Abstract
Objective:
The present study investigates whether associations between telomere length (TL) and cognitive performance across multiple domains are moderated by poverty status and race.
Methods:
Participants were 325 African American and White urban-dwelling adults (M age = 47.9 years; 49.5% African American; 50.2% female; 48.9% living in poverty) from the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. TL was assayed from peripheral blood mononuclear cells using quantitative polymerase chain reactions. Multivariable regression analyses examined interactions of TL, poverty status, and race with performance on the following cognitive tests: Trail-Making Test Parts A and B, Digit Span Forward and Backward, semantic verbal fluency, Brief Test of Attention, Benton Visual Retention Test (BVRT), and California Verbal Learning Test-II total learning, short-delay free recall, and long-delay free recall scores. Analyses adjusted for age, sex, and high school-or-greater educational attainment.
Results:
Significant three-way interactions of TL × Poverty Status × Race revealed that, among White participants living in poverty, shorter TL was associated with worse performance on Digit Span Forward and Backward (p’s<.05). Additionally, significant two-way interactions of TL × Poverty Status revealed that, among all participants living in poverty, shorter TL was associated with worse performance on the Trail-Making Test Part B and the BVRT (p’s<.05).
Conclusions:
TL may be differentially associated with aspects of attention, executive functioning, and memory among individuals living in poverty, who may be uniquely vulnerable to adverse effects of shorter telomeres. Replication of these findings is needed to determine their generalizability.
Keywords: telomere length, poverty, race, cognitive function, aging
Telomeres are the caps on the ends of chromosomes that are crucial for protecting genetic material. Telomeres shorten during the process of mitosis, and experience cumulative shortening over the lifespan of cells. Because telomere attrition is associated with cellular dysfunction, senescence, and apoptosis, TL has been proposed as a primary indicator of cellular aging (Chan & Blackburn, 2004). In humans, shorter TL has been linked with all-cause mortality (Cawthon, Smith, O’Brien, Sivatchenko, & Kerber, 2003; Kimura et al., 2008), as well as several age-related chronic diseases, including type-II diabetes mellitus (Zhao, Miao, Wang, Ding, & Wen Wang, 2013), cardiovascular disease (Haycock et al., 2014; Serrano & Andrés, 2004), and reduced immune response to influenza vaccine (Najarro et al., 2015). With regard to clinical brain health endpoints, several studies have found that individuals with Alzheimer’s disease and other forms of dementia have shorter TL than healthy controls (Grodstein et al., 2008; Hochstrasser, Marksteiner, & Humpel, 2012; Kume et al., 2012; von Zglinicki et al., 2000).
Telomere Length and Cognitive Function
Several studies have also examined relations between TL and cognitive functioning in individuals without dementia, although to date, the evidence is equivocal. Some studies have found relations between longer TL and better performance on the Mini Mental State Examination (Folstein, Folstein, & McHugh, 1975) and other global cognitive screening measures (Ma et al., 2013; Martin-Ruiz et al., 2006; Yaffe et al., 2011), while others did not (Bendix et al., 2011; Harris et al., 2006). Using a more extensive neuropsychological battery, a cross-sectional study of 382 non-demented women revealed that shorter TL was associated with poorer learning and episodic memory, recognition memory for nonverbal items, and working memory (Valdes et al., 2010). However, at least two studies using ample cognitive measures did not find significant associations between TL and baseline cognitive functioning or cognitive decline (Harris, Martin-Ruiz, von Zglinicki, Starr, & Deary, 2012; Mather et al., 2010). These equivocal findings may result, at least in part, from methodological variability across studies. For example, studies have examined different populations and estimated cognitive functioning differently (i.e., some have utilized composites, whereas others have examined specific domains of function).
Prior inconsistencies in the literature may be explained, in part, by variable relations of TL and cognitive function across sociodemographic groups. In that regard, the TL-cognition association may be only found, or may be more pronounced, among members of socially disadvantaged groups. A review of the literature on sociodemographic factors, TL, and cognitive function reveals poverty status and race as potentially key moderating variables. One might posit that greater burden of biopsychosocial risk factors and fewer protective factors among individuals living in poverty and/or African Americans might yield a greater vulnerability to poorer cognitive outcomes as a result of shorter TL in these subgroups. The present study explores this possibility by examining whether poverty status and race moderate associations between TL and cognitive functioning across a range of domains. This methodological approach is consistent with recommendations by Williams and colleagues (2012) to consider both independent and interactional contributions of race and socioeconomic status (SES) in health disparities research. To our knowledge, this is the first study to examine interactive relations among TL, poverty status, and race with cognitive functioning.
Telomere Length and Sociodemographic Factors
Sociodemographic moderation of TL-cognition associations could occur due to TL variability between groups. Although TL attrition occurs with normal aging, exposure to repeated or prolonged psychological stress has been shown to accelerate shortening of telomeres (Epel et al., 2004). Therefore, it is plausible that sociodemographic groups exposed to greater rates of chronic stress may have accelerated TL attrition compared other groups with less stress exposure (Geronimus et al., 2010). To that end, previous studies have examined race- and poverty-related disparities in TL, though findings have been inconsistent. Some studies found that African Americans have longer TL than Whites (Aviv et al., 2009; Brown, Needham, & Ailshire, 2017; Hunt et al., 2008; Lynch et al., 2016; Zhu et al., 2011), whereas at least one study found that African Americans have shorter TL than their White counterparts (Diez Roux et al., 2009). Findings on poverty-related disparities in TL are also mixed. Some studies report significant associations between poverty and shortened TL (Theall, Brett, Shirtcliff, Dunn, & Drury, 2013), while others have found non-significant associations between income and TL (Cherkas et al., 2006; Needham et al., 2013; Steptoe et al., 2011).
As Geronimus and colleagues (2015) note, few studies examining socioeconomic characteristics and TL have racially/ethnically diverse samples. Likewise, TL studies examining racial variation have sometimes neglected to include socioeconomic measures in their analyses altogether (Hunt et al., 2008). To remediate these methodological limitations, Geronimus and colleagues (2015) examined interactive relations among race/ethnicity and poverty with TL. They found that poor Whites had shorter TL than non-poor Whites, whereas poor and non-poor African Americans had statistically equivalent TL.
Alternatively, sociodemographic moderation of TL-cognition associations could occur as a result of increased vulnerability of select, disadvantaged groups, such as those living in poverty and African Americans, to shortened TL due to other causes. Socioeconomic status and race are social constructs that influence health, including cognitive functioning, through complex, multilevel pathways (G. W. Evans & Kantrowitz, 2002; Glymour & Manly, 2008). For example, those of lower SES are more likely than their higher SES counterparts to experience chronic stressors, neighborhood deprivation, and exposure to environmental toxins (Baum, Garofalo, & Yali, 1999; G. W. Evans & Kantrowitz, 2002). Likewise, African Americans are more likely to be of lower socioeconomic position, and be exposed to geographic segregation, poorer material conditions, inadequate nutrition, and interpersonal discrimination than Whites (Glymour & Manly, 2008). Given the multitude of vulnerability factors present in these groups that may influence cognitive health, it is plausible that shortened TL will have more profound influence on the neurocognitive health of those of living in poverty and/or African Americans.
Potential Role of Cardiometabolic, Inflammatory, and Psychosocial Risk Factors
Associations between TL and cognitive function may be explained, at least in part, by correlated age-related diseases and risk factors, among them inflammation and cardiometabolic diseases, such as hypertension and diabetes (Wang et al., 2016; Yeh & Wang, 2016). As discussed, TL shortening is a driver of cellular aging and senescence, which increases risk for age-related diseases and chronic inflammatory states (Blackburn, Epel, & Lin, 2015). Cumulative burden of cardiovascular disease and inflammation is also associated with cognitive aging and may contribute to dementia onset (Breteler, Claus, Grobbee, & Hofman, 1994; Knopman et al., 2001; Newman et al., 2005). Given these relations, it is plausible that cardiometabolic and inflammatory risk factors partially explain TL-cognition associations. Likewise, psychiatric illness, substance abuse, and domestic abuse are considered risk factors for lower cognitive functioning (Bruijnen et al., 2019; Fioravanti, Bianchi, & Cinti, 2012; Ramey & Regier, 2019; Rock, Roiser, Riedel, & Blackwell, 2014; Stein, Kennedy, & Twamley, 2002) and may also be linked to shorter TL (Darrow et al., 2016; Drury et al., 2014; Pavanello et al., 2011; Yang et al., 2013). Therefore, the present study includes sensitivity analyses that examine whether significant effects of TL or its interaction with poverty status and/or race were eliminated following adjustment for several key cardiometabolic (obesity, diabetes, hypertension), inflammatory (high-sensitivity C-reactive protein [CRP]), and psychosocial (psychiatric, substance use, and domestic abuse history) risk factors.
