Abstract
Little data are available regarding the determinants and prognostic significance of serum albumin in Heart Failure with Preserved Ejection Fraction (HFpEF). We sought to examine the phenotypic correlates of albumin and its independent prognostic implications in HFpEF. We analyzed data from 3,254 subjects enrolled the TOPCAT trial. We stratified subjects according to tertiles of albumin and examined differences in various phenotypic traits between these strata, including 8 protein biomarkers selected ad hoc and measured from frozen samples available in a subset of participants (n=372). We also assessed the relationship between albumin and the trial primary endpoint. Lower albumin was associated with older age, black race, and greater prevalence of NYHA class III-IV, peripheral arterial disease, atrial fibrillation and diabetes mellitus. Lower albumin was also associated with increased levels of several inflammatory biomarkers, markers of liver fibrosis, albuminuria, and greater arterial stiffness, diastolic dysfunction and pulmonary hypertension. Albumin was a strong predictor of the primary trial endpoint, even after adjustment for the MAGGIC risk score (HR 0.72, CI 0.67-0.78; P<0.0001) and pre-specified traditional risk factors (HR 0.78, CI 0.71-0.85; P<0.0001). Lower albumin was strongly associated with a worse prognosis even well within normal ranges (>3.5 g/dL), with a sharp increase in risk between 4.6 and 3.6 g/dL. In conclusion, albumin is an integrated marker of various adverse processes in HFpEF, including inflammation, subclinical liver disease, arterial stiffness and renal disease. Albumin is a powerful risk predictor independent of traditional risk prediction models, even within normal ranges.
Keywords: HFpEF, serum albumin, pathophysiology, risk prediction
Introduction
Hypoalbuminemia occurs in at least one quarter of patients with HF and is a powerful predictor of adverse outcomes, particularly in systolic HF. 1 The significance of serum albumin in HFpEF has been less well studied. In non-HFpEF populations, low albumin levels have been shown to result from systemic inflammation2, macroalbuminuria3, and liver disease with synthetic dysfunction4, but the correlates of albumin in HFpEF are poorly understood. Systemic inflammation may mediate cardiac and other target organ dysfunction in HFpEF.5 Low albumin levels may reflect systemic inflammation, renal dysfunction with albuminuria, or liver dysfunction from unrecognized non-alcoholic fatty liver disease. Few studies have investigated the association of low albumin with outcomes in patients with HFpEF and have shown conflicting results.6,7 Accordingly, the goal of the current study is to determine phenotypic correlates of albumin in a contemporary HFpEF cohort and to investigate prognostic implications of low albumin in this population independent of current risk prediction models.
Methods
The design of the TOPCAT study and the general characteristics of the trial population have been described in previous publications. 8,9 The parent TOPCAT dataset was acquired from the National Heart, Lung, and Blood Institute (NHLBI) via the Biolincc website. Briefly, TOPCAT was a multi-center, international, randomized, double-blind, placebo-controlled trial of spironolactone in adults with HFpEF. The trial was funded by the NHLBI. The primary results of the trial, as well as inclusion and exclusion criteria, have been previously published in detail.8,9 All study participants provided written informed consent. The primary goal of the trial was to determine if spironolactone was associated with a reduction in the composite outcome of cardiovascular mortality, aborted cardiac arrest, or HF hospitalization. All HF hospitalizations were adjudicated by a blinded clinical end point committee at Brigham and Women’s Hospital, as previously described.8 Echocardiographic data for a subset of participants was analyzed in a central core laboratory at the Brigham and Women’s Hospital as previously described.10 Albumin and other blood laboratory parameters were measured at baseline. Laboratory measurements for albumin and microalbuminuria were performed locally at the enrolling site. Participants with albumin >10 g/dL (n=11) likely representing errors in data entry and/or measurement error, were excluded, as were subjects without an available value (n=177). The current analysis therefore included 3,254 subjects.
We obtained stored plasma samples from NIH Biolincc for all available participants who had stored plasma from the baseline examination (n=379), of which 372 also had available serum albumin levels at baseline. We measured proteins of interest using a Luminex ® Bead-Based multiplexed assay (Bristol Myers-Squibb; Ewing Township, NJ), including: biomarkers of inflammation (C-reactive protein, IL-1, IL-6, tumor necrosis factor [TNF]-alpha, TNR-Receptor 1 [TNF-RI] and 2 [TNF-RII]), NT-proBNP levels, as well as YLK-40, a biomarker that correlates with liver fibrosis in non-alcoholic fatty liver disease (NAFLD).11 We also analyzed 2 common liver fibrosis scores: the Fib-4 score (which correlates with liver fibrosis of multiple etiologies) and the NAFLD score, which correlates with liver fibrosis from NAFLD.4,12 Since the NAFLD score includes albumin, a modified version of the NAFLD score, without including albumin in the calculation, was utilized.
