Abstract
Background:
Smoking cessation is especially critical for smokers with diabetes to reduce their overall markedly elevated risk of cardiovascular disease (CVD) and premature mortality, although the role of weight change in the long-term health consequence of smoking cessation remains to be characterized. This study aimed to examine smoking cessation and subsequent weight change in relation to incident cardiovascular events and all-cause and cause-specific mortality among adults with diabetes.
Methods:
This prospective analysis included 10,809 men and women with diabetes, who were either current smokers or never smokers without CVD or cancer at diabetes diagnosis. Information on demographics, newly diagnosed diseases, medical history, and lifestyle factors, including smoking status and weight change, was updated every two years through validated questionnaires.
Findings:
During 153,166 and 152,811 person-years of follow-up, 2,580 incident cases of CVD and 3,827 deaths occurred among participants with diabetes. Compared with those who continued to smoke, recent quitters (2–6 consecutive years since smoking cessation) without weight gain within the first 6 years of quitting had a significantly lower risk of CVD and CHD. The multivariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for CVD were 0.83 (0.70–0.99) among all recent quitters, 0.77 (0.62–0.95) among recent quitters without weight gain, 0.99 (0.70–1.41) among recent quitters with weight gain of 0.1 to 5.0 kg, 0.89 (0.65–1.23) among recent quitters with weight gain of more than 5.0 kg, and 0.72 (0.61–0.84) among longer-term quitters (>6 consecutive years since smoking cessation). In addition, weight gain within 6 years following smoking cessation did not attenuate the inverse relationship between long-term quitting and mortality among diabetes patients. The multivariate-adjusted HRs (95% CIs) for mortality were 0.69 (0.58–0.82) among long-term quitters without weight gain within 6 years following cessation, 0.57 (0.45–0.71) among long-term quitters with weight gain of 0.1 to 5.0 kg, and 0.51 (0.42–0.62) among long-term quitters with weight gain of more than 5.0 kg. Similar results were observed for CVD and cancer mortality.
Interpretation:
Smoking cessation without subsequent weight gain is significantly associated with a lower CVD incidence and mortality among smokers with diabetes. Weight gain following smoking cessation attenuates the reductions in the risk of developing CVD, but not premature death, among diabetic smokers who quit smoking.
Introduction
Lifestyle modification plays a fundamental role in the prevention and management of diabetes.1 In particular, for diabetes patients who smoke, the risk of developing cardiovascular disease (CVD) and other morbidities is significantly boosted by both smoking and hyperglycemia.1,2 Therefore, smoking cessation is strongly advised for this group of patients.1,3 However, smoking cessation is often accompanied by weight gain,4 which is a potential health concern for diabetes patients because it might result in poor diabetes control and increased risk of developing diabetes complications.5,6 The knowledge base is limited regarding whether weight gain following smoking cessation attenuates the long-term reductions in the risk of primary diabetes complications associated with quitting, and previous studies generated inconsistent findings.7,8 Nonetheless, these existing studies were limited by small sample size7,8 and the use of point prevalence abstinence which could not reflect dynamic changes in smoking status during follow-up.8
To address the limitations in previous studies and to further elucidate the role of weight gain in smoking cessation and CVD risk and mortality in diabetes patients, we investigated smoking cessation and weight change in relation to subsequent risk of total CVD, CHD, and stroke, and all-cause and cause-specific mortality among adults with diabetes who participated in the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS).
Methods
Study Population
The NHS, established in 1976, is an ongoing prospective cohort study with an enrollment of 121,700 female nurses aged 30 to 55 years from 11 U.S. states.9 The HPFS, initiated in 1986, is an ongoing prospective cohort study with an enrollment of 51,529 male health professionals aged 40 to 75 years from 50 U.S. states.10 Detailed information on lifestyle factors and medical conditions was updated every two years through validated questionnaires. Follow-up rates exceeded 90% in both cohorts. More details have been described previously.11,12
For the current study, we included participants with prevalent diabetes in the beginning of the cohorts (1976 for the NHS and 1986 for the HPFS), and incident diabetes cases diagnosed during follow-up through 2014. Given our aim of evaluating weight change and disease risk upon incident smoking cessation after diabetes diagnosis, we only considered diabetes patients who were either current smokers or never smokers at diabetes diagnosis, and excluded past smokers at diabetes diagnosis. We excluded participants who had cancer, CVD, or chronic obstructive pulmonary disease (COPD) before diabetes diagnosis. The exclusion of these diseases helps to reduce the probability that participants quit smoking due to existing diseases. For all analyses, participants with missing smoking information in two or more consecutive cycles were also excluded. Finally, 10,809 and 9,688 participants were included in the incident CVD analysis and mortality analysis, respectively (Supplementary Figure 1).
The present study was approved by the Institutional Review Boards at the Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital, and the return of completed questionnaires was considered implied consent.
Assessment of Smoking Status and Weight Change
In each two-year survey cycle, we identified quitters as those who reported as smokers in the previous cycle but as past smokers in the current cycle, assuming the beginning of previous cycle as the onset of quitting. In a sensitivity analysis, the start of quitting was defined as the beginning of the cycle that participants first reported being past smokers. Smoking cessation duration was counted from the onset of quitting to the relapse of smoking, occurrence of study outcomes, or the end of follow-up (Supplementary Table 1). Previous studies have demonstrated that weight gain related with smoking cessation primarily occurs in approximately the first 6 years since quitting.13,14 Based on this knowledge, quitters’ status was mutually-exclusively defined as transient quitters (past smokers who reported as current smokers in the immediately-preceding and the immediately-following cycle), recent quitters (quitting 2–6 consecutive years) and long-term quitters (>6 consecutive years). Of note, all analyses were based on person-times in these “time windows” since quitting. We replaced missing smoking information by valid assessments in the previous cycle only to ensure that changes in smoking status were recent. In our cohorts, self-reported smoking status and body weight have been demonstrated to be highly accurate.15,16
We focused on weight change within 6 years after quitting, which was most relevant to smoking cessation.13,14 In a sensitivity analysis, weight change within 4 years or 8 years after quitting was also examined. Missing body weight estimates were replaced with last available values (<5%). Weight changes were grouped into ≤0 kg, 0.1–5.0 kg, and >5.0 kg.14 In a sensitivity analysis, we grouped participants by tertiles of weight change to test the robustness of associations.
