Abstract
Unlike mammals, Xenopus laevis tadpoles have a high regenerative potential. To characterize this regenerative response, we performed single-cell RNA sequencing (scRNA-seq) following tail amputation. By comparing naturally-occurring regeneration-competent and incompetent tadpoles, we identified a previously unrecognized cell type that we term the regeneration-organizing cell (ROC). ROCs are present in the epidermis during normal tail development, and specifically relocalize to the amputation plane of regeneration-competent tadpoles, forming the wound epidermis. Genetic ablation or manual removal of ROCs blocks regeneration, whereas transplantation of ROC-containing grafts induces ectopic outgrowths in early embryos. Transcriptional profiling revealed that ROCs secrete ligands associated with key regenerative pathways, signaling to progenitors to reconstitute lost tissue. These findings reveal the cellular mechanism through which ROCs form the wound epidermis and ensure successful regeneration.
Appendage regeneration involves coordinated changes in many cell types, and has been largely characterized by morphological assessments, lineage tracing studies, and low-throughput gene investigations. As a result, regeneration is broadly divided into three essential steps: the formation of a specialized wound epidermis, blastema/regenerative bud formation, and outgrowth via proliferation (1, 2). However, a comprehensive understanding of the changes in cell types, transcriptional dynamics, and cellular mechanisms accompanying these processes is lacking. For example, the first morphological change upon amputation is the formation of the specialized wound epidermis, a ligand-expressing structure covering the wound that is essential in many different regeneration scenarios in different species (e.g. zebrafish, axolotl) (1, 3, 4). However, it is not clear which cell types are present in the specialized wound epidermis, what is its origin, what is the broad catalogue of ligands expressed from it, and why is it crucial for regeneration. To answer such questions and identify essential regulators of regeneration, we focused on Xenopus tadpoles, with their naturally occurring regeneration-competent and -incompetent developmental stages, making them an ideal system for comparative studies (5).
To assess comprehensively the transcriptional dynamics and cell type changes that occur during regeneration, we took advantage of high-throughput scRNA-seq to target Xenopus laevis tails at various stages following amputation in both regeneration-competent and incompetent tadpoles, as well as uninjured (intact) tails at the same developmental stage (Fig. 1A). We sequenced >13,000 cells, with at least 2 biological replicates per condition, with an average of ~2,300 genes detected per cell (table S1). Cells from all samples were pooled and visualized by the dimensionality reduction method, UMAP (6) (Fig. 1A). Cluster identity was assigned using multiple known markers and revealed a total of 46 putative cell types (some rare and others uncharacterized) encompassing the immune system, skin, nervous system and somites, emphasizing the cellular heterogeneity of the tail (Fig. 1B, and fig. S1). Biological replicates showed a similar distribution of cell types, confirming the reproducibility of the atlas (fig. S2). Computational inference of cell cycle state confirmed that progenitor cell populations are mostly positioned in G2/M and S phases, whereas terminally differentiated cells are in G1 (fig. S3). Our comprehensive cell atlas can be viewed using the interactive platform (marionilab.cruk.cam.ac.uk/XenopusRegeneration/).
Fig. 1. Pooled transcriptional cell state atlas of the Xenopus laevis tail before and after amputation.
(A) Samples were prepared for single-cell RNA-seq analysis from regeneration-competent and incompetent tadpoles, collecting either intact tails, or tails at various stages following amputation: 1-3 days post amputation (dpa) for regeneration-competent, and 1 dpa for regeneration-incompetent tadpoles. Developmental timing is indicated for each sample (days post-fertilisation, dpf). Samples were processed separately for sequencing and then pooled for UMAP visualisation (Methods). Each dot represents a single cell; colour indicates main tissue group (n≥2 for each sample). (B) Cluster identities based on established cell type markers. For details of cluster annotations, see main text, fig. S1 and Methods.
