Abstract
Objective:
Substantial research indicates that fluid and crystallized abilities are highly correlated throughout the adult lifespan. However, recent proposals suggest that a large discrepancy between these two abilities, defined as crystallized performance minus fluid performance, indicates heightened risk for Alzheimer’s disease (AD).
Method:
In 266 cognitively healthy older adults, the present study tested linear and quadratic relationships between an ability discrepancy score and early AD neuropathology indexed via in vivo measures of beta-amyloid deposition and cortical thickness in AD-vulnerable regions. We also tested the extent that alternative forms of this ability discrepancy measure (e.g., subdomain discrepancies, verbal-visual discrepancies) and an episodic memory composite might also be sensitive markers of early AD pathology.
Results:
An overall ability discrepancy was linearly and positively correlated with beta-amyloid. A quadratic relationship was found between the overall ability discrepancy score and cortical thickness such that a small positive correlation was found at lower discrepancy levels (fluid > crystallized), but at higher discrepancy (crystallized > fluid) levels a negative relationship was found (i.e., an inverted-U pattern). Similar patterns were found across each subdomain of cognition, but the effects were weaker than the overall ability discrepancy score. Importantly, inclusion of episodic memory (the gold standard) did not alter any of the effects, suggesting that an ability discrepancy confers unique predictiveness of AD biomarkers.
Conclusions:
These findings replicate previous findings and increase the confidence in their usefulness to predict AD biomarkers. Longitudinal validation is needed to clearly relate an ability discrepancy to specific stages of preclinical AD.
Keywords: ability discrepancy, aging, Alzheimer’s disease, beta-amyloid, cortical thickness
Introduction
Predicting which older adults will receive a diagnosis of Alzheimer’s disease (AD) later in life is critical to maximize their healthspan—the length of time one lives a happy and cognitively intact life (Rowe & Kahn, 1987). Early identification can encourage changes in lifestyle factors (e.g., exercise, mindfulness, cognitive stimulation) that might reduce the risk of dementia (Deckers et al., 2015; Livingston et al., 2017). Furthermore, early detection can facilitate the targeting of pharmaceutical treatments for both research and clinical use (Sperling et al., 2014). Current methods of identifying preclinical stages of AD rely on biomarkers that are expensive, often invasive, and are not accessible by all members of a given community (Sperling et al., 2011b). One way to mitigate these issues is by developing a cognitive marker that is sensitive to these AD biomarkers and that can predict future cognitive impairment (Mortamais et al., 2017). Moreover, it has been recommended that such a cognitive marker should be “personally relevant” and corrects test scores for peak prior level of cognitive ability (Rentz & Weintraub, 2000; Weintraub et al., 2018). The present study aims to provide increased validation for and clarification of a potentially sensitive cognitive marker of preclinical AD that takes into account peak levels of cognitive ability: a crystallized-fluid ability discrepancy score.
This ability discrepancy score is calculated by subtracting one’s fluid ability score from their crystallized ability score (e.g., Dierckx et al 2008; Lezak, 1995; McCarthy et al., 2005). Fluid ability assesses the online efficiency of a cognitive system and is usually measured using a variety of different cognitive domains such as processing speed, executive function, episodic memory, and reasoning (Wechsler, 1944, 1997). These tasks often are intentionally created to make minimal use of prior knowledge or learned skills (Johnson et al., 2004; Kaszniak, 1986; Lezak, 1995; Wechsler, 1944). Conversely, crystallized ability encompasses that which is learned through experience and education–generally measured using vocabulary (Ekstrom et al., 1976; Wechsler, 1944; Zachary & Shipley, 1986) or word pronunciation tasks (Blair and Spreen, 1989).1 Critically, these two abilities are highly correlated with one another across the adult lifespan (Cattell, 1965; Kaufman et al., 1996), such that individuals with higher crystallized ability should have higher fluid ability. Therefore, a large difference between these two abilities (in the direction of crystallized > fluid) suggests an abnormal decline selectively in fluid ability.
The logic behind this measure is that fluid and crystallized abilities enforce restrictions on one another. Fluid ability influences the efficiency of learning, which further constrains the accumulation of crystallized intelligence (Cattell, 1971; Kaufman, Kaufman, Liu, & Johnson, 2009). However, throughout the progression of AD, fluid abilities often begin to decline earlier than crystallized abilities (O’Carroll & Gilleard, 1986; Wechsler, 1944). This differential timing leads to large discrepancies between the two abilities, the rate of which is indicative of the rate of AD symptom progression (Albert et al., 2011; Bastin and Salmon, 2014; McKhann et al., 2011; Schmid et al., 2013).
Evidence for the usefulness of this measure has been mounting. An ability discrepancy score is larger in those with AD than in cognitively healthy adults and increases as disease severity worsens (e.g., Dierckx et al., 2008; Lezak, 1995; McCarthy et al., 2005). Evidence supporting this score also comes from cognitively healthy adults in the preclinical AD stage. Specifically, McDonough and colleagues (2016) showed that an ability discrepancy score greater than 0 was associated with greater accumulation of beta-amyloid and smaller cortical thickness in brain regions known to decline in the AD process. This study suggested that an ability discrepancy score around zero (or less) might be indicative of healthy aging, whereas an increasingly positive score is suggestive of greater progression of the AD pathology. Interestingly, a lower ability discrepancy score also has been associated with greater social and physical activity (O’Shea et al., 2018) that, in turn, has been associated with successful aging and greater levels of cognitive reserve (e.g., Christensen & Mackinnon, 1993; Fratiglioni, Paillard-Borg, & Winblad, 2004; Karp et al., 2006). Together, a lower ability discrepancy score appears to be a sign of successful or non-pathological aging.
While these results are promising, they are still several concerns for relying on an ability discrepancy score for clinical and research purposes. First, an ability discrepancy relies on a composite of different fluid abilities, not all of which might decline at the same rate in AD. For example, longitudinal studies have shown that episodic memory often declines first, followed by executive function and processing speed (Boraxbekk et al., 2015; Grober et al., 2008; Mistridis et al. 2015; Schmid et al., 2013). Second, inconsistent support has been found for associations between AD biomarkers and specific domains of cognition. For example, a meta-analysis revealed very weak but significant associations between beta-amyloid and episodic memory, executive function, and global cognition (Hedden et al., 2013). Notably, many cross-sectional studies have been unsuccessful at finding significant associations with episodic memory (e.g., Amariglio et al., 2012; Doherty et al., 2015; Johnson et al., 2014; Perrotin et al., 2012; Song et al., 2016). Moreover, in early models of the AD process, subtle cognitive declines are not expected to be detectable until very late in the preclinical AD stage (Sperling et al., 2011a) or early mild cognitive impairment (Jack et al., 2013). Thus, it is not clear how effective a cognitive marker ultimately would be to detect preclinical AD.
One critical disadvantage that the above studies share is that baseline or cross-sectional performance covaries with lifelong level of overall ability (e.g., g or IQ). Individuals starting out with a lower level of cognition in any domain might then appear to be on the path towards AD (or other related dementia), despite no actual change in ability since young adulthood, and thus no evidence of abnormal cognitive declines. Thus, the advantage of an ability discrepancy score over single measures of cognition is that it helps to control for individual differences in overall ability, thus serving as a proxy for within-individual changes in cognition. The implication is that the aforementioned longitudinal studies might have shown different levels of sensitivity of their measures (stronger or weaker) had they accounted for individual differences in crystallized ability.
Complicating interpretations further are the findings that the relationships between crystallized and fluid abilities differ in the context of cross-sectional “healthy” aging studies. In comparison with younger adults, some studies have found that the correlation between fluid and crystallized abilities is weaker in older than younger adults (e.g., Cunningham, Clayton, & Overton, 1975; Eisdorfer et al., 1959; Rabbitt, 1993). Such findings are accompanied by mean level differences between younger and older adults such that younger adults have greater fluid abilities than crystallized abilities whereas older adults have greater crystallized than fluid abilities (e.g., Park et al., 2002; Salthouse, 2010). These findings suggest that healthy aging also might be accompanied by an ability discrepancy.
Contrasting these claims, other studies have found stronger correlations between fluid and crystallized abilities in old age relative to younger adults (Cunningham, 1980; Lindenberger & Baltes, 1997; McHugh, & Owens, 1954; Reinert, 1970). These studies have given rise to the cognitive dedifferentiation hypothesis (Baltes et al., 1980; Reinert, 1970) in which cognitive abilities become more inter-related in old age, perhaps due to an increased reliance on common underlying processes. Although longitudinal analyses have suggested that fluid and crystallized abilities are quite stable in old age (Salthouse, 2010), it has also been noted that many cross-sectional studies with older adults likely contain older adults in preclinical stages of AD when large declines in cognition have not yet been detected (Sliwinski et al., 1996). As suggested above, it is not clear whether fluid abilities longitudinally decline to a faster extent than crystallized abilities in healthy aging. Regardless, it seems likely that any such discrepancies that might occur in healthy aging would be much smaller than the differences found in AD.
