Abstract
Background.
Relapsing-onset MS typically starts in early- to mid-adulthood, yet the trajectory of disease activity over the subsequent lifetime remains poorly defined. Previous studies have not quantified the age-specific portion of decreases in annualized relapse rates (ARR).
Objective.
To determine, under a range of disease-related assumptions, the age-specific component of decreases in ARR over time among adults with relapsing-onset MS.
Methods:
We used a simulation modeling approach to examine a range of assumptions about changes in ARR due to age versus disability status. Scenarios included variations in initial ARR and rate of worsening on the Expanded Disability Status Scale. Model parameters were developed through analysis of MS patients in British Columbia, Canada, and literature review.
Results:
We found a substantial age-specific decrease in ARR in all simulated scenarios, independent of disability worsening. Under a range of clinically plausible assumptions, 88–97% of the decrease was attributed to age and 3–13% to disability. The age-specific decrease ranged from 22–37% per 5 years for a wide range of initial ARR (0.33–1.0).
Conclusion:
Decreases in ARR were due mostly to age rather than disability status. To facilitate informed decision making in MS, it is important to quantify the dynamic relationship between relapses and age.
Search terms: Multiple sclerosis, Relapsing/remitting, Decision support techniques, Deprescriptions, Aging, Epidemiology
Introduction
MS is a chronic neurological condition with no known cure. Life expectancy is only modestly affected in MS,1 yet changes in disease activity across the lifetime are not well understood. The most common form of MS is relapsing-onset MS, and the average age of individuals living with MS is approximately 55–64 years in North America.2,3 However, while participants in key clinical trials typically have relapsing-onset MS, they are considerably younger and largely in their mid to late 30s; adults aged 55 or 60 and older are often ineligible.4
Increasing age is associated with decreasing disease activity, including fewer relapses and new brain lesions measured by MRI.5–9 Past studies have found a particularly low risk of relapses beyond ages 55 or 60.6–8,10 Medications that target the immune system (disease-modifying drugs [DMDs]) may be more efficacious in younger individuals (<40 years).11 DMDs are effective at reducing the number of relapses and new lesions, which characterize the early phase of relapsing-onset MS. As these decrease with age, DMDs may provide less absolute benefit. Therefore, quantifying age-related changes in annualized relapse rates (ARR), as distinct from disability-related changes, could help inform decisions about when DMDs might be stopped. Previous studies have described overall age-related decreases in ARR,6,7 but have not examined the relationship between relapses, age, and disability status.
The objective of our study was to determine, under a range of disease-related assumptions, the age-specific component of the decline in ARR over time among adults with relapsing-onset MS. An understanding of how the ARR changes with age, independently from disease worsening, is important for people of all ages with MS, and their providers, to support healthcare decision making throughout their lives.
Methods
Study design.
We used a simulation modeling approach to quantify the age-specific component of decreases in ARR. This allowed us to examine different assumptions about the relationship between relapses, disability, and age, and to separately quantify the decreases in ARR associated with age versus disability. This study included secondary analyses of existing or aggregated data, as approved by the relevant Institutional Review Boards.
Markov model.
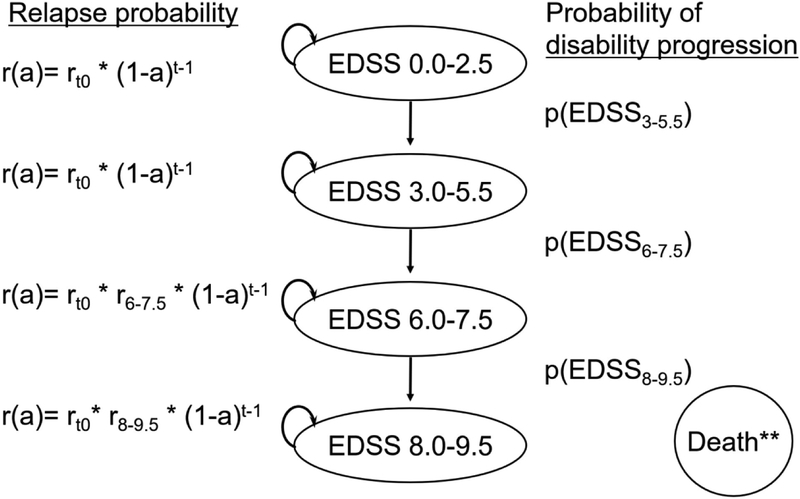
We developed a Markov model to simulate a cohort of people with relapsing-onset MS (Figure 1). We characterized disability by four categories based on the Expanded Disability Status Scale (EDSS), as has been done in prior Markov models designed to project long-term outcomes among this population.12,13 Our model simulated people over their lifetime from MS symptom onset at age 30, approximately the average onset age.14 People progressed through the model only in the direction of increasing disability, which is a simplifying assumption. Because advanced disability is associated with increased mortality risk,15 the risk of MS-related death (beyond age-specific background mortality)16 was only introduced at the highest level of disability represented in the model (EDSS 8–9.5; Table 1). We used TreeAge Pro 2018, R2.0 software.
