Abstract
Radiation therapy is an essential intervention used in the treatment plan of more than half of cancer patients. With the increasing use of hypofractionated radiation regimens, concurrent use of radiation and chemotherapy, targeted agents and immunotherapy, the risk of radiation-induced toxicities is increased. Hematopoietic toxicity caused by radiation limits a patient’s options for subsequent systemic therapy. However, much remains unknown about the molecular underpinnings responsible for radiation-induced hematopoietic toxicity. MicroRNA (miRNA) are small, non-coding RNA involved in post-transcriptional regulation of gene expression and are associated with essential cellular processes. miR-21 is a potent oncomiR that is dysregulated in a significant fraction of human malignancies, and its overexpression is linked to poor overall survival, chemoresistance, and radioresistance in several human cancers. However, the contribution of miR-21 in governing radiation sensitivity in normal, untransformed cells, and the impact of silencing this miRNA in normal tissues remains largely unexplored. We determined ionizing radiation significantly increased miR-21 levels in radio-sensitive tissues, but not in radio-insensitive tissues, suggesting it may have a critical function in the normal tissue response to radiation. To test this concept, we generated miR-21 knockout mice. Mice lacking one or both alleles of miR-21 showed reduced numbers of hematopoietic stem and progenitor cells (HSPCs) and increased sensitivity to an LD50/30 dose of total body irradiation (TBI) with evidence of bone marrow failure compared to wild-type mice. Transplantation of wild-type bone marrow into irradiated miR-21-deficient mice rescued the mice from death. Thus, our data identify miR-21 as a critical component of HSPC viability and essential for bone marrow recovery following irradiation. Further investigation is warranted to determine if miR-21 can be used to stratify patients at risk for hematopoietic toxicity following irradiation.
Summary
Here we report miR-21, which is commonly overexpressed in malignant cells, was induced in radio-sensitive tissues and was essential for maintaining hematopoiesis following ionizing radiation. Loss of miR-21 conferred sensitivity to mice exposed to whole body ionizing radiation, resulting in bone marrow failure and death. These results increase our understanding of the endogenous factors governing radiation sensitivity in normal cells, and the potential use of miR21 to identify patients at risk for increased radiation toxicity.
Introduction
Of the ~1.6 million newly diagnosed cancer patients in the United States this year, over half will receive radiation therapy during their cancer treatment (1,2). Ionizing radiation is increasingly administered to cancer patients with either altered fractionation schedules or in conjunction with systemic therapy. Hypofractionated radiation regimens, where slightly more radiation is given per day, have become the treatment of choice for breast, prostate, lung, and pancreatic cancers, as clinical trials have demonstrated equivalent or improved cancer control with less financial toxicity to the patient (3–7). However, evidence demonstrates a propensity for long-term toxicity with these regimens (8,9). The use of radiation to augment systemic agents such as immunotherapy and other targeted agents is also increasing, but data are scarce on toxicity, particularly the long-term side effects (10,11).
Normal tissue toxicity from radiation may limit subsequent local and/or systemic treatment regimens for a patient, thereby limiting potential increases in overall survival that might otherwise be realized (12). One such example, bone marrow toxicity, may prevent administration of systemic therapies which may also cause hematopoietic toxicities due to the overlapping toxicity profile (13). Therefore, understanding the endogenous factors that govern normal tissue toxicity, and delineating which patients may be more susceptible, is crucial when considering these regimens.
MicroRNA (miRNA) are small, non-coding RNA that regulate gene expression post-transcriptionally. miRNA have significant functions in many cellular processes (14) and in regulating the cellular response to multiple stresses, including ionizing radiation, tumor development, and metastases (15–18). miR-21 is overexpressed in the majority of human malignancies (19) and has been implicated in radiation resistance in cancer cells through its regulation of DNA damage response (DDR) genes and genes promoting apoptosis (19–23). Thus, targeting miR-21 in malignant cells represents an attractive avenue for therapeutic intervention to increase tumor cell sensitivity to radiation-induced DNA damage and reduce tumor burden. However, the role of miR-21 in the radiation response in normal tissues remains largely unexplored and poorly understood.
Here we report the generation of miR-21 knockout mice and show that loss of miR-21 sensitizes mice to whole-body irradiation, resulting in reduced overall survival. Our data indicate this sensitivity results from intrinsic defects in HSPCs in miR-21-deficient mice and a subsequent inability to maintain normal hematopoiesis following irradiation, leading to bone marrow failure. Thus, our results reveal an essential function of miR-21 in normal HSPCs and their response to radiation.
Materials and methods
Mice
Using standard procedures, miR-21 knockout mice were generated at the University of North Carolina Animal Models Core. The targeted embryonic stem cells were verified by PCR and Southern blot. Deletion of the Neo cassette followed by global miR-21 knockout were produced by crossing mice to flipase and Ella-Cre mice. See Supplemental Material for details on the generation and verification of the mice. Mice were backcrossed onto two different genetic backgrounds (FVB and Ola129/B6). Male and female littermates (6–12 week old) were used for experiments. All experiments with the mice were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University.
