Abstract
Objective:
To investigate the role time of day plays in perioperative outcomes we examined intraoperative transfusion rates throughout the day in adult cardiac surgery patients. We hypothesized that the rate of transfusion changes with later case start times in scheduled cardiac surgery.
Design:
Retrospective observational study
Setting:
Single academic medical center
Participants:
Adults undergoing cardiac surgery involving cardiopulmonary bypass
Interventions:
None
Measurements and Main Results:
The primary outcome was a composite variable of transfusion. The association between the time of day and the rate of transfusion was explored with a multivariate logistic regression to fit the effect of starting time as a cubic spline. 1,421 cases met inclusion criteria. 1,220 cases were matched for modeling. The estimated probability of a patient receiving a transfusion changed significantly with later case start times in the multivariable model after adjusting for initial hemoglobin, age, gender, height, ideal body weight, diabetes, peripheral vascular disease, stroke, chronic kidney disease, chronic obstructive pulmonary disease, duration of cardiopulmonary bypass, aortic cross clamp time, attending surgeon, and attending anesthesiologist (p = 0.032, c statistic = 0.807, n = 1220). The estimated probability of receiving an intraoperative red blood cell transfusion increased with later case start times in the multivariable model (p = 0.027, c statistic = 0.902, n = 1220). There was no difference in the probability of transfusion for plasma, cryoprecipitate, or platelets.
Conclusions:
The observed rate of intraoperative blood product transfusion changed with later case start times in a multivariable model of scheduled cardiac surgery.
Keywords: transfusion, cardiopulmonary bypass, timing of surgery, decision-fatigue, decision-making
Introduction
The time of day a patient presents to the operating room may exert an influence on certain perioperative outcomes as demonstrated in previous clinical investigations, with some suggesting a possible association between later case start times and the rate of observed adverse outcomes1, 2. The impact of case scheduling on patient outcomes remains unclear as prior work has demonstrated mixed results2, 3. These findings have led some investigators to hypothesize that individual variables influenced by time of day including cognitive fatigue, provider handoffs, patient hormone levels, or even circadian fluctuations may influence the delivery of care4–6. Decisions regarding intraoperative blood product transfusions are complex with a myriad of variables culminating in the eventual administration of a product to a patient. It is plausible that similar patient and provider related factors may impact the blood product utilization rate.
Allogeneic blood products are a scarce but essential resource for the perioperative care of the cardiac surgery patient. Blood transfusions have the potential for precipitating both immediate complications and negatively influencing long-term outcomes. Prior work has demonstrated an association between blood product transfusion and increases in morbidity, postoperative mortality, ICU length of stay, time to extubation, and resource utilization in the cardiac surgery population7–10. Evidence supports a directly-proportional and dose-dependent relationship between the number of packed red blood cell units administered and patient mortality11.
Patients undergoing cardiac surgery are reported to consume up to 10% to 15% of the available blood supply nationwide. While published rates of transfusion during cardiac surgery vary widely the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database reports that 50% of cardiac surgical patients receive a blood transfusion12–17. The debate surrounding optimal transfusion strategies, thresholds, and protocols in cardiac surgery continues without a definitive consensus in the literature particularly as it pertains to certain vulnerable subpopulations including geriatric patients18–21. Recent evidence supports the non-inferiority of a restrictive approach to transfusion in cardiac surgery22, 23. Applying these findings to real-time transfusion decisions as an anesthesiologist faced with an individual, high-risk, actively bleeding patient rather than an aggregate study population remains challenging in the rapidly changing intraoperative environment.
We sought to further investigate the potential relationship between the time of day a surgery occurs and patient outcomes by focusing specifically on the influence of case start time on allogeneic blood product transfusion rates. We hypothesized that adults undergoing nonemergent cardiac surgery involving cardiopulmonary bypass (CPB) with surgical start times later in the day are more likely to receive intraoperative blood product transfusions.