Another important factor to consider is the differential predictability of specific cognitive domains for AD. That is, discrepancies between crystallized ability and specific cognitive domains might yield differential sensitivity to markers of preclinical AD. Recently, Takaiwa et al. (2018) investigated an ability discrepancy in a sample of older adults with mild cognitive impairment (MCI). They found that 74% of their sample had an ability discrepancy using a composite score of fluid measures. In addition to measuring ability discrepancy using this fluid composite, these researchers also calculated domain-specific ability discrepancies with the measure of crystallized ability constant across all measures. They found that the most frequent discrepancies occurred in the domains of immediate verbal memory (66% of the MCI patients) and attention (60% of the MCI patients)—notably the RBANS tests of attention assess retention span, executive function, and processing speed. Although Takaiwa et al. (2018) used fluid and crystallized abilities to form their ability discrepancy scores, other types of discrepancy scores also have been reported to be a cognitive marker of preclinical AD. For example, differences between verbal and visuospatial abilities have been found in preclinical AD (Jacobson et al., 2002), in cognitively healthy older adults with genetic risk for AD (Fine et al., 2008; Houston et al., 2005; Jacobson et al., 2005), and in Alzheimer’s disease (Strite et al., 2007). Together, these findings hint at the possibility that many types of cognitive discrepancies may exist in the AD process, but not all may be equally sensitive to AD biomarkers.
Aims of the Current Study
In the present study, we used data from the Harvard Aging Brain Study (Dagley et al., 2017) to test four aims. First, we sought to bolster support for a crystallized-fluid ability discrepancy to be used as a marker of preclinical AD in cognitively healthy older adults. We aimed to do so by replicating previously found correlations between this score and biological markers of preclinical AD—beta-amyloid in the precuneus and cortical thickness in Alzheimer’s disease signature regions. To mitigate the potential influence of a discrepancy occurring as a part of normative aging, chronological age was statistically controlled in all analyses consistent with prior studies using an ability discrepancy (McDonough et al., 2016; O’Shea et al., 2018). Importantly, we also acknowledge the recent criticisms of psychology and neuroscience for failing to replicate key findings (Ioannidis, 2005; Open Science Collaboration, 2015), in part due to neuroimaging studies being underpowered (Button et al., 2013). These issues underscore the importance of replication in independent and large samples.
Second, we aimed to better understand the relationship between a “positive” (crystallized > fluid) or “negative” (fluid > crystallized) ability discrepancy score. Previously, it was found that greater ability discrepancy scores (with larger values signaling poorer cognitive outcomes) with absolute scores greater than zero (a positive discrepancy) were associated with both increases in beta-amyloid and decreases in cortical thickness. However, McDonough et al. (2016) found that for individuals who had absolute scores less than zero (a negative discrepancy), a greater discrepancy score was only associated with increases in beta-amyloid and not cortical thickness. In other words, a significant relationship between ability discrepancy and beta-amyloid was found regardless of whether the absolute score was positive or negative. However, a significant relationship with cortical thickness was only found for individuals with a positive discrepancy (i.e., a subsample of the data with crystallized > fluid). While McDonough et al. (2016) conducted separate analyses for positive and negative scores, we tested whether these two different patterns stemmed from 1) a linear positive relationship between ability discrepancy scores and beta-amyloid and 2) a quadratic relationship between ability discrepancy scores and cortical thickness (i.e., an inverted-U pattern) using spline regression techniques.
Our third aim was to assess the extent to which domain-specific discrepancy scores would differentially predict biological markers of preclinical AD (beta-amyloid and cortical thickness). Based on the findings by Takaiwa et al. (2018), we predicted that memory discrepancy scores and attention/processing speed discrepancy scores would be more strongly associated with beta-amyloid and cortical thickness than executive function discrepancy scores. We also conducted sensitivity analyses to test the extent that a language discrepancy and verbal-visual discrepancy would show similar patterns.
Lastly, we tested the extent to which the average ability discrepancy measure (across fluid domains) was more sensitive to AD biomarkers than a composite score of episodic memory, which is the current gold standard for identifying early cognitive deficits in the AD process (McKhann et al., 2011; Sperling et al., 2011a).
Method
Study Details
Data were taken from version 1.0 of the Harvard Aging Brain Study (HABS) and led by Drs. Sperling and Johnson at Massachusetts General Hospital/Harvard Medical School in Boston, MA (P01AG036694; http://nmr.mgh.harvard.edu/lab/harvardagingbrain). The initial description of the study, which includes recruitment criteria and measures, can be found in Dagley et al. (2015). For the purposes of the present study, we were interested in data that consisted of PIB-PET scanning, MRI scanning, and neuropsychological testing. The local IRB has approved the use of this data.
Participants
The sample consisted of 266 cognitively healthy older adults (159 females) aged between 62 and 90 (M = 73.59; SD = 6.15) that completed all variables of interest. Participants were excluded from the study if they reported a history of alcoholism, drug abuse, head trauma, or current serious medical or psychiatric illness. The sample consisted primarily of non-Hispanic Whites with 43 Blacks and 4 that did not indicate their ethnoracial category. Participants were highly educated (M = 15.79, SD = 3.03), all had a Clinical Dementia Rating (CDR) score of 0, a high Mini-Mental Status Exam (M = 29.03, SD = 1.06), and low depression symptomology based on the Geriatric Depression Scale (M = 3.064, SD = 2.80).
Neuropsychological Testing
Seven cognitive tasks were included in the study. These tasks spanned both crystallized and fluid ability domains. The specific tasks were the American National Adult Reading Test that assessed pronunciation of words with irregular spelling and often serves as a proxy for education, literacy, and overall intellectual ability (Ryan and Paolo, 1992), the 30-item Boston Naming Test that assessed word retrieval through the naming of objects of varying difficulty level (Kaplan et al., 1983), Verbal Fluency that assessed word retrieval for different phonological categories within a limited time span (Benton, 1983), Logical Memory I and II that assessed immediate and delayed memory for details from a short story read aloud (Wechsler, 1997), Digit Symbol Coding that assessed how fast one can use a symbol-digit key to transcribe a page full of symbols with the missing digit (Wechsler, 1981), Trail Making Test A & B that assessed how fast one can draw a line to sequential letters and then alternate between sequential letters and numbers (Reitan, 1979). Descriptive statistics for each measure can be found in Table 1.
Table 1.
Descriptive Statistics of Brain and Cognitive Measures.
| Measure | Mean | SD | Min | Max |
|---|---|---|---|---|
| Beta-Amyloid | 1.29 | 0.26 | 0.96 | 2.35 |
| Cortical Thickness | 2.67 | 0.12 | 2.33 | 2.93 |
| Trails A | 36.36 | 12.18 | 11 | 100 |
| Trails B | 91.74 | 49.21 | 31.79 | 300 |
| Digit Symbol | 47.30 | 10.63 | 19 | 86 |
| Logical Memory Immediate | 15.04 | 3.25 | 8 | 24 |
| Logical Memory Delayed | 13.77 | 3.28 | 6 | 25 |
| FAS | 44.67 | 13.54 | 13 | 92 |
| Category Fluency | 44.61 | 9.84 | 19 | 79 |
| Boston Naming Test | 27.73 | 2.55 | 15 | 30 |
| AMNART | 121.07 | 9.16 | 78 | 132 |
| MMSE | 29.03 | 1.06 | 25 | 30 |
| Average Ability Discrepancy | 0.00 | 0.69 | −1.77 | 1.97 |
| Speed Discrepancy | 0.00 | 0.83 | −2.75 | 2.61 |
| Memory Discrepancy | 0.00 | 1.08 | −3.56 | 2.78 |
| Executive Function Discrepancy | 0.00 | 0.95 | −2.59 | 4.16 |
| Language Discrepancya | 0.00 | 0.84 | −3.55 | 2.03 |
| Verbal-Visual Discrepancy | 0.00 | 0.67 | −2.07 | 2.24 |
| Logical Memory Composite | 0.00 | 0.95 | −2.27 | 2.94 |
Only the AMNART was used to represent crystallized ability in this discrepancy score.
Structural MRI
MRI scanning was completed at the MGH Martinos Center using a Siemens TIM Trio 3T System with a 12-channel head coil. Two different acquisitions were used to acquire structural MRI scans: ADNI1 MPRAGE (TR/TE/TI = 2300/2.98/900 ms, flip angle = 9°, 1 × 1 × 1.2 mm resolution, 0 acceleration) or ADNI2GO MPRAGE (TR/TE/TI = 2300/2.95/900 ms, flip angle = 9°, 1.1 × 1.1 × 1.2 mm resolution, 2× GRAPPA acceleration). These two acquisitions are considered interchangeable.
Cortical thickness was calculated using FreeSurfer v5.1. From the whole-brain calculations, cortical regions of interest (ROIs) were defined using the Desikan-Killiany atlas. As indicated by Dagley et al. (2015), the quality of the Freesurfer estimates were visually inspected and either manually edited or corrected by adjusting the watershed threshold. An index of neurodegeneration was created by extracting and averaging ROIs that closely corresponded to AD signature regions (Bakkour et al., 2009; Dickerson et al., 2009): entorhinal cortex, inferior temporal gyrus, temporal pole, inferior parietal cortex, superior parietal lobe, supramarginal gyrus, precuneus, superior frontal gyrus, and parsopercularis. Descriptive statistics for the mean cortical thickness measure can be found in Table 1.
PET imaging
A Siemens ECAT EXACT HR + PET scanner was used to collect beta-amyloid using the C11-PIB tracer. Before injection, 10-min transmission scans for attenuation correction were collected. After injection of 8.5–15 mCi PIB, 60-min of dynamic data were acquired in 3D acquisition mode. Data were manually evaluated and corrected for motion. Then, a mean image was created by averaging across the first 8 min of data acquisition and used for co-registration. Each PET image was coregistered to that subjects T1 Freesurfer processed structural image and mapped into native PET space. The native space labels were then used to make ROI measurements computed using the Logan plot method with cerebellar grey matter as the reference region. The same atlas for extracting cortical thickness was used to extract mean DVR values from ROIs (i.e., the Desikan-Killiany atlas). Specifically, left and right precuneus ROIs were extracted as a proxy measure for beta-amyloid load. This region was chosen because it accumulates beta-amyloid early in AD (Jagust, 2009), shows reliable PIB binding (Mintun et al., 2006; Rowe et al., 2007), has high inter-rater reliability (Rosario et al., 2011), and has been found to be highly correlated with ability discrepancy scores (McDonough et al. 2016). Descriptive statistics for the beta-amyloid can be found in Table 1.