Ascertainment of Type 2 Diabetes
Participants who reported a physician’s diagnosis of diabetes on any of the biennial questionnaires were mailed a validated supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy. Prior to the release of the American Diabetes Association criteria in 1997, the National Diabetes Data Group criteria were used to diagnose diabetes. Hemoglobin A1c ≥6.5% was further included in the diagnosis criteria after 2010. More details have been documented elsewhere.17
Ascertainment of CVD and Mortality
Incident CVD was defined as fatal and non-fatal CHD (including nonfatal myocardial infarction [MI] and coronary artery bypass graft surgery) or percutaneous coronary intervention (PCI) and fatal and non-fatal stroke. Permission was requested to review medical records when participants reported non-fatal CVD events on any biennial questionnaires. All medical records were reviewed by physicians, who were blinded to the participant questionnaire data, to confirm or refute non-fatal CVD events. Deaths were identified by reports by next of kin, U.S. postal authorities, or searching the National Death Index. Fatal CVD events were identified and confirmed/refuted through reviewing hospital records, death certificates, or autopsy reports. CVD mortality was defined using ICD-9 (International Classification of Diseases-Ninth Revision) codes of 390–459 and cancer mortality using ICD-9 codes of 140–208. More details have been described previously.18
Assessment of Covariates
In the biennial follow-up questionnaires, information was updated on demographics, physical activity, alcohol consumption, menopausal status and use of postmenopausal hormones (women only), medical history (including use of aspirin and lipid-lowering medication), family history of MI or cancer, presence of hypertension, hypercholesterolemia, CVD, cancer, or other diseases. Body mass index (BMI) was calculated as self-reported weight in kilograms divided by the square of height in meters (kg/m2). Physical activity was estimated as metabolic equivalents (METs) per week based on the average hours spent on various activities, weighted by the intensity level.17 Dietary intake was assessed using validated semi-quantitative food frequency questionnaire (FFQ) with approximately 131 food items administered every 2–4 years.17 The overall diet quality was assessed using the Alternative Health Eating Index (AHEI) score (ranging from 10–110, with higher scores indicating a healthier diet).
Statistical Analysis
The primary analyses consisted of analyses that evaluated CVD incidence and those examined mortality. For incident CVD analyses, we focused on CVD risk during both recent quitting time window (2–6 consecutive years since quitting) and long-term quitting time window (>6 consecutive years of quitting). For mortality analysis, to minimize the potential, serious reverse causation bias, i.e., deaths due to some severe diseases that result in both smoking cessation and weight loss, we focused on mortality in the long-term quitting time window and excluded participants who died within 6 years after quitting smoking, leaving 9,688 individuals for mortality analyses (Supplementary Figure 1). As such, we explicitly addressed the question “Does weight change incurred by smoking cessation influence long-term survival among diabetes patients who have survived the first 6 years of quitting?” In a secondary analysis, we nevertheless examined mortality during the first 2–6 years after quitting, by weight change during the same period.
Person-time was calculated from the date of a diabetes diagnosis to occurrence of study outcomes, last return of a valid follow-up questionnaire, or the end of follow-up (June 30, 2014 for the NHS and January 30, 2014 for the HPFS), whichever came first. For CVD analysis, follow-up was further censored on the incidence of cancer because this disease is likely to lead to changes in both smoking status and body weight. For mortality analysis, we stopped updating smoking status after diagnosis of CVD, cancer, or COPD to minimize the reverse-causation bias caused by the “ill quitter effect”.
Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of smoking cessation and weight change with total CVD, CHD, and stroke incidence, and all-cause and cause-specific mortality. In multivariate models, we adjusted for age (years), diabetes duration (years), sex (men or women), Caucasian (yes/no), BMI assessed shortly before diabetes diagnosis (<25.0, 25.0–29.9, 30.0–35.0, >35.0 kg/m2), physical activity (in quintiles), alcohol consumption (0, 0.1–4.9, 5.0–14.9, ≥15.0 g/day), total energy (in quintiles), AHEI (in quintiles), family history of MI before 60 years of age (yes, no), family history of cancer (yes, no; for mortality analysis only), current aspirin use (yes, no), current multivitamin use (yes, no), presence of hypertension (yes, no), presence of hypercholesterolemia (yes, no), and diabetes medication use (insulin, oral medication, or others). Time-varying covariates were considered in the multivariate models, except sex, race, BMI assessed shortly before diabetes diagnosis, family history of MI and cancer, for which baseline assessment was adjusted. In the current study the proportional hazards assumption was tested by using a likelihood ratio test comparing models with and without multiplicative interaction terms between exposure and calendar year, and we did not find evidence of violation of the proportional hazards assumption. A cubic spline regression model with 3 knots was fitted to examine the association between smoking cessation duration and study outcomes. Tests for nonlinearity were based on the likelihood ratio test comparing two models: one with only the linear term and the other with the linear and the cubic spline terms.
Several sensitivity analyses were conducted to test the robustness of our findings. First, although most women in our study were postmenopausal, menopausal status and use of postmenopausal hormones (premenopausal, postmenopausal never users, postmenopausal past users, or postmenopausal current users) were further included in the model. Second, we further adjusted for other medication use, including anti-hypertensive drugs and lipid-lowering agents. Finally, we excluded participants who lost more than 30 kg of body weight after smoking cessation within 6 years to minimize the potential reverse causation bias.
All statistical analyses were performed with SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina). Two-sided P<0.05 was considered statistically significant.