Materials and Methods
We conducted a retrospective analysis of adult, elective cardiac surgical procedures requiring cardiopulmonary bypass at a single academic medical center. Institutional review board approval was obtained prior to the initiation of this study. Electronic medical records for all cardiothoracic surgery cases occurring after the implementation of Epic EMR Clarity DataStore software were extracted. All cases occurred between July 2013 and December 2016.
Included cases were those that required cardiopulmonary bypass for scheduled, nonemergent coronary artery bypass graft (CABG), valvular repair/replacement, or combined procedures. Exclusion criteria were age <18 years old or adult congenital heart surgery, off-pump CABG, minimally invasive techniques, emergency or “add-on” case posting status, cases utilizing deep hypothermic circulatory arrest (DHCA), surgeries involving a repeat sternotomy, durable mechanical ventricular assist device implantation, cardiac transplantation, cases missing anesthesia start time, and patients with the following preexisting comorbidities: end-stage renal disease (ESRD), thrombocytopenia, or cirrhosis. Initial exclusions were performed using the case posting. It was noted on primary quality assurance review that the recorded case posting did not consistently reflect all components of the procedure performed. Consequently, a manual chart review was conducted for each case, and a trained abstracter recorded all elements of the procedure performed based upon the operative note generated by the attending cardiothoracic surgeon, a cardiothoracic surgery fellow, or a physician assistant involved in the case. Figure 1 provides the cohort and case exclusion information.
Figure 1:
Flow diagram of study inclusion and exclusion criteria. LVAD, left ventricular assist device. DHCA, deep hypothermic circulatory arrest. ESRD, end-stage renal disease.
The primary endpoint determined a priori was a composite variable reflecting whether any allogenic blood product was administered to a patient. Allogenic blood products were defined as packed red blood cells (pRBC), plasma, platelets, and cryoprecipitate. The administration of any amount of one or more of those products was considered an intraoperative transfusion.
Secondary outcomes were also determined a priori and include transfusion of individual blood products and the amount of individual product administered. While conducting the manual chart review of the entire cohort to examine the operative notes we also reviewed the amounts of blood products administered for the first 250 charts (18% of the cohort) and found that automated software-based extraction (Epic EMR Clarity DataStore) and manual review had 100% interrater agreement. Software-based electronic medical record (EMR) extraction was then employed for obtaining blood product information in the remainder of the study sample.
Patient demographic information was obtained from the EMR and represents data present in the chart at the time of surgery. Age, sex, body mass index (kg/m2), and body surface area (m2) were collected for analysis. Patient comorbidities were determined by examination of the EMR problem list on the day of surgery to capture comorbidities present on arrival for surgery. The day-of-surgery problem list was also utilized for exclusion of patients with a diagnosis of ESRD, thrombocytopenia, or cirrhosis.
Case specific characteristics were determined by analysis of defined events present within the anesthesia record based on EMR reminders of the events as recorded by the anesthesiology team providing care to the patient. Case duration was defined as the difference in minutes between the marking of “anesthesia stop time” and “anesthesia start time” by the anesthesia provider. Similarly, cardiopulmonary bypass duration and aortic cross clamp duration were determined based upon intraoperative markers within the anesthesia record.
The initial hemoglobin was determined by manual chart review of the first arterial blood gas sample obtained after “anesthesia start time” was marked by the provider. All patients at our institution have a baseline arterial blood gas and activated clotting time drawn after induction of anesthesia and prior to initiation of CPB allowing a consistently timed hemoglobin baseline to be compared across patients.
Our institution routinely utilizes an intraoperative antifibrinolytic infusion for cardiac surgery. Due to the timing of our study period, administration of both aminocaproic acid and tranexamic acid occurred within our sample. The presence or absence of any antifibrinolytic was determined based upon medication administration record review.
Coagulation factor concentrate administration was determined by manual chart review of the anesthetic medication administration record. Specific coagulation factors available at our institution include recombinant Factor VIIa, Factor IX complex, 4-factor prothrombin complex concentrate (PCC), Anti-inhibitor coagulant complex, and Factor VIII.
