Abstract
IgG therapeutics directed against solid tumor antigens can be effective in preventing disseminated cancer spread in mouse models, but are typically ineffective in eradicating established solid tumor masses. Here, we have studied the anti-tumor activity of the recombinant murine IgG2a antibody TA99, directed against a melanoma antigen. As previously reported, IgG2a(TA99) was extremely efficacious in preventing the growth of B16 lung metastases. However, the same antibody only mediated a minimal tumor growth retardation, when used to treat established neoplastic masses. The therapeutic activity of IgG2a(TA99) could be substantially enhanced by co-administration with an antibody-cytokine fusion (TA99-mTNF), consisting of the TA99 antibody in scFv format fused to murine TNF. This fusion protein efficiently killed endothelial cells in vitro, while displaying only minimal activity against B16 melanoma cells. In vivo, TA99-mTNF boosted the influx of NK cells into B16 melanoma lesions. Therapy studies with two different administration schedules revealed that the combination of TA99-mTNF and IgG2a(TA99) was superior to the individual products used as single agents. The combination treatment converted most of the tumor mass into a necrotic lesion, but a vital tumor rim eventually regrew. The treatment modality described in this article may be applicable for the treatment of melanoma patients, given the specificity of the gp75 antigen and its conservation across species. Addition of a third combination partner may be required, in order to eradicate the last tumor cells, which survive treatment.
Keywords: Antibody-dependent cell-mediated cytotoxicity, cancer immunotherapy, immunocytokines, Tumor necrosis factor, melanoma
Introduction
Intact antibodies in IgG format can be efficacious for the selective depletion of certain lymphocyte populations and for the treatment of hematological malignancies [1–3]. However, antibody-dependent cell-mediated cytotoxicity (ADCC) is much less efficient against solid tumors [4, 5]. An efficient implementation of ADCC would be highly desirable for pharmaceutical applications. In principle, if the target antigen was specifically expressed on tumor cells, IgG’s capable of connecting tumor cells with the killing power of NK cells would come close to an embodiment of those “magic bullets” (Zauberkugeln), originally envisaged by Paul Ehrlich [6].
The cell membrane gp75 antigen is selectively expressed in melanocytes and in melanoma cells, but is otherwise undetectable in all other normal tissues [7, 8]. This antigen, which is conserved from mouse to man [9], represents an ideal target for antibody-based pharmaceutical applications and an excellent model system to study ADCC. The group of Jeffrey Ravetch had clearly demonstrated that the simultaneous intravenous administration of B16 melanoma cells and of the gp75-specific TA99 antibody in murine IgG2a format could efficiently prevent formation of lung metastases in immunocompetent mice [10]. The antibody isotype was important, as murine IgG variants with lower affinity towards the cognate FcγRIV and FcγRI did not display a similar anti-cancer activity [10–12]. Other reports, performed using IgG2a(TA99), had shown that this agent could not eradicate melanoma lesions, once the tumors had established as small subcutaneous solid nodules [13–15]. With our work, we aimed at characterizing the tumor-homing properties and therapeutic activity of murine IgG2a(TA99), alone or in combination with other modalities.
Cytokine-based therapeutics can substantially boost ADCC activity in mouse models of cancer. There is a considerable biomedical interest in the use of engineered cytokines [16–20].The recent transaction for a PEGylated version of interleukin-2 (NKTR-214) has been dubbed “the largest licensing deal in the pharmaceutical industry” [21]. Wittrup and colleagues have recently reported that the ADCC activity of the intact immunoglobulin TA99 could be substantially enhanced by combination with an Fc fusion of murine interleukin-2 [15]. Similarly, we have previously described that the ADCC activity of IgG therapeutics can be boosted by the antibody-based delivery of interleukin-2 in mouse models of cancer [22, 23] and in patients [24, 25].
In addition to interleukin-2, also the antibody-based delivery of tumor necrosis factor (TNF) to the tumor environment holds promises for the implementation of an efficient anti-cancer therapy. TNF may display a direct anti-cancer activity against certain cancer cell types and against endothelial cells [26, 27]. This strong pro-inflammatory cytokine may also favor the uptake of therapeutics into the solid tumor mass [28, 29]. We have recently shown that the antibody-based delivery of murine TNF to splice variants of fibronectin leads to the complete eradication of soft-tissue sarcoma in immunocompetent mouse models [30–32]. Interestingly, cured mice were able to reject subsequent challenges of heterologous tumor cells from the same mouse strain, in a mechanism that depended on CD8+ T cells, recognizing an endogenous retroviral antigen [32]. Encouraged by these results and by the tolerability of TNF fusions in patients [33, 34], the L19-TNF fusion protein has recently progressed to Phase III clinical trials, for the treatment of patients with metastatic soft-tissue sarcoma [NCT02076620]. Antibody-TNF fusions capable of selective localization at the tumor site promote hemorrhagic necrosis of neoplastic lesions and favor the influx of leukocytes (including NK cells and macrophage) into the tumor mass [27, 29, 35]. Collectively, these findings suggest that the targeted delivery of TNF may be considered as a strategy for ADCC potentiation.
In this article, we have characterized the tumor homing properties and anti-cancer activity of murine IgG2a(TA99) in B16 melanoma, both as single agent and in combination with the recombinant TA99-mTNF fusion protein. The combination treatment was extremely efficacious in converting the tumor mass into a necrotic lesion, but few tumor cells survived the treatment and eventually regrew. These findings may open new clinical applications for the management of melanoma patients, since the gp75 antigen is conserved from mouse to man.