Results
Table 1 shows the characteristics according to person-years by smoking status for incident CVD analysis. Compared with current smokers’ person-time, quitters’ person-time was associated with older age, a higher prevalence of hypertension, a higher physical activity level, and a better diet quality. Among recent quitters, compared with those who did not gain weight within 6 years after quitting, patients who gained weight tended to be older, and had a higher prevalence of hypercholesterolemia, a lower prevalence of family history of MI, and a lower diet quality (Table 1). Supplementary Table 2 demonstrates the characteristics for mortality analysis.
Table 1.
Characteristics of person-years according to smoking status among participants with diabetes in the NHS and HPFS
| Characteristic | Current smokers | Recent quitters according to weight gain within 6 years after quitting | Long-term quitters | Transient quitters | Never smoked | ||
|---|---|---|---|---|---|---|---|
| ≤0 kg | 0.1–5.0 kg | >5.0 kg | |||||
| Person-years* | 20,983 | 8,471 | 1,818 | 2,154 | 16,742 | 600 | 102,398 |
| Age (years) | 59.2 (9.9) | 59.9 (11.2) | 61.2 (9.6) | 61.6 (9.0) | 66.7 (9.6) | 59.5 (9.5) | 66.0 (10.6) |
| BMI shortly before diabetes diagnosis (kg/m2) | 28.7 (6.0) | 29.8 (6.6) | 27.8 (5.8) | 29.4 (6.3) | 29.6 (5.9) | 29.4 (5.6) | 30.7 (6.0) |
| Race, % | |||||||
| White | 95.7 | 94.8 | 94.7 | 95.1 | 94.1 | 91.4 | 92.4 |
| Black | 1.3 | 1.6 | 1.8 | 1.8 | 2.0 | 3.0 | 2.5 |
| Asian | 1.6 | 1.8 | 2.0 | 2.1 | 1.9 | 2.8 | 2.7 |
| Other | 1.4 | 1.8 | 1.5 | 1.0 | 2.0 | 2.7 | 2.4 |
| Self-reported hypertension, % | 63.4 | 64.6 | 64.4 | 68.4 | 67.7 | 72.6 | 70.1 |
| Self-reported high cholesterol, % | 58.1 | 50.6 | 57.3 | 67.3 | 60.9 | 58.0 | 60.0 |
| Family history of MI, % | 29.9 | 28.5 | 28.0 | 26.6 | 29.8 | 27.3 | 26.5 |
| Multivitamin use, % | 36.4 | 35.0 | 40.9 | 38.1 | 43.7 | 36.4 | 41.4 |
| Physical activity (MET-hours/week) | 5.0 (1.0,15.2) | 7.5 (2.3,20.6) | 8.4 (2.5,20.2) | 5.9 (1.5,15.4) | 7.7 (2.0,20.2) | 4.2 (0.9,15.4) | 7.4 (2.1,18.7) |
| Alternative healthy eating index | 51.3 (11.4) | 53.8 (12.2) | 52.8 (11.0) | 52.0 (10.9) | 55.3 (11.8) | 52.4 (10.5) | 53.9 (11.8) |
| Alcohol consumption (g/day) | 0.0 (0.0,4.7) | 0.0 (0.0,5.3) | 0.0 (0.0,5.5) | 0.0 (0.0,2.7) | 0.0 (0.0,3.8) | 0.0 (0.0,3.2) | 0.0 (0.0,1.2) |
| Total energy intake (kcal/day) | 1624.1 (581.5) | 1672.7 (566.9) | 1675.0 (535.0) | 1669.1 (561.8) | 1704.5 (560.8) | 1573.1 (579.8) | 1694.9 (568.1) |
Person-years are based on the analyses for CVD. Values are means (SD), median (interquartile range) or percentages; NHS: Nurses’ Health Study; HPFS: Health Professionals’ Follow-Up Study; MI: myocardial infarction; MET: metabolic equivalent tasks. Recent quitters were defined as patients who had quitted smoking for 2–6 consecutive years. Long-term quitters were defined as patients who had quitted smoking for more than 6 consecutive years. Transient quitters were defined as patients who reported being past smokers in the current 2-year survey cycle but being current smokers in the previous and next cycles.
During 153,166 person-years of follow-up, we documented 2,580 incident cases of CVD (including 607 stroke cases). In all study participants, 2,633 (24.3%) were current smokers at diabetes diagnosis. Among these individuals, the percentage of quitting smoking at least once was 72.4% in the NHS (<6% quit more than once) and 64.5% in the HPFS (<2% quit more than once). The median post-cessation weight gain within 6 years was 3.2 (interquartile range: −0.9, 6.8) kg. Compared with those who continued to smoke, recent quitters without weight gain within the 2–6 years since quitting had a significantly lower risk of CVD (Table 2). After multivariate adjustment including diabetes duration, BMI assessed shortly before diabetes diagnosis, other lifestyle and dietary factors, and medication use, the HRs (95% CIs) for CVD were 0.83 (0.70–0.99) among all recent quitters, 0.77 (0.62–0.95) among recent quitters without weight gain, 0.99 (0.70–1.41) among recent quitters with weight gain of 0.1 to 5.0 kg, 0.89 (0.65–1.23) among recent quitters with weight gain of more than 5.0 kg, and 0.72 (0.61–0.84) among longer-term quitters. Supplementary Table 3 shows the association between long-term quitting and CVD risk according to weight change within 6 years after quitting. Inverse associations were observed across groups with various weight change, although some of the associations did not achieve statistical significance probably due to lower statistical power. Figure 1 shows a linear inverse relationship between duration of smoking cessation and CVD incidence among participants with diabetes (P linearity <0.01). Similar patterns of associations were observed for CHD and stroke (Table 2), although some of the associations did not achieve statistical significance, likely due to limited power.
Table 2.