Statistical Analysis
Stata Version 15.1 (StataCorp, College Station, TX, USA) was used for all statistical analysis other than model fitting; SAS version 9.4 (SAS Institute, Cary, NC, USA) was used to fit the models over time. The data were extracted from Epic EMR Clarity DataStore. Cases were identified by the recorded surgery classification. Product administration within the cohort was analyzed based on blood product type (pRBC, plasma, platelets, and cryoprecipitate) received. A statistical significance test using logistic regression and fitting the effect of starting time as a cubic spline was performed to determine the univariate and multivariable statistical significance of likelihood of receiving each blood product based on start time and controlling for other covariates, respectively, including initial hemoglobin, age, gender, height, ideal body weight, diabetes, peripheral vascular disease, stroke, chronic kidney disease, COPD (chronic obstructive pulmonary disease), duration of CPB, duration of aortic cross clamping, attending surgeon, and attending anesthesiologist. The formal test for the start of anesthesia time is the test of the effect of the natural cubic spline of start time. P-values <0.05 were considered significant.
Results
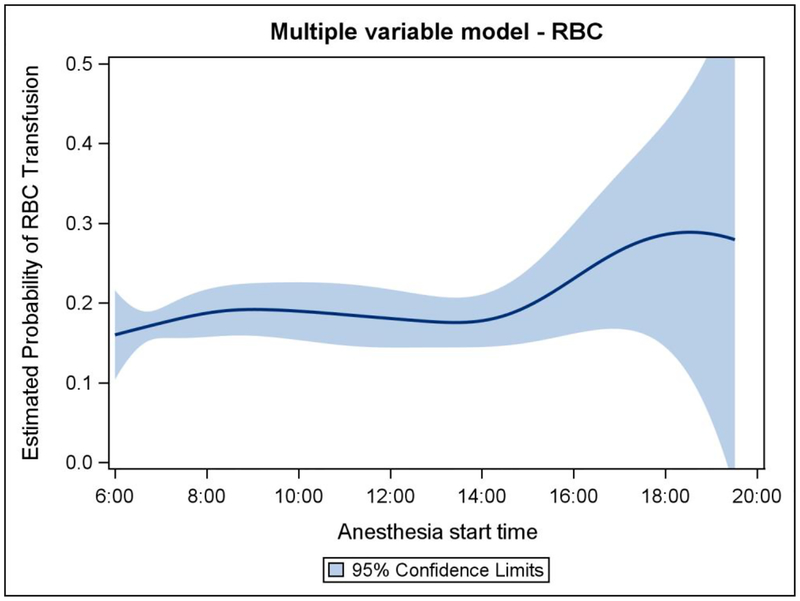
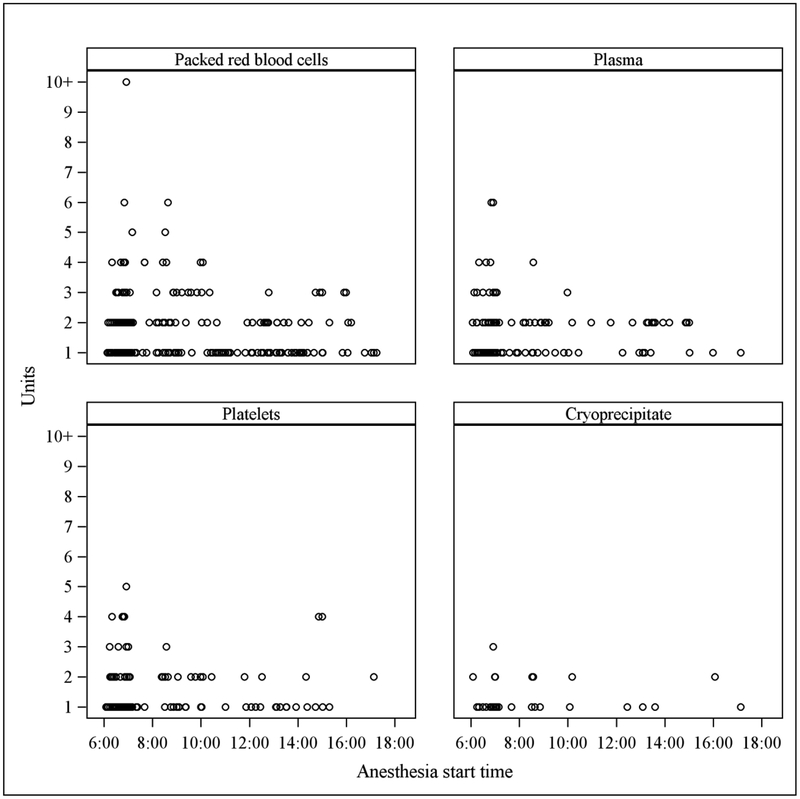
1421 eligible cardiac surgery cases met inclusion criteria for analysis (Figure 1), 1220 of which were matched for modeling. The demographics and case characteristics of matched cases are represented in Table 1. The distribution of case start times within the modeled cohort is demonstrated in Figure 2. The estimated probability of a patient receiving an intraoperative allogeneic transfusion changed significantly with later case start times in the multivariable model after adjusting for initial hemoglobin, age, gender, height, ideal body weight, diabetes, peripheral vascular disease, stroke, chronic kidney disease, chronic obstructive pulmonary disease, duration of CPB, duration of aortic cross clamping, attending surgeon, and attending anesthesiologist (p = 0.032, c statistic = 0.807, n = 1220). Figure 3 shows the estimated probability of