Radiation
Mice were subjected to total body irradiation (TBI) at a dose of 6.5 Gy using a Pantak H-320 (320kV) Precision X-Ray. Mice were sacrificed at pre-specified times or at the humane endpoint following irradiation.
Genotyping PCR
Genomic DNA from tail clips and bone marrow was isolated using the REDExtract-N-Amp Tissue PCR kit (Sigma-Aldrich). PCR was performed using the following primers: 5’-TTTATGACGCATTGCACACCCTC (forward), 5’-CACAGAGAAGTAAGCTTCCACCTGTTAAAG (WT reverse); 5’-AATAAGACTTATGAGATGGAGTCAGAAGGC (KO reverse). The expected product sizes were 492 and 578 bp for wild-type and knockout alleles respectively.
RNA isolation and qRT-PCR
Total RNA was extracted from tissues or tails with Trizol (Invitrogen) according to manufacturer’s instructions. Reverse transcription was performed using miScript II RT kit (Qiagen). qRT-PCR for miR-21–5p was performed (triplicate) using miScript SYBR Green PCR Kit (Qiagen) or ABI7500 fast system (Applied Biosystems, Life Technologies). miR-21–5p expression was compared to snord95 expression, a small RNA endogenous control. For experiments evaluating the effect of radiation, total RNA was isolated from tissues using Trizol per manufacturer’s instructions 12 hours after radiation, and miRNA were enriched as previously described (24). TaqMan microRNA assays (Applied Biosystems) for miR-21–5p and miR-21–3p expression were performed (triplicate) and was compared to sno202 expression, a small RNA endogenous control. Data are presented as 2−ΔCt.
Northern blotting
Total RNA was collected from tails and fractionated on 15% polyacrylamide-urea gels (Bio-Rad) and transferred onto Immobilon-N+ membranes. End-labelled pri-, pre-, and mature miR-21 LNA-probes (Exiqon) were hybridized in ULTRAhyb-Oligo hybrization buffer (Ambion). A 5s probe was used for normalization.
Complete blood count (CBC)
Blood was collected from mice in MiniCollect K3 EDTA tubes (Greiner Bio-One). Samples were analyzed on a Genesis veterinary hematology system (Oxford Science).
Histology/Pathology
Sternums were collected from unirradiated mice or at the humane endpoint following irradiation exposure. Sternums were fixed in 10% formalin for 72 hours, de-calcified, embedded in paraffin, sectioned, and H&E stained. Images were captured on a Cytation5 instrument (BioTek) using a 4X or 10X objective or with a BX41 microscope (Olympus).
Flow cytometry HSPC analysis
Spleen and bone marrow were harvested from littermates, processed into single cell suspensions, and immunophenotyping for mature lymphoid, myeloid and HSPC cell lineages was performed as previously described (25,26). The following antibodies were used: biotin mouse lineage panel (BD; includes CD11b, Gr1, CD3, B220, Ter119), biotin-CD4 (RM4–5; eBioscience), biotin-CD8a (53–6.7; eBioscience), biotin-CD19 (eBio1D3; eBioscience), pacific blue-conjugated streptavidin (Invitrogen), BUV395 or FITC Sca1 (D7; BD), APC cKit (ACK2; eBioscience), PE Flt3 (A2F10; BioLegend), PECy7 CD150 (TC150–12F12.2; BioLegend), APC-Cy7 CD48 (HM48–1, BD). Samples were run on a Fortessa or Celesta instrument (BD Biosciences) and analyzed using FlowJo software. HSPC populations were defined as follows: lineage negative (CD19−, B220−, CD4−, CD8−, CD3−, Gr1−, CD11b−, Ter119−), LSK (lineage−, cKit+, Sca1+), MPP (LSK, Flt3+), ST-HSC (LSK, Flt3−, CD48−, CD150−), and LT-HSC (LSK, Flt3−, CD48−, CD150+).
Bone marrow transplantation
Bone marrow harvested from unirradiated leg bones (femurs and tibias) of donor mice was processed into single-cell suspensions. miR-21+/+ total bone marrow cells (5×106) were injected (intravenous, retro-orbit) into irradiated (6.5 Gy, approximately 16 hours prior to transplantation) miR-21+/− and miR-21−/− mice. Mice were monitored, and 35 days after transplantation, any mice still alive were sacrificed and bone marrow collected.
Statistical analysis
GraphPad Prism software was used to perform Student’s t-tests paired, two tailed (Fig. 1), Student’s t-tests unpaired, two tailed (2B, 3B, 4B-E), or log-rank test (Figs. 3A, 5A, Supplemental Fig. 2).