Hazard ratios for association between smoking cessation and the incidence of CVD, CHD, and stroke among individuals with diabetes
| Current smokers | Recent quitters | Recent quitters according to weight gain within 6 years after quitting | Long-term quitters (> 6 years) | Transient quitters | Never smoked | |||
|---|---|---|---|---|---|---|---|---|
| ≤0 kg | 0.1–5.0 kg | >5.0 kg | ||||||
| CVD | ||||||||
| Person-years | 20,983 | 12,443 | 8,471 | 1,818 | 2,154 | 16,742 | 600 | 102,398 |
| Cases | 454 | 231 | 143 | 40 | 48 | 389 | 9 | 1,497 |
| Crude incident rate per 1000 person-years | 21.64 | 18.56 | 16.88 | 22.00 | 22.28 | 23.23 | 15.00 | 14.62 |
| Multivariable adjusted | 1.00 | 0.83 (0.70, 0.99) | 0.77 (0.62, 0.95) | 0.99 (0.70, 1.41) | 0.89 (0.65, 1.23) | 0.72 (0.61, 0.84) | 0.63 (0.30, 1.32) | 0.59 (0.53, 0.67) |
| CHD | ||||||||
| Cases | 355 | 185 | 119 | 33 | 33 | 319 | 8 | 1,134 |
| Crude incident rate per 1000 person-years | 16.92 | 14.87 | 14.05 | 18.15 | 15.32 | 19.05 | 13.33 | 11.07 |
| Multivariable adjusted | 1.00 | 0.82 (0.67, 1.00) | 0.80 (0.63, 1.00) | 1.02 (0.69, 1.50) | 0.75 (0.51, 1.11) | 0.78 (0.66, 0.94) | 0.82 (0.38, 1.73) | 0.58 (0.50, 0.66) |
| Stroke | ||||||||
| Cases | 102 | 49 | 26 | 8 | 15 | 70 | 2 | 383 |
| Crude incident rate per 1000 person-years | 4.86 | 3.93 | 3.07 | 4.40 | 6.96 | 4.18 | 3.33 | 3.74 |
| Multivariable adjusted | 1.00 | 0.87 (0.60, 1.27) | 0.70 (0.44, 1.12) | 1.03 (0.48, 2.23) | 1.30 (0.72, 2.37) | 0.51 (0.36, 0.73) | 0.33 (0.04, 2.53) | 0.67 (0.52, 0.86) |
Adjusted for age (years), diabetes duration (years), sex (male, female), race (white, non-white), body mass index shortly before diabetes diagnosis (<25.0, 25.0–29.9, 30.0–35.0, or >35.0 kg/m2), physical activity (in quintiles), alcohol intake (0, <5.0, 5.0–14.9, or ≥15.0 g/day), total energy (in quintiles), Alternative Healthy Eating Index score (in quintiles), family history of diabetes (yes, no), family history of myocardial infarction (yes, no), current aspirin use (yes, no), and current multivitamin use (yes, no), presence of hypertension (yes, no), presence of hypercholesterolemia (yes, no), and diabetes medication use (oral hypoglycemic medication, insulin, or other).
Figure 1. Association between duration of smoking cessation and CVD incidence among individuals with diabetes*.
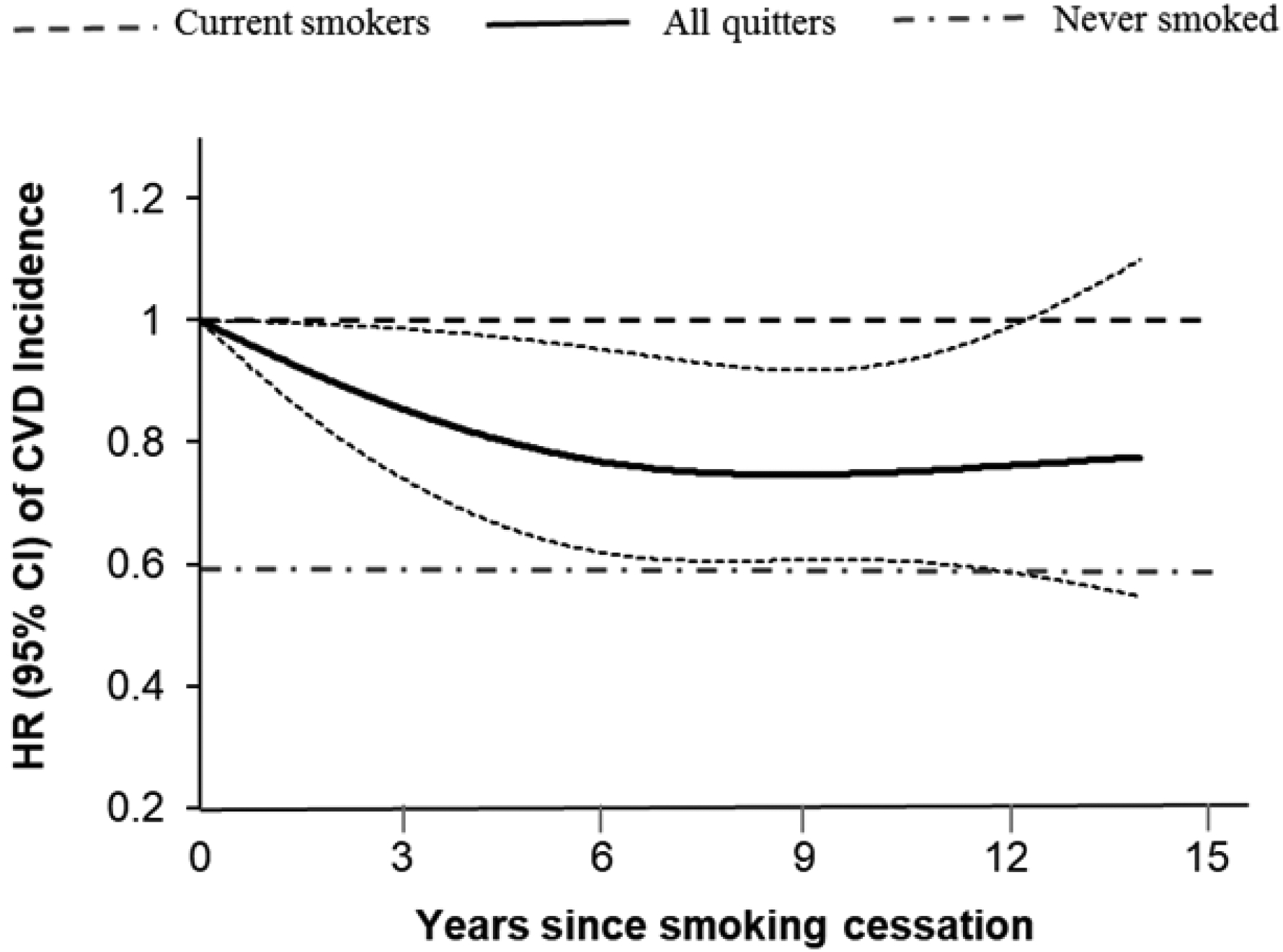
*Adjusted for age (years), diabetes duration (years), sex (male, female), race (white, non-white), BMI shortly before diabetes diagnosis (<25.0, 25.0–29.9, 30.0–35.0, or >35.0 kg/m2), physical activity (in quintiles), alcohol intake (0, <5.0, 5.0–14.9, or ≥15.0 g/day), total energy (in quintiles), Alternative Healthy Eating Index score (in quintiles), family history of diabetes (yes, no), family history of myocardial infarction (yes, no), current aspirin use (yes, no), current multivitamin use (yes, no), presence of hypertension (yes, no), presence of hypercholesterolemia (yes, no), and diabetes medication use (oral hypoglycemic medication, insulin, or other).