Having established the atlas, we then questioned what transcriptional and cell type changes are associated specifically with tail regeneration. By comparing samples, we could make a distinction between developmental (Fig. 2A), amputation-specific (Fig. 2B), and regeneration-specific (Fig. 2C) effects. Most cell types were found in all samples (fig. S4A). Consistent with lineage tracing studies (7), we found no evidence for the emergence of a multipotent progenitor population during regeneration, nor did we observe “intermediate” cell states reflective of transdifferentiation. Indeed, the only new cell type to emerge following amputation was an uncharacterized motor neuron-like cell type that expressed genes associated with spinal cord injury (e.g. Fgf10 (8)) and metabolic hormones (e.g. Leptin (9, 10)) (Fig. 2B). This phenotype was also observed in regeneration-incompetent tadpoles (Fig. 2B). Hence, we considered it an amputation-response, and focused instead on regeneration-specific changes.
Fig. 2. Comparison of scRNA-seq samples discriminate gene expression and cell state changes that take place during development from those associated with the response to amputation or regeneration.
Examples of cell-specific gene expression changes that take place (A) during development, (B) in response to amputation, and (C) in response to regeneration. Grey dots: cells from samples at all respective time points; red dots: cells from selected time point and condition. Black and white filled arrows indicate presence and absence of populations, respectively, when comparing intact tail to 1 dpa samples. Panel A shows a continuous change in the gene expression profile of Goblet cells that takes place during development both in regeneration-competent and incompetent tail; panel B shows gene expression changes that take place in motor neurons in response to amputation, both in regeneration-competent and incompetent tail; and panel C shows differential gene expression changes that take place in epidermis between regeneration-competent and incompetent tail, identifying a cell state change specific to regeneration.
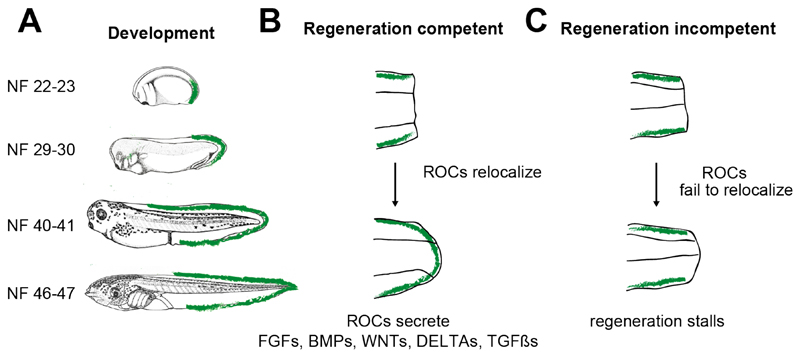
Surveying the range of single-cell data, we found that the most significant cell-type change specific to regeneration was related to a previously unidentified cell type of the epidermis (fig. S4A and B), which (for reasons that will become clear) we designated as the regeneration-organizing cell (ROC). Notably, based on the scRNA-seq data, ROCs were found to be present in both intact and regenerating tails, but were observed following tail amputation only in regeneration-competent tadpoles (Fig. 2C). As this cell population distinguishes the amputation response of regeneration–competent tadpoles from incompetent ones, and expresses multiple genes that support regeneration (e.g. Wnt5a (11), Fgf10 (12), Fgf20 (13), Msx1 and Bmpr1a (5)), we hypothesized that ROCs may represent an essential component of the regenerative response.
To assess the function of ROCs, we first investigated their location using marker genes identified by scRNA-seq. Using published in situ data from Xenbase, we found that >25 ROC marker genes were expressed along the midline edge of the epidermis (e.g. Fgf7, Msx2, C3, Wnt3a), from the posterior trunk towards the tail tip (table S2). We further confirmed the localization of ROCs using a Lef1 reporter line (pbin7LEF:GFP (14)), in combination with TP63 immunolabelling. Whilst Lef1 is expressed in multiple cell types, only ROCs express high levels of both Lef1 and Tp63 (Fig. 3A and fig. S5A). Therefore, we identified ROCs as LEF1+/TP63+ cells and confirmed that they are localized to the edge of the epidermis (Fig. 3B and fig. S5B). ROCs are present in this location in both regeneration-competent and incompetent tadpoles and, immediately following amputation, this population is largely removed from the amputation plane, but remains along the posterior trunk (Fig. 3C, left hand column). During successful regeneration, ROCs reappear in the amputation plane within 24 hours; however, they remain notably absent at the amputation plane of regeneration-incompetent tadpoles (Fig. 3C and D, fig. S5C). Our Lef1 data were consistent with published in situ data of ROC marker genes seen in the tails of amputated regeneration-competent tadpoles (Fgf9, Fgf10, Wnt5a, Wnt3a, Msx1, Msx2 (5, 15), C3 (16)) and lacking in regeneration-incompetent tadpoles (Msx1, Msx2 (5, 15)). The gene expression profile and location of ROCs at the amputation plane further suggest that they are in fact a single cell type that defines the specialized wound epidermis, which specifically forms in regeneration-competent tadpoles to trigger the regenerative response.