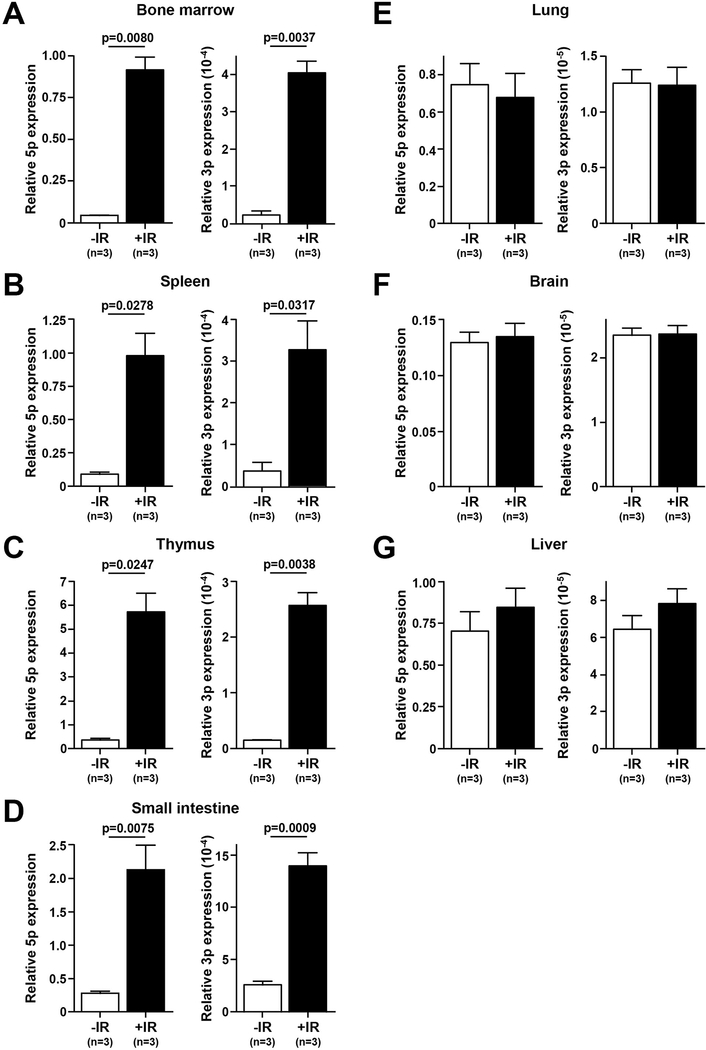
Figure 1. miR-21 is upregulated in radiation sensitive tissues following sub-lethal irradiation.
A-G) qRT-PCR quantification of miR-21–5-p and miR-21–3-p levels in the tissues indicated from wild-type FVB mice (3 mice each) without or 12 hours following a single dose of radiation (6.5 Gy); S.E.M., Student’s t-tests (paired, two-tailed).
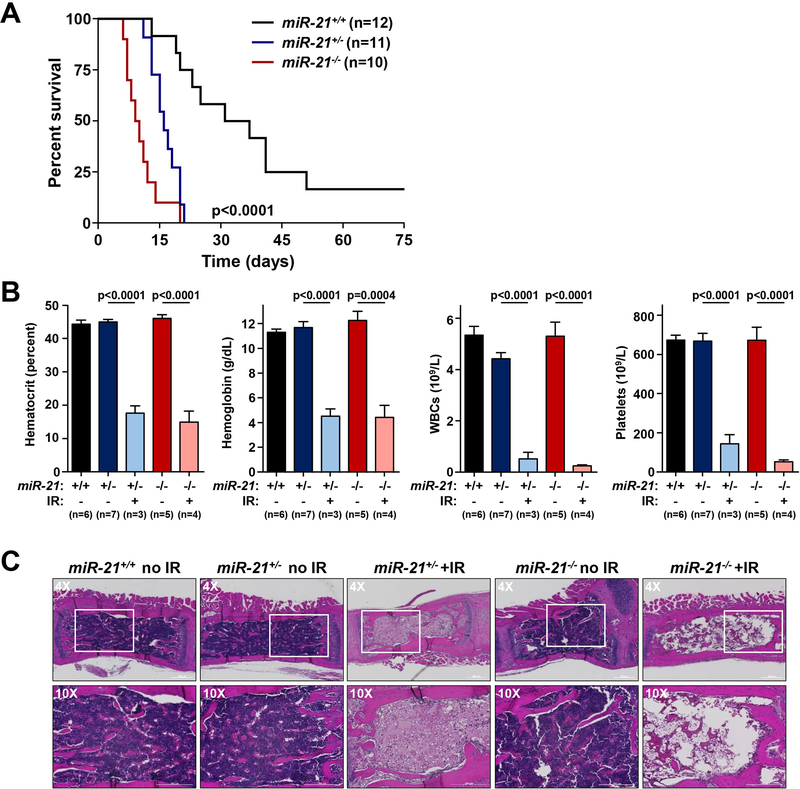
Figure 3. miR-21-deficiency sensitizes mice to irradiation and induces bone marrow failure.
A) Kaplan-Meier survival curve of a cohort of FVB mice of the indicated genotypes following exposure to a single 6.5 Gy dose of irradiation (p<0.0001 miR21+/+ vs miR-21+/−; p<0.0001 miR21+/+ vs miR-21−/−, log-rank tests). B) Quantification of hematocrit, hemoglobin, total white blood cells (WBCs), and platelets in littermates at baseline or at humane endpoints following a single dose of 6.5 Gy radiation; S.E.M; Student’s t-tests (unpaired, two-tailed). C) Representative H&E images of sternums from littermate-matched mice at baseline or following a single dose of 6.5 Gy radiation.