During 152,811 person-years of follow-up, we documented a total of 3,827 deaths. Of note, in this analysis, we focused on long-term quitters, i.e., quitting for more than 6 years, and their mortality after they survived the first 6 consecutive years of quitting. Weight gain within 6 years after smoking cessation did not attenuate the inverse association between long-term quitting and mortality (Table 3). Compared with those who continued to smoke, the multivariate-adjusted HRs (95% CIs) for all-cause mortality were 0.69 (0.58–0.82) among long-term quitters without weight gain within 6 years after quitting, 0.57 (0.45–0.71) among long-term quitters with weight gain of 0.1 to 5.0 kg, and 0.51 (0.42–0.62) among long-term quitters with weight gain of more than 5.0 kg (Table 3). In a sensitivity analysis, person-time upon smoking cessation was grouped into weight gain ≤0 kg or >0 kg, and similar results were observed: compared with those who continued to smoke, the multivariate-adjusted HRs (95% CIs) for all-cause mortality were 0.69 (0.58–0.82) among long-term quitters without weight gain within 6 years after quitting, 0.54 (0.45–0.63) among long-term quitters with weight gain. Figure 2 shows a linear relationship between duration of smoking cessation and all-cause mortality among participants with diabetes (P linearity <0.001).
Table 3.
Hazard ratios for association between smoking cessation and all-cause and cause-specific mortality among individuals with diabetes after excluding those who died within 6 years after quitting*
| Current smokers | Long-term quitters according to weight gain within 6 years after quitting | Never smoked | |||
|---|---|---|---|---|---|
| ≤0 kg | 0.1–5.0 kg | >5.0 kg | |||
| All-cause mortality | |||||
| Person-years | 23,921 | 5,184 | 2,839 | 3,806 | 117,061 |
| Cases | 721 | 269 | 121 | 156 | 2,560 |
| Crude incident rate per 1000 person-years | 30.14 | 51.89 | 42.62 | 40.99 | 21.87 |
| Multivariable adjusted | 1.00 | 0.69 (0.58, 0.82) | 0.57 (0.45, 0.71) | 0.51 (0.42, 0.62) | 0.40 (0.36, 0.44) |
| CVD mortality | |||||
| Cases | 242 | 98 | 45 | 45 | 874 |
| Crude incident rate per 1000 person-years | 10.11 | 18.90 | 15.85 | 11.82 | 7.47 |
| Multivariable adjusted | 1.00 | 0.84 (0.63, 1.12) | 0.66 (0.46, 0.95) | 0.47 (0.32, 0.67) | 0.45 (0.38, 0.53) |
| Cancer mortality | |||||
| Cases | 205 | 58 | 24 | 36 | 507 |
| Crude incident rate per 1000 person-years | 8.57 | 11.19 | 8.45 | 9.46 | 4.33 |
| Multivariable adjusted | 1.00 | 0.71 (0.50, 0.99) | 0.53 (0.33, 0.84) | 0.55 (0.37, 0.83) | 0.33 (0.27, 0.39) |
There were no cases among transient quitters.
Adjusted for age (years), diabetes duration (years), sex (male, female), race (white, non-white), BMI shortly before diabetes diagnosis (<25.0, 25.0–29.9, 30.0–35.0, or >35.0 kg/m2), physical activity (in quintiles), alcohol intake (0, <5.0, 5.0–14.9, or ≥15.0 g/day), total energy (in quintiles), Alternative Healthy Eating Index score (in quintiles), family history of diabetes (yes, no), family history of myocardial infarction (yes, no), family history of cancer (yes, no), current aspirin use (yes, no), and current multivitamin use (yes, no), presence of hypertension (yes, no), presence of hypercholesterolemia (yes, no), and diabetes medication use (oral hypoglycemic medication, insulin, or other).
Figure 2. Association between duration of smoking cessation and all-cause mortality among individuals with diabetes*.