Fig. 3. Regeneration-organizing cells (ROCs) characterize the specialized wound epidermis in regeneration competent tadpole.
(A) ROCs express high Lef1 mRNA level, and reappear after amputation specifically in regeneration-competent tadpoles. Grey dots: TP63 positive epidermal clusters; circled dots: selected sample. Relative Lef1 expression visualized for each cell. (B) ROCs (TP63+/LEF1+ cells, denoted by asterisks) are localized along the midline edge of the epidermis in intact tails. Green, pbin7Lef; Red, TP63. Scale bar: 500 μm. (C) ROCs (LEF1+) remain along the posterior trunk following amputation (asterisks), but are removed from the amputation plane (empty arrowheads). ROCs specifically reappear in the amputation plane of 1 dpa regeneration-competent tadpoles (filled arrowhead). hpa: hours post-amputation. Green, pbin7LEF:GFP. Scale bars: 250 μm; a total of ≥3 tadpoles per conditions were imaged from 2 biological replicates. (D) Quantification of TP63+/LEF1+ cells at the amputation plane (mean ± standard deviation) shows a significant reduction in regeneration-incompetent tadpoles at 1 dpa (n=12 and n=11 for competent and incompetent samples, respectively, both from 2 biological replicates). *: p < 0.001.
As the presence of ROCs at the amputation plane correlates with regenerative outcome, we tested whether these cells are required for regeneration. We first performed NTR/MTZ based genetic ablation (17) of ROCs using F0-transgenic tadpoles expressing Nitroreductase (NTR) under the control of the Krt.L promoter, a member of the keratin gene family that is expressed in a highly specific manner in ROCs in stages where regeneration is assessed (Fig. 4A, and fig. S6A to D; Krt.L is also known as Krt70.L). Upon Metronidazole (MTZ) treatment, we were able to specifically ablate ROCs, as confirmed by the disappearance of GFP positive cells in pbin7LEF:GFP /Krt.L:NTR F0 transgenic tadpoles (Fig. 4A), together with the observation of no apparent gross off-target effects in other tissue types (fig. S6E). Ablation of ROCs in regeneration-competent tadpoles led to drastically reduced tail regeneration (Fig. 4B and fig. S7A-D), demonstrating that ROCs are indeed required for regeneration.
Fig. 4. Regeneration-organizing cells relocation to the amputation area mediates tail regeneration.
(A) Nitroreductase (NTR)/Metronidazole-mediated ablation of ROCs during regeneration. pbin7LEF:GFP/Krt.L:NTR F0 transgenic tadpoles (bottom) show successful cell ablation at 3 dpa: GFP positive ROCs are present in control (plain arrowhead) but lost in MTZ treated animal (empty arrowhead). Scale, 1 mm. (B) ROC-ablated tadpoles cannot regenerate (n=11 from 2 biological replicates). (C) Schematic of ROCs-containing region manual removal protocol. Green colored area indicate ROCs localization. (D) Manual removal of posterior trunk ROCs at the same time as tail amputation reduces regeneration (n= 45 from 5 biological replicates), but manual removal of posterior trunk ROCs 12-16 hours post amputation does not negatively affect regeneration (n= 95 from 3 biological replicates). (E) Time-lapse images of ROCs relocating to the amputation plane, as assessed by pbin7LEF:GFP. Asterisks denote cells with brighter GFP that can be tracked (n=8 from 3 biological replicates). Scale bar, 500 μm.