The Present Study
This study sought to clarify the divergent findings in the literature by examining the relation of TL and cognitive function across poverty lines and racial groups. Additionally, we utilized an extensive neuropsychological battery to better elucidate the specific cognitive domains that may be particularly vulnerable to the impact of poverty status, race, and TL. Domains assessed were attention, working memory, executive functioning, psychomotor speed, semantic verbal fluency, verbal learning and memory, and nonverbal immediate memory. Here, we examined interactive relations among TL, poverty status, and race with cognitive performance in a socioeconomically and racially diverse sample of community-dwelling adults. We hypothesized relatively shorter TL would have stronger negative effects on cognitive function among African Americans and those living in poverty. Given inconsistencies in the literature, three-way interactive relations among TL, poverty status, and race with cognitive function were exploratory. Lastly, we computed sensitivity analyses to examine whether significant effects were eliminated following adjustment for several cardiometabolic, inflammatory, and psychosocial risk factors.
Methods
Parent Study Procedure and Participants
As previously described elsewhere (M. K. Evans et al., 2010), Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) is an ongoing longitudinal investigation of age-related disparities in health and disease attributable to race and SES. Briefly, the HANDLS sample is a fixed cohort of urban-dwelling adults living initially within 13 neighborhoods (contiguous census tracts) in the city of Baltimore, MD. The neighborhoods were pre-determined for their likelihood of yielding representative samples of participants who were African American and White, men and women, and with annual household incomes above and below 125% of the 2004 federal poverty guidelines. All HANDLS participants self-identified their race/ethnicity as African American or White and were between the ages of 30–64 years at baseline. The Institutional Review Board at the National Institute of Environmental Health Sciences approved the HANDLS protocol.
The first wave of HANDLS occurred between 2004–2009. Data collection took place within participants’ households and on medical research vehicles (MRV) located within participants’ neighborhoods, where they completed a medical history assessment, physical examinations, biological sample collections, cognitive testing, and other assessments. After initial selection, participants were excluded from further participation in HANDLS if they met any of the following criteria at baseline: (1) were unable to provide informed consent, (2) were pregnant, (3) were within six months of active cancer treatment (i.e., chemotherapy, radiation, or biological treatments), (4) self-reported a diagnosis of acquired immune deficiency syndrome, (5) were unable to provide valid government-issued identification, or did not have a verifiable address.
There were 3,720 participants who enrolled in the study, of whom 2,799 completed the initial MRV visit. All but 69 of those who completed the MRV visit consented to genetic analyses. Of these participants, 360 with DNA in the biorepository from Waves 1 and 3 of HANDLS (i.e., baseline and the next in-person follow-up examination) were randomly selected from a cross of race, sex, and baseline age (median-split) for telomere assays. The present study’s overall analytic sample included 325 participants with valid data for TL, sociodemographic characteristics (poverty status, race, age, sex, and high school-or-greater educational attainment), sensitivity covariates (obesity, diabetes, hypertension, systemic inflammation, psychiatric history, substance use history, and domestic abuse exposure), and at least one cognitive test at baseline. Analysis-specific samples varied due to unequal missing data across the cognitive tests, ranging from 273–320 participants. In the analytic sample, age, race, sex, and poverty status were not associated with completion of any of the cognitive tests (all p’s > .05).
Telomere Assay
TL was measured by the quantitative polymerase chain reaction (qPCR)-based method described previously by Cawthon (2002). Briefly, 10 ng of DNA isolated from peripheral blood mononuclear cells (PBMC) was used in each PCR reaction in triplicates for each participant. Both telomere (T) and a single copy gene (36B4) (S) were included in the same 384-well plate using SYBR master mix on an Applied Biosystem 7900HT system (ThermoFisher). The average cycle threshold (Ct) values of T and S were calculated from the triplicates to generate the average T/S ratio of each sample. To convert T/S ratio into TL in kilobases (kb), we measured 130 samples by both qPCR and the Southern method (Lin et al., 2015) and used the resulting conversion equation to calculate TL in kb from the T/S ratio.
Sociodemographic Information
Participants reported their age in years, biological sex (0 = women, 1 = men), and self-identified race (0 = White, 1 = African American). Annual household income (adjusted for household size) was used to classify participants as living above (0) or below (1) 125% of the 2004 Health and Human Services Poverty Guidelines. Educational attainment was dichotomized as ≥ high school diploma/graduate equivalency diploma (GED) (0) and < high school diploma/GED (1).
Cognitive Test Outcomes
Participants completed a cognitive test battery during the MRV visit. The present study used participants’ scores on the following tests: Trail-Making Test Parts A and B; Digit Span Forward and Digit Span Backward from the Wechsler Adult Intelligence Scale-Revised (WAIS-R); Brief Test of Attention; semantic verbal fluency; Benton Visual Retention Test (BVRT) total errors; and the California Verbal Learning Test-II (CVLT) total learning, short-delay free recall, and long-delay free recall scores.
Trail-Making Test Parts A and B.
The Trail-Making Test is a test of attention, sequencing, mental flexibility, visual search, and motor functioning (Strauss, Sherman, & Spreen, 2006). Part A requires the participant to make connections between 25 encircled numbers randomly arranged on a page, in sequential order. Part B requires them to connect 25 encircled numbers and letters in alternating order. The outcome variable is time in seconds; errors count only in slower performance time. While the normative time cutoff for the Trail Making Test Part B is 300 seconds (Strauss et al., 2006), we extended the cutoff to 600 seconds to allow for greater variability in task performance. Given skewness in this variable, it was log-transformed to normalize the distribution.
WAIS-R Digit Span Forward and Backward.
Total scores on Digit Span Forward and Digit Span Backward, subtests of the WAIS-R, were used to measure attention and working memory, respectively (Lezak, Howieson, Bigler, & Tranel, 2012). For Digit Span Forward, participants listened to a series of numbers read aloud by an examiner and then repeated the numbers back in the same order. For Digit Span Backward, participants were instructed to repeat the series of digits in reverse order. Each test was discontinued when participants failed to complete two trials of the same span length. The score on each test is the number of correct span trials.
Brief Test of Attention.
Total score on the Brief Test of Attention was used as an additional measure of auditory attention. Following standard administration (for the manual, see Schretlen, Bobholz, & Brandt, 2007), participants were presented with two parallel forms of numbers and letters via audio recording that contained ten lists increasing from 4–18 items. During administration of the first form, participants were instructed to listen and count the numbers while disregarding the letters. During administration of the second form, they were instructed to count the letters while disregarding the numbers. The score is the number of correctly monitored lists summed across both forms.
Verbal fluency.
Semantic verbal fluency was assessed by evaluating the spontaneous production of words of a given semantic category within a minute. Participants were instructed to name as many animals as fast as they can. The score is the total number of admissible words (Strauss et al., 2006).
Benton Visual Retention Test.
The BVRT was used to measure nonverbal immediate memory (for the manual, see Sivan, 1992). Participants were presented with ten designs, one at a time, for five seconds. The first two designs contained one geometric shape, whereas the latter eight designs contained two larger figures and one smaller figure. After the five-second presentation, the designs were withdrawn, and the participants were then instructed to draw them from memory. Figures were scored according to the manual instructions, and two examiners independently scored the figures to ensure interrater agreement. Errors in the drawings were coded as omissions, distortions, perseverations, rotations, misplacements, and incorrect size. In the proposed study, total number of errors across the ten figures was used to measure nonverbal immediate memory, such that lower scores indicated fewer errors and therefore better performance.
California Verbal Learning Test-II.
The CVLT was used to measure verbal learning and memory. HANDLS adapted the CVLT by using three trials of a 16-word list (versus five trials in standard administration; for the manual, see Delis, Kramer, Kaplan, & Ober, 2000). Three outcome variables from the CVLT were assessed. First, verbal learning was assessed with the CVLT learning trials score, which measured the total number of correctly recalled words over the three learning trials. Second, short-term verbal memory was assessed with the CVLT short-delay free recall score, which measured the total number of correctly recalled words following a short delay that consisted of administration of an alternate word list for the purposes of interference (consistent with standard administration). Lastly, long-term verbal memory was assessed with the CVLT long-delay free recall score, which measured the total number of correctly recalled words following a 25-minute delay.