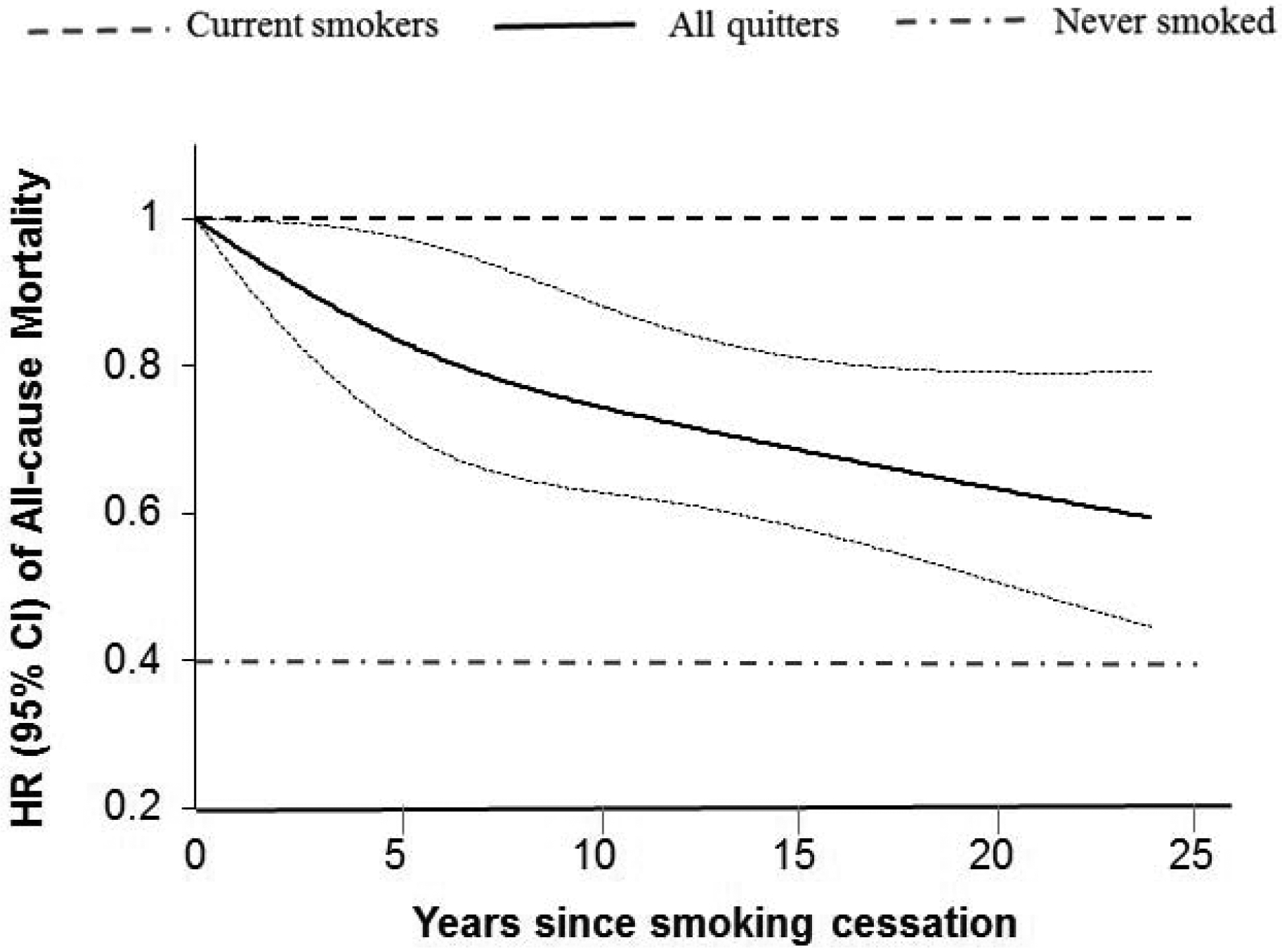
*Adjusted for age (years), diabetes duration (years), sex (male, female), race (white, non-white), BMI shortly before diabetes diagnosis (<25.0, 25.0–29.9, 30.0–35.0, or >35.0 kg/m2), physical activity (in quintiles), alcohol intake (0, <5.0, 5.0–14.9, or ≥15.0 g/day), total energy (in quintiles), Alternative Healthy Eating Index score (in quintiles), family history of diabetes (yes, no), family history of myocardial infarction (yes, no), family history of cancer (yes, no), current aspirin use (yes, no), current multivitamin use (yes, no), presence of hypertension (yes, no), presence of hypercholesterolemia (yes, no), and diabetes medication use (oral hypoglycemic medication, insulin, or other).
In a secondary analysis among recent quitters, we modeled the association between smoking cessation and mortality within the first 2–6 years since quitting (without excluding those who died within 6 years after quitting) (Supplementary Table 4). Compared with those who continued to smoke, the multivariate-adjusted HRs (95% CIs) for all-cause mortality were 0.84 (0.71–0.99) among recent quitters without weight gain within 6 years after quitting, 0.73 (0.43–1.24) among recent quitters with weight gain of 0.1 to 5.0 kg, and 0.46 (0.26–0.83) among recent quitters with weight gain of more than 5.0 kg. Similar patterns of associations were observed for CVD and cancer mortality (Table 3).
Similar results were observed when recent quitters were defined as quitting for 2–4 or 2–8 consecutive years rather than 2–6 years (Supplementary Tables 5 and 6), when tertiles of weight change after cessation were used (Supplementary Tables 7 and 8), when the start of cessation was defined as the beginning of the cycle that participants first reported being past smokers (Supplementary Tables 9 and 10), when menopausal status and use of postmenopausal hormones, or anti-hypertensive drugs and lipid-lowering agents were further adjusted, or participants who lost more than 30 kg of body weight after smoking cessation within 6 years were excluded (data not shown).
Discussion
In two large prospective cohort studies among U.S. men and women with diabetes, smoking cessation without weight gain was associated with a lower risk of incident CVD and all-cause and cause-specific mortality. Post-cessation weight gain attenuated the inverse associations with CVD incidence, although the long-term benefits of quitting on lowering mortality were independent of weight change following smoking cessation. These associations were independent of established risk factors, including diabetes duration, BMI before diabetes diagnosis, lifestyle and dietary factors, and medication use.