Materials and Methods
Cell lines, animals, and tumor models
CHO cells and L-M fibroblasts were obtained from the ATCC between 2015 and 2017, expanded, and stored as cryopreserved aliquots in liquid nitrogen. The B16F10 melanoma cell line was kindly provided by the group of Prof. Michael Detmar (Swiss Federal Institute of Technology, Zurich) and Mouse pancreatic islet endothelial cells (MS-1) cells by the group of Prof. Cornelia Halin (Swiss Federal Institute of Technology, Zurich). Cells were grown according to the manufacturer's protocol. Authentication of the cell lines also including check of post-freeze viability, growth properties and morphology, test for mycoplasma contamination, isoenzyme assay, and sterility test were performed by the cell bank before shipment.
Production, purification and in vitro characterization of IgG2a(TA99) and TA99-mTNF
Sequences of the variable region of the light and heavy chain (VH and VL) of the anti-gp75 antibody TA99 were taken from Boross, P. et al [12]. The genes encoding for TA99 VH, VL and the constant regions of the IgG2a immunoglobulin were PCR amplified, PCR assembled, and cloned into the mammalian expression vector pMM137. The pMM137 vector was provided by Philochem AG, and has been previously described [36]. The same cloning was performed with VH and VL of the KSF antibody (binding the irrelevant hen egg lysozyme antigen) used as negative control [37]. The fusion protein TA99-mTNF contains the antibody TA99 as single-chain variable fragment (scFv) fused to murine TNF at the C-terminus by a 15-amino-acid linker [31]. The gene encoding for TA99-scFv and the gene encoding murine TNF were assembled through PCR, and cloned into the mammalian expression vector pcDNA3.1(+) (Invitrogen). IgG2a(TA99) and TA99-mTNF were expressed using transient gene expression in CHO cells as described previously [37, 38] and purified from the cell culture medium to homogeneity by protein L chromatography (Thermo Scientific). Quality control of the proteins was performed by SDS-PAGE, size exclusion chromatography (Superdex200 10/300GL, GE Healthcare) and Mass Specrometry.
The biological activity of murine TNF was determined on L-M fibroblasts, MS-1 endothelial cells and B16F10 melanoma cells, as described previously [31]. Images of the cells incubated with 10-7 M (L-M fibroblasts and MS-1 cells) or 10-8 M TA99-mTNF for 24 hours were taken with the Axiovert 200 M microscope (Carl Zeiss).
Ex-vivo immunofluorescences
TA99-mTNF, IgG2a(TA99) and IgG2a(KSF) were labelled with fluorescein isothiocyanate (FITC) as described in the manufacturer's protocol (Sigma). For ex vivo immunofluorescence analysis, C57/BL6 mice bearing s.c. B16F10 tumors were injected with 300μg FITC-labelled IgG2a(TA99) or IgG2a(KSF) respectively. Mice were sacrificed 24 hours after injection. The organs were excised, embedded in cryoembedding medium (Thermo Scientific) and cryostat sections (8 mm) were stained using rabbit anti-FITC (BioRad; 4510-780) and rat anti-CD31 (BD Biosciences; 553370) as primary antibody and donkey anti-rabbit Alexa488 (Invitrogen; A21206) and anti-rat Alexa594 (Invitrogen; A21209) as secondary antibodies. Slides were mounted with fluorescent mounting medium (Dako) and analyzed with Axioscop2 mot plus microscope (Zeiss).
Immunofluorescence studies
Infiltration of NK cells and macrophages in the B16F10 tumor masses was assessed by immunofluorescence studies on ice-cold acetone-fixed 8μm thick tumor sections. Rabbit anti-Asialo GM1 (Wako Chemicals) and rat F4/80 (BM8, eBiosciences) were detected with anti-rabbit Alexa488 (Invitrogen; A21206) and anti-rat Alexa 488 (Invitrogen; A21208), respectively. Blood vessels were stained with goat anti-CD31 (R&D Systems; AF3628) and anti-goat AlexaFluor594 (Invitrogen; A11058) antibodies. Slides were mounted with fluorescent mounting medium and analyzed with Axioskop2 mot plus microscope (Zeiss).
Flow cytometry
B16F10 cells cultured in a T-150 flask were detached with 2mM EDTA and stained with IgG2a(TA99) or FITC-labelled TA99-mTNF. IgG2a(TA99) was detected using anti-mouse AlexaFluor488 (Invitrogen, A21202). Rabbit anti-FITC (BioRad; 4510-780) and anti-rabbit AlexaFluor488 (Invitrogen; A21206) were used to detect TA99-mTNF binding. All staining and washing steps were performed in 2 mM EDTA 0.5% BSA in PBS. Cells were sorted by FACS (CytoFLEX, Beckman Coulter) and analyzed using FlowJo software.
Animal studies
6-7 weeks old C57BL/6 mice were obtained from Janvier. B16F10 melanoma cells were injected intravenously (1 x 105 cells) through the tail vein or implanted subcutaneously in the flank (1 x 106 cells). Mice injected intravenously with B16F10 cells were perfused with 1% PFA through the pulmonary artery and the aorta after euthanasia. Lungs were excised and fixed for 1h. The volume of subcutaneous tumors was measured with a caliper (Volume = Length x Width2 x 0.5). Animals were sacrificed when tumors volumes reached a maximum of 2000 mm3 or body weight loss exceeded 15%. Experiments were performed under project licenses issued by the Veterinäramt des Kantons Zürich, Switzerland (Bew. Nr. 027/15).