Sensitivity Variables
Obesity, diabetes, hypertension, systemic inflammation, psychiatric history, substance use history, and domestic abuse exposure were assessed as additional adjustment variables in sensitivity analyses. Obesity was dichotomized from body mass index (BMI), which was computed as weight divided by height squared (kg/m2) using height and weight obtained with calibrated equipment by a trained technician. Participants with BMI < 30 were categorized as not obese (0), whereas those with BMI ≥ 30 were categorized as obese (1). Diabetes was determined by a fasting blood glucose level of > 126 mg/dl (assessed by standard laboratory methods at Quest Diagnostics in Chantilly, VA; http://www.questdiagnostics.com), self-reported history, or use of relevant medications. Hypertension was determined by self-reported history, use of anti-hypertensive medications, or resting systolic or diastolic blood pressures > 140 mm Hg or > 90 mm Hg, respectively. Diabetes and hypertension were then dichotomized into absent (0) or present (1). Inflammation was assessed with high-sensitivity CRP levels, which were measured from blood samples by immunoassay at the National Institute on Aging or Quest Diagnostics using similar equipment and reagents.
Psychiatric and substance use history were assessed with separate, dichotomous composite variables. Participants self-reported their psychiatric diagnoses and substance use history during the medical history assessment on the MRV. The psychiatric history composite indicated the absence (0) or presence (1) of any of the following mental health diagnoses: depression, anxiety disorder(s), bipolar disorder, and/or schizophrenia. For the substance use history composite, participants were classified as having Never used regularly (0) or Ever used regularly (1) one or more of the following substances: cigarettes, alcohol, marijuana, cocaine/crack, and/or opiates. Finally, history of exposure to domestic abuse was assessed with a dichotomous variable (0 = no exposure, 1 = exposed).
Statistical Approach
Statistical analyses were conducted with the Statistical Package for the Social Sciences (SPSS) version 25. Multivariable regression analyses examined higher-order effects up to three-way interactions, which included (1) TL, (2) poverty status, and (3) race. All analyses began with one three-way interaction term, all two-way interaction terms nested beneath it, as well as main effects and adjustment variables (i.e., participants’ age, sex, and high school-or-greater educational attainment).
Data analysis proceeded by backward elimination of nonsignificant interactions, which guides the removal of non-significant, higher-level interaction terms from regression analyses (Morrell, Pearson, & Brant, 1997). As such, any non-significant three-way interaction terms were removed first, after which analyses were rerun, followed by the removal of non-significant two-way interaction terms while significant interactions were retained. The final regression analysis for each set included (1) the highest-order significant interaction term, (2) lower-order interaction terms nested beneath it, as well as (3) main effects and adjustment variables.
The PROCESS macro for SPSS, Version 2.16 (Hayes, 2013) was used to test and visualize significant conditional effects. PROCESS is a useful statistical tool for examining the conditional effects of two- and three-way interactions in moderated linear regression.
Sensitivity analyses were conducted by adding obesity, diabetes, hypertension, and CRP as adjustment variables into the aforementioned models that yielded significant effects of TL with cognitive performance. Sensitivity covariates were added into the models separately to evaluate each variable’s unique influence on the significance of the TL effects. Due to missing data for CRP (n = 15 missing), data were imputed using a predictive mean matching method within the ‘mice’ package for R version 3.5.2 (R Core Team, 2018; van Buuren & Groothuis-Oudshoorn, 2011).
Results
Demographics and Variable Characteristics
There were no significant differences in age, sex, obesity, diabetes, CRP, substance use history, domestic abuse exposure, or TL by race and poverty status (Table 1). Individuals living above the poverty line and African Americans were more likely to have a high school diploma or GED than those living in poverty and Whites, respectively (p’s < .05). African Americans were more likely to be diagnosed with hypertension than Whites, χ2(1) = 4.95, p = .026, whereas Whites were more likely to be diagnosed with a psychiatric illness than African Americans, χ2(1) = 14.31, p < .001. Those living above the poverty line were more likely to be employed in the last month than those living in poverty, χ2(1) = 25.76, p < .001.
Table 1.
Demographic Characteristics in the Overall Sample and Stratified by Race and Poverty Status
| Variable | White (n = 164) |
African American (n = 161) |
sig. | Above Poverty (n = 166) |
Below Poverty (n = 159) |
sig. | Overall (N = 325) |
|---|---|---|---|---|---|---|---|
| Age, M (SD) | 48.28 (8.13) | 47.62 (9.63) | 47.66 (9.47) | 48.22 (8.33) | 47.93 (8.91) | ||
| Female, % | 50.0% | 50.3% | 50.6% | 49.7% | 50.2% | ||
| African American, % | — | — | 50.0% | 49.1% | 49.5% | ||
| Below Poverty, % | 49.4% | 48.4% | — | — | 48.9% | ||
| < High school/GED, % | 33.5% | 16.8% | *** | 18.1% | 32.7% | ** | 25.2% |
| Obesity, % | 40.2% | 38.5% | 41.1% | 37.7% | 39.4% | ||
| Diabetes, % | 14.0% | 15.5% | 15.7% | 13.8% | 14.8% | ||
| Hypertension, % | 33.5% | 45.3% | * | 36.7% | 42.1% | 39.4% | |
| C-reactive protein, M (SD) | 4.34 (7.84) | 5.68 (14.97) | 4.22 (7.76) | 5.82 (15.07) | 5.00 (11.92) | ||
| Currently not employed, % | 39.0% | 38.1% | 75.3% | 47.4% | *** | 59.4% | |
| Psychiatric diagnosis, % a | 39.0% | 19.9% | *** | 25.3% | 34.0% | 29.5% | |
| Substance use, % b | 81.7% | 84.5% | 81.3% | 84.9% | 83.1% | ||
| Domestic abuse, % | 7.3% | 6.8% | 5.4% | 8.8% | 7.1% | ||
| Telomere length, M (SD) | 5.66 (0.72) | 5.63 (0.70) | 5.69 (0.65) | 5.59 (0.76) | 5.64 (0.71) |
Note. Independent samples t-tests and chi-square tests of independence were used to assess differences between race and poverty groups,
p < .05,
p < .01,
p < .001
Percentage of participants with any of the following psychiatric diagnoses: depression, anxiety disorder(s), bipolar disorder, or schizophrenia
Percentage of participants who ever regularly used any of the following substances: cigarettes, alcohol, marijuana, cocaine/crack, and/or opiate.
Age, sex, and poverty status were not associated with consenting for genetic analyses. White participants were more likely to consent to genetic analyses than African Americans (OR = 2.2, 95% CI [1.28,3.94], p = .006).
Moderation Analyses
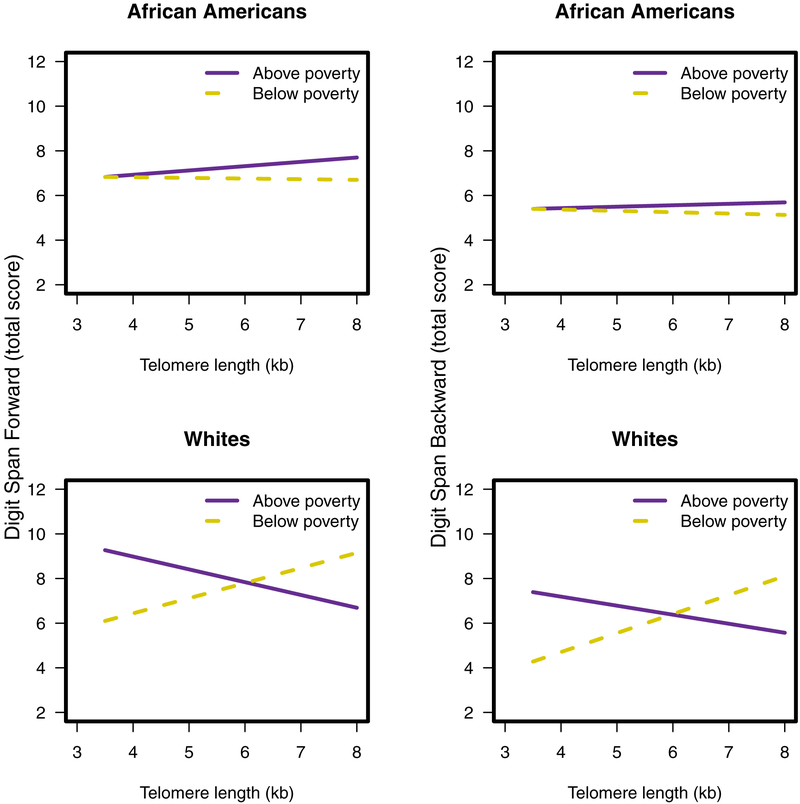
There were significant three-way interactions of TL × Poverty Status × Race with Digit Span Forward, b = −1.46, p = .036, sr2 = .01, and Digit Span Backward, b = −1.34, p = .046, sr2 = .01 (Table 2). Among Whites in poverty, greater TL was associated with better performance on Digit Span Forward, b = 0.67, p = .033, and Digit Span Backward, b = 0.83, p = .007 (Figure 1). There were no other three-way interaction effects, therefore the three-way interaction term was removed from subsequent analyses.
