only modestly reduce HbA1c levels (0.15–0.2%), insulin doses and weight in subjects with T1D andat the expense of increased hypoglycemia and hyperglycemia with ketosis (Mathieu et al., 2016). Other trials in T1D have shown similar reductions of body weight and insulin requirements, but treatment effect on HbA1C compared to placebo is not always seen (Frandsen et al., 2015) (Dejgaard et al., 2016). Most recently, data using liraglutide 1.8mg for 52 weeks in addition to standard insulin therapy in newly diagnosed people with T1D resulted in reduced insulin doses and improved β cell function as evidenced by increased secreted C-peptide in the liraglutide group vs placebo, effects that disappeared 6 weeks after the final dose of liraglutide (DEJGAARD et al., 2019). It is possible the modest effect of GLP-1 RA on glycemic control is due to only certain populations, such as those with some degree of residual insulin/C-peptide secretion or overweight individuals with a concurrent amount of insulin resistance, reaping the majority of the benefit from these drugs.
Directly suppressing glucagon’s actions has been tested in T1D and shows promise. Proof of the therapeutic potential of glucagon inhibition was demonstrated clearly in preclinical animal studies including glucagon receptor knockout mice that fail to develop diabetes with destruction of their β-cells, and normalization of glucose with glucagon receptor blockade in streptozocin induced diabetes (Lee et al., 2012). The first clinical trial in occurred in 1978 and showed patients with T1D treated with an infusion of somatostatin that suppressed glucagon levels markedly lower insulin requirements, glycosuria and hyperglycemia, but prohibitive side effects have prevented its long term use (Raskin and Unger, 1978). Approximately 40 years later, targeted blockade of the glucagon axis in T1D was tested using REMD-477, a human IgG2 monoclonal antibody against the human glucagon receptor (Pettus et al., 2018). In their Phase 1 trial, one dose of REMD-477 leads to decreased insulin requirements by 14% with improved decreased glycemic variability and average glucose levels. A Phase 2 trial is currently underway looking at whether REMD-477 can decrease daily insulin requirements and improvement glycemic control after 12 weeks in T1D ().
Improving Glucose excretion
SGLTs are found in the mucosa of the small intestine (SGLT1) and proximal tubules of the kidney (SGLT2 >> SGLT1), and their inhibition allows for increased glucose excretion. Because the glucose lowering effect is insulin-independent, they represent a promising class of adjunct therapy in T1D to insulin. Selective SGLT2 inhibitors (empagliflozin, dapagliflozin, and canagliflozin) and dual SGLT1/SGLT2 inhibitor sotagliflozin are FDA approved for T2D. Clinical trials in T1D have shown they improve glycemic control, reduce body weight and total daily insulin doses (Chen et al., 2017; Fattah and Vallon, 2018). Phase 3 clinical trials using dapagliflozin (DEPICT), empagliflozin (EASE) and sotagliflozin (inTandem) found placebo corrected decrease in HbA1c (0.3%−0.5%), increase in weight loss (2.0–3.5kg) and time in range (2–3 hours) while reducing risk of hypoglycemia (Goldenberg et al., 2019). Unfortunately, concerning risk of diabetic ketoacidosis (DKA), likely an effect of indirect (insulin dose reduction and volume contraction) and direct (increased glucagon secretion, increased ketogenesis, increased renal tubular reabsorption of acetoacetate) causes, was a primary reason for sotagliflozin’s rejection by the FDA in the US for T1D (FDA, 2019). However, in April 2019, the European Commission approved Sotagliflozin for adults with T1D and BMI ≥27 kg/m2. Similarly, dapagliflozin received approval for T1D in Europe, but not by the FDA. Methods to mitigate this risk developed from a Consensus Report by an international group of experts were recently developed and emphasized selecting patients with willingness and ability to monitor ketones and follow medication instructions (Danne et al., 2019). More prospective data will be needed to validate their approach and define a safe strategy for using SGLT inhibitors in T1D.
Conclusions
Advances in our understanding of T1D pathogenesis is allowing for more specific targets that may positively impact those living with this disease. Immunotherapies targeting T cells, specifically the effector CD4+ and CD8+ response to Tregs, show the most promise. Improved understanding of insulin and glucose physiology is also allowing for the development of better insulins that will be able to improve life for those already diagnosed when used in combination with CGMS and computer technology. Finally, SGLT2 inhibitors may be the most powerful adjuvant therapy to insulin in T1D, if used cautiously and with proper guidance to minimize the risk of DKA. Recent advances in T1D management have progressed significantly since the original discovery of insulin in 1922 by Banting and Best, and new technologies will continue to allow us to not only treat disease, but also prevent and reverse it in the future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
J.A.B. is an advisor to Juno Therapeutics (a Celgene company); a member of the Scientific Advisory Boards of Arcus, Celsius, and Quentis. JAB is a member of the Board of Directors of Rheos as well as Provention, a company actively involved in the development of Teplizumab and other T1D-directed therapies. M.S.A. holds stock in Medtronic and Merck.