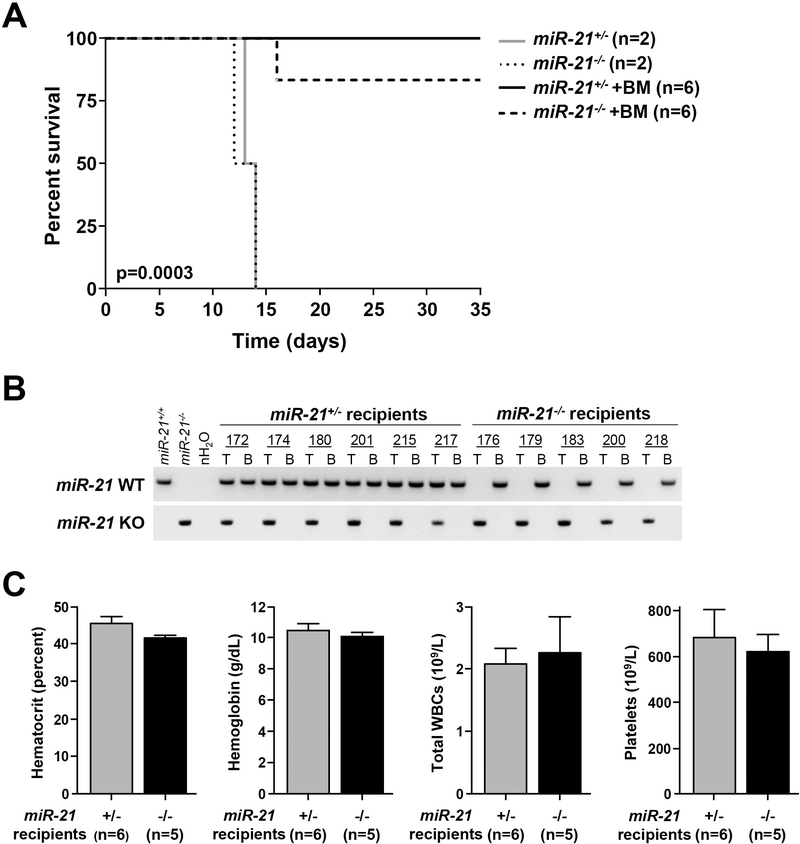
Figure 5. Bone marrow transplantation rescues miR-21-deficient mice from radiation-induced death.
A) Kaplan-Meier survival curve of irradiated miR-21+/− and miR-21−/− mice that received wild-type bone marrow (+BM) or a sham; log-rank test. B) PCR performed for miR-21 wild-type (WT) or knockout (KO) alleles from genomic DNA isolated from tail (T) or bone marrow (B) of mice in (A) following transplantation. C) Quantification of hematocrit, hemoglobin, total white blood cells (WBCs) and platelets of the mice in (A) at 35 days post-transplant; S.E.M.
Results
miR-21 is upregulated in radio-sensitive tissues is mice following radiation
Overexpression of miR-21 is linked to chemo- and radio-resistance in multiple cancer types (22,23,27); however, much remains unknown about the contribution of miR-21 in radiation sensitivity in normal cells. To determine if miR-21 is involved in the radiation response in normal cells, we subjected wild-type FVB mice to LD50/30 TBI (6.5 Gy). We evaluated miR-21 expression in a panel of tissues at baseline and 12 hours following TBI. We detected a significant increase (ranging from 5–21 fold) in expression of both the dominant miRNA arm (miR-21–5p) and the minor miRNA arm (miR-21–3p) in radiation sensitive tissues, including spleen, thymus, bone marrow, and small intestine (Figs. 1A–1D). No significant increase in expression of miR-21–5p or miR-21–3p was observed in liver, lung, and brain tissues (Figs. 1E–1G). Thus, miR-21 is selectively upregulated in radio-sensitive hematopoietic and intestinal tissue following whole-body irradiation, suggesting it may have an important function in modulating the normal tissue response to irradiation.
miR-21-deficient mice are sensitive to whole body radiation
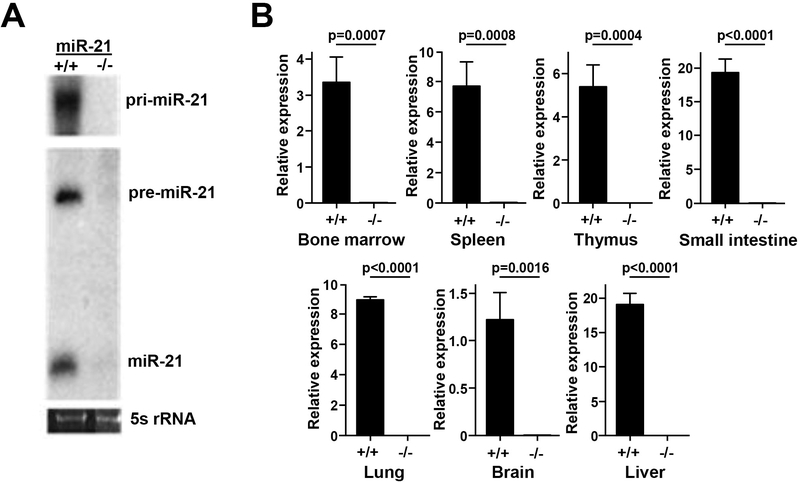
To further investigate the functions of miR-21 in normal cells, we generated miR-21 knockout mice (Supplemental Fig. 1A). Northern blots of RNA isolated from tails of mice showed expression of pri-miR-21, pre-miR-21, and mature miR-21–5p in wild-type mice, whereas no expression of the miR-21 transcripts were detected in the miR-21-null mice (Fig. 2A). To validate that miR-21 was globally knocked out, we performed qRT-PCR for miR-21 on a panel of tissues from miR-21+/+ and miR-21−/− mice. miR-21–5p levels were undetectable in all tissues evaluated (Figs. 2B), confirming the complete loss of expression of miR-21 in the miR-21 knockout mice. Because miR-21 is encoded in the 3’-untranslated region of Tmem49, we evaluated Tmem49 expression by qRT-PCR and Western blot. miR-21 deletion did not alter Tmem49 expression (Supplemental Fig. 1B).