Figure 1.
Schematic representation of the Markov model for a cohort of adults with relapsing-onset MS. Key: r= annualized relapse rate (ARR); a= age-specific decrease in ARR; rt0= ARR at MS symptom onset; t= years post onset; EDSS= Expanded Disability Status Scale; p(EDSS)= average annual probability of progression (EDSS scores categorized as: 0.0–2.5, 3.0–5.5, 6.0–7.5, 8.0–9.5); r6.0–7.5= ARR at EDSS scores 6.0–7.5 relative to EDSS 0–5.5; r8.0–9.5= ARR at EDSS scores 8.0–9.5 relative to EDSS 0–5.5. ** Age-specific background mortality was applied to simulated individuals at all EDSS scores, with an additional risk of death from MS for individuals at EDSS scores of 8.0–9.5.
Table 1.
Summary parameters for the Markov model simulating a cohort of adults with relapsing-onset MS.
| Model parameter | Estimate | Sources |
|---|---|---|
| Annual probability of progression by EDSS score category | Analyses of a cohort of MS patients from the BCMS database (1980–2009) plus data from the existing MS literature12,13,20,21 | |
| -EDSS 0–2.5 to 3.0–5.5 | 0.0418 | |
| -EDSS 3.0–5.5 to 6.0–7.5 | 0.0843 | |
| -EDSS 6.0–7.5 to 8.0–9.5 | 0.0456 | |
| Annual probability of MS-specific mortality at EDSS scores 8.0–9.5, beyond age-specific background mortality | 0.0675 | Model calibration based on a previously published finding that BCMS patients had a 6-year decrease in life expectancy compared with the general population1 |
| Percent of simulated cohort by EDSS score category during first annual Markov model cycle | Analyses of a cohort of MS patients from the BCMS database | |
| EDSS 0.0–2.5 | 95% | |
| EDSS 3.0–5.5 | 5% | |
| ARR during 5 years post MS symptom onset | 0.51 | Analyses of a cohort of MS patients from the BCMS database |
Key: ARR= annualized relapse rate; BC= British Columbian; EDSS= Expanded Disability Status Scale.
While previous models have typically assumed that the ARR is constant over EDSS scores 0–5.5, followed by no relapses at EDSS scores 6–9.5,12,13,17,18 our model allowed for an annual age-specific decrease in ARR that could also vary by disability status. We developed three versions of the Markov model representing different assumptions about the extent to which the decreases in ARR are due to age versus disability status. In the default model, we assumed that decreases in ARR over time were mostly age-specific. In the secondary model we assumed relatively more of the decreases in ARR were associated with worsening disability. In the replication model, we replicated the structural assumptions used in Markov models in several past studies,12,13,17,18 with a constant ARR from EDSS scores 0–5.5 and no relapses after reaching EDSS 6. The replication was a modified version of the approach in earlier modeling studies because we allowed an age-related decrease in order to fit the decreased ARR observed among older adults.
The structure of the default and secondary models was informed by observational data through an iterative process of model calibration (Appendix). We derived calibration targets from a cohort of MS clinic patients by accessing the British Columbian MS (BCMS) database (details under ‘Cohort and data’). These targets included the ARR in the 5 years post-onset, the ARR by age (focusing on ages 50+) and the ARR by disability status (Tables 1 and 2). Cross sectional analyses of the ARR by age were assumed to be representative of an aging cohort. Observation of the ARR during EDSS 0–2.5 was limited by left censoring, thus we did not include this as a calibration target. After calibration, both models included an ARR that decreased with both increasing age and worsening disability.