Given that circulating albumin levels are potentially dependent on synthetic function, pathologic excretion, and inhibition of hepatic synthesis from inflammatory cytokines, we assessed these pathophysiologic domains using various ad hoc phenotypic indicators (Supplemental Table 1). We also assessed the relationship between albumin and established measures of target organ damage, including estimated glomerular filtration rate (eGFR), albuminuria, echocardiographic measures of diastolic function, pulmonary hypertension, and indices of arterial stiffness. For the latter, we assessed pulse pressure and the stroke volume/pulse pressure ratio (indexed for body surface area), which is a metric of total arterial compliance.
Participant characteristics were summarized using mean (SD) for normally distributed variables and median (interquartile range) for non-normally distributed continuous variables. Categorical variables are expressed as counts (percentages). We stratified the sample according to tertiles of albumin and compared various clinical characteristics between the strata. We used analysis of variance (ANOVA) for normally-distributed variables, the Kruskal-Wallis test for non-normally distributed variables, and the chi-square of Fisher’s exact test, as appropriate, for categorical data. We identified significant general differences between the strata and compared key phenotypic traits, adjusting for demographic differences using analysis of covariance (ANCOVA) with post-hoc pairwise comparisons with Bonferroni correction. For these comparisons, normality was assessed with the Anderson-Darling test and log-transformations were applied as needed to improve normality prior to constructing ANCOVA models. In all cases, means and 95% CIs are expressed in the native (linear) scale.
We assessed the relationship between albumin and the risk of: (1) the primary outcome (cardiovascular death, aborted cardiac arrest, or HF hospitalization); (2) all-cause mortality, and; (3) the composite endpoint of death and HF hospital admission. Cox regression was used to assess the relationship between albumin and outcomes, in unadjusted and models that adjusted for confounders, including: (1) the MAGGIC risk score, which incorporates multiple demographic, clinical and laboratory variables (Model 1); 13 (2) The MAGGIC risk score plus NT-proBNP levels (Model 2); (3) Important individual clinical covariates chosen a priori, including age, sex, diabetes mellitus, eGFR, systolic and diastolic blood pressure, New York Heart Association (NYHA) class III/IV, body mass index (BMI), history of atrial fibrillation, COPD and myocardial infarction.
To better resolve the graded relationship between albumin and outcomes, we computed Kaplan-Meier survival curves in tertiles of albumin and compared them with the log-rank test. Finally, we computed the relationship between the continuum of albumin levels and the risk of outcomes using spline-based hazard ratio curves, computed with the R package smoothHR, as previously described.14 Statistical significance was defined as a 2-tailed P value<0.05. All probability values presented are 2-tailed. Analyses were performed using the Matlab statistics and machine learning toolbox (Matlab 2016b, the Mathworks; Natwick, MA) and R Statistical Software v3.5.2 (Foundation for Statistical Computing, Vienna, Austria),
Results
General characteristics of the study population stratified by albumin are shown in Table 1. Participants with lower albumin demonstrated older age, higher proportions of black race, and higher body mass index. Additionally, they exhibited a greater prevalence of NYHA class III-IV HF symptoms, atrial fibrillation and diabetes. Hypoalbuminemia (<3.5 g/dL) was present in 332 subjects (10.7%).
Table 1.
