Abstract
Background
Neutrophils and allergen-specific T-cells accumulate in allergic late-phase reactions (LPR). Their presence is associated with severe inflammation. Cytokines like GM-CSF, IFN-γ, and IL-3, typically found in allergic LPR, have been proposed to convert neutrophils into antigen-presenting cells (APC).
Objective
To assess the antigen-processing and presenting capacity of neutrophils from allergic patients.
Methods
Neutrophils were isolated from peripheral blood of birch pollen-allergic donors and stimulated with GM-CSF, IFN-γ, and IL-3. Viability and expression of HLA-DR, CD80, and CD86 were assessed by flow cytometry. HLA-DM expression was analysed by immunoblotting. Allergen-uptake was studied with fluorescence-labelled major birch pollen allergen Bet v 1. Bet v 1 was digested with neutrophilic endolysosomal extracts and resulting fragments were sequenced using mass spectrometry. Neutrophils were used as APC in co-culture experiments with autologous HLA-DR-restricted Bet v 1-specific T-cell clones reactive with epitopes in different regions of the allergen. In all experiments, monocytes were used for comparison. Fluids from suction blisters formed on top of LPR induced by intradermal allergen injection were assessed for HLA-DR-positive neutrophils by flow cytometry.
Results
The cytokines significantly enhanced the survival, allergen uptake and expression of HLA-DM and HLA-DR of neutrophils. Neutrophils rapidly degraded Bet v 1 into fragments containing all relevant T-cell epitopes. Cytokine-activated, allergen-pulsed neutrophils induced proliferative and cytokine responses of Bet v 1-specific T-cells irrespective of epitope specificity confirming that they fully processed and presented the allergen. HLA-DR-positive neutrophils were detected in cutaneous allergic LPR.
Conclusion
Neutrophils may serve as APC for local allergen-specific effector T-cells in allergic LPR.
Keywords: antigen-presentation, neutrophils, IgE-mediated allergy, allergic inflammation, allergic late-phase response, T-cells, T-cell-activation
Introduction
Re-exposure of allergic individuals to either the sensitizing or a cross-reactive allergen causes an acute response, seen in the skin as wheal and flare reaction, or, after inhalation, as rhinitis and airway constriction 1, 2. This immediate reaction is considered to result from the release of histamine and other preformed inflammatory mediators from mast cells upon cross-linking of adjacent FcεRI-bound IgE molecules on their surface 3. The late-phase reaction (LPR) follows 3-48 h after allergen challenge, resulting in the skin as swelling and induration, in the nose as continued nasal blockage, and in the lung as late asthmatic reaction (LAR) 1, 2, 4. LPR are accompanied by inflammatory cell infiltration, in particular by eosinophils and Th2 cells 5–9, and have been used as models of chronic allergic inflammation 10, 11. The pivotal role of allergen-specific CD4+ T-cells in LPR has been underlined by the finding that LAR can be provoked by T-cell-activation independently of the IgE response after administration of peptides containing T-cell epitopes but incapable of cross-linking IgE 12.
Neutrophils are the first innate immune cells that migrate to inflammatory sites and rapidly exert manifold effector functions 13. They also accumulate in allergic LPR 9, 14 and their presence has been linked to more severe inflammation 15–17. Bronchoalveolar lavage fluids of patients with allergic LPR contain larger numbers of neutrophils than those of individuals experiencing no LPR 18, 19. Furthermore, severe asthma has been associated with elevated numbers of neutrophils in sputum, tracheal aspirates and in the bronchial mucosa 20–22. Activated neutrophils account for enzymes and other cytotoxic agents and release chemokines and cytokines that attract immature dendritic cells (DC), T-cells, monocytes and macrophages and instruct the interaction of these cells types 23, 24. Additionally, evidence for a direct cross-talk of neutrophils with T-cells has been provided 25.
Recently, it was reported that human neutrophils acquire HLA-DR-dependent antigen-presenting capacity when co-cultured with autologous antigen-specific CD4+ memory T-cells plus antigen 26. Evidence for the antigen-presenting capacity of human neutrophils had already been indicated before, mainly because these professional phagocytes express HLA-DR when exposed to GM-CSF, IFN-γ, and IL-3, both in vitro 27, 28 and in vivo 29, 30. These cytokines are released by specific CD4+ T-cells and sensitized mast cells in response to natural allergen exposure or the injection of allergy vaccines 3, 5, 31–33. This tempted us to speculate that the cytokine milieu in allergic LPR may convert neutrophils into antigen-presenting cells (APC). To address this hypothesis, we stimulated neutrophils from allergic patients with a combination of GM-CSF, IFN-γ, and IL-3, and characterized key features of antigen-presentation, namely upregulation of HLA-DR, HLA-DM, CD80, and CD86, as well as antigen internalization, processing, and presentation to T-cells by utilizing the major birch pollen allergen Bet v 1 as model protein. This clinically highly relevant allergen has been thoroughly characterized regarding its T-cell-reactivity 34–36. Neutrophils were compared to monocytes which represent another type of phagocytes and are professional APC. Finally, we explored whether HLA-DR-positive neutrophils are actually found in allergen-induced LPR in the skin of allergic individuals.
Material and Methods
Allergic patients
Birch pollen-allergic patients showed rhinoconjunctivitis in spring, positive skin prick tests to birch pollen extract (ALK-ABELLO, Hørsholm, Denmark) and birch pollen-specific IgE of >0.35 kUA/l (ImmunoCAP; Thermo Fisher Scientific, Uppsala, Sweden). Grass pollen-allergic patients showed rhinoconjunctivitis in summer, positive skin prick tests to grass pollen extract (ALK-ABELLO) and grass pollen-specific IgE of >0.35 kUA/l (ImmunoCAP). The study was approved by the ethics committees of the Medical University of Vienna (EK-1488/2017) and Lower Austria (GS1-EK-4/317-2015) and conducted in accordance with the Declaration of Helsinki. Patients gave written informed consent.
Allergens
Recombinant Bet v 1 and Hev b 3 were purchased from Biomay (Vienna, Austria). Bet v 1 was conjugated to pHrodo-succinimidyl ester (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Birch pollen extract was prepared as described 37. Grass pollen extract for intradermal administration was purchased from ALK-ABELLO.
Cell preparations
Neutrophils were isolated as described 38. The procedure included a density gradient centrifugation step of whole blood on Ficoll-Hypaque (Pharmacia Diagnostics, Uppsala, Sweden), dextran sedimentation (4% dextran T 500, Carl ROTH, Germany), red cell osmotic lysis of the granulocyte pellet, and a final immunomagnetic negative selection step using the human MACSxpress® Neutrophil Isolation Kit (Miltenyi Biotec GmbH, Bergisch-Gladbach, Germany) and resulted repeatedly in >99% pure CD16+CD66b+CCR3-HLA-DR-CD19-CD3-CD14- cells. Neutrophils (1x106/ml) were cultured in RPMI 1640 (Sigma Aldrich, Darmstadt, Germany) supplemented with 10% autologous plasma alone or stimulated with GM-CSF (100 pg/ml), IFN-γ (10 ng/ml) and IL-3 (30 ng/ml; all from Peprotech, Rocky Hill, USA) as described 28. Monocytes (>98% CD14+CCR3-CD19-CD3-) were obtained by using CD14 MicroBeads (Miltenyi Biotec) according to the manufacturer´s instructions.