We then eliminated ROCs in a spatially localized manner, by manually removing ROCs in the posterior trunk that remain directly after amputation (Fig. 4C, and fig. S7E). When these regions were removed at the same time as the amputation, we observed a reduction in Lef1+ cells at the amputation plane, and correspondingly reduced regeneration (Fig 4D, and fig. S7E). However, when removed 12-16 hours after amputation, Lef1+ expression was maintained at the amputation plane, and regeneration could proceed (Fig 4C and D, and fig. S7E), indicating that there is a critical time window during which posterior trunk ROCs are required to initiate regeneration. This suggests that, soon after amputation, existing ROCs may have to relocate to the amputation area to initiate the regenerative response. To test this hypothesis, we traced ROCs following amputation with the Lef1 reporter, and observed the mobilization of resident ROCs from the posterior trunk towards the amputation area within 2-8 hours (Fig. 4E). Together, these observations suggest that mobilization of ROCs to the amputation area is a necessary step in wound epidermis formation and subsequent regeneration, in contrast to previous suggestions that the wound epidermis is a novel state that differs from normal epithelium and appears upon amputation (2).
Next, we asked whether inhibition of pathways that are necessary for wound epidermis formation, which are rapidly upregulated upon injury, interfere with the mobilization of ROCs to the amputation plane. Indeed chemical inhibition of reactive oxygen species (ROS) production (13, 18, 19) or the TGFβ pathway (20) resulted in significantly reduced ROC migration (fig. S8). In contrast, inhibition of the FGF pathway, which is known to not affect wound epidermis formation (15), had no effect on ROC migration (fig. S8). Hence, wound-induced ROS production and TGFβ pathway activation are necessary for ROCs to migrate to the amputation plane, where they form the wound epidermis.
To understand the essential role played by ROCs during regeneration, we used our scRNA-seq data to dissect their transcriptional signature. Ligands of signalling pathways that are known to be required for regeneration and increase proliferating cell numbers, including FGF (15), BMP (5, 21), WNT (15), NOTCH (5) and TGFβ (20), are simultaneously expressed in ROCs, but not in any other cell type. In contrast, receptors for these pathways are mostly expressed in progenitor cell types (Fig. 5A, and fig. S9). Moreover, we found that progenitor populations at the amputation site showed an increase in the fraction of cells in G2/M and S phases during regeneration (Fig. 5B). These results suggest that ROCs function as a signalling centre by secreting factors that promote progenitor proliferation in multiple tissues. Such an increase in proliferation can explain how tissue loss can be reconstituted from progenitors of the tail without requiring the emergence of a new multipotent cell state or states.
Fig. 5. ROCs act as a signalling centre coordinating progenitor outgrowth during tail regeneration.
(A) Expression of FGF ligands (Top) and receptor (Bottom) shown for selected cell types as a boxplot (outliers not shown). (B) Bar plot indicating the change in the fraction of cells in G2/M and S phases between regeneration-competent 2 dpa and incompetent intact tail samples, all taken at 5 dpf. (C) (Left) Removal of large or small ROC-containing tissues causes tail development defects in donors (n= 20), (Right) grafting these regions to the trunk enables tail-enriched or fin-enriched distal growth in hosts, respectively. Matching donor-acceptor pairs are shown 2 days post-grafting (n= 20 from 3 biological replicates). (D) Non-labelled grafts to CMV:GFP positive embryos induce outgrowth containing GFP positive cells; donor tissues are at the tip of the ectopic structure (n=12 from 3 biological replicates). Green, CMV:GFP. Scale bars: full tadpoles, 1 mm; zoomed grafts and merged graft images, 500 μm.