References
- (2019). 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2019. Diabetes care 42, S90–S102. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Cerosaletti K, James E, Long SA, Mannering S, Speake C, Nakayama M, Tree T, Roep BO, Herold KC, et al. (2019). Standardizing T-Cell Biomarkers in Type 1 Diabetes: Challenges and Recent Advances. Diabetes 68, 1366–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadj Ali M, Liu YF, Arif S, Tatovic D, Shariff H, Gibson VB, Yusuf N, Baptista R, Eichmann M, Petrov N, et al. (2017). Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Science translational medicine 9. [DOI] [PubMed] [Google Scholar]
- Ambery P, Donner TW, Biswas N, Donaldson J, Parkin J, and Dayan CM (2014). Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study. Diabet Med 31, 399–402. [DOI] [PubMed] [Google Scholar]
- Anderson MS, and Bluestone JA (2005). The NOD mouse: a model of immune dysregulation. Annual review of immunology 23, 447–485. [DOI] [PubMed] [Google Scholar]
- Aronson R, Gottlieb PA, Christiansen JS, Donner TW, Bosi E, Bode BW, and Pozzilli P (2014). Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes care 37, 27462754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babon JA, DeNicola ME, Blodgett DM, Crevecoeur I, Buttrick TS, Maehr R, Bottino R, Naji A, Kaddis J, Elyaman W, et al. (2016). Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nature medicine 22, 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach J-F (2002). The Effect of Infections on Susceptibility to Autoimmune and Allergic Diseases. New England Journal of Medicine 347, 911–920. [DOI] [PubMed] [Google Scholar]
- Bakh NA, Cortinas AB, Weiss MA, Langer RS, Anderson DG, Gu Z, Dutta S, and Strano MS (2017). Glucose-responsive insulin by molecular and physical design. Nature Chemistry 9, 937. [DOI] [PubMed] [Google Scholar]
- Banting FG, Best CH, Collip JB, Campbell WR, and Fletcher AA (1922). Pancreatic Extracts in the Treatment of Diabetes Mellitus. Canadian Medical Association journal 12, 141–146. [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, et al. (2015). Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Science translational medicine 7, 315ra189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Herold K, and Eisenbarth G (2010). Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464, 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacio E, and Ziegler AG (2010). Advances in the prediction and natural history of type 1 diabetes. Endocrinology and metabolism clinics of North America 39, 513–525. [DOI] [PubMed] [Google Scholar]
- Bottazzo GF, Florin-Christensen A, and Doniach D (1974). Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet (London, England) 2, 1279–1283. [DOI] [PubMed] [Google Scholar]
- Bretzel RG, Brandhorst D, Brandhorst H, Eckhard M, Ernst W, Friemann S, Rau W, Weimar B, Rauber K, Hering BJ, et al. (1999). Improved survival of intraportal pancreatic islet cell allografts in patients with type-1 diabetes mellitus by refined peritransplant management. J Mol Med (Berl) 77, 140–143. [DOI] [PubMed] [Google Scholar]
- C. FN, W. BR, M. MK, A. CM, R. RM, A. DL, M. MD, V. TW, Richard B, Elizabeth S, et al. (2019). State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technology & Therapeutics 21, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caladrius Biosciences, I. (2019). Caladrius Biosciences Reports Top-Line Data for the Phase 2a Sanford Project: T-Rex Trial of CLBS03 for Recent Onset Type 1 Diabetes.