Flow cytometry
Analyses were performed with a FACS Canto II using FACS Diva (BD Biosciences, San Jose, CA, USA) and FlowJo software (TreeStar, Inc., Ashland, OR, USA). The following anti-human antibodies were used: HLA-DR-BV421™, HLA-DR-APC (clone L243), CD80-Alexa647, CD3-APC-Cy7, CD16-PE (clone 3G8), CD15-PE-Cy7, CD15-PerCp-Cy5, CCR3-APC and PerCP-labelled CD14, CD19, CD56, CD123, and CCR3 (all from Biolegend, San Diego, CA), CD4-PE and CD86-PE, CD45-FITC (all from BD Biosciences) and CD3-PerCP and CD8-PerCP (both from eBioscience Inc., San Diego, CA). Viability was determined by using the Fixable Viability Dye eFluor® 780 (eBioscience Inc.). Annexin V-FITC (BD Pharmingen, San Jose, CA, USA) was used to determine cells undergoing apoptosis. For internalization experiments, cells (1x106/ml) were incubated with and without pHrodo-labelled Bet v 1 (1.5 μg/ml) in the presence of GM-CSF/IFN-γ/IL-3.
Detection of HLA-DM
Cell lysates were prepared from neutrophils and monocytes (each 10x106) as described 35 immediately after their isolation and from HLA-DR-negative (3x106) and HLA-DR-positive (1.8x106) neutrophils sorted by a FACSAria III (BD Biosciences) after stimulation with GM-CSF/IFN-γ/IL-3 for 48 h. Cell lysates (30 μl) were separated by 15% SDS-PAGE and transferred onto nitrocellulose membranes. After saturation with PBS/Tween 20 supplemented with 5% skim milk overnight at 4°C immunoblotting was performed with a monoclonal mouse anti-HLA-DM antibody (Novus Biological, Abingdon, UK) and a horseradish peroxidase-conjugated anti-mouse IgG (CST, Massachusetts, USA). As loading control GAPDH was detected with a rabbit anti-GAPDH (Sigma, St. Louis, MO, USA) and a horseradish peroxidase-conjugated ECL anti-rabbit IgG (Amersham, Buckinghamshire, UK). After developing with the ECL Prime Western blotting Detection Reagent, the nitrocellulose was exposed to a high performance autoradiography film (both GE Healthcare, Buckinghamshire, UK).
Endocytosis and endolysosomal degradation assays of Bet v 1
Endolysosomal microsomes were isolated from neutrophils and monocytes as described 36. Microsomes (2 μg) were incubated with Bet v 1 (2 μg) at 37°C in a final volume of 20 μl containing 100 mM citrate buffer (pH 4.8) and 2 mM dithiothreitol. At indicated time points samples were collected, denaturated for 5 min at 95°C, loaded on a 15% SDS gel and visualized by Coomassie staining. Remaining peptides were analyzed using an ESI-QTOF mass spectrometer fitted with a capillary reversed phase HPLC (Waters, Milford, MA, USA).
The quantity of intact Bet v 1 was determined by an in-house capture ELISA. Briefly, microtiter plates (Maxisorp, Nunc, Denmark) were coated with the Bet v 1-specific monoclonal antibody BIP1 (20 μg/ml) in carbonate buffer (pH 9.6) overnight at 4°C. After saturation with 1% BSA in PBS/0.05% Tween 20 for 6 h at room temperature, samples were incubated overnight at 4°C. Non-digested Bet v 1 was used to generate a standard curve. After washing, sera from birch pollen-allergic patients containing Bet v 1-specific IgE were incubated for 1 h at 37°C and 1 h at 4°C. Bound IgE antibodies were detected with alkaline phosphatase-labelled anti-human IgE Ab (BD Biosciences). All samples were tested in duplicate at various dilutions.
Allergen-specific T-cell cultures
Allergen-specific T-cell lines (TCL) were expanded from PBMC of allergic patients as described 34. Cryopreserved HLA-DR-restricted Bet v 1-specific T-cell clones (TCC) with known epitope specificity were thawed and expanded. T-cells (2-5×104) were stimulated with purified irradiated (60 Gray) autologous neutrophils or monocytes (5x104), cultured in the absence or presence of Bet v 1 (5 μg/ml), birch pollen extract (20 μg/ml) or Hev b 3 (5 μg/ml) in GM-CSF/IFN-γ/IL-3 for 24 h. All experiments were performed in duplicate. After 48 h supernatants were assessed for cytokine levels by bead array using the Luminex System 100 (Luminex, Austin, TX, USA). After 48 h proliferative responses were determined by 3H-thymidine incorporation assay.
Allergic LPR and suction blisters
Grass pollen extract (ALK-ABELLO) was injected intradermally into the left volar forearm of grass pollen-allergic individuals according to the manufacturer´s instructions. The diluent was administered into the right volar forearm as negative control. All individuals developed immediate reactions within 15 min. Provided the individuals developed visible cutaneous LPR, skin suction blisters were formed on top of the injection sites after 20-24 h by using a low pressure instrument from Electronic Diversities (Ridge Road, Finksburg, MD, USA) by mounting 2 sterile, 5-hole (5 mm diameter per hole) skin suction plates. Low pressure (150–200 mmHg) was applied over a time period of 2 h, and adjusted according to blister size to prevent blood vessel trauma through excessive mechanical stress. Fluids from intact and hemoglobin-free blisters were collected using a Micro-Fine syringe (BD Biosciences), pooled, centrifuged at 1000 x g for 5 min at 4°C, and analyzed by flow cytometry. The total numbers of neutrophils detected in the blister fluids after injection of allergen ranged from 282-2646. No neutrophils were detected in blister fluids after injection of the diluent.
Statistics
Data were analyzed using IBM SPSS 20.0 (SPSS, Chicago, IL, USA). Statistical differences were determined by the Wilcoxon signed ranks test and considered statistically significant for P<0.05.
Results
Neutrophils from allergic donors acquire features of APC upon stimulation with GM-CSF, IFN-γ, and IL-3
Neutrophils from six allergic individuals were incubated with either titrated concentrations of GM-CSF, IFN-γ, or IL-3, or their combination 28 for up to 48 h and HLA-DR expression was assessed subsequently (see Figure E1A in the Online Repository). Similar to what has been reported for neutrophils from non-allergic individuals 27, GM-CSF was the most potent HLA class II stimulus, followed by IFN-γ and IL-3. All three cytokines in combination induced highest percentages of HLA-DR+ neutrophils when compared to medium control (see Figure E1B in the Online Repository). As all three cytokines are present in the microenvironment of allergic LPR we used the cytokine mix for further in vitro experiments.