Table 2.
Interactive Relations of Telomere Length, Poverty Status, and Race with Digit Span Forward and Backward, Final Models
| (a) Digit Span Forward (n = 317) | ||||
|---|---|---|---|---|
| Variable | b | se | p | sr2 |
| Telomere length × Poverty Status × Race * | −1.46 | 0.69 | .036 | .01 |
| Telomere Length × Poverty Status * | 1.26 | 0.49 | .010 | .02 |
| Telomere Length × Race | 0.78 | 0.52 | .131 | .01 |
| Poverty Status × Race * | 8.16 | 3.94 | .039 | .01 |
| Telomere length | −0.59 | 0.38 | .117 | .01 |
| Poverty status ** | −7.47 | 2.79 | .008 | .02 |
| Race | −5.11 | 2.96 | .085 | .01 |
| Age | −0.02 | 0.01 | .130 | .01 |
| Sex | −0.14 | 0.25 | .575 | <.01 |
| Education ** | −0.83 | 0.29 | .004 | .03 |
| (b) Digit Span Backward (n = 317) | ||||
| Variable | b | se | p | sr2 |
| Telomere Length × Poverty Status × Race * | −1.34 | 0.67 | .046 | .01 |
| Telomere Length × Poverty Status ** | 1.26 | 0.47 | .008 | .02 |
| Telomere Length × Race | 0.47 | 0.50 | .349 | <.01 |
| Poverty Status × Race * | 7.66 | 3.82 | .046 | .01 |
| Telomere length | −0.43 | 0.37 | .236 | .01 |
| Poverty status ** | −7.43 | 2.71 | .006 | .01 |
| Race | −3.59 | 2.87 | .212 | .02 |
| Age | −0.02 | 0.01 | .115 | .01 |
| Sex | −0.01 | 0.24 | .979 | <.01 |
| Education *** | −1.46 | 0.28 | <.001 | .08 |
Note. Education = High school-or greater educational attainment sr2 = semipartial correlations squared;
p < .05,
p < .01,
p < .001
Figure 1.
Three-way interactions between TL, poverty status, and race with Digit Span Forward (left) and Digit Span Backward (right).
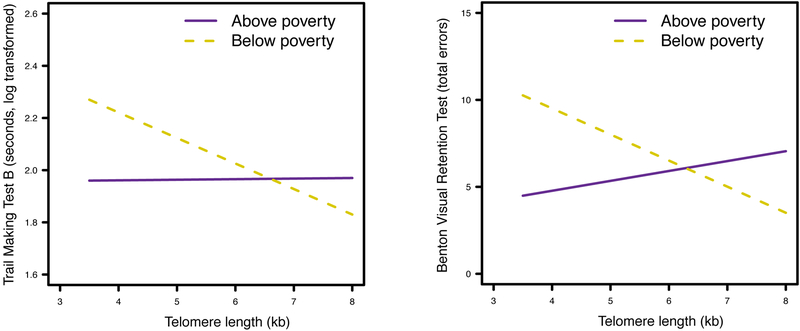
Findings then revealed significant two-way interactions of TL × Poverty Status with the Trail-Making Test Part B, b = −0.10, p = .041, sr2 = .01, and the BVRT, b = −2.06, p = .008, sr2 = .02 (Table 3). Among those living in poverty, greater TL was associated with faster performance on the Trail-Making Test Part B, b = −0.10, p = .003, and fewer total errors on the BVRT, b = −1.48, p = .005 (Figure 2). No significant interactions or main effects of TL were found for the other cognitive tests (all p’s > .05; see Supplementary Tables 1–6 for all regression models with the Trail Making Test Part A, Brief Test of Attention, semantic verbal fluency, and all CVLT subtests).
Table 3.
Interactive Relations of Telomere Length, Poverty Status, and Race with the Trail Making Test Part B and Benton Visual Retention Test, Final Models
| (a) Trail Making Test Part B (n = 320) | ||||
|---|---|---|---|---|
| Variable | b | se | p | sr2 |
| Telomere Length × Poverty Status ** | −1.00 | 0.05 | .041 | .01 |
| Telomere length | 0.003 | 0.04 | .935 | <.01 |
| Poverty status ** | 0.67 | 0.28 | .017 | .02 |
| Race | 0.17 | 0.04 | <.001 | .07 |
| Age | 0.01 | 0.002 | .008 | .02 |
| Sex | 0.28 | 0.04 | .412 | <.01 |
| Education *** | 0.15 | 0.04 | <.001 | .04 |
| (b) Benton Visual Retention Test (n = 319) | ||||
| Variable | b | se | p | sr2 |
| Telomere Length × Poverty Status ** | −2.06 | 0.77 | .008 | .02 |
| Telomere length | 0.58 | 0.58 | .314 | <.01 |
| Poverty status ** | 12.81 | 4.36 | .004 | .03 |
| Race | 1.12 | 0.55 | .041 | .01 |
| Age | 0.11 | 0.03 | .001 | .04 |
| Sex | −1.25 | 0.54 | .022 | <.02 |
| Education *** | 2.38 | 0.64 | < .001 | .04 |
Note. Education = High school-or greater educational attainment sr2 = semipartial correlations squared;
p < .05,
p < .01,
p < .001
Figure 2.
Two-way interactions between TL and poverty status with the Trail Making Test Part B (left) and the BVRT (right).
Sensitivity Analyses
Sensitivity analyses examined whether previously observed significant effects attenuated following adjustment for key risk factors, namely obesity, diabetes, hypertension, systemic inflammation, psychiatric history, substance use history, or domestic abuse exposure (examined separately). The two-way interaction of TL × Poverty Status with the Trail-Making Test Part B became nonsignificant (i.e., p ≥ .05) following adjustment for (a) obesity, b = −0.10, p = .053, sr2 = .01, (b) hypertension, b = −0.10, p = .051, sr2 = .01, and (c) CRP, b = −0.10, p = .050, sr2 = .01 (Table 4). Conversely, the interaction of TL × Poverty Status with the Trail Making Test Part B remained significant following adjustment for diabetes, b = −0.10, p = .045, sr2 = .01. In addition, the three-way interactions of TL × Poverty Status × Race with Digit Span Forward and Digit Span Backward and the two-way interaction of TL × Poverty Status with the BVRT remained significant following adjustment for hypertension, diabetes, obesity, systemic inflammation, psychiatric history, substance use history, or domestic abuse exposure (p’s < .05).
Table 4.