Statistical analysis
Fluid and crystallized measures were calculated using factor analysis. Specifically, the seven neuropsychological measures were z-transformed and submitted to an exploratory factor analysis using direct oblimin rotation (Kline, 1994). The number of factors was chosen based on a Parallel Analysis (Valle et al., 1999). This method consists of creating factor analysis models based on two matrices. The first matrix is composed of the original data and the second matrix is a random and uncorrelated data with the same number of observations and variables as the original data set. A correlation matrix is then computed to determine the eigenvalues for each of the two matrices. When the eigenvalues from the random matrix are smaller than the eigenvalues from the factor analysis in the original data, it is assumed that those factors stem mostly from noise in the data.
Using the factor analysis results as a guide, we then averaged each of the standardized measures together to form each composite score. These composite scores were then used to create the average fluid ability score and the domain ability scores (including the crystallized ability score). Discrepancy scores for each subject were calculated by subtracting each fluid composite score from the crystallized composite score. Greater scores represented a larger discrepancy in ability, representing a precipitous decline in cognitive functioning (e.g., Kaufman, 1990; Matarazzo & Herman, 1985; Schretlen et al., 1994).
To better understand the independence of each of the ability discrepancy subdomains. Pearson correlations were first calculated between each of the ability discrepancy scores. To replicate and extend the ability discrepancy results (averaged across all fluid measures), multivariate analyses of covariance (MANCOVAs) were conducted using the “manova” package in R. This analysis was chosen because we had two dependent variables (beta-amyloid and mean cortical thickness), thus making testing more efficient by having a single multivariate statistic to test significance. In this analysis, the average ability discrepancy score (averaging across all fluid domains) and the squared average ability discrepancy score served as the primary independent variables to test for linear and quadratic relationships, respectively. The covariates that were included in the model included chronological age, race (non-White = 1, White = 0), sex (female = 1, male = 0), and Geriatric Depression Scale score (continuous). The two dependent variables were beta-amyloid and averaged cortical thickness. If the multivariate statistic was significant, the results were then broken down by univariate tests of each independent variable. Following the analysis of the average ability discrepancy, the same MANCOVAs were conducted separately for each ability discrepancy subdomain (speed, episodic memory, and executive function). Specifically, each subdomain and its squared term served as the independent variables, beta-amyloid and cortical thickness were the dependent variables, and the same covariates were included. Lastly, to characterize any quadratic effects, spline regression was used to find the point (or knot) in which a large change in the direction of the effects begin to occur. The points of change in the regression line were calculated using the “spline” package in R.
Results
Factor analysis of the neuropsychological assessment
Based on the intersection of the simulated eigenvalues and actual eigenvalues, the factor analysis yielded four factors that cumulatively explained 70.2% of the variance in the data (see Table 2). The first factor was labeled executive function (Trails B and Trails B – Trails A) and explained 20.9% of the variance. The second factor was labeled episodic memory (logical memory immediate and delayed) and explained 17.3% of the variance. The third factor was labeled crystallized ability (AMNART, Boston Naming Test, Category Fluency, and FAS) and explained 18.3% of the variance. The final factor was labeled processing speed/attention (Trails A and Digit Symbol Coding) and explained 13.7% of the variance. Note that the Digit Symbol Coding task had similar loadings to the crystallized factor. An average fluid composite score was then calculated by taking the mean of the three fluid factors (executive function, episodic memory, and processing speed). Consistent with prior work (e.g., Cattell, 1965; Kaufman et al., 1996), the crystallized ability and average fluid ability scores were positively correlated with one another, r(264) = 0.58, p < 0.001.
Table 2.
Factor Loadings for Exploratory Factor Analysis of Cognitive Variables.
| Measure | Factor 1 (Executive Function) | Factor 2 (Episodic Memory) | Factor 3 (Crystallized Ability) | Factor 4 (Speed/Attention) |
|---|---|---|---|---|
| Trails B-A | 1.030 | −0.004 | −0.006 | −0.107 |
| Trails B | 0.928 | −0.002 | −0.006 | 0.154 |
| Logical Memory Delayed | 0.009 | 0.992 | −0.023 | 0.008 |
| Logical Memory Immediate | −0.011 | 0.825 | 0.031 | 0.010 |
| FAS | −0.017 | −0.045 | 0.821 | 0.101 |
| Category Fluency | 0.041 | 0.050 | 0.681 | −0.077 |
| AMNART | −0.114 | 0.086 | 0.497 | −0.163 |
| Boston Naming Test | −0.187 | 0.084 | 0.313 | −0.175 |
| Digit Symbol | −0.060 | 0.067 | 0.396 | −0.358 |
| Trails A | 0.013 | 0.006 | −0.002 | 0.993 |
Note. Numbers in bold represent the highest loading measures for each factor.
Calculating ability discrepancy scores and partitioning groups
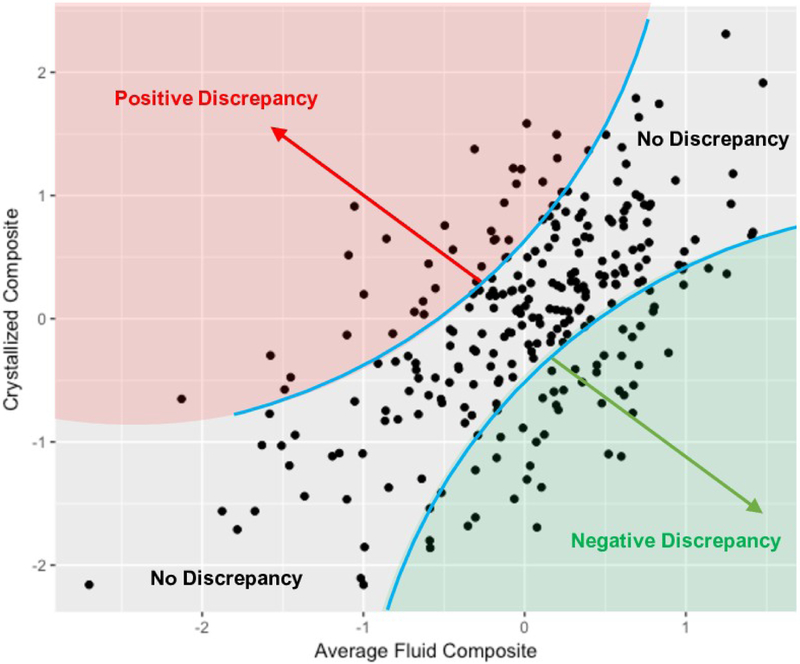
Subtracting the average fluid composite score from the crystallized ability score resulted in an ability discrepancy score for each subject that ranged from −1.77 to 1.97. We propose that higher scores represent a greater risk for AD. The relationship between traditional measures of fluid and crystallized ability and discrepancy scores can be found in Figure 1, which also highlights that an ability discrepancy is conceptually independent of overall (mean) ability level. Table 4 contains the correlations between each of the discrepancy measures. The discrepancy scores in the primary subdomains highly correlated with the total discrepancy score (r’s ranged from .69 - .76), whereas the discrepancy subdomains exhibited small to moderate correlations among each other (r’s ranged from .27 - .32), suggesting that each domain exhibited a moderate degree of independence.
Figure 1.
Scatterplot of the average fluid ability composite against the crystallized ability composite. Both fluid and crystallized composite scores are on a standardized scale. A clear positive relationship between fluid and crystallized ability can be seen. We argue that Quadrants I and III indicate people with the cognitively healthy range. In contrast, people within Quadrants II and IV are at risk for abnormal cognitive functioning. Of most relevance here, individuals with a positive discrepancy (in red) might be in the preclinical Alzheimer’s disease stage because they demonstrate abnormally low fluid abilities given their level of crystallized ability.
Table 4.
MANCOVA Results as a function of Ability Discrepancy on AD Markers
| Average | Speed | Memory | Executive Function | |||||
|---|---|---|---|---|---|---|---|---|
| Factor | Pillai’s Trace | F (2,254) | Pillai’s Trace | F (2,254) | Pillai’s Trace | F (2,254) | Pillai’s Trace | F (2,254) |
| Ability Discrepancy | .039 | 5.16** | .041 | 5.44** | .061 | 8.18*** | .006 | .77 |
| Ability Discrepancy Squared | .028 | 3.71* | .003 | .39 | .020 | 2.55 | .041 | 5.50** |
| Age | .090 | 12.53*** | .094 | 13 24*** | .086 | 11.99*** | .088 | 12 18*** |
| Race (White) | .109 | 15.48*** | .116 | 16.68*** | .097 | 13.60*** | .098 | 13.84*** |
| Sex (Male) | .016 | 2.07 | .015 | 1.89 | .025 | 3.25* | .019 | 2.51 |
| GDS | .004 | .52 | .003 | .39 | .003 | .36 | .003 | .41 |
Notes.
p<.05;
p<.01;
p<.001;
GDS = Geriatric Depression Scale.