transfusion of any blood product with respect to the case start time. The estimated probability of receiving allogeneic red blood cells changed significantly with later start times (p = 0.027, c statistic = 0.902) (Figure 4) demonstrating an increase over the course of the day. There was no observed difference in probability of transfusion for plasma (p = 0.86, c statistic = 0.782) cryoprecipitate (p = 0.46, c statistic = 0.867), or platelets (p = 0.86, c statistic = 0.778). The amount of individual blood products administered to each patient in the modeled cohort in respect to case start time is represented graphically in Figure 5.
Table 1:
Characteristics and demographics of modeled cases
| Characteristic (mean ± SD unless otherwise specified) | |
|---|---|
| Demographics | |
| Age (years) | 64.8 (11.2) |
| Sex | |
| Male | 879 (72.05%) |
| Female | 341 (27.95%) |
| IBW (kg) | 65.2 (7.9) |
| Chronic Medical Conditions | |
| Chronic Kidney Disease | 130 (10.66%) |
| COPD | 105 (8.61%) |
| Diabetes | 449 (36.80%) |
| Peripheral Vascular Disease | 38 (3.11%) |
| Cerebrovascular Accident | 104 (8.52%) |
| Case Specific Characteristics | |
| CPB duration (min) | 110 (48.3) |
| Cross clamp duration (min) | 76 (34.4) |
| Initial hemoglobin (g/dL) | 13.0 (1.9) |
| Procedure Category | |
| CABG + multiple valves | 5 (0.41%) |
| CABG +/− Maze | 777 (63.69%) |
| CABG + single valve +/− Maze | 129 (10.57%) |
| Single valve +/− Maze | 260 (21.31%) |
| Multiple valves +/− Maze | 49 (4.02%) |
Figure 2:
Histogram demonstrating the distribution of cases in the modeled cohort in respect to case start time.
Figure 3:
Multiple variable model representing the estimated probability of transfusion of any blood product (red blood cells, plasma, platelets, cryoprecipitate) with respect to case start time controlling for other covariates.
Figure 4:
Multiple variable model representing the estimated probability of transfusion of red blood cells with respect to case start time controlling for other covariates.
Figure 5:
Scatterplots of blood products received by patients who were transfused in the modeled cohort with respect to case start time. Each individual circle represents a patient.