- Chaillous L, Lefevre H, Thivolet C, Boitard C, Lahlou N, Atlan-Gepner C, Bouhanick B, Mogenet A, Nicolino M, Carel JC, et al. (2000). Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet (London, England) 356, 545–549. [DOI] [PubMed] [Google Scholar]
- Chapman I, Parker B, Doran S, Feinle-Bisset C, Wishart J, Strobel S, Wang Y, Burns C, Lush C, Weyer C, et al. (2005). Effect of pramlintide on satiety and food intake in obese subjects and subjects with type 2 diabetes. Diabetologia 48, 838–848. [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Thervet E, Primo J, and Bach JF (1994). Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proceedings of the National Academy of Sciences 91, 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Fan F, Wang JY, Long Y, Gao CL, Stanton RC, and Xu Y (2017). The efficacy and safety of SGLT2 inhibitors for adjunctive treatment of type 1 diabetes: a systematic review and meta-analysis. Scientific reports 7, 44128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, et al. (2016). Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440. [DOI] [PubMed] [Google Scholar]
- Concannon P, Rich SS, and Nepom GT (2009). Genetics of Type 1A Diabetes. New England Journal of Medicine 360, 1646–1654. [DOI] [PubMed] [Google Scholar]
- Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simoes BP, et al. (2009a). C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. Jama 301, 1573–1579. [DOI] [PubMed] [Google Scholar]
- Couri CEB, Oliveira MCB, Stracieri ABPL, Moraes DA, Pieroni F, Barros GMN, Madeira MIA, Malmegrim KCR, Foss-Freitas MC, Simões BP, et al. (2009b). C-Peptide Levels and Insulin Independence Following Autologous Nonmyeloablative Hematopoietic Stem Cell Transplantation in Newly Diagnosed Type 1 Diabetes Mellitus. Jama 301, 1573–1579. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, Bell R, Badaru A, Talton JW, Crume T, et al. (2014). Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. Jama 311, 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danne T, Garg S, Peters AL, Buse JB, Mathieu C, Pettus JH, Alexander CM, Battelino T, Ampudia-Blasco FJ, Bode BW, et al. (2019). International Consensus on Risk Management of Diabetic Ketoacidosis in Patients With Type 1 Diabetes Treated With Sodium-Glucose Cotransporter (SGLT) Inhibitors. Diabetes care 42, 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goffau MC, Fuentes S, van den Bogert B, Honkanen H, de Vos WM, Welling GW, Hyoty H, and Harmsen HJ (2014). Aberrant gut microbiota composition at the onset of type 1 diabetes in young children. Diabetologia 57, 1569–1577. [DOI] [PubMed] [Google Scholar]
- Dejgaard TF, Frandsen CS, Hansen TS, Almdal T, Urhammer S, Pedersen-Bjergaard U, Jensen T, Jensen AK, Holst JJ, Tarnow L, et al. (2016). Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. The lancet. Diabetes & endocrinology 4, 221–232. [DOI] [PubMed] [Google Scholar]
- DEJGAARD TF, FRANDSEN CS, KIELGAST U, ANDERSEN HU, THORSTEINSSON B, KRARUP T, HOLST JJ, and MADSBAD S (2019). 59-OR: Liraglutide Preserved Insulin Secretion in Adults with Newly Diagnosed Type 1 Diabetes: The NewLira Trial. Diabetes 68, 59–OR. [Google Scholar]
- Delong T, Wiles TA, Baker RL, Bradley B, Barbour G, Reisdorph R, Armstrong M, Powell RL, Reisdorph N, Kumar N, et al. (2016). Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science (New York, N.Y.) 351, 711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ (2018). Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell metabolism 27, 740–756. [DOI] [PubMed] [Google Scholar]
- Dunne JL, Richardson SJ, Atkinson MA, Craig ME, Dahl-Jorgensen K, Flodstrom-Tullberg M, Hyoty H, Insel RA, Lernmark A, Lloyd RE, et al. (2019). Rationale for enteroviral vaccination and antiviral therapies in human type 1 diabetes. Diabetologia 62, 744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman S, Garg S, Frias J, Maggs D, Wang Y, Zhang B, Strobel S, Lutz K, and Kolterman O (2006). A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes care 29, 2189–2195. [DOI] [PubMed] [Google Scholar]
- Eisenbarth GS (1986). Type I Diabetes Mellitus. New England Journal of Medicine 314, 1360–1368. [DOI] [PubMed] [Google Scholar]
- Ergun-Longmire B, Marker J, Zeidler A, Rapaport R, Raskin P, Bode B, Schatz D, Vargas A, Rogers D, Schwartz S, et al. (2004). Oral insulin therapy to prevent progression of immune-mediated (type 1) diabetes. Ann N Y Acad Sci 1029, 260–277. [DOI] [PubMed] [Google Scholar]
- Erlich H, Valdes AM, Noble J, Carlson J, Varney M, Concannon P, Mychaleckyj JC, Todd J, Bonella P, Fear AL, et al. (2008). HLA DR-DQ Haplotypes and Genotypes and Type 1 Diabetes Risk. Diabetes 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattah H, and Vallon V (2018). The Potential Role of SGLT2 Inhibitors in the Treatment of Type 1 Diabetes Mellitus. Drugs 78, 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA (2019). U.S. Food and Drug Administration. 2019 Meeting Materials, January 17, 2019 Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee [Google Scholar]