Results
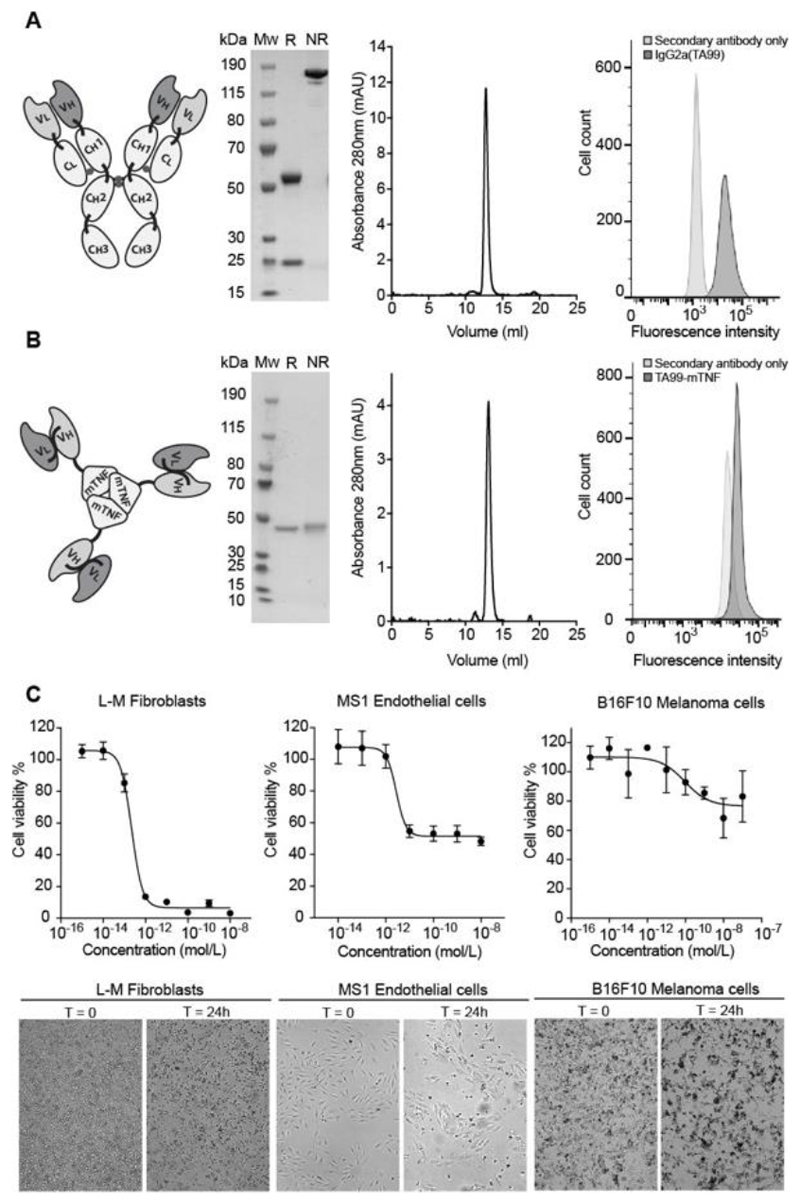
Figure 1 shows the biochemical properties of the recombinant antibody-based therapeutics used for this study. Murine IgG2a(TA99) and the non-covalent homotrimeric TA99-mTNF fusion migrated as a single band in non-reducing SDS-PAGE analysis, eluted as a single peak in gel-filtration and bound to B16F10 melanoma cells. TA99-mTNF efficiently killed fibroblasts and endothelial cells, but did not display a potent biocidal activity against B16 melanoma in vitro [Figure 1C,D].
Figure 1. Cloning expression and characterization of IgG2a(TA99) and TA99-mTNF.
A, Starting from left: schematic representation of the TA99 antibody in the murine IgG2a format, SDS-page analysis of the protein product (MW, molecular weight; R, reducing conditions; NR, nonreducing conditions), size exclusion chromatography profile, FACS analysis for binding of IgG2a(TA99) to B16F10 melanoma cells. B, Starting from left: schematic representation of the TA99-mTNF fusion protein, SDS-page analysis of the protein product, size exclusion chromatography profile, FACS analysis for binding FITC-labelled TA99-mTNF to B16F10 melanoma cells. C, TA99-mTNF in vitro killing assay on L-M fibroblasts, MS-1 endothelial cells and B16F10 melanoma cells. Bottom: representative pictures of cells before and 24 hours after incubation with TA99-mTNF (10-8 M for L-M fibroblasts and MS-1, 10-7 M for B16F10).
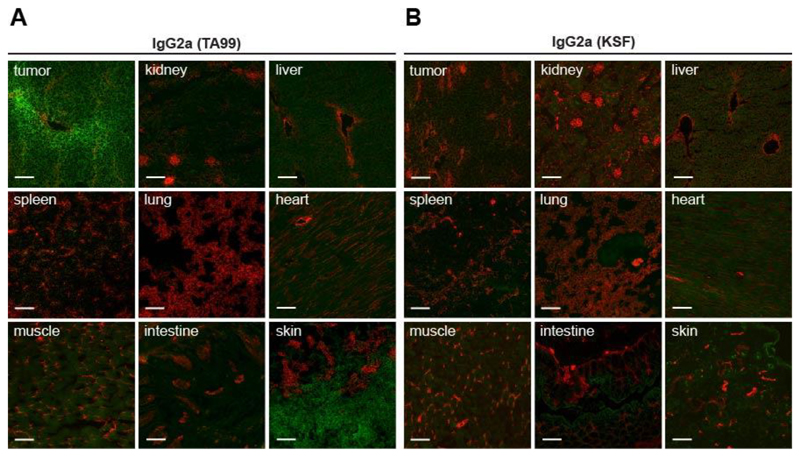
We then assessed the tumor-homing properties of the TA99 antibody in immunocompetent C57/BL6 mice, bearing subcutaneously-grafted B16 melanomas. An ex vivo immunofluorescence analysis, performed 24 hours after intravenous administration of IgG2a(TA99), revealed a selective and homogenous homing of the antibody to the tumor cells and to melanocytes, consistently with the very specific pattern of gp75 antigen expression[7, 8]. By contrast, an IgG2a of irrelevant specificity (KSF, directed against hen egg lysozyme) [37] did not exhibit any preferentially tumor homing [Figure 2].