Having established the central role of ROCs during regeneration, we then asked whether they resemble other cell types associated with early development. ROCs express markers of limb development and appendage growth (fig. S10), including the limb Apical Ectodermal Ridge (AER) regulating transcription factors Sp8 and Sp9 (22). The AER is a structure formed at limb bud tips, and plays an essential role in limb growth and patterning by sending extracellular signals to underlying tissues (23). Moreover, in urodele limb regeneration, a similar structure, the Apical Epithelial Cap (AEC), is shown to be necessary for limb regeneration (1). Although ROCs resemble AER and AEC transcriptionally (e.g., Sp9, Msx2, Wnt5a), we were not able to detect their well-known regulators (e.g. Fgf2, Fgf4, Fgf8, and Cx43(Gja1)) (fig. S10A). Despite this, we hypothesized that ROCs could play an instructive role during tail growth by secreting growth factors and extracellular cues, similar to the AER during limb growth, and aimed to test this using transplantation assays.
In order to isolate potential ROCs for grafting, we investigated where the transcriptional signature of ROCs is first detected during early embryonic development. Marker genes of ROCs first appear at the early tailbud tip (NF stage 23), and later expand posteriorly along the midline edge of the epidermis (table S2). We grafted different sizes of posterior tailbud tissues, which contain ROCs, to the surface of different regions of trunk of a host embryo (Fig. 5C). All grafts induced ectopic outgrowth, regardless of graft size or implantation location. Larger grafts induced tail-like structures, with a corresponding defect in donor tail growth; whereas smaller grafts, composed of only skin layers, induced fin-like structures, without significantly impacting the donor (Fig. 5C, and fig. S11A and B). In contrast, control grafts, in which dissected trunk skin tissues were transplanted, did not result in outgrowths and instead were incorporated into the host trunk (fig. S11C). To further pinpoint the cell type responsible for the outgrowths, we repeated our grafting experiments whilst simultaneously removing ROCs using the MTZ/NTR system. The ablation of Krt.L expressing cells in the donor graft significantly reduced the length of the ectopic outgrowths (fig. S11D and E), suggesting that ROCs are involved in the outgrowth phenomenon, although other cell types may also contribute.
If these distal growths are induced by the organizing abilities of the grafted ROCs, we would expect the transplanted cells to localize to the tip of the ectopic outgrowths. Indeed, when we tested this using GFP-labelled donor or host embryos, we found that most of the donor tissues were located at the tip of the ectopic structures. Moreover, host cells contributed significantly to the ectopic structures in all grafts, indicating that ROCs can stimulate outgrowth of both donor and host cells (Fig. 5D, and fig. S11F and G). Together, these results suggest that ROCs act as an instructive signalling centre that induces outgrowth during both the development and regeneration of the tail.
Overall, our comprehensive analysis of cell types in the regenerating Xenopus tail provides a mechanistic understanding of the initiation and organization of tail regeneration via the re-establishment of a ROC-signalling centre. By acting as the primary source of major growth factors and instructive signals, ROCs promote proliferation of underlying progenitors to regenerate tissue following amputation (Fig. 6). Our data also suggests that ROCs are a single cell type that characterizes the wound epidermis, a structure that is crucial for regeneration in many contexts (2). Investigation of other species (e.g. neonatal mouse, salamander) will indicate whether a ROC-based mechanism is a conserved feature of specialized wound epidermis formation and appendage regeneration.
Fig. 6. ROC-based model of tail regeneration.
Transcriptional signature of ROCs first appears in NF stage 22-23 embryos at the tip of the tail-bud, then expand towards the edges of the epidermis midline from the tail tip to the posterior trunk during development (table S2). Relocalization of ROCs to the wound area forms the specialized wound epidermis and is a hallmark of successful tail regeneration.
Finally, signatures of the specialized wound epidermis formation are absent in non-regenerating animals such as birds, adult mice and adult frogs. However, reintroduction of molecules secreted from the specialized wound epidermis can re-initiate cell cycle entry to some degree in these animals (24, 25, 26). The discovery of a single cell type defining the wound epidermis offers a new perspective on cell replacement therapies, suggesting that “organizer grafts” may perhaps one day serve as a potential alternative to full organ replacement in regenerative therapies.
Supplementary Material
This Excel file contains a summary of each single-cell RNA-sequencing experiment. Samples are labelled depending on their developmental stage reflective of their regenerative ability and days post amputation status. Sequencing batch, numbers of sequenced cells, mean reads per cell, median genes per cell, median UMI counts per cell, total genes detected, and fraction of reads in cells are reported.