- Ferrannini E (2017). Sodium-Glucose Co-transporters and Their Inhibition: Clinical Physiology. Cell metabolism 26, 27–38. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Mari A, Nofrate V, Sosenko JM, and Skyler JS (2010). Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes 59, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feutren G, Assan R, Karsenty G, Du Rostu H, Sirmai J, Papoz L, Vialettes B, Vexiau P, Rodier M, Lallemand A, et al. (1986). CYCLOSPORIN INCREASES THE RATE AND LENGTH OF REMISSIONS IN INSULIN-DEPENDENT DIABETES OF RECENT ONSET: Results of a Multicentre Double-blind Trial. The Lancet 328, 119–124. [DOI] [PubMed] [Google Scholar]
- Fisher MM, Watkins RA, Blum J, Evans-Molina C, Chalasani N, DiMeglio LA, Mather KJ, Tersey SA, and Mirmira RG (2015). Elevations in Circulating Methylated and Unmethylated Preproinsulin DNA in New-Onset Type 1 Diabetes. Diabetes 64, 3867–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourlanos S, Perry C, Gellert SA, Martinuzzi E, Mallone R, Butler J, Colman PG, and Harrison LC (2011). Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes 60, 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen CS, Dejgaard TF, Holst JJ, Andersen HU, Thorsteinsson B, and Madsbad S (2015). Twelve-Week Treatment With Liraglutide as Add-on to Insulin in Normal-Weight Patients With Poorly Controlled Type 1 Diabetes: A Randomized, Placebo-Controlled, Double-Blind Parallel Study. Diabetes care 38, 2250–2257. [DOI] [PubMed] [Google Scholar]
- Gaglia JL, Harisinghani M, Aganj I, Wojtkiewicz GR, Hedgire S, Benoist C, Mathis D, and Weissleder R (2015). Noninvasive mapping of pancreatic inflammation in recent-onset type-1 diabetes patients. Proceedings of the National Academy of Sciences of the United States of America 112, 2139–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi A, Sherr J, VanName M, Carria L, Zgorski M, Tichy E, Weyman K, Cengiz E, Weinzimer S, and Tamborlane W (2018). Pramlintide but Not Liraglutide Suppresses Meal-Stimulated Glucagon Responses in Type 1 Diabetes. The Journal of clinical endocrinology and metabolism 103, 1088–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JM, Posselt AM, Wisel S, Mashirani U, Szot G, Nguyen V, Johnson K, McElroy J, Tang Q, and Stock PG (2018). Ten Year Insulin-Independence in Select Islet Transplant Recipients Receiving CNI-Sparing Immunosuppression with Either Costimulation Blockade or Anti-LFA1. Transplantation 102, S374. [Google Scholar]
- Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, Pozzilli P, Gesty-Palmer D, Lapuerta P, Simo R, et al. (2017). Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. The New England journal of medicine 377, 2337–2348. [DOI] [PubMed] [Google Scholar]
- Gepts W (1965). Pathologic Anatomy of the Pancreas in Juvenile Diabetes Mellitus. Diabetes 14, 619–633. [DOI] [PubMed] [Google Scholar]
- Gepts W, and De Mey J (1978). Islet cell survival determined by morphology. An immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes 27 Suppl 1, 251–261. [DOI] [PubMed] [Google Scholar]
- Giannoukakis N, Phillips B, Finegold D, Harnaha J, and Trucco M (2011). Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes care 34, 2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman SE, and Bluestone JA (2016). Regulatory T cell therapy for type 1 diabetes: May the force be with you. Journal of autoimmunity 71, 78–87. [DOI] [PubMed] [Google Scholar]
- Goldenberg RM, Gilbert JD, Hramiak IM, Woo VC, and Zinman B (2019). Sodium-glucose co-transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: The STOP DKA Protocol. Diabetes, obesity & metabolism. [DOI] [PubMed] [Google Scholar]
- Gu Y, Gong C, Peng X, Wei L, Su C, Qin M, Wang X, and Li F (2014). Autologous hematopoietic stem cell transplantation and conventional insulin therapy in the treatment of children with newly diagnosed type 1 diabetes: long term follow-up. Chinese medical journal 127, 2618–2622. [PubMed] [Google Scholar]
- Hagerkvist R, Makeeva N, Elliman S, and Welsh N (2006). Imatinib mesylate (Gleevec) protects against streptozotocin-induced diabetes and islet cell death in vitro. Cell Biol Int 30, 1013–1017. [DOI] [PubMed] [Google Scholar]
- Hagerkvist R, Sandler S, Mokhtari D, and Welsh N (2007). Amelioration of diabetes by imatinib mesylate (Gleevec): role of beta-cell NF-kappaB activation and anti-apoptotic preconditioning. Faseb j 21, 618–628. [DOI] [PubMed] [Google Scholar]
- Hagopian W, Ferry RJ, Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC, et al. (2013). Teplizumab Preserves C-Peptide in Recent-Onset Type 1 Diabetes. Two-Year Results From the Randomized, Placebo-Controlled Protégé Trial 62, 3901–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller MJ, Long SA, Blanchfield JL, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Geyer SM, Warnock MV, Miller JL, et al. (2019). Low-Dose Anti-Thymocyte Globulin Preserves C-Peptide, Reduces HbA1c, and Increases Regulatory to Conventional T-Cell Ratios in New-Onset Type 1 Diabetes: Two-Year Clinical Trial Data. Diabetes 68, 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller MJ, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Miller JL, Atkinson MA, Becker DJ, Baidal D, DiMeglio LA, et al. (2018). Low-Dose Anti-Thymocyte Globulin (ATG) Preserves β-Cell Function and Improves HbA1c in New-Onset Type 1 Diabetes. Diabetes care 41, 1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harjutsalo V, Sund R, Knip M, and Groop PH (2013). Incidence of type 1 diabetes in Finland. Jama 310, 427–428. [DOI] [PubMed] [Google Scholar]