Regression coefficients of Interaction Effects After Adjustment for Additional Covariates
| (a) Interaction of Telomere Length × Poverty Status × Race with Digit Span Forward and Backward | ||||
|---|---|---|---|---|
| Digit Span Forward (n = 317) | Digit Span Backward (n = 317) | |||
| Covariate | b | sr2 | b | sr2 |
| Previous model a | −1.46* | .01 | −1.34* | .01 |
| Obesity | −1.46* | .01 | −1.34* | .01 |
| Diabetes | −1.46* | .01 | −1.33* | .01 |
| Hypertension | −1.51* | .01 | −1.36* | .01 |
| CRP | −1.47* | .01 | −1.45* | .01 |
| Psychiatric history | −1.46* | .01 | −1.33* | .01 |
| Substance use history | −1.46* | .01 | −1.33* | .01 |
| Domestic abuse | −1.46* | .01 | −1.35* | .01 |
| (b) Interaction of Telomere Length × Poverty Status with Trail Making Test Part B and the BVRT | ||||
| Trail Making Test Part B (n = 320) | BVRT (n = 319) | |||
| Covariate | b | sr2 | b | sr2 |
| Previous model b | −0.10* | .01 | −2.06** | .02 |
| Obesity | −0.10 | .01 | −1.98** | .02 |
| Diabetes | −0.10* | .01 | −2.03** | .02 |
| Hypertension | −0.10 | .01 | −2.00** | .02 |
| CRP | −0.10 | .01 | −1.97** | .02 |
| Psychiatric history | −0.10* | .01 | −2.14** | .03 |
| Substance use history | −0.10* | .01 | −2.05** | .02 |
| Domestic abuse | −0.10* | .01 | −2.12** | .02 |
Discussion
In the present study we investigated whether the relation between TL and cognitive function was moderated by poverty status and race in a socioeconomically and racially diverse sample of urban-dwelling adults. Consistent with our hypotheses, we found that those living in poverty with shorter telomeres performed worse on well-validated measures of executive function (Trail Making Test Part B, a measure of set-shifting and cognitive flexibility) and nonverbal immediate memory (BVRT). However, contrary to our hypotheses, White, but not African American, participants living in poverty with shorter telomeres performed worse on tests of executive function (Digit Span Backward, a measure of working memory) and attention (Digit Span Forward). It is also important to note that there were no significant relations of TL or its interaction with race or poverty status for tests of psychomotor speed (Trail-Making Test Part A), verbal learning and memory (all CVLT subtests), or semantic verbal fluency, nor for another measure of attention (Brief Test of Attention).
Previous studies examining relations of TL and cognition have reported equivocal findings. Although some studies found associations between longer TL and better performance on global cognitive screening measures (Ma et al., 2013; Martin-Ruiz et al., 2006; Yaffe et al., 2011), as well as specific cognitive domains (Valdes et al., 2010), there were others that found null associations (Bendix et al., 2011; Harris et al., 2006; Mather et al., 2010; Yaffe et al., 2011). This lack of consistency across studies may be attributable to a combination of methodological variability, sampling biases, and even random chance. However, our current findings further suggest that these inconsistencies may partially reflect that (a) relations between TL and cognitive functioning may vary across SES and racial groups, and (b) associations may only exist for particular subdomains of cognitive functioning.
Importantly, without independent verification it is not possible to know whether our findings among those living in poverty, particularly Whites, are unique to characteristics of the HANDLS sample. For example, HANDLS is one of few epidemiologic studies to explicitly recruit lower-SES White participants (M. K. Evans et al., 2010). In addition, the urban environment of Baltimore, Maryland may have influenced our findings. Replication in other samples is necessary to establish the validity of our results. It is currently unknown if the present study findings would be generalizable to other samples (e.g., those of other ages, racial/ethnic backgrounds, and persons living in non-urban environments).
In addition to replication of study findings, an important avenue for future research is exploration of potential biopsychosocial mechanisms that may explain TL-cognition associations among at-risk groups. In the following sections, we propose a number of potential pathways to be examined in future studies.
Potential Explanations for Sociodemographic Variability in Telomere Length-Cognition Associations
Our findings revealed that shorter TL was associated with poorer performance on tests of executive function among participants living in poverty, particularly Whites. One possible explanation for these differences may be attributed to the higher prevalence of biopsychosocial risk factors among those living in poverty. For instance, higher rates of cardiovascular risk factors and diseases (Winkleby, Kraemer, Ahn, & Varady, 1998), chronic stress (G. W. Evans & Schamberg, 2009), and neighborhood deprivation (Lang et al., 2009), which may influence cognitive function in their own right, may increase this subgroup’s sensitivity to the negative effects of TL shortening on cognitive function. Importantly, most of the present study’s findings remained significant following adjustment for several cardiometabolic and inflammatory risk factors. However, it is important to note that there were many other potentially relevant risk factors that were not assessed (e.g., pro-inflammatory cytokines, oxidative stress, cortisol reactivity), indicating that further exploration of potential biomedical, as well as psychosocial, mediators and/or moderators is warranted.
Contrary to our expectations, we found no associations between TL and cognitive function among African Americans, regardless of their poverty status. Although exposure to risk factors might vary as a function of poverty status among Whites, African Americans across poverty levels experience a wide range of risk factors, including discrimination, geographical segregation, and chronic stress (Glymour & Manly, 2008). Given the cumulative burden of these risk factors experienced by African Americans, regardless of their poverty status, longer TL may not confer advantages for cognitive health in this group. Likewise, given the multitude of advantages experienced by Whites living above poverty, shorter TL may not confer risk for poor cognitive outcomes in this group. In summary, the unique social position occupied by urban Whites living in poverty, characterized by concurrent racial privilege and socioeconomic disadvantage, might make this group disproportionately susceptible to the effects of shortened TL on cognition. As stated previously, replication of our findings among individuals living in poverty is necessary to determine their validity and broader generalizability. Given that Whites were more than twice as likely than African Americans to consent to genetic analyses, replication of the racial differences observed in this study will also be important. African Americans who consented to genetic analyses might have had better health and functioning and/or fewer risk factors than those who did not, which could have influenced the race-related differences in this study.
Nonetheless, our findings among Whites are in line with growing literature that suggests declining health for Whites of lower SES during middle adulthood. Case and Deaton (2015) demonstrated that Whites of lower SES (defined as those having a high school degree or less) are experiencing increased rates of midlife morbidity and mortality. These trends have emerged rapidly during the 21st century, but are thought to reflect “cumulative disadvantage” building over several generations among poor Whites, and concurrent declines in self-reported mental health and increased use of prescription opioid medications, among other trends (Case & Deaton, 2017). HANDLS investigators also found greater risk for overall mortality was associated with lower SES in Whites and African Americans (Zonderman, Mode, Ejiogu, & Evans, 2016). Of note, adjustment for history of psychiatric illness and substance use (including opiate use) did not significantly alter the findings, nor did a history of exposure to domestic abuse. Future studies should use more detailed assessments of these and related variables (e.g., other forms of abuse or trauma) to determine whether they explain, in part, the present findings among those living in poverty, particularly Whites. For example, assessing psychiatric diagnostic status and quantity and frequency of substance misuse via structured interview would allow for more sensitive examination of these questions.
Our findings suggest that for a subset of people, shorter TL is associated with poorer performance on tests measuring executive functioning, attention, and nonverbal immediate memory. Executive function is conceptualized as a group of higher-order cognitive abilities that permit individuals to complete goal-oriented behaviors. Attention has been consistently identified as one of the key cognitive processes involved in executive functioning (Strauss et al., 2006). Interestingly, using principal component analyses, Trail Making Test Part B and the Digit Span tests have been found to load onto a single factor (Mirsky, 1989), suggesting some unity across these measures. Given the overlapping elements of these three measures, it is possible that TL is predominantly related to the executive functioning domain in a subgroup of community-dwelling adults.
It is unclear why associations were not found between TL or interactions with poverty status and race and the Brief Test of Attention, which is another measure of basic auditory attention. One possibility is that the Brief Test of Attention is less dependent on working memory than Digit Span Forward, given that it was developed to reduce the influence of confounding factors on the measurement of attention (Strauss et al., 2006). This suggests that associations between TL and cognitive function may only be found for tasks that place more complex demands on attention. In addition, the smaller analysis sample size for the Brief Test of Attention (n = 273) compared to that for Digit Span Forward (n = 317) may have influenced the results due to reduced statistical power.
Findings also demonstrated that shorter TL was associated worse performance on the BVRT, but not the CVLT, which are measures of nonverbal and verbal memory, respectively. The BVRT is widely considered to be a test of nonverbal immediate memory; however, other cognitive capacities are also involved, such as visual-spatial perception and psychomotor response (Lezak et al., 2012). Therefore, it is plausible that shortened TL is specifically implicated in nonverbal memory performance, as well as aspects visual-spatial functioning. In addition, participants may have relied on other strategies unique to verbal learning and memory on the CVLT, such as semantic clustering (Lezak et al., 2012), which could explain why TL was not associated with any of the CVLT subtests.
Potential Pathways Between Telomere Length, Executive Function, and Memory
Medical conditions associated with shorter TL have adverse effects on cognitive function. In cross-sectional comparisons, shorter TL is associated with factors traditionally related to cardiovascular disease, such as diabetes, blood pressure, carotid intima-medial thickness and atherosclerosis (Fitzpatrick et al., 2007; Serrano & Andrés, 2004), all of which are risk-factors for poorer cognitive outcomes (Knopman et al., 2001; Novak & Hajjar, 2010; Pugh, Kiely, Milberg, & Lipsitz, 2003; Waldstein, Wendell, Hosey, Seliger, & Katzel, 2010), and specifically executive and memory dysfunction (for a review, see Waldstein & Wendell, 2010). Therefore, it is plausible that TL is related to executive function and memory partly via cumulative cardiovascular risk.