Figure 2. IgG2a(TA99) specifically accumulates in s.c. B16F10 tumors.
Microscopic fluorescence analysis of organs from B16F10 tumor bearing mice, 24 hours after intravenous administration of FITC labelled IgG2a(TA99) (A) or IgG2a(KSF) (B) (green, Alexa Fluor 488). Blood vessels stained with anti-CD31 (red, Alexa Fluor 594). Magnification 10x, scale bar = 100μm.
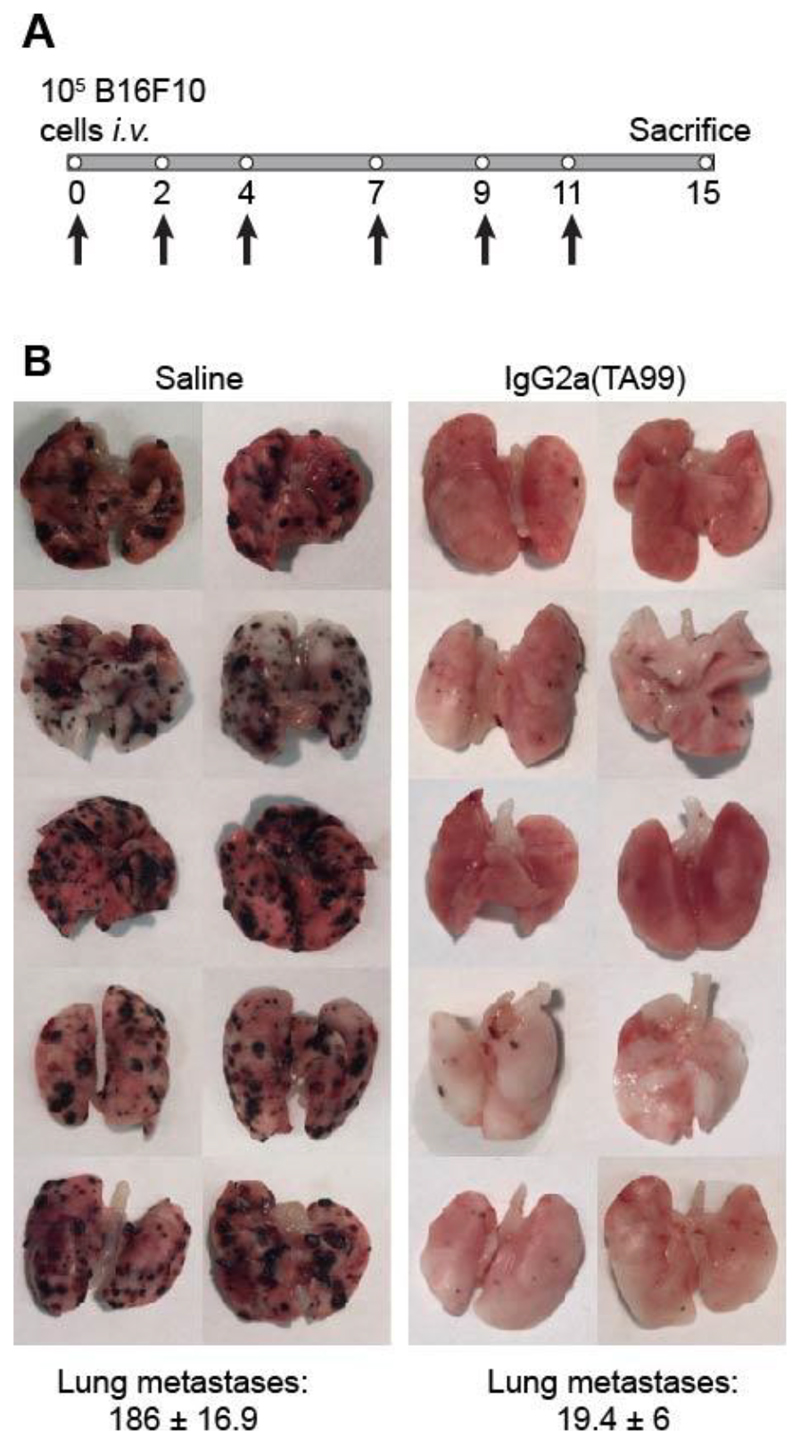
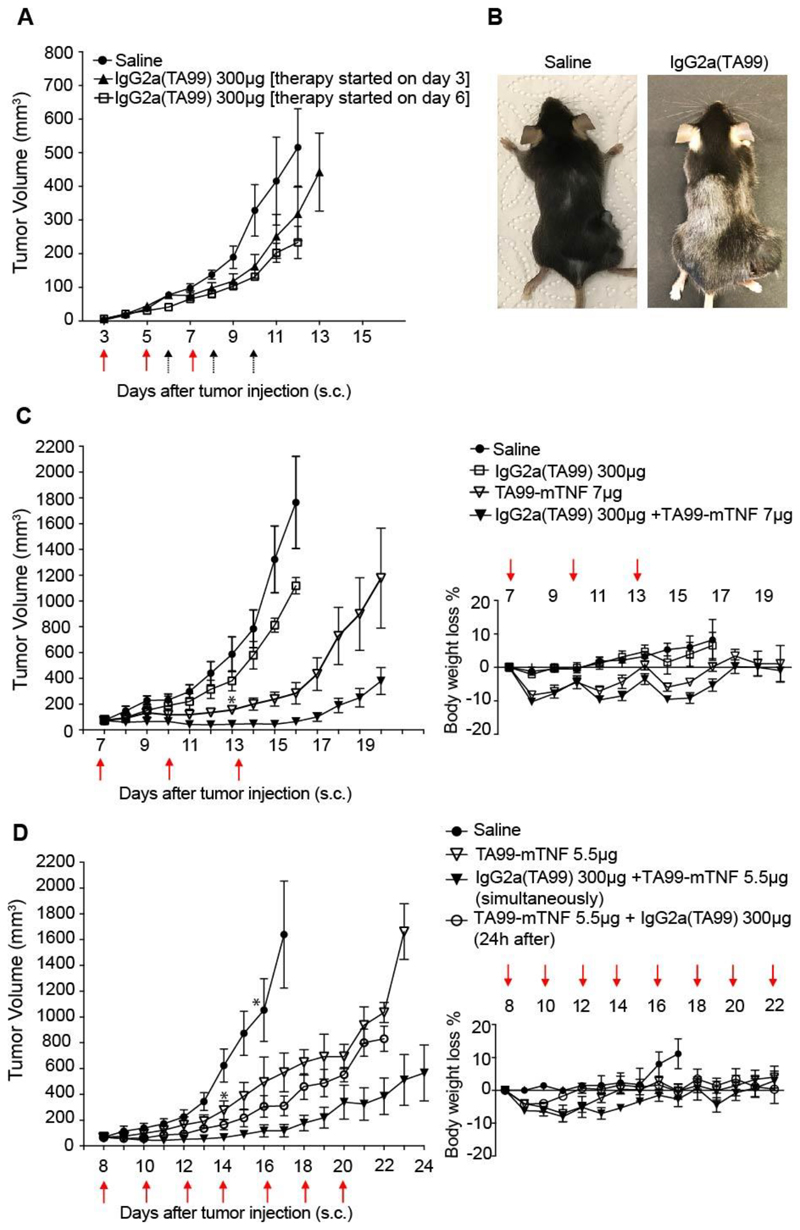
In keeping with what had previously been reported by the Ravetch group [10], IgG2a(TA99) efficiently prevented B16 lung metastasis formation, when the antibody was administered one hour after intravenous injection of B16 melanoma cells [Figure 3]. However, when the product was administered to mice bearing small tumors (i.e., 3 or 6 days after subcutaneous injection of B16 cells), only a minimal tumor growth retardation was observed [Figure 4A]. The IgG2a(TA99) was biologically active in this setting, as evidenced by the discoloration of the mouse coat [Figure 4B].
Figure 3. IgG2a(TA99) prevents B16F10 lung metastases formation in C57/BL6 mice.
A, Representative scheme of the therapy schedule indicating i.v. B16F10 cells injection in C57/BL6 mice followed by 200 μg IgG2a(TA99) or saline i.v. injection at day 0, 2, 4, 7, 9 and 11. B, Images of lungs for each treatment group with B16 lung metastases as black masses in the lungs tissue. Lung metastases count ± SEM.
Figure 4. TA99-mTNF is able to boost the therapeutic effect of IgG2a(TA99) on B16F10 tumors.
A, Therapy in B16F10 melanoma bearing C57/BL6 mice with IgG2a(TA99) as single agent. Mice were injected three times intravenously every 48 hours with 300 μg IgG2a(TA99) or saline (arrows). Therapy started at day 3 (red arrows) or at day 6 (black arrows) after tumor implantation. Data represent mean tumor volume ± SEM, n = 5 mice per group.