Discussion
Perioperative transfusion practices and patterns are highly varied; this variation likely reflects the heterogeneous nature of patient populations, surgical techniques, institutional guidelines, and physician preferences among a myriad of other potential factors. We sought to delineate a sample of our population that was representative of a “standard” risk for intraoperative transfusion. We elected to confine our study to patients who were scheduled for a procedure utilizing CPB via a median sternotomy and to exclude procedures with known increased risk for hemorrhage including DHCA, repeat sternotomy, and emergency or “add-on” case posting. We excluded these cases with high bleeding risk because of the observation that these cases have a specific distribution during the day; dissections, heart transplants, and emergent revascularization procedures on patients who have not had appropriate wash-out time for antiplatelet agents are typically performed in the afternoon or evening, whereas scheduled redo sternotomies and DHCA cases are often performed as first starts. We also excluded patients with preexisting comorbidities known to increase likelihood of transfusion: ESRD, cirrhosis, and thrombocytopenia (defined by presence on the patient’s problem list on the day of surgery and not by a specific preoperative quantitative platelet assessment). To some degree this approach limits the generalizability of our results. However we believe the potential benefit of implementing these exclusions outweighs the limitations as this step may help limit the impact of potential unidentified confounders, an inherent risk of any retrospective analysis.
Initial institution data analysis (not shown) demonstrated several discrepancies in characteristics of cases based upon time of day that could represent important confounders. For example, at our institution we found that the rate of atrial fibrillation as a preexisting comorbidity was higher earlier in the day, corresponding with a higher rate of a concurrent MAZE procedure. These patients are more likely to be chronically anticoagulated and a Maze procedure prolongs CPB; both could convey a higher risk of transfusion. Additionally, there were statistically significant differences in case duration, CPB duration, aortic cross clamp duration, procedure performed, and attending surgeon based upon the time of day. This heterogeneous distribution of case and patient characteristics necessitated a statistical approach to attempt to control for this variability.
In designing this model, we attempted to include covariates related to the patient (age, gender, height, ideal body weight, initial hemoglobin, and pertinent comorbidities), the procedure (CPB duration, aortic cross clamp duration), and the involved physicians (attending cardiothoracic anesthesiologist, attending cardiothoracic surgeon).
A perioperative algorithm incorporating point-of-care testing and emphasizing the need for multidisciplinary input effectively assists clinical decision making, facilitates perioperative blood conservation efforts, and is jointly supported by the Society of Cardiovascular Anesthesiologists and the STS as a Class I recommendation with a Level of Evidence Rating of A14. Further, the 2017 European Association for Cardio-Thoracic Surgery and the European Association for Cardiothoracic Anaesthesiology Guidelines on patient blood management for adult cardiac surgery recommend the implementation of a patient blood management protocol for the bleeding patient (Class I, Level of Evidence Rating of C)15. Given the strength of the evidence, such a tool is in place at our institution that existed in its current form for the entire cohort presented in this paper. However, it should be emphasized that these tools are present to supplement clinical decision-making and is not a substitute for physician judgment. In addition, given that medical decision-making can be time-sensitive, there are expected deviations from such decision support tools. In the event of an acute large-volume hemorrhage, a massive transfusion strategy is implemented for immediate resuscitation. Finally, for this study we did not seek to determine adherence or divergence from this algorithm, thus it can serve only as a rough guide to the principles driving transfusion practices at our institution. Figure 6 provides our institution’s guideline for transfusion decision-making.
Figure 6:
Institutional transfusion guidelines for adult cardiac surgery employed during the study period.
We obtained our sample from a multi-year population of cardiac surgery patients, allowing for a large sample size and the consequent ability to match patient and case characteristics in a multivariable model. By excluding cases and patients with known high risk for bleeding, we attempted to isolate a clinically relevant sample of scheduled cases whose transfusion requirements would be anticipated to be similar. To assure the quality of our data we utilized a manual chart review to determine the starting hemoglobin, the procedure performed as delineated in the operative note, and to confirm the accuracy of the EMR in capturing transfusions.
There are several limitations inherent to our study design and methodology. The retrospective nature of this investigation precludes determination of causality. Despite our attempts to control for potential confounding variables the lack of prospective randomization introduces a risk of unrecognized confounders not accounted for in our model. Our sample includes patients from a single, academic institution, thus potentially limiting the generalizability of our results. This study is limited to intraoperative transfusion rates, and it did not include the incidence or timing of postoperative transfusions in the cardiothoracic intensive care unit. It is possible that patients who did not receive a transfusion intraoperatively were transfused shortly after arrival to the intensive care unit and this is an important area for investigation in future studies.