General characteristics of the study sample, stratified according to tertiles of serum albumin. Numbers represent Mean (SD), Median (IQR) or counts (%)
| Serum Albumin (g/dl) | ||||
|---|---|---|---|---|
| <=3.9 (n=990) |
>3.9-4.3 (n=1102) |
>4.3 (n=1173) |
P value | |
| Variable | ||||
| Age (years) | 71 (63,78) | 69 (62,76) | 66 (60,73) | <0.0001 |
| Men | 467 (47.17%) | 509 (46.19%) | 580 (49.91%) | 0.1842 |
| Race | <0.0001 | |||
| White | 788 (79.60%) | 1002 (90.93%) | 1097 (94.41%) | |
| Black | 161 (16.26%) | 79 (7.17%) | 50 (4.30%) | |
| Asian | 12 (1.21%) | 1 (0.09%) | 4 (0.34%) | |
| Other | 37 (3.74%) | 21 (1.91%) | 10 (0.86%) | |
| BMI (Kg/m2) | 32.2 (27.6,38.2) | 30.9 (27.2,35.6) | 30.1 (26.9,34) | <0.0001 |
| Heart rate (bpm) | 68 (61,76) | 68 (60,75) | 69 (62,76) | 0.0238 |
| Systolic BP (mmHg) | 130 (120,139) | 130 (120,139) | 130 (120,140) | 0.0008 |
| Diastolic BP (mmHg) | 72 (63,80) | 79 (70,80) | 80 (75,85) | <0.0001 |
| Estimated GFR (mL/min) | 62 (60.9 to 63.2) | 65.1 (64 to 66.2) | 66.8 (65.7 to 68) | <0.0001 |
| NYHA class III-IV | 366 (37.04%) | 341 (30.94%) | 355 (30.55%) | 0.0020 |
| Myocardial Infarction | 209 (21.11%) | 298 (27.07%) | 319 (27.45%) | 0.0010 |
| Stroke | 75 (7.58%) | 86 (7.81%) | 87 (7.49%) | 0.9565 |
| Chronic obstructive | 144 (14.55%) | 144 (13.08%) | 98 (8.43%) | <0.0001 |
| pulmonary disease Hypertension | 895 (90.40%) | 1011 (91.83%) | 1076 (92.60%) | 0.1804 |
| Peripheral Arterial Disease | 117 (11.82%) | 103 (9.36%) | 71 (6.11%) | <0.0001 |
| Atrial Fibrillation | 398 (40.20%) | 370 (33.61%) | 387 (33.30%) | 0.0010 |
| Diabetes Mellitus | 411 (41.52%) | 347 (31.52%) | 292 (25.13%) | <0.0001 |
| Medication Use | ||||
| Beta Blockers | 800 (80.89%) | 813 (73.77%) | 910 (78.31%) | 0.0004 |
| Calcium Channel | 379 (38.32%) | 433 (39.29%) | 409 (35.20%) | 0.1097 |
|
Blockers Diuretics |
854 (86.35%) | 916 (83.12%) | 917 (78.92%) | <0.0001 |
| Glucose-lowering agents | 360 (36.40%) | 303 (27.50%) | 241 (20.74%) | <0.0001 |
| ACE Inhibitors or ARBs | 806 (81.50%) | 913 (82.85%) | 1017 (87.52%) | 0.0003 |
| Statins | 566 (57.23%) | 569 (51.63%) | 569 (48.97%) | 0.0006 |
| Warfarin | 295 (29.83%) | 234 (21.23%) | 220 (18.93%) | <0.0001 |
Phenotypic differences in study participants stratified by albumin are shown in Table 2. Participants with lower albumin demonstrated increased levels of several inflammatory markers. Similar results were obtained even after exclusion of patients with clinical hypoalbuminemia (<3.5 g/dL) (Supplemental Table 2). As albumin levels decreased, there were stepwise increases in the white blood cell count and various inflammatory protein biomarkers (IL-6, TNF, TNFRI, TNFRII), as shown in Figure 1A. There were no significant differences in levels of CRP or IL-1 across the albumin tertiles.
Table 2.