B, Example of coat depigmentation in C57/BL6 mouse treated with IgG2a(TA99). Mice were shaved on day -1 over the right flank and on the back. C, Left: Therapy in B16F10 melanoma bearing C57/BL6 mice with IgG2a(TA99), TA99-mTNF as single agents or in combination. Mice were injected three times intravenously every 72 hours with 300 μg IgG2a(TA99), 7 μg TA99-mTNF or saline (red arrows). Data represent mean tumor volume ± SEM. Right: Toxicity monitoring by alterations in body weight change during therapy represented as mean % weight change ± SEM, n = 5 mice per group for IgG2a(TA99) + TA99-mTNF combination and TA99-mTNF monotherapy, n = 4 mice per group for Saline, IgG2a(TA99) monotherapy and TA99-mTNF monotherapy after day 13 (*). D, Left: Therapy in B16F10 melanoma bearing C57/BL6 mice with IgG2a(TA99), TA99-mTNF as single agents or in combination. Mice were injected three times intravenously every 48 hours with 300 μg IgG2a(TA99), 5.5 μg TA99-mTNF or saline (red arrows). For the combination treatment TA99-mTNF was injected either 24 hours before or immediately after IgG2a(TA99) administration. Data represent mean tumor volume ± SEM. Right: Toxicity monitoring by alterations in body weight change during therapy represented as mean % weight change ± SEM, n = 5 mice per group, n = 4 mice per group for Saline, TA99-mTNF monotherapy after day 16 and day 13 respectively (*).
Treatment of mice with B16 melanoma using TA99-mTNF inhibited tumor growth and this anti-cancer activity was potentiated by combination with IgG2aTA99 [Figure 4C]. At the dose used (7 μg), TA99-mTNF caused a transient body weight loss of 10%. In an attempt to improve activity and tolerability, a more frequent dosing of the product (5.5 μg) was tested. While in a combination group (i.e., the one with simultaneous injection of the two biopharmaceuticals) tumor progression could be substantially delayed, neoplastic masses eventually regrew [Figure 4D].