Table 2.
ARR by age and by EDSS among adults with relapsing-onset MS in British Columbia, Canada (1980–2009).
| ARR | |
|---|---|
| Age category | |
| Age 50–54 | 0.13 |
| Age 55–59 | 0.09 |
| Age 60–64 | 0.06 |
| Age 65–69 | 0.05 |
| Age 70+ | 0.04 |
| EDSS score categon | |
| EDSS 3.0–5.5 | 0.19 |
| EDSS 6.0–7.5 | 0.12 |
| EDSS 8.0–9.5 | 0.04 |
Key: ARR= annualized relapse rate; EDSS= Expanded Disability Status Scale.
Cohort and data.
The BCMS database represents one of the largest sources of clinical information collected on people with MS prior to the availability of DMDs. The provincial government’s coverage of the first MS-specific DMD (beta-interferon-1b) began in 1996.14 Prospective data collection started from the initial MS clinic visit. These population-based data included, an estimated 80% of people with MS in British Columbia, Canada during the study period.19 The data for this study were available for adults who were first registered with a BCMS clinic from 1980 to 2004, with follow-up data, such as EDSS scores, available through the end of 2009. The cohort (n=2,203) included adults (age ≥18 at their initial visit) with MS confirmed by an MS specialist neurologist based on the prevailing criteria at the time of diagnosis, with a relapsing onset disease course and at least two available disability assessments. In order to reduce potential biases due to excluding people who died or moved away from the sample, no minimum follow-up time was required. For the primary analyses, the study period was 1980–2009.
Relapses.
Relapses were defined by new or worsening symptoms lasting over 24 hours in the absence of fever or infection, as recorded by an MS specialist neurologist at each clinic visit; these included relapses from onset which were captured at the first MS clinic visit. Relapse symptoms occurring within 30 days of a prior relapse were counted as a single relapse. We analyzed the data to describe the ARR in the first 5 years after MS symptom onset (excluding the onset relapse), based on subjects with at least one clinic visit occurring 5 or more years after onset. We tabulated the ARR by 5-year age ranges based on person-years and required a minimum of 1 year of observed data per age group (Table 2). Similarly, we estimated the ARR by disability status with a minimum of 1 year of observed data per disability category.
Disability scores.
During routine MS clinic visits, the neurologist measured disability using the EDSS. We analyzed the BCMS cohort to estimate model parameters for the average probabilities of progression between the disability categories represented in the Markov model (Table 1). Model development was supported by prior literature,12,13,20,21 particularly due to limitations (left censoring) in observing the transition from EDSS 0–2.5 to 3.0–5.5. The primary analytic outcome was the median time from MS onset to EDSS 6.0 (sustained, with no subsequent EDSS scores below 6.0), which is a disability milestone in MS.14 We performed Kaplan-Meier survival analyses of the time from symptom onset to EDSS 6.0 (sustained) and EDSS 8.0 (first occurrence). In the subsamples of individuals who had reached EDSS 3.0 (first occurrence) or EDSS 6.0 (sustained), we analyzed the time from EDSS 3.0 to 6.0 and from EDSS 6.0 to 8.0, respectively. The initial EDSS category of the simulated cohort during the first model cycle was estimated by examining subsamples of people by length of delay between MS onset and their first EDSS measurement.
Age-specific decrease in relapses.
In all three models, we derived an age-specific decline in ARR (“a” in Figure 1), independent of EDSS level. We used the observed ARR among older adults (Table 2) and a range of estimates for the ARR during the 5 years post-onset. The range was based on our descriptive analysis and prior literature, including reviews of the ARR among people in the placebo arms of clinical trials,22,23 decision-analytic models including relapse outcomes,12,13 and longitudinal studies of the ARR among people with relapsing-onset MS.6 The age-specific decrease was assumed to be a linear decrease in ARR with increasing age.
Sensitivity analyses.
For descriptive analyses of the ARR post-onset, we truncated the data at December 1995 to examine the impact of excluding follow-up time during which the ARR may have been influenced by DMD exposure. We varied the rate of disability progression based on previous observations from MS natural history studies and examined a shorter or longer median time to sustained EDSS 6.0 (23 and 36 years, respectively).14
Results
Disability-specific decrease in ARR.