Smoking is a leading cause of many chronic diseases and premature death, and quitting smoking substantially reduces these risks.19,20 Smoking cessation is particularly important for smokers with diabetes because their risk of developing CVD or other morbidities is substantially augmented by both smoking and insulin resistance or glycemia.1,2 However, smoking cessation is often accompanied by weight gain (e.g., mean weight gain of 4.0–5.0kg after 1 year of abstinence),4 which is a risk factor for cardiometabolic diseases and might dilute the health benefits of quitting.6 Previous studies among apparently healthy individuals found that excessive weight gain (e.g., 5 kg or more) following smoking cessation could lead to a short-term (e.g., 5–7 years after quitting) increase in developing diabetes,14,21 deterioration in cardiovascular risk factors,22 and attenuation of the protective effect on cancer,23 although other studies suggested that weight gain after quitting did not modify the associations of smoking cessation with MI, stroke, and mortality in the general population.14,24,25 Our previous analysis in largely healthy individuals demonstrated that weight gain was associated with a transient, increased risk of developing type 2 diabetes risk, although the reduction in CVD mortality and total mortality was independent of weight gain.14
Evidence regarding the inter-relationship between smoking cessation, weight change, and cardiometabolic consequences in diabetes patients is relatively sparse. In comparison with the general population, diabetes patients are at a particularly high risk of developing cardiovascular disease, which is the primary diabetes complication, as well as premature deaths.26 Some studies have suggested that among diabetes patients smoking cessation could also result in short-term weight gain, temporary deterioration in glycemic control and worsening of some diabetic symptoms.27 Evidence regarding whether weight gain following cessation would attenuate the benefits of quitting smoking on CVD events among diabetes patients is inconsistent.7,8 Among 445 adults with diabetes from the Framingham Offspring Study, Clair et al., found that compared with participants who continued to smoke, recent quitters (≤4 years) and long-term quitters (>4 years) had a non-significant lower risk of CVD events, and the results were largely unchanged when further adjusting for post-cessation weight change.7 In contrast, among 6,338 postmenopausal women with diabetes who participated in the Women’s Health Initiative cohort, Luo et al., found that weight gain after smoking cessation mitigated the association between quitting and CHD, especially for diabetes patients who gained 5 kg or more, although only 8 CHD cases were identified among recent quitters.8 In the current study, with longitudinal, repeated assessments of smoking status and body weight during three decades of follow-up in two large prospective cohort studies, we observed inverse association between smoking cessation and CVD risk among quitters who did not gain weight, whereas among quitters who gained body weight this association was not apparent. This observation is in line with accumulating evidence suggesting that excess weight gain could result in alterations in lipids, blood pressure, coagulation, and inflammation, and subsequently endothelial dysfunction and atherosclerosis.28
To date, no study has examined whether weight gain after smoking cessation would influence the reductions in all-cause and cause-specific mortality observed among smokers with diabetes. Considering the potential reverse causation bias in mortality analysis (i.e., diabetes patients who died soon after quitting were likely to have a severe disease that resulted in both smoking cessation and weight loss), our analysis was conducted among long-term quitters who had survived the first 6 years after quitting. We found that long-term quitters had a substantial lower all-cause mortality and CVD and cancer mortality, regardless of weight gain within the first 6 years after quitting. Interestingly, long-term quitters with weight gain >5 kg seemed to have a lower mortality than those with weight gain of 0.1–5 kg or without weigh gain, although a cautious interpretation is warranted as this may still reflect reverse causality to certain extent. Nevertheless, these data suggested that weight gain did not attenuate the reductions in mortality observed following long-term quitting, and preventing excessive weight gain might maximize the health benefits of smoking cessation on reducing CVD complications among diabetes patients. These findings are of great public health significance, given that the fear of post-cessation weight gain is the main reason for not attempting to quit smoking or for relapsing after a quick attempt.6,29
The strengths of the present study include a prospective design, relatively large study populations comprised of both men and women, repeated assessments of smoking status and body weight after diabetes diagnosis, long-term follow-up with a high retention rate, careful adjustments for a multitude of potential risk factors, and analyses of several adjudicated disease outcomes including total CVD, all-cause mortality, and cause-specific mortality.
Several limitations should be considered as well. First, our study participants were all health professionals, and most were Caucasians. Although the relative homogeneity in socioeconomic status and race/ethnicity potentially minimizes confounding by these factors, it also limits generalizability of our findings to other groups with different racial/ethnic or other characteristics. Second, measurement errors in self-reported assessments of smoking status and body weight were inevitable, although the high accuracy of the self-reports has been demonstrated in our validation studies.15,16 Third, the exact date of quitting smoking was not available and therefore the assessments of smoking cessation duration and related weight changes were subject to misclassification. In this prospective study, such measurement errors were likely to be non-differential and more likely to bias the associations toward the null,30 although we cannot rule out the possibility that errors in the measurement confounders may also bias the true association away from the null. Fourth, if quitters underreported weight gain or if quitters relapsed but reported continued cessation due to social-desirability bias, the true association may have been attenuated towards the null because these participants still had an increased disease risk. In addition, we might not capture relapses between two examinations. Fifth, our study did not have direct measurements of glycemic control and severity of diabetes, although the results did not change significantly when we adjusted for duration of diabetes or use of insulin and hypoglycemic medications. In addition, the current study did not acquire information of nicotine use, vaping, or other smoking cessation interventions, the impact of which on associations of interest requires further investigations. Sixth, we did not have enough power to test whether sex difference exists, which warrants further investigation. Lastly, the role of confounding by genetic susceptibility or psychosocial stress, residual confounding due to measurement errors of covariates, or chance in the present study could not be entirely excluded.
Conclusions
Findings from these two large prospective cohort studies suggest that smoking cessation without subsequent weight gain is significantly associated with a lower risk of total CVD and CHD, as well as premature death, among individuals with diabetes. In addition, weight gain after smoking cessation attenuates the inverse association with CVD incidence, although the reduction of all-cause and cause-specific mortality persists in quitters who gain weight. These data provide evidence that not only supports the overall health benefits of quitting smoking in the prevention of morbidity and mortality among individuals with diabetes, but also emphasizes the importance of weight management after smoking cessation to maximize the health benefits of smoking cessation.
Supplementary Material
Research in context.
Evidence before this study:
We searched PubMed, Web of Knowledge, and Google Scholar for articles published up to March 31, 2019. In two existing studies thus far, one study reported that smoking cessation was non-significantly associated with a lower risk of CVD among diabetes patients, which was independent of weight change, whereas the other study demonstrated that the association with coronary heart disease was attenuated by post-cessation weight gain. These existing studies shared some common limitations, such as small sample size and use of point prevalence abstinence, which could not reflect dynamic changes in smoking status during follow-up.