- Heptulla RA, Rodriguez LM, Bomgaars L, and Haymond MW (2005). The role of amylin and glucagon in the dampening of glycemic excursions in children with type 1 diabetes. Diabetes 54, 1100–1107. [DOI] [PubMed] [Google Scholar]
- Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, et al. (2019). An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. New England Journal of Medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, et al. (2013). Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62, 3766–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Usmani-Brown S, Ghazi T, Lebastchi J, Beam CA, Bellin MD, Ledizet M, Sosenko JM, Krischer JP, and Palmer JP (2015). beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. The Journal of clinical investigation 125, 1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D, Holz A, Bot A, Coon B, Wolfe T, Petersen J, Dyrberg TP, Grusby MJ, and von Herrath MG (1999). Autoreactive CD4+ T cells protect from autoimmune diabetes via bystander suppression using the IL-4/Stat6 pathway. Immunity 11, 463–472. [DOI] [PubMed] [Google Scholar]
- Hull CM, Nickolay LE, Estorninho M, Richardson MW, Riley JL, Peakman M, Maher J, and Tree TI (2017). Generation of human islet-specific regulatory T cells by TCR gene transfer. Journal of autoimmunity 79, 63–73. [DOI] [PubMed] [Google Scholar]
- Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, Lawrence JM, Liese AD, Liu LL, Mayer-Davis EJ, et al. (2012). Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes care 35, 2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, et al. (2015). Staging Presymptomatic Type 1 Diabetes: A Scientific Statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes care 38, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison BL, Neef T, Goodspeed A, Bradley B, Baker RL, Miller SD, and Haskins K (2019). Nanoparticles Containing an Insulin–ChgA Hybrid Peptide Protect from Transfer of Autoimmune Diabetes by Shifting the Balance between Effector T Cells and Regulatory T Cells. The Journal of Immunology, ji1900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Hattersley AT, and Flanagan SE (2016). Monogenic autoimmune diseases of the endocrine system. The lancet. Diabetes & endocrinology 4, 862–872. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, et al. (2005). Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. The New England journal of medicine 352, 2598–2608. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Walter M, Mathieu C, Kaufman L, Gorus F, Hilbrands R, Vandemeulebroucke E, Van de Velde U, Crenier L, De Block C, et al. (2010). Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia 53, 614–623. [DOI] [PubMed] [Google Scholar]
- Kidron MH, Kenneth; Neutel Joel (2018). Lowered glucose levels and exogenous insulin requirements in T2DM and T1DM patients treated with oral insulin (ORMD-0801): Phase 2, randomized, placebo-controlled evaluations In Cardiometabolic Health Congress (Boston, MA: ). [Google Scholar]
- Kielgast U, Holst JJ, and Madsbad S (2011). Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual beta-cell function. Diabetes 60, 1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knip M, Akerblom HK, Al Taji E, Becker D, Bruining J, Castano L, Danne T, de Beaufort C, Dosch HM, Dupre J, et al. (2018). Effect of Hydrolyzed Infant Formula vs Conventional Formula on Risk of Type 1 Diabetes: The TRIGR Randomized Clinical Trial. Jama 319, 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda JE, Fineman M, Rink TJ, Dailey GE, Muchmore DB, and Linarelli LG (1992). Amylin concentrations and glucose control Lancet (London, England: ) 339, 1179–1180. [DOI] [PubMed] [Google Scholar]
- Kostic AD, Gevers D, Siljander H, Vatanen T, Hyotylainen T, Hamalainen AM, Peet A, Tillmann V, Poho P, Mattila I, et al. (2015). The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell host & microbe 17, 260–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, and Mathis D (2011). Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proceedings of the National Academy of Sciences of the United States of America 108, 11548–11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krischer JP (2013). The use of intermediate endpoints in the design of type 1 diabetes prevention trials. Diabetologia 56, 1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krischer JP, Liu X, Vehik K, Akolkar B, Hagopian WA, Rewers MJ, She J-X, Toppari J, Ziegler A-G, and Lernmark Å (2019). Predicting Islet Cell Autoimmunity and Type 1 Diabetes: An 8-Year TEDDY Study Progress Report. Diabetes care 42, 1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krischer JP, Schatz DA, Bundy B, Skyler JS, and Greenbaum CJ (2017). Effect of Oral Insulin on Prevention of Diabetes in Relatives of Patients With Type 1 Diabetes: A Randomized Clinical Trial. Jama 318, 1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug AW, Visser SAG, Tsai K, Kandala B, Fancourt C, Thornton B, Morrow L, Kaarsholm NC, Bernstein HS, Stoch SA, et al. (2019). Clinical Evaluation of MK-2640: An Insulin Analog With Glucose-Responsive Properties. Clinical pharmacology and therapeutics 105, 417–425. [DOI] [PubMed] [Google Scholar]