In the default model, in order to match the calibration targets, the ARR decreased only at the highest category of disability represented in the model. Relative to EDSS scores of 0–5.5, we found no decrease in ARR at 6.0–7.5 and a decrease of 66% at 8.0–9.5 (Table 3). In the secondary model, in which we maximized the decrease in ARR due to disability rather than age, the results were similar to those of the default model, with a decrease in ARR of only 6% at EDSS scores 6.0–7.5 and 71% for EDSS scores 8.0–9.5, relative to 0–5.5.
Table 3.
Comparison of model assumptions about annualized relapse rates by age and disability, informed by model calibration.
| EDSS category | Default model | Secondary model | Replication model |
| EDSS 0.0–2.5 | r(a) | r(a) | r(a) |
| EDSS 3.0–5.5 | r(a) | r(a) | r(a) |
| EDSS 6.0–7.5 | r(a) | 0.94r(a) | N/A |
| EDSS 8.0–9.5 | 0.44r(a)b | 0.29r(a) | N/A |
Key: EDSS= Expanded Disability Status Scale; r(a)= annualized relapse rate (ARR) as a function of age-specific decreases in ARR; N/A= no relapses.
These results express the decrease in ARR at higher EDSS score categories as a ratio proportional to the ARR at EDSS 0–5.5. For example: in the default model, the ARR was 44% of the base rate at EDSS scores 8.0–9.5, i.e., the ARR decreased 66% at EDSS scores 8.0–9.5 relative to 0–5.5, beyond age-related decreases).
Age-specific decrease in ARR.
In the default model, for an initial ARR of 0.51, we found an age-specific decrease in ARR of 28% per 5 years (Table 4). Annually, this represents a decrease in ARR of approximately 6% per year. In the secondary model, the age-specific decrease in ARR was similar, at 27% per 5 years. Using the default model, the age-specific decrease in ARR per 5 years remained within 22–37% over a wide range of initial ARR, and results were similar for the secondary model (Table 4). Compared with the other models, the replicated model (no relapses at EDSS scores 6.0–9.5) resulted in a reduced age-specific decrease.
Table 4.
Age-related decrease in ARR per 5 years among simulated cohorts of adults with relapsing-onset MS.
| Initial ARR during the 5 years following MS symptom onset | Default model, reduced relapses by age and EDSS scores 8.0–9.5 | Secondary model, reduced relapses by age and EDSS scores 6.0–9.5 | Replication model, reduced relapses by age and no relapses at EDSS scores 6.0–9.5 |
|---|---|---|---|
| 0.33a | 22% | 21% | 14% |
| 0.51b | 28% | 27% | 21% |
| 0.60c | 30% | 29% | 23% |
| 1.00d | 37% | 36% | 30% |
Key: ARR= annualized relapse rate; EDSS= Expanded Disability Status Scale.
Estimate from a previously published study including a cohort of MS patients aged 30-<40 at onset,6 and near the lower end of the range of ARR reported in placebo arms of clinical studies.22,23
Analyses of a cohort of MS patients from the British Columbian MS database (1980–2009).
Overall decrease in ARR.
Decreases in ARR were predominantly associated with age, rather than disability status. Across simulated scenarios, with varied initial ARR, the portion of the overall decrease in ARR attributed to age was 92–97% in the default model and 88–95% in the secondary model. Accordingly, 3–8% of the decrease in ARR was attributed to disability status in the default model and 5–13% in the secondary model. For example, a simulated cohort starting from an ARR of 0.51 (over the 5 years onset at age 30) and decreasing to 0.06 at 60–64 (Table 2) showed an overall decrease of 29% per 5-year interval. With a 28% age-specific 5-year decrease (Table 4), 97% of the overall decrease in ARR was attributed to age, rather than disability status.
Sensitivity analyses.
Truncating the data did not substantially change our post-onset ARR estimate (0.51 [Table 1] versus 0.57 when truncated), which remained within the range of post-onset ARR we modeled (0.33–1.0). Changing the rate of disability progression had little impact on the findings from the default or secondary models (Supplementary Table S4). In the replication model, variation in the rate of disability progression had a more substantial impact on age-specific decreases in ARR, since relapses stopped after reaching EDSS 6.0.