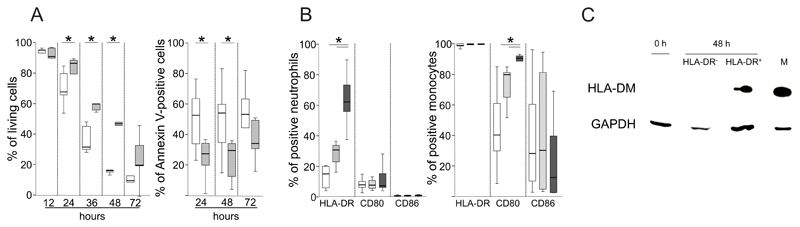
First, we monitored the effect of GM-CSF/IFN-γ/IL-3 on the survival of neutrophils from allergic individuals for up to 72 h. The cytokines significantly delayed apoptosis and prolonged neutrophil survival and after 48 h around 50% of the cells were still alive (Fig. 1A). Next, we assessed the expression of HLA class II and key costimulatory molecules on neutrophils and monocytes in the presence of GM-CSF/IFN-γ/IL-3 after 3, 6, 12, 24 and 48 h. None of these molecules were detected after 3 and 6 h, respectively (data not shown). After 12 h, activated neutrophils significantly upregulated HLA-DR but neither CD80 nor CD86 (Fig. 1B). The constitutive expression of HLA-DR on monocytes was not affected by GM-CSF/IFN-γ/IL-3. However, monocytes significantly upregulated CD80 and slightly downregulated CD86 in the presence of the cytokines (Fig. 1B). The cytokine-induced expression of HLA-DM was detected in lysates from HLA-DR+ neutrophils and absent in cytokine-stimulated HLA-DR- and freshly isolated neutrophils (Fig. 1C). As expected, monocytes showed strong HLA-DM expression.
Figure 1. Survival and key antigen-presentation molecules of activated neutrophils from allergic individuals.
(A) Neutrophils from six birch pollen-allergic subjects were stimulated with GM-CSF/IFN-γ/IL-3 (gray) or cultured in medium alone (white box plots). Vital and Annexin-V-positive cells were assessed at the indicated time points by flow cytometry; (B) Neutrophils or monocytes from seven birch pollen-allergic subjects were stimulated with GM-CSF/IFN-γ/IL-3 and the expression of HLA-DR, CD80 and CD86 was assessed after 14 (white), 24 (light gray) and 48 h (dark gray); *P<0.05, Wilcoxon Signed Ranks test; (C) Lysates of freshly isolated neutrophils (0 h), monocytes (M), or neutrophils stimulated with GM-CSF/IFN-γ/IL-3 for 48 h sorted into HLA-DR+ and HLA-DR- cells were assessed for HLA-DM and GAPDH by immunoblotting;
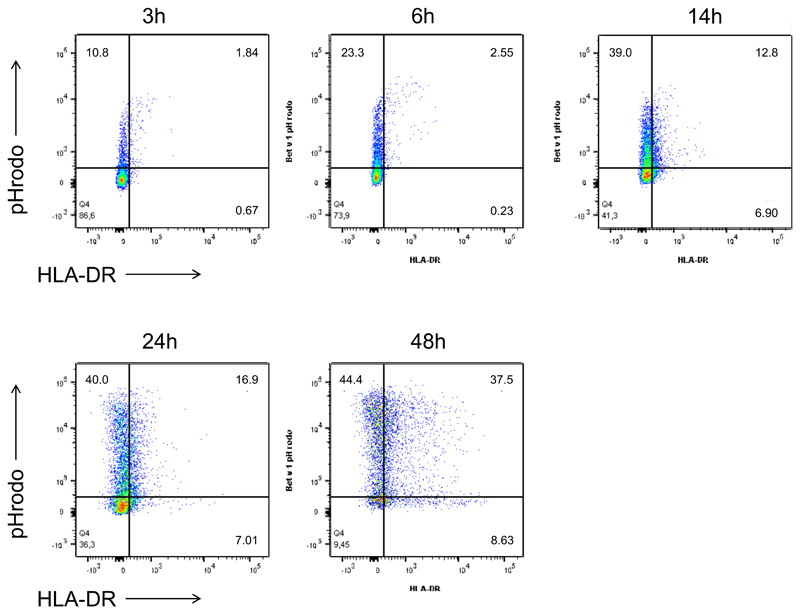
Neutrophils internalize and rapidly degrade Bet v 1 to fragments that contain relevant T-cell epitopes of the major birch pollen allergen
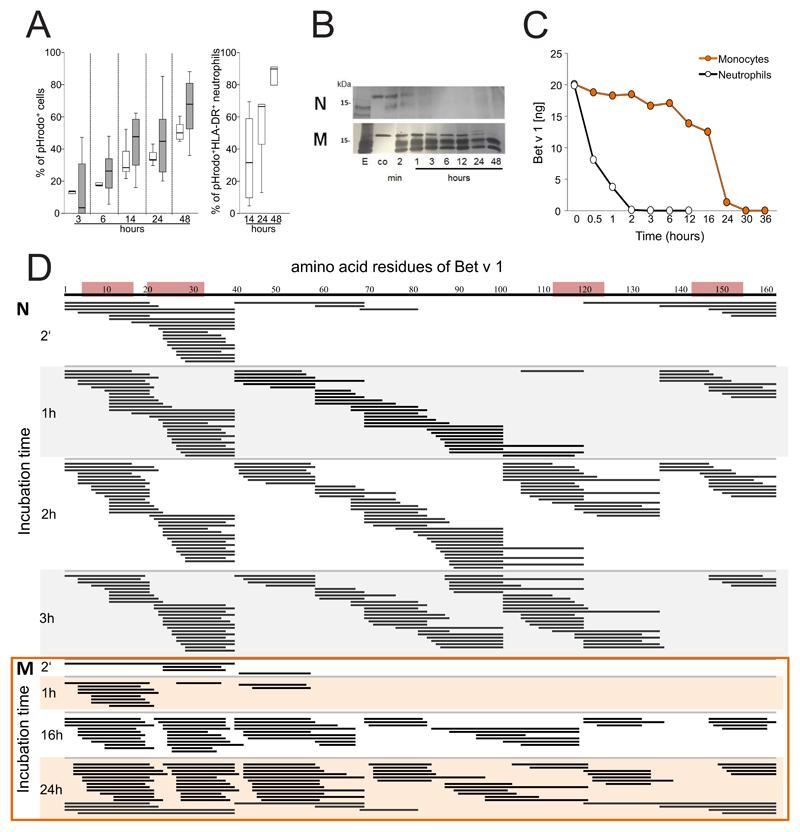
To study allergen uptake, neutrophils and monocytes were incubated with GM-CSF/IFN-γ/IL-3 and Bet v 1 labelled with pHrodo, a dye that emits fluorescence only when it reaches the acidic endolysosomes. The percentage of pHrodo+ cells was assessed during a time course of 3-48 h of culture. Both cell types efficiently internalized Bet v 1 (Fig. 2A). Co-staining of HLA-DR revealed that after 24 h more than 60% of HLA-DR+ neutrophils had internalized Bet v 1 (Fig. 2A and Figure E2 in the Online repository). To evaluate antigen-processing capacity, lysosomal extracts were prepared from either cell type and incubated with Bet v 1. SDS-PAGE analysis revealed that neutrophilic proteases fully degraded Bet v 1 within 6 h whereas monocytic enzymes required more than 24 h for this process (Fig. 2B). The more rapid proteolysis of Bet v 1 by neutrophilic extracts was confirmed by means of capture ELISA. No intact allergen was found after 2 h (Fig. 2C) while 10% of intact Bet v 1 were still detected after 24 h of incubation with monocytic proteases. The Bet v 1-fragments resulting from degradation by either cell type were characterized by tandem mass spectrometry (Fig. 2D). Already after 2 minutes of incubation with neutrophilic proteases, several peptide clusters derived from the N-terminus Bet v 11-38, the central region Bet v 139-79 and the C-terminus Bet v 1103-159 were identified. At the same time point, only N-terminal fragments located within the region Bet v 11-79 were found in samples digested by monocytes. After 1 hour, additional peptides originating from the region Bet v 180-103 were detected in neutrophilic samples. A similar fragmentation was achieved by monocytic extracts with a delayed kinetics. Importantly, the peptide clusters matched frequently recognized T-cell-activating regions such as Bet v 14-15, Bet v 119-33, Bet v 1112-123, and Bet v 1142-153 that have been identified in birch pollen-allergic donors 34 (Fig. 2D).