Linear and quadratic associations with average ability discrepancy
Across the whole sample, we conducted a MANCOVA with both linear and quadratic effects of the average ability discrepancy on beta-amyloid and cortical thickness, controlling for chronological age, sex, race, and depression. This analysis resulted in a significant multivariate linear effect of ability discrepancy (Pillai’s Trace = .039, F (2,254) = 5.16, p = .0064) and a quadratic effect of ability discrepancy (Pillai’s Trace = .028, F (2,254) = 3.71, p = .026). Significant covariates included an effect of age (Pillai’s Trace = .090, F (2,254) = 12.53, p < .0001) and an effect of race (Pillai’s Trace = .11, F (2,254) = 15.48, p < .0001). Table 4 shows the full multivariate results.
Univariate analyses stemming from the MANCOVA can be found in Table 5. A linear effect of average ability discrepancy was found for beta-amyloid, F(1,255) = 6.87, p = .009, and a quadratic effect was found for cortical thickness, F(1,255) = 7.42, p = .007. As can be seen in Figure 2, a larger average ability discrepancy was associated with a greater accumulation of beta-amyloid. At the same time, a larger average ability discrepancy was also associated with greater cortical thickness, but only for lower (mostly negative) ability discrepancy scores (fluid > crystallized). For higher (positive) scores (crystallized > fluid), a larger discrepancy was associated with smaller cortical thickness.2 This inverted-U pattern is reminiscent of the results found in McDonough et al. (2016). Using spline regression techniques, we determined that the discrepancy score at which this relationship changed was 0.71. Thus, it appears as if a threshold must be reached before an ability discrepancy score is sensitive to neurodegeneration in the AD signature regions. Note that the strong covariates effects in the MANCOVA were driven by cortical thickness: age was strongly negatively correlated with cortical thickness, F(1,255) = 25.14, p < .0001, and being White was associated with greater cortical thickness, F(1,255) = 22.74, p < .0001. A marginal effect of race was found for beta-amyloid, suggesting a slightly more elevated level of beta-amyloid for Whites than Blacks, F(1,255) = 3.22, p = .074.
Table 5.
Univariate ANCOVA Results as a function of Ability Discrepancy on AD Markers
| Beta-Amyloid | Cortical Thickness | |||
|---|---|---|---|---|
| Factor | Sum Squares | F(1,255) | Sum Squares | F(1,255) |
| Average Ability Discrepancy | .45 | 6.87** | .02 | 1.59 |
| Average Ability Discrepancy Squared | .04 | .59 | .09 | 7.42** |
| Speed Ability Discrepancy | .67 | 10.39*** | .00 | .001 |
| Speed Ability Discrepancy Squared | .05 | .69 | .00 | .012 |
| Memory Ability Discrepancy | .19 | 2.83 | .12 | 10.43*** |
| Memory Ability Discrepancy Squared | .02 | .24 | .06 | 5.12* |
| Executive Function Ability Discrepancy | .07 | 1.05 | .01 | .83 |
| Executive Function Ability Discrepancy Squared | .17 | 2.63 | .07 | 6.23* |
Notes.
p<.05;
p<.01;
p<.001;
Figure 2.
Scatter plot and spline regressions for the average ability discrepancy score. A linear and positive relationship was found for the average ability discrepancy score and beta-amyloid (left plot). A quadratic relationship was found for the average ability discrepancy score and cortical thickness (right plot) such that there is a slight positive trend at mostly negative levels of ability discrepancy (in green), but a large negative relationship at mostly positive levels of ability discrepancy (in red).
Linear and quadratic associations with ability discrepancy within subdomains
Separate MANCOVAs were calculated for each ability discrepancy subdomain: speed/attention, episodic memory, and executive function (Table 4). As with the average ability discrepancy, these subdomain discrepancies are conceptually independent from their mean level counterpart. Overall, the patterns were similar in these discrepancies within subdomains as compared with the average discrepancy results. The one exception, as described below, was in regard to the speed/attention discrepancy, which did not show a relationship with cortical thickness.
For the speed ability discrepancy, we found a significant multivariate linear effect of ability discrepancy (Pillai’s Trace = .041, F (2,254) = 5.44, p = .0048). No quadratic effect was found (p = .68). Significant covariates included an effect of age (Pillai’s Trace = .094, F (2,254) = 13.24, p < .0001) and an effect of race (Pillai’s Trace = .116, F (2,254) = 16.68, p < .0001). Breaking down these multivariate effects (Table 5) revealed a significant linear speed discrepancy effect on beta-amyloid, F(1,255) = 10.39, p = .001, but no significant effects on cortical thickness (p=.97 and p=.92, for linear and quadratic effects, respectively). Because no quadratic trends were found for the speed discrepancy score, no spline regressions were conducted.
For the memory discrepancy, we found a significant multivariate linear effect of ability discrepancy (Pillai’s Trace = .061, F (2,254) = 8.18, p < .0001). A marginal quadratic effect was found (Pillai’s Trace = .020, F (2,254) = 2.55, p = .080). Significant covariates included an effect of age (Pillai’s Trace = .086, F (2,254) = 11.99, p < .0001) and an effect of race (Pillai’s Trace = .097 F (2,254) = 13.60, p < .0001). Breaking down these multivariate effects revealed a marginal linear memory discrepancy effect on beta-amyloid, F(1,255) = 2.83, p = .094, and a significant quadratic effect on cortical thickness, F(1,255) = 5.12, p = .025. Spline regressions indicated that the discrepancy score at which this relationship with cortical thickness changed was 1.00, which was slightly greater than the average ability score.
For the executive function ability discrepancy, we found no multivariate linear effect of ability discrepancy (p = .77), but we did find a significant quadratic effect (Pillai’s Trace = .041, F (2,254) = 5.50, p = .0046). Significant covariates included an effect of age (Pillai’s Trace = .088, F (2,254) = 12.18, p < .0001) and an effect of race (Pillai’s Trace = .098, F (2,254) = 13.84, p < .0001). Breaking down these multivariate effects revealed no significant effects on beta-amyloid (p=.31 and p=.11 for linear and quadratic effects, respectively). A significant quadratic effect was found for executive function discrepancy on cortical thickness, F(1,255) = 6.23, p = .013. Spline regressions indicated that the discrepancy score at which this relationship with cortical thickness changes was .63, which was slightly smaller than the average ability score.
Sensitivity Analyses
We next conducted sensitivity analyses a) to address the unique explanatory power that an ability discrepancy score has compared to other cognitive indices, and b) to better understand the role that the specific cognitive tasks play in defining the crystallized ability factor.
Comparing ability discrepancy and episodic memory to AD biomarkers
As outlined previously, an episodic memory composite score is conceptually independent from the memory discrepancy score used in the earlier analyses, and so we would expect unique effects for each measure. Using two different methods, we next tested the extent that the ability discrepancy measure was more sensitive to AD biomarkers than an episodic memory composite score. First, we conducted the same MANCOVA on the average ability discrepancy score but added the episodic memory composite (immediate and delayed logical memory) as a covariate to determine if an ability discrepancy score (linear and squared) would continue to predict AD biomarkers. As can be seen in Table 6 (left), this analysis resulted in a significant multivariate linear effect of ability discrepancy (Pillai’s Trace = .039, F (2,253) = 5.14, p = .0064) and a quadratic effect of ability discrepancy (Pillai’s Trace = .029, F (2,253) = 3.76, p = .025). The size and significance of these effects were nearly identical to the original analysis when episodic memory was not included in the model, suggesting that an ability discrepancy score predicts AD biomarkers above and beyond traditional episodic memory measures. Notably, the episodic memory score was not significant (p = .135).
Table 6.
MANCOVA Results as a function of Ability Discrepancy and Episodic Memory on AD Markers
| Average Score | Episodic Memory Composite | |||
|---|---|---|---|---|
| Factor | Pillai’s Trace | F (2,253) | Pillai’s Trace | F (2,253) |
| Ability Discrepancy | .039 | 5.14** | .003 | .44 |
| Ability Discrepancy Squared | .029 | 3.76* | .016 | 2.04 |
| Episodic Memory Composite Linear | .016 | 2.02 | - | - |
| Age | .091 | 12.67*** | .095 | 13.40*** |
| Race (White) | .109 | 15.54*** | .128 | 18.74*** |
| Sex (Male) | .016 | 2.07 | .018 | 2.28 |
| GDS | .004 | .52 | .003 | .37 |
Notes.
p<.05;
p<.01;
p<.001;
GDS = Geriatric Depression Scale.
In a second analysis, we conducted a similar MANCOVA but replaced the linear and squared ability discrepancy score with a linear and squared episodic memory composite score (Table 6, right). In this analysis, neither memory scores significantly predicted AD biomarkers (p = .646 and p = .133 for linear and squared scores, respectively). Thus, composite episodic memory scores did not predict AD biomarkers at all in this sample.
Comparing alternative ability discrepancy scores to AD biomarkers
In our factor analysis, we found that the standard measure of premorbid/crystallized ability (i.e., the AMNART) shared a large degree of covariance with other language-based tasks such as the Boston Naming Test and tests of fluency. Consistent with this notion, both knowledge and fluency have been represented as broad domains of crystallized ability (e.g., Lindenberger & Baltes, 1997). Although each of these tasks do involve previously learned knowledge, they also rely on other cognitive processes such as executive function (e.g., Hedden, Lautenschlager, & Park, 2005) and have been shown to decline in preclinical AD (e.g., Twamley et al., 2006). Thus, despite the potential advantage that composite scores have for reducing error-related variance, the crystallized composite score used in the present study may not be ideal if some of these measures show cognitive decline similar to the fluid ability measures.