Discussion
We observed that the majority of the decreases in ARR in MS were due to age rather than disability status. By applying a comprehensive simulation modeling approach, we found that 88–97% of the decreases in the ARR were attributed to age. The remaining 3–13% were attributed to worsening disability. Describing the ARR over the lifetime will help inform decision making among people with relapsing-onset MS.
In all of our simulated scenarios, we found that a substantial age-specific decrease in ARR was needed to model the relatively low ARR observed among older adults with MS. We explored a range of assumptions about the relationship between relapses, disability, and age, and modeled a wide range of initial ARR (0.33–1.0). In the default model, an age-specific 22–37% decrease in ARR per 5 years was needed to reflect the ARR observed among older adults. Findings were similar in the secondary model, which assumed that more of the decrease in ARR was associated with disability, and in sensitivity analyses. The replication model produced a smaller age-specific decrease.
Given the uncertainty about the mechanisms underlying observed decreases in relapses in MS, we used a simulation modeling approach that enabled us to specify how relapses may be affected by age and disability status. For comparable onset ages, our results were consistent with a prior study from British Columbia, Canada, which did not incorporate changes in ARR by disability status.6 They described age-related ARR decreases by onset age, with similar results for age or versus time since disease onset. we focused on age, rather than time from onset, and explored the impact of varied initial ARR, rather than differing onset ages. Our estimated range of 5-year ARR decreases for initial ARR 0.33–1.0 (14–37%, Table 4) is similar to the range this previous study estimated for onset ages 20–40+ (14.3–35.9%). Similar to previously published MS Markov models,12,13,17,18 our model does not include additional clinical variables that may be associated with ARR, such as the time from first attack, relapse severity, and outcomes from MRI (e.g., new gadolinium enhancing lesions). Our results could be synthesized through modeling or patient-focused prognostic tools,24 to facilitate dialogue between individuals and their neurologists. Future studies could extend our work to model the impact of patient characteristics such as sex, onset age, and early clinical history.
MS Markov models described in previous publications12,13,17,18 that assumed relapses stop once EDSS 6 is reached would likely overestimate relapse frequency among older adults who have not reached this disability milestone. Because these prior Markov models did not incorporate an age-related decrease in ARR, they may overestimate the potential benefits of DMDs by extrapolating the results of clinical trials of DMDs to older populations (that are typically excluded from trials). Similar to prior Markov models, a limitation of our model is that the relative risk of disability progression was not modeled as age-dependent. If the risk of disability progression drops after a certain age, our model may overestimate disability among older individuals. Accurate information about treatment benefits is important particularly because compared with providers, people with MS tend to focus more on the benefits of treatment than the risks.25,26 The trajectory of the ARR over the lifetime is relevant to questions about the timing of DMD discontinuation.8,10,27–30 Our work can improve simulation models that examine the potential risks and benefits of the DMDs, particularly among older adults with relapsing-onset MS.
A key limitation of our study is the dearth of published ARR estimates specific to older adults. Our estimates of the ARR among older adults (Table 2) may be conservative. Among 178 people over age 60 who discontinued DMDs, only one had a relapse during up to two years of follow-up after discontinuing DMDs.8 Relapsing-onset MS has been theorized to “burn out” eventually, with relapses no longer occurring due to changes in the underlying disease pathology from inflammatory to degenerative.31 However, currently we cannot identify people who are no longer at risk for relapses. A strength of our historical cohort was the limited DMD exposure. The ARR among older adults today could be affected by DMD exposure, which makes contemporary natural history studies impossible. Whether relapse rates have changed in contemporary cohorts is unclear. While ARR appear to have decreased within the placebo arms of clinical trials,32 early ARR have not decreased in a recent cohort, even as the overall disability progression slowed.33 Either way, longitudinal data collected prior to widespread use of DMDs are valuable for gaining an understanding of disease outcomes in people with relapsing-onset MS.
Study limitations include the measurement of disability and relapse. The EDSS measure of disability has known limitations,14 but it remains widely used. Relapses may be underreported or overreported, and if this occurs at different rates at different ages, this could affect estimates of age-related decreases. For example, one survey of people with MS who were within 3 years of initiating a DMD revealed that nearly half (46%) had experienced a relapse in the past without reporting it to their neurologist.34 Our analyses were limited to clinical data, which could bias our findings if people who did not go to a clinic (or fit our inclusion criteria) were more or less likely to experience relapses than our sample. For example, if older adults who did not experience a relapse were less likely to go to an MS clinic, we could have overestimated relapses in this population. Our model focused on the relative contributions of aging and disability status to changes in ARR. Future studies could explore interactions with other patient characteristics, such as whether the relative decreases in ARR differ by sex.