We were unable to determine timing or dosages of antiplatelet or anticoagulant therapy in the preoperative setting. Our population contains a significant number of external transfers as well as same day surgical admissions and thus accurate determinations of the time of last antiplatelet or anticoagulant dose were not consistently attainable on retrospective review. Further, the reliance on the EMR for determination of comorbidities requires an assumption that coexisting diagnoses were accurately entered into the patient’s problem list. The attending anesthesiologist used in our analysis was the attending anesthesiologist who started the case. The number of case handoffs between attending physicians is a potential factor in quality of care that is related to the time of day and could not be accounted for in our study. In addition, despite the existence of a transfusion algorithm as discussed above, different anesthesiologists have different blood product administration tendencies and these individual discrepancies may not be fully accounted for by our model’s inability to discern which anesthesiologist was responsible for a transfusion decision. Finally, this data was collected retrospectively leading to a discrepancy of several years between the care of the first patients in our dataset and the present time. Surgical techniques may have improved over the course of this study. Despite this potential shortcoming, institutional transfusion practices have not changed significantly in the intervening time and the same three experienced surgeons operated on all patients in this cohort.
This study highlights that anesthesiologists and researchers should consider the role time of day plays in the delivery of care by the perioperative team and the response of the patient to various procedures and inventions. Recent work in the field of chronobiology and the role time of day may play in perioperative outcomes elicited a call for additional research in this arena24. Our study provides another potential incentive for further work to explore these complex relationships.
Medical decision making requires complex cognitive processing and our understanding of the impact external influences have on cognition and decision making is still being elucidated25. One specific deleterious effect on cognitive processing related to time of day may partially explain our findings. Decision fatigue describes the psychological loss of self-control with repeated decision-making events26. In one study, researcher found that surgery is less likely to be scheduled for a patient when the patient is examined at the end of the clinician’s shift27. Professionals outside of the medical field are also subject to these forces including judges, who tend towards simplification of decisions to preserve the status quo after a prolonged session in court28. In the operating room this phenomenon would be expected to increase the risk of medical error and the likelihood of patient harm and is an area of recent interest for both quality improvement research and healthcare economics. In our study, decision fatigue may bias the physician to make what is arguably the “easier” decision to transfuse and avoid the perceived or unconscious “risk” of not transfusing. Similar to a judge subconsciously denying parole at the end of the workday due to the “ease” of maintaining the status quo and avoidance of the “risk” of releasing a potential repeat offender. It is interesting to note that we did not observe a collinear increase in the utilization rates of other blood products aside from red blood cells. One possible explanation is that physicians consider platelets, plasma, and cryoprecipitate higher risk products than red blood cells. Therefore, the conscious or unconscious cognitive risk assessment underpinning the transfusion decision biases physicians in two different directions. Thus increasing the likelihood of ordering a red blood cell transfusion as the perceived risk of adverse consequences is outweighed by the perceived benefit of transfusion while conversely decreasing the likelihood of the transfusion of other blood components where the physician has mentally assigned a greater risk of adverse events that outweigh the benefit of transfusion.
With high monetary and societal costs associated with allogeneic transfusion along with the potential risks posed to the recipient, it is important that anesthesiologists are cognizant of recommended transfusion practices. Perhaps more importantly, we should remain aware of how closely the care we deliver aligns with the best available evidence. Human, circumstantial, and physiologic factors may result in variations in the care delivered to patients undergoing cardiac surgery, perioperative transfusion practice is similarly subject to potential variability1. Provider fatigue, system-level time pressures, case burden, and healthcare economics all contribute to produce a unique scenario within which the physician must make challenging clinical decisions. On top of these more intangible factors is the ever-evolving patient complexity and surgical intricacy present within the cardiac operating rooms. Due to the lack of previous publication regarding cardiac surgical case time of day and transfusion rate, it is difficult to say if our results represent a generalizable phenomenon. We believe that the potential for increased red blood cell transfusion later in the day is something that clinicians should keep in mind in order to optimize blood management and patient care.