To address this issue, we created a new ability discrepancy score using the same average fluid composite but replaced the crystallized composite with the single standardized AMNART score (i.e., standardized AMNART – fluid ability composite). We then retested the linear and quadratic relationships between this new ability discrepancy score and the AD biomarkers. This alternative ability discrepancy score was strongly correlated with the original ability discrepancy score, r(263) = .72, p < .001. As can be seen in Table 7, this analysis resulted in a significant multivariate linear effect of ability discrepancy (Pillai’s Trace = .026, F (2,253) = 3.31, p = .038), but not a significant quadratic effect of ability discrepancy (Pillai’s Trace = .016, F (2,253) = 2.05, p = .13). The univariate effects revealed a linear relationship between ability discrepancy and beta-amyloid (p = .040) and a trending quadratic relationship with cortical thickness (p = .077). In summary, using only the AMNART score to represent crystallized ability revealed similar pattern as the crystallized factor score, consistent with the high correlation between the two ability scores. However, the relationships were quite a bit weaker as evidenced by the non-significant quadratic relationship with cortical thickness.
Table 7.
MANCOVA and Univariate Results as a function of Ability Discrepancy using the AMNART only on AD Markers
| Average Scorea | Beta-Amyloid | Cortical Thickness | ||||
|---|---|---|---|---|---|---|
| Factor | Pillai’s Trace | F (2,253) | Sum Squares | F (2,254) | Sum Squares | F (2,254) |
| Ability Discrepancy | .026 | 3.31* | .28 | 4.26* | .01 | 1.17 |
| Ability Discrepancy Squared | .016 | 2.05 | .12 | 1.75 | .04 | 3.16 |
| Age | .091 | 12.60*** | .06 | .97 | .30 | 25.30*** |
| Race (White) | .105 | 14.85*** | .16 | 2.44 | .27 | 22.95*** |
| Sex (Male) | .020 | 2.63 | .14 | 2.06 | .03 | 2.12 |
| GDS | .004 | .45 | .00 | .04 | .01 | .91 |
Notes.
p<.05;
p<.01;
p<.001;
Average ability discrepancy was calculated by subtracting the fluid composite score across all cognitive domains from the standardized AMNART score;
GDS = Geriatric Depression Scale.
We next directly tested the hypothesis that the non-AMNART measures of language measures might provide an alternative subdomain ability discrepancy. As mentioned above, this possibility might be predicted by studies that have found semantic memory to be an early predictor of preclinical AD (e.g., Twamley et al., 2006). We created a subdomain ability score by standardizing and averaging the three language tasks together (Boston Naming, Category Fluency, and Verbal Fluency) to form a language fluid composite and subtracted this composite from the standardized AMNART score. The language discrepancy score was not correlated with the average ability discrepancy score using the crystallized factor, r(263) = .066, p = .29. This initial correlation suggests that the two discrepancy scores are capturing very different sets of variances. The MANCOVA assessing the relationships with AD biomarkers confirm this conclusion (Table 8). Neither the multivariate linear nor quadratic effects approached significance (p’s > .25). The univariate effects revealed non-significant relationships with the language ability discrepancy score and beta-amyloid (p’s > .53) but a trend for a quadratic relationship with cortical thickness (p = .86 and .09 for linear and quadratic effects, respectively). Overall, the language ability discrepancy score showed the poorest predictability of AD biomarkers relative to the other ability discrepancy subdomains. Similarly, Takaiwa et al. (2018) found that their language discrepancy score was one of the poorest indicators of MCI.
Table 8.
MANCOVA and Univariate Results as a function of Language Ability Discrepancy on AD Markers
| Average Scorea | Beta-Amyloid | Cortical Thickness | ||||
|---|---|---|---|---|---|---|
| Factor | Pillai’s Trace | F (2,253) | Sum Squares | F (2,254) | Sum Squares | F (2,254) |
| Language Ability Discrepancy | .002 | .24 | .03 | .38 | .00 | .03 |
| Language Ability Discrepancy Squared | .011 | 1.38 | .02 | .28 | .03 | 2.73 |
| Age | .089 | 12 42*** | .12 | 1.73 | .30 | 24.83*** |
| Race (White) | .110 | 15.70*** | .20 | 3.05 | .28 | 23.79*** |
| Sex (Male) | .018 | 2.27 | .12 | 1.73 | .02 | 1.91 |
| GDS | .004 | .46 | .01 | .08 | .01 | .92 |
Notes.
p<.05;
p<.01;
p<.001;
Average ability discrepancy was calculated by subtracting the language composite score from the standardized AMNART score;
GDS = Geriatric Depression Scale.
Lastly, we tested the extent that differences between verbal and visuospatial abilities might be associated with AD biomarkers. Accordingly, we created standardized composite scores for verbal ability (Logical Memory Immediate, Logical Memory Delayed, FAS, Category Fluency, AMNART, Boston Naming) and visual ability (Trails A, Trails B, Trails B-A, Digit Symbol). A verbal-visual discrepancy score was then calculated such that greater scores represented higher verbal than visual abilities. The direction of these effects was in a similar direction as the original ability discrepancy scores (crystallized > fluid ability) given that all the crystallized measures were verbal and most of the fluid abilities were visuospatial. The verbal-visual discrepancy score was positively correlated with the original ability discrepancy score, r(264) = .52, p < .001. As can be seen in Table 9, this analysis resulted in a significant multivariate linear effect of verb-visual discrepancy (Pillai’s Trace = .031, F (2,254) = 4.10, p = .018), but not a significant quadratic effect of verb-visual discrepancy (Pillai’s Trace = .004, F (2,254) = .49, p = .62). The univariate effects reveal no significant association with beta-amyloid (p=.078 and .34 for linear and quadratic effects, respectively), but a significant (negative) linear association with cortical thickness (p = .01 and .95 for linear and quadratic effects, respectively). Interestingly, when linear and quadratic verb-visual discrepancy terms were entered into the same MANCOVA with linear and quadratic average ability discrepancy scores, the patterns remained the same for both types of discrepancy scores (not shown), suggesting that these two measures might represent different abnormalities. This joint analysis should be interpreted with caution because the measures were not independent.
Table 9.
MANCOVA and Univariate Results as a function of Verbal/Visual Discrepancy on AD Markers
| Average Scorea | Beta-Amyloid | Cortical Thickness | ||||
|---|---|---|---|---|---|---|
| Factor | Pillai’s Trace | F (2,253) | Sum Squares | F (2,254) | Sum Squares | F (2,254) |
| Verbal-Visual Discrepancy | .031 | 4.10* | .21 | 3.14 | .08 | 6.56* |
| Verbal-Visual Discrepancy Squared | .004 | .49 | .06 | .91 | .00 | .00 |
| Age | .080 | 11.04*** | .10 | 1.49 | .26 | 22.07*** |
| Race (White) | .112 | 15.96*** | .23 | 3.57 | .28 | 23.66*** |
| Sex (Male) | .020 | 2.56 | .10 | 1.45 | .03 | 2.71 |
| GDS | .003 | .31 | .00 | .03 | .01 | .63 |
Notes.
p<.05;
p<.01;
p<.001;
Average ability discrepancy was calculated by subtracting a composite of the visual tasks from a composite of the verbal tasks;
GDS = Geriatric Depression Scale.
Discussion
The present study sought to better understand the relationships between ability discrepancy scores and biomarkers for AD. An ability discrepancy score is conceptually independent from a single or composite score of a cognitive domain because it builds in a correction for peak levels of cognitive performance. Beta-amyloid deposition is one requirement for AD diagnosis postmortem (McKhann et al., 2011). Beta-amyloid may be deposited in cognitively healthy aging as well, but to a larger and faster degree in those on the AD trajectory (Sliwinski et al., 1996). Our findings show that average ability discrepancy scores are linearly and positively associated with beta-amyloid deposition in the precuneus, a region commonly associated with AD pathology (e.g., Braak and Braak, 1991). This finding converges with a similar analysis in a different sample conducted by McDonough et al. (2016). In that study, they found a similar relationship with beta-amyloid in both positive (crystallized > fluid) and negative discrepancy scores (fluid > crystallized).
Excessive cortical thinning is another key indicator of the AD process (Dickerson et al., 2009) and is negatively related to beta-amyloid deposition (Dickerson et al., 2009). We found that negative discrepancy scores (fluid > crystallized) showed a numerically positive association with cortical thickness, while positive scores (crystallized > fluid) showed clear negative associations with cortical thickness. This finding suggests that only positive ability discrepancy scores are sensitive to declines in cortical thickness, which are suggestive of neurodegeneration.