Importantly, the present study’s sensitivity analyses revealed that adjustment for several common cardiometabolic risk factors attenuated the significant interaction effect of TL × Poverty Status on Trail Making Test Part B performance. However, the size of the interaction effect was unchanged following adjustment for these factors (sr2 = .01, with and without adjustment), and other significant effects did not attenuate. Nonetheless, the attenuated effect could suggest that cardiometabolic and inflammatory burden associated with shortened TL explains, at least in part, associations between TL and Trail Making Test Part B performance among those living in poverty. Further, there were many cardiometabolic risk factors that we did not examine in the present study that may have been more impactful. Longitudinal research examining mediational pathways could further elucidate these potential mechanisms.
There is also well-established literature in adult populations linking chronic, low-grade inflammation, characterized by inflammatory markers, such as interleukin-6 (IL-6) and CRP, with adverse effects on cognitive function (Weaver et al., 2002; Yaffe et al., 2003). Higher levels of IL-6 and CRP have been linked to worse executive functioning (Schram et al., 2007) and memory (Walker et al., 2019), which were the cognitive domains implicated in our study. As previously discussed, elevated levels of inflammatory markers have also been linked to telomere erosion (von Zglinicki, 2002), although these associations may be bidirectional. That is, in addition to the shortening effects of inflammation on TL, cells with shortened TL are more likely to release inflammatory cytokines such as IL-6, thereby accelerating inflammation (Rodier et al., 2009). Therefore, it is plausible that shorter TL indirectly causes poorer executive function and memory, in part, through elevated release of inflammatory cytokines.
As with the cardiometabolic risk factors, adjustment for CRP in the present study’s sensitivity analyses only eliminated the significant interaction effect of TL × Poverty Status with the Trail Making Test Part B. Similarly, the size of the interaction effect for this outcome was not reduced following adjustment for these factors (sr2 = .01, with and without adjustment). We were unable to examine other potentially important inflammatory markers, such IL-6, which may have been more impactful. Future research should examine additional inflammatory parameters. Further, longitudinal studies examining telomere dynamics, cognitive functioning, medical comorbidities, and inflammation would provide valuable information about the influential and directional relations of these variables.
Strengths and Limitations
The present study has notable strengths. First, the HANDLS study utilized an area probability sample of urban-dwelling adults, designed to address the respective contributions of self-identified race and poverty status to health outcomes. Our sample had nearly equal representation of participants across racial, sex, and poverty groups, as well as uniform distribution of African Americans and Whites across poverty levels. The diversity of our sample allowed us to investigate the relative contribution of TL to cognitive functioning across sociodemographic groups, which has not been examined previously. In addition, the present study included an extensive neuropsychological test battery that allowed for an in-depth examination of cognitive domains that may be vulnerable to shortened TL. Nonetheless, future studies in this area should examine additional cognitive domains, such as information processing speed, constructional praxis, and visuo-spatial processing.
This study also has several limitations. Given the equivocal literature on TL and cognitive functioning, it is possible that our findings are spurious. Also, the present work may not generalize to racial/ethnic groups beyond African Americans and Whites as well as non-urban populations. Replication is therefore necessary to determine the validity and generalizability of the findings. Further, given that Whites were more likely than African Americans to consent to genetic analyses, the findings might not completely generalize to the overall HANDLS sample. As described previously, differences between African Americans who consented to genetic analyses (versus those who did not) might have influenced the racial differences observed in this study.
We were unable to characterize income as a continuous variable due to difficulty obtaining annual household income through self-report. Nonetheless, we chose to examine dichotomous poverty status as a primary moderator, given its importance in the HANDLS study design and recruitment (M. K. Evans et al., 2010); the inconsistent literature linking poverty and TL; and, because of poverty’s unique role in cognitive health (Mani, Mullainathan, Shafir, & Zhao, 2013). Although we adjusted for high school-or-greater education, future studies should expand on this work by examining the role of other relevant SES indicators, such as literacy, occupational status, and wealth. Future work should also examine sex differences in TL-cognition associations. The study’s cross-sectional design does not allow us to infer directionality. Future research should apply longitudinal designs to better elucidate the temporal associations between TL and cognitive function across sociodemographic groups. Additionally, direct examination of mediating factors, such as cardiovascular risk factors and inflammatory markers, within these prospective relations is warranted.
We were unable to examine other potentially important biomedical risk factors, such as markers of stress physiology (e.g., cortisol reactivity), which may have influenced the findings. Our psychiatric composite variable was based on self-reported history of mental health diagnoses that may have underestimated the prevalence of these disorders due to barriers to healthcare access within the HANDLS sample. We also were unable to adjust for other mental health diagnoses (e.g., PTSD and related symptoms) or social factors (e.g., family structure) that may be impactful. Therefore, future studies should consider a wider range of biopsychosocial risk factors that may be implicated in TL-cognition associations.
Summary and Conclusions
In this study, we found that TL was associated with cognitive test performance, namely executive function and nonverbal immediate memory, among those living below 125% of the 2004 federal poverty level, particularly Whites. To our knowledge, this was the first study to demonstrate that the TL-cognition relation is domain-specific and varies as a function of sociodemographic factors. Future studies should aim to replicate the present findings in unique samples from other urban and nonurban settings. Should the findings be replicated, examination of potential biopsychosocial mediators and contributing factors described previously in the present study is warranted. Furthermore, investigations should determine how living in poverty might increase vulnerability to the negative effects of shortened TL on executive functioning and nonverbal memory. Finally, longitudinal studies are needed to examine whether and how telomere shortening influences cognitive decline across the life span as a function of race and poverty status. Ultimately, clarification of the various biological indicators involved in sociodemographic health disparities may pave the way for the prevention and treatment of premature aging and disease.
Supplementary Material
Public Health Significance:
In this study, we found that associations between peripheral blood mononuclear cell telomere length and cognitive test performance among those living in poverty, particularly Whites. Our findings suggest that poverty, particularly among Whites, may confer greater vulnerability to adverse effects of shortened telomere length on neurocognitive health. Ultimately, further clarification of the biopsychosocial mechanisms underlying sociodemographic health disparities may pave the way for the prevention and treatment of premature cognitive aging and disease.
Sources of Funding:
This research was supported by the National Institute on Aging’s Intramural Research Program of the National Institutes of Health (ZIAAG000513), NIH grants R01AG034161 (Waldstein) and K01AG043581 (Beatty Moody), and the University of Maryland Claude D. Pepper Older Americans Independence Center (P30AG028747). The funding sources were not involved in the conduct of the research and preparation of the article.
Acronyms:
- BVRT
Benton Visual Retention Test
- CVLT
California Verbal Learning Test-II
- CRP
high-sensitivity C-reactive protein
- DNA
deoxyribonucleic acid
- HANDLS
Healthy Aging in Neighborhoods of Diversity across the Life Span
- IL-6
interleukin-6
- mPFC
medical prefrontal cortex
- MRV
medical research vehicles
- SES
socioeconomic status
- SPSS
Statistical Package for the Social Sciences
- TL
telomere length
- WAIS-R
Wechsler Adult Intelligence Scale-Revised
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
Ethics Approval: Approval for data collection was obtained from the National Institutes of Health, National Institute of Environmental Health Services Institutional Review Board (protocol 09-AG-N248). All participants provided written informed consent.