Key phenotypic comparisons between participants in each tertile of serum albumin
| Tertile | P value | |||
|---|---|---|---|---|
| 1 Mean (95%CI) |
2 Mean (95%CI) |
3 Mean (95%CI) |
||
| Variable | ||||
| WBC count (k/uL) | 6.96 (6.84 to 7.09) | 6.7 (6.59 to 6.81) | 6.59 (6.48 to 6.7) | <0.0001 * # |
| CRP (mg/dL)1 | 10.51 (5.76 to 15.27) | 14.14 (8.02 to 20.26) | 11.77 (6.53 to 17.01) | 0.5267 |
| IL6 (pg/mL)1 | 0.233 (0.148 to 0.319) | 0.215 (0.14 to 0.29) | 0.107 (0.069 to 0.146) | 0.0007 # $ |
| TNF alpha (pg/mL)1 | 1.5 (1.28 to 1.72) | 1.21 (1.03 to 1.4) | 1.06 (0.88 to 1.24) | 0.0061 # |
| TNFRI (pg/mL)1 | 2208 (1940 to 2477) | 1909 (1686 to 2132) | 1531 (1348 to 1715) | <0.0001 # $ |
| TNFRII (pg/mL)1 | 207103 (161439 to 252766) | 167890 (132317 to 203463) | 130722 (102289 to 159155) | 0.0059 # |
| IL1 (pg/mL)1 | 0.088 (0.026 to 0.15) | 0.129 (0.067 to 0.191) | 0.121 (0.058 to 0.184) | 0.6022 |
| Modified NAFLD score | 2.78 (2.69 to 2.88) | 2.61 (2.52 to 2.7) | 2.35 (2.27 to 2.44) | <0.0001 * # $ |
| Fib-4 score | 1.55 (1.5 to 1.6) | 1.52 (1.47 to 1.57) | 1.45 (1.41 to 1.49) | 0.0045 # |
| YLK-40 (pg/mL)1 | 104184 (77737 to 130632) | 89041 (67331 to 110751) | 56896 (42650 to 71141) | 0.0006 # $ |
| Urine albumin/creatinine ratio2 | 35.6 (29.3 to 41.8) | 27.8 (22.5 to 33.2) | 11.8 (9.2 to 14.4) | <0.0001 # $ |
| Urine albumin/creatinine ratio2,3 | 21.5 (18.3 to 24.7) | 17.8 (14.9 to 20.6) | 7.9 (6.4 to 9.3) | <0.0001 # $ |
| Estimated GFR (ml/min/1.73 m2) | 62 (60.9 to 63.2) | 65.1 (64 to 66.2) | 66.7 (65.6 to 67.9) | <0.0001 * # |
| E/e’, septal4 | 15.6 (14.7 to 16.5) | 14.2 (13.2 to 15.2) | 12.1 (11 to 13.1) | <0.0001 # $ |
| E/e’, lateral4 | 11.3 (10.6 to 12) | 11.2 (10.4 to 12) | 8.9 (8.1 to 9.7) | <0.0001 # $ |
| LA volume index (ml/m2)4 | 28.5 (27.3 to 29.7) | 26 (24.8 to 27.3) | 24.8 (23.5 to 26.1) | <0.0001 * # |
| LV Ejection Fraction | 59.4 (58.4 to 60.3) | 59.3 (58.3 to 60.4) | 58.7 (57.6 to 59.8) | 0.6127 |
| Global Longitudinal Strain (%) | −15.4 (−15.9 to −14.8) | −15.5 (−16.1 to −15) | −16 (−16.7 to −15.3) | 0.3253 |
| PASP (mmHg) | 31.7 (30.3 to 33.1) | 30.2 (28.6 to 31.8) | 27 (25 to 28.9) | 0.0006 # $ |
| RV fractional area change | 0.49 (0.48 to 0.50) | 0.49 (0.48 to 0.50) | 0.48 (0.47 to 0.49) | 0.5896 |
| Arterial Stiffness | ||||
| Pulse pressure (PP)4 | 54 (53.3 to 54.8) | 52.2 (51.5 to 52.9) | 50.4 (49.7 to 51) | <0.0001 * # $ |
| Stroke volume Index (SVI)/PP ratio4 | 0.49 (0.47 to 0.50) | 0.51 (0.49 to 0.54) | 0.55 (0.53 to 0.58) | 0.01 |
Including only subjects with available biomarker data (n=372);
Including only subjects with available urine microalbumin data (1457);
Including only those without macroalbuminuria (subsample n=1297);
Including only subset with available echocardiographic data (LVEF=811 Stroke volume index (SV)/PP ratio=808; LAVI=768; E/e’ lateral=452; E/e’ septal=464; global longitudinal strain=420; PASP=427; RV fractional area change=631).
Significant post-hoc pairwise comparisons are indicated by the symbols:
tertile 1 vs. 2 is significant
tertile 1 vs 3 is significant; tertile 2 vs 3 is significant.
Figure 1.