It may be of value for future studies to quantify how measurements of relapses and disability interact with the aging process. Among older adults, some forms of MS-related EDSS disability might be hard to distinguish from “normal” age-related disability. Measurements of relapses may also be impacted by the aging process or accumulation of disability. For example, at high levels of disability, relapses may be hard to distinguish from pseudo-relapses (worsening neurological symptoms associated with pre-existing MS damage plus new stressors, such as infection, as opposed to new MS pathology occurring during a relapse). Conversely, with advanced disability, relapses may not cause an appreciable change in symptoms or disability and thus be undetected. Contemporary studies of DMD discontinuation may help address the possibility of pseudo-relapses and strengthen knowledge about relapses among older adults by reporting both relapses and MRI data in the absence of DMD use.
This study contributes to what is known about how the ARR changes from the onset of relapsing MS throughout the lifetime. Our work will facilitate more accurate modeling of the natural history of relapsing-onset MS, including long-term outcomes for relapses. Our findings include quantification of ARR changes over time, with decreases largely driven by age rather than advanced disability status. This information is important for informed clinical decisions among people with relapsing-onset MS and their providers.
Supplementary Material
Acknowledgments
The BeAMS Study group: Long-term Benefits and Adverse Effects of Beta-interferon for Multiple Sclerosis: Shirani A.; Zhao Y.; Evans C.; van der Kop M.L.; Gustafson G; Petkau J; Oger J. Role: facilitated gaining funding and creation of the study cohort used in the simulation-related analyses in the current manuscript [funding: Canadian Institutes of Health Research (CIHR) [MOP-93646] and the US National MS Society [#RG 4202-A-2]; 2009–12; PI: Tremlett).
We gratefully acknowledge contributions to this research project including but not limited to the following individuals:
BCMS clinic neurologists who contributed to the study through patient examination and data collection (current members at the time of data extraction listed here by primary clinic): UBC MS Clinic: A. Traboulsee, MD, FRCPC (UBC Hospital MS Clinic Director and Head of the UBC MS Programs); A-L. Sayao, MD, FRCPC; V. Devonshire, MD, FRCPC; S. Hashimoto, MD, FRCPC (UBC and Victoria MS Clinics); J. Hooge, MD, FRCPC (UBC and Prince George MS Clinic); L. Kastrukoff, MD, FRCPC (UBC and Prince George MS Clinic); J. Oger, MD, FRCPC. Kelowna MS Clinic: D. Adams, MD, FRCPC; D. Craig, MD, FRCPC; S. Meckling, MD, FRCPC Prince George MS Clinic: L. Daly, MD, FRCPC. Victoria MS Clinic: O. Hrebicek, MD, FRCPC; D. Parton, MD, FRCPC; K Atwell-Pope, MD, FRCPC
UBC research support: Tom Duggan, Feng Zhu, and the Pharmacoepidemiology in MS Research Group (https://epims.med.ubc.ca/)
We are indebted to all of the people with MS who contributed to the data used in this study.
The views expressed in this study do not necessarily reflect the views of each individual acknowledged.
Funding and Disclosure
This study was funded in part by a Thesis Research Travel Grant from the University of Minnesota and the University of Minnesota’s NIH Clinical and Translational Science Award: UL1TR002494 (Advanced Research Program Scholar). The funding sources played no role in the design, methods, data, or interpretation of the results of the study.
Helen Tremlett is the Canada Research Chair for Neuroepidemiology and Multiple Sclerosis. Current research support received from the National Multiple Sclerosis Society, the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada and the Multiple Sclerosis Scientific Research Foundation. In addition, in the last five years, has received research support from the Multiple Sclerosis Society of Canada (Don Paty Career Development Award); the Michael Smith Foundation for Health Research (Scholar Award) and the UK MS Trust; speaker honoraria and/or travel expenses to attend CME conferences from the Consortium of MS Centres (2013, 2018), the National MS Society (2014, 2016, 2018), ECTRIMS (2013, 2014, 2015, 2016, 2017, 2018, 2019), Biogen Idec (2014), American Academy of Neurology (2013, 2014, 2015, 2016, 2019). All speaker honoraria are either declined or donated to an MS charity or to an unrestricted grant for use by HT’s research group.