References
- Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, … Berenson GS (2009). Leukocyte telomere dynamics: Longitudinal findings among young adults in the Bogalusa Heart Study. American Journal of Epidemiology, 169, 323–329. doi: 10.1093/aje/kwn338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, & Yali AM (1999). Socioeconomic status and chronic stress. Does stress account for SES effects on health? Annals of the New York Academy of Sciences, 896, 131–144. doi: 10.1111/j.1749-6632.1999.tb08111.x [DOI] [PubMed] [Google Scholar]
- Bendix L, Gade MM, Staun PW, Kimura M, Jeune B, Hjelmborg J. v. B., … Christensen K (2011). Leukocyte telomere length and physical ability among Danish Twins age 70+. Mechanisms of Ageing and Development, 132, 568–572. doi: 10.1016/j.mad.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, & Lin J (2015). Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science, 350, 1193–1198. doi: 10.1126/science.aab3389 [DOI] [PubMed] [Google Scholar]
- Breteler MMB, Claus JJ, Grobbee DE, & Hofman A (1994). Cardiovascular disease and distribution of cognitive function in elderly people: The Rotterdam study. BMJ, 308, 1604–1608. doi: 10.1136/bmj.308.6944.1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Needham B, & Ailshire J (2017). Telomere length among older U.S. adults: Differences by race/ethnicity, gender, and age. Journal of Aging and Health, 29, 1350–1366. doi: 10.1177/0898264316661390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnen CJWH, Dijkstra BAG, Walvoort SJW, Markus W, VanDerNagel JEL, Kessels RPC, & DE Jong CAJ (2019). Prevalence of cognitive impairment in patients with substance use disorder. Drug and Alcohol Review, 38, 435–442. doi: 10.1111/dar.12922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, & Deaton A (2015). Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proceedings of the National Academy of Sciences, 112, 15078–15083. doi: 10.1073/pnas.1518393112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, & Deaton A (2017). Mortality and morbidity in the 21st century. Brookings Papers on Economic Activity, 2017, 397–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM (2002). Telomere measurement by quantitative PCR. Nucleic Acids Research, 30, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, & Kerber RA (2003). Association between telomere length in blood and mortality in people aged 60 years or older. Lancet, 361, 393–395. doi: 10.1016/S0140-6736(03)12384-7 [DOI] [PubMed] [Google Scholar]
- Chan SRWL, & Blackburn EH (2004). Telomeres and telomerase. Philosophical Transactions of the Royal Society B: Biological Sciences, 359, 109–121. doi: 10.1098/rstb.2003.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, … Spector TD (2006). The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell, 5, 361–365. doi: 10.1111/j.1474-9726.2006.00222.x [DOI] [PubMed] [Google Scholar]
- Darrow SM, Verhoeven JE, Révész D, Lindqvist D, Penninx BWJH, Delucchi KL, … Mathews CA (2016). The Association Between Psychiatric Disorders and Telomere Length: A Meta-Analysis Involving 14,827 Persons. Psychosomatic Medicine, 78, 776–787. doi: 10.1097/PSY.0000000000000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). California verbal learning test, second edition, adult version. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, & Seeman T (2009). Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell, 8, 251–257. doi: 10.1111/j.1474-9726.2009.00470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Mabile E, Brett ZH, Esteves K, Jones E, Shirtcliff EA, & Theall KP (2014). The association of telomere length with family violence and disruption. Pediatrics, 134, e128–37. doi: 10.1542/peds.2013-3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, & Cawthon RM (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the Unites States of America, 101, 17312–17315. doi: 10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, & Schamberg MA (2009). Childhood poverty, chronic stress, and adult working memory. Proceedings of the National Academy of Sciences of the United States of America, 106, 6545–6549. doi: 10.1073/pnas.0811910106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans Gary W., & Kantrowitz E (2002). Socioeconomic status and health: The potential role of environmental risk exposure. Annual Review of Public Health, 23, 303–331. doi: 10.1146/annurev.publhealth.23.112001.112349 [DOI] [PubMed] [Google Scholar]
- Evans MK, Lepkowski JM, Powe NR, LaVeist T, Kuczmarski MF, & Zonderman AB (2010). Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS): Overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethnicity & Disease, 20, 267–275. [PMC free article] [PubMed] [Google Scholar]
- Fioravanti M, Bianchi V, & Cinti ME (2012). Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry, 12, 64. doi: 10.1186/1471-244X-12-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, … Aviv A (2007). Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology, 165, 14–21. doi: 10.1093/aje/kwj346 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). ‘Mini-mental State’: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, & Dawson Cruz T (2010). Do US Black women experience stress-related accelerated biological aging?: A novel theory and first population-based test of Black-White differences in telomere length. Human Nature, 21, 19–38. doi: 10.1007/s12110-010-9078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Pearson JA, Linnenbringer E, Schulz AJ, Reyes AG, Epel ES, … Blackburn EH (2015). Race-ethnicity, poverty, urban stressors, and telomere length in a Detroit community-based sample. Journal of Health and Social Behavior, 21, 19–38. doi: 10.1177/0022146515582100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, & Manly JJ (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18, 223–254. doi: 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Grodstein F, Van Oijen M, Irizarry MC, Rosas HD, Hyman BT, Growdon JH, & De Vivo I (2008). Shorter telomeres may mark early risk of dementia: Preliminary analysis of 62 participants from the Nurses’ Health Study. PLoS ONE, 3, e1590. doi: 10.1371/journal.pone.0001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, … Shiels PG (2006). The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neuroscience Letters. doi: 10.1016/j.neulet.2006.07.055 [DOI] [PubMed] [Google Scholar]
- Harris SE, Martin-Ruiz C, von Zglinicki T, Starr JM, & Deary IJ (2012). Telomere length and aging biomarkers in 70-year-olds: The Lothian Birth Cohort 1936. Neurobiology of Aging, 33, 1486.e3–8. doi: 10.1016/j.neurobiolaging.2010.11.013 [DOI] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, & Willeit P (2014). Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta- Analysis. BMJ, 349, g4227. doi: 10.1136/bmj.g4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: The Guilford Press. [Google Scholar]
- Hochstrasser T, Marksteiner J, & Humpel C (2012). Telomere length is age-dependent and reduced in monocytes of Alzheimer patients. Experimental Gerontology, 47, 160–163. doi: 10.1016/j.exger.2011.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, … Aviv A (2008). Leukocyte telomeres are longer in African Americans than in whites: The National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell, 7, 451–458. doi: 10.1111/j.1474-9726.2008.00397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Hjelmborg JVB, Gardner JP, Bathum L, Brimacombe M, Lu X, … Christensen K (2008). Telomere length and mortality: A study of leukocytes in elderly danish twins. American Journal of Epidemiology, 7, 451–458. doi: 10.1093/aje/kwm380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, … Folsom AR (2001). Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology, 56, 42–48. doi: 10.1212/WNL.56.1.42 [DOI] [PubMed] [Google Scholar]
- Kume K, Kikukawa M, Hanyu H, Takata Y, Umahara T, Sakurai H, … Iwamoto T (2012). Telomere length shortening in patients with dementia with Lewy bodies. European Journal of Neurology, 19, 905–910. doi: 10.1111/j.1468-1331.2011.03655.x [DOI] [PubMed] [Google Scholar]
- Lang IA, Hubbard RE, Andrew MK, Llewellyn DJ, Melzer D, & Rockwood K (2009). Neighborhood deprivation, individual socioeconomic status, and frailty in older adults. Journal of the American Geriatrics Society, 57, 1776–1780. doi: 10.1111/j.1532-5415.2009.02480.x [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, & Tranel D (2012). Neuropsychological assessment (5th ed.). New York, NY, US: Oxford University Press. [Google Scholar]
- Lin Y, Damjanovic A, Metter EJ, Nguyen H, Truong T, Najarro K, … Weng N (2015). Age-associated telomere attrition of lymphocytes in vivo is co-ordinated with changes in telomerase activity, composition of lymphocyte subsets and health conditions. Clinical Science, 128, 367–377. doi: 10.1042/cs20140481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SM, Peek MK, Mitra N, Ravichandran K, Branas C, Spangler E, … Riethman H (2016). Race, ethnicity, psychosocial factors, and telomere length in a multicenter setting. PLoS ONE, 11, e0146723. doi: 10.1371/journal.pone.0146723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SL, Lau ESS, Suen EWC, Lam LCW, Leung PC, Woo J, & Tang NLS (2013). Telomere length and cognitive function in southern Chinese community-dwelling male elders. Age and Ageing, 42, 450–455. doi: 10.1093/ageing/aft036 [DOI] [PubMed] [Google Scholar]