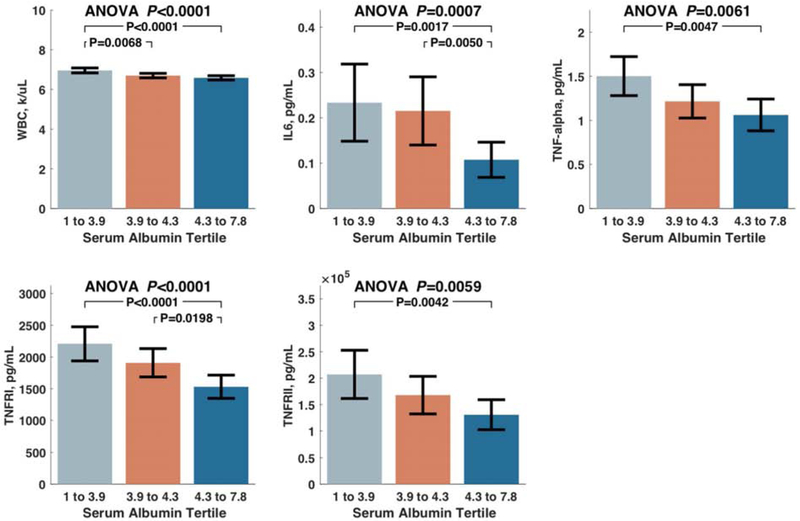

A. Levels of white blood cell count and various inflammatory protein biomarkers that differed between tertiles of albumin. 1B. Liver fibrosis scores and YLK-40 levels among subjects stratified by tertiles of albumin.
Participants with lower albumin levels demonstrated increased liver fibrosis scores, with stepwise increases in both the modified NAFLD score and the Fib-4 score. Additionally, levels of YLK-40, a protein biomarker associated with liver fibrosis, were increased in participants with lower albumin (Figure 1B).
Lower serum albumin levels were associated with several measures of target organ dysfunction, including renal dysfunction, left ventricular diastolic dysfunction, pulmonary hypertension. and increased arterial stiffness. With decreasing albumin levels, participants had similar stepwise increases in albuminuria, as well was decrease in renal function. Worsening diastolic dysfunction was seen across the groups, along with greater levels of pulmonary artery systolic pressure measured by echocardiography. This was despite no significant differences in global longitudinal strain or ejection fraction between the groups. Furthermore, systemic pulse pressure was increased in participants with albumin, whereas the stroke volume index/PP ratio, a surrogate for arterial compliance, was decreased across tertiles of albumin, indicating increased arterial stiffness. Of note, most of these phenotypic differences were already present when comparing the middle (3.9 to 4.3 g/dL) vs. the upper (>4.3 g/dL), all of whom exhibited albumin levels well within the normal range.
Associations of albumin with clinical outcomes are shown in Table 3. Albumin was independently associated with the trial’s primary endpoint of cardiovascular death, aborted cardiac arrest, or HF hospitalization in unadjusted models, as well as after controlling for both the MAGGIC risk score (Adjusted Model 1) and several important clinically relevant covariates (Adjusted Model 2). Albumin was also independently associated with the composed outcome of death or HF hospitalization, in both unadjusted and adjusted models.
Table 3.
Hazard Ratios per SD increase in Albumin
| Model | Standardized Hazard Ratio |
95% CI, LB | 95%CI, UB |
P value |
|---|---|---|---|---|
| Primary Endpoint | ||||
| Unadjusted | 0.68 | 0.63 | 0.73 | <0.0001 |
| Adjusted Model 1 | 0.72 | 0.67 | 0.78 | <0.0001 |
| Adjusted Model 2 | 0.78 | 0.71 | 0.85 | <0.0001 |
| Death or HF admission | ||||
| Unadjusted | 0.67 | 0.63 | 0.72 | <0.0001 |
| Adjusted Model 1 | 0.72 | 0.67 | 0.78 | <0.0001 |
| Adjusted Model 2 | 0.78 | 0.72 | 0.84 | <0.0001 |
Adjusted Model 1: adjusted for the MAGGIC risk score.
Adjusted Model 2: adjusted for age, sex, diabetes mellitus, estimated glomerular filtration rate, systolic and diastolic blood pressure, NYHA class III/IV, body mass index, history of atrial fibrillation, COPD and myocardial infarction.
Figure 2 shows Kaplan-Meier survival plots for the primary endpoint (A,C) and death/HFA (B,D) among participants stratified by tertiles (A,B) or quintiles (C,D) of albumin values. It can be appreciated that even well-within the normal range of albumin, decreased levels were associated with a progressively increased risk of reaching these endpoints.
Figure 2.