The average ability discrepancy score used in the present study differed from that used by a previous study investigating relationships between ability discrepancy scores and AD biomarkers (McDonough et al., 2016). In fact, none of the cognitive tests were the same between the two studies. We believe that this convergence provides strong evidence for the power and flexibility of the concept of a crystallized-fluid ability discrepancy measure. Indeed, large scale studies have shown that the specific tasks used to measure general cognitive abilities are interchangeable and what is important is the underlying construct (for review, see Deary, 2012). Nevertheless, no single measure is “process pure” and we acknowledge that some of the crystallized measures used in the present study not only rely on previously learned knowledge, but also on executive function abilities (e.g., Hedden, Lautenschlager, & Park, 2005) and have been shown to decline in preclinical AD (e.g., Twamley et al., 2006). We first evaluated this issue by assessing the relationship with AD biomarkers and ability discrepancy when only using the AMNART, which is a more acceptable measure of crystallized ability. This analysis yielded similar, albeit weaker, patterns as to when the composite crystallized ability was used, suggesting that using tasks like the Boston Naming Test and the two fluency tasks did not strongly compromise the construction of a crystallized composite. To the extent that those naming tasks would evidence similar relationships with AD biomarkers as the other fluid ability scores, we would expect to find that a language discrepancy score (using the AMNART as the only crystallized measure) would be linearly related to beta-amyloid and quadratically related to cortical thickness. However, neither of these patterns were found. We believe that the most parsimonious explanation is that a similar crystallized factor underlies each of the language measures (i.e., previously learned knowledge) and therefore can be a suitable measure of crystallized ability. This conclusion is also supported by the factor analysis in that other executive function tasks were not highly related to these language measures. We note that other samples may evidence different patterns of inter-correlations and a composite score of vocabulary tests and/or word pronunciation tasks are still preferred to provide a less contested crystallized ability factor.
These relationships provide support for the use of an average ability discrepancy score as a risk factor for the development of AD, potentially indicating that a person has preclinical AD status and provide evidence for differences in staging dependent on the level of discrepancy. According to the newest framework for classifying the different stages of the AD continuum, an individual can be classified with different biomarker profiles that are predictive of syndromal cognitive stages (Jack et al. 2018). Specifically, one can assess the likelihood of having preclinical AD depending on the presence or absence of beta-amyloid (A), tau (T), and neurodegeneration (N). For example, being negative on the three measures, A-T-(N)-, would be associated with a “cognitively healthy” profile. In contrast, four profiles are suggestive of the Alzheimer’s continuum: A+T-(N)-, A+T+(N)-, A+T+(N)+, A+T-(N)+. Our study suggests that a person with a small (but positive) ability discrepancy score (crystallized > fluid), even when mean levels of cognition are in the cognitively healthy range, might be classified as A+T*N-, which represents preclinical Alzheimer’s pathologic change. A person with a large (and positive) ability discrepancy score might be classified as being in either the A+T+(N)+ or A+T-(N)+ categories, which represents preclinical AD or the combination of Alzheimer’s and suspected non-Alzheimer’s pathologic change, respectively.
Importantly, this framework also outlines six stages of clinical and cognitive transitions. Whereas Stage 1 represents the absence of any cognitive decline or neurobehavioral symptoms, Stage 2 represents a point of transition where cognitive performance is within the expected range, but cognition (not necessarily episodic memory) begins to decline. The recommendations to test for such a decline is through subjective report or concern (by the individual or an informant), longitudinal change in cognition over 1–3 years that is persistent for longer than 6 months, or longitudinal change in subjective report of cognition. The present study offers the next step for validating an ability discrepancy to be added as an objective measure that has the potential to capture changes in cognition while having cognitive tests within the cognitively healthy range and that does not require long-term testing. An ability discrepancy measure offers a strong alternative to use for staging given that subjective reports in cognition level and change can be influenced by current levels of well-being, depressive symptoms, anxiety, and even one’s personality (for reviews, see Mitchell et al., 2014; McDonough et al., 2019). Although the ability discrepancy has strong potential, further longitudinal validation is strongly needed.
Our results concerning subdomain ability discrepancy scores also shed light on the relationship between fluid ability and AD biomarkers. We used four cognitive domains as measures of fluid ability: processing speed/attention, episodic memory, executive function, and language (the latter of which only used the AMNART to assess crystallized ability). While each discrepancy subdomain except the language discrepancy was significantly correlated with the average ability discrepancy score, they were not strongly correlated with one another; additionally, they had varying relationships to beta-amyloid deposition and cortical thickness. Episodic memory discrepancy and executive function discrepancy scores showed strong quadratic patterns with cortical thickness, but they showed no significant associations with beta-amyloid. In contrast, weaker or no relationships were found for processing speed discrepancy and language discrepancy. On the one hand, these differences suggest that not all facets of fluid ability have the same relationship to AD biomarkers. On the other hand, the most predictive ability discrepancy score was the average of all fluid domains. Thus, an ability discrepancy score averaging across many fluid domains might be the most robust and reliable measure to detect individuals in the preclinical AD stage instead of using subdomain ability discrepancy scores. Indeed, it has been argued that composite scores might be more sensitive to a diverse constellation of symptoms and that solely relying on episodic memory may miss critical declines in cognition associated with AD (Jack et al., 2012; Mortamais et al., 2017).
When comparing the cut-off scores within each cognitive subdomain, one might be tempted to interpret the executive function discrepancy as being the most sensitive to cortical thickness differences because the cut-off score was the closest to 0, meaning that only slight deviations in the discrepancy score are sensitive to neurodegeneration. While this might be true, more research is needed to bolster confidence in this interpretation. First, executive function is a broad domain of cognition and only the Trails Making Test, which is closely tied to task switching abilities, was used to infer this ability. This finding is consistent with prospective studies that have found the Trails Making Test to differentiate preclinical AD from healthy controls (Blacker et al., 2007; Chen et al., 2001; Rajan et al., 2015; Rapp et al., 2005). A recent review on cognitive differences from healthy aging to AD also indicated that executive function was likely to become impaired in the AD trajectory more than other attentional measures (McDonough, Wood, & Miller, 2019). It could be the case that breakdowns in executive function lead to downstream consequences of poor episodic memory (Mortamais et al., 2017).
In the present study, we also found that relying on episodic memory composite would not have detected elevations in amyloid or declines in cortical thickness. While an ability discrepancy score, especially when averaging across subdomains of cognition, might not be able to specify exact levels of beta-amyloid nor provide regional variation in cortical thickness declines, it might be used as an inexpensive and sensitive behavioral measure for determining who is at risk for being in the preclinical stages of AD. To be used most effectively in clinical settings, additional research is needed to create cut-off scores based on age-adjusted population norms. In this way, clinicians could take advantage of commonly collected neuropsychological tests and compare a patient’s standardized discrepancy score with the population to determine their risk of AD. Of course, to the extent that this measure can be combined with other inexpensive measures (e.g., odor detection or EEG), neuroimaging markers may not be necessary for at least basic recommendations to engage in serious lifestyle changes to reduce risks for developing dementia.
On a conceptual level, using an ability discrepancy rather than a single or composite measure of cognition fundamentally alters how researchers and clinicians might think about how to best distinguish between cognitively healthy and at-risk individuals. At early stages of the AD process, many people might be miscategorized as being at risk for dementia instead of as being cognitively healthy. For example, Figure 1 reveals that there was one individual with an average fluid composite score more than 2.5 standard deviations away from the mean (lower left point in the scatterplot), which according to some calculations, would classify this person as having possible (multi-domain) MCI and being at high risk for developing dementia. However, that same individual also had a crystallized ability score that was more than 2 standard deviations below the mean, suggesting that while some decline in fluid abilities might be present, this person might actually fall within a range of cognition healthy for them. The opposite also is true. A different individual in the sample would be classified as “healthy” using the average fluid composite score because their average fluid ability score fell within healthy range (i.e., −0.31 standard deviations from the mean). However, because this individual also had a high crystalized ability score (1.38), their discrepancy score of 1.69 is suggestive of a high level of beta-amyloid accumulation and neurodegeneration (far surpassing the .71 spline regression threshold). While all the participants in this sample had a CDR score of 0 and were classified as cognitively healthy, this person might be in late preclinical stages and soon diagnosed with MCI according to their ability discrepancy score.
Conclusion
Understanding the differences between healthy and abnormal aging is vital to ensuring improved quality of life for older adults. This study provides novel research into understanding how a discrepancy between fluid and crystallized ability might be an indicator of preclinical AD and fundamentally challenges who we consider to be cognitively healthy. Such a measure takes advantage of the reliability of ability measures across the lifespan (Deary, Alison, & Starr, 2013; Deary, Whalley, Lemmon, Crawford, & Starr, 2000) and conceptually serves as a proxy for within-subject change in cognition beyond the effects of declining cognition with age. By using this measure alone or in conjunction with other measures, detection of preclinical AD might be improved and the question of who might be targeted for preclinical AD treatments can be better understood.
Figure 3.
Scatter plot and spline regressions for each ability discrepancy subdomain score. Positive linear trends were found for each ability discrepancy subdomains and beta-amyloid (left plots) including speed (top plot), episodic memory (middle plot), and executive function (bottom plot). However, only the speed subdomain showed a significant relationship. Quadratic relationships were found for the memory discrepancy and executive function discrepancy subdomains (right plots) such that there is a slight positive trend at mostly negative levels of each ability discrepancy subdomain (in green), but a large negative relationship at mostly positive levels of each ability discrepancy subdomain (in red).
Table 3.
Correlation Matrix as a Function of Discrepancy Ability Type and Logical Memory Composite.
| Discrepancy Score Type | 1. | 2. | 3. | 4. | 5. | 6. | 7. |
|---|---|---|---|---|---|---|---|
| 1. Average Ability Discrepancy | - | ||||||
| 2. Speed Discrepancy | .69* | - | |||||
| 3. Memory Discrepancy | .76* | .29* | - | ||||
| 4. Executive Function Discrepancy | .72* | .31* | .27* | - | |||
| 5. Language Discrepancya | .07 | .08 | .03 | .04 | - | ||
| 6. Verbal-Visual Discrepancy | .52* | .71* | −.14 | .68* | −.04 | - | |
| 7. Logical Memory Composite | −.34* | .04 | −.69* | .00 | .06 | .33* | - |
Notes.
p < .001;
Only the AMNART was used to represent crystallized ability in this discrepancy score.