Figure 2. Generation and validation of miR-21 knockout mice.
A) Northern blot of RNA from wild-type and miR-21−/− tails using probes for pri-miR-21, pre-miR-21, and mature miR-21–5p. B) qRT-PCR of miR-21–5p in the tissue indicated from wild-type and miR-21−/− mice; S.E.M, Student’s t-tests (unpaired, two-tailed).
Since miR-21 was significantly overexpressed in multiple radio-sensitive tissues in wild-type mice following TBI (Fig. 1), we tested whether mice deficient in miR-21 would have altered sensitivity to ionizing radiation. We subjected a cohort of miR-21+/+, miR-21+/−, and miR-21−/− FVB mice to TBI (LD50/30; 6.5 Gy) and monitored survival. Mice deficient in miR-21 had significantly reduced survival (p<0.0001, log-rank test). All miR-21−/− mice became ill and died within 20 days of TBI, whereas 75% of the wild-type mice were alive at day 20 (Fig. 3A). Similarly, we also observed that mice lacking a single allele of miR-21 also became ill following irradiation and all died by day 21 (Fig. 3A). Wild-type mice had a mean survival of 38 days whereas miR-21−/− and miR-21+/− mice had mean survivals of 10 and 16 days, respectively. To test if the radiation sensitivity of the miR-21-deficient mice was strain-specific, we backcrossed the FVB miR-21 knockout mice onto the Ola129/B6 background and subjected these mice to 6.5 Gy TBI. As with the FVB mice, miR-21-null mice had significantly reduced survival compared to wild-type controls (p=0.0006, log-rank test) (Supplemental Fig. 2). Thus, the data indicate expression of both alleles of miR-21 are essential for mouse survival following TBI.
To determine the cause of death in irradiated miR-21-deficient mice, we evaluated tissues from heterozygous and knockout mice at the humane endpoint following TBI. Prior to irradiation exposure, we observed no differences in hematocrit, hemoglobin, white blood cells (WBCs), or platelets in the blood of miR-21+/− and miR-21−/− mice compared to miR-21+/+ mice (Fig. 3B). However, following radiation, moribund miR-21-deficient mice were pale and had significant reductions in each of these blood components compared to unirradiated controls (Fig. 3B). Histological analysis of bone marrow showed moribund irradiated miR-21+/− and miR-21−/− mice had wide-spread bone marrow hypocellularity, a hallmark of bone marrow failure (Fig. 3C). In contrast, prior to irradiation, there were no detectable histological differences between wild-type, miR-21-heterozygous, and miR-21-null bone marrow (Fig. 3C). Since we also observed miR-21 overexpression in small intestine following TBI (Fig. 1D), we performed histological evaluation of the small intestine of miR-21−/− mice following TBI. Here we observed relatively mild villous blunting and slightly increased cleaved caspase 3 levels by immunohistochemistry (Supplemental Figs. 3A, 3B). Thus, mice deficient in miR-21 are significantly more sensitive to radiation than wild-type controls, have impaired hematopoiesis following TBI, and die due to bone marrow failure within 20 days, whereas apoptosis of intestinal cells appears to be a minor factor.