- Kuhadiya ND, Dhindsa S, Ghanim H, Mehta A, Makdissi A, Batra M, Sandhu S, Hejna J, Green K, Bellini N, et al. (2016). Addition of Liraglutide to Insulin in Patients With Type 1 Diabetes: A Randomized Placebo-Controlled Clinical Trial of 12 Weeks. Diabetes care 39, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachin JM, McGee P, and Palmer JP (2014). Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes 63, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Berglund ED, Wang MY, Fu X, Yu X, Charron MJ, Burgess SC, and Unger RH (2012). Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proceedings of the National Academy of Sciences of the United States of America 109, 14972–14976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libman IM, and LaPorte RE (2005). Changing trends in epidemiology of type 1 diabetes mellitus throughout the world: how far have we come and where do we go from here. Pediatr Diabetes 6, 119–121. [DOI] [PubMed] [Google Scholar]
- Libman IM, Miller KM, DiMeglio LA, Bethin KE, Katz ML, Shah A, Simmons JH, Haller MJ, Raman S, Tamborlane WV, et al. (2015). Effect of Metformin Added to Insulin on Glycemic Control Among Overweight/Obese Adolescents With Type 1 Diabetes: A Randomized Clinical Trial. Jama 314, 2241–2250. [DOI] [PubMed] [Google Scholar]
- Long SA, Rieck M, Sanda S, Bollyky JB, Samuels PL, Goland R, Ahmann A, Rabinovitch A, Aggarwal S, Phippard D, et al. (2012). Rapamycin/IL-2 Combination Therapy in Patients With Type 1 Diabetes Augments Tregs yet Transiently Impairs β-Cell Function. Diabetes 61, 2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, Zhu S, Weiss A, and Bluestone JA (2008). Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proceedings of the National Academy of Sciences of the United States of America 105, 18895–18900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson J, Krisky D, Casas R, Battelino T, Castano L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert JJ, et al. (2012). GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. The New England journal of medicine 366, 433–442. [DOI] [PubMed] [Google Scholar]
- Maahs DM, West NA, Lawrence JM, and Mayer-Davis EJ (2010). Epidemiology of type 1 diabetes. Endocrinology and metabolism clinics of North America 39, 481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmegrim KC, de Azevedo JT, Arruda LC, Abreu JR, Couri CE, de Oliveira GL, Palma PV, Scortegagna GT, Stracieri AB, Moraes DA, et al. (2017). Immunological Balance Is Associated with Clinical Outcome after Autologous Hematopoietic Stem Cell Transplantation in Type 1 Diabetes. Frontiers in immunology 8, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek-Trzonkowska N, Mysliwiec M, Iwaszkiewicz-Grzes D, Gliwinski M, Derkowska I, Zalinska M, Zielinski M, Grabowska M, Zielinska H, Piekarska K, et al. (2016). Factors affecting long-term efficacy of T regulatory cell-based therapy in type 1 diabetes. J Transl Med 14, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño E, Silveira PA, Stolp J, and Grey ST (2011). B cell-directed therapies in type 1 diabetes. Trends in immunology 32, 287–294. [DOI] [PubMed] [Google Scholar]
- Masteller EL, Warner MR, Tang Q, Tarbell KV, McDevitt H, and Bluestone JA (2005). Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice Journal of immunology (Baltimore, Md. : 1950) 175, 3053–3059. [DOI] [PubMed] [Google Scholar]
- Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, and Quattrin T (2009). Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes care 32, 1244–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C, Bode BW, Franek E, Philis-Tsimikas A, Rose L, Graungaard T, Birk Osterskov A, and Russell-Jones D (2018). Efficacy and safety of fast-acting insulin aspart in comparison with insulin aspart in type 1 diabetes (onset 1): A 52-week, randomized, treat-to-target, phase III trial. Diabetes, obesity & metabolism 20, 1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C, Zinman B, Hemmingsson JU, Woo V, Colman P, Christiansen E, Linder M, and Bode B (2016). Efficacy and Safety of Liraglutide Added to Insulin Treatment in Type 1 Diabetes: The ADJUNCT ONE Treat-To-Target Randomized Trial. Diabetes care 39, 1702–1710. [DOI] [PubMed] [Google Scholar]
- Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, et al. (2017). Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. The New England journal of medicine 376, 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Richardson CC, Ravishankar A, Brigatti C, Liberati D, Lampasona V, Piemonti L, Morgan D, Feltbower RG, and Christie MR (2016). Identification of Tetraspanin-7 as a Target of Autoantibodies in Type 1 Diabetes. Diabetes 65, 1690–1698. [DOI] [PubMed] [Google Scholar]
- Michels AW, Landry LG, McDaniel KA, Yu L, Campbell-Thompson M, Kwok WW, Jones KL, Gottlieb PA, Kappler JW, Tang Q, et al. (2017). Islet-Derived CD4 T Cells Targeting Proinsulin in Human Autoimmune Diabetes. Diabetes 66, 722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MC, Kelley DE, Camacho RC, Zafian P, Ye T, Lin S, Kaarsholm NC, Nargund R, Kelly TM, Van Heek M, et al. (2018). Superior Glycemic Control With a Glucose-Responsive Insulin Analog: Hepatic and Nonhepatic Impacts. Diabetes 67, 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Greenbaum CJ, Herold KC, Marks JB, Raskin P, et al. (2013). Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials Lancet (London, England: ) 381, 1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, et al. (2005). Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435, 220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onkamo P, Vaananen S, Karvonen M, and Tuomilehto J (1999). Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia 42, 1395–1403. [DOI] [PubMed] [Google Scholar]
- Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, et al. (2011). Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial Lancet (London, England: ) 378, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathiraja V, Kuehlich JP, Campbell PD, Krishnamurthy B, Loudovaris T, Coates PT, Brodnicki TC, O’Connell PJ, Kedzierska K, Rodda C, et al. (2015). Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes 64, 172–182. [DOI] [PubMed] [Google Scholar]
- Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, Rami-Merhar B, Soltesz G, Svensson J, Parslow RC, et al. (2019). Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia 62, 408–417. [DOI] [PubMed] [Google Scholar]
- Paun A, Yau C, Meshkibaf S, Daigneault MC, Marandi L, Mortin-Toth S, Bar-Or A, Allen-Vercoe E, Poussier P, and Danska JS (2019). Association of HLA-dependent islet autoimmunity with systemic antibody responses to intestinal commensal bacteria in children. Science Immunology 4, eaau8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescovitz MD, Greenbaum CJ, Bundy B, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, Moran A, Raskin P, et al. (2014). B-lymphocyte depletion with rituximab and beta-cell function: two-year results. Diabetes care 37, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie JR, Chaturvedi N, Ford I, Brouwers M, Greenlaw N, Tillin T, Hramiak I, Hughes AD, Jenkins AJ, Klein BEK, et al. (2017). Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. The lancet. Diabetes & endocrinology 5, 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus J, Reeds D, Cavaiola TS, Boeder S, Levin M, Tobin G, Cava E, Thai D, Shi J, Yan H, et al. (2018). Effect of a glucagon receptor antibody (REMD-477) in type 1 diabetes: A randomized controlled trial. Diabetes, obesity & metabolism 20, 1302–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin P, and Unger RH (1978). Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. The New England journal of medicine 299, 433–436. [DOI] [PubMed] [Google Scholar]
- Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, Strobel SA, Weyer C, and Kolterman OG (2004). Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med 21, 1204–1212. [DOI] [PubMed] [Google Scholar]
- Redondo MJ, Jeffrey J, Fain PR, Eisenbarth GS, and Orban T (2008). Concordance for islet autoimmunity among monozygotic twins. The New England journal of medicine 359, 2849–2850. [DOI] [PubMed] [Google Scholar]
- Rigby MR, Harris KM, Pinckney A, DiMeglio LA, Rendell MS, Felner EI, Dostou JM, Gitelman SE, Griffin KJ, Tsalikian E, et al. (2015). Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. The Journal of clinical investigation 125, 3285–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roep BO, Solvason N, Gottlieb PA, Abreu JRF, Harrison LC, Eisenbarth GS, Yu L, Leviten M, Hagopian WA, Buse JB, et al. (2013). Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8(+) T cells in type 1 diabetes. Science translational medicine 5, 191ra182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safari F, Farajnia S, Arya M, Zarredar H, and Nasrolahi A (2018). CRISPR and personalized Treg therapy: new insights into the treatment of rheumatoid arthritis. Immunopharmacology and Immunotoxicology 40, 201–211. [DOI] [PubMed] [Google Scholar]
- Shapiro AMJ, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. (2006). International Trial of the Edmonton Protocol for Islet Transplantation. New England Journal of Medicine 355, 1318–1330. [DOI] [PubMed] [Google Scholar]