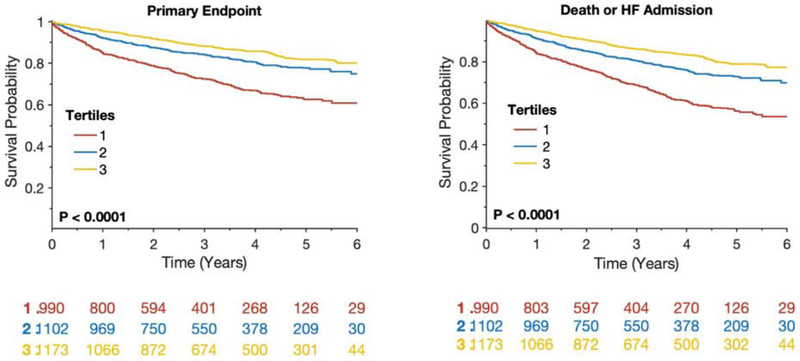
Kaplan-Meier survival plots for the primary endpoint (A, C) and death/HFA (B, C) among participants stratified by tertiles (A, B) and quintiles (C, D) of albumin values.
Figure 3 shows a spline model which demonstrates the (ln) hazard ratio for the primary endpoint (3A) and death/HFA (3B) according to albumin levels. It can be seen that the risk of adverse outcomes starts to increase at albumin levels well above the lower limit of normal (3.5 g/dL). Indeed, the lowest risk for these endpoints corresponded to 4.6 g/dL, with a sharp increase in risk between 4.6 and 3.7 g/dL. Accordingly, even when subjects with hypoalbuminemia (albumin <3.5 g/dL) were excluded, albumin was predictive of the primary endpoint (Standardized HR=0.34; 95%CI=0.22-0.52; P<0.0001) and of death/HFA (Standardized HR=0.51; 95%CI=0.35-0.75; P=0.0006).
Figure 3.

Spline modeling of albumin against the ln (Hazard Ratio) for the primary endpoint (A) and death/HFA (B). An albumin level of 4.6 was associated with the lowest risk and is used as the reference.
Figure 4 shows a volcano plot in which the standardized hazard ratios for albumin and other well-known predictors of outcomes in HFpEF are shown. It can be seen that albumin was more predictive of these endpoints than most well-established risk markers.
Figure 4.
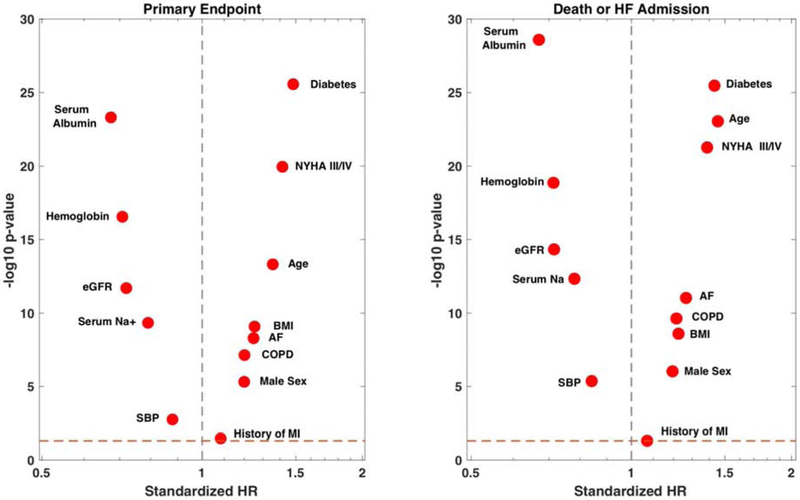
Volcano plot of standardized hazard ratios (a measure of effect size) vs. the −log 10-P value (a measure of statistical significance) for albumin and other well-known predictors of outcomes in HFpEF. Biomarkers with a greater effect size and greater statistical significance are further away from the vertical dashed line (HR=1) and closer to the top of the plot, respectively
Discussion
We studied the phenotypic correlates of albumin and its relationship with outcomes in HFpEF. We demonstrate that lower albumin is associated with older age, more symptomatic HF, and increased prevalence of several comorbidities. Lower albumin was also associated with increased levels of inflammatory biomarkers and target organ dysfunction. Albumin was a strong independent predictor of adverse outcomes even after adjustment for several risk scores, and compared favorably as a predictor relative to other well-known risk markers in HFpEF. Interestingly, the risk of adverse outcomes increased at albumin levels well above the lower limit of normal (3.5 g/dL).