Public Significance Statement:
Without a cure for Alzheimer’s disease or related dementias, lifestyle modifications for older adults at risk for developing are the only option to prevent or delay these diseases. The present study provided further validation for an inexpensive and non-invasive cognitive measure that might help detect risk for dementia by showing the relationship with this measure and brain markers of Alzheimer’s disease. This measure should serve as a proxy for longitudinal cognitive change and provides the foundation for creating a standardized method that can be implemented in memory clinics.
Footnotes
One might wonder whether measuring years of education would be a sufficient and easier measure of premorbid or peak levels of cognitive ability. Several reasons argue against this idea. First, an objective measure of ability assesses life-long experiences and habits (e.g., reading, cognitive engagement), not only those accrued during formative years of education. Second, at a given level of education (e.g., college level), the cognitive ability between individuals can still vary greatly, thus making an objective test using more fine-grained differentiation a more sensitive measure to differences in ability. Lastly, research indicates the years of education mask differences in the quality of education, which is better captured by tests that measure crystallized ability (Crowe et al., 2012; Liu et al., 2015).
Years of education was weakly (but significantly) correlated with the average ability discrepancy score, r(264) = .19, p = .002). Entering years of education as an additional covariate did not change the significance or strength of the results.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, & Snyder PJ (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, … & Johnson KA (2012). Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia, 50(12), 2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, & Dickerson BC (2009). The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology 72, 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB, Cornelius SW, Spiro A, Nesselroade JR, & Willis SL (1980). Integration versus differentiation of fluid/crytallized intelligence in oldage. Developmental Psychology, 16(6), 625. [Google Scholar]
- Bastin C, & Salmon E (2014). Early neuropsychological detection of Alzheimer’s disease. Eur. J. Clin. Nutr 68, 1192–1199. [DOI] [PubMed] [Google Scholar]
- Benton AL (1983). Contributions to Neuropsychological Assessment: A Clinical Manual. Oxford University Press, New York. [Google Scholar]
- Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, … & Albert M (2007). Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Archives of neurology, 64(6), 862–871. [DOI] [PubMed] [Google Scholar]
- Blair JR, & Spreen O (1989). Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin. Neuropsychol 3, 129–136. [Google Scholar]
- Boraxbekk C-J, Lundquist A, Nordin A, Nyberg L, Nilsson L-G, & Adolfsson R (2015). Free recall episodic memory performance predicts dementia ten years prior to clinical diagnosis: Findings from the Betula Longitudinal Study. Dementia and Geriatric Cognitive Disorders, 5(2), 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, & Braak E (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol, 82, 239–259. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Nagel IE, Preuschhof C, Gluth S, Bӓckman L, Li S, Lindenberger U, & Heekeren HR (2011). Cortical thickness is linked to executive functioning in adulthood and aging. Human Brain Mapping, 33(7), 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, & Munafò MR (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience, 14(5), 365. [DOI] [PubMed] [Google Scholar]
- Cattell RB (1971). Abilities: Their structure, growth, and action. Boston, MA: Houghton Mifflin. [Google Scholar]
- Cattell RB (1965). The Scientific Analysis of Personality. Penguin Books, Baltimore. [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, & Ganguli M (2001). Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Archives of general psychiatry, 58(9), 853–858. [DOI] [PubMed] [Google Scholar]
- Christensen H, & Mackinnon A (1993). The association between mental, social and physical activity and cognitive performance in young and old subjects. Age and Ageing, 22(3), 175–182. [DOI] [PubMed] [Google Scholar]
- Crowe M, Clay OJ, Martin RC, Howard VJ, Wadley VG, Sawyer P, & Allman RM (2012). Indicators of childhood quality of education in relation to cognitive function in older adulthood. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 68(2), 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WR (1980). Age comparative factor analysis of ability variables in adulthood and old age. Intelligence, 4(2), 133–149. [Google Scholar]
- Cunningham WR, Clayton V, & Overton W (1975). Fluid and crystallized intelligence in young adulthood and old age. Journal of Gerontology, 30(1), 53–55. [DOI] [PubMed] [Google Scholar]
- Dagley A, LaPoint M, Huijbers W, Hedden T, McLaren DG, Chatwal JP, Papp KV, Amariglio RE, Blacker D, Rentz DM, Johnson KA, Sperling RA, & Schultz AP (2017). Harvard Aging Brain Study: Dataset and accessibility. NeuroImage 144B, 255–258. 10.1016/j.neuroimage.2015.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ (2012). Intelligence. Annual Review of Psychology, 63, 453–482. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Alison P, & Starr JM (2013). The stability of intelligence from age 11 to age 90 years: the Lothian birth cohort of 1921. Psychol Sci. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whalley LJ, Lemmon H, Crawford JR, & Starr JM (2000). The stability of individual differences in mental ability from childhood to old age: follow-up of the 1932 Scottish Mental Survey. Intelligence, 28, 49–55. [Google Scholar]
- Deckers K, van Boxtel MPJ, Schiepers OJG, de Vugt M, Sánchez JLM, Anstey KJ, Brayne C, Dartigues J-F, Engedal K, Kivipelto M, Ritchie K, Starr JM, Yaffe K, Irving K, Verhey FRJ, & Köhler S (2015). Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. International Journal of Geriatric Psychiatry, 30(3), 234–246. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, & Sperling RA (2009). The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex 19, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierckx E, Engelborghs S, De Raedt R, Van Buggenhout M, De Deyn PP, Verleye G, Verte D, & Ponjaert-Kristoffersen I (2008). Differentiation between dementia and depression among older persons: can the difference between actual and premorbid intelligence be useful? J. Geriatr. Psychiatry Neurol 21, 242–249. [DOI] [PubMed] [Google Scholar]
- Doherty BM, Schultz SA, Oh JM, Koscik RL, Dowling NM, Barnhart TE, … & LaRue A (2015). Amyloid burden, cortical thickness, and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 1(2), 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisdorfer C, Busse EW, & Cohen LD (1959). The WAIS performance of an aged sample: The relationship between verbal and performance IQs. Journal of gerontology, 14(2), 197–201. [DOI] [PubMed] [Google Scholar]
- Ekstrom R, French J, Harman H, & Dermen D (1976). Manual for Kit of Factor Referenced Cognitive Tests. Educational Testing Service, Princeton. [Google Scholar]
- Fine EM, Delis DC, Wetter SR, Jacobson MW, Jak AJ, McDonald CR, … & Bondi MW (2008). Cognitive discrepancies versus APOE genotype as predictors of cognitive decline in normal-functioning elderly individuals: a longitudinal study. The American Journal of Geriatric Psychiatry, 16(5), 366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, & Winblad B (2004). An active and socially integrated lifestyle in late life might protect against dementia. The Lancet Neurology, 3(6), 343–353. [DOI] [PubMed] [Google Scholar]
- Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, & Kawas C (2008). Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Society, 14(2), 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Lautenschlager G, & Park DC (2005). Contributions of processing ability and knowledge to verbal memory tasks across the adult life-span. The Quarterly Journal of Experimental Psychology Section A, 58(1), 169–190. [DOI] [PubMed] [Google Scholar]
- Hedden T, Oh H, Younger AP, & Patel TA (2013). Meta-analysis of amyloidcognition relations in cognitively normal older adults. Neurology 80, 1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston WS, Delis DC, Lansing A, Jacobson MW, Cobell KR, Salmon DP, & Bondi MW (2005). Executive function asymmetry in older adults genetically at-risk for Alzheimer’s disease: verbal versus design fluency. Journal of the International Neuropsychological Society, 11(7), 863–870. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP (2005). Why most published research findings are false. PLoS Med 2 (124). doi: 10.1371/journal.pmed.0020124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, … & Roberts RO (2012). An operational approach to National Institute on Aging–Alzheimer’s Association criteria for preclinical Alzheimer disease. Annals of neurology, 71(6), 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, … & Lesnick TG (2013). Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. The Lancet Neurology, 12(2), 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MW, Delis DC, Bondi MW, & Salmon DP (2002). Do neuropsychological tests detect preclinical Alzheimer’s disease: Individual-test versus cognitive-discrepancy score analyses. Neuropsychology, 16(2), 132. [DOI] [PubMed] [Google Scholar]
- Jacobson MW, Delis DC, Bondi MW, & Salmon DP (2005). Asymmetry in auditory and spatial attention span in normal elderly genetically at risk for Alzheimer’s disease. Journal of clinical and experimental neuropsychology, 27(2), 240–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W (2009). Amyloid+ activation= Alzheimer’s?. Neuron, 63(2), 141–143. [DOI] [PubMed] [Google Scholar]
- Johnson W, Bouchard TJ, Krueger RF, McGue M, & Gottesman II (2004). Just one g: consistent results from three test batteries. Intelligence 32, 95–107. [Google Scholar]
- Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, … & Hall LT (2014). Amyloid burden and neural function in people at risk for Alzheimer’s disease. Neurobiology of Aging, 35(3), 576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, & Fratiglioni L (2006). Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dementia and geriatric cognitive disorders, 21(2), 65–73. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (1983). The Boston Naming Test: Assessment of aphasia and related disorders. Philadelphia, PA: Lea & Febiger, 2. [Google Scholar]