- Sharp SA, Rich SS, Wood AR, Jones SE, Beaumont RN, Harrison JW, Schneider DA, Locke JM, Tyrrell J, Weedon MN, et al. (2019). Development and Standardization of an Improved Type 1 Diabetes Genetic Risk Score for Use in Newborn Screening and Incident Diagnosis. Diabetes care 42, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr., Bode B, Aronoff S, Holland C, Carlin D, et al. (2011). Teplizumab for treatment of type 1 diabetes (Protege study): 1-year results from a randomised, placebo-controlled trial Lancet (London, England: ) 378, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, and Herold KC (2006). Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 55, 3238–3245. [DOI] [PubMed] [Google Scholar]
- Sims EK, Bahnson HT, Nyalwidhe J, Haataja L, Davis AK, Speake C, DiMeglio LA, Blum J, Morris MA, Mirmira RG, et al. (2019). Proinsulin Secretion Is a Persistent Feature of Type 1 Diabetes. Diabetes care 42, 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stene LC, Oikarinen S, Hyöty H, Barriga KJ, Norris JM, Klingensmith G, Hutton JC, Erlich HA, Eisenbarth GS, and Rewers M (2010). Enterovirus Infection and Progression From Islet Autoimmunity to Type 1 Diabetes. The Diabetes and Autoimmunity Study in the Young (DAISY) 59, 3174–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, and Bluestone JA (2004). In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. The Journal of experimental medicine 199, 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, and Hattersley AT (2018). Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. The lancet. Diabetes & endocrinology 6, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PJ, Shah A, Ntranos V, Van Gool F, Atkinson M, and Bhushan A (2019). Targeted Elimination of Senescent Beta Cells Prevents Type 1 Diabetes. Cell metabolism 29, 1045–1060.e1010. [DOI] [PubMed] [Google Scholar]
- Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, Lernmark A, Hagopian WA, Rewers MJ, She JX, et al. (2018). The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature 562, 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella S, Buetow L, Royle P, Livingstone S, Colhoun HM, and Petrie JR (2010). The use of metformin in type 1 diabetes: a systematic review of efficacy. Diabetologia 53, 809–820. [DOI] [PubMed] [Google Scholar]
- Virostko J, Williams J, Hilmes M, Bowman C, Wright JJ, Du L, Kang H, Russell WE, Powers AC, and Moore DJ (2019). Pancreas Volume Declines During the First Year After Diagnosis of Type 1 Diabetes and Exhibits Altered Diffusion at Disease Onset. Diabetes care 42, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann H, and Cobbold S (1998). How do monoclonal antibodies induce tolerance? A role for infectious tolerance? Annual review of immunology 16, 619–644. [DOI] [PubMed] [Google Scholar]
- Walter M, Philotheou A, Bonnici F, Ziegler AG, and Jimenez R (2009). No effect of the altered peptide ligand NBI-6024 on beta-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes care 32, 2036–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yu J, Zhang Y, Zhang X, Kahkoska AR, Chen G, Wang Z, Sun W, Cai L, Chen Z, et al. (2019). Charge-switchable polymeric complex for glucose-responsive insulin delivery in mice and pigs. Science Advances 5, eaaw4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Ehlers MR, Harris KM, Kanaparthi S, Long A, Phippard D, Weiner LJ, Jepson B, McNamara JG, Koulmanda M, et al. (2018). Alpha-1 antitrypsin treatment of new-onset type 1 diabetes: An open-label, phase I clinical trial (RETAIN) to assess safety and pharmacokinetics. Pediatr Diabetes 19, 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, et al. (2008). Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455, 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Zhou Z, Guo L, Zhu D, Ji L, Luo X, Mu Y, and Jia W (2018). Incidence of type 1 diabetes in China, 2010–13: population based study BMJ (Clinical research ed.) 360, j5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Herold KC, Marks JB, et al. (2011). Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial Lancet (London, England: ) 378, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse F, Kruger DF, Fineman M, Shen L, Ruggles JA, Maggs DG, Weyer C, and Kolterman OG (2002). A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes care 25, 724–730. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Chau W, Callaway MP, and Dayan CM (2007). Magnetic resonance imaging: a reliable method for measuring pancreatic volume in Type 1 diabetes. Diabet Med 24, 35–40. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Thrower SL, Sequeiros IM, Ward A, Bickerton AS, Triay JM, Callaway MP, and Dayan CM (2012). Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. The Journal of clinical endocrinology and metabolism 97, E2109–2113. [DOI] [PubMed] [Google Scholar]
- Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, et al. (2013). Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. Jama 309, 2473–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoso A, Serafini P, Lanzoni G, Peixoto E, Messinger S, Mantero A, Padilla-Tellez ND, Baidal DA, Alejandro R, Ricordi C, et al. (2016). G-CSF and Exenatide Might Be Associated with Increased Long-Term Survival of Allogeneic Pancreatic Islet Grafts. PloS one 11, e0157245. [DOI] [PMC free article] [PubMed] [Google Scholar]