Figure 2. Uptake and degradation of Bet v 1 by neutrophils.
(A) Neutrophils (white box plots) and monocytes (gray) from six birch pollen-allergic individuals were incubated with pHrodo-labelled Bet v 1 in GM-CSF/IFN-γ/IL-3. The percentage of pHrodo+ cells and pHrodo+HLA-DR+ neutrophils was assessed at the indicated time points using flow cytometry; (B) SDS-PAGE analysis of the degradation of Bet v 1 by lysosomal extracts from neutrophils (N) or monocytes (M), E, lysosomal extract, co: Bet v 1 without lysosomal extracts; (C) degradation of Bet v 1 quantified by capture ELISA; (D) peptides (black lines) generated over time by incubation of Bet v 1 with lysosomal extracts from neutrophils (N) or monocytes (M) were characterized by mass spectrometry. Frequently recognized T-cell-activating regions in the amino acid sequence of Bet v 1 on top are highlighted in red. The results in B and C are representative of two and three independent experiments, respectively.
Neutrophils fully activate allergen-specific effector T-cells
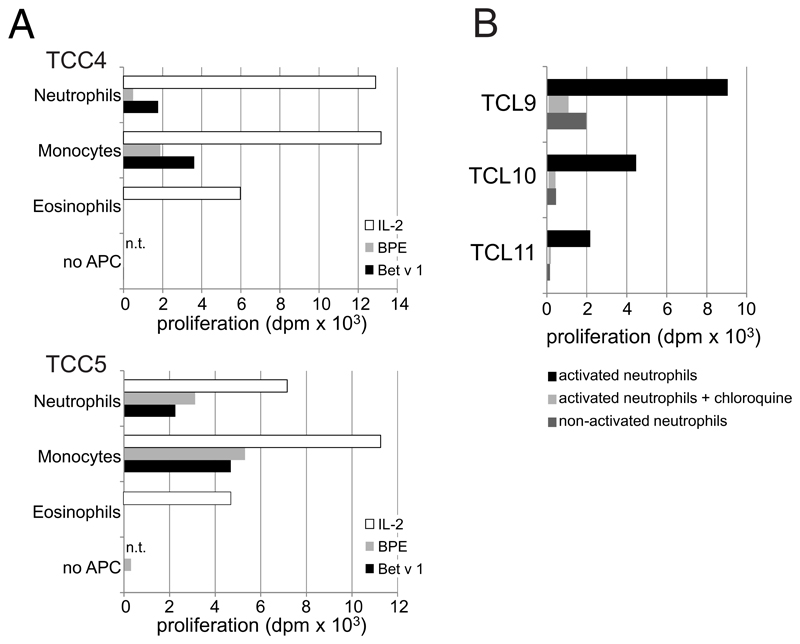
To assess whether neutrophils are actually able to present the processed peptides to specific effector T-cells, we first co-cultured Bet v 1-specific TCL from different birch pollen-allergic individuals with autologous neutrophils or monocytes that had been stimulated with GM-CSF/IFN-γ/IL-3 plus allergen. These polyclonal T-cell cultures proliferated in response to activated neutrophils that had been incubated with Bet v 1 but not with the control allergen Hev b 3 (Table 1). Neutrophils also induced T-cell proliferation when incubated with birch pollen extract containing native Bet v 1. Bet v 1-specific TCL also proliferated in response to allergen-pulsed APC, i.e. neutrophils and monocytes that had been washed after stimulation with GM-CSF/IFN-γ/IL-3 plus allergen (Table 1). As expected, the proliferative responses were more pronounced when monocytes served as APC.
Table 1. Proliferative responses of Bet v 1-specific T-cell cultures.
| APC | Neutrophils | Monocytes | |||||
|---|---|---|---|---|---|---|---|
| Stimulus | Bet v 1 | Birch pollen | Hev b 3 | Bet v 1 | Birch pollen | Hev b 3 | HLA-DR phenotype |
| TCL1 | 13.3a | n.t. | 1.8 | 21.6 | n.t. | 1.7 | DRB1*0301/1401 |
| TCL2 | 21.6 | 14.1 | n.t. | 6.7 | 4.4 | n.t. | DRB1*0101/0301 |
| TCL3 | 8.8 | 4.4 | <0.01 | 16.2 | 14.9 | <0.01 | DRB1*0301/0801 |
| TCL4 | 1.2 | 3.4 | 0.02 | 11.6 | 12.1 | <0.01 | n.t. |
| TCL5 | 3.6 | 0.1 | 0.04 | 17.7 | 4.7 | 0.08 | DRB1*0701/1501 |
| TCL6 | 1.2 | n.t. | <0.01 | 9.9 | n.t. | <0.01 | DRB1*0701/1501 |
| TCL7 | 1.6 | n.t. | n.t. | 12.1 | n.t. | n.t. | n.t. |
| TCL8 | 1.8 | n.t. | n.t. | 10.7 | n.t. | n.t. | DRB1*0701/1501 |
| TCL9 | 7.2 | n.t. | n.t. | 33.2 | n.t. | n.t. | DRB1*0301/1401 |
dpm × 103 are shown; n.t., not tested; TCL7-9 were stimulated with allergen-pulsed APC
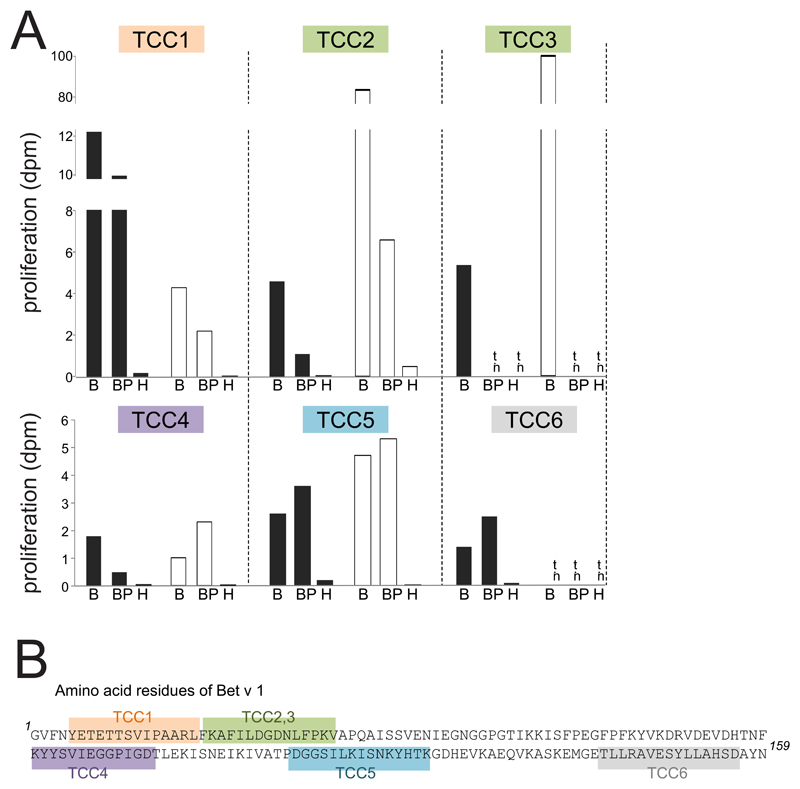
Bet v 1 contains multiple T-cell epitopes distributed over its entire sequence 34 and thus was also used to test whether neutrophils were able to present peptides derived from all parts of the protein. To this end, we stimulated HLA-DR-restricted Bet v 1-reactive TCC specific for different epitopes spread over the entire amino acid sequence with cytokine-activated neutrophils or monocytes incubated with either Bet v 1 or birch pollen extract. All TCC proliferated in the presence of either cell type irrespective of their epitope recognition (Fig. 3). Again, monocytes were in general more potent APC than neutrophils. The control allergen Hev b 3 did not induce proliferative responses in the tested clones. We also determined the cytokine response of TCC3 and TCC5, both showing a Th2-phenotype with production of high amounts of IL-4, IL-13 and IL-5 and low IFN-γ, to stimulation by neutrophils. Both clones released Th2 cytokines comparably to stimulation by Bet v 1-pulsed monocytes (Table 2) indicating their full activation by allergen-pulsed neutrophils. In line with the reduced proliferative response (Fig. 3A), the levels of the individual cytokines produced by TCC3 in response to allergen-pulsed neutrophils were lower than those induced by allergen-pulsed monocytes.