Reduced numbers of HSPCs in miR-21-deficient mice
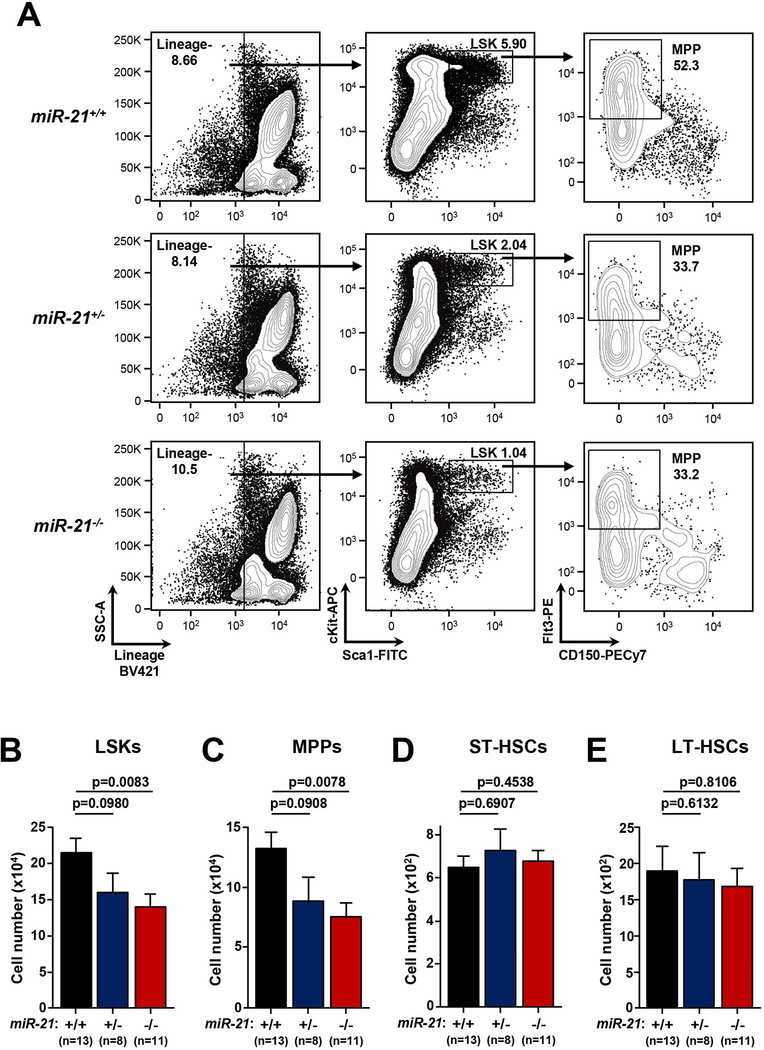
Compensatory hematopoiesis by HSPCs is required following TBI to repopulate lymphoid organs depleted by radiation and ensure maintenance of normal hematopoietic cell populations (28). To determine whether miR-21 loss affects HSPC compartments in mice in the absence of radiation, we performed immunophenotyping for multiple HSPC populations in unirradiated miR-21+/+, miR-21+/−, and miR-21−/− mice. HSPC populations are highly enriched in the lineage negative cKit+ Sca1+ (LSK) compartment (29,30). In miR-21+/− and miR-21−/− mice, we detected a significant decrease in the fraction and total number of LSK cells in the bone marrow, showing that miR-21 loss leads to reduced HSPCs at baseline (Fig. 4A, 4B). Further characterization of the LSK population showed that the defect was confined to the multi-potent progenitor population (MPP; LSK, Flt3+; Fig. 4A, 4C), which retain multi-lineage differential potential but have reduced self-renewal capacity and are required for emergency hematopoiesis following stress (31). We did not observe any difference in the most basal, short-term (ST-HSC; LSK, Flt3−, CD48−, CD150−; Fig. 4D) and long-term (LT-HSC; LSK, Flt3−, CD48−, CD150+; Fig. 4E) stem cell populations. Additionally, mature hematopoietic cells were unaffected by miR-21 loss, as we did not observe any changes in total cell number (Supplemental Figs. 4A, 4B), B cells (Supplemental Figs. 4C, 4G), T cells (Supplemental Figs. 4D, 4E, 4H), myeloid lineage cells (Supplemental Figs. 4F, 4I), or erythrocytes (Supplemental Fig. 4J) in the spleen and bone marrow of miR-21-deficient mice. Moreover, in the absence of irradiation, numbers of lymphocytes, neutrophils, monocytes, eosinophils, and basophils in the blood were within normal ranges in mice lacking miR-21 (Supplemental Fig 4k). Therefore, miR-21 loss leads to reduced numbers of HSPCs at baseline, further suggesting miR-21-deficient mice have an impaired ability to hematopoietically recover following irradiation exposure.
Figure 4. miR-21 knockout mice have reduced numbers of hematopoietic stem and progenitor cells.
A) Representative contour plots from unirradiated mice of the indicated genotypes showing HSPC populations and gating strategy to define LSK and MPP populations. B-E) Quantification of the total number of LSK (B), MPP (C), ST-HSC (D), and LT-HSC (E) cells per femur from mice of the indicated genotypes; S.E.M, Student’s t-tests (unpaired, two-tailed).
Bone marrow transplantation rescues irradiated miR-21-deficient mice from death
To further test whether irradiated miR-21+/− and miR-21−/− mice have defects in hematopoiesis following TBI resulting in their death, we performed bone marrow transplantation experiments. miR-21-deficient mice were transplanted with wild-type bone marrow cells following a single dose (6.5 Gy) of TBI. The sham non-transplanted miR-21-deficient mice all died by day 14 post-irradiation (Fig. 5A). However, all six miR-21+/− and five of six of the miR-21−/− mice that were transplanted with wild-type bone marrow were still alive five weeks after irradiation (Fig. 5A). To evaluate engraftment of the wild-type bone marrow in the miR-21-deficient mice that survived, we performed PCR genotyping analysis of their bone marrow. We only detected the presence of the wild-type miR-21 allele in all surviving miR-21-deficient mice, demonstrating complete engraftment of the wild-type donor bone marrow (Fig. 5B). Moreover, analysis of peripheral blood of the transplanted mice at the time of sacrifice revealed normal levels of hematocrit, hemoglobin, WBCs, and platelets (Fig. 5C), thereby demonstrating the engrafted bone marrow was fully functional and able to support normal hematopoiesis. Therefore, the rescue from death induced by a sub-lethal dose of irradiation of the miR-21-deficient mice indicated that mice lacking one or two miR-21 alleles die due to impaired hematopoiesis and bone marrow failure. Thus, our data show miR-21 expression is required for the normal hematopoietic cell response to irradiation and is a critical factor for maintaining hematopoietic cell viability when mice are subjected to ionizing radiation.