Lower albumin has been shown to predict incident HF among patients without cardiovascular disease.15 Once HF is manifest, studies have generally shown albumin levels to predict worse outcomes.1,6 Unfortunately most studies on the prognostic role of albumin in HF either excluded patients with HFpEF or did not distinguish the type of HF. Only two studies have examined the impact of albumin levels in HFpEF and showed conflicting results.6,7 Our study revealed that lower albumin was associated with increased levels of various inflammatory markers. Elevated levels of inflammatory markers have been shown to correlate with incident HFpEF and to distinguish patients with hypertensive heart disease who subsequently develop HFpEF.16,17 Systemic inflammation may serve as a primary driver of myocardial disease in HFpEF.5 Endomyocardial biopsies from patients with HFpEF demonstrate increased collagen deposition and increased inflammatory cell infiltrates, which correlate with parameters of diastolic dysfunction on echocardiography.18,19
Albumin is known to decrease in chronic liver disease.20 In patients with NAFLD, albumin levels are associated with more advanced fibrosis, and accordingly are part of the NAFLD fibrosis scoring system.4 In the current study, we found that low albumin was associated with increased NAFLD score (modified to exclude albumin) as well the Fib-4 score, both of which correlate strongly with liver fibrosis. We also demonstrate a relationship between albumin and YLK-40, a biomarker of liver fibrosis in NAFLD.21 Our findings regarding the association between albumin and liver dysfunction, and between albumin and a high risk of adverse outcomes, are consistent with recent work showing correlations between liver fibrosis scores and a higher risk of all-cause mortality in HFpEF.22 It is unclear whether the liver dysfunction in patients with HFpEF is causally related to elevated right sided filling pressures, primary liver disease from NAFLD, or both.
Finally, low albumin was strongly associated with markers of micro- and macrovascular dysfunction including lower GFR and albuminuria, arterial stiffness, and worse diastolic dysfunction. Albuminuria is considered a marker of renal damage and endothelial dysfunction, and is independently associated with increased LV mass, lower global longitudinal strain, worse RV systolic function 23 and adverse outcomes in HFpEF.24 Endothelial dysfunction, which correlates with albuminuria, may also contribute to impaired coronary flow reserve, which in turn is associated with higher myocardial stiffness and adverse clinical outcomes in HFpEF.25 Whether albumin is related to systemic or coronary endothelial dysfunction in HFpEF remains to be determined in future studies.
Albumin was among the strongest univariate predictors of adverse outcomes, even at levels that are not considered clinically abnormal. Albumin was within normal limits in the vast majority (approximately 90%) of TOPCAT participants. Yet, there was a strong association between albumin and outcomes even within the normal range. It is therefore relevant to understand the phenotypic correlates and potential mechanisms of reduced albumin in HFpEF, and our study increases our understanding of this issue.
Our study has some limitations. First, TOPCAT enrolled a carefully selected population of HFpEF patients who were considered suitable candidates for spironolactone therapy, which may reduce generalizability of our results. Secondly, there were neither invasive hemodynamics nor liver histologic or imaging data to better ascertain liver fibrosis in our study. Accordingly, we utilized non-invasive biomarker scores which have been shown to be strongly related to the presence of histologic hepatic fibrosis. A final limitation is that protein biomarkers and echocardiographic data were available only in a subset of participants.
Our study increases our understanding of albumin in HFpEF. We demonstrate that albumin is an integrated marker of systemic inflammation, renal and liver dysfunction, and more advanced diastolic dysfunction and arterial stiffness. Albumin was a strong independent clinical predictors of adverse outcomes in this population above and beyond traditional risk factors and composite prediction models. Further work is needed to determine whether albumin can be used to monitor the progression of organ dysfunction and/or systemic inflammation in HFpEF.
Supplementary Material
Acknowledgements:
This manuscript was prepared using TOPCAT (Treatment of Pre- served Cardiac Function Heart Failure With an Aldosterone Antagonist) Trial research materials obtained from the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the TOPCAT Trial or the National Heart, Lung, and Blood Institute.
Sources of Funding
This study was funded by R01 HL121510-01A1 (JAC) and by a research grant from Bristol-Myers-Squibb (JAC). Zamani is funded by 1-K23-HL-130551-01.