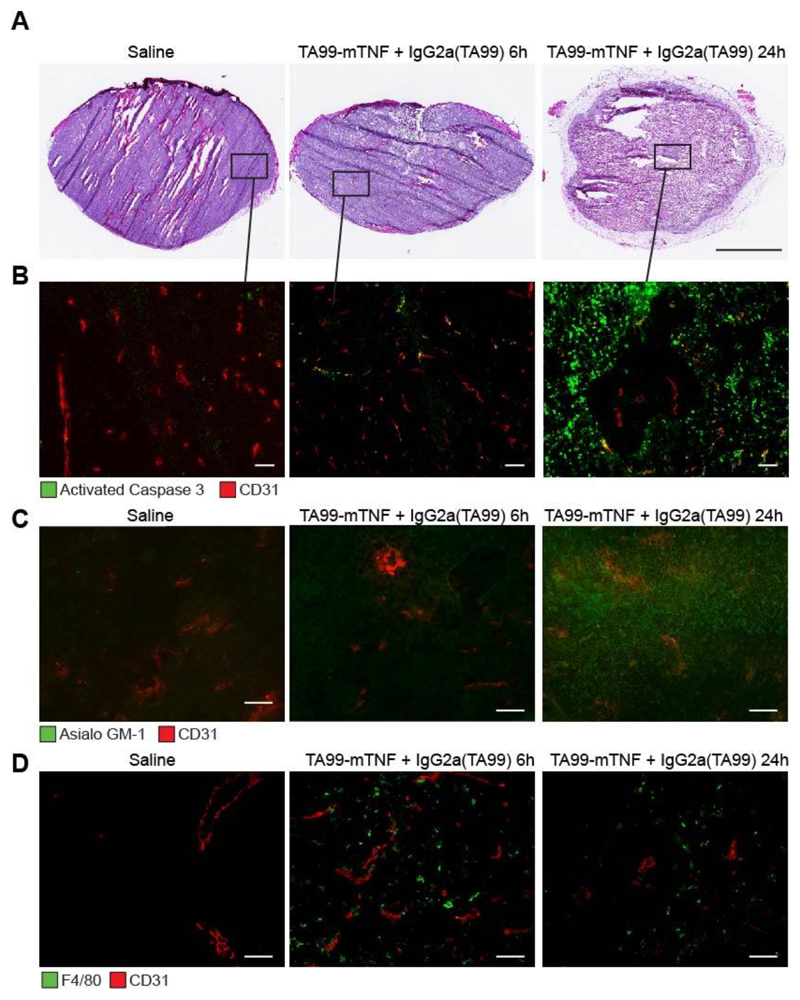
Analysis of tumor sections (H&E and caspase 3 stainings) in mice treated with saline or with the combination of TA99-mTNF and IgG2a(TA99) revealed that the combination treatment rapidly transformed the neoplastic mass into a largely necrotic scab [Figure 5A,B]. However, a few areas with vital tumor cells, surrounding tumor blood vessels, could still be detected [Figure 5B]. Prior to therapy, macrophages and NK cells were virtually undetectable within the tumor mass, thus hindering ADCC activity. The combination of TA99-mTNF and IgG2a(TA99) led to a substantial influx of these cells into the solid tumor mass, as detected by F4/80 and asialo-GM1 staining [Figure 5C,D].
Figure 5. The combination of IgG2a(TA99) and TA99-mTNF kills most of the neoplastic mass and boosts infiltration of NK cells and macrophages.
A, H&E analysis of tumor sections 24 hours after administration of IgG2a(TA99) and TA99-mTNF. Scale bar = 2.5 mm. Detection of activated caspase-3 (B), Asialo GM1 on NK cells (C) and F4/80 on macrophages (D) by immunofluorescence (green, Alexa Fluor 488) in B16F10 tumor sections 24 hours after combination treatment with IgG2a(TA99) and TA99-mTNF. Blood vessels stained stained with anti-CD31 (red, Alexa Fluor 594). Magnification 20x, scale bar = 100 μm
Discussion
In keeping with previous reports, IgG2a(TA99) efficiently prevented B16 melanoma lung metastasis [10] but could not eradicate solid tumor masses, even of small size [13–15] [Figures 3 and 4]. The antibody could mediate ADCC in vivo against melanocytes, as revealed by discoloration of the black coat of C57/BL6 mice [Figure 4B]. Thus, there are mechanisms which prevent an efficient ADCC within the solid tumor mass.
In order to achieve ADCC in vivo, a few prerequisites are needed: (i) the antibody needs to efficiently localize to tumor cells; (ii) a sufficiently high density of effector cells must be present; (iii) the tumor cell must be sensitive to components of cytotoxic granules (e.g., perforin and granzymes). Ex vivo detection of tumor targeting clearly revealed a selective and homogenous homing of the IgG2a antibody to B16 melanoma cells [Figure 2]. The inhibition of lung metastasis formation confirms that the tumor cells could be killed by ADCC [Figure 3]. Collectively, the results of this article suggest that the main limitation for an efficient implementation of ADCC against solid B16 melanoma masses relates to the lack of active NK cells or macrophages within the neoplastic mass. [Figure 5C,D].
Antibody-cytokine fusions, capable of selective localization within the tumor mass, can substantially increase the local density of NK cells and other leukocytes [18, 30, 35, 39, 40]. The antibody-based delivery of TNF to components of the tumor extracellular matrix can mediate a hemorrhagic necrosis of the tumor mass [27], followed by activation of the immune system against the (few) residual tumor cells [32, 35]. This process may be boosted by chemotherapy [31, 41] or by other combination modalities (e.g., other tumor-homing immunocytokines) [28, 35, 41].