Footnotes
The Authors declare that there is no conflict of interest.
Contributor Information
NA Schwehr, Division of Health Policy and Management, University of Minnesota School of Public Health, Minneapolis, MN.
KM Kuntz, Division of Health Policy and Management, University of Minnesota School of Public Health, Minneapolis, MN.
M Butler, Division of Health Policy and Management, University of Minnesota School of Public Health, Minneapolis, MN.
EA Enns, Division of Health Policy and Management, University of Minnesota School of Public Health, Minneapolis, MN.
ND Shippee, Division of Health Policy and Management, University of Minnesota School of Public Health, Minneapolis, MN.
E Kingwell, Medicine (Neurology), University of British Columbia and The Djavad Mowafaghian Centre for Brain Health, Vancouver, BC, Canada.
H Tremlett, Medicine (Neurology), University of British Columbia and The Djavad Mowafaghian Centre for Brain Health, Vancouver, BC, Canada.
AF Carpenter, Department of Neurology, University of Minnesota Medical School and Brain Sciences Center, VA Medical Center, Minneapolis MN..
References
- 1.Kingwell E, van der Kop M, Zhao Y, et al. Relative mortality and survival in multiple sclerosis: findings from British Columbia, Canada. J Neurol Neurosurg Psychiatry 2012; 83: 61–66. [DOI] [PubMed] [Google Scholar]
- 2.Kingwell E, Zhu F, Marrie RA, et al. High incidence and increasing prevalence of multiple sclerosis in British Columbia, Canada: findings from over two decades (1991–2010). J Neurol 2015; 262: 2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States A population-based estimate using health claims data. 2019; 0: 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corboy JR. Disease-modifying therapies can be safely discontinued in an individual with stable relapsing-remitting MS – Commentary. Mult Scler J 2017; 23: 1192–1193. [DOI] [PubMed] [Google Scholar]
- 5.Filippi M, Wolinsky JS, Sormani MP, et al. Enhancement frequency decreases with increasing age in relapsing-remitting multiple sclerosis. Neurology 2001; 56: 422–423. [DOI] [PubMed] [Google Scholar]
- 6.Tremlett H, Zhao Y, Joseph J, et al. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 2008; 79: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 7.Soldán MMP, Novotna M, Zeid NA, et al. Relapses and disability accumulation in progressive multiple sclerosis. Neurology 2015; 84: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua LH, Fan TH, Conway D, et al. Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler J 2018; 1–10. [DOI] [PubMed] [Google Scholar]
- 9.Tortorella C, Bellacosa A, Paolicelli D, et al. Age-related gadolinium-enhancement of MRI brain lesions in multiple sclerosis. J Neurol Sci 2005; 239: 95–99. [DOI] [PubMed] [Google Scholar]
- 10.Birnbaum G Stopping disease-modifying therapy in nonrelapsing multiple sclerosis: Experience from a clinical practice. Int J MS Care 2017; 19: 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Signori A, Schiavetti I, Gallo F, et al. Subgroups of multiple sclerosis patients with larger treatment benefits: A meta-analysis of randomized trials. Eur J Neurol 2015; 22: 960–966. [DOI] [PubMed] [Google Scholar]
- 12.Prosser L, Kuntz K. Cost Effectiveness of Interferon Beta-1a, Interferon Beta-1b, and Glatiramer Acetate in Newly Diagnosed Non-primary Progressive Multiple Sclerosis. Value Heal 2004; 7: 554–568. [DOI] [PubMed] [Google Scholar]
- 13.Bell C, Graham J, Earnshaw S, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm 2007; 13: 245–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tremlett H, Zhao Y, Rieckmann P, et al. New perspectives in the natural history of multiple sclerosis. Neurology 2010; 74: 2004–2015. [DOI] [PubMed] [Google Scholar]
- 15.Scalfari A, Knappertz V, Cutter G, et al. Mortality in patients with multiple sclerosis. Neurology 2013; 81: 184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canada Statistics. Life tables, Canada Provinces and Territories. Reference Period: 2000 to 2002, (updated July 2006).