Figure 3. T-cell proliferation to allergen-pulsed neutrophils and monocytes.
(A) Proliferation of six T-cell clones (TCC) to rBet v 1 (B), birch pollen extract (BP) or Hev b 3 (H) and either neutrophils (black bars) or monocytes (white bars) as APC; dpm x 103 are shown; n.t., not tested; (B) Epitopes recognized by the TCC are highlighted in different colours in the amino acid sequence of Bet v 1.
Table 2. Cytokine response of Bet v 1-specific Th2 clones to Bet v 1-pulsed APC.
| Cytokines [pg/ml] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APC | Stimulus | IL-4 | IL-5 | IL-9 | IL-10 | IL-13 | IL-22 | IL-17A | IFN-γ | GM-CSF | TNF-α | |
| TCC 3 | Neutrophils | Medium | 62.8 | 1456 | <4.5 | 0.1 | 1039 | <4.5 | <4.5 | 231 | 109 | 240 |
| Neutrophils | Bet v 1 | 3483 | 4461 | 51.3 | 0.0 | 2716 | <4.5 | <4.5 | 319 | 164 | 1866 | |
| Monocytes | Medium | 476.2 | 1830 | <4.5 | <4.5 | 1074 | <4.5 | <4.5 | <4.5 | <4.5 | 888 | |
| Monocytes | Bet v 1 | 16770 | 8450 | 395 | 20.6 | 23891 | <4.5 | <4.5 | 726 | 13059 | 9644 | |
| TCC 5 | Neutrophils | Medium | 483 | 4799 | <4.5 | 5.6 | 3694 | <4.5 | <4.5 | 52.4 | <4.5 | 1715 |
| Neutrophils | Bet v 1 | 14415 | 8796 | 184 | 1281 | 13939 | 184 | 39.9 | 1246 | 7957 | 5943 | |
| Monocytes | Medium | 717 | 4816 | <4.5 | 6.2 | 3843 | <4.5 | <4.5 | 50.2 | <4.5 | 369 | |
| Monocytes | Bet v 1 | 15073 | 8816 | 194 | 1297 | 15571 | 194 | 37.3 | 1246 | 7957 | 6004 | |
A series of control experiments was performed to prove that activated neutrophils were indeed the responsible APC. First, we confirmed that Bet v 1-specific TCC cultured with Bet v 1 in the absence of APC did not proliferate (see Figure E3A in the Online Repository). Second, we employed autologous eosinophils (CD66b+CCR3+CD16-) as APC, as these cells were detected as the sole contaminating cell type in our 99% pure neutrophil preparations. Eosinophils did not induce T-cell proliferation under the chosen conditions (see Figure E3A in the Online Repository). Third, neutrophils incubated with Bet v 1 in the absence of GM-CSF/IFN-γ/IL-3 failed to induce T-cell-proliferation (see Figure E3B in the Online Repository). Finally, the addition of chloroquine, an inhibitor of acidification of endosomal/lysosomal compartments and antigen-processing, to cytokine-stimulated neutrophils during the priming with Bet v 1 prevented T-cell proliferation (see Figure E3B in the Online Repository).
HLA-DR-positive neutrophils are present in cutaneous allergic LPR
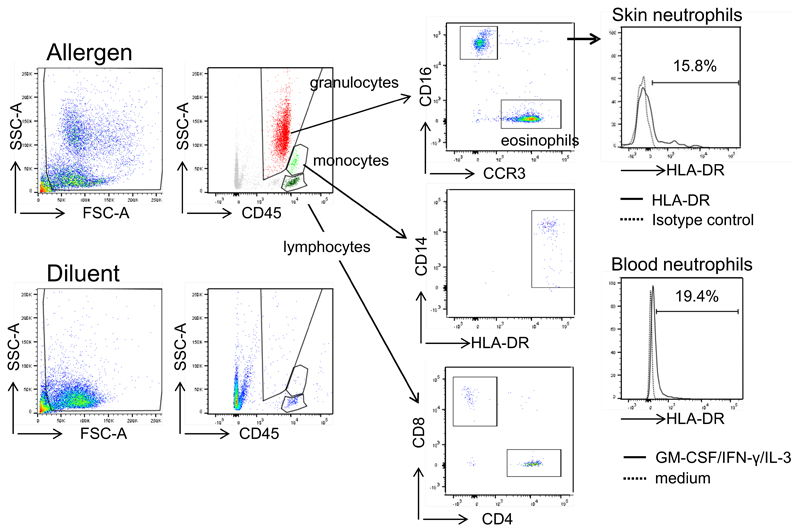
To assess whether HLA-DR-positive neutrophils are present in allergic LPR in vivo, we employed suction blister technology 39. To achieve a reliable induction of LPR we intradermally injected extract from grass pollen because it contains more major allergens than birch pollen. Suction blisters were induced at the initial intradermal injection sites after 20-24 h. While blister fluids at sites of diluent-injection contained T lymphocytes but neither granulocytes nor monocytes, blisters at sites of allergen-injection contained CD4+ and CD8+ T-cells, monocytes, eosinophils, and neutrophils, but were devoid of B cells (Fig. 4). We analyzed the neutrophils ex vivo for HLA-DR expression. In five independent experiments we detected 11-25% HLA-DR-positive neutrophils in the blister fluids from allergen-induced LPR (Fig. 4). In addition, neutrophils from two individuals were isolated from peripheral blood collected at the day of suction blistering. As expected, freshly isolated blood neutrophils were HLA-DR-negative (data not shown). However, when these neutrophils were incubated with GM-CSF/IFN-γ/IL-3 for 24 h, similar percentages of HLA-DR-positive neutrophils to those in suction blister fluids were detected (Fig. 4). Neutrophils cultured in medium alone remained HLA-DR-negative.