There has been an intense industrial effort in potentiating the ADCC activity of antibodies by mutagenesis of the Fc portion [42] or by glycoengineering [43]. The use of antibody-cytokine fusions, capable of boosting the influx and activation of certain leukocytes into the tumor cells mass, may represent a valuable complementary approach. Two clinical trials, feature the combination of L19-IL2 (a fusion protein directed against the alternatively-spliced EDB domain of fibronectin) with rituximab (NCT02957019), or the combination of F16-IL2 with the anti-CD33 antibody BI 836858 (NCT03207191) [24, 25]. While two TNF fusions are currently being investigated in advanced clinical trials [44, 45], we are not aware of on-going combination trials with IgG-based therapeutics.
In preclinical models of cancer, the main activity of targeted TNF products appears to be associated with a rapid induction of hemorrhagic necrosis [27]. In patients, similar effects on superficial melanoma lesions have been reported in isolated limb perfusion procedures both with recombinant TNF [46] and with the L19-TNF fusion [33]. When considering visceral metastases or internal solid tumor masses, advances in perfusion MRI methodologies [47] may allow to detect whether necrotic processes, similar to the ones observed in mice, can also be induced in patients with cancer.
ADCC remains an attractive and elegant avenue for exploiting the potential of the immune system, against target antigens of choice. Biocidal events are only manifested when the IgG molecule engages in a binding interaction with a suitable marker on the surface of target cells. ADCC applications have not been very successful for the treatment of disseminated solid tumors, but the successful application of trastuzumab for the adjuvant treatment of patients with breast cancer is due, at least in part, to ADCC mechanisms [4]. ADCC may even play a role in the growing field of immune check-point inhibitors [48, 49].
Gp75, the target of the TA99 antibody, is one of the “cleanest” tumor-associated antigens described so far, being only expressed in melanocytes and in melanoma lesions. The excellent quality of this target can be easily confirmed by inspection of the Protein Atlas database [8, 50]. We believe that molecular strategies directed against the gp75 antigen may be ideally suited, in order to investigate whether ADCC approaches can be efficient for the management of disseminated solid tumors. Based on the results of our study, a fully-human IgG1 product, specific to gp75, may deserve industrial and clinical development, especially if used in combination with antibody-cytokine products.
Acknowledgements
We thank Dr. Lothar Dietrich and Peter Runge for providing the B16F10 and MS-1 cell lines. We acknowledge the positive and constructive criticisms and intellectual contributions made by Dr. Roberto De Luca, Dr. Samuele Cazzamalli and Dr. Giovanni Pellegrini. The authors gratefully acknowledge financial support from ETH Zürich, the ERC Advanced Grant “Zauberkugel” (Grant Agreement 670603) and the he Swiss National Science Foundation (Projects Nr. 310030B_163479/1, SINERGIA CRSII2_160699/1).
Footnotes
Conflict of interest disclosure: Prof. Dr. Dario Neri is board member and shareholder of Philogen AG. Dr. Mattia Matasci is an employee of Philochem AG. The authors have no additional conflict of interest.
References
- 1.Maloney DG, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84(8):2457–66. [PubMed] [Google Scholar]
- 2.Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341(6151):1192–8. doi: 10.1126/science.1241145. [DOI] [PubMed] [Google Scholar]
- 3.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6(5):343–57. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann SH. Immunology's foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol. 2008;9(7):705–12. doi: 10.1038/ni0708-705. [DOI] [PubMed] [Google Scholar]
- 7.Takechi Y, et al. A melanosomal membrane protein is a cell surface target for melanoma therapy. Clin Cancer Res. 1996;2(11):1837–42. [PubMed] [Google Scholar]
- 8.Uhlen M, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220) doi: 10.1126/science.1260419. p. 1260419. [DOI] [PubMed] [Google Scholar]
- 9.Vijayasaradhi S, Bouchard B, Houghton AN. The melanoma antigen gp75 is the human homologue of the mouse b (brown) locus gene product. J Exp Med. 1990;171(4):1375–80. doi: 10.1084/jem.171.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310(5753):1510–2. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 11.Nimmerjahn F, et al. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci U S A. 2010;107(45):19396–401. doi: 10.1073/pnas.1014515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boross P, et al. Anti-tumor activity of human IgG1 anti-gp75 TA99 mAb against B16F10 melanoma in human FcgammaRI transgenic mice. Immunol Lett. 2014;160(2):151–7. doi: 10.1016/j.imlet.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Moynihan KD, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2016;22(12):1402–1410. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sockolosky JT, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci U S A. 2016;113(19):E2646–54. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzeng A, et al. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proc Natl Acad Sci U S A. 2015;112(11):3320–5. doi: 10.1073/pnas.1416159112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arenas-Ramirez N, et al. Improved cancer immunotherapy by a CD25-mimobody conferring selectivity to human interleukin-2. Sci Transl Med. 2016;8(367) doi: 10.1126/scitranslmed.aag3187. p. 367ra166. [DOI] [PubMed] [Google Scholar]
- 17.Charych DH, et al. NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models. Clin Cancer Res. 2016;22(3):680–90. doi: 10.1158/1078-0432.CCR-15-1631. [DOI] [PubMed] [Google Scholar]