This Excel file lists ROCs marker genes that have reported mRNA in situ hybridization results in the literature. These reported findings are provided with their PMID, or PMCID, or DOI, or Xenbase link.
This Excel file contains the gene lists that are used for cell cycle analysis, and scGSEA analysis. It also includes ROCs marker genes.
One Sentence Summary.
Regeneration-organizing-cells play an essential role in coordinating the regeneration of Xenopus tail following amputation.
100 words Summary.
Some vertebrae show a remarkable, if sometimes restricted, ability to regenerate lost appendages. By utilizing single-cell mRNA sequencing and comparing naturally-occurring regeneration competent and -incompetent Xenopus laevis tadpoles, Aztekin et al. identified a new cell type, termed regeneration organizing cells (ROCs) that coordinate tail regeneration. Relocation of ROCs from the body to the amputation plane enables specialized wound epidermis formation and subsequent regeneration. ROCs simultaneously express many different ligands that can induce proliferation of different progenitor cell populations. Hence, by signaling to underlying progenitors, ROCs act as a center that orchestrates the growth of a new appendage.
Acknowledgments
We thank the Cambridge Institute Genomics Core for their support with this work on the 10X-Genomics and sequencing library preparations. The transgenic testes that are used in this study, and pTransgenesis vectors were obtained from the European Xenopus Resource Centre, curated with funding from the Wellcome Trust/BBSRC and maintained by the University of Portsmouth, School of Biological Sciences. Anti-PCNA and anti-PHH3 antibodies were kind gifts from the Brand Lab. CFP-NTR construct was a kind gift from J. Mumm and M. Zuber. We would like to thank H. Ma and M. Huch for use of their stereoscopes. We would like to thank A. Lun for advice on scRNA-seq analysis; J. Griffiths for assisting with the creation of the website; C. Baker and M. Minarik for their help during revision period; B. Steventon, J. Robert, J. Kaufman, N. McGovern and D. Wagner for general discussion of the single-cell data. We are grateful to A. Philpott, V. Gaggioli and E. Rawlins for their critical reading of the manuscript.
Funding
C.A. is funded by University of Cambridge and Cambridge Trust. J.J. and J.B.G. are funded by a grant from the Wellcome Trust (101050/Z/13/Z). T.W.H., J.C.M and B.D.S are funded as part of a Wellcome Strategic Award to study cell fate decisions (105031/D/14/Z). T.W.H. is also supported by an EMBO Long Term Fellowship (ALTF 606-2018). B.D.S also acknowledges funding from the Royal Society E.P. Abraham Research Professorship (RP\R1\180165) and Wellcome Trust (098357/Z/12/Z). J.C.M. acknowledges core funding from the European Molecular Biology Laboratory and Cancer Research UK (A17197). This work is funded by a grant from the Wellcome Trust (101050/Z/13/Z) and supported by the Gurdon Institute core grant from Cancer Research UK (C6946/A14492) and the Wellcome Trust (092096/Z/10/Z).
Footnotes
Author Contributions
Conceptualization: C.A., J.J.; Experiments: C.A. with assistance from J.J. with grafting; Computational Analysis: T.W.H.; Data interpretation: C.A., T.W.H., J.J.; Writing – Original, Draft: C.A., T.W.H., J.J.; Writing – Review & Editing: all authors; Supervision: J.C.M., B.D.S., J.B.G. contributed to general supervision, and J.J. mainly supervised the project.
Competing interests
The authors declare no competing interests.
Data and materials availability
Materials and codes are freely available and requests should be addressed to J.J. (jj256@gurdon.cam.ac.uk) and B.D.S. (bds10@cam.ac.uk).