Discussion
Radiation therapy is integral to improving cancer outcomes, but unfortunately also induces normal tissue toxicity (12). Systemic therapy following radiation hinges upon adequate end organ function, including bone marrow and the hematopoietic stem cell population. Therefore, understanding the endogenous factors that govern normal tissue toxicity, and delineating which patients may be more susceptible, is crucial when considering these regimens. Here we report that the oncomiR, miR-21, is essential for hematopoietic cell viability following irradiation exposure. Mice that were deficient for either one or both alleles of miR-21 were unable to survive TBI and developed bone marrow failure. We determined this was due to intrinsic defects in HSPC populations in miR-21-deficient mice, which caused bone marrow failure following irradiation exposure. Moreover, we were able to rescue this phenotype by transplanting miR-21-deficient mice with wild-type bone marrow after TBI. Thus, the experiments demonstrate that miR-21 is essential for maintaining normal HSPC populations and for the continued hematopoiesis following cellular stress from ionizing radiation.
miR-21 is one of the most studied miRNA. It is overexpressed in many human malignancies, including acute myeloid leukemia (AML), and lung, brain and breast cancers (19). miR-21 has been implicated in mediating chemo- and radiation-resistance in cancer cells (20,22,32), and has been proposed to be a potential therapeutic target that could be used to sensitize cancer cells to radiation (23). miR-21 has also been linked to cardiovascular and pulmonary diseases, and specifically cardiac and pulmonary fibrosis, which radiation can induce (33,34). Multiple oncogenic functions have been attributed to miR-21, including modulation of numerous tumor suppressor genes associated with proliferation and invasion as well as genes involved in the DNA damage response and apoptosis (19–23). Our data support these studies and suggest miR-21 has an essential function in promoting hematopoietic cell viability, proliferation, and/or differentiation in mice at baseline prior to exposure to cytotoxic stressors. Mice lacking one or both alleles of miR-21 had reduced numbers of LSKs and MPP cells, which are progenitor populations essential for emergency hematopoiesis. These data indicate miR-21 is necessary for normal hematopoiesis and is required for emergency hematopoiesis.
Due to its oncogenic properties, miR-21 is postulated to be an ideal therapeutic target for cancer (32), but our results demonstrate that targeting miR-21 could lead to radiation-induced hematopoietic toxicity. However, miR-21 has also been shown to be overexpressed in hematopoietic malignancies, such as AML (35–38). By showing that miR-21-deficient mice have HSPC defects at baseline and are highly sensitive to radiation, targeting miR-21 could prove to be beneficial in the treatment of AML or other blood cancers by eliminating all residual cancer cells in patients prior to bone marrow transplantation or improving targeted therapies against malignant hematopoietic cells.
Conclusions
Overall, our data significantly improve the understanding of the radiation response in normal cells and further expands our knowledge of the role of miR-21. Through the use of genetic mouse models, we established that miR-21 is essential for normal hematopoiesis and that mice lacking miR-21 are sensitive to whole-body irradiation due to the development of bone marrow failure. In the future, we can harness this knowledge to more effectively design treatment regimens for human malignancies and mitigate the impact of radiation toxicity on normal cells
Supplementary Material
Acknowledgements
The authors thank members of the Eischen and Simone labs for helpful discussions. This work was supported by F30CA189433 (MVP), R01CA226432 (CME), R01CA227479 (NLS) and the NCI Cancer Center Grant P30CA056036 for supporting the Flow Cytometry, Translational Research/Pathology, and Lab Animals core facilities.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Baskar R, Lee KA, Yeo R, et al. Cancer and radiation therapy: Current advances and future directions. Int J Med Sci 2012;9:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol 2015;1:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegemann NS, Guckenberger M, Belka C, et al. Hypofractionated radiotherapy for prostate cancer. Radiat Oncol 2014;9:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beli I, Koukourakis G, Platoni K, et al. Hypofractionated radiotherapy in non small cell lung cancer: A review of the current literature. Rev Recent Clin Trials 2010;5:103–11. [DOI] [PubMed] [Google Scholar]
- 6.Kim KS, Shin KH, Choi N, et al. Hypofractionated whole breast irradiation: New standard in early breast cancer after breast-conserving surgery. Radiat Oncol J 2016;34:81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bari B, Porta L, Mazzola R, et al. Hypofractionated radiotherapy in pancreatic cancer: Lessons from the past in the era of stereotactic body radiation therapy. Crit Rev Oncol Hematol 2016;103:49–61. [DOI] [PubMed] [Google Scholar]