Figure 4. Detection of HLA-DR-positive neutrophils in cutaneous allergic LPR.
Allergen or diluent was injected intradermally and suction blisters were performed at the injection sites after 20-24 h. Fluids were analyzed for T-cells, monocytes, eosinophils and neutrophils by flow cytometry. Skin neutrophils were assessed for HLA-DR expression. One representative of five experiments is shown. In two patients, also blood neutrophils were isolated, cultured in medium or GM-CSF/IFN-γ/IL-3 for 24 h and analyzed for HLA-DR expression by flow cytometry.
Discussion
Large numbers of neutrophils infiltrate the airways and skin during allergen-induced LPR 5, 18, 32, 40, 41. In addition to this recruitment which is mainly mediated by IgE-activated mast cells 3, certain pollen species have been reported to attract neutrophils to the airways in a toll-like receptor 4, myeloid differentiation protein-2, and chemokine (C-X-C motif) receptor (CXCR) 2-dependent manner 42. Recruited neutrophils are generally considered to contribute to the allergic inflammation by producing compounds with pathogenic potential, e.g. reactive oxygen species, elastase, leukotrienes, and cytokines (reviewed in 15 and 17). Herein, we propose a novel role for neutrophils in allergic inflammation: in the local cytokine environment they convert into functional APC and activate allergen-specific effector CD4+ T-cells.
Evidence for the upregulation of HLA class II molecules on human neutrophils in the presence of GM-CSF, IFN-γ and IL-3 in vitro and in vivo had already been indicated in the past (reviewed in 43). We confirm that also neutrophils from allergic donors show prolonged survival, suppressed apoptosis and HLA-DR expression in the presence of these cytokines. Beyond that, we demonstrate that these neutrophils express HLA-DM, an intracellular molecular chaperone that orchestrates the loading of peptides into HLA class II molecules. As the capacity to fully degrade antigens and properly load antigenic peptides onto HLA II molecules is essential for antigen presentation, we addressed these features by a sophisticated experimental approach involving highly purified neutrophils, a clinically relevant and immunologically well-characterized allergen, and allergen-specific HLA-DR-restricted TCC with defined epitope reactivity. Neutrophils efficiently internalized and fully cleaved the major birch pollen allergen. The resulting proteolytic fragments encompassed its relevant T-cell-activating regions. Proper loading and presentation of the peptides was confirmed in T-cell-activation assays. Notably, T-cells specific for epitopes spreading over the entire amino acid sequence of Bet v 1 and restricted by different HLA-DR alleles responded to allergen-loaded neutrophils. These results strongly indicate that neutrophils show no limitation regarding antigen-processing and subsequent T-cell activation. Collectively, our in vitro findings provide strong evidence for the genuine antigen-presenting capacity of neutrophils in the cytokine environment of allergic LPR. This evidence was further supported by the in vivo identification of HLA-DR-positive neutrophils in cutaneous allergic LPR.
The comparison of endolysosomal processing by neutrophils and monocytes revealed no major differences in the quality of the peptides of Bet v 1. However, remarkable differences in the kinetics of antigen proteolysis by both types of phagocytes became evident. The rapid cleavage of Bet v 1 by neutrophils resembled the degradation behaviour described for macrophages 44. Hev b 3 was also rapidly degraded (data not shown). In contrast, significant amounts of Bet v 1 remained intact during endolysosomal proteolysis by monocytes and resembled the processing behaviour of DC 35. The slow degradation of internalized antigens has been considered to guarantee that DC can still generate and present proteolytic fragments to naive T-cells after migration to the lymph nodes 44. Thus, efficient priming of naive T-cells may depend on the prevention of premature intracellular antigen degradation. This ability would not apply to neutrophils which rapidly fully digest internalized antigens, even though they have been shown to transport antigen to lymphatic compartments 26, 45. Furthermore, even upon optimum stimulation with GM-CSF, IFN-γ and IL-3, neutrophils did not express high levels of CD80 and CD86 which are relevant for full activation of naïve T lymphocytes. In support of these results, Vono et al demonstrated that human neutrophils failed to activate allogeneic naive T-cells 26. In contrast to naïve T cells, previously activated T cells also respond to APC lacking costimulatory molecules, although less strongly 46. Together, we conclude that human neutrophils upon appropriate stimulation are highly efficient in collecting antigens and subsequent activation of effector and memory CD4+ T-cells that do not require high expression of HLA class II and costimulatory molecules.
In summary, we propose a compelling new role for neutrophils in the pathophysiology of IgE-mediated allergy. Upon natural exposure or injection of allergens during allergen-specific immunotherapy, activated mast cells induce an influx of leukocytes with neutrophil infiltration as relatively early event 8. The local cytokine microenvironment prolongs their lifespan and fosters them to internalize, process and present allergen to specific CD4+ effector T-cells. Activated T-cells then execute various effector mechanisms, for example release IL-5 which recruits and activates eosinophils, that further contribute to the allergic inflammation 5. Indeed, eosinophil infiltration of asthmatic lungs after allergen challenge has been reported to be delayed compared to the accumulation of CD4+ T-cells and neutrophils 8. Elevated expression of HLA-DR in cutaneous cellular infiltrates at sites of allergen-induced LPR has been described 47. Here, we show that HLA-DR-positive neutrophils are present at such inflammatory sites as well as CD4+ and CD8+ T-cells, monocytes, and eosinophils. These results match the cell populations that have been detected in biopsies of late local skin reactions obtained 24 h after subcutaneous injections of allergen vaccines for specific immunotherapy 48. In contrast to human basophils 49 and eosinophils (see Figure E3B in the Online Repository) neutrophils are able to present allergens and activate T-cells. Hence, it is tempting to speculate that neutrophils act as magnifiers of the local allergic T-cell-mediated inflammation following natural exposure or vaccination during allergen-specific immunotherapy.
Extended Data
Figure E1. Expression of HLA-DR on cytokine-activated neutrophils.
(A) Purified neutrophils from six birch pollen-allergic individuals were cultured in medium alone (0) or stimulated with titrated concentrations 10(1), 1(2), 0.1(3), and 0.01(4) ng/ml) of GM-CSF, IFN-γ, and IL-3. The percentage of HLA-DR-positive cells was assessed by flow cytometry at 24 (white), 36 (light gray) and 48 h (dark gray box plots); (B) Neutrophils were left in medium alone (white) or stimulated with a cocktail of GM-CSF (100 pg/ml), IFN-γ (10 ng/ml) and IL-3 (30 ng/ml, gray) according to published protocols 1. The percentage of HLA-DR-positive cells was assessed at indicated time points; (C) The percentage of HLA-DR-positive cells expressing CD80 and CD86 at 24 h, the dot blot shows one representative experiment. *P<0.05, Wilcoxon Signed Ranks test;
Figure E2. HLA-DR expression of Bet v 1-positive neutrophils.
Purified neutrophils from birch pollen-allergic individuals were incubated with pHrodo-labelled Bet v 1 in a cocktail of GM-CSF (100 pg/ml), IFN-γ (10 ng/ml) and IL-3 (30 ng/ml, gray). At the indicated time points the percentage of HLA-DR+ neutrophils that had internalized Bet v 1 was assessed using flow cytometry; the dot blot shows one representative experiment of six.
Figure E3. Allergen-induced proliferation of Bet v 1-specific T cells in the presence of different cell types.