References and Notes
- 1.Tanaka EM. The Molecular and Cellular Choreography of Appendage Regeneration. Cell. 2016;165:1598–1608. doi: 10.1016/j.cell.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 2.Stoick-Cooper CL, Moon RT, Weidinger G. Advances in signaling in vertebrate regeneration as a prelude to regenerative medicine. Genes & Development. 2007;21:1292–1315. doi: 10.1101/gad.1540507. [DOI] [PubMed] [Google Scholar]
- 3.Chen C-H, Poss KD. Regeneration Genetics. Annu Rev Genet. 2017;51:63–82. doi: 10.1146/annurev-genet-120116-024554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Zhang S, Amaya E. The cellular and molecular mechanisms of tissue repair and regeneration as revealed by studies in Xenopus. Regeneration. 2016;3:198–208. doi: 10.1002/reg2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck CW, Christen B, Slack JMW. Molecular Pathways Needed for Regeneration of Spinal Cord and Muscle in a Vertebrate. Developmental Cell. 2003;5:429–439. doi: 10.1016/s1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- 6.McInnes L, Healy J, Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. 2018:1–51. https://arxiv.org/pdf/1802.03426.pdf. [Google Scholar]
- 7.Gargioli C, Slack JMW. Cell lineage tracing during Xenopus tail regeneration. Development. 2004;131:2669–2679. doi: 10.1242/dev.01155. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, et al. Neuron and microglia/macrophage-derived FGF10 activate neuronal FGFR2/PI3K/Akt signaling and inhibit microglia/macrophages TLR4/NF-κB-dependent neuroinflammation to improve functional recovery after spinal cord injury. Cell Death and Disease. 2017:1–12. doi: 10.1038/cddis.2017.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang J, et al. Modulation of tissue repair by regeneration enhancer elements. Nature. 2016;532:201–206. doi: 10.1038/nature17644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Love NR, et al. Genome-wide analysis of gene expression during Xenopus tropicalis tadpole tail regeneration. BMC Dev Biol. 2011;11:70. doi: 10.1186/1471-213X-11-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugiura T, Tazaki A, Ueno N, Watanabe K, Mochii M. Xenopus Wnt-5a induces an ectopic larval tail at injured site, suggesting a crucial role for noncanonical Wnt signal in tail regeneration. Mechanisms of Development. 2009;126:56–67. doi: 10.1016/j.mod.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi Y, Sugiura T, Tazaki A, Watanabe K, Mochii M. Spinal cord is required for proper regeneration of the tail in Xenopus tadpoles. Develop Growth Differ. 2008;50:109–120. doi: 10.1111/j.1440-169X.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- 13.Love NR, et al. Amputation-induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nature Cell Biology. 2013;15:222–228. doi: 10.1038/ncb2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thi Tran H, Sekkali B, Van Imschoot G, Janssens S, Vleminckx K. Wnt/β-catenin signaling is involved in the induction and maintenance of primitive hematopoiesis in the vertebrate embryo. Proc Natl Acad Sci USA. 2010:1–6. doi: 10.1073/pnas.1007725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin G, Slack JMW. Requirement for Wnt and FGF signaling in Xenopus tadpole tail regeneration. Developmental Biology. 2008;316:323–335. doi: 10.1016/j.ydbio.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Tazaki A, et al. Macroarray-based analysis of tail regeneration inXenopus laevis larvae. Dev Dyn. 2005;233:1394–1404. doi: 10.1002/dvdy.20472. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-De Luna RI, Zuber ME. Rod-Specific Ablation Using the Nitroreductase/Metronidazole System to Investigate Regeneration in Xenopus. Cold Spring Harb Protoc. 2018:1–9. doi: 10.1101/pdb.prot100974. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira F, Luxardi G, Reid B, Zhao M. Early bioelectric activities mediate redox-modulated regeneration. Development. 2016;143:4582–4594. doi: 10.1242/dev.142034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira F, Raghunathan V, Luxardi G, Zhu K, Zhao M. Early redox activities modulate Xenopus tail regeneration. Nature Communications. 2018:1–15. doi: 10.1038/s41467-018-06614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho DM, Whitman M. TGF-β signaling is required for multiple processes during Xenopus tail regeneration. Developmental Biology. 2008;315:203–216. doi: 10.1016/j.ydbio.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck CW, Christen B, Barker D, Slack JMW. Temporal requirement for bone morphogenetic proteins in regeneration of the tail and limb of Xenopus tadpoles. Mechanisms of Development. 2006;123:674–688. doi: 10.1016/j.mod.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Kawakami Y. Sp8 and Sp9, two closely related buttonhead-like transcription factors, regulate Fgf8 expression and limb outgrowth in vertebrate embryos. Development. 2004;131:4763–4774. doi: 10.1242/dev.01331. [DOI] [PubMed] [Google Scholar]