- 17.Sanchez-De La Rosa R, Sabater E, Casado M, et al. Cost-effectiveness analysis of disease modifiying drugs (interferons and glatiramer acetate) as first line treatments in remitting-relapsing multiple sclerosis patients. J Med Econ 2012; 15: 424–433. [DOI] [PubMed] [Google Scholar]
- 18.Bin Sawad A, Seoane-Vazquez E, Rodriguez-Monguio R, et al. Cost – effectiveness of different strategies for treatment relapsing-remitting multiple sclerosis. J Comp Eff Res 2017; 6: 97–108. [DOI] [PubMed] [Google Scholar]
- 19.Shirani A, Zhao Y, Karim ME, et al. Association between use of beta-interferon and progression of disability in patients with relapsing-remitting multiple sclerosis. Jama 2012; 308: 247–56. [DOI] [PubMed] [Google Scholar]
- 20.Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology 2006; 66: 172–177. [DOI] [PubMed] [Google Scholar]
- 21.Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010; 133: 1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inusah S, Sormani MP, Cofield SS, et al. Assessing changes in relapse rates in multiple sclerosis. Mult Scler 2010; 16: 1414–1421. [DOI] [PubMed] [Google Scholar]
- 23.Uitdehaag BMJ, Barkhof F, Coyle PK, et al. The changing face of multiple sclerosis clinical trial populations. Curr Med Res Opin 2011; 27: 1529–1537. [DOI] [PubMed] [Google Scholar]
- 24.Heesen C, Gaissmaier W, Nguyen F, et al. Prognostic Risk Estimates of Patients with Multiple Sclerosis and Their Physicians: Comparison to an Online Analytical Risk Counseling Tool. PLoS One 2013; 8: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieckmann P, Centonze D, Elovaara I, et al. Unmet needs , burden of treatment , and patient engagement in multiple sclerosis : A combined perspective from the MS in the 21st Century Steering Group. Mult Scler Relat Disord 2018; 19: 153–160. [DOI] [PubMed] [Google Scholar]
- 26.Kremer IEH, Evers SMAA, Jongen PJ, et al. Comparison of preferences of healthcare professionals and MS patients for attributes of disease- - modifying drugs : A best- - worst scaling. 2018; 171–180. [DOI] [PMC free article] [PubMed]
- 27.Bonenfant J, Bajeux E, Deburghgraeve V, et al. Can we stop immunomodulatory treatments in secondary progressive multiple sclerosis? Eur J Neurol 2016; 1–8. [DOI] [PubMed] [Google Scholar]
- 28.Bsteh G, Feige J, Ehling R, et al. Discontinuation of disease-modifying therapies in multiple sclerosis - Clinical outcome and prognostic factors. Mult Scler J 2016; 1–8. [DOI] [PubMed] [Google Scholar]
- 29.Kister I, Spelman T, Alroughani R, et al. Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study. J Neurol Neurosurg Psychiatry 2016; 87: 1133–1137. [DOI] [PubMed] [Google Scholar]
- 30.National US Library of Medicine. Discontinuation of Disease Modifying Therapies (DMTs) in Multiple Sclerosis (MS) (DISCOMS). ClinicalTrials.gov Identifier: NCT03073603, https://clinicaltrials.gov/ct2/show/NCT03073603 (2017, accessed 26 October 2018). [Google Scholar]
- 31.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 2015; 14: 183–193. [DOI] [PubMed] [Google Scholar]
- 32.Steinvorth SM, Röver C, Schneider S, et al. Explaining temporal trends in annualised relapse rates in placebo groups of randomised controlled trials in relapsing multiple sclerosis: Systematic review and meta-regression. Mult Scler J 2013; 19: 1580–1586. [DOI] [PubMed] [Google Scholar]
- 33.Beiki O, Frumento P, Bottai M, et al. Changes in the Risk of Reaching Multiple Sclerosis Disability Milestones in Recent Decades: A Nationwide Population-Based Cohort Study in Sweden. JAMA Neurol 2019; 76: 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duddy M, Lee M, Pearson O, et al. The UK patient experience of relapse in Multiple Sclerosis treated with first disease modifying therapies. Mult Scler Relat Disord 2014; 3: 450–456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.