Footnotes
Disclosures: J.A.C. has received consulting honoraria from Sanifit, Microsoft, Fukuda-Denshi, Bristol-Myers Squibb, OPKO Healthcare, Ironwood Pharmaceuticals, Pfizer, Akros Pharma, Merck and Bayer. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol-Myers Squibb and Microsoft. J.A.C. is named as inventor in a University of Pennsylvania invention disclosure for the use of circulating fibrosis biomarkers in HFpEF and in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of HFpEF. L.Z, M.E.C., M.B, T.A, Z.L., M.Y., Z.W., D.S., and D.A.G. are employees of Bristol-Myers Squibb. The remaining authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 2008;155:883–889. [DOI] [PubMed] [Google Scholar]
- 2.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–454. [DOI] [PubMed] [Google Scholar]
- 3.Keller C, Katz R, Sarnak MJ, Fried LF, Kestenbaum B, Cushman M, Shlipak MG, study CHS. Inflammatory biomarkers and decline in kidney function in the elderly: the Cardiovascular Health Study. Nephrol Dial Transplant 2010;25:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- 5.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 6.Liu M, Chan CP, Yan BP, Zhang Q, Lam YY, Li RJ, Sanderson JE, Coats AJ, Sun JP, Yip GW, Yu CM. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2012;14:39–44. [DOI] [PubMed] [Google Scholar]
- 7.Uthamalingam S, Kandala J, Daley M, Patvardhan E, Capodilupo R, Moore SA, Januzzi JL Jr. Serum albumin and mortality in acutely decompensated heart failure. Am Heart J 2010;160:1149–1155. [DOI] [PubMed] [Google Scholar]
- 8.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O'Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 2011;162:966–972 e910. [DOI] [PubMed] [Google Scholar]
- 9.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 10.Shah AM, Shah SJ, Anand IS, Sweitzer NK, O'Meara E, Heitner JF, Sopko G, Li G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD, Investigators T. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ Heart Fail 2014;7:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumagai E, Mano Y, Yoshio S, Shoji H, Sugiyama M, Korenaga M, Ishida T, Arai T, Itokawa N, Atsukawa M, Hyogo H, Chayama K, Ohashi T, Ito K, Yoneda M, Kawaguchi T, Torimura T, Nozaki Y, Watanabe S, Mizokami M, Kanto T. Serum YKL-40 as a marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Scientific Repons 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265–1269. [DOI] [PubMed] [Google Scholar]
- 13.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN, Meta-Analysis Global Group in Chronic Heart F. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–1413. [DOI] [PubMed] [Google Scholar]
- 14.Meira-Machado L, Cadarso-Suarez C, Gude F, Araujo A. smoothHR: an R package for pointwise nonparametric estimation of hazard ratio curves of continuous predictors. Comput Math Methods Med 2013;2013:745742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filippatos GS, Desai RV, Ahmed MI, Fonarow GC, Love TE, Aban IB, Iskandrian AE, Konstam MA, Ahmed A. Hypoalbuminaemia and incident heart failure in older adults. Eur J Heart Fail 2011;13:1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collier P, Watson CJ, Voon V, Phelan D, Jan A, Mak G, Martos R, Baugh JA, Ledwidge MT, McDonald KM. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur J Heart Fail 2011;13:1087–1095. [DOI] [PubMed] [Google Scholar]
- 17.Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, Kritchevsky SB, Harris TB, Butler J, Health ABCSI Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol 2010;55:2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 2011;4:44–52. [DOI] [PubMed] [Google Scholar]
- 19.Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kuhl U, Schultheiss HP, Tschope C. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardiol 2011;57:977–985. [DOI] [PubMed] [Google Scholar]
- 20.Kaysen GA, Dubin JA, Muller HG, Rosales L, Levin NW, Mitch WE, NIDDK HSG. Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int 2004;65:1408–1415. [DOI] [PubMed] [Google Scholar]
- 21.Kumagai E, Mano Y, Yoshio S, Shoji H, Sugiyama M, Korenaga M, Ishida T, Arai T, Itokawa N, Atsukawa M, Hyogo H, Chayama K, Ohashi T, Ito K, Yoneda M, Kawaguchi T, Torimura T, Nozaki Y, Watanabe S, Mizokami M, Kanto T. Serum YKL-40 as a marker of liver fibrosis in patients with non-alcoholic fatty liver disease. Sci Rep 2016;6:35282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail 2018;5:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz DH, Burns JA, Aguilar FG, Beussink L, Shah SJ. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail 2014;2:586–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvaraj S, Claggett B, Shah SJ, Anand I, Rouleau JL, O'Meara E, Desai AS, Lewis EF, Pitt B, Sweitzer NK, Fang JC, Pfeffer MA, Solomon SD. Prognostic Value of Albuminuria and Influence of Spironolactone in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail 2018;11:e005288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