(A) Autologous neutrophils (CD66b+CD16+CCR3-) and eosinophils (CD66b+CCR3+CD16-) were sorted from peripheral blood cells by FACSAria III (BD Biosciences). Monocytes were isolated by using CD14 MicroBeads (Miltenyi Biotec). All three cell types (purity >99%) were incubated with GM-CSF/IFN-γ/IL-3 plus/minus Bet v 1 (5 μg/ml), birch pollen extract (20 μg/ml), or IL-2 (2 U/ml) for Online Repository: Polak et al 3 24 h, irradiated (60 Gray) and co-cultured with two Bet v 1-specific T cell clones (TCC); (B) neutrophils (purity >99%) were incubated without (non-activated) or with GM-CSF/IFN-γ/IL-3 plus/minus Bet v 1 (5 μg/ml) in the presence or absence of chloroquine diphosphate salt (100 μg/ml, Sigma Aldrich, Darmstadt, Germany), washed, irradiated (60 Gray) and added to three different Bet v 1-specific T cell lines (TCL). In all experiments proliferation was assessed after 48 h, dpm = cpm in response to stimulus - cpm in medium controls; n.t., not tested.
Key messages.
-
-
Cytokine-stimulated neutrophils activate allergen-specific T-cells to proliferate and synthesize cytokines
-
-
HLA-DR-positive neutrophils are found in cutaneous allergic late-phase reactions in vivo
Capsule summary.
Neutrophils may serve as antigen-presenting cells in the allergic late-phase reaction.
Acknowledgments
This work was supported by the Austrian Science Fund, projects W1248 and SFB F4610, and by the Medical University of Vienna. We are greatful for helpful discussions with Johannes Stöckl and Georg Stingl. We kindly thank Ingrid Faé for HLA-typing.
Abbreviations
- aa
amino acid
- Ab
antibody
- APC
antigen-presenting cell
- BPE
birch pollen extract
- cpm
counts per minute
- DC
dendritic cell
- dpm
delta-cpm
- GM-CSF
granulocyte macrophage colony-stimulating factor
- h
hour
- IL
interleukin
- LAR
late asthmatic reaction
- LPR
late-phase response
- PBMC
peripheral blood mononuclear cells
- TCC
T-cell clone
- TCL
T-cell line
- TCR
T-cell receptor
- Th
T helper
- TNF
tumor necrosis factor
Footnotes
D. Polak, A. Elbe-Bürger, P. Briza. C. Kitzmüller, N. Samadi, M. Gschwandtner and B. Jahn-Schmid have nothing to disclose. C. Hafner reports personal fees from Thermo Fischer Scientific and Novartis, non-financial support from HAL Allergy, outside the submitted work; W. Pfützner reports grants and personal fees from ALK-Abello, personal fees from Novartis and Thermo Fisher Scientific, outside the submitted work; G. J. Zlabinger reports personal fees from Alexion, Bristol-Myers Squibb, MedAhead, Austrian Chamber of Physicians, Pfizer, UCB Pharma, Merck Sharp & Dohme, GlaxoSmith Kline, and AbbVie, outside the submitted work; B. Bohle reports grants from Austrian Science Funds and from Medical University of Vienna during the conduct of the study; personal fees from Allergen Online Database and Christian Doppler Research Organisation, non-financial support from Paul Ehrlich Institute, outside the submitted work;
References
- 1.Bloemen K, Verstraelen S, Van Den Heuvel R, Witters H, Nelissen I, Schoeters G. The allergic cascade: review of the most important molecules in the asthmatic lung. Immunol Lett. 2007;113:6–18. doi: 10.1016/j.imlet.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016;46:1139–51. doi: 10.1111/cea.12780. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frew AJ, Kay AB. The pattern of human late-phase skin reactions to extracts of aeroallergens. J Allergy Clin Immunol. 1988;81:1117–21. doi: 10.1016/0091-6749(88)90878-0. [DOI] [PubMed] [Google Scholar]
- 5.Frew AJ, Kay AB. The relationship between infiltrating CD4+ lymphocytes, activated eosinophils, and the magnitude of the allergen-induced late phase cutaneous reaction in man. J Immunol. 1988;141:4158–64. [PubMed] [Google Scholar]
- 6.Gaga M, Frew AJ, Varney VA, Kay AB. Eosinophil activation and T lymphocyte infiltration in allergen-induced late phase skin reactions and classical delayed-type hypersensitivity. J Immunol. 1991;147:816–22. [PubMed] [Google Scholar]
- 7.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 8.Lommatzsch M, Julius P, Kuepper M, Garn H, Bratke K, Irmscher S, et al. The course of allergen-induced leukocyte infiltration in human and experimental asthma. J Allergy Clin Immunol. 2006;118:91–7. doi: 10.1016/j.jaci.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Leaker BR, Malkov VA, Mogg R, Ruddy MK, Nicholson GC, Tan AJ, et al. The nasal mucosal late allergic reaction to grass pollen involves type 2 inflammation (IL-5 and IL-13), the inflammasome (IL-1beta), and complement. Mucosal Immunol. 2017;10:408–20. doi: 10.1038/mi.2016.74. [DOI] [PubMed] [Google Scholar]
- 10.Verstraelen S, Bloemen K, Nelissen I, Witters H, Schoeters G, Van Den Heuvel R. Cell types involved in allergic asthma and their use in in vitro models to assess respiratory sensitization. Toxicol In Vitro. 2008;22:1419–31. doi: 10.1016/j.tiv.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Gauvreau GM, El-Gammal AI, O'Byrne PM. Allergen-induced airway responses. Eur Respir J. 2015;46:819–31. doi: 10.1183/13993003.00536-2015. [DOI] [PubMed] [Google Scholar]
- 12.Haselden BM, Kay AB, Larche M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–94. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 14.Varney VA, Jacobson MR, Sudderick RM, Robinson DS, Irani AM, Schwartz LB, et al. Immunohistology of the nasal mucosa following allergen-induced rhinitis. Identification of activated T lymphocytes, eosinophils, and neutrophils. Am Rev Respir Dis. 1992;146:170–6. doi: 10.1164/ajrccm/146.1.170. [DOI] [PubMed] [Google Scholar]
- 15.Monteseirin J. Neutrophils and asthma. J Investig Allergol Clin Immunol. 2009;19:340–54. [PubMed] [Google Scholar]
- 16.Pelaia G, Vatrella A, Busceti MT, Gallelli L, Calabrese C, Terracciano R, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/879783. 879783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosoki K, Itazawa T, Boldogh I, Sur S. Neutrophil recruitment by allergens contribute to allergic sensitization and allergic inflammation. Curr Opin Allergy Clin Immunol. 2016;16:45–50. doi: 10.1097/ACI.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz P, Gonzalez MC, Galleguillos FR, Ancic P, Cromwell O, Shepherd D, et al. Leukocytes and mediators in bronchoalveolar lavage during allergen-induced late-phase asthmatic reactions. Am Rev Respir Dis. 1989;139:1383–9. doi: 10.1164/ajrccm/139.6.1383. [DOI] [PubMed] [Google Scholar]
- 19.Lopuhaa CE, Out TA, Jansen HM, Aalberse RC, van der Zee JS. Allergen-induced bronchial inflammation in house dust mite-allergic patients with or without asthma. Clin Exp Allergy. 2002;32:1720–7. doi: 10.1046/j.1365-2222.2002.01542.x. [DOI] [PubMed] [Google Scholar]
- 20.Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: Clinical and biologic significance. Am J Respir Crit Care Med. 2000;161:1185–90. doi: 10.1164/ajrccm.161.4.9812061. [DOI] [PubMed] [Google Scholar]