Added value of this study:
With a relatively large sample size and repeated assessments of smoking status and body weight after diabetes diagnosis, our study showed that smoking cessation without subsequent weight gain was significantly associated with lower CVD and CHD incidence, as well as lower mortality, among smokers with diabetes. Weight gain following smoking cessation attenuated the beneficial relationship with CVD incidence, but not all-cause and cause-specific mortality, among diabetes patients.
Implications:
These data emphasize the importance of weight control upon smoking cessation in maximizing the cardiovascular health benefits of quitting among smokers with diabetes. Meanwhile, this study also highlights the long-term benefits of smoking cessation on lowering mortality among diabetes patients, regardless of weight change following cessation.
Acknowledgement:
We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-Up Study that contributed data for their valuable contributions.
Funding: This study was sponsored by the U.S. National Institutes of Health, CA186107, CA176726, CA167552, DK082486, HL35464, HL088521, DK058845, U01 CA167552, and HL034594.
Role of the Sponsor: The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. GL, GZ, and AP were former postdoctoral fellows at Harvard T.H. Chan School of Public Health. No disclosure was reported.
Reference
- 1.Cardiovascular Disease and Risk Management. Sec. 9. In Standards of Medical Care in Diabetes-2017. Diabetes Care 2017;40:S75–S87. [DOI] [PubMed] [Google Scholar]
- 2.Al-Delaimy WK, Manson JE, Solomon CG, et al. Smoking and risk of coronary heart disease among women with type 2 diabetes mellitus. Arch Intern Med 2002;162:273–9. [DOI] [PubMed] [Google Scholar]
- 3.Pan A, Wang Y, Talaei M, Hu FB. Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation 2015;132:1795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 2012;345:e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia 2009;52:65–73. [DOI] [PubMed] [Google Scholar]
- 6.Harris KK, Zopey M, Friedman TC. Metabolic effects of smoking cessation. Nat Rev Endocrinol 2016;12:684. [DOI] [PubMed] [Google Scholar]
- 7.Clair C, Rigotti NA, Porneala B, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA 2013;309:1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo J, Rossouw J, Margolis KL. Smoking cessation, weight change, and coronary heart disease among postmenopausal women with and without diabetes. JAMA 2013;310:94–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willett WC, Green A, Stampfer MJ, et al. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med 1987;317:1303–9. [DOI] [PubMed] [Google Scholar]
- 10.Colditz GA, Rimm EB, Giovannucci E, Stampfer MJ, Rosner B, Willett WC. A prospective study of parental history of myocardial infarction and coronary artery disease in men. Am J Cardiol 1991;67:933–8. [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–8. [DOI] [PubMed] [Google Scholar]
- 13.O’Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol 1998;148:821–30. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Zong G, Liu G, et al. Smoking Cessation, Weight Change, Type 2 Diabetes, and Mortality. N Engl J Med 2018;379:623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Delaimy WK, Stampfer MJ, Manson JE, Willett WC. Toenail nicotine levels as predictors of coronary heart disease among women. Am J Epidemiol 2008;167:1342–8. [DOI] [PubMed] [Google Scholar]
- 16.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19:570–2. [PubMed] [Google Scholar]
- 17.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7. [DOI] [PubMed] [Google Scholar]
- 18.Liu G, Li Y, Hu Y, et al. Influence of Lifestyle on Incident Cardiovascular Disease and Mortality in Patients With Diabetes Mellitus. J Am Coll Cardiol 2018;71:2867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 2013;368:341–50. [DOI] [PubMed] [Google Scholar]
- 20.Duncan MS, Freiberg MS, Greevy RA Jr, Kundu S, Vasan RS, Tindle HA. Association of Smoking Cessation With Subsequent Risk of Cardiovascular Disease. JAMA 2019;322:642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo J, Rossouw J, Tong E, et al. Smoking cessation, weight gain, and risk of type 2 diabetes mellitus among postmenopausal women. Arch Intern Med 2012;172:438–40. [DOI] [PubMed] [Google Scholar]
- 22.Yoon C, Goh E, Park SM, Cho B. Effects of smoking cessation and weight gain on cardiovascular disease risk factors in Asian male population. Atherosclerosis 2010;208:275–9. [DOI] [PubMed] [Google Scholar]
- 23.Kim K, Choi S, Lee G, et al. Cancer risk among young men with weight gain after smoking cessation: A population-based cohort study. Cancer Epidemiol 2019;60:86–92. [DOI] [PubMed] [Google Scholar]
- 24.Dinh PC, Schrader LA, Svensson CJ, Margolis KL, Silver B, Luo J. Smoking cessation, weight gain, and risk of stroke among postmenopausal women. Prev Med 2019;118:184–90. [DOI] [PubMed] [Google Scholar]
- 25.Kim K, Park SM, Lee K. Weight gain after smoking cessation does not modify its protective effect on myocardial infarction and stroke: evidence from a cohort study of men. Eur Heart J 2018;39:1523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med 2011;171:404–10. [DOI] [PubMed] [Google Scholar]
- 27.Lycett D, Nichols L, Ryan R, et al. The association between smoking cessation and glycaemic control in patients with type 2 diabetes: a THIN database cohort study. Lancet Diabetes Endocrinol 2015;3:423–30. [DOI] [PubMed] [Google Scholar]
- 28.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006;444:875–80. [DOI] [PubMed] [Google Scholar]
- 29.Klesges RC, Brown K, Pascale RW, Murphy M, Williams E, Cigrang JA. Factors associated with participation, attrition, and outcome in a smoking cessation program at the workplace. Health Psychol 1988;7:575–89. [DOI] [PubMed] [Google Scholar]
- 30.Gullen WH, Bearman JE, Johnson EA. Effects of misclassification in epidemiologic studies. Public Health Rep 1968;83:914–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