- 23.Petit F, Sears KE, Ahituv N. Limb development: a paradigm of gene regulation. Nature Publishing Group. 2017;18:245–258. doi: 10.1038/nrg.2016.167. [DOI] [PubMed] [Google Scholar]
- 24.Kostakopoulou K, Vogel A, Brickell P, Tickle C. ‘Regeneration’ of wing bud stumps of chick embryos and reactivation of Msx-l and Shh expression in response to FGF-4 and ridge signals. Mechanisms of Development. 1996:119–131. doi: 10.1016/0925-4773(95)00492-0. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, et al. BMP signaling induces digit regeneration in neonatal mice. Development. 2010;137:551–559. doi: 10.1242/dev.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin G, Chen Y, Slack JMW. Imparting Regenerative Capacity to Limbs by Progenitor Cell Transplantation. Developmental Cell. 2013;24:41–51. doi: 10.1016/j.devcel.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoppler S, Vize PD. Xenopus Protocols. Methods in Molecular Biology. 2012:917. [Google Scholar]
- 28.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) North-Holland Publishing Company; Amsterdam: 1967. [Google Scholar]
- 29.Pandey S, Shekhar K, Regev A, Schier AF. Comprehensive Identification and Spatial Mapping of Habenular Neuronal Types Using Single-Cell RNA- Seq. Current Biology. 2018;28:1052–1065.e7. doi: 10.1016/j.cub.2018.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilicic T, et al. Classification of low quality cells from single-cell RNA-seq data. Genome Biol. 2016:1–15. doi: 10.1186/s13059-016-0888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McInnes L, Healy J, Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. 2018:1–51. https://arxiv.org/pdf/1802.03426.pdf. [Google Scholar]
- 32.Lun ATL, McCarthy DJ, Marioni JC. A step-by-step workflow for low-level analysis of single-cell RNA-seq data. F1000Res. 2016;5:2122–68. doi: 10.12688/f1000research.9501.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Session AM, et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature. 2016;538:336–343. doi: 10.1038/nature19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lun ATL, Richard AC, Marioni JC. Testing for differential abundance in mass cytometry data. Cell Death and Disease. 2017:1–5. doi: 10.1038/nmeth.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashburner M, et al. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aibar A, et al. SCENIC: single-cell regulatory network inference and clustering. Nat Meth. 2017;14:1083–1086. doi: 10.1038/nmeth.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg T, et al. A draft network of ligand-receptor-mediated multicellular signalling in human. Nature Communications. 2016;6:1–11. doi: 10.1038/ncomms8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler A, Hoffman P, Smibert P, Papalexi W, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tirosh I, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2019:1–10. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Love NR, et al. pTransgenesis: a cross-species, modular transgenesis resource. Development. 2011;138:5451–5458. doi: 10.1242/dev.066498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Curado S, et al. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- 43.Ogino H, McConnell WB, Grainger RM. High-throughput transgenesis in Xenopus using I-SceI meganuclease. Nat Protoc. 2006;1:1703–1710. doi: 10.1038/nprot.2006.208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This Excel file contains a summary of each single-cell RNA-sequencing experiment. Samples are labelled depending on their developmental stage reflective of their regenerative ability and days post amputation status. Sequencing batch, numbers of sequenced cells, mean reads per cell, median genes per cell, median UMI counts per cell, total genes detected, and fraction of reads in cells are reported.
This Excel file lists ROCs marker genes that have reported mRNA in situ hybridization results in the literature. These reported findings are provided with their PMID, or PMCID, or DOI, or Xenbase link.
This Excel file contains the gene lists that are used for cell cycle analysis, and scGSEA analysis. It also includes ROCs marker genes.