- 21.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–63 e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricciardolo FLM, Sorbello V, Folino A, Gallo F, Massaglia GM, Favata G, et al. Identification of IL-17F/frequent exacerbator endotype in asthma. J Allergy Clin Immunol. 2017;140:395–406. doi: 10.1016/j.jaci.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Megiovanni AM, Sanchez F, Robledo-Sarmiento M, Morel C, Gluckman JC, Boudaly S. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J Leukoc Biol. 2006;79:977–88. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- 24.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–9. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–43. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 26.Vono M, Lin A, Norrby-Teglund A, Koup RA, Liang F, Lore K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood. 2017;129:1991–2001. doi: 10.1182/blood-2016-10-744441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gosselin EJ, Wardwell K, Rigby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993;151:1482–90. [PubMed] [Google Scholar]
- 28.Smith WB, Guida L, Sun Q, Korpelainen EI, van den Heuvel C, Gillis D, et al. Neutrophils activated by granulocyte-macrophage colony-stimulating factor express receptors for interleukin-3 which mediate class II expression. Blood. 1995;86:3938–44. [PubMed] [Google Scholar]
- 29.Mudzinski SP, Christian TP, Guo TL, Cirenza E, Hazlett KR, Gosselin EJ. Expression of HLA-DR (major histocompatibility complex class II) on neutrophils from patients treated with granulocyte-macrophage colony-stimulating factor for mobilization of stem cells. Blood. 1995;86:2452–3. [PubMed] [Google Scholar]
- 30.Reinisch W, Tillinger W, Lichtenberger C, Gangl A, Willheim M, Scheiner O, et al. In vivo induction of HLA-DR on human neutrophils in patients treated with interferon-gamma. Blood. 1996;87:3068. [PubMed] [Google Scholar]
- 31.Cembrzynska-Nowak M, Szklarz E, Inglot AD, Teodorczyk-Injeyan JA. Elevated release of tumor necrosis factor-alpha and interferon-gamma by bronchoalveolar leukocytes from patients with bronchial asthma. Am Rev Respir Dis. 1993;147:291–5. doi: 10.1164/ajrccm/147.2.291. [DOI] [PubMed] [Google Scholar]
- 32.Werfel S, Massey W, Lichtenstein LM, Bochner BS. Preferential recruitment of activated, memory T lymphocytes into skin chamber fluids during human cutaneous late-phase allergic reactions. J Allergy Clin Immunol. 1995;96:57–65. doi: 10.1016/s0091-6749(95)70033-1. [DOI] [PubMed] [Google Scholar]
- 33.Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol. 2002;109:694–700. doi: 10.1067/mai.2002.123236. [DOI] [PubMed] [Google Scholar]
- 34.Jahn-Schmid B, Radakovics A, Luttkopf D, Scheurer S, Vieths S, Ebner C, et al. Bet v 1142-156 is the dominant T-cell epitope of the major birch pollen allergen and important for cross-reactivity with Bet v 1-related food allergens. J Allergy Clin Immunol. 2005;116:213–9. doi: 10.1016/j.jaci.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Mutschlechner S, Egger M, Briza P, Wallner M, Lackner P, Karle A, et al. Naturally processed T cell-activating peptides of the major birch pollen allergen. J Allergy Clin Immunol. 2010;125:711–8. doi: 10.1016/j.jaci.2009.10.052. 8 e1–8 e2. [DOI] [PubMed] [Google Scholar]
- 36.Kitzmuller C, Zulehner N, Roulias A, Briza P, Ferreira F, Fae I, et al. Correlation of sensitizing capacity and T-cell recognition within the Bet v 1 family. J Allergy Clin Immunol. 2015;136:151–8. doi: 10.1016/j.jaci.2014.12.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deifl S, Zwicker C, Vejvar E, Kitzmuller C, Gadermaier G, Nagl B, et al. Glutathione-S-transferase: a minor allergen in birch pollen due to limited release from hydrated pollen. PLoS One. 2014;9:e109075. doi: 10.1371/journal.pone.0109075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calzetti F, Tamassia N, Arruda-Silva F, Gasperini S, Cassatella MA. The importance of being "pure" neutrophils. J Allergy Clin Immunol. 2017;139:352–5 e6. doi: 10.1016/j.jaci.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Akbar AN, Reed JR, Lacy KE, Jackson SE, Vukmanovic-Stejic M, Rustin MH. Investigation of the cutaneous response to recall antigen in humans in vivo. Clin Exp Immunol. 2013;173:163–72. doi: 10.1111/cei.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durham SR. Mechanisms of mucosal inflammation in the nose and lungs. Clin Exp Allergy. 1998;28(Suppl 2):11–6. [PubMed] [Google Scholar]
- 41.Gauvreau GM, Evans MY. Allergen inhalation challenge: a human model of asthma exacerbation. Contrib Microbiol. 2007;14:21–32. doi: 10.1159/000107052. [DOI] [PubMed] [Google Scholar]
- 42.Hosoki K, Aguilera-Aguirre L, Brasier AR, Kurosky A, Boldogh I, Sur S. Facilitation of Allergic Sensitization and Allergic Airway Inflammation by Pollen-Induced Innate Neutrophil Recruitment. Am J Respir Cell Mol Biol. 2016;54:81–90. doi: 10.1165/rcmb.2015-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takashima A, Yao Y. Neutrophil plasticity: acquisition of phenotype and functionality of antigen-presenting cell. J Leukoc Biol. 2015;98:489–96. doi: 10.1189/jlb.1MR1014-502R. [DOI] [PubMed] [Google Scholar]
- 44.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–4. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 45.Duffy D, Perrin H, Abadie V, Benhabiles N, Boissonnas A, Liard C, et al. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity. 2012;37:917–29. doi: 10.1016/j.immuni.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229:5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eberlein-Konig B, Jung C, Rakoski J, Ring J. Immunohistochemical investigation of the cellular infiltrates at the sites of allergoid-induced late-phase cutaneous reactions associated with pollen allergen-specific immunotherapy. Clin Exp Allergy. 1999;29:1641–7. doi: 10.1046/j.1365-2222.1999.00671.x. [DOI] [PubMed] [Google Scholar]
- 48.Jung CM, Funk A, Rakoski J, Ring J. Immunohistochemical analysis of late local skin reactions during rush venom immunotherapy. Allergy. 1997;52:717–26. doi: 10.1111/j.1398-9995.1997.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 49.Kitzmuller C, Nagl B, Deifl S, Walterskirchen C, Jahn-Schmid B, Zlabinger GJ, et al. Human blood basophils do not act as antigen-presenting cells for the major birch pollen allergen Bet v 1. Allergy. 2012;67:593–600. doi: 10.1111/j.1398-9995.2011.02764.x. [DOI] [PubMed] [Google Scholar]
References
- 1.Smith WB, Guida L, Sun Q, Korpelainen EI, van den Heuvel C, Gillis D, et al. Neutrophils activated by granulocyte-macrophage colony-stimulating factor express receptors for interleukin-3 which mediate class II expression. Blood. 1995;86:3938–44. [PubMed] [Google Scholar]