- Kaszniak AW (1986). The neuropsychology of dementia In: Grant I, & Adams KM (Eds.), Neuropsychological Assessment of Neuropsychiatric Disorders. Oxford University Press, Oxford, pp. 172–220. [Google Scholar]
- Kaufman AS, Kaufman JC, Liu X, & Johnson CK (2009). How do educational attainment and gender relate to fluid intelligence, crystallized intelligence, and academic skills at ages 22–90 years? Archives of Clinical Neuropsychology, 24(2), 153–163. [DOI] [PubMed] [Google Scholar]
- Kaufman AS (1990). Assessing Adolescent and Adult Intelligence Allyn and Bacon, Boston. [Google Scholar]
- Kaufman AS, McLean JE, & Lincoln A (1996). The relationship of the Myers-Briggs Type Indicator (MBTI) to IQ level and the fluid and crystallized IQ discrepancy on the Kaufman Adolescent and Adult Intelligence Test (KAIT). Assessment, 3(3), 225–239. [Google Scholar]
- Kline P (1994). An Easy Guide to Factor Analysis. Routledge, New York. [Google Scholar]
- Lezak MD, 1995. Neuropsychological Assessment, third ed Oxford University Press, New York. [Google Scholar]
- Lindenberger U, & Baltes PB (1997). Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychology and Aging, 12(3), 410. [DOI] [PubMed] [Google Scholar]
- Liu SY, Manly JJ, Capistrant BD, & Glymour MM (2015). Historical differences in school term length and measured blood pressure: contributions to persistent racial disparities among US-born adults. PloS one, 10(6), e0129673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, … & Cooper C (2017). Dementia prevention, intervention, and care. The Lancet, 390(10113), 2673–2734 [DOI] [PubMed] [Google Scholar]
- Matarazzo JD, & Herman DO (1985). Clinical uses of the WAIS-R: Base rates of differences between VIQ and PIQ in the WAIS-R standardization sample Handbook of intelligence: Theories, measurements, and applications, 899–932. [Google Scholar]
- McCarthy F, Burns WJ, & Sellers AH (2005). Discrepancies between premorbid and current IQ as a function of progressive mental deterioration. Percept. Mot. Skills 100, 69–76. [DOI] [PubMed] [Google Scholar]
- McDonough IM, Bischof GN, Kennedy KM, Rodrigue KM, Farrell ME, & Park DC (2016). Discrepancies between fluid and crystallized ability in healthy adults: a behavioral marker of preclinical Alzheimer’s disease. Neurobiology of aging, 46, 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough IM, McDougall GJ, LaRocca M, Dalmida SG, & Arheart KL (2019). Refining the metamemory in adulthood questionnaire: a 20-item version of change and capacity designed for research and clinical settings. Aging & mental health, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough IM, Wood MM, & Miller WS Jr (2019). Focus: Attention Science: A Review on the Trajectory of Attentional Mechanisms in Aging and the Alzheimer’s Disease Continuum through the Attention Network Test. The Yale Journal of Biology and Medicine, 92(1), 37. [PMC free article] [PubMed] [Google Scholar]
- McHugh RB, & Owens WA (1954). Age changes in mental organization—a longitudinal study. Journal of Gerontology, 9, 296–302. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, … & Mohs RC (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia, 7(3), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, … & Morris JC (2006). [11C] PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology, 67(3), 446–452. [DOI] [PubMed] [Google Scholar]
- Mistridis P, Krumm S, Monsch AU, Berres M, & Taylor KI (2015). The 12 years preceding mild cognitive impairment due to Alzheimer’s disease: the temporal emergence of cognitive decline. Journal of Alzheimer’s Disease, 48(4), 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, & Stubbs B (2014). Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatrica Scandinavica, 130(6), 439–451. [DOI] [PubMed] [Google Scholar]
- Mortamais M, Ash JA, Harrison J, Kaye J, Kramer J, Randolph C, … & Ritchie K (2017). Detecting cognitive changes in preclinical Alzheimer’s disease: A review of its feasibility. Alzheimer’s & Dementia, 13(4), 468–492. [DOI] [PubMed] [Google Scholar]
- O’Carroll RE, & Gilleard CJ (1986). Estimation of premorbid intelligence in dementia. Br. J. Clin. Psychol 25, 157–158. [DOI] [PubMed] [Google Scholar]
- O’Shea DM, Fieo R, Woods A, Williamson J, Porges E, & Cohen R (2018). Discrepancies between crystallized and fluid ability are associated with frequency of social and physical engagement in community dwelling older adults. Journal of Clinical and Experimental Neuropsychology, 1–8. [DOI] [PubMed] [Google Scholar]
- Open Science Collaboration (2015). Estimating the reproducibility of psychological science. Science 349(6251, aac4716). doi: 10.1126/science.aac4716 [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, & Smith PK (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and aging, 17(2), 299–320. [PubMed] [Google Scholar]
- Perrotin A, Mormino EC, Madison CM, Hayenga AO, & Jagust WJ (2012). Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Archives of neurology, 69(2), 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitt P (1993). Does it all go together when it goes? The Nineteenth Bartlett Memorial Lecture. The Quarterly Journal of Experimental Psychology, 46(3), 385–434. [DOI] [PubMed] [Google Scholar]
- Rajan KB, Wilson RS, Weuve J, Barnes LL, & Evans DA (2015). Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology, 85(10), 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp MA, & Reischies FM (2005). Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE). The American Journal of Geriatric Psychiatry, 13(2), 134–141. [DOI] [PubMed] [Google Scholar]
- Reinert G (1970). Comparative factor analytic studies of intelligence throughout the human life-span In Life-span developmental psychology (pp. 467–484). Academic Press. [Google Scholar]
- Reitan R (1979). Manual for administration of neuropsychological test batteries for adults and children. Reitan Neuropsychology Laboratories, Tucson, AZ. [Google Scholar]
- Rentz DM, & Weintraub S (2000). Neuropsychological detection of early probable Alzheimer’s disease In Early diagnosis of Alzheimer’s disease (pp. 169–189). Humana Press, Totowa, NJ. [Google Scholar]
- Rosario BL, Weissfeld LA, Laymon CM, Mathis CA, Klunk WE, Berginc MD, … & Price JC (2011). Inter-rater reliability of manual and automated region-of-interest delineation for PiB PET. Neuroimage, 55(3), 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, & Kahn RL (1987). Human aging: usual and successful. Science, 237(4811), 143–149. [DOI] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, … & Smith C (2007). Imaging β-amyloid burden in aging and dementia. Neurology, 68(20), 1718–1725. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, & Paolo AM (1992). A screening procedure for estimating premorbid intelligence in the elderly. The Clinical Neuropsychologist, 6(1), 53–62. [Google Scholar]
- Salthouse TA (2010). Selective review of cognitive aging. Journal of the International neuropsychological Society, 16(5), 754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid NS, Taylor KI, Foldi NS, Berres M, & Monsch AU (2013). Neuropsychological signs of Alzheimer’s disease 8 years prior to diagnosis. J. Alzheimers Dis. 34, 537–546. [DOI] [PubMed] [Google Scholar]
- Schretlen D, Benedict RH, & Bobholz JH (1994). Composite reliability and standard errors of measurement for a seven-subtest short form of the Wechsler Adult Intelligence Scale—Revised. Psychological Assessment, 6(3), 188. [Google Scholar]
- Sliwinski M, Lipton RB, Buschke H, & Stewart W (1996). The effects of preclinical dementia on estimates of normal cognitive functioning in aging. J. Gerontol. B. Psychol. Sci. Soc. Sci 51, 217–225. [DOI] [PubMed] [Google Scholar]
- Song Z, McDonough IM, Liu P, Lu H, & Park DC (2016). Cortical amyloid burden and age moderate hippocampal activity in cognitively-normal adults. NeuroImage: Clinical, 12, 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … & Park DC (2011a). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia, 7(3), 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Jack CR Jr., & Aisen PS (2011b). Testing the right target and right drug at the right stage. Sci. Transl. Med 3, 111–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Mormino E, & Johnson K (2014). The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron, 84(3), 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strite D, Massman PJ, Cooke N, & Doody RS (1997). Neuropsychological asymmetry in Alzheimer’s disease: Verbal versus visuoconstructional deficits across stages of dementia. Journal of the International Neuropsychological Society, 3(5), 420–427. [PubMed] [Google Scholar]
- Takaiwa A, Kuwayama N, Akioka N, Kashiwazaki D, & Kuroda S (2018). Discrepancy analysis between crystallized and fluid intelligence tests: a novel method to detect mild cognitive impairment in patients with asymptomatic carotid artery stenosis. European Journal of Neurology, 25(2), 313–319. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Ropacki SAL, & Bondi MW (2006). Neuropsychological and neuroimaging changes in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Society, 12(5), 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle S, Li W, & Qin SJ (1999). Selection of the number of principal components: the variance of the reconstruction error criterion with a comparison to other methods. Industrial & Engineering Chemistry Research, 38(11), 4389–4401. [Google Scholar]
- Wechsler D (1944). The Measurement of Adult Intelligence. Williams and Wilkins, Baltimore. [Google Scholar]
- Wechsler D (1981). WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; Harcourt Brace Jovanovich. [Google Scholar]
- Wechsler D (1997). Wechsler Adult Intelligence Scale (WAIS-iii). Psychological Corporation, New York. [Google Scholar]
- Weintraub S, Carrillo MC, Farias ST, Goldberg TE, Hendrix JA, Jaeger J, … & Sikkes SA (2018). Measuring cognition and function in the preclinical stage of Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 4, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary RA, & Shipley WC (1986). Shipley Institute of Living Scale: Revised Manual. Western Psychological Services, Los Angeles. [Google Scholar]