- Mani A, Mullainathan S, Shafir E, & Zhao J (2013). Poverty impedes cognitive function. Science, 341, 976–980. doi: 10.1126/science.1238041 [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, & von Zglinicki T (2006). Telomere length predicts poststroke mortality, dementia, and cognitive decline. Annals of Neurology, 60, 174–180. doi: 10.1002/ana.20869 [DOI] [PubMed] [Google Scholar]
- Mather KA, Jorm AF, Anstey KJ, Milburn PJ, Easteal S, & Christensen H (2010). Cognitive performance and leukocyte telomere length in two narrow age-range cohorts: A population study. BMC Geriatrics, 10, 62. doi: 10.1186/1471-2318-10-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky AF (1989). The neuropsychology of attention: Elements of a complex behavior In Perecman Ellen (Ed.), Integrating Theory and Practice in Clinical Neuropsychology (pp. 75–91). Hillsdale, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Morrell CH, Pearson JD, & Brant LJ (1997). Linear transformations of linear mixed-effects models. American Statistician, 51, 338–343. doi: 10.1080/00031305.1997.10474409 [DOI] [Google Scholar]
- Najarro K, Nguyen H, Chen G, Xu M, Alcorta S, Yao X, … Weng NP (2015). Telomere length as an indicator of the robustness of B- and T-cell response to influenza in older adults. Journal of Infectious Diseases, 212, 1261–1269. doi: 10.1093/infdis/jiv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, & Epel ES (2013). Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Social Science & Medicine, 85, 1–8. doi: 10.1016/j.socscimed.2013.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, … Kuller LH (2005). Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the cardiovascular health study cohort. Journal of the American Geriatrics Society, 53, 1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x [DOI] [PubMed] [Google Scholar]
- Novak V, & Hajjar I (2010). The relationship between blood pressure and cognitive function. Nature Reviews Cardiology, 7, 686–698. doi: 10.1038/nrcardio.2010.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A, … Baccarelli A (2011). Shortened telomeres in individuals with abuse in alcohol consumption. International Journal of Cancer, 129, 983–992. doi: 10.1002/ijc.25999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KG, Kiely DK, Milberg WP, & Lipsitz LA (2003). Selective impairment of frontal-executive cognitive function in African Americans with cardiovascular risk factors. Journal of the American Geriatrics Society, 51, 1439–1444. doi: 10.1046/j.1532-5415.2003.51463.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2018). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ramey T, & Regier PS (2019). Cognitive impairment in substance use disorders. CNS Spectrums, 24, 102–113. doi: 10.1017/S1092852918001426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, & Blackwell AD (2014). Cognitive impairment in depression: a systematic review and meta-analysis. Psychological Medicine, 44, 2029–2040. doi: 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- Rodier F, Coppé JP, Patil CK, Hoeijmakers WAM, Muñoz DP, Raza SR, … Campisi J (2009). Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nature Cell Biology, 11, 973–979. doi: 10.1038/ncb1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram MT, Euser SM, De Craen AJM, Witteman JC, Frölich M, Hofman A, … Westendorp RGJ (2007). Systemic markers of inflammation and cognitive decline in old age. Journal of the American Geriatrics Society, 55, 708–716. doi: 10.1111/j.1532-5415.2007.01159.x [DOI] [PubMed] [Google Scholar]
- Schretlen D, Bobholz JH, & Brandt J (2007). Development and psychometric properties of the Brief Test of Attention. The Clinical Neuropsychologist, 10, 80–89. doi: 10.1080/13854049608406666 [DOI] [Google Scholar]
- Serrano AL, & Andrés V (2004). Telomeres and cardiovascular disease: Does size matter? Circulation Research, 94, 575–584. doi: 10.1161/01.RES.0000122141.18795.9C [DOI] [PubMed] [Google Scholar]
- Sivan AB (1992). Benton Visual Retention Test (5th ed.). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Stein MB, Kennedy CM, & Twamley EW (2002). Neuropsychological function in female victims of intimate partner violence with and without posttraumatic stress disorder. Biological Psychiatry, 52, 1079–1088. doi: 10.1016/S0006-3223(02)01414-2 [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Butcher L, Lin J, Brydon L, Kivimäki M, … Erusalimsky JD (2011). Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain, Behavior, and Immunity, 25, 1292–1298. doi: 10.1016/j.bbi.2011.04.010 [DOI] [PubMed] [Google Scholar]
- Strauss EH, Sherman EMS, & Spreen O (2006). A compendium of neuropsychological Tests: Administration norms and commentary (3rd ed.). New York, NY, US: Oxford University Press. [Google Scholar]
- Theall KP, Brett ZH, Shirtcliff EA, Dunn EC, & Drury SS (2013). Neighborhood disorder and telomeres: Connecting children’s exposure to community level stress and cellular response. Social Science & Medicine, 85, 50–58. doi: 10.1016/j.socscimed.2013.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Deary IJ, Gardner J, Kimura M, Lu X, Spector TD, … Cherkas LF (2010). Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiology of Aging, 31, 986–992. doi: 10.1016/j.neurobiolaging.2008.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, & Groothuis-Oudshoorn K (2011). mice : Multivariate Imputation by Chained Equations in R. Journal of Statistical Software, 45, 1–67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- von Zglinicki T (2002). Oxidative stress shortens telomeres. Trends in Biochemical Sciences, 27, 339–344. doi: 10.1016/S0968-0004(02)02110-2 [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Serra V, Lorenz M, Saretzki G, Lenzen-Großimlighaus R, Geßner R, … Steinhagen-Thiessen E (2000). Short telomeres in patients with vascular dementia: An indicator of low antioxidative capacity and a possible risk factor? Laboratory Investigation, 80, 1739–1747. doi: 10.1038/labinvest.3780184 [DOI] [PubMed] [Google Scholar]
- Waldstein SR, & Wendell CR (2010). Neurocognitive function and cardiovascular disease. Journal of Alzheimer’s Disease, 20, 833–842. doi: 10.3233/JAD-2010-091591 [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Wendell CR, Hosey MM, Seliger SL, & Katzel LI (2010). Cardiovascular disease and neurocognitive function In Armstrong CL & Morrow L (Eds.), Handbook of Medical Neuropsychology (pp. 69–99). doi: 10.1007/978-1-4419-1364-7_5 [DOI] [Google Scholar]
- Walker KA, Gottesman RF, Wu A, Knopman DS, Gross AL, Mosley TH, … Windham BG (2019). Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology, 92, e1256–e1267. doi: 10.1212/WNL.0000000000007094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dong X, Cao L, Sun Y, Qiu Y, Zhang Y, … Zhong L (2016). Association between telomere length and diabetes mellitus: A meta-analysis. Journal of International Medical Research, 44, 1156–1173. doi: 10.1177/0300060516667132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, & Seeman TE (2002). Interleukin-6 and risk of cognitive decline: Macarthur studies of successful aging. Neurology, 59, 371–378. doi: 10.1212/WNL.59.3.371 [DOI] [PubMed] [Google Scholar]
- Williams DR, John DA, Oyserman D, Sonnega J, Mohammed SA, & Jackson JS (2012). Research on discrimination and health: An exploratory study of unresolved conceptual and measurement issues. American Journal of Public Health, 102, 975–978. doi: 10.2105/AJPH.2012.300702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkleby MA, Kraemer HC, Ahn DK, & Varady AN (1998). Ethnic and socioeconomic differences in cardiovascular disease risk factors: Findings for women from the third national health and nutrition examination survey, 1988–1994. Journal of the American Medical Association, 280, 356–362. doi: 10.1001/jama.280.4.356 [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Kluse M, Cawthon R, Harris T, Hsueh WC, … Cummings SR (2011). Telomere length and cognitive function in community-dwelling elders: Findings from the Health ABC Study. Neurobiology of Aging, 32, 2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx, Simonsick EM, Pahor M, Kritchevsky S, … Harris T (2003). Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology, 61, 76–80. doi: 10.1212/01.WNL.0000073620.42047.D7 [DOI] [PubMed] [Google Scholar]
- Yang Z, Ye J, Li C, Zhou D, Shen Q, Wu J, … Liu Y (2013). Drug addiction is associated with leukocyte telomere length. Scientific Reports, 3, 1542. doi: 10.1038/srep01542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JK, & Wang CY (2016). Telomeres and telomerase in cardiovascular diseases. Genes, 7, 58. doi: 10.3390/genes7090058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Miao K, Wang H, Ding H, & Wen Wang D (2013). Association between telomere length and Type 2 diabetes mellitus: A meta-analysis. PLoS ONE, 8, e79993. doi: 10.1371/journal.pone.0079993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang X, Gutin B, Davis CL, Keeton D, Thomas J, … Dong Y (2011). Leukocyte telomere length in healthy Caucasian and African-American adolescents: Relationships with race, sex, adiposity, adipokines, and physical activity. Journal of Pediatrics, 158, 215–220. doi: 10.1016/j.jpeds.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonderman AB, Mode NA, Ejiogu N, & Evans MK (2016). Race and poverty status as a risk for overall mortality in community-dwelling middle-aged adults. JAMA Internal Medicine, 176, 1394–1395. doi: 10.1001/jamainternmed.2016.3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.