- 18.Lode HN, et al. Immunocytokines: a promising approach to cancer immunotherapy. Pharmacol Ther. 1998;80(3):277–92. doi: 10.1016/s0163-7258(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 19.Neri D, Sondel PM. Immunocytokines for cancer treatment: past, present and future. Curr Opin Immunol. 2016;40:96–102. doi: 10.1016/j.coi.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasche N, Neri D. Immunocytokines: a novel class of potent armed antibodies. Drug Discov Today. 2012;17(11–12):583–90. doi: 10.1016/j.drudis.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Taylor N. Bristol-Myers Squibb makes history with major multibillion-dollar Nektar drug pact. [cited 2018 February 14];Fierce Biotech. [Google Scholar]
- 22.Borschel N, et al. Potentiating the activity of rituximab against mantle cell lymphoma in mice by targeting interleukin-2 to the neovasculature. Leuk Res. 2015;39(7):739–48. doi: 10.1016/j.leukres.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Schliemann C, et al. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood. 2009;113(10):2275–83. doi: 10.1182/blood-2008-05-160747. [DOI] [PubMed] [Google Scholar]
- 24.Gutbrodt KL, et al. Antibody-based delivery of interleukin-2 to neovasculature has potent activity against acute myeloid leukemia. Sci Transl Med. 2013;5(201) doi: 10.1126/scitranslmed.3006221. p. 201ra118. [DOI] [PubMed] [Google Scholar]
- 25.Schliemann C, et al. Targeting interleukin-2 to the bone marrow stroma for therapy of acute myeloid leukemia relapsing after allogeneic hematopoietic stem cell transplantation. Cancer Immunol Res. 2015;3(5):547–56. doi: 10.1158/2326-6066.CIR-14-0179. [DOI] [PubMed] [Google Scholar]
- 26.Fajardo LF, et al. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol. 1992;140(3):539–44. [PMC free article] [PubMed] [Google Scholar]
- 27.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11(4):397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 28.Halin C, et al. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res. 2003;63(12):3202–10. [PubMed] [Google Scholar]
- 29.Khawli LA, Miller GK, Epstein AL. Effect of seven new vasoactive immunoconjugates on the enhancement of monoclonal antibody uptake in tumors. Cancer. 1994;73(3 Suppl):824–31. doi: 10.1002/1097-0142(19940201)73:3+<824::aid-cncr2820731312>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Borsi L, et al. Selective targeted delivery of TNFalpha to tumor blood vessels. Blood. 2003;102(13):4384–92. doi: 10.1182/blood-2003-04-1039. [DOI] [PubMed] [Google Scholar]
- 31.Hemmerle T, et al. The antibody-based targeted delivery of TNF in combination with doxorubicin eradicates sarcomas in mice and confers protective immunity. Br J Cancer. 2013;109(5):1206–13. doi: 10.1038/bjc.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Probst P, et al. Sarcoma Eradication by Doxorubicin and Targeted TNF Relies upon CD8(+) T-cell Recognition of a Retroviral Antigen. Cancer Res. 2017;77(13):3644–3654. doi: 10.1158/0008-5472.CAN-16-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papadia F, et al. Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J Surg Oncol. 2013;107(2):173–9. doi: 10.1002/jso.23168. [DOI] [PubMed] [Google Scholar]
- 34.Spitaleri G, et al. Phase I/II study of the tumour-targeting human monoclonal antibody-cytokine fusion protein L19-TNF in patients with advanced solid tumours. J Cancer Res Clin Oncol. 2013;139(3):447–55. doi: 10.1007/s00432-012-1327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Luca R, et al. Potency-matched Dual Cytokine-Antibody Fusion Proteins for Cancer Therapy. Mol Cancer Ther. 2017;16(11):2442–2451. doi: 10.1158/1535-7163.MCT-17-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putelli A, et al. A fibrin-specific monoclonal antibody from a designed phage display library inhibits clot formation and localizes to tumors in vivo. J Mol Biol. 2014;426(21):3606–18. doi: 10.1016/j.jmb.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Pasche N, et al. Cloning and characterization of novel tumor-targeting immunocytokines based on murine IL7. J Biotechnol. 2011;154(1):84–92. doi: 10.1016/j.jbiotec.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Rajendra Y, et al. A simple high-yielding process for transient gene expression in CHO cells. J Biotechnol. 2011;153(1–2):22–6. doi: 10.1016/j.jbiotec.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Halin C, et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20(3):264–9. doi: 10.1038/nbt0302-264. [DOI] [PubMed] [Google Scholar]
- 40.Moschetta M, et al. Paclitaxel enhances therapeutic efficacy of the F8-IL2 immunocytokine to EDA-fibronectin-positive metastatic human melanoma xenografts. Cancer Res. 2012;72(7):1814–24. doi: 10.1158/0008-5472.CAN-11-1919. [DOI] [PubMed] [Google Scholar]
- 41.Pretto F, et al. Preclinical evaluation of IL2-based immunocytokines supports their use in combination with dacarbazine, paclitaxel and TNF-based immunotherapy. Cancer Immunol Immunother. 2014;63(9):901–10. doi: 10.1007/s00262-014-1562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Presta LG. Molecular engineering and design of therapeutic antibodies. Curr Opin Immunol. 2008;20(4):460–70. doi: 10.1016/j.coi.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Umana P, et al. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17(2):176–80. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 44.Danielli R, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immunother. 2015;64(8):999–1009. doi: 10.1007/s00262-015-1704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoro A, et al. Phase II study of NGR-hTNF, a selective vascular targeting agent, in patients with metastatic colorectal cancer after failure of standard therapy. Eur J Cancer. 2010;46(15):2746–52. doi: 10.1016/j.ejca.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Lejeune FJ, et al. Isolated limb perfusion: the European experience. Surg Oncol Clin N Am. 2001;10(4):821–32, ix. [PubMed] [Google Scholar]
- 47.Li SP, Padhani AR. Tumor response assessments with diffusion and perfusion MRI. J Magn Reson Imaging. 2012;35(4):745–63. doi: 10.1002/jmri.22838. [DOI] [PubMed] [Google Scholar]
- 48.Ingram JR, et al. Anti-CTLA-4 therapy requires an Fc domain for efficacy. Proc Natl Acad Sci U S A. 2018;115(15):3912–3917. doi: 10.1073/pnas.1801524115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simpson TR, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Human Protein Atlas. [cited 2018 April 15th];2018 http://www.proteinatlas.org/