- 8.Cozzarini C, Fiorino C, Deantoni C, et al. Higher-than-expected severe (grade 3–4) late urinary toxicity after postprostatectomy hypofractionated radiotherapy: A single-institution analysis of 1176 patients. Eur Urol 2014;66:1024–30. [DOI] [PubMed] [Google Scholar]
- 9.Youssef A, Stanford J. Hypofractionation radiotherapy vs. Conventional fractionation for breast cancer: A comparative review of toxicity. Cureus 2018;10:e3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asna N, Livoff A, Batash R, et al. Radiation therapy and immunotherapy-a potential combination in cancer treatment. Curr Oncol 2018;25:e454–e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma V, Cushman TR, Tang C, et al. Toxicity of radiation and immunotherapy combinations. Adv Radiat Oncol 2018;3:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat Rev Cancer 2009;9:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Probin V, Zhou D. Cancer therapy-induced residual bone marrow injury-mechanisms of induction and implication for therapy. Curr Cancer Ther Rev 2006;2:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartel DP. Metazoan micrornas. Cell 2018;173:20–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rupaimoole R, Slack FJ. Microrna therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017;16:203–222. [DOI] [PubMed] [Google Scholar]
- 16.Esteller M Non-coding rnas in human disease. Nat Rev Genet 2011;12:861–74. [DOI] [PubMed] [Google Scholar]
- 17.Iorio MV, Croce CM. Microrna dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med 2017;9:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rupaimoole R, Calin GA, Lopez-Berestein G, et al. Mirna deregulation in cancer cells and the tumor microenvironment. Cancer Discov 2016;6:235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krichevsky AM, Gabriely G. Mir-21: A small multi-faceted rna. J Cell Mol Med 2009;13:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu B, Wang X, Hu S, et al. Mir-21-mediated radioresistance occurs via promoting repair of DNA double strand breaks. J Biol Chem 2017;292:3531–3540. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Liu ZL, Wang H, Liu J, et al. Microrna-21 (mir-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting pten. Mol Cell Biochem 2013;372:35–45. [DOI] [PubMed] [Google Scholar]
- 22.Chao TF, Xiong HH, Liu W, et al. Mir-21 mediates the radiation resistance of glioblastoma cells by regulating pdcd4 and hmsh2. J Huazhong Univ Sci Technolog Med Sci 2013;33:525–529. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Zhu H, Yang X, et al. Microrna-21 is a novel promising target in cancer radiation therapy. Tumour Biol 2014;35:3975–9. [DOI] [PubMed] [Google Scholar]
- 24.McGirt LY, Adams CM, Baerenwald DA, et al. Mir-223 regulates cell growth and targets proto-oncogenes in mycosis fungoides/cutaneous t-cell lymphoma. J Invest Dermatol 2014;134:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puccetti MV, Fischer MA, Arrate MP, et al. Defective replication stress response inhibits lymphomagenesis and impairs lymphocyte reconstitution. Oncogene 2017;36:2553–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arrate MP, Vincent T, Odvody J, et al. Microrna biogenesis is required for myc-induced bcell lymphoma development and survival. Cancer Res 2010;70:6083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng J, Lei W, Fu JC, et al. Targeting mir-21 enhances the sensitivity of human colon cancer ht-29 cells to chemoradiotherapy in vitro. Biochem Biophys Res Commun 2014;443:789–95. [DOI] [PubMed] [Google Scholar]
- 28.Dainiak N Hematologic consequences of exposure to ionizing radiation. Exp Hematol 2002;30:513–28. [DOI] [PubMed] [Google Scholar]
- 29.Okada S, Nakauchi H, Nagayoshi K, et al. In vivo and in vitro stem cell function of c-kit- and sca-1-positive murine hematopoietic cells. Blood 1992;80:3044–50. [PubMed] [Google Scholar]
- 30.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science 1988;241:58–62. [DOI] [PubMed] [Google Scholar]
- 31.Zhao JL, Baltimore D. Regulation of stress-induced hematopoiesis. Curr Opin Hematol 2015;22:286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan X, Wang ZX, Wang R. Microrna-21: A novel therapeutic target in human cancer. Cancer Biol Ther 2010;10:1224–32. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Friggeri A, Yang Y, et al. Mir-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med 2010;207:1589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y, Zhang C. Microrna-21 in cardiovascular disease. J Cardiovasc Transl Res 2010;3:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulci V, Chiaretti S, Goldoni M, et al. Quantitative technologies establish a novel microrna profile of chronic lymphocytic leukemia. Blood 2007;109:4944–51. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Wang H, Chen H, et al. Clinical significance and detection of microrna-21 in serum of patients with diffuse large b-cell lymphoma in chinese population. Eur J Haematol 2014;92:407–12. [DOI] [PubMed] [Google Scholar]
- 37.Jones K, Nourse JP, Keane C, et al. Plasma microrna are disease response biomarkers in classical hodgkin lymphoma. Clin Cancer Res 2014;20:253–64. [DOI] [PubMed] [Google Scholar]
- 38.Riccioni R, Lulli V, Castelli G, et al. Mir-21 is overexpressed in npm1-mutant acute myeloid leukemias. Leuk Res 2015;39:221–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.