Conclusion
The observed rate of intraoperative allogeneic transfusion is influenced by case start times in a multivariable model of scheduled adult cardiac surgery cases utilizing cardiopulmonary bypass with later cases demonstrating a higher rate of pRBC transfusion. Further investigation of the role patient, provider, and system-level factors play in transfusion decisions is warranted.
Acknowledgments:
The authors would like to thank Amit K. Saha, PhD, Assistant Professor of Anesthesiology, Wake Forest University Department of Anesthesiology for his contributions to the data collection process and Emma O’Hagan, MLIS, Assistant Professor and Clinical Research and Education Librarian, UAB Department of Anesthesiology and Perioperative Medicine for editorial and reference assistance.
Funding Statement:
Dr. Addis is supported by the National Institutes of Health under award number T32HL129948. We would like to acknowledge the statistical analysis support of the Wake Forest Clinical and Translational Science Institute (WF CTSI), which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001420. Additional support was provided solely from the Wake Forest School of Medicine Department of Anesthesiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior Presentations: A matter of time: The effect of case start time on intraoperative transfusion rates in adult cardiac surgery, a single-center retrospective analysis. Abstract and poster presentation. 40th Annual Meeting – The Society of Cardiovascular Anesthesiologists. Sunday, April 29, 2018, Phoenix, Arizona.
Conflicts of Interest:
The authors declare no competing interests.
References
- 1.Montaigne D, Marechal X, Modine T, et al. : Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbalpha antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet. 2018; 391:59–69. [DOI] [PubMed] [Google Scholar]
- 2.Kelz RR, Freeman KM, Hosokawa PW, et al. : Time of day is associated with postoperative morbidity: an analysis of the national surgical quality improvement program data. Annals of surgery. 2008; 247:544–552. [DOI] [PubMed] [Google Scholar]
- 3.Turrentine FE, Wang H, Young JS, et al. : What is the safety of nonemergent operative procedures performed at night? A study of 10,426 operations at an academic tertiary care hospital using the American College of Surgeons national surgical quality program improvement database. The Journal of trauma. 2010; 69:313–319. [DOI] [PubMed] [Google Scholar]
- 4.Wright MC, Phillips-Bute B, Mark JB, et al. : Time of day effects on the incidence of anesthetic adverse events. Quality & safety in health care. 2006; 15:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelz RR, Tran TT, Hosokawa P, et al. : Time-of-day effects on surgical outcomes in the private sector: a retrospective cohort study. Journal of the American College of Surgeons. 2009; 209:434–445.e432. [DOI] [PubMed] [Google Scholar]
- 6.Tan PJ, Xu M, Sessler DI, et al. : Operation timing does not affect outcome after coronary artery bypass graft surgery. Anesthesiology. 2009; 111:785–789. [DOI] [PubMed] [Google Scholar]
- 7.Scott BH, Seifert FC, Grimson R: Blood transfusion is associated with increased resource utilisation, morbidity and mortality in cardiac surgery. Annals of cardiac anaesthesia. 2008; 11:15–19. [DOI] [PubMed] [Google Scholar]
- 8.Bhaskar B, Dulhunty J, Mullany DV, et al. : Impact of blood product transfusion on short and long-term survival after cardiac surgery: more evidence. The Annals of thoracic surgery. 2012; 94:460–467. [DOI] [PubMed] [Google Scholar]
- 9.Rady MY, Ryan T, Starr NJ: Perioperative determinants of morbidity and mortality in elderly patients undergoing cardiac surgery. Critical care medicine. 1998; 26:225–235. [DOI] [PubMed] [Google Scholar]
- 10.Hajjar LA, Vincent JL, Galas FR, et al. : Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. Jama. 2010; 304:1559–1567. [DOI] [PubMed] [Google Scholar]
- 11.Santos AA, Sousa AG, Piotto RF, et al. : Mortality risk is dose-dependent on the number of packed red blood cell transfused after coronary artery bypass graft. Revista brasileira de cirurgia cardiovascular : orgao oficial da Sociedade Brasileira de Cirurgia Cardiovascular. 2013; 28:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snyder-Ramos SA, Mohnle P, Weng YS, et al. : The ongoing variability in blood transfusion practices in cardiac surgery. Transfusion. 2008; 48:1284–1299. [DOI] [PubMed] [Google Scholar]
- 13.Bennett-Guerrero E, Zhao Y, O’Brien SM, et al. : Variation in use of blood transfusion in coronary artery bypass graft surgery. Jama. 2010; 304:1568–1575. [DOI] [PubMed] [Google Scholar]
- 14.Ferraris VA, Brown JR, Despotis GJ, et al. : 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. The Annals of thoracic surgery. 2011; 91:944–982. [DOI] [PubMed] [Google Scholar]
- 15.Boer C, Meesters MI, Milojevic M, et al. : 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. Journal of cardiothoracic and vascular anesthesia. 2018; 32:88–120. [DOI] [PubMed] [Google Scholar]
- 16.Diegeler A, Borgermann J, Kappert U, et al. : Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. The New England journal of medicine. 2013; 368:1189–1198. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann E, Zhu R, Ogami T, et al. : Intraoperative Autologous Blood Donation Leads to Fewer Transfusions in Cardiac Surgery. The Annals of thoracic surgery. 2019; 10.1016/j.athoracsur.2019.06.091 [DOI] [PubMed] [Google Scholar]
- 18.Curley GF, Shehata N, Mazer CD, et al. : Transfusion triggers for guiding RBC transfusion for cardiovascular surgery: a systematic review and meta-analysis*. Critical care medicine. 2014; 42:2611–2624. [DOI] [PubMed] [Google Scholar]
- 19.Reeves BC, Pike K, Rogers CA, et al. : A multicentre randomised controlled trial of Transfusion Indication Threshold Reduction on transfusion rates, morbidity and health-care resource use following cardiac surgery (TITRe2). Health technology assessment (Winchester, England). 2016; 20:1–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura RE, Vincent JL, Fukushima JT, et al. : A liberal strategy of red blood cell transfusion reduces cardiogenic shock in elderly patients undergoing cardiac surgery. The Journal of thoracic and cardiovascular surgery. 2015; 150:1314–1320. [DOI] [PubMed] [Google Scholar]
- 21.Simon GI, Craswell A, Thom O, et al. : Outcomes of restrictive versus liberal transfusion strategies in older adults from nine randomised controlled trials: a systematic review and meta-analysis. The Lancet. Haematology. 2017; 4:e465–e474. [DOI] [PubMed] [Google Scholar]
- 22.Mazer CD, Whitlock RP, Fergusson DA, et al. : Restrictive or Liberal Red-Cell Transfusion for Cardiac Surgery. The New England journal of medicine. 2017; 377:2133–2144. [DOI] [PubMed] [Google Scholar]
- 23.Shehata N, Mistry N, da Costa BR, et al. : Restrictive compared with liberal red cell transfusion strategies in cardiac surgery: a meta-analysis. European heart journal. 2019; 40:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin D, McKenna H, Galley H: Rhythm and cues: role of chronobiology in perioperative medicine. British journal of anaesthesia. 2018; 121:344–349. [DOI] [PubMed] [Google Scholar]
- 25.Stiegler MP, Tung A: Cognitive processes in anesthesiology decision making. Anesthesiology. 2014; 120:204–217. [DOI] [PubMed] [Google Scholar]
- 26.Vohs KD, Baumeister RF, Schmeichel BJ, et al. : Making choices impairs subsequent self-control: a limited-resource account of decision making, self-regulation, and active initiative. Journal of personality and social psychology. 2008; 94:883–898. [DOI] [PubMed] [Google Scholar]
- 27.Persson E, Barrafrem K, Meunier A, et al. : The effect of decision fatigue on surgeons’ clinical decision making. Health economics. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danziger S, Levav J, Avnaim-Pesso L: Extraneous factors in judicial decisions. Proceedings of the National Academy of Sciences of the United States of America. 2011